Abstract
Background and Objectives: The objective of our clinical trial was to determine the effectiveness of the deep dry needling technique (DDN) (neuromuscular deprogramming) as a first step in the treatment of temporomandibular disorders. Methods and Materials: The double-blind randomized clinical trial comprised 36 patients meeting the inclusion criteria who had signed the corresponding informed consent form. The participants were randomly distributed into two groups, the Experimental group (Group E) and the Control group (Group C). Group E received bilateral DDN on the masseter muscle, while Group C received a simulation of the technique (PN). All the participants were evaluated three times: pre-needling, 10 min post-needling, and through a follow-up evaluation after 15 days. These evaluations included, among other tests: pain evaluation using the Visual Analog Scale (VAS) and bilateral muscle palpation with a pressure algometer; evaluation of the opening pattern and range of the mouth, articular sounds and dental occlusion using T-scans; and electromyography, which was used to evaluate the muscle tone of the masseter muscles, in order to control changes in mandibular position. Results: Digital control of occlusion using Tec-Scan (digital occlusion analysis) showed a significant reduction both in the time of posterior disclusion and in the time needed to reach maximum force in an MI position after needling the muscle, which demonstrated that there were variations in the static position and the trajectory of the jaw. The symmetry of the arch while opening and closing the mouth was recovered in a centric relation, with an increase in the opening range of the mouth after the procedure. Conclusions: facial pain is significantly reduced and is accompanied by a notable reduction in muscle activity after needling its trigger points.
Keywords: temporomandibular dysfunction, deep dry needling trigger points, myofacial pain, randomized clinical trial
1. Background and Objectives
The temporomandibular joint (TMJ), a form of bicondylar diarthrosis that establishes a double connection between the jaw and the temporal bone working symmetrically, is one of the most complex articulations in the body. According to a recent study evaluating historical case series of representative samples of the Spanish population, 12% of adults and elderly patients in Spain experience pain-related symptoms in relation to the muscles of mastication and/or the TMJ [1].
At present, the origin of temporomandibular disorders is considered to be multifactorial, and a series of predisposing or precipitating factors has been described in relation to anatomy, occlusion, parafunction, trauma, and psycho-emotional conditions [2]. These disorders are frequently found to be accompanied by tension-type headaches and other neurological episodes (depression, anxiety) [3]. Furthermore, there is evidence of greater prevalence in women [4], which seems to be a significant prognostic factor in all the historical case series evaluated in Spain in the latest national surveys on oral health [1].
Multiple therapeutic strategies have been implemented to alleviate the pain and discomfort associated with musculoskeletal pathology in the orofacial area, with different degrees of invasiveness (splints, medications (infiltration of corticosteroids), physiotherapy, regenerative infiltrations (although though the effectiveness of platelet concentrates is at the center of a recent academic debate, since injections of Plasma Rich in Growth Factors or PRGF modulate the inflammatory response and have regenerative activity in damaged joint components of the TMJ), or surgery) [5,6,7,8,9,10]. Nevertheless, there is still little certainty on the matter. At present, the most consensual approach is to only treat conditions that impact on quality of life through non-invasive means, except when the general repercussions of the clinical presentation worsen, in which case combined and progressively more invasive strategies should be implemented when previous efforts have failed [11].
In this sense, the dry needling technique could be a promising therapy for presentations with chronic muscle involvement (myalgia, referred myofacial pain, or direct myofacial pain) [12]. This minimally invasive technique is based on the insertion of a low-caliber needle, without any additional substances, into myofascial trigger points, which are irritable nodules of a tensed band composed of hypertonic muscle fibers [12]. Two types of dry needling technique exist, based on the depth to which the needle is inserted: superficial needling, or Baldry’s Technique, in which the needle is inserted up to the subcutaneous cellular tissue overlying the myofacial trigger point; and deep needling, in which the needle is inserted into the muscle with the intention of reaching the myofacial trigger point [13]. The process behind this technique is the generation of controlled microspasms in the affected muscle area, which alternate with periods of muscle relaxation, with various studies supporting its therapeutic effectiveness [14,15,16].
In the orofacial area, several authors have studied the effectiveness of dry needling on the muscles of mastication in order to increase the pain threshold to pressure and to maximize the free-of-pain opening of the mouth [17,18]. The study conducted by Luis-Miguel Gonzalez-Perezse et al., focusing on the application of DDN in the lateral pterygoid muscle, which should always be treated under strict ultrasound control due to its complex accessibility and management, reported a reduction in pain and an improvement in maximum mouth opening mobility, jaw protrusion and laterality [18]. This was in agreement with the results obtained in a different DDN study on the temporal and masseter muscles, where the effects measured immediately after and a week after the procedure were evaluated [19]. However, other authors attributed these effects to placebo, although their small sample size (n = 10 per group) could have limited their statistical inference capacity [20,21].
Our study offers an affordable, swift, and easily applicable alternative, which is useful not only as an isolated treatment for muscle dysfunction, but to facilitate or enable the use of other types of intervention carried out by odontologists, such as the reversion to the baseline physiological position of the jaw in centric relation (CR).
The purpose of this study was to evaluate DDN’s effectiveness in the treatment of myogenous forms of temporomandibular joint disorder by monitoring the activity of the masseter muscle, bite force, mouth opening range and symmetry, as well as changes in the position of the jaw after applying DDN.
2. Materials and Methods
The sample size necessary to undertake our study was calculated with 95% confidence and 80% statistical power.
We conducted a randomized double-blind clinical trial with a total sample size of 36 patients. Subjects in the sample were recruited from patients attending the Clinical Odontology Consultation of the Faculty of Medicine of the University of Salamanca, as well as university students and other external individuals who wished to participate and met the following inclusion criteria: subjects between the ages of 18 and 40 years, with myofacial pain due to temporomandibular dysfunction at the time, diagnosed using the Research Diagnostic Criteria (RDC) [21], who had teeth or carried partial fixed prostheses, and showed signs of temporomandibular articular pathology (without determining the degree of TMJ involvement). Patients had not received any other form of treatment related to TMJ disorders.
The exclusion criteria were: a confirmed or suspected diagnosis of an inflammatory disorder (arthralgia), the presence of an oral or dental infection, a confirmed or suspected diagnosis of neurological disorders, a history of physical trauma in the head or face, intake of anticoagulants or drugs for circulation disorders, allergies to metals, and patients with cognitive and/or communication impairments that could hamper necessary data collection [22].
At the beginning of the study, and in order to verify the inclusion criteria, all participants completed a self-administered questionnaire (ANNEX 1) based on the guidelines of the Research Diagnostic Criteria (RDC) for temporomandibular disorders [23].
2.1. Intervention
Each patient was asked to fill in a specific questionnaire on the level of pain suffered before starting the procedure. Pain was evaluated using a visual analog scale, thus allowing the patients to express their own sensation of pain.
The sample was divided into two groups:
Group E: intervention group, who received the deep dry needling technique.
Group C: placebo group, on whom deep dry needling was not performed.
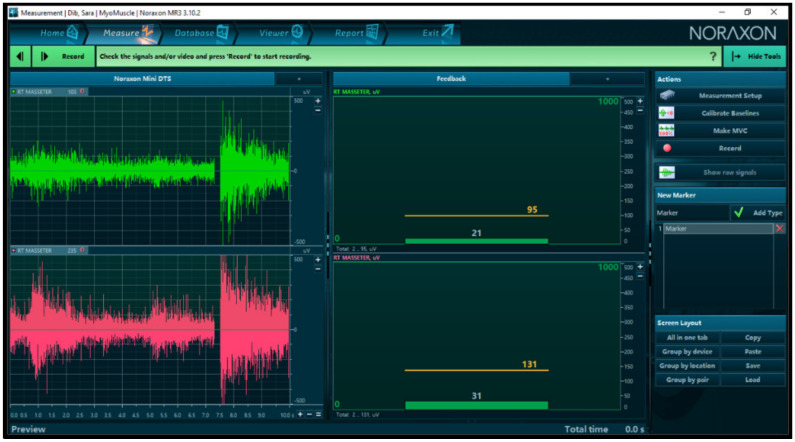
Next, digital occlusal registration was carried out using a T-scan to determine the occlusal contact points, the percentage of occlusal force applied on each tooth, the time needed to reach maximum occlusal force, and the time of posterior disclusion, parameters directly associated with the conditions of the muscles, and which determine postural characteristics at the same time. The electrical activity was registered at baseline and at the maximum intercuspation position (MI) in the masseter muscle after placing the corresponding electrodes in the mandibular angle in parallel to the fibers of the masseter muscle on both sides.
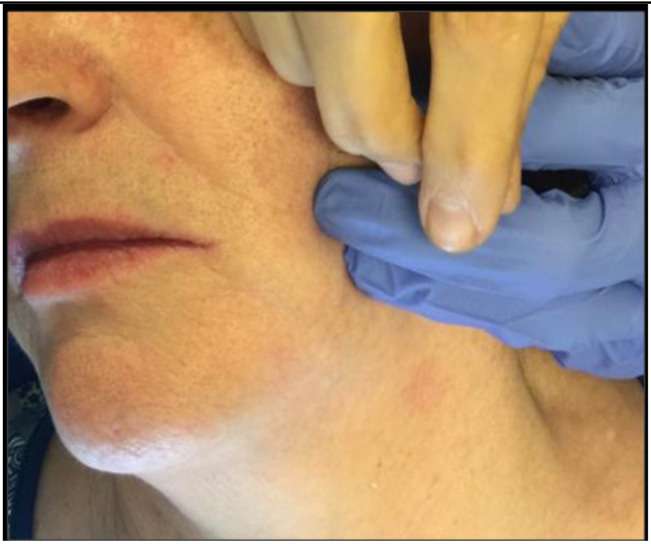
Group E received the DDN treatment in both masseter muscles, using 0.30 × 0.30 mm AGUPUNT acupuncture needles with guides. The technique requires the patient to rest in a supine position, with their eyes closed and with the head rotated towards the right when treating the left masseter muscle and towards the left for the right masseter muscle. The trigger point is identified and marked with a dermographic pen (pain evaluation was carried out through the palpation of different points according to RDC guidelines, using an algometer to determine the exact amount of exerted pressure (ANNEX 3).
The skin was then cleaned and the electromyographic electrodes are placed at the origin and the insertion of the masseter muscle. An antiseptic was subsequently applied on the needling area and the needle was inserted under the aseptic conditions required for the technique. During needling, the physical response of the patient was observed at all times, with the objective of controlling local spasm responses in each masseter muscle Figure 1.
Figure 1.
Patient lying in the required position for needling.
Next, the opening pattern and the measurements of the opening of the mouth were evaluated using a digital caliper, and the joint was auscultated on both sides during the opening and closing movements to assess the characteristics of potential sounds (such as clicks, pops, and so on, as well as their volume and frequency) (Figure 2 and Figure 3).
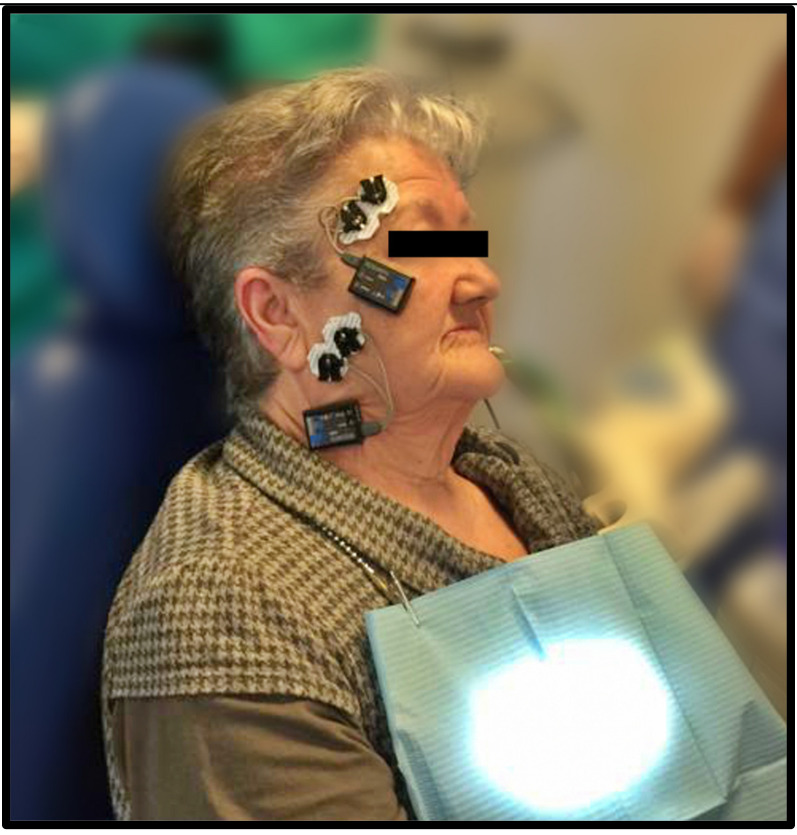
Figure 2.
Electromyography surface electrode positioning.
Figure 3.
Pre-needling registry of the activity in MI position.
Ten minutes after the procedure, a new evaluation was conducted (post-needling), which included all the above-mentioned change variables.
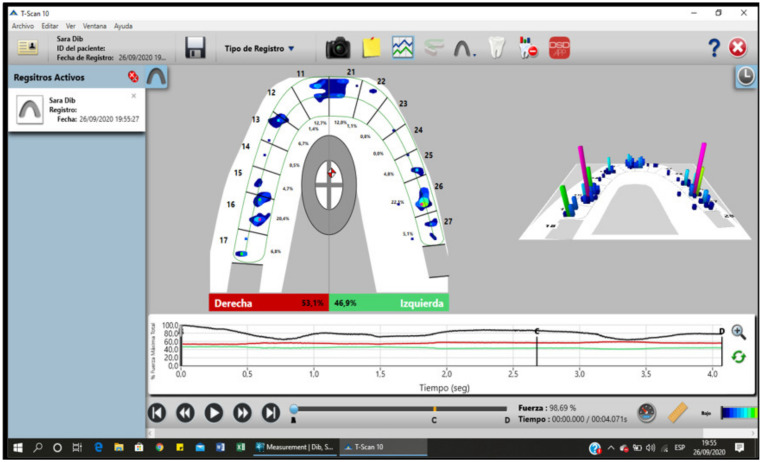
Group C received a simulation of the DDN treatment. The patient was set in the same position, using the same needle and guide with the same characteristics, and for the same time as estimated for group E, although in this case the safety piece was not removed so as not to needle the patient. All the aforementioned records were obtained out for muscle activity, mandibular position, mandibular opening and closing patterns, and joint noises, both before and after the simulation of the dry needling maneuver (Figure 4).
Figure 4.
Digital occlusal analysis determining the characteristics of the jaw position.
After conducting the technique, the patient was left to rest for 10 min for the needling to take effect, and afterwards the same evaluation registrations described above were repeated, evaluating the post-needling pain levels. The patient was then asked to fill in the same pain level questionnaire completed at the beginning.
Subsequently, 15 days after the procedure, a new evaluation (follow-up evaluation) was conducted, including, in addition to the change variables registered during the post-needling evaluation, a new, self-administered questionnaire with aspects related to pain and functional activity.
2.2. Data Analysis
The data were analyzed using the IBM.SPSS Statistics program, version 23 and were presented with a 95% confidence level.
3. Results
Table 1 offers the variations of the masseter muscle activity values in both sides, comparing electric activity results during the pre-needling and post-needling phases.
Table 1.
Mean muscle activity value comparison in microvolts (µV) of the masseter muscle in different jaw positions, where RM is relaxed mandible, MI is maximum intercuspation position, CR is jaw in centric relation according to Dawson’s bimanual manipulation, CR-L is centric relation measured using Long’s laminae, CR-P is centric relation obtained in the position of the first dental contact, DT is jaw disclusion time, P is jaw protrusion position and T-MI is time needed to reach the position of maximum intercuspation. We were able to clearly observe a reduction in activity in the right (19 µV) and left (20.36 µV) masseter muscles in a relaxed jaw position before needling the trigger points. After waiting 10 min post-needling and repeating the same measurement, we obtained values of 23.71 µV/17.65 µV, respectively.
| Mean Pre- | Deviation Pre- | Mean Post- | Deviation Post- | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Cont. | Interv. | Cont. | Interv. | |||||
| Right | Left | Right | Left | Right | Left | Right | Left | |||||
| RM | 19 | 20.36 | 19.31 | 25.05 | 21 | 15.8 | 23.71 | 17.65 | 20.06 | 12.76 | 40.27 | 18.04 |
| MI | 130.73 | 125.55 | 227.83 | 248.22 | 229 | 228.75 | 112.86 | 90.71 | 402.1 | 402.25 | 222.34 | 168.37 |
| CR | 94 | 85 | 73.5 | 68.38 | 94 | 70.50 | 37.71 | 34.14 | 150.70 | 106.34 | 58.71 | 49.14 |
| CR-L | 42.91 | 36.91 | 66.96 | 53.86 | 92 | 70 | 30.50 | 28.3 | 64.3 | 52.5 | 35.3 | 30.45 |
| CR-P | 36.91 | 33 | 54.83 | 46.77 | 35 | 30 | 24.4 | 20 | 50.30 | 40.23 | 45.5 | 35.45 |
| DT | 3.59 | 1.74 | 3.45 | 2.09 | 0.5 | 1.3 | ||||||
| P | 94.82 | 81 | 147.46 | 160.41 | 174.50 | 98.75 | 64.57 | 51.43 | 255.03 | 147.54 | 123.92 | 87.74 |
| T-MI | 0.87 | 1.21 | 0.25 | 0.6 | ||||||||
The activity of the same muscle in its maximum intercuspation position was also registered following the same protocol, finding changes in the muscle’s electric activity though without statistical significance. These were probably due to the clinical trial’s sample size. We registered the following values: 130.73 µV in the right masseter and 125.55 µV in the left masseter pre-needling; and 112.86 µV and 90.71 µV post-needling. The reduction in activity is notable, especially in the left side. This was probably due to prematurity and interferences in the right side, which were evidenced after analyzing the T-scan results. This situation was no longer noticeable in the different CR positions, where the occlusal irregularities that can affect the electric activity of the muscle were nullified.
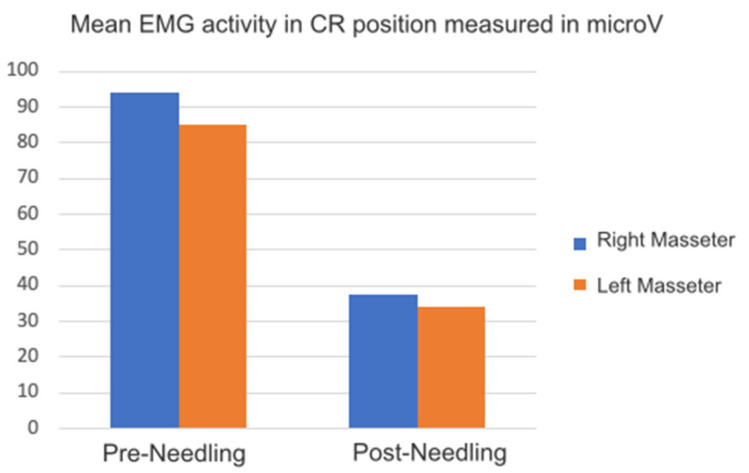
According to the protocol, regarding the EMG values registered in centric relation positions with different techniques, we found a significant reduction in muscle activity between pre- and post-needling, of > 0.05. Dawson’s bimanual technique CR was associated with the highest reduction percentage of the activity of the right 94 µV and left 85 µV muscles before, as well as 31.71 µV right and 34.14 µV left after needling (Figure 5). This striking reduction, corresponding to CR measurement using jaw induction after achieving a relaxed state, is the only technique that does not entail any dental contact, a situation that favors an increase in muscle activity.
Figure 5.
Graph of EMG activity in CR position compared with other techniques (CR-P, CR-L), Table 1.
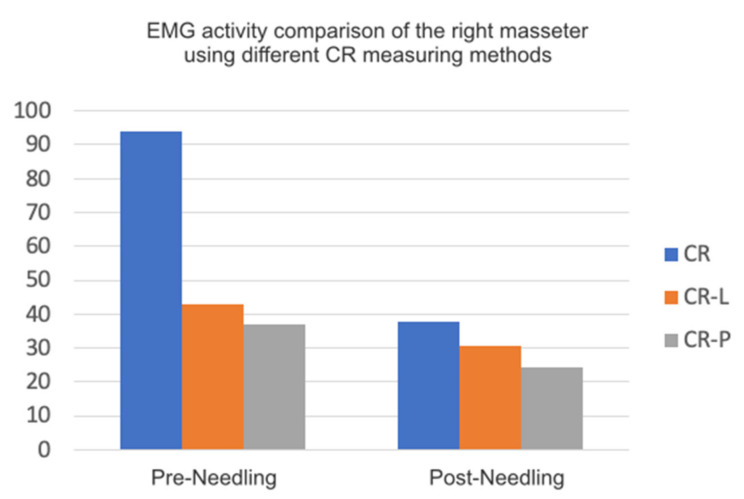
Considering the EMG, we can sort the methods used for measuring centric relation according to the masseter muscle’s activity based on the reduction in EMG activity between the pre- and post-needling states in the following order:
CR according to Dawson’s bimanual technique.
CR-P, where centric relation is determined by the closing arch of the jaw up to the first dental contact, with a mean pre-needling activity of 36.91 µV in the right/33 µV in the left masseter and of 24.4 µV in the right/20 µV in the left masseter post-needling.
CR-L, where the centric relation is measured with Long’s laminae with a mean pre-needling activity of 42.91 µV in the right/36.91 µV in the left masseter and of 30.5 µV in the right/28.3 µV in the left masseters post-needling.
Figure 6 shows the comparison between the three methods of determining the central relation.
Figure 6.
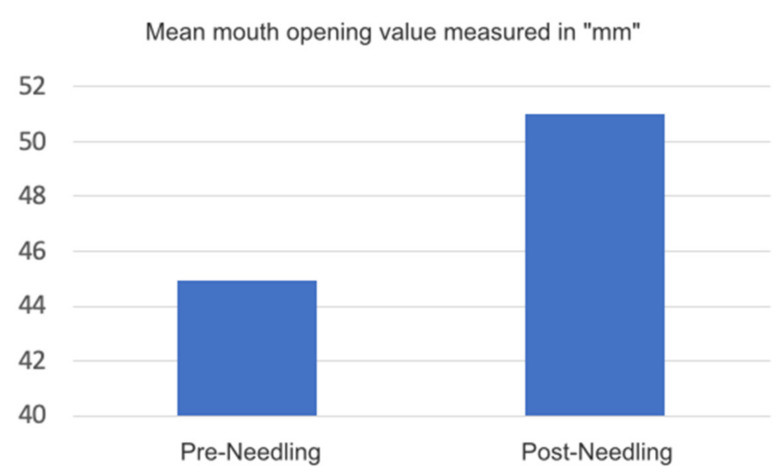
Graph EMG activity comparison of the right masseter using different CR measuring methods. Regarding the opening of the mouth and its symmetry, we found a significant change, of >0.05, in the opening, with a mean value of 44.92 mm pre-needling, measured between the incisal borders of the superior and inferior centrals.
The mean post-needling distance is 51 mm. Figure 7.
Figure 7.
Graph mean mouth opening value measured in mm.
For the symmetry of the arch when opening and closing, we registered significant changes. In certain situations, the symmetry was affected by intra-articular morphological and structural changes. These modifications where mostly motivated by masseter muscle relaxation after trigger point needling (Table 2).
Table 2.
Percentage symmetry of opening and closing mouth.
| Presence Percentage | Asymmetry | Symmetry |
|---|---|---|
| Pre- | 75% | 25% |
| Post- | 37.5% | 62.5% |
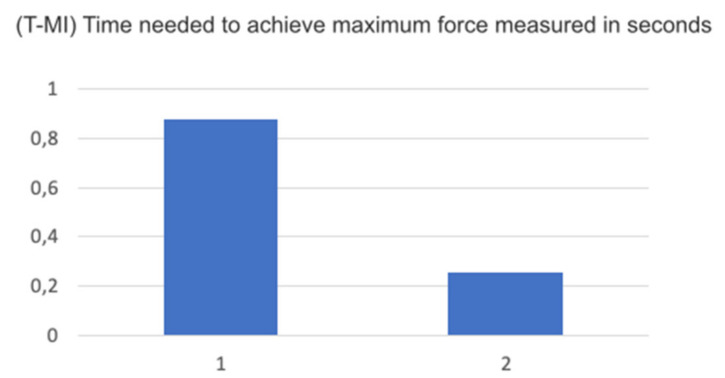
For the variable of the time needed to achieve maximum force in a maximum intercuspation position, disregarding the determining factor of the muscle due to the needling technique, and measuring using the T-scan, we found a considerable reduction in the amount of time needed, possibly due to the postural change of the jaw after the procedure, avoiding premature reactions secondary to muscle issues (Figure 8).
Figure 8.
Graph T-Mi, time needed to achieve maximum force measured in seconds.
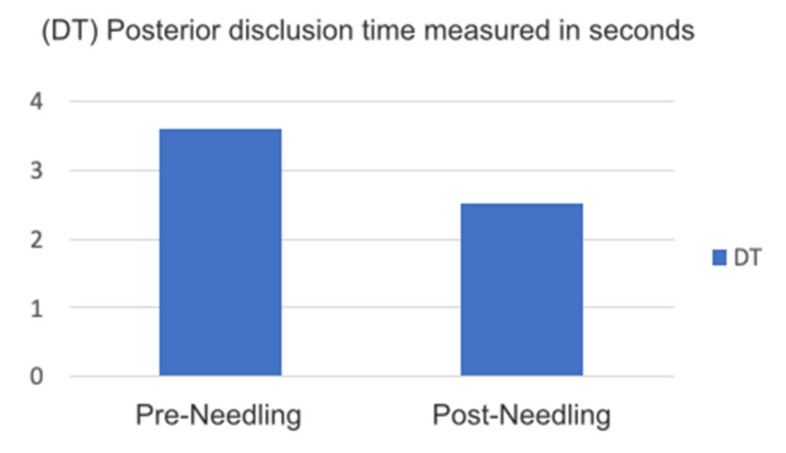
The posterior disclusion time, a major characteristic of optimal functional occlusion, is usually affected in certain circumstances by the situation of the muscle and vice versa, since delayed posterior disclusion is one of the main reasons for increases in facial pain and EMG activity. According to our results, the reduction in DT was significant: >0.05 (Figure 9).
Figure 9.
Graph DT posterior disclusion time measured in seconds.
Regarding both articular and facial pain, we registered significant mean values, especially for facial pain, a variable that is directly subject to the state of the muscle. These results are shown in Table 3.
Table 3.
Articular and facial pain.
| Mean Pre- | Dev. Pre- | Mean Post- | Dev. Post- | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | Cont. | Interv. | ||
| Mouth Opening | 44.92 | 7.36 | 51.75 | 51 | 2.36 | 2.64 | |||
| Facial Pain | 7.75 | 8.57 | 0.95 | 0.97 | 0.5 | 1.5 | 0.57 | 0.97 | |
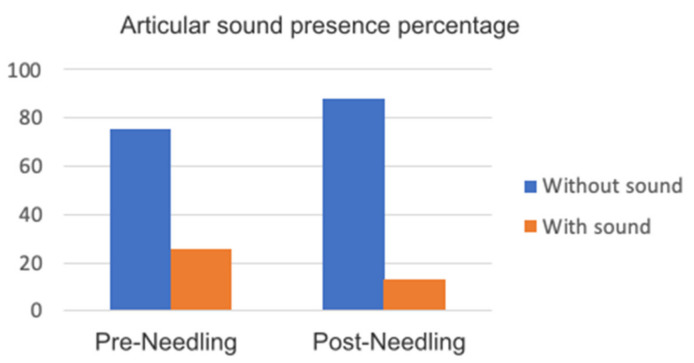
| Articular Sound | No | 75% | 75% | 100% | 87.5% | ||||
| Yes | 25% | 25% | 0 | 12.5% | |||||
| TMJ pain | 2.50 | 3 | 1.73 | 2.77 | 1 | 2.63 | 2 | 3.24 | |
Compared with the results on articular pain measured through VAS in both variables, we observed a smaller reduction in articular pain between the pre- and post-needling states, though intra-articular factors; changes affected this variable more than the effects of the muscle itself.
The articular sounds showed a noticeable improvement at 10 min after needling of the masseter muscle, considering that the muscle factor plays an important role in adjusting the trajectory that the jaw follows in its movement. The results can be found in Figure 10.
Figure 10.
Graph articular sound presence percentage.
4. Discussion
At present, the effectiveness of DDN is more than evident thanks to numerous research studies that have highlighted the importance of ischemic compression and eccentric load in muscle exercises following DDN [21,22].
The first use of DDN therapy was in the 1940s and, since then, numerous studies have tried to analyze this technique in all its diversity. Two meta-analyses comparing the effects of dry needling with wet needling using lidocaine concluded that short-term results are similar [23,24,25,26]. In 2016, the Canadian Agency for Drugs and Technologies in Health accepted the use of DDN in the public health system for the treatment of different musculoskeletal pain syndromes [27,28,29,30]. A recent meta-analysis study on the use of DDN in temporomandibular disorders concluded that the technique in question considerably reduces pain intensity compared with sham therapy [31,32]. In their meta-analysis, Hall et al. concluded that DDN effectiveness is low at the muscular level, but is higher at the neurological level [33,34].
Gatte et al. proved that DDN effectiveness was low-to-moderate in relation to physiotherapy treatment for musculoskeletal pain in the short- or medium-term [35,36,37,38,39]. Gerwin and Shah proved that DDN is capable of interrupting dysfunction at the terminal of the motor end plate, increasing muscle length and reducing the superposition of actin and myosin fibers [40,41,42]. Lui Q.G. reports that DDN helps reduce the range and electric frequency at the terminal of the motor end plate, reducing acetylcholine levels [43,44]. Chou and Hsieh report that the spontaneous reduction in electric activity is associated with a cascade of muscle contractions during the DDN procedure [45,46]. This phenomenon leads to a reduction in acetylcholine levels, causing an increase in t blood flow and, in turn, in local oxygenation levels, leading to muscle relaxation in the area [47].
Butts reports that, from a neurophysiological standpoint, DDN reduces both peripheral and central sensitivity by neutralizing nociceptors in the area, modeling de activity of the dorsal spine through the inhibition of the activity of central pain pathways [48,49]. In their studies, Shah et al proved that with DDN, there is an immediate concentration in the area of neurotransmitters, such as calcitonin, as well as of various cytokines and interleukins, both outside and in cellular fluids [50,51]. Hsieh et al. confirmed that DDN models chemical mediators associated with pain and inflammation by increasing B-endorphins [52,53]. It is evident that DDN cannot be the only therapeutic option when treating chronic pain but must be accompanied by other therapeutic techniques [54,55], such as physical exercise, psychological treatment, and the treatment of sleep disorders [56,57]. Woolf considers that afferent signals and their transmission pathways, as well as nociceptor sensors, constitute the most frequent etiology for myofacial pain [58,59,60]. Fernández de la Peña reports that trigger points can be considered peripheral sensors for nociception that contribute to pain propagation [61,62]. This theory suggests that the interactions are bi-directional. Their conclusions concur with our results regarding trigger point needling, which deactivates nociceptors, deprograms the affected muscles, and allows mandibular movements free of muscle conditioning [63,64,65,66].
Clinically, in situations of chronic pain, a proper understanding by the patient of the mechanism behind the pain is considered crucial [63,64,65]. Secondly, understanding the role of trigger point needling, its interventions in nociceptors, their pathways, and the effects of the technique on pain relief are also important [66]. Thirdly, the combination of DDN with a good understanding of the mechanism behind its effects is key in reducing kinesiophobia [67,68].
From our point of view, and in light of the results of our study and the duration of DDN effectiveness, we consider the use of the technique as a neuromuscular de-programmer as the first step in the multidisciplinary therapeutic process of myofacial pain.
5. Conclusions
Although, at present, there is no consensus on the effects of DDN, various studies consider that this technique offers swift pain relief despite the short duration of its effects. We observed:
A significant reduction in facial pain and a reduction in muscle activity after needling trigger points.
A significant variation in the static position and in the trajectory of the movement of the jaw, determined through digital occlusion control using Tec-Scan (occlusal digital analysis).
A reduction in the asymmetry of the arch when opening and closing the mouth in the centric relation with an increase in the maximum mouth opening after needling.
Abbreviations
| CR | Central relationship |
| CR-L | Central Relationship through long sheets |
| CR-P | Central Relationship through the first point of occlusal contact |
| DDN | Deep dry puncture |
| DT | Mandibular disclusion time |
| EMG | Electro-miography |
| EVA | Visual Analogue scale |
| MI | Maximum intercuspidation |
| P | Protrusive position |
| PP | Placebo puncture |
| RDC | Research diagnostic criteria |
| RM | Mandibular rest |
| TMJ | Temporomandibular joint |
| T-MI | Time to reach maximum occlusive force |
| Uv | Micro-volt |
Author Contributions
Conceptualization, I.D.-Z. and J.F.-F.; methodology, J.D.-Z. and J.M.-M.; software, S.D.-Z.; validation, I.D.-Z.; formal analysis, J.M.-M.; investigation, J.D.-Z. and S.D.-Z.; resources, J.D.-Z. and J.F.-F.; data curation, J.M.-M.; writing—original draft preparation, J.D.-Z.; writing—review and editing, J.F.-F.; visualization, I.D.-Z.; supervision, J.M.-M.; project administration, J.F.-F. All authors have read and agreed to the published version of the manuscript.
Funding
Expenses arising from the data processing and logistics were borne by Avances en Salud Oral Group (Salamanca, Spain).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University of Salamanca (27 September 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare not to have any conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montero J., Llodra J.C., Bravo M. Prevalence of the signs and symptoms of temporomandibular disorders among Spanish adults and seniors according to five national surveys performed between 1993 and 2015. J. Oral Facial Pain Headache. 2018;32:349–357. doi: 10.11607/ofph.2085. [DOI] [PubMed] [Google Scholar]
- 2.Lobbezoo F., Naeije M. Bruxism is mainly regulated centrally, not peripherally. J. Oral Rehabil. 2001;28:1085–1091. doi: 10.1046/j.1365-2842.2001.00839.x. [DOI] [PubMed] [Google Scholar]
- 3.Conti P.C., Costa Y.M., Gonçalves D.A., Svensson P. Headaches and myofascial temporomandibular disorders: Overlapping entities, separate managements? J. Oral Rehabil. 2016;43:702–715. doi: 10.1111/joor.12410. [DOI] [PubMed] [Google Scholar]
- 4.Carlsson G.E. Epidemiology and treatment need for temporomandibular disorders. J. Orofac. Pain. 1999;13:232–237. [PubMed] [Google Scholar]
- 5.Mehta N.R., Correa L.P. Oral appliance therapy and temporomandibular disorders. Sleep Med. Clin. 2018;13:513–519. doi: 10.1016/j.jsmc.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Liapaki A., Thamm J.R., Ha S., Monteiro J.L.G.C., McCain J.P., Troulis M.J., Guastaldi F.P.S. Is there a difference in treatment effect of different intra-articular drugs for temporomandibular joint osteoarthritis? A systematic review of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2021;50:1233–1243. doi: 10.1016/j.ijom.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira N., Masterson D., Lopes de Lima R., de Souza B., Oliveira A.T., Kelly da Silva Fidalgo T., Carvalho A.C.P., DosSantos M.F., Grossmann E. Efficacy of viscosupplementation with hyaluronic acid in temporomandibular disorders: A systematic review. J. Craniomaxillofac. Surg. 2018;46:1943–1952. doi: 10.1016/j.jcms.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Awan K.H., Patil S. The role of transcutaneous electrical nerve stimulation in the management of temporomandibular joint disorder. J. Contemp. Dent. Pract. 2015;16:984–986. doi: 10.5005/jp-journals-10024-1792. [DOI] [PubMed] [Google Scholar]
- 9.Zotti F., Albanese M., Rodella L.F., Nocini P.F. Platelet-rich plasma in treatment of temporomandibular joint dysfunctions: Narrative review. Int. J. Mol. Sci. 2019;20:277. doi: 10.3390/ijms20020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renapurkar S.K. Surgical versus nonsurgical management of degenerative joint disease. Oral Maxillofac. Surg. Clin. N. Am. 2018;30:291–297. doi: 10.1016/j.coms.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Temporomandibular Joint Surgeons Guidelines for diagnosis and management of disorders involving the temporomandibular joint and related musculoskeletal structures. Cranio. 2003;21:68–76. doi: 10.1080/08869634.2003.11746234. [DOI] [PubMed] [Google Scholar]
- 12.Fricton J. Myogenous temporomandibular disorders: Diagnostic and management considerations. Dent. Clin. N. Am. 2007;51:61–83. doi: 10.1016/j.cden.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Mayoral Del Moral O. Dry needling treatments for myofascial trigger points. J. Musculoskelet. Pain. 2010;18:411–416. doi: 10.3109/10582452.2010.502632. [DOI] [Google Scholar]
- 14.Karakurum B., Karaalin O., Coskun O., Dora B., Ucler S., Inan L. The ‘dry-needle technique’: Intramuscular stimulation in tension-type headache. Cephalalgia. 2001;21:813–817. doi: 10.1046/j.1468-2982.2001.218238.x. [DOI] [PubMed] [Google Scholar]
- 15.Hong C.-Z. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am. J. Phys. Med. Rehabil. 1994;73:256–263. doi: 10.1097/00002060-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Chen J.T., Chung K.C., Hou C.R., Kuan T.S., Chen S.M., Hong C.Z. Inhibitory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am. J. Phys. Med. Rehabil. 2001;80:729–735. doi: 10.1097/00002060-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Carnero J., La Touche R., Ortega-Santiago R., Galan-del-Rio F., Pesquera J., Ge H.Y., Fernández-de-Las-Peñas C. Short-term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J. Orofac. Pain. 2010;24:106–112. [PubMed] [Google Scholar]
- 18.Gonzalez-Perez L.M., Infante-Cossio P., Granados-Nunez M., Urresti-Lopez F.J., Lopez-Martos R., Ruiz-Canela-Mendez P. Deep dry needling of trigger points located in the lateral pterygoid muscle: Efficacy and safety of treatment for management of myofascial pain and temporomandibular dysfunction. Med. Oral Patol. Oral Cir. Bucal. 2015;20:e326–e333. doi: 10.4317/medoral.20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasco-Bonora P.M., Martín-Pintado-Zugasti A. Effects of myofascial trigger point dry needling in patients with sleep bruxism and temporomandibular disorders: A prospective case series. Acupunct. Med. 2017;35:69–74. doi: 10.1136/acupmed-2016-011102. [DOI] [PubMed] [Google Scholar]
- 20.McMillan A.S., Nolan A., Kelly P.J. The efficacy of dry needling and procaine in the treatment of myofascial pain in the jaw muscles. J. Orofac. Pain. 1997;11:307–314. [PubMed] [Google Scholar]
- 21.Lopez-Martos R., Gonzalez-Perez L.M., Ruiz-Canela-Mendez P., Urresti-Lopez F.J., Gutierrez-Perez J.L., Infante-Cossio P. Randomized, double-blind study comparing percutaneous electrolysis and dry needling for the management of temporomandibular myofascial pain. Med. Oral Patol. Oral Cir. Bucal. 2018;23:e454–e462. doi: 10.4317/medoral.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Perez L.M., Infante-Cossio P., Granados-Nuñez M., Urresti-Lopez F.J. Treatment of temporomandibular myofascial pain with deep dry needling. Med. Oral Patol. Oral Cir. Bucal. 2012;17:e781–e785. doi: 10.4317/medoral.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dib A., Montero J., Sanchez J.M., López-Valverde A. Electromyographic and patient-reported outcomes of a computer-guided occlusal adjustment performed on patients suffering from chronic myofascial pain. Med. Oral Patol. Oral Cir. Bucal. 2015;20:e135–e143. doi: 10.4317/medoral.20272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffman E., Ohrbach R., Truelove E., Look J., Anderson G., Goulet J.P., List T., Svensson P. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache. 2014;28:6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doig G.S., Simpson F. Randomization and allocation concealment: A practical guide for researchers. J. Crit. Care. 2005;20:187–191. doi: 10.1016/j.jcrc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis of the Behavioural Sciences. 2nd ed. Lawrence Erlbaum; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 27.Fernández-Carnero J., Gilarranz-de-Frutos L., León-Hernández J.V., Pecos-Martin D., Alguacil-Diego I., Gallego-Izquierdo T., Martín-Pintado-Zugasti A. Effectiveness of different deep dry needling dosages in the treatment of patients with cervical myofascial pain: A pilot RCT. Am. J. Phys. Med. Rehabil. 2017;96:726–733. doi: 10.1097/PHM.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 28.Abbaszadeh-Amirdehi M., Ansari N.N., Naghdi S., Olyaei G., Nourbakhsh M.R. Neurophysiological and clinical effects of dry needling in patients with upper trapezius myofascial trigger points. J. Bodyw. Mov. Ther. 2017;21:48–52. doi: 10.1016/j.jbmt.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Pintado-Zugasti A., Rodríguez-Fernández A.L., Fernandez-Carnero J. Postneedling soreness after deep dry needling of a latent myofascial trigger point in the upper trapezius muscle: Characteristics, sex differences and associated factors. J. Back Musculoskelet. Rehabil. 2016;29:301–308. doi: 10.3233/BMR-150630. [DOI] [PubMed] [Google Scholar]
- 30.Dar G., Hicks G.E. The immediate effect of dry needling on multifidus muscles´ function in healthy individuals. J. Back Musculoskelet. Rehabil. 2016;29:273–278. doi: 10.3233/BMR-150624. [DOI] [PubMed] [Google Scholar]
- 31.Fuentes-Gallardo I., Perez-Muñoz M., Mayoral-Del-Moral O., Lluch-Girbés E., Prieto-Valiente L., Falla D. Effectiveness of dry needling for chronic nonspecific neck pain: A randomizes, single-blinded, clinical trial. Pain. 2016;157:1905–1917. doi: 10.1097/j.pain.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 32.Cerezo-Téllez E., Lacomba M.T., Fuentes-Gllardo I., Mayoral-del-Moral O., Rodrigo-Medina B., Gutiérrez Ortega C. Dry needling of the trapezius muscle in office workers with neck pain: A randomized clinical trial. J. Man. Manip. Ther. 2016;24:223–232. doi: 10.1179/2042618615Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calvo-Lobo C., Pacheco-da-Costa S., Hita-Herranz E. Efficacy of deep dry needling on latent myofascial trigger points in older adults with nonspecific shoulder pain: A randomized, controlled clinical trial pilot study. J. Geriatr. Phys. Ther. 2017;40:63–73. doi: 10.1519/JPT.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez-Mila Z., Salom-Moreno J., Fernandez-de-las-Peñas C. Effects of dry needling on post-stroke spasticity, motor function and stability limits: A randomized clinical trial. Acupunct. Med. 2018;36:358–366. doi: 10.1136/acupmed-2017-011568. [DOI] [PubMed] [Google Scholar]
- 35.Gattie E., Cleland J.A., Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017;47:133–149. doi: 10.2519/jospt.2017.7096. [DOI] [PubMed] [Google Scholar]
- 36.Arias-Buría J.L., Fernández-de-Las-Peñas C., Palacios-Ceña M., Koppenhaver S.L., Salom-Moreno J. Exercises and dry needling for subacromial pain syndrome: A randomized parallel-group trial. J. Pain. 2017;18:11–18. doi: 10.1016/j.jpain.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Arias-Buría J.L., Martín-Saborido C., Cleland J., Koppenhaver S.L., Plaza-Manzano G., Fernández-de-Las-Peñas C. Cost-effectiveness evaluation of the inclusion of dry needling into an exercise program for subacromial pain syndrome: Evidence from a randomized clinical trial. Pain Med. 2018;19:2336–2347. doi: 10.1093/pm/pny021. [DOI] [PubMed] [Google Scholar]
- 38.Espí-López G.V., Serra-Añó P., Vicent-Ferrando J., Sánchez-Moreno-Giner M., Arias-Buría J.L., Cleland J., Fernandez-De-Las-Penas C. Effectiveness of inclusion of dry needling in a multimodal therapy program for patellofemoral pain: A randomized parallel-group trial. J. Orthop. Sports Phys. Ther. 2017;47:392–440. doi: 10.2519/jospt.2017.7389. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez-Romero E.A., Pecos-Martín D., Calvo-Lobo C., Ochoa-Sáez V., Burgos-Caballero V., Fernández-Carnero J. Effects of dry needling in an exercise program for older adults with knee osteoarthritis: A pilot clinical trial. Medicine. 2018;97:e11255. doi: 10.1097/MD.0000000000011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunning J., Butts R., Young I., Mourad F., Galante V., Bliton P., Tanner M., Fernández-de-Las-Peñas C. Periosteal electrical dry needling as an adjunct to exercise and manual therapy for knee osteoarthritis: A multicenter randomized clinical trial. Clin. J. Pain. 2018;34:1149–1158. doi: 10.1097/AJP.0000000000000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cagnie B., Dewitte V., Barbe T., Timmermans F., Delrue N., Meeus M. Physiologic effects of dry needling. Curr. Pain Headache Rep. 2013;17:348. doi: 10.1007/s11916-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 42.Gerwin R.D., Dommerholt J., Shah J.P. An expansion of Simons‘ integrated hypothesis of trigger point formation. Curr. Pain Headache Rep. 2004;8:468–475. doi: 10.1007/s11916-004-0069-x. [DOI] [PubMed] [Google Scholar]
- 43.Chou L.W., Hsieh Y.L., Kao M.J., Hong J.Z. Remote influences of acupuncture on the pain intensity and the amplitude changes of endplate noise in the myofascial trigger point of the upper trapezius muscle. Arch. Phys. Med. Rehabil. 2009;90:905–912. doi: 10.1016/j.apmr.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q.G., Liu L., Huang Q.M., Nguyen T.T., Ma Y.T., Zhao J.M. Decreased spontaneous electrical activity and acetylcholine at myofascial trigger spots after dry needling treatment: A pilot study. Evid.-Based Complementary Alternat. Med. 2017;2017:3938191. doi: 10.1155/2017/3938191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbaszadeh-Amirdehi M., Ansari N.N., Naghdi S., Olyaei G., Nourbakhsh M.R. Therapeutic effects of dry needling in patients with upper trapezius myofascial trigger points. Acupunct. Med. 2017;35:85–92. doi: 10.1136/acupmed-2016-011082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cagnie B., Barbe T., De Ridder E., Van Oosterwijck J., Cools A., Danneels L. The influence of dry needling of the trapezius muscle on muscle blood flow and oxygenation. J. Manip. Physiol. Ther. 2012;35:685–691. doi: 10.1016/j.jmpt.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Dommerholt J. Dry needling: Peripheral and central considerations. J. Man. Manip. Ther. 2011;19:223–237. doi: 10.1179/106698111X13129729552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butts R., Dunning J., Perreault T., Mourad F., Grubb M. Peripheral and spinal mechanisms of pain and dry needling mediated analgesia: A clinical resource guide for health care professionals. Int. J. Phys. Med. Rehabil. 2016;4:2. doi: 10.4172/2329-9096.1000327. [DOI] [Google Scholar]
- 49.Chou L.W., Kao M.J., Lin J.G. Probable mechanisms of needling therapies for myofascial pain control. Evid.-Based Complementary Alternat. Med. 2012;2012:705327. doi: 10.1155/2012/705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsieh Y.L., Yang S.A., Yang C.C., Chou L.W. Dry needling at myofascial trigger spots of rabbit skeletal muscles modulates the biochemicals associated with pain, inflammation, and hypoxia. Evid.-Based Complementary Alternat. Med. 2012;2012:342165. doi: 10.1155/2012/342165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsieh Y.L., Kao M.J., Kuan T.S., Chen S.M., Chen J.T., Hong C.Z. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am. J. Phys. Med. Rehabil. 2007;86:397–403. doi: 10.1097/PHM.0b013e31804a554d. [DOI] [PubMed] [Google Scholar]
- 52.Tsai C.T., Hsieh L.F., Kuan T.S., Kao M.J., Chou L.W., Hong C.Z. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am. J. Phys. Med. Rehabil. 2010;89:133–140. doi: 10.1097/PHM.0b013e3181a5b1bc. [DOI] [PubMed] [Google Scholar]
- 53.Hsieh Y.L., Chou L.W., Joe Y.S., Hong C.Z. Spinal cord mechanism involving the remote effects of dry needling on the irritability of myofascial trigger spots in rabbit skeletal muscle. Arch. Phys. Med. Rehabil. 2011;92:1098–1105. doi: 10.1016/j.apmr.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Hsieh Y.L., Yang S.A., Liu S.Y., Chou L.W., Honc C.Z. Remote dose-dependent effects of dry needling at distant myofascial trigger spots of rabbit skeletal muscles on reduction of substance P levels of proximal muscle and spinal cords. BioMed Res. Int. 2014;2014:982121. doi: 10.1155/2014/982121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srbely J.Z., Dickey J.P., Lee D., Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J. Rehabil. Med. 2010;42:463–468. doi: 10.2340/16501977-0535. [DOI] [PubMed] [Google Scholar]
- 56.Audette J.F., Wang F., Smith H. Bilateral activation of motor unit potentials with unilateral needle stimulation of active myofascial trigger points. Am. J. Phys. Med. Rehabil. 2004;83:368–374. doi: 10.1097/01.PHM.0000118037.61143.7C. [DOI] [PubMed] [Google Scholar]
- 57.Chae Y., Chang D.S., Lee S.H., Jung W.M., Lee I.S., Jackson S., Kong J., Lee H., Park H.J., Lee H., et al. Inserting needles into the body: A meta-analysis of brain activity associated with acupuncture needle stimulation. J. Pain. 2013;14:215–222. doi: 10.1016/j.jpain.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Niddam D.M., Chan R.C., Lee S.H., Yeh T.C., Hsieh J.C. Central modulation of pain evoked from myofascial trigger point. Clin. J. Pain. 2007;23:440–448. doi: 10.1097/AJP.0b013e318058accb. [DOI] [PubMed] [Google Scholar]
- 59.Louw A., Zimney K., Puentedura E.J., Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: A systematic review of the literature. Physiother. Theory Pract. 2016;32:332–355. doi: 10.1080/09593985.2016.1194646. [DOI] [PubMed] [Google Scholar]
- 60.Geneen L.J., Moore R.A., Clarke C., Martin D., Colvin L.A., Smith B.H. Physical activity and exercise for chronic pain in adults: An overview of cochrane reviews. Cochrane Database Syst. Rev. 2017;4:CD011279. doi: 10.1002/14651858.CD011279.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunner E., De Herdt A., Minguet P., Baldew S.S., Probst M. Can cognitive behavioural therapy-based strategies be integrated into physiotherapy for the prevention of chronic low back pain? A systematic review. Dis. Rehabil. 2013;35:1–10. doi: 10.3109/09638288.2012.683848. [DOI] [PubMed] [Google Scholar]
- 62.Williams A.C., Eccleston C., Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nijs J., Mairesse O., Neu D., Leysen L., Danneels L., Cagnie B., Meeus M., Moens M., Ickmans K., Goubert D. Sleep disturbances in chronic pain: Neurobiology, assessment and treatment in physical therapist practice. Phys. Ther. 2018;98:325–335. doi: 10.1093/ptj/pzy020. [DOI] [PubMed] [Google Scholar]
- 64.Lluch Girbes E., Meeus M., Baert I., Balancing N.J. “Hands-on” with “hands-off” physical therapy interventions for the treatment of central sensitization pain in osteoarthritis. Man. Ther. 2015;20:349–352. doi: 10.1016/j.math.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Woolf C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kosek E., Cohen M., Baron R., Gebhart G.F., Mico J.A., Rice A.S., Rief W., Sluka A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157:1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 67.Fernández de Las Peñas C., Dommerholt J. Myofascial trigger points: Peripheral or central phenomenon? Curr. Rheumatol. Rep. 2014;16:395. doi: 10.1007/s11926-013-0395-2. [DOI] [PubMed] [Google Scholar]
- 68.Malfliet A., Kregel J., Coppieters I., De Pauw R., Meeus M., Roussel N., Cagnie B., Danneels L., Nijs J. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain: A randomized clinical trial. JAMA Neurol. 2018;75:808–817. doi: 10.1001/jamaneurol.2018.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.