Abstract
BACKGROUND:
The ApoE e4 allele is a well-known risk factor for Alzheimer’s disease (AD). Previous research argues that higher education helps to preserve cognition in older adults with AD pathology because of its key role in cognitive reserve and resilience.
OBJECTIVE:
To test if higher educational level buffers the effect of ApoE e4 on cognition among older non-Hispanic Blacks.
METHODS:
Participants were 849 non-demented older non-Hispanic Blacks (38.3% ApoE e4+), who underwent a comprehensive neuropsychological evaluation. Multiple linear regression models tested the relationship between ApoE e4 status and twelve cognitive measures with education (up to high school and beyond high school) as a moderator.
RESULTS:
Education buffered the effects of the ApoE e4 allele, such that there was no impact of ApoE e4 status on word-list memory retention and working memory among participants with more than a high school degree. This pattern was not observed for ten other cognitive measures of verbal and visual episodic memory, semantic memory, executive function, and processing speed—although a similar trend was observed for switching ability in executive functioning. The buffering effect of education was stronger among women than men.
CONCLUSION:
Our findings suggest that genetic effects on late-life cognition may be modified by environmental factors such as educational attainment. These results are consistent with the framework of cognitive reserve such that engaging in cognitively enriching activities and acquiring skills and knowledge with more years of education may increase the capacity to maintain cognitive function despite high genetic risk for impairment.
Keywords: ApoE, genetic risk, Alzheimer’s disease, episodic memory, neuropsychological evaluation, educational attainment, cognitive reserve, African American
INTRODUCTION
Risk factors of cognitive impairment and Alzheimer’s disease (AD) range from genetics to environmental influences. Apolipoprotein e4 (ApoE e4) is a well-known genetic risk factor for AD [1] and on average, older individuals with the e4 allele show decreased cognitive functioning compared to non-carriers [2]. Education attainment is a modifiable environmental factor and has a protective effect against cognitive impairment and dementia in late life [3, 4]. However, the increased risk of cognitive impairment by having either the ApoE e4 allele or lower educational attainment both differ substantially across race and ethnicity [3, 5, 6]. However, the intersection of these two risk factors in minority populations has been relatively unexplored. Due to the growth of the United States’ aging population, it is important to understand risk factors of cognitive impairment in late life in minority populations, including their potential to be modified to reduce risk and decrease health disparities.
In minority populations, the strength of the effects of ApoE e4 on dementia risk [5], as well as those of education on cognitive performance [7, 8], differ from non-Hispanic Whites. While the association between ApoE e4 and dementia is less strong among non-Hispanic Blacks than non-Hispanic Whites, the effect of ApoE e4 is still relevant [9, 10]. In a meta-analysis by Farrer, et al. [11], the odds ratios to develop AD having the e4 allele compared to the APOE e3/e3 genotype in non-Hispanic Blacks were 1.8 (e2/e4), 1.1 (e3/e4), and 5.7 (e4/e4). In addition to AD risk, ApoE e4 has been demonstrated to be a risk factor for cognitive impairment in healthy older adults as well [12–16]. For example, our previous work demonstrated lower scores on semantic memory in dementia-free non-Hispanic Black ApoE e4 carriers compared to non-carriers [17].
In parallel, higher educational attainment reduces the risk of cognitive decline among older individuals [18, 19]. Shadlen, et al. [6] showed that the risk of dementia among non-Hispanic Blacks was 2.6 times smaller for those with high education (>10 years) compared to those with low education (≤10 years). More years of education may serve as a protective factor against clinical symptoms of dementia in the presence of neuropathology, through a phenomenon called cognitive reserve [20–22]. Apart from years of education, the quality of education that non-Hispanic Black older adults received differs from that of non-Hispanic Whites [23]. During legal racial segregation in the United States, when Black older adults grew up, the school quality in Black schools was substantially lower than that in White schools. The difference in school quality was due to the difference in amount of resources allocated to the school systems, shorter school years, and other social inequalities [24, 25]. Because the construct of education may be inequivalent across racial groups, it is important to characterize within-group effects of education on cognitive aging among non-Hispanic Black older adults.
At the intersection of the risk factors of education and ApoE e4, educational attainment may moderate the risk of ApoE e4 on cognitive decline. Winnock, et al. [12] showed that the effect of ApoE e4 on cognition disappeared after adjusting for education. In non-Hispanic Blacks, more years of education was an important predictor of less cognitive change over time among ApoE e4 carriers [26]. Besides years of education, Kaup, et al. [26] also found female sex to be a predictor of cognitive resilience among non-Hispanic Black ApoE e4 carriers. Both Winnock, et al. [12] and Kaup, et al. [26] investigated the effect of education on the relation between ApoE e4 and general cognitive status, measured with the Mini-Mental State Examination [MMSE; 27] and its modified version [3MS; 28], respectively. The MMSE/3MS, being a cognitive screener, is only a gross measure of cognition and does not characterize functioning across different cognitive domains. Little is known about the role of educational attainment within the relation between ApoE genotype and late-life cognitive functioning in non-Hispanic Blacks across multiple cognitive domains, including memory (e.g., episodic memory, semantic memory) and non-memory domains (e.g., executive function, processing speed).
This study investigated the relationship between presence of the ApoE e4 allele and cognition in multiple domains among older non-Hispanic Blacks, and tested whether number of years of formal education moderates this association. We hypothesized 1) that ApoE e4 carriers would have lower cognitive scores than non-carriers across domains and 2) that ApoE e4 and education would interact, such that the effect of ApoE e4 on cognition is stronger in those with lower educational attainment than in those with higher educational attainment. In addition, we were interested in whether the relation of ApoE e4, cognition, and education would differ across sex/gender and age. Based on the findings by Kaup, et al. [26], we hypothesized that the moderating effect of education on the relationship between ApoE e4 and cognition would be stronger among women compared to men. Given the larger variability in cognitive impairment at older age, we expected that the main effect of ApoE e4 on cognition and the interaction by education would be stronger among the oldest-old compared to young-old adults.
METHODS
Participants
Participants were 849 self-identified non-Hispanic Blacks in a multi-center study investigating genetic and environmental pathways of AD pathogenesis among non-Hispanic Blacks [29]. Inclusion criteria for the current study were being nondemented and having available ApoE e4 status and reported years of education. All individuals were dementia-free as evaluated at a consensus conference by standard research criteria [30–33], based on information from neurological, neuropsychological, medical, and functional assessments. A subset of the individuals was identified as having amnestic mild cognitive impairment (MCI), non-amnestic MCI, or impairment but not MCI (n = 276, 32.5%). Among all participants, 325 (38.3%) were ApoE e4 carriers (298 heterozygotes, 27 homozygotes) and 524 were non-carriers.
Race and ethnicity, sex/gender, and years of education were self-reported based on U.S. Census criteria [34]. All participants endorsed English as their primary language. Quality of education was assessed with the Wide Range Achievement Test 3 (WRAT-3) Reading performance subtest [35, 36]. Participants were excluded from participation if they reported a past history of psychosis, epilepsy, electroconvulsive therapy, Parkinson’s disease, or Huntington’s disease. ApoE genotyping was performed as described by Hixon and Vernier with slight modification [37]. Individuals were categorized as ApoE e4 positive or negative based on the presence of at least one e4 allele.
Participants were recruited for a multi-site case-control study on genetic and environmental risk factors for Alzheimer’s disease among non-Hispanic Blacks between April 2008 and December 2013 (AA Genetics Study; R01 AG028786). Participants were drawn from the Greater New York area, North Carolina, South Carolina, Virginia, Georgia, Tennessee, Kentucky, and Alabama. Recruitment took place through educational presentations at churches, community centers, and local chapters of African American organizations, and advertisements in the media (radio and television, and newspapers).
Participants were tested at one of four sites; Columbia University, North Carolina A&T (NC A&T) State University, Vanderbilt University, and University of Miami. Participants were assessed using neuropsychological tests, a neurological evaluation, and assessment of daily functioning; additionally, blood was collected, and all participants were screened for and asked to participate in a structural MRI scan. The project was approved by the Institutional Review Boards of all participating sites.
Cognitive assessment
Individuals underwent an extensive neuropsychological assessment that evaluated verbal and visual episodic memory, semantic memory, working memory, executive function, and processing speed [38]. Episodic memory was assessed with the California Verbal Learning Test (CVLT) [39], the delayed free recall score of the Logical memory subtest of the Wechsler Memory Scale Revised [40], and the delayed recall of the Rey-Osterrieth Complex Figure (ROCF) [41]. On the CVLT, a verbal learning score was calculated as the score on Trial 5 minus the score on Trial 1, and a CVLT memory retention score was calculated as the delayed recall score divided by the score on Trial 5. On the Logical Memory subtest, a Logical Memory retention score was calculated as the delayed recall score divided by the immediate recall score. On ROCF, a visual memory ROCF retention score was calculated as the delayed recall score divided by the immediate recall score. Auditory working memory was measured with the Digit Span Backward subtest of the Wechsler Memory Scale-Revised. Semantic memory was evaluated with picture naming using the 30-item Boston Naming Test (BNT) [42], and with semantic fluency of animals. Executive function was measured with the Trail Making Test [43] and letter fluency (the total sum correct across three trials). On the Trail Making Test, the score was measured as the difference in seconds between performing Trails A (max. 120 seconds) and Trails B (max. 300 seconds). Processing speed was tested using the Digit Symbol test of the Wechsler Adult Intelligence Scale-Revised [44].
Statistical Analysis
Participant characteristics were analyzed using descriptive statistics, general linear models, and chi-square tests. All cognitive scores were standardized. We performed multiple imputation to account for missing data of cognitive scores and WRAT-3 scores. A missing value analysis revealed that these scores were Missing At Random (MAR). All variables to be imputed were continuous variables, which were handled with predictive mean matching to avoid linearity assumptions regarding imputation. Predictors in the imputation model included diagnosis, age and its dichotomization, sex, site, years of education and its dichotomization, ApoE e4 status and number of alleles, all cognitive scores, WRAT-3, (Modified) Mini-Mental State Examination (MMSE) score [27, 28], Geriatric Depression Scale (GDS) score [45], and the interaction term of ApoE e4 status and education groups. We used Fully Conditional Specification as imputation method with 10 imputations and 20 iterations.
We performed multiple linear regression models to examine the relationship between ApoE e4 status and cognition, with years of education as a moderator. Years of education was dichotomized as up to high school (coded as 1) and beyond high school (coded as 0 as the reference category). First, we tested models of main effects with ApoE e4 status (carrier = 1 vs. non-carrier = 0) and education group as predictors and each cognitive measure as an outcome, adjusted for age, sex/gender, and testing site. Subsequently, we added the interaction term of ApoE e4 and education group to test if education moderates the effect of ApoE e4 on each cognitive measure. We then performed the same models but with the inclusion of quality of education, as measured by WRAT-3 performance, as a covariate. Lastly, we stratified the models by sex/gender and by age group (median split at 68.0 years). We performed sensitivity analyses of main and interaction effects by excluding those with cognitive impairment to assess if the observed patterns remained similar in the restricted sample.
Multiple comparisons were corrected for with a False Discovery Rate (FDR) approach using the Benjamini–Hochberg procedure [46]. In short, p-values of the effect of interest were ordered from smallest to largest and ranked i = 1 through i = 12, respectively. The Benjamini-Hochberg critical value was calculated as (i/m)Q where i is the rank, m is the total number of tests (i.e., 12), and Q is the false discovery rate. The largest p-value in the ranked order that is smaller than the critical value plus all p-values preceding it in rank are considered significant. The Q-value was set at .10 for main effects and .20 for interaction effects, as the statistical power to detect interactions is typically much lower than the power for main effects [47, 48]. All analyses were performed in SPSS Version 25 [49].
RESULTS
Sample characteristics.
The mean age of the participants was 69.3 years (SD = 7.6; range = 44–93). In our sample, 80.1% of the individuals were women, and 39.0% had completed no more than high school. An overview of participant characteristics per ApoE e4 status group is presented in Table 1. ApoE e4 status was not associated with age, and did not differ across sex/gender, education group, or testing site. The prevalence of ApoE e4 did not differ among all the diagnosis groups (normal, amnestic MCI, non-amnestic MCI, or impaired but not MCI), nor when contrasting normal versus all three forms of cognitive impairment (χ2 = 2.432, p = .069).
Table 1.
Participant characteristics
| ApoE e4− | ApoE e4+ | p | ||
|---|---|---|---|---|
| (n = 524) | (n = 325) | |||
| Age (mean (SD)) | 69.36 (7.6) | 69.32 (7.5) | 0.942 | |
| Sex/gender (% women) | 80.9% | 78.8% | 0.446 | |
| Education (% <= high school) | 38.7% | 39.4% | 0.852 | |
| Testing site (%) | Columbia University | 50.0% | 51.1% | 0.996 |
| NC A&T University | 16.2% | 15.1% | ||
| Vanderbilt University | 13.4% | 12.9% | ||
| University of Miami | 20.4% | 20.9% | ||
| Diagnosis (%) | Normal | 69.5% | 64.3% | 0.294 |
| Amnestic MCI | 15.6% | 20.6% | ||
| Non-amnestic MCI | 9.9% | 10.5% | ||
| Impaired, not MCI | 5.0% | 4.6% | ||
Multiple Imputation of cognitive scores.
There were 460 participants (54.2%) with at least one missing value on any of the twelve cognitive measures or WRAT-3. Values were missing for 2.6% of the scores on CVLT verbal learning score, 2.2% on CVLT memory retention, 13.3% on delayed recall of Logical Memory, 13.7% on Logical Memory retention, 12.8% on Digit Span Backward, 41.3% on ROCF delayed recall, 41.7% on ROCF retention, 1.3% on semantic fluency, 13.2% on BNT, 18.7% on Trail Making Test, 13.4% on letter fluency, 14.0% on Digit Symbol, and 5.2% on WRAT-3. A comparison of the characteristics of participants with any missing value and those with completely observed data is provided in Table 2, supporting the MAR assumption by missingness being related to observed characteristics.
Table 2.
Distribution of variables among participants without and with missing values (100%: n=849)
| No missings | ≥ 1 missing | p1 | ||
|---|---|---|---|---|
| (n = 389; 45.8%) | (n = 460; 54.2%) | |||
| ApoE e4 status (% carrier) | 40.6% | 36.3% | .203 | |
| Age (mean (SD)) | 68.0 (7.1) | 70.5 (7.8) | < .001 | |
| Sex/gender (% women) | 80.5% | 79.8% | .863 | |
| Education (% <= high school) | 29.8% | 46.7% | < .001 | |
| Testing site (%) | Columbia University | 82.0% | 23.7% | < .001 |
| NC A&T University | 7.7% | 22.6% | ||
| University of Miami | 5.1% | 33.7% | ||
| Vanderbilt University | 5.1% | 20.0% | ||
| Diagnosis (%) | Normal | 745% | 62% | .001 |
| Amnestic MCI | 13.1% | 21.35% | ||
| Non-amnestic MCI | 9.3% | 10.9% | ||
| Impaired, not MCI | 3.6% | 5.9% | ||
| CVLT verbal learning score (mean (SD)) | .116 (1.009) | −.103 (.982) | .002 | |
| CVLT memory retention (mean (SD)) | .129 (.898) | −.114 (1.070) | < .001 | |
| Logical Memory recall (mean (SD)) | .195 (.963) | −.218 (.997) | < .001 | |
| Logical Memory retention (mean (SD)) | .063 (.935) | −.072 (1.065) | .068 | |
| Digit Span Backward (mean (SD)) | .073 (1.017) | −.081 (.976) | .037 | |
| ROCF recall (mean (SD)) | .103 (.994) | −.368 (.937) | < .001 | |
| ROCF retention (mean (SD)) | .036 (1.102) | −.133 (.441) | .121 | |
| Semantic fluency (mean (SD)) | .12 (.998) | −.104 (.991) | .001 | |
| BNT (mean (SD)) | .205 (.814) | −.229 (1.132) | < .001 | |
| Trail Making Test (mean (SD)) | −.142 (.967) | .184 (1.013) | < .001 | |
| Letter fluency (mean (SD)) | .115 (.978) | −.129 (1.010) | .001 | |
| Digit Symbol (mean (SD)) | .248 (.944) | −.282 (.988) | < .001 | |
| WRAT-3 (mean (SD)) | 45.43 (5.88) | 44.35 (7.29) | .021 | |
Note.
Calculated with chi-square tests and t-tests;
CVLT = California Verbal Learning Test; ROCF = Rey-Osterrieth Complex Figure; BNT = Boston Naming Test; WRAT-3 = Wide Range Achievement Test 3; all cognitive scores were standardized.
ApoE e4, education, and cognition.
Table 3 shows the relationships of ApoE e4 and education with each cognitive measure in models adjusted for age, sex/gender, and testing site. Individuals with ApoE e4 perform worse on CVLT memory retention, but not on any of the other eleven measures. Lower education is associated with lower scores on every cognitive measure except for Logical Memory retention and ROCF retention. Excluding subjects with cognitive impairment (remaining ApoE e4−: n = 364, ApoE e4+: n = 209) yielded largely the same patterns in beta values but lead to a loss of power due to the reduced sample size (Table 3).
Table 3.
Adjusted estimates of main effects of ApoE e4 status and education group
| Whole sample | Restricted sample (sensitivity analysis) | ||||||
|---|---|---|---|---|---|---|---|
| Cognitive measure | B | 95% CI | p | B | 95% CI | p | |
| CVLT verbal learning | ApoE e4 | −.028 | [−.164; .108] | .687 | −.040 | [−.202; .122] | .627 |
| Education | −.169 | [−.307; −.032] | .016* | −.067 | [−.236; .102] | .435 | |
| CVLT memory retention | ApoE e4 | −.189 | [−.324; −.054] | .006* | −.125 | [−.254; .004] | .057 |
| Education | −.305 | [−.442; −.169] | < .001* | −.100 | [−.237; .036] | .150 | |
| Logical Memory Delay | ApoE e4 | −.099 | [−.235; .037] | .153 | −.064 | [−.233; .106] | .461 |
| Education | −.503 | [−.655; −.352] | < .001* | −.449 | [−.628; −.269] | < .001* | |
| Logical Memory retention | ApoE e4 | −.026 | [−.183; .131] | .745 | .011 | [−.176; .198] | .907 |
| Education | −.074 | [−.220; .073] | .325 | −.078 | [−.251; .095] | .379 | |
| Digit Span Backward | ApoE e4 | −.037 | [−.184; .110] | .619 | −.019 | [−.203; .165] | .837 |
| Education | −.400 | [−.538; −.261] | < .001* | −.361 | [−.543; −.179] | < .001* | |
| ROCF Delay | ApoE e4 | −.037 | [−.211; .137] | .672 | .005 | [−.208; .217] | .965 |
| Education | −.419 | [−.587; −.251] | < .001* | −.346 | [−.545; −.147] | .001* | |
| ROCF retention | ApoE e4 | .123 | [−.044; .291] | .147 | .135 | [−.078; .349] | .212 |
| Education | −.041 | [−.227; .146] | .663 | −.066 | [−.285; .153] | .552 | |
| Semantic fluency | ApoE e4 | −.121 | [−.251; .009] | .067 | −.020 | [−.169; .129] | .791 |
| Education | −.277 | [−.409; −.146] | < .001* | −.216 | [−.371; −.062] | .006* | |
| BNT | ApoE e4 | −.056 | [−.194; .082] | .429 | −.078 | [−.225; .068] | .292 |
| Education | −.454 | [−.587; −.321] | < .001* | −.399 | [−.548; −.250] | < .001* | |
| Trails | ApoE e4 | .048 | [−.088; .185] | .488 | .043 | [−.105; .191] | .569 |
| Education | .274 | [.135; .412] | < .001* | .352 | [.198; .507] | < .001* | |
| Letter fluency | ApoE e4 | −.025 | [−.166; .117] | .731 | .042 | [−.124; .208] | .621 |
| Education | −.487 | [−.625; −.348] | < .001* | −.346 | [−.514; −.177] | < .001* | |
| Digit Symbol Test | ApoE e4 | .005 | [−.118; .128] | .934 | .051 | [−.095; .197] | .491 |
| Education | −.578 | [−.705; −.450] | < .001* | −.441 | [−.598; −.284] | < .001* | |
Note. Reference category ApoE e4 status = non-carriers; B = unstandardized coefficient; CI = confidence interval;
FDR-corrected significant; adjusted for age, sex/gender, and testing site;
CVLT = California Verbal Learning Test; ROCF = Rey-Osterrieth Complex Figure; BNT = Boston Naming Test; WRAT-3 = Wide Range Achievement Test 3; ; all cognitive scores were standardized; restricted sample excluded participants with cognitive impairment.
Moderation by education.
Table 4 shows the estimates for ApoE e4, adjusted for age, sex/gender, testing site, in individuals with low versus high education. In individuals with lower educational attainment, ApoE e4 carriers performed worse than non-carriers on CVLT memory retention, but ApoE e4 groups performed similarly in those with higher educational attainment. Formally testing interactions of ApoE e4 status with education confirmed an interaction effect for CVLT memory retention (B = −.369, CI [−.648; −.091], p = .009). A similar pattern was observed for Digit Span Backward: among those with lower educational attainment, ApoE e4 carriers performed worse than non-carriers, but ApoE e4 groups performed similarly in those with higher educational attainment. There was no ApoE e4 by education interaction across the other ten cognitive measures. Figure 1 illustrates the relationships between ApoE e4 status and cognition by education group, adjusted for covariates. The pattern of an interaction effect between ApoE e4 and education on CVLT memory retention remained after excluding subjects with cognitive impairment (B = −.314, CI [−.595; −.032], p = .029) but not for Digit Span Backward (B = −.297, CI [−.658; .064], p = .107) because of a loss of power due to the reduced sample size.
Table 4.
Estimates for the effect of ApoE e4 status on cognition in education strata
| Education group | ||||||
|---|---|---|---|---|---|---|
| High school or less | Beyond high school | |||||
| Cognitive measure | B | 95% CI | p | B | 95% CI | p |
| CVLT verbal learning | −.013 | [−.236; .211] | .911 | −.048 | [−.221; .124] | .582 |
| CVLT memory retention | −.433 | [−.675; −.191] | < .001 | −.050 | [−.209; .109] | .536 |
| Logical Memory | −.044 | [−.258; .170] | .687 | −.153 | [−.332; .027] | .096 |
| Digit Span Backward | −.257 | [−.472; −.042] | .019 | .083 | [−.108; .274] | .394 |
| Rey Figure | −.087 | [−.387; .213] | .559 | −.003 | [−.191; .186] | .976 |
| Semantic fluency | −.058 | [−.272; .156] | .594 | −.164 | [−.328; .001] | .052 |
| BNT | −.081 | [−.326; .164] | .516 | −.057 | [−.217; .102] | .481 |
| Trails | .202 | [−.043; .447] | .106 | −.051 | [−.212; .111] | .537 |
| Letter fluency | −.109 | [−.325; .107] | .324 | .007 | [−.177; .192] | .938 |
| Digit Symbol Test | .013 | [−.181; .206] | .899 | −.012 | [−.175; .152] | .890 |
Note. Reference category ApoE e4 status = non-carriers; B = unstandardized coefficient; CI = confidence interval;
FDR-corrected significant; adjusted for age, sex/gender, and testing site;
CVLT = California Verbal Learning Test; ROCF = Rey-Osterrieth Complex Figure; BNT = Boston Naming Test; WRAT-3 = Wide Range Achievement Test 3; all cognitive scores were standardized
Figure 1.
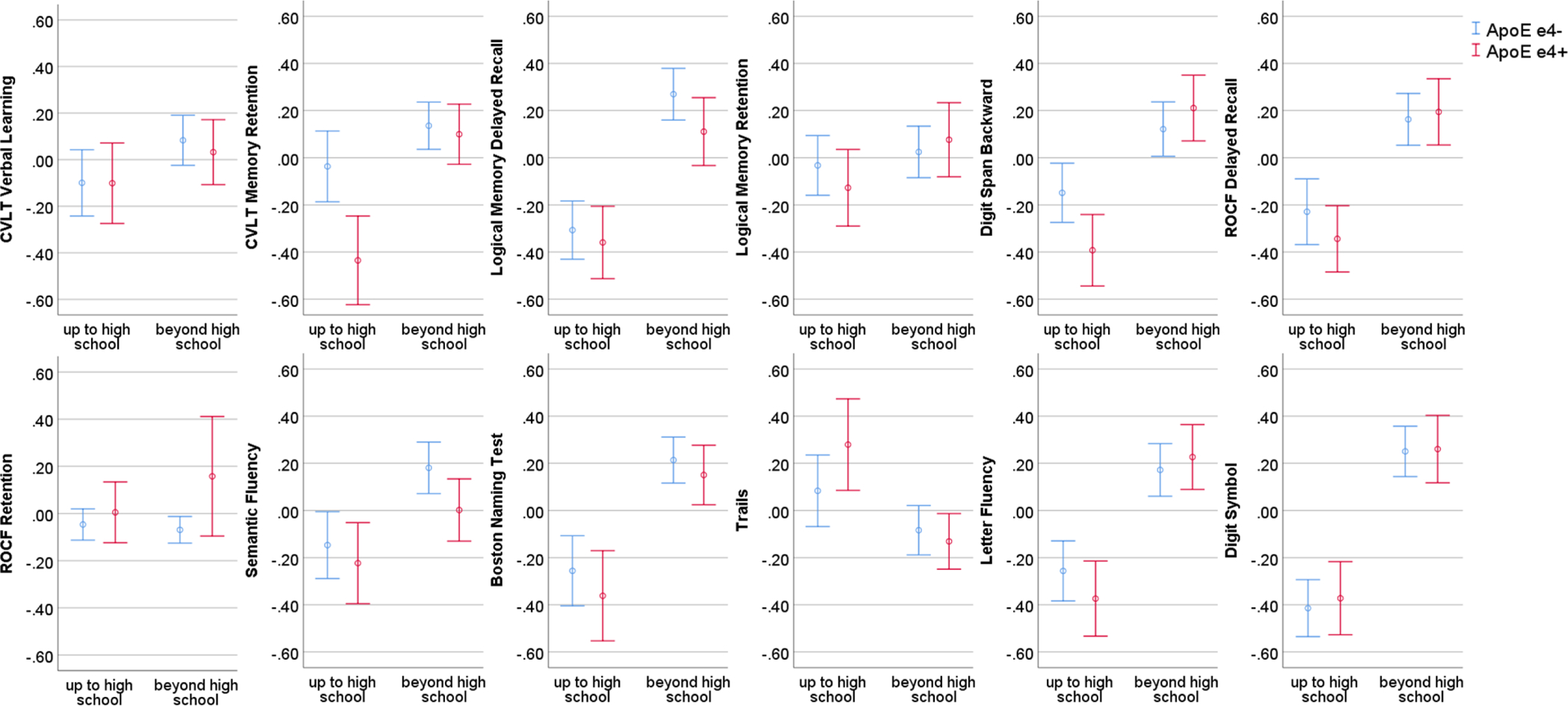
Relationships between ApoE e4 status and cognitive test by education group. Cognitive test scores are standardized residuals after adjusting for age, sex/gender, and testing site; bars represent 95% confidence intervals; figures are based on imputation set 10.
Stratification by sex/gender and age.
To investigate if moderation by education on the relationship between ApoE e4 status and cognition differed as a function of sex/gender or age, we performed analyses for separate strata of men versus women and by age group stratified at the median split (≤ 68 years versus >68 years; Table 5). For CVLT memory retention, main effects of ApoE e4 status on CVLT memory retention scores were stronger for women than men; correspondingly, the interaction between ApoE e4 and education was only present in women. Notably, an effect of ApoE e4 status was present on Digit Span Backward performance in men, but not women. However, the interaction between ApoE e4 and education on Digit Span Backward was only present in women. In age strata, the main effect of ApoE e4 on CVLT memory retention was observed in the group older than 68 years but not in the group younger than 68. No interaction effects between ApoE e4 status and education in age groups survived FDR correction. No other cognitive measures showed interaction effects of ApoE e4 with education in either stratum of sex/gender or age.
Table 5.
Effect estimates of ApoE e4 status, education group, and their interaction on cognitive measures across strata of sex/gender and age groups
| Sex/Gender | Age Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Up to 68 years | Older than 68 years | ||||||||||
| Cognitive measure | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| CVLT verbal learning | ApoE e4 | −.156 | [−.466; .153] | .322 | .000 | [−.153; .154] | .999 | −.081 | [−.272; .109] | .402 | −.007 | [−.205; .191] | .947 |
| Education | −.144 | [−.447; .159] | .350 | −.167 | [−.324..011] | .036* | −.027 | [−.221; .167] | .786 | −.313 | [−.510; −.116] | .002* | |
| ApoE e4*Education | −.336 | [−.947; .275] | .281 | .157 | [−.162; .475] | .335 | .020 | [−.380; .420] | .923 | .056 | [−.346; .458] | .784 | |
| CVLT memory retention | ApoE e4 | −.252 | [−.612; .108] | .171 | −.192 | [−.337; −.048] | .009* | −.074 | [−.249; .101] | .408 | −.312 | [−.522; −.101] | .004* |
| Education | −.104 | [−.459; .251] | .566 | −.351 | [−.496; −.205] | < .001* | −.322 | [−.503; −.142] | < .001* | −.323 | [−.531; −.114] | .002* | |
| ApoE e4*Education | −.005 | [−.721; .712] | .990 | −.481 | [−.778; −.184] | .001* | −.347 | [−.707; .013] | .059 | −.385 | [−.810; .039] | .075 | |
| Logical Memory | ApoE e4 | −.216 | [−.519; .087] | .163 | −.084 | [−.235; .067] | .273 | −.026 | [−.220; .168] | .791 | −.182 | [.376; .012] | .067 |
| Education | −.245 | [−.551; .060] | .115 | −.565 | [−.742; −.389] | < .001* | −.463 | [−.657; −.269] | < .001* | −.571 | [−.809; −.333] | < .001* | |
| ApoE e4*Education | .328 | [−.274; .931] | .285 | .064 | [−.260; .389] | .697 | .251 | [−.168; .670] | .239 | −.002 | [−.417; .412] | .992 | |
| Logical Memory retention | ApoE e4 | .008 | [−.358; .374] | .996 | −.037 | [−.206; .132] | .666 | .135 | [−.072; .343] | .201 | −.200 | [−.419; .020] | .074 |
| Education | −.013 | [−.395; .369] | .947 | −.094 | [−.264; .077] | .280 | −.088 | [−.292; .117] | .401 | −.093 | [−.322; .137] | .426 | |
| ApoE e4*Education | −.209 | [−.956; .538] | .582 | −.035 | [−.383; .312] | .841 | .113 | [−.316; .541] | .606 | −.219 | [−.694; .256] | .363 | |
| Digit Span Backward | ApoE e4 | −.330 | [−.637; −.023] | .035* | .028 | [−.143; .199] | .748 | −.101 | [−.304; .103] | .332 | .012 | [−.188; .213] | .906 |
| Education | −.192 | [−.506..123] | .232 | −.442 | [−.692; −.282] | < .001* | −.288 | [−.494; −.081] | .006* | −.520 | [−.725; −.315] | < .001* | |
| ApoE e4*Education | −.195 | [−.833; .443] | .549 | −.346 | [−.671; −.020] | .037* | −.431 | [−.843; −.019] | .040 | −.231 | [−.650; .188] | .280 | |
| ROCF delay | ApoE e4 | .150 | [−.229; .528] | .434 | −.081 | [−.266; .104] | .383 | .062 | [−.190; .314] | .622 | −.150 | [−.365; .065] | .171 |
| Education | −.447 | [−.869; −.025] | .038* | −.420 | [−.609; −.232] | < .001* | −.431 | [−.661; −.202] | < .001* | −.430 | [−.653; −.207] | < .001* | |
| ApoE e4*Education | −.031 | [−.764; .702] | .933 | −.124 | [−.496; .248] | .509 | −.228 | [−.675; .219] | .314 | −.067 | [−.374; .508] | .764 | |
| ROCF retention | ApoE e4 | .093 | [−.353; .538] | .674 | .130 | [−.051; .310] | .159 | .162 | [−.080; .403] | .189 | .093 | [−.128; .313] | .403 |
| Education | .007 | [−.399; .413] | .973 | −.053 | [−.239; .134] | .578 | −.100 | [−.347; .146] | .424 | .013 | [−.261; .287] | .923 | |
| ApoE e4*Education | .039 | [−.904; .981] | .933 | −.178 | [−.532; .176] | .324 | −.253 | [−.791; .284] | .353 | .003 | [−.394; .399] | .989 | |
| Semantic fluency | ApoE e4 | −.408 | [−.750; −.065] | .020* | −.050 | [−.190; .089] | .481 | −.168 | [−.362; .027] | .091 | −.077 | [−.256; .102] | .400 |
| Education | −.188 | [−.525; .148] | .273 | −.289 | [−.431; −.148] | < .001* | −.236 | [−.434; −.037] | .020* | −.335 | [−.514; −.157] | < .001* | |
| ApoE e4*Education | −.059 | [−.741; .624] | .866 | .181 | [−.108; .471] | .220 | .019 | [−.384; .422] | .927 | .166 | [−.200; .531] | .374 | |
| BNT | ApoE e4 | −.002 | [−.307; .303] | .990 | −.081 | [−.237; .075] | .308 | −.055 | [−.226; .116] | .528 | −.071 | [−.290; .147] | .522 |
| Education | −.269 | [−.568; .030] | .078 | −.503 | [−.651; −.355] | < .001* | −.411 | [−.582; −.240] | < .001* | −.516 | [−.735; −.297] | < .001* | |
| ApoE e4*Education | .100 | [−.495; .696] | .741 | −.063 | [−.380; .254] | .697 | −.143 | [−.489; .202] | .416 | .122 | [−.340; .583] | .604 | |
| Trails | ApoE e4 | .108 | [−.215; .432] | .512 | .038 | [−.113; .189] | .623 | .041 | [−.142; .224] | .663 | .049 | [−.157; .255] | .642 |
| Education | .188 | [−.130; .505] | .246 | .294 | [.139; .448] | < .001* | .311 | [.123; .499] | .001* | .247 | [.041; .452] | .019* | |
| ApoE e4*Education | .470 | [−.169; 1.108] | .149 | .195 | [−.120; .511] | .225 | .323 | [−.057; .703] | .096 | .182 | [−.238; .601] | .396 | |
| Letter fluency | ApoE e4 | −.071 | [−.393; .251] | .667 | −.024 | [−.179; .132] | .767 | −.137 | [−.335; .062] | .176 | .073 | [−.130; .276] | .480 |
| Education | −.279 | [−.601; .042] | .089 | −.541 | [−.697; −.384] | < .001* | −.386 | [−.586; −.185] | < .001* | −.577 | [−.774; .380] | < .001* | |
| ApoE e4*Education | −.322 | [−.948; .305] | .315 | −.050 | [−.369; .269] | .759 | −.316 | [−.719; .087] | .124 | .085 | [−.323; .493] | .682 | |
| Digit Symbol Test | ApoE e4 | .052 | [−.239; .342] | .727 | −.016 | [−.152; .121] | .822 | .020 | [−.162; .202] | .830 | −.018 | [−.204; .167] | .847 |
| Education | −.511 | [−.808; −.215] | .001* | −.594 | [−.737; −.450] | < .001* | −.532 | [−.716; −.347] | < .001* | −.651 | [−.828; −.474] | < .001* | |
| ApoE e4*Education | −.313 | [−.865; .239] | .267 | .113 | [−.179; .406] | .447 | −.191 | [−.576; .193] | .329 | .225 | [−.130; .580] | .213 | |
Note. Reference category ApoE e4 status = non-carriers; B = unstandardized coefficient; CI = confidence interval;
FDR-corrected significant; adjusted for age, sex/gender, and testing site;
CVLT = California Verbal Learning Test; ROCF = Rey-Osterrieth Complex Figure; BNT = Boston Naming Test; WRAT-3 = Wide Range Achievement Test 3; all cognitive scores were standardized.
Adjustment for quality of education.
To explore the influence of quality of education, we repeated the models for main effects of ApoE e4 status and education, and their interaction effect, adjusted for covariates as well as WRAT-3 score. Addition of WRAT-3 did not change the results for CVLT memory retention, with similar main effects of ApoE e4 status (B = −.189, CI [−.324; −.054], p = .006) and education (B = −.273, CI [−.418; −.127], p < .001), as well as their interaction effect (B = −.362, CI [−.641; −.084], p = .011). For Digit Span Backward, the model with WRAT-3 showed a similar but weaker pattern compared to a model without WRAT-3, without main effects of ApoE e4 status (B = −.038, CI [−.174; .099], p = .587) or education (B = −.079, CI [−.215; .056], p = .251), and their interaction effect not surviving FDR correction (B = −.252, CI [−.519; .016], p = .065).
DISCUSSION
We found that among older non-Hispanic Black adults studied cross-sectionally, education buffered the negative effects of the ApoE e4 allele, such that there was no impact of e4 status on CVLT memory retention and Digit Span Backward among participants with more than a high school degree. This pattern was stronger in women compared to men. The moderation of education on the relation of ApoE e4 on cognition was only observed for CVLT memory retention and Digit Span Backward, but not for other memory and non-memory measures—although a similar trend was observed for switching ability in executive functioning.
Main effects of ApoE e4 [13, 17, 50] and education [18, 51] on cognition have been well-established in the literature. Education is arguably the most important non-biological predictor of cognitive performance in old age [52]. Our findings suggest a gene-environment interaction between ApoE e4 and education on cognition, and replicates prior studies [12, 26]. The modifying effect of education on CVLT memory retention and Digit Span Backward in ApoE e4 carriers is consistent with the framework of cognitive reserve such that higher educational attainment may provide cognitively enriching activities and acquisition of skills and knowledge that enables the ability to maintain cognitive function in the presence of disease risk [53]. Future research should replicate our findings in a larger cohort and test whether the moderation effect of education on the relation between ApoE e4 and CVLT memory retention differs among race/ethnicity groups, e.g., non-Hispanic Blacks, non-Hispanic Whites, and Hispanics.
There may be additional circumstances and correlates of lower versus higher education throughout the life course (e.g., childhood socio-economic status, income in adulthood) that may contribute to the observed effect that should be investigated. For example, the health benefits of education may be undermined by structural discrimination in non-Hispanic Black men [54]. These structural limitations that frame the context of life-course social factors in non-Hispanic Blacks highlight the need to deconstruct what ‘genetic risk for cognitive impairment’ means in minority populations, when there are so many strong social factors that can affect health [55]. Additional directions for future research are to investigate to what extent modification by social and environmental factors on genetic risk for cognitive impairment differs among race/ethnicity groups, and which factors specifically contribute to potential dissimilarities.
Not all studies confirm a protective effect of education on genetic risk for cognitive decline. For example, Seeman, et al. [56] did not find any modifying effect of education on baseline global cognition by ApoE e4 risk. In our study, we expected education to buffer the effects of ApoE e4 on all cognitive measures (e.g., semantic memory, executive function, and processing speed), but the moderation effect was only on CVLT memory retention and Digit Span Backward. The effect of CVLT memory retention, however, was the only measure to uniquely and consistently show the main effects of ApoE e4 and its interaction effect with years of education, even independent of quality of education. A possible explanation may be that difficulty in retention of a word list is typically one of the first—if not the first—cognitive manifestations in the cascade of AD-related cognitive impairment [57–59]. Thus, in our sample of individuals without dementia, we were only able to observe the effect of ApoE e4 and education moderation robustly on this sensitive indicator of word-list memory.
In our sample, 32.5% of the individuals were classified in consensus conference as having cognitive impairment but not dementia (of whom 85.1% with MCI). Mild signs of cognitive impairment are often considered to put an individual at increased risk of developing dementia [60]. However, not all individuals with MCI progress to dementia; a meta-analysis by Bruscoli and Lovestone [61] reported annual conversion rates ranging from 10.9–31.1%. Our sensitivity analysis that excluded individuals with mild impairment mirrored a similar pattern as was observed in the whole sample for the relation between ApoE e4, education, and CVLT memory retention. Importantly, the prevalence of ApoE e4 in our sample did not differ between those who were cognitively normal and those that had some mild form of cognitive impairment.
Stratified analyses by sex/gender revealed a stronger main effect of ApoE e4 on CVLT memory retention in women than men, a finding that is in line with previous findings [11, 26], particularly between ages 65 and 75 [62]. While the interaction between ApoE e4 and education on memory has not been previously studied across sex/gender, prior work shows that women tend to outperform men in measures of episodic memory [51, 63, 64], and that sex/gender and education interact such that men with fewer years of education perform worse on verbal memory than women with both high and low education attainment [65]. We also found that the effects of ApoE e4 on CVLT memory retention were particularly present in the group that was older than 68 years. The absence of a main effect of ApoE e4 in our participants of 68 years and younger extends previous observations that the effect of ApoE e4 on cognition was not yet expressed in 45–55 year-olds [66].
We did not consider local ancestry of the APOE haplotype in this analysis, and if years of education is associated with local African ancestry in our cohort, the moderation of schooling on the effect of ApoE e4 could be due to confounding. Prior research indicates that within admixed groups, such as Hispanics and African Americans, those who inherit a genetic region around the ApoE allele of African ancestry are at lower risk for AD than those who inherit a European ApoE region [67, 68]. Marden, et al. [69] demonstrated a relationship between global African genetic ancestry and social factors such as years of education. A next step in this line of research will be to determine whether local African ancestry surrounding the ApoE gene is a common cause of both educational attainment (through structural racism that has denied Black people educational opportunities throughout the history of the United States) and protective genetic variants that surround the E4 allele for those with African ancestral background.
Our study has strengths and limitations. Strengths include a large sample of non-Hispanic Blacks that had been genotyped and underwent an extensive neuropsychological battery of cognitive tasks. Within-group examinations of non-Hispanic Blacks and Hispanics are crucial to advance aging research in minorities because they can 1) reveal the degree of within-group heterogeneity of psychological processes and how meaningful social variables contribute to this variability, and 2) potentially expose processes underlying specific behaviors or measurement differences that may be lost in between-group comparisons [70]. One limitation includes the low percentage of men among our participants. This gender gap is in the same direction—yet more extreme—as in the general population, with women making up 60% of the population of non-Hispanic Blacks aged 65 and older in 2014 [71]. The limited number of men in stratified analyses may explain the absence of a significant main effect of ApoE e4 on CVLT memory retention for men, as the effect size (indicated by the beta estimate) was approximately equal for men and women. Another limitation is that the low number of homozygote ApoE e4 carriers (n = 23) prevented us from performing analyses stratified by number of alleles. Our hypothesis would have been that the main effect of ApoE e4 as well as its interaction effect with education would be expressed more strongly in homozygotes than heterozygotes. Future research should investigate the possibility of a dose effect by number of alleles. Lastly, a limitation is that our sample was not community-based but volunteer-based, with recruitment via flyers and advertisements in newspapers and online. This recruitment process can introduce a participation bias. We observe the consequence of this recruitment process in the mean 13.2 years of education among our participants, which is higher than reported for older non-Hispanic Blacks in community-based samples, including means that range from 7.5–11.7 years [72–74]. Future studies should replicate our findings in a community-based cohort.
In sum, our findings suggest that genetic effects on late-life cognition may be modified by environmental factors such as education. Importantly, this effect was observed in a sample of non-Hispanic Blacks, a minority population that is consistently shown to have higher prevalence of ApoE e4, fewer years of education, and higher dementia rates compared to non-Hispanic Whites. Therefore, these findings underscore the large potential that lays in targeting modifiable risk factors such as education early in life with public policy to reduce risk of cognitive decline and decrease health disparities in later life.
Acknowledgments
This work was supported by National Institutes of Health/National Institute on Aging Grant R01 AG028786. We thank Larry Deon Adams, Jovita Inciute, Tsvyatko P. Dorovski, Raquel Cabo, Josina Habegger, Karmen Louie, and Elizabeth Allocco for their help with recruitment, data collection, and/or data management, and Gelan Ying for her help in organizing statistical output.
REFERENCES
- [1].Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature Reviews Neurology 9, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hyman BT, Gomez-Ilsa T, Briggs M, Chung H, Nichols S, Kohout F, Wallace R (1996) Apolipoprotein E and cognitive change in an elderly population. Annals of Neurology 40, 55–66. [DOI] [PubMed] [Google Scholar]
- [3].Gross AL, Mungas DM, Crane PK, Gibbons LE, MacKay-Brandt A, Manly JJ, Mukherjee S, Romero H, Sachs B, Thomas M (2015) Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychology and aging 30, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zahodne LB, Stern Y, Manly JJ (2015) Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology 29, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tang MX, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, Andrews H, Feng L, Tycko B, Mayeux R (1998) The APOE-e4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755. [DOI] [PubMed] [Google Scholar]
- [6].Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S (2006) Education, cognitive test scores, and black‐white differences in dementia risk. Journal of the American Geriatrics Society 54, 898–905. [DOI] [PubMed] [Google Scholar]
- [7].Manly JJ, Jacobs DM, Sano M, Bell K, Merchant CA, Small SA, Stern Y (1998) Cross-cultural comparison of neuropsychological test performance and diagnosis of dementia. Neurology 50, 91. [Google Scholar]
- [8].Scarmeas N, Albert S, Manly J, Stern Y (2006) Education and rates of cognitive decline in incident Alzheimer’s disease. Journal of Neurology, Neurosurgery & Psychiatry 77, 308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tang MX, Maestre G, Tsai WY, Liu XH, Feng L, Chung WY, Chun M, Schofield P, Stern Y, Tycko B, Mayeux R (1996) Relative risk of Alzheimer’s disease and age-at-onset distributions, based on APOE genotypes among elderly African-Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet 58, 574–584. [PMC free article] [PubMed] [Google Scholar]
- [10].Barnes LL, Bennett DA (2015) Cognitive resilience in APOE ε4 carriers—is race important? Nature Reviews Neurology 11, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM (1997) Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama 278, 1349–1356. [PubMed] [Google Scholar]
- [12].Winnock M, Letenneur L, Jacqmin-Gadda H, Dallongeville J, Amouyel P, Dartigues J-F (2002) Longitudinal analysis of the effect of apolipoprotein E ε4 and education on cognitive performance in elderly subjects: the PAQUID study. Journal of Neurology, Neurosurgery & Psychiatry 72, 794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R (1998) Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 29, 388–398. [DOI] [PubMed] [Google Scholar]
- [14].Caselli RJ, Reiman E, Osborne D, Hentz J, Baxter L, Hernandez J, Alexander G (2004) Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology 62, 1990–1995. [DOI] [PubMed] [Google Scholar]
- [15].Liu F, Pardo LM, Schuur M, Sanchez-Juan P, Isaacs A, Sleegers K, de Koning I, Zorkoltseva IV, Axenovich TI, Witteman JC (2010) The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiology of aging 31, 1831–1833. [DOI] [PubMed] [Google Scholar]
- [16].Fillenbaum GG, Landerman LR, Blazer DG, Saunders AM, Harris TB, Launer LJ (2001) The relationship of APOE genotype to cognitive functioning in older African‐American and Caucasian community residents. Journal of the American Geriatrics Society 49, 1148–1155. [DOI] [PubMed] [Google Scholar]
- [17].Vonk JMJ, Flores RJ, Rosado D, Qian C, Cabo R, Habegger J, Louie K, Allocco E, Brickman AM, Manly JJ (2019) Semantic network function captured by word frequency in nondemented APOE ε4 carriers. Neuropsychology 33(2), 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anstey K, Christensen H (2000) Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology 46, 163–177. [DOI] [PubMed] [Google Scholar]
- [19].Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, Roberts RO, Geda YE, Rocca WA, Petersen RC (2014) Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA neurology 71, 1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB (1997) Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. The American Journal of Psychiatry 154, 165–172. [DOI] [PubMed] [Google Scholar]
- [21].Stern Y, Alexander GE, Prohovnik I, Mayeux R (1992) Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Annals of Neurology 32, 371–375. [DOI] [PubMed] [Google Scholar]
- [22].Valenzuela MJ, Sachdev P (2006) Brain reserve and dementia: a systematic review. Psychological medicine 36, 441–454. [DOI] [PubMed] [Google Scholar]
- [23].Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y (2002) Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society 8, 341–348. [DOI] [PubMed] [Google Scholar]
- [24].Aiken-Morgan AT, Gamaldo AA, Sims RC, Allaire JC, Whitfield KE (2014) Education desegregation and cognitive change in African American older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 70, 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aiken-Morgan AT, Marsiske M, Whitfield KE (2007) Characterizing and explaining differences in cognitive test performance between African American and European American older adults. Experimental aging research 34, 80–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaup AR, Nettiksimmons J, Harris TB, Sink KM, Satterfield S, Metti AL, Ayonayon HN, Yaffe K (2015) Cognitive resilience to apolipoprotein E ε4: contributing factors in black and white older adults. JAMA neurology 72, 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [28].Teng EL, Chui HC (1987) The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry 48, 314–318. [PubMed] [Google Scholar]
- [29].Hamilton JL, Brickman AM, Lang R, Byrd GS, Haines JL, Pericak-Vance MA, Manly JJ (2014) Relationship between depressive symptoms and cognition in older, non-demented African Americans. Journal of the International Neuropsychological Society 20, 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, American Psychiatric Press, Washington, DC. [Google Scholar]
- [31].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JL, Garcia JH, Amaducci LA, Orgogozo JM, Brun A, Hofman A (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260. [DOI] [PubMed] [Google Scholar]
- [33].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56, 303–308. [DOI] [PubMed] [Google Scholar]
- [34].U.S. Census Bureau (2003) 2000 Census of Population and Housing. In Population and Housing Unit Counts, PHC-3–50 West Virginia Washington, D.C. [Google Scholar]
- [35].Wilkinson GS (1993) Wide Range Achievement Test 3–Administration Manual, Jastak Associates, Inc, Wilmington, DE. [Google Scholar]
- [36].Manly J, Jacobs DM, Sano M, Small SA, Merchant C, Touradji P, Stern Y (1999) Quality of education and neuropsychological test performance among nondemented community-dwelling elders. Neurology 52, A433. [Google Scholar]
- [37].Hixson JE, Vernier DT (1990) Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. Journal of lipid research 31, 545–548. [PubMed] [Google Scholar]
- [38].Meier IB, Manly JJ, Provenzano FA, Louie KS, Wasserman BT, Griffith EY, Hector JT, Allocco E, Brickman AM (2012) White matter predictors of cognitive functioning in older adults. Journal of the International Neuropsychological Society 18, 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Delis DC, Kramer JH, Kaplan E, Ober BA (1987) California Verbal Learning Test: Research edition, Psychological Corporation, San Antonio. [Google Scholar]
- [40].Wechsler D (1945) A standardized memory scale for clinical use. Journal of Psychology 19, 87–95. [Google Scholar]
- [41].Osterrieth PA (1944) Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. Archives de psychologie. [Google Scholar]
- [42].Kaplan E, Goodglass H, Weintraub S (1983) Boston Naming Test, Lea & Febiger, Philadelphia, PA. [Google Scholar]
- [43].Reitan R (1978) Manual for Administration of Neuropsychological Test Batteries for Adults and Children, Reitan Neuropsychology Laboratories, Inc, Tuscon, AZ. [Google Scholar]
- [44].Wechsler D (1981) Wechsler Adult Intelligence Scale-Revised, The Psychological Corporation, New York, NY. [Google Scholar]
- [45].Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO (1982) Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [46].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 57, 289–300. [Google Scholar]
- [47].Aguinis H (1995) Statistical power problems with moderated multiple regression in management research. Journal of Management 21, 1141–1158. [Google Scholar]
- [48].McClelland GH, Judd CM (1993) Statistical difficulties of detecting interactions and moderator effects. Psychological bulletin 114, 376. [DOI] [PubMed] [Google Scholar]
- [49].IBM Corp (2017) IBM SPSS Statistics for Windows, Version 25, IBM Corp., Armonk, NY. [Google Scholar]
- [50].Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D (2007) Cognitive domain decline in healthy apolipoprotein E ε4 homozygotes before the diagnosis of mild cognitive impairment. Archives of neurology 64, 1306–1311. [DOI] [PubMed] [Google Scholar]
- [51].Van Hooren SAH, Valentijn AM, Bosma H, Ponds R, Van Boxtel MPJ, Jolles J (2007) Cognitive functioning in healthy older adults aged 64–81: a cohort study into the effects of age, sex, and education. Aging, Neuropsychology, and Cognition 14, 40–54. [DOI] [PubMed] [Google Scholar]
- [52].Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN (2009) Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology 72, 2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society 8, 448–460. [PubMed] [Google Scholar]
- [54].Glymour MM, Manly JJ (2018) Compulsory Schooling Laws as quasi-experiments for the health effects of education: Reconsidering mechanisms to understand inconsistent results. Social science & medicine (1982) 214, 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hicken MT, Kravitz-Wirtz N, Durkee M, Jackson JS (2018) Racial inequalities in health: Framing future research. Social science & medicine (1982) 199, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, Guralnik JM (2005) Education and APOE-e4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. The journals of gerontology, Series B: Psychological sciences and social sciences 60, 74–83. [DOI] [PubMed] [Google Scholar]
- [57].Hodges JR, Patterson K (1995) Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia 33, 441–459. [DOI] [PubMed] [Google Scholar]
- [58].Albert SM, Del Castillo-Castaneda C, Sano M, Jacobs DM, Marder K, Bell K, Bylsma F, Lafleche G, Brandt J, Albert M, Stern Y (1996) Quality of life in patients with Alzheimer’s disease as reported by patient proxies. Journal of the American Geriatrics Society 44, 1342–1347. [DOI] [PubMed] [Google Scholar]
- [59].Bäckman L, Jones S, Berger A-K, Laukka EJ, Small BJ (2005) Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology 19, 520–531. [DOI] [PubMed] [Google Scholar]
- [60].Angevaare MJ, Geerlings MI, Vonk JMJ, Bertola L, Zahodne LB, Watson CWM, Schupf NS, Mayeux R, Manly JJ (in preparation) Predictors of incident MCI and its course in an ethnically diverse population-based cohort. [DOI] [PMC free article] [PubMed]
- [61].Bruscoli M, Lovestone S (2004) Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 6, 129 – 140. [DOI] [PubMed] [Google Scholar]
- [62].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang L-S, Romero K, Arneric SP, Redolfi A (2017) Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA neurology 74, 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].De Frias CM, Nilsson L-G, Herlitz A (2006) Sex differences in cognition are stable over a 10-year period in adulthood and old age. Aging, Neuropsychology, and Cognition 13, 574–587. [DOI] [PubMed] [Google Scholar]
- [64].Avila JF, Vonk JMJ, Verney SP, Witkiewitz K, Arce Rentería M, Schupf N, Mayeux R, Manly JJ (in press) Sex/Gender Differences in Cognitive Trajectories Vary as a Function of Race/Ethnicity. Alzheimer’s & Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Perlmutter M (1978) What is memory aging the aging of? Developmental Psychology 14, 330. [Google Scholar]
- [66].Evans S, Dowell NG, Tabet N, Tofts PS, King SL, Rusted JM (2014) Cognitive and neural signatures of the APOE E4 allele in mid-aged adults. Neurobiology of aging 35, 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pericak-Vance M, Rajabli F, Feliciano-Astacio B, Celis K, Hamilton-Nelson K, Adams L, Rodgriguez A, Byrd G, Vance J, Cuccuro M (2017) African haplotypic background mitigates the effect of apoe ε4 risk allele in alzheimer disease. Journal of the Neurological Sciences 381, 1137. [Google Scholar]
- [68].Rajabli F, Feliciano BE, Celis K, Hamilton-Nelson KL, Whitehead PL, Adams LD, Bussies PL, Manrique CP, Rodriguez A, Rodriguez V (2018) Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS genetics 14, e1007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Marden JR, Walter S, Kaufman JS, Glymour MM (2016) African ancestry, social factors, and hypertension among non-Hispanic Blacks in the Health and Retirement Study. Biodemography and social biology 62, 19–35. [DOI] [PubMed] [Google Scholar]
- [70].Whitfield KE, Allaire JC, Belue R, Edwards CL (2008) Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups? The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 63, P301–P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Population Reference Bureau, Table 1. Sex Ratio at Older Ages (65+), by Race/Ethnicity, 2000 and 2014 (Source: U.S. Census Bureau; ), https://www.prb.org/us-oldage-gendergap-smoking/, Accessed March 24 2019. [Google Scholar]
- [72].Vonk JMJ, Arce Rentería M, Avila JF, Schupf N, Noble JM, Mayeux R, Brickman AM, Manly JJ (in press) Secular trends in cognitive trajectories of diverse older adults. Alzheimer’s & Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Barnes LL, Wilson RS, Hebert LE, Scherr PA, Evans DA, Mendes de Leon CF (2011) Racial differences in the association of education with physical and cognitive function in older blacks and whites. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 66, 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sachs-Ericsson N, Blazer DG (2005) Racial differences in cognitive decline in a sample of community-dwelling older adults: the mediating role of education and literacy. The American journal of geriatric psychiatry 13, 968–975. [DOI] [PubMed] [Google Scholar]


