Abstract
Plant-associated fungi (endophytic fungi) are a biodiversity-rich group of microorganisms that are normally found asymptomatically within plant tissues or in the intercellular spaces. Endophytic fungi promote the growth of host plants by directly producing secondary metabolites, which enhances the plant’s resistance to biotic and abiotic stresses. Additionally, they are capable of biosynthesizing medically important “phytochemicals” that were initially thought to be produced only by the host plant. In this review, we summarized some compounds from endophyte fungi with novel structures and diverse biological activities published between 2011 and 2021, with a focus on the origin of endophytic fungi, the structural and biological activity of the compounds they produce, and special attention paid to the exploration of pharmacological activities and mechanisms of action of certain compounds. This review revealed that endophytic fungi had high potential to be harnessed as an alternative source of secondary metabolites for pharmacological studies.
Keywords: endophytic fungi, secondary metabolites, structural feature, biological activities, drug discovery
1. Introduction
The term “endophytic fungi” refers to fungi that live in plant tissues throughout the entire or partial life cycle by establishing a mutually beneficial symbiotic relationship with its host plant without causing any adverse effect or disease [1,2]. They are natural components of the plant micro-ecosystem that positively affect the physiological activities of the host plant in several ways, including producing hormones such as indoleacetic acid, biosynthesizing and acquiring nutrients for plant growth and development, secreting stress-adaptor metabolites to protect the host plant from the invasion of herbivores, pathogens, and improving the host’s adaptability to abiotic stressors. In return, plants provide habitats and nutrients for endophytic fungi [3,4]. Endophytic fungi are capable of producing a rich variety of bioactive substances and can produce compounds that are identical or similar to pharmacological activities identified from plants [5]. They produce a range of metabolites of different chemical classes, including alkaloids, flavonoids, steroids, terpenoids, and phenolic compounds. Some compounds show pleiotropic and interesting pharmacological activities, including antimicrobial, antioxidant, anti-diabetic, anti-malarial, and antitumor properties. The discovery of these structurally novel and diverse active compounds provides a valuable resource for studying natural medical products from the microbiome [6,7,8]. In the search for bioactive molecules as pro-drug compounds or in the development of medicines, endophytic fungi can serve as an alternative source for valuable active plant compounds. Endophytic fungi can be harnessed to produce bioactive compounds for human pharmaceutical use when the bioactive secondary metabolites are not commercially available, derived from slow-growing or rare and endangered plants, and difficult to synthesize due to heavy molecular weight or structural complexity. Endophytic fungal secondary metabolites have drawn extensive attention among medicinal plants, mangroves, and marine microorganisms [9,10].
Endophytic fungi are a highly biodiverse and versatile microbial community that seems to be ubiquitous in nature. Studies have shown that almost all plants contain endophytic fungi, including colonized plants in the Arctic and Antarctic regions, deserts, oceans, and tropical rainforests [11,12]. They have been isolated and cultured from the roots and above-ground parts of various plants, including algae, mosses, ferns, gymnosperms, and angiosperms. Evidence from microorganism’s records in the fossil plant tissue indicated that the plant-endophytic fungal interactions have existed for approximately 400 million years, and during this time, endophytic fungi co-evolved unique biosynthetic pathways and metabolic mechanisms to synthesize complex secondary metabolites [13]. To date, only 5% of 1.5 million fungal species on Earth have been described in detail, and out of this percentage (69,000 fungal species), only 16% (11,500 species) have been cultured and studied. About 0.035–5.1 million fungal species have been found on Earth according to results from next-generation sequencing technologies [14]. Approximately 300,000 known species of higher plants exist on Earth, and each of which is a host for one or more obligate endophytic fungi. The high number of bioactive secondary metabolites found in endophytic fungi is due to their rich species diversity [15,16]. Endophytic fungi have been studied for more than 100 years, with the first endophytic strain isolated from the seeds of ryegrass (Lolium temulentum L.) by Vogl et al. in 1898 [17]. Stierle et al. [18] discovered the paclitaxel-producing endophytic fungus (Taxomyces andreanae) from the Pacific yew and then from other plant species successively. This discovery aroused the attention of mycologists and pharmaceutical chemists on endophytic fungi as a new source of bioactive substances and stimulated the interest in endophytic fungi as a sustainable source of plant metabolites. As shown in Table 1, many compounds that were isolated from endophytic fungi were also identified in some plant species as well as exhibited similar biological activity even though there were isolated from different sources, confirming endophytic fungi as an alternative source of bioactive compounds [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. An overview of the recent literature surveys revealed that 51% of the bioactive substances isolated from endophytic fungi were previously unknown, with about 38% being isolated from soil microbiota [19]. Over the past decade, there has been a surge in the number of patents for endophytic fungi with new molecular secondary metabolites, which play a key role in the pharmaceutical industry, phytoremediation, and biomedicine [20,21]. Researchers are now searching for an economical, environmentally safe, and sustainable way to obtain new bioactive secondary metabolites from endophytic fungi.
Table 1.
Several endophytic fungi of host plants have been reported to produce compounds with similar activity.
| No. | Endophytic Fungus | Host Plant | Regions/Countries | Compound | Biological Activity | Ref. |
|---|---|---|---|---|---|---|
| 1 | Lophiostoma sp. | Eucalyptus exserta | Guangzhou, China. | Scorpinone | Antibacterial | [22] |
| 2 | Mycosphaerella sp. | Myrciaria floribunda | Amazon rainforest, Brazil. | Myriocin | Antifungal | [23] |
| 3 | Mucor sp. | Centaurea stoebe | Idaho, USA | Terezine E | Antifungal and cytotoxicity | [24] |
| 4 | Aspergillus calidoustus | Acanthospermum australe | Jalapao State Park, Tocantins, Brazil. | Ophiobolin K 6-epi-ophiobolin K |
Antifungal, trypanocidal and cytotoxicity | [25] |
| 5 | Phomopsis sp. | Garcinia kola (Heckel) nut | Yaounde, Cameroon | Cytochalasins H | Antibacterial and cytotoxicity | [26] |
| 6 | Aspergillus nidulans | Nyctanthes arbor-tristis Linn | Karachi, Pakistan | Sterigmatocystin | Antiproliferative activity | [27] |
| 7 | Trichoderma asperellum and Trichoderma brevicompactum | Vinca herbacea | Hamedan, Iran | 4b-hydroxy-12,13-epoxytrichothec-9-ene | Antimicrobial and antiproliferative activity |
[28] |
| 8 | Phyllosticta elongata | Cipadessa baccifera | Western Ghats, India | Camptothecin | Anticancer agent | [29] |
| 9 | Fusarium verticillioides | Huperzia serrata | Gucheng Mountain, Sichuan, China | Huperzine A | Treatment of Alzheimer’s disease | [30] |
| 10 | Fusarium solani | Cassia alata | Bangladesh | Napthaquinones Azaanthraquinones |
Cytotoxicity, antimicrobial and antioxidant activity |
[31] |
| 11 |
Fusarium sp. and Lasiodiplodia theobromae |
Avicennia lanata | Terengganu, Malaysia | Anhydrofusarubin dihydrojavanicin |
Antitrypanosomal | [32] |
| 12 | Corynespora cassiicola | Gongronema latifolium | Nigeria | Corynesidone D | Anti-inflammatory/anticancer agent | [33] |
| 13 | Pestalotiopsis theae | Camellia sinensis Theaceae | Hangzhou, China | punctaporonin H | Antibacterial and cytotoxicity | [34] |
| 14 | Phialocephala fortinii | Podophyllum peltatum | Tamilnadu, India | Podophyllotoxin | Antiviral, antioxidant, and antirheumatic activities | [35] |
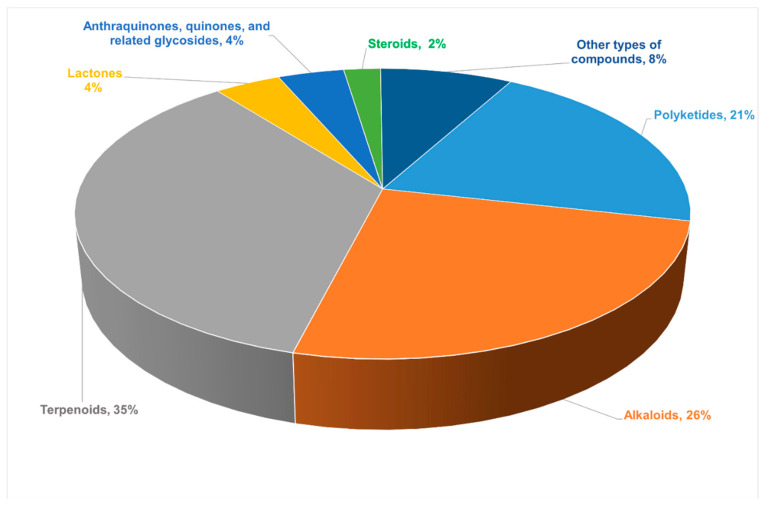
This article reports 220 new compounds with rare or novel structures or skeleton structures from endophytic fungi from 82 journal articles between 2011 and 2021 and briefly describes the sources of endophytic fungi, chemical structures, and biological activities of these compounds. Among all the new compounds reported in this review, terpenoids (35%) were largest in proportion, followed by alkaloids (26%). The proportion of different types of compounds among all the new compounds are presented in Figure 1. These new compounds were obtained from different species of endophytic fungi, which had diverse chemical skeletons and exhibited diverse and interesting biological activities. Additionally, the most common pharmacological activities these compounds showed were antimicrobial and antitumor activities. However, some of the compounds showed anti-angiogenic, anti-phytotoxic, and α-glucosidase inhibitory effects. Therefore, this review summarized different insights into the prospects and challenges of endophytic fungi as an alternative source of plant-derived bioactive compounds for drug development. In addition, this review will affirm that endophytic fungi produce similar bioactive compounds just as their host plants to give knowledge for the development of drug candidates from endophytic fungi using different strategies, thus making Endophytic fungi a treasure trove of new secondary metabolites.
Figure 1.
The proportion of different types of compounds among all new compounds.
2. Bioactive New Metabolites Isolated from Endophytic Fungi and Their Biological Activities
2.1. Polyketides
2.1.1. Chromones
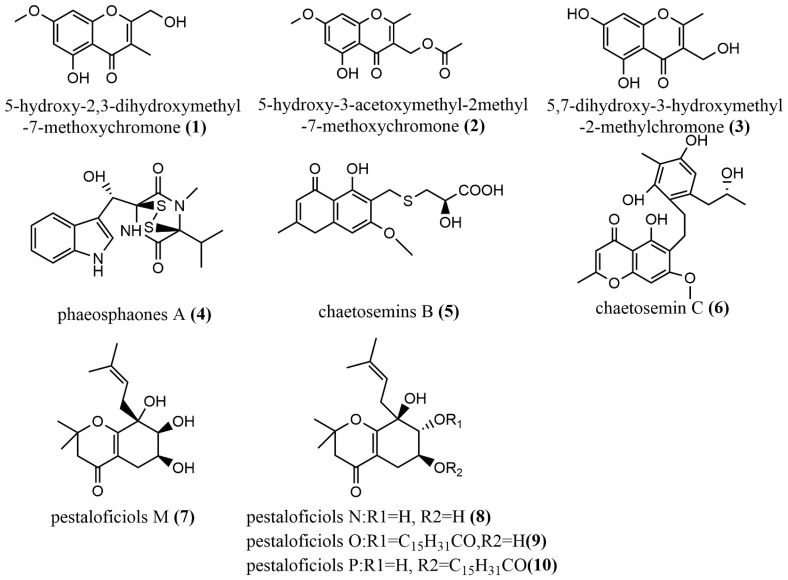
The induction of endophyte metabolism by adding Host components was used to add the same phytocomponents (2R, 3R)-3, 5, 7- trihydroxyflavanone 3-acetate in Botryosphaeria ramosa L29 potato dextrose broth culture to induce the production of 5-hydroxy2,3-dihydroxymethyl-7-methoxychromone 1 (Figure 2), 5-hydroxy-3-acetoxymethyl-2-methyl-7- methoxychromone 2 (Figure 2) and 5,7-dihydroxy-3-hydroxymethyl-2-methylchromone 3 (Figure 2), where Compounds 1–3 displayed acceptable antimicrobial activities against Fusarium oxysporum with MIC values of 50 μg/mL, 50 μg/mL, and 6.25 μg/mL, respectively. These values were superior compared to those of the positive drug—triadimefon—for the antimicrobial test (with an MIC value of 100 μg/mL) [36]. This indicated that the induction of endophytes metabolism to produce bioactive components of interest might be an ideal strategy for easy identification of drug candidates from these microbes; however, there is the need for long-term studies on how specific components influence endophytes metabolism and the bioactive compounds there are linked with. Phaeosphaonesa A 4 (Figure 2), isolated from Phaeosphaeria fuckelii, contains a β-(oxy)thiotryptophan motif structure that is rare in nature. Compound 4 showed stronger inhibition activity of mushroom tyrosinase than the positive control kojic acid (IC50 value of 40.4 μM) at 100 μM concentration, with an IC50 value of 33.2 μM [37]. Two aromatic chromones, Chaetosemins B–C 5–6 (Figure 2), were isolated from Chaetomium seminudum brown rice cultures, and compounds 5–6 contained L-cysteine and D-cysteine units, respectively. Compound 5 showed antifungal activity against Magnaporthe oryzae and Gibberella saubinetti, with MIC values of 6.25 μM and 12.5 μM, respectively. Compound 6 showed significant antioxidant activity at a concentration of 50 μM with a DPPH radical scavenging rate of 50.7% [38]. Pestaloficiols M–P 7–10 (Figure 2), which are new isoprenylated chromone derivatives, were isolated from brown rice culture extract of the plant endophytic fungus Pestalotiopsis fici. The structures of these compounds were elucidated primarily by MS and NMR techniques. Compounds 7–8 displayed inhibitory effects on HIV-1 replication in C8166 cells, with EC50 values of 56.5 μM and 10.5 μM, respectively (the EC 50 value of the positive control Indinavir Sulfate was 8.2 μM), whereas compounds 9–10 showed cytotoxic activity against the human tumor cell line HeLa, with IC50 values of 56.2 μM and 74.9 μM, respectively (the positive control 5-fluorouracil has an IC50 of 10.0 μM). Compound 10 exhibited a potent antifungal activity against Aspergillus fumigatus at IC50 = 7.35 μM) [39].
Figure 2.
Chemical structures of chromones.
2.1.2. α-Pyrones
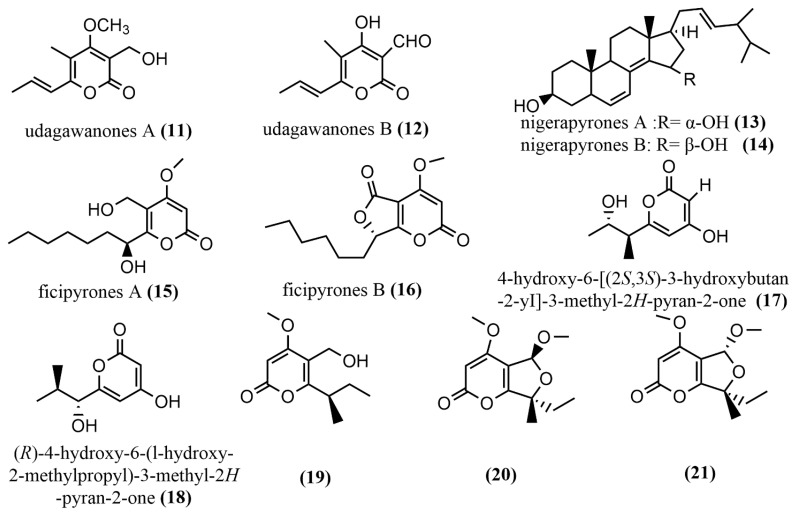
Two tetrasubstituted α-pyrone derivatives—Neurospora udagawae udagawanones A-B 11–12 (Figure 3)—were isolated from oak endophytic fungi, with both containing unique oxidation functional groups at the C-2 position. Compound 11 exhibited potent antifungal activity against Rhodoturula glutinis with MIC = 66 μg/mL). Additionally, compounds 11 and 13 showed moderate cytotoxic activity against KB3.1 cells with IC50 = 27 μg/mL [40]. The study revealed moderate activity of compounds 11 and 12 against fungi and mammalian cells, and this may be as a result of the method (serial dilution antimicrobial assay) used; therefore, it is suggested that other biological tests be employed to verify these findings. The nigerapyrones A–B 13–14 (Figure 3) were obtained from Aspergillus niger MA-132, which was isolated from the mangrove plant Avicennia marina. Compounds 13–14 both showed potent antifungal activities against two tumor cell lines (HL60 and A549), with IC50 values ranging from 0.3 to 5.41 μM [41]. The ficipyrones A–B 15–16 (Figure 3) were isolated from solid cultures of Pestalotiopsis fici. Compound 15 showed significant antifungal activity against Gibberella zeae CGMCC 3.2873, with an IC50 value of 15.9 μM, but had no activity against Fusarium culmorum CGMCC 3.4595 and Verticillium aiboatrum CGMCC 3.4306 [42]. The endophytic fungus Aspergillus oryzae was isolated from the rhizome of Paris polyphylla in Dali, Yunnan, China, and 4-hydroxy-6-[(2S, 3S)-3-hydroxybutan-2-yI]-3-methyl-2H-pyran-2-one 17 (Figure 3) and (R)-4-hydroxy-6-(l-hydroxy-2-methylpropyl)-3-methyl-2H-pyran-2-one 18 (Figure 3) were obtained from this fungi. However, the biological activities of these compounds were not tested in the study; hence, investigating the biological activities of these compounds is needed, as it may yield a very important source of drug activity [43].The pyran-2-one scaffold compounds 19–21 (Figure 3) were isolated by adding 10 mg/L DNA methyltransferase inhibitor 5-aza-2-deoxycytidine to Penicillium herquei liquid cultures, whereas the MTT method was used to measure the cytotoxicity of all compounds in MDA-ME-231 and MV-411 cell lines. Compounds 19–21 showed weak cytotoxicity only against the MV4-11 cell line with IC50 values of 90.09 µM, 74.16 µM, and 70.00 µM, respectively [44].
Figure 3.
Chemical structures of α-pyrone compounds.
2.1.3. Other Polyketides
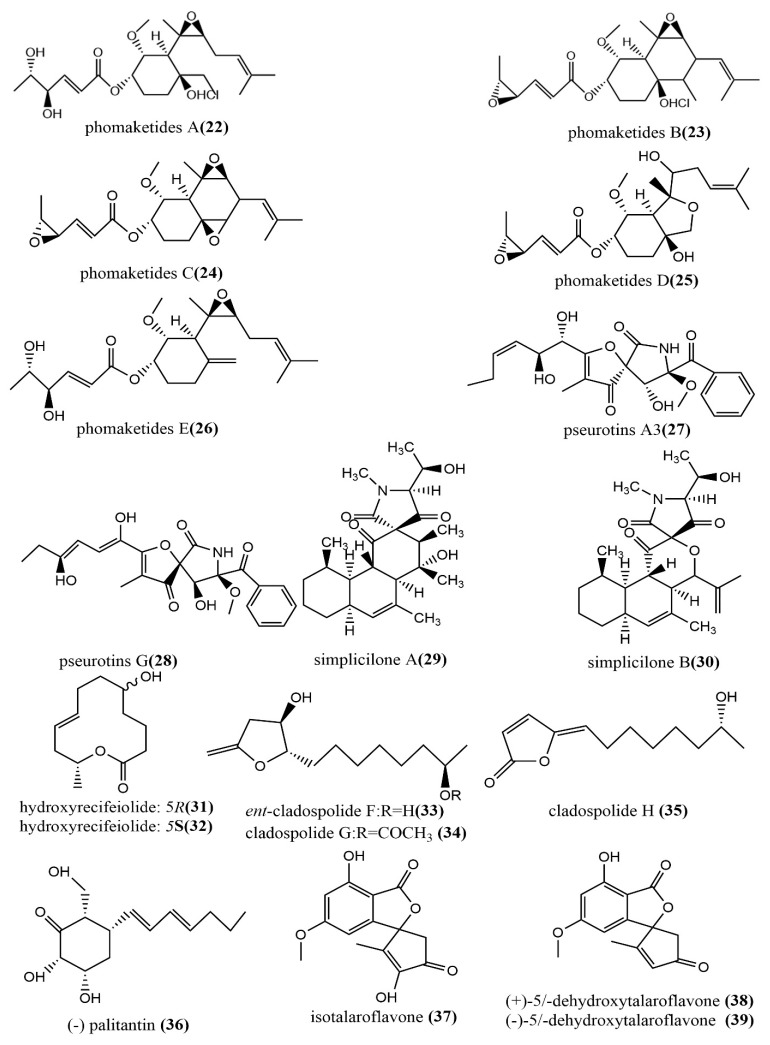
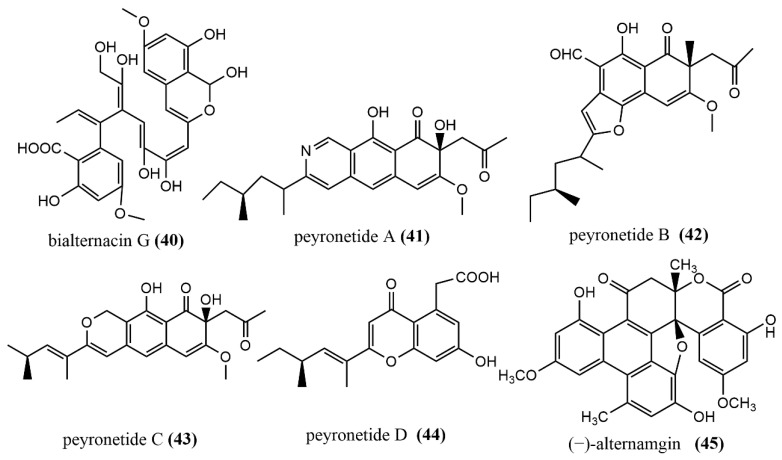
The phomaketides A–E 22–26 (Figure 4), pseurotins A3 27 (Figure 4), and pseurotins G 28 (Figure 4) were isolated from fermentation broth and mycelial extracts of the marine red algae endophytic fungus Phoma sp. NTOU4195. The mouse macrophages RAW264 were induced using the endothelial progenitor cells of human umbilical cord blood, lipopolysaccharide (LPS), to assess the anti-angiogenic and anti-inflammatory activities of all compounds. Compound 22 showed potent anti-angiogenic activity by inhibiting endothelial cell proliferation, with an IC50 value of 8.1 μM. Compound 24 at the concentration of 20 μM induced effective nitric oxide (NO) inhibition activity against LPS-induced RAW264.7 cells, with an IC50 value of 8.8 μM [45]. There were two tetracyclic polyketide compounds, simplicilone A–B 29–30 (Figure 4), containing helical centers obtained from the broth culture of the endophytic fungus Simplicillium sp., which was isolated from the bark of the medicinal plant Duguetia staudtii (Engl. and Diels) Chatrou in the Cameroon region. Compounds 29–30 showed weak cytotoxic activities against the KB3.1 cell line, with IC50 values of 1.25 μg/mL and 2.29 μg/mL, respectively, but had no antimicrobial activity against the tested bacteria (Staphylococcus aureus DSM 346 and Bacillus subtilis DSM 10) [46]. 5R-hydroxyrecifeiolide 31 (Figure 4), 5S-hydroxyrecifeiolide 32 (Figure 4), and ent-cladospolide F–H 33–35 (Figure 4) were also isolated from the endophytic fungal strain Cladosporium cladosporioides MA-299, which was obtained from the leaves of the mangrove plant Bruguiera gymnorrhiza from Hainan Island, China. Compounds 31–35 showed potent antimicrobial activities against Escherichia coli and Staphylococcus aureus, with MIC values ranging from 1.0 to 64 μg/mL. Compound 33 showed moderate inhibition activity against acetylcholinesterase, with an IC50 value of 40.26 μM [47]. The antimicrobial polyketide compound, palitantin 36 (Figure 4), was obtained from Aspergillus fumigatiaffnis and isolated from healthy leaves of Tribulus terrestris L. In addition, compound 36 showed effective antimicrobial activity against the multi-drug-resistant pathogens Enterococcus faecalis UW 2689 and Streptococcus pneumoniae 25697, both with an MIC value of 64 μg/mL [48]. The four polyketide derivatives—isotalaroflavone 37 (Figure 4), (+/−)-50-dehydroxytalaroflavone 38–39 (Figure 4), and bialternacin G 40 (Figure 4)—were obtained from the endophytic fungus Alternaria alternata ZHJG5 isolated from the leaves of Cercis chinensis, which was collected from the Nanjing Botanical Garden, Nanjing, China. They exhibited potent antimicrobial activity against Xanthomonas oryzae pv. oryzicola (Xoc) and Ralstonia solanacearum, with MIC values ranging from 0.5 to 64 μg/mL. Compound 37 at the concentration of 200 μg/mL showed a significant protective effect against the bacterial blight of rice caused by Xanthomonas oryzae pv. oryza, with a protection rate of 75.1% [49]. Four polyketide derivatives containing the benzoisoquinoline-9-one moiety structure peyronetides A–D 41–44 (Figure 4) were isolated from the mycelial crude acetone extract of Peyronellaea sp. FT431. Compounds 41–42 showed moderate to weak cytotoxic activity against human kidney cancer cell line TK10 and human ovarian cancer cell line A2780cisR, with IC50 values ranging from 6.7 to 29.2 μM [50]. The aromatic polyketide compound, (−)alternamgin 45 (Figure 4), was obtained from potato dextrose broth cultures of the endophytic fungus Alternaria sp. MG1 isolated from Vitis quinquangularis. This compound was of particular interest because it had the rare dibenzopyrone functionality of 6/6/6/6/5/6/6/6 heptacyclic backbone. Compound 45 displayed a weak cytotoxic activity against cells from two tested cell lines (Hela and HepG2), both with IC50 values exceeding 20 μM [51].
Figure 4.
Chemical structures composition of other polyketides.
In summary, Polyketides, such as chromones and α-pyrone, and their derivatives identified from plant sources have also been found in endophytic fungi in recent studies. Chromones and their derivatives isolated from both plant and endophytic fungi sources all showed antimicrobial properties against specific pathogens; therefore, chromones from endophytic fungus can be used in the development of antimicrobials in the place of plant chromones to reduce the depletion of plants’ resources in the ecosystem.
2.2. Alkaloids
2.2.1. Cytochalasin
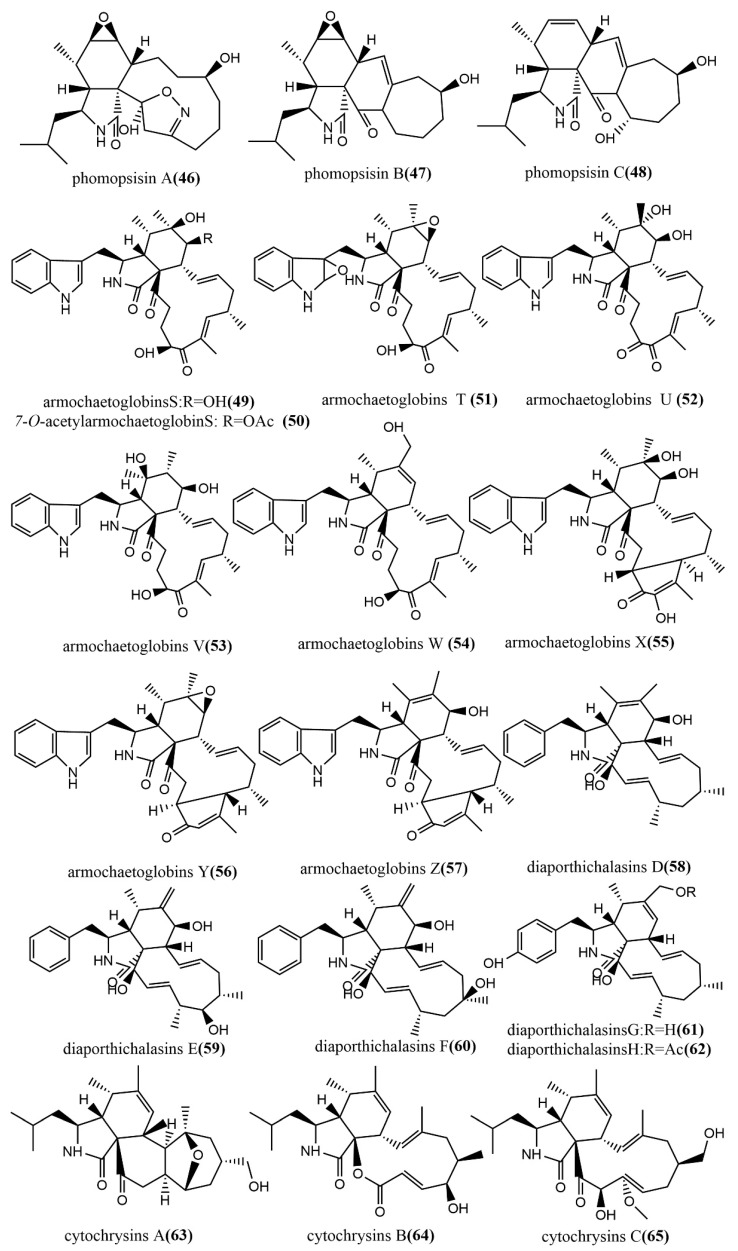
The methylation-deficient backbone, Phomopsisin A–C 46–48 (Figure 5), was obtained from brown rice cultures of Phomopsis sp. sh917, which was isolated from Isodon eriocalyx var. laxiflora stems. Compound 46 contained an unusual 5/6/11/5 tetracyclic ring system 2H-isoxazole moiety and showed significant inhibition activity against LPS-induced NO production in RAW264.7 cells, with an IC50 value of 32.38 μM, which was more potent than the positive control L-NMMA (IC50 value of 42.34 μM) [52]. The highly oxidized cytochalasin alkaloids—armochaetoglobins S–Z 49–57 (Figure 5) and 7-O-acetylarmochaetoglobin S 50 (Figure 5)—were identified and isolated from Chaetomium globosum TW1-1. The effects of all compounds on five tested human cancer cell lines (HL-60, A-549, SMMC-7721, MCF-7, and SW-480) were measured using the MTT method. Compounds 56–57 showed potent cytotoxic activities, with IC50 values ranging from 10.45 to 30.42 µM [53]. Furthermore, diaporthichalasins D–H 58–62 (Figure 5) were obtained from solid cultures of the endophytic fungus Diaporthe sp. SC-J0138 isolated from the leaves of the pteridophyte Cyclosorus parasiticus, and the MTS method was used to evaluate the cytotoxic activities of these compounds on four human cancer cell lines (A549, HeLa, HepG2, and MCF-7). Compound 58 exhibited significant cytotoxic activity against all tested human cancer cell lines; compounds 59–62 exhibited selective cytotoxic activities against some cell lines [54]. Cytochrysins A–C 63–65 (Figure 5) were obtained from rice cultures of Cytospora chrysosperma HYQZ-931, an endophytic fungus isolated from the desert plant Hippophae rhamnoides. Compound 63 showed significant antimicrobial activity to Enterococcus faecium, with an MIC value of 25 μg/mL. Compound 65 showed potent antimicrobial activity to Staphylococcus aureus, with an MIC value of 25 μg/mL [55].
Figure 5.
Chemical structures composition of cytochalasins.
2.2.2. Indole Alkaloids
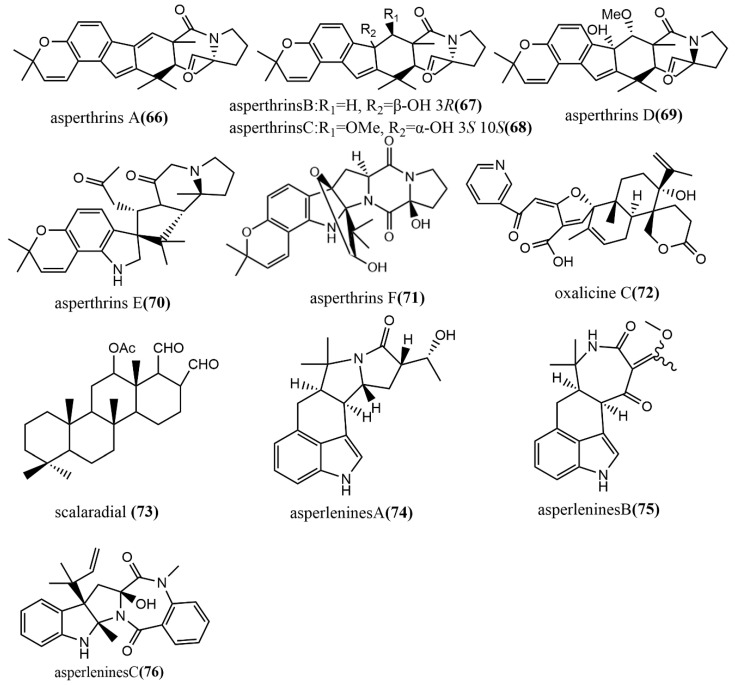
Six prenylated indole alkaloids, asperthrins A–F 66–71 (Figure 6), were derived from the marine endophytic fungus Aspergillus sp. YJ191021. Compound 66 showed moderate antimicrobial activity against Vibrio anguillarum, with an MIC value of 8 μg/mL. Additionally, the compounds 66 and 69 showed potent–weak anti-inflammatory activities against propionibacterium acnes-induced human mononuclear cell line (THP-1), with IC50 values of 1.46 μΜ and 30.5 μΜ, respectively, while compound 66 showed higher anti-inflammatory activity than the positive control Tretinoin at an IC50 value of 3.38 μM [56]. The α-pyrone meroterpenoid-type alkaloid, oxalicine C 72 (Figure 6), was obtained from Penicillium chrysogenum XNM-12, which was isolated from the marine brown algae Leathesia nana. Compound 72 showed potent antimicrobial activity against the phytopathogenic fungus Ralstonia solanacearum, with an MIC of 8 μg/mL [57]. Scalarane 73 (Figure 6) was isolated from Hypomontagnella monticulosa Zg15SU through the potato dextrose liquid culture. Compound 73 showed potent cytotoxic activity against cancer cell lines Panc-1, NBT-T2, and HCT116, with IC50 values of 0.05, 0.75, and 0.05 μg/mL, respectively [58]. Asperlenines A–C 74–76 (Figure 6) were isolated from Aspergillus lentulus DTO 327G5 cultures, and the antimicrobial activity of all compounds was evaluated using the broth-microdilution method against five tested agricultural pathogens (Xanthomonas oryzae pv. Oryzae, Xanthomonas oryzae pv. Oryzicola, Rhizoctonia solani, Fusarium oxysporum, and Colletotrichum gloeosporioides). Compounds 74–76 showed moderate to weak antimicrobial activities against Xanthomonas oryzae pv. Oryzae and Xanthomonas oryzae pv. Oryzicola, with MIC values ranging from 25 to 100 μg/mL [59].
Figure 6.
Chemical structures of indole alkaloids.
2.2.3. Diketopiperazine Derivatives
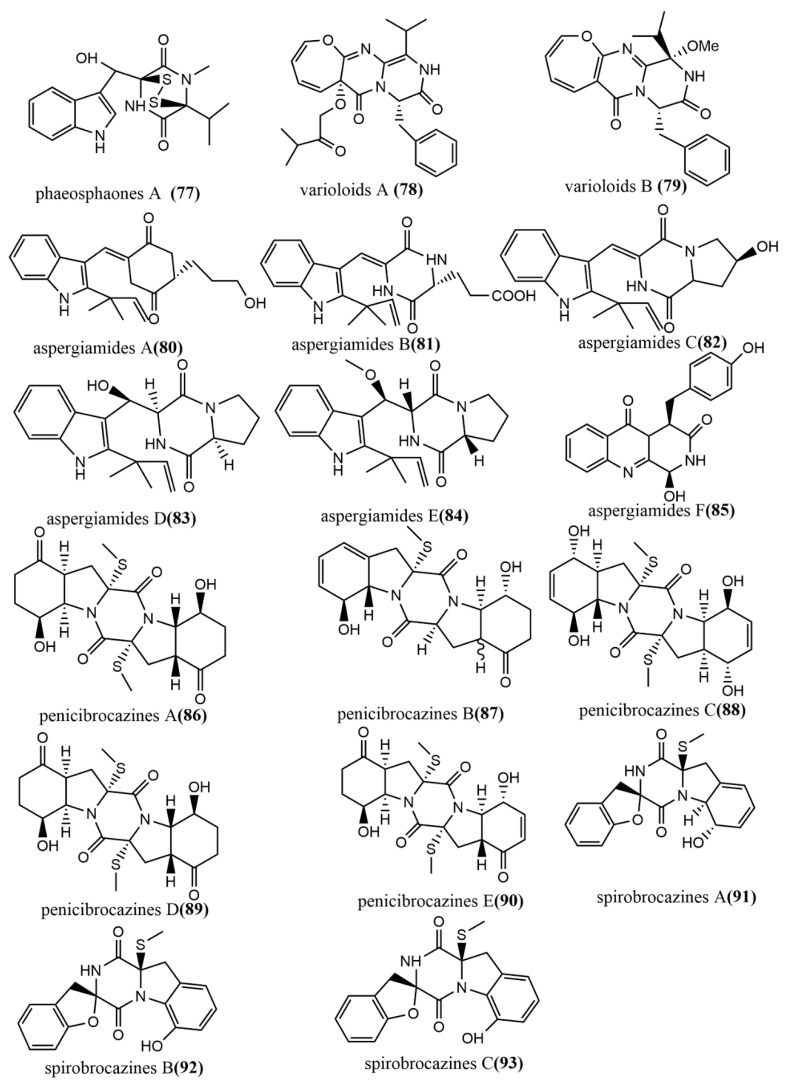
The thiodiketopiperazine alkaloid, phaeosphaones D 77 (Figure 7), featuring an unusual β-(oxy) thiotryptophan motif, was obtained from endophytic fungus Phaeosphaeria fuckelii isolated from the medicinal plant Phlomis umbrosa. Compound 77 showed stronger mushroom tyrosinase inhibition activity than the positive control kojic acid (IC50 value of 40.4 μM), with an IC50 value of 33.2 μM. [60]. The oxepine-containing diketopiperazine-type alkaloids, varioloids A-B 78–79 (Figure 7), were obtained from Paecilomyces variotii EN-291, which was isolated from the marine red alga Grateloupia turuturu. Compounds 78–79 showed potent antifungal effects against Fusarium graminearum, with MIC values of 8 μg/mL and 4 μg/mL, respectively [61]. Aspergiamides A–F 80–85 (Figure 7) were isolated from the endophytic fungus Aspergillus sp. 16-5 of mangroves, and all compounds were evaluated for their inhibition activities against protein-tyrosine phosphatase 1B (PTP1B) and α-glucosidase. Compounds 80 and 81 showed potent to moderate α-glucosidase inhibition activities, with IC50 values of 18.2 µM and 40.7 µM, respectively. Compounds 80–85 did not show significant PTP1B inhibition activities (<10% inhibition) at 100 µg/mL [62]. Five sulfide diketopiperazines derivatives, penicibrocazines A–E 86–90 (Figure 7), were obtained from the endophytic fungus Penicillium brocae MA-231 isolated from the mangrove plant Avicennia marina. The antimicrobial effects of all compounds were evaluated by the agar diffusion method against five tested pathogens (Aeromonas hydrophilia, Escherichia coli, Staphylococcus aureus, Vibrio arveyi, and V. parahaemolyticus). Compounds 86–90 showed potent antimicrobial activities against S. aureus, with MIC values ranging from 0.25 to 32 μg/mL [63]. Spirobrocazines A–C 91–93 (Figure 7) were isolated from the mangrove-derived Penicillium brocae MA-231. Compounds 91–93 contained a 6/5/6/5/6 cyclic system with a rare spirocyclic center at C-2. All compounds showed moderate antimicrobial activities against S. aureus, Aeromonas hydrophilia, and Vibrio harveyi, with MIC values ranging from 16 to 64 μg/mL [64].
Figure 7.
Chemical structures of diketopiperazine derivatives.
2.2.4. Other Types of Alkaloids
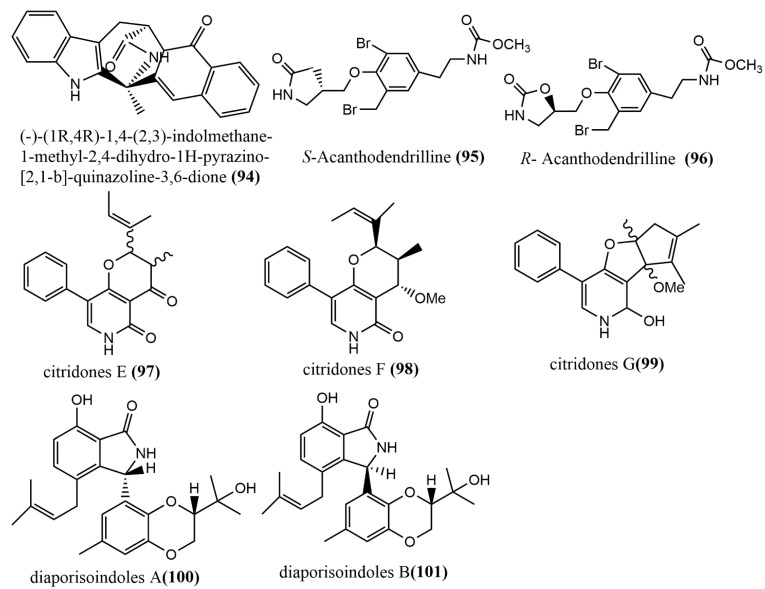
The quinazoline alkaloid (-)-(1R,4R)-1,4-(2,3)-indolmethane-1-methyl-2,4-dihydro-1H-pyrazino-[2,1-b]-quinazoline-3,6-dione 94 (Figure 8) was obtained from the endophytic fungus Penicillium vinaceum X1, which was isolated from corms of Crocus sativus (Iridaceae). The in vitro cytotoxicity of compound 94 was evaluated against three human tumor cell lines (A549, LOVO, and MCF-7), to which compound 94 showed weak cytotoxic activities against all human tumor cell lines, with IC50 values of 76.83, 68.08, and 40.55 μg/mL, respectively [65]. The enantiomeric bromotyrosine alkaloids S-Acanthodendrilline 95 (Figure 8) and R-Acanthodendrilline 96 (Figure 8) were isolated from the ethyl acetate extract of the sponge endophytic fungus Acanthodendrilla sp. The cytotoxic activities of compounds 95–96 against human non-small cell lung cancer H292 and normal human immortalized fibroblast HaCaT cell lines were evaluated using the MTT method. Compound 95 (IC50 value of 58.5 µM) was approximately three times more potent than compound 96 (IC50 value of 173.5 µM) against the H292 cell line. Compounds 95–96 exhibited efficient and selective cytotoxic activities against H292 and HaCaT cell lines, with IC50 values ranging from 58.5 to 173.5 µM and >400 µM, respectively [66]. Three phenylpyridone derivatives, citridones E–G 97–99 (Figure 8), were obtained from the endophytic fungal strain Penicillium sumatrense GZWMJZ-313 9, which was isolated from the leaves of Garcinia multiflora. These compounds showed moderate to weak antimicrobial activities against Staphylococcus aureus ATCC6538, Pseudomonas aeruginosa ATCC10145, and Escherichia coli ATCC11775, with MIC values ranging from 32 to 128 μg/mL [67]. Two isoprenylisoindole alkaloids, diaporisoindoles A-B 100–101 (Figure 8), were obtained from the endophytic fungus Diaporthe sp. SYSU-HQ3, which was isolated from a fresh branch of the mangrove plant Excoecaria agallocha. Compound 100 showed potent inhibition activity against Mycobacterium tuberculosis protein-tyrosine phosphatase B, with an IC50 value of 4.2 µM [68].
Figure 8.
Chemical structure of other types of alkaloids.
In a nutshell, anti-angiogenic and anti-inflammatory activities were the main activities of alkaloids in both plants and endophytic fungi. In addition, phomaketides and their derivatives that were isolated from fungal endophytes possess antimicrobial activity just as those isolated in plants; therefore, alkaloids producing endophytic fungi can be used in the development of anti-angiogenic, anti-inflammatory, and antimicrobial drugs for both human and animal use.
2.3. Terpenoids
2.3.1. Sesquiterpenoids and Their Derivatives
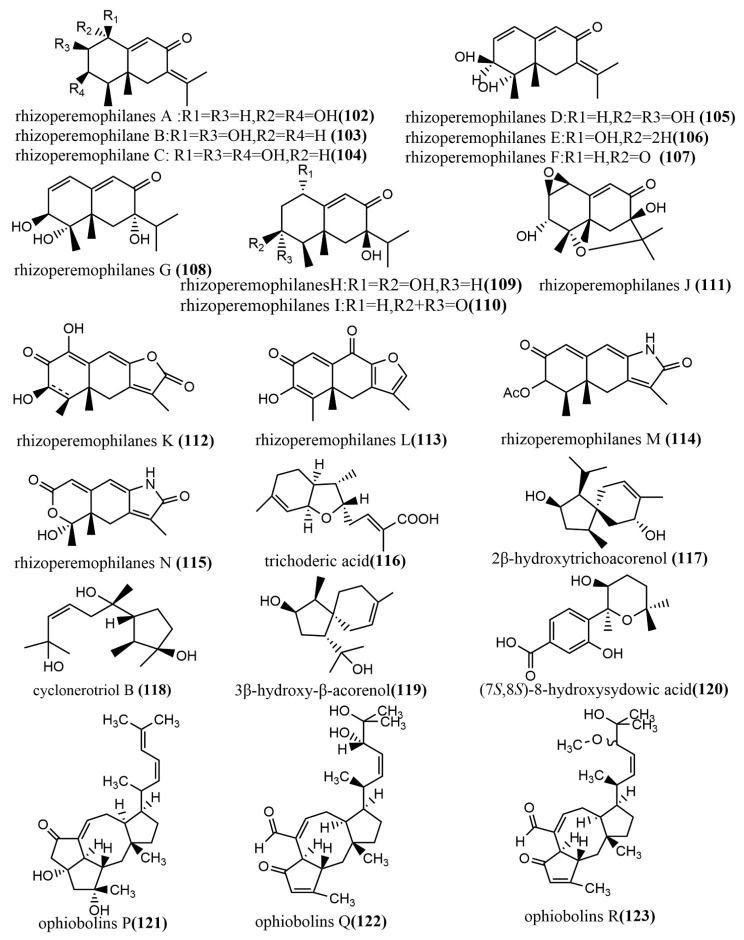
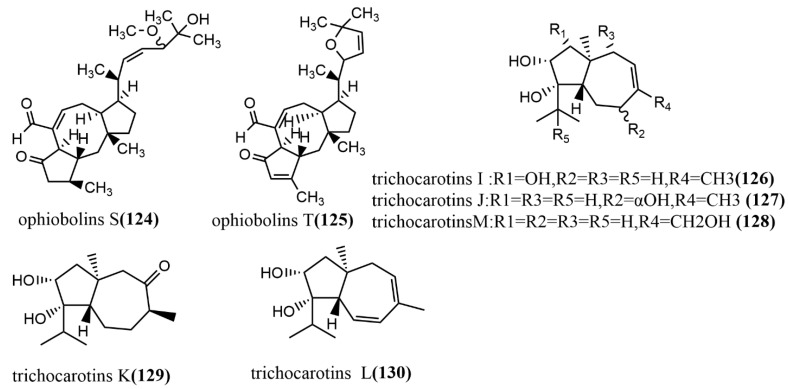
The 1-methoxypestabacillin B 107 (Figure 9) was obtained from brown rice cultures of endophytic fungus Diaporthe sp. SCSIO 41011 isolated from the stem of the mangrove plant Rhizophora stylosa. Compound 107 was evaluated for the reversal of HIV incubation period and anti-influenza A virus activities, to which compound 107 did not show antiviral activity. However, its structure could serve as the backbone for the synthesis of more potent antiviral compounds [69]. The eremophilane-type sesquiterpenoids rhizoperemophilanes A-N 102–115 (Figure 9) were isolated from the ethyl acetate extract of Rhizopycnis vagum Nitaf22. Compound 111 contained a C-4/C-11 epoxide, and compound 115 had a 3-nor-eremophilane lactone-lactam skeleton. All compounds were evaluated for their cytotoxic activities against five tested human cancer cells (BGC823, Daoy, HCT116, HepG2, and NCI-H1650) and inhibition activities against radicle growth in rice seedlings. Compound 115 showed high selective cytotoxicity against NCI-H1650 and BGC823 cell lines, with IC50 values of 15.8 µM and 48.2 µM, respectively, while no significant cytotoxic activity was observed for other compounds at IC50 > 50 μm. Compounds 106–107 and 113–114 showed strong phytotoxic activities against radicle growth in rice seedlings at a concentration of 200 µg/mL, where the inhibition exceeded 50% [70]. The bisabolane-type sesquiterpene, trichoderic acid 116, (Figure 9) and acorane-type sesquiterpene, 2β-hydroxytrichoacorenol 117 (Figure 9), were obtained from Trichoderma sp. PR-35 culture, an endophytic fungus isolated from stems of Paeonia delavayi. Compounds 116–117 were tested for antimicrobial activity against two pathogens (Escherichia coli, and Shigella sonnei) using an agar diffusion method. Compounds 116–117 showed moderate to weak antimicrobial activities, with MIA values ranging from 50 to 175 µg/mL [69]. The ring flores aurantii alkane-type sesquiterpene, cyclonerotriol B 118 (Figure 9), and the α-pinene skeleton-containing sesquiterpene, 3β-hydroxy-β-acorenol 119 (Figure 9), were obtained from Fusarium proliferatum AF-04 isolated from Chlorophytum comosum roots via a combination of high-performance liquid chromatography (HPLC) and a bioassay-guided method. Compounds 118–119 showed weak antimicrobial activities (MIC values > 100 μg/mL) against Bacillus subtilis, Clostridium perfringens, E. coli, and methicillin-resistant Staphylococcus aureus (MRSA) [71]. The aromatic bisabolene-type sesquiterpene (7S, 8S)-8-hydroxysydowic acid 120 (Figure 9) was obtained from the brown rice culture of the endophytic fungus Aspergillus sydowii EN-434 isolated from the marine red alga Symphyocladia latiuscula from Qingdao, China. Compound 120 showed potent DPPH radical scavenging activity, with an IC50 value of 113.5 μmol/L [72]. The ophiobolane sesquiterpenes ophiobolins P–T 121–125 (Figure 9) were isolated from the acetone extract of the endophytic fungus Ulocladium sp. using the one-strain many-compound (OSMAC) strategy. Compounds 121–125 were evaluated for their cytotoxicity and antibacterial activities against two tested human cancer cell lines (KB and HepG2 cell lines) and three tested pathogens (Bacillus subtilis, MRSA, and Bacille Calmette-Guerin). Compounds 121–125 showed moderate antimicrobial activities against B. subtilis and multi-drug-resistant S. aureus, with MIC values ranging from 15.6 to 62.5 μM. Compound 125 showed moderate antimicrobial activity against Bacille Calmette-Guerin, with an MIC value of 31.3 μM. Additionally, compound 125 showed potent cytotoxic activity against the HepG2 cell line, with an IC50 value of 0.24 μM, which was stronger than the positive control etoposide (IC50 value of 2.02 μM) [73]. The daucane-type sesquiterpenes trichocarotins I-M 126–130 (Figure 9) were obtained from Trichoderma virens QA-8 isolated from the roots of Artemisia argyi H. Lév. and Vaniot, and these compounds showed significant antimicrobial activities against E. coli EMBLC-1, with MIC values ranging from 0.5 to 16 μg/mL [74].
Figure 9.
Chemical structures of sesquiterpenoids and derivatives.
2.3.2. Diterpenoids
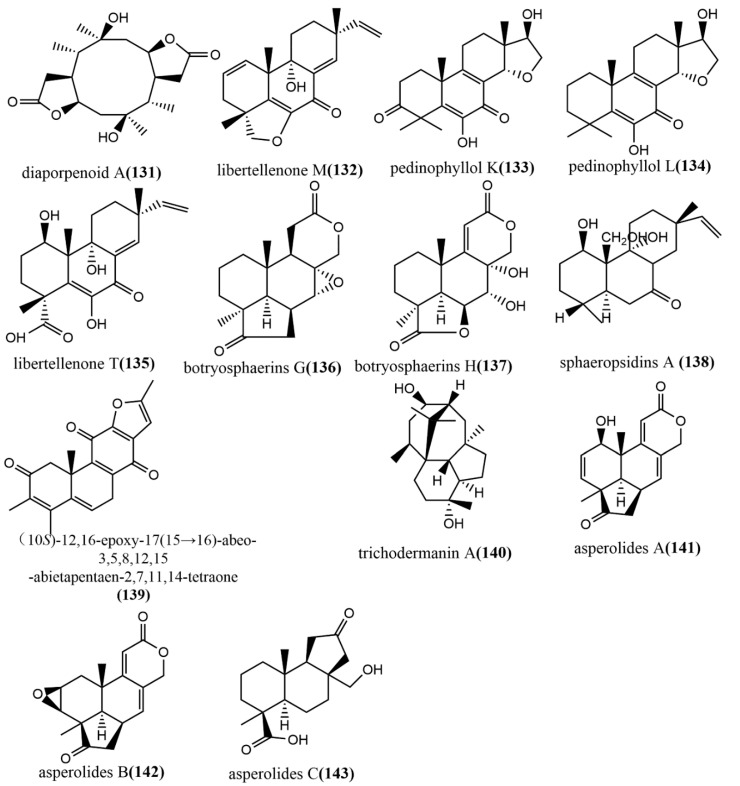
The ring diterpene diaporpenoid A 131 (Figure 10), containing a 5/10/5-fused tricyclic ring system, was isolated from the MeOH extract obtained from cultures of the mangrove endophytic fungus Diaporthe sp. QYM12. Compound 131 showed significant anti-inflammatory activity by inhibiting LPS-induced NO production in a mouse macrophage cell line RAW264.7, with an IC50 value of 21.5 μM [75]. The pimarane-type diterpene Libertellenone M 132 (Figure 10) was isolated from the marine source endophytic fungus Phomopsis sp. S12. Compound 132 inhibited pro-inflammatory cytokines IL1β and IL-18 mRNA expression in colon tissue, significantly reduced the cleavage of pro-caspase1, and dose-dependently inhibited the NF-κB nuclear translocation in macrophages. Clinical indications of acute colitis induced by 3% dextran sulphate sodium in mice were attenuated by intravenous administration of different doses of compound 132 (10 or 20 mg/kg), which is a potent inhibitor of NLRP3 inflammatory vesicles and may be a new medicine for treating acute colitis [76]. Three pimarane-type diterpenoids—pedinophyllol K 133 (Figure 10), pedinophyllol L 134 (Figure 10), and libertellenone T 135 (Figure 10)—were isolated from the endophytic fungal Phomopsis sp. S12 culture using the OSMAC strategy. The anti-inflammatory activities of all compounds were assessed using an LPS-induced inflammation model of mouse macrophage RAW264.7. Compound 135 dose-dependently inhibited the expression of inflammatory factors IL-1β and IL-6 at the mRNA level. Additionally, the anti-inflammatory activity of compounds 133–134 was similar to that of compound 135 in terms 0f IL-6 inhibition [77]. Two tetranorlabdane diterpenoids botryosphaerins G–H 136–137 (Figure 10) were obtained from the ethyl acetate extract of Botryosphaeria sp. P483 isolated from the branches of the herb Huperzia serrata (Thunb.) Trev. and tested for their antifungal activities against Gaeumannomyces graminis, Fusarium solani, and Pyricularia oryzae by the disk diffusion method. Compound 137 showed effective antifungal activity at a concentration of 100 μg/disk with an inhibitory zone diameter of 9 mm. (The inhibitory zone diameter of positive control carbendazim was 15–18 mm.) Compounds 136–137 were evaluated for their nematicidal activities against Panagrellus redivivus and Caenorhabditis elegans and showed weak nematicidal activities, with 30% and 28% fatality rates at a 24h action concentration of 400 mg/L, respectively [78]. The isopimarane diterpene sphaeropsidin A 138 (Figure 10) was isolated from the ethyl acetate extract of the endophytic fungus Smardaea sp. AZ0432 of Ceratodon purpureus. The in vitro cytotoxic activities of compound 138 against five human cancer cell lines (NCI- H460, MDA-MB-231, MCF-7, PC-3M, and SF-268) and human embryonic lung fibroblast cell line WI-38 were evaluated using the resazurin colorimetric assay. The results showed that compound 138 showed a high cell selectivity when it was applied at a concentration of 10 μM for 72 h and inhibited the migration of MDA-MB-231 cells by 50% at a subcytotoxic concentration of 1.5 μM [79]. (10S)-12,16-epoxy-17(15→16)-abeo-3,5,8,12,15-abietapentaene-2,7,11,14-tetraone 139 (Figure 10) was obtained from the cultures of the endophytic fungus Pestalotiopsis adusta isolated from stems of the medicinal plant Clerodendrum canescens. The cytotoxicity of compound 139 to the HL-60 tumor cell line was evaluated using the MTT assay, by which compound 139 showed moderate cytotoxic activity, with an IC50 value of 12.54 μM [80]. (The IC50 value of the positive control cisplatin was 9.20 μM.) The trichodermanin A 140 (Figure 10), a diterpene containing a 6-5-6-6 ring system, was obtained from the endophytic fungus Trichoderma atroviride S361 of Cephalotaxus fortunei and was not tested for any biological activities [81]. Therefore, further studies are needed to identify the potential biological activity of this compound in the future. The new tetranorlabdane diterpenoids, asperolides A–C 141–143 (Figure 10), were isolated from the ethyl acetate extract of the marine brown alga Aspergillus wentii EN-48 and the cytotoxic activities of compounds 141–143 to seven tested human cancer cell lines (NCI-H460, MDA-MB-231, HeLa, MCF-7, SMMC-7721, HepG2, and SW1990) were evaluated using the MTT method. Compounds 141–143 showed moderate cytotoxic activities, with IC50 values ≤ 10 Μm [82].
Figure 10.
Chemical structures of diterpenoids and derivatives.
2.3.3. Triterpenoids
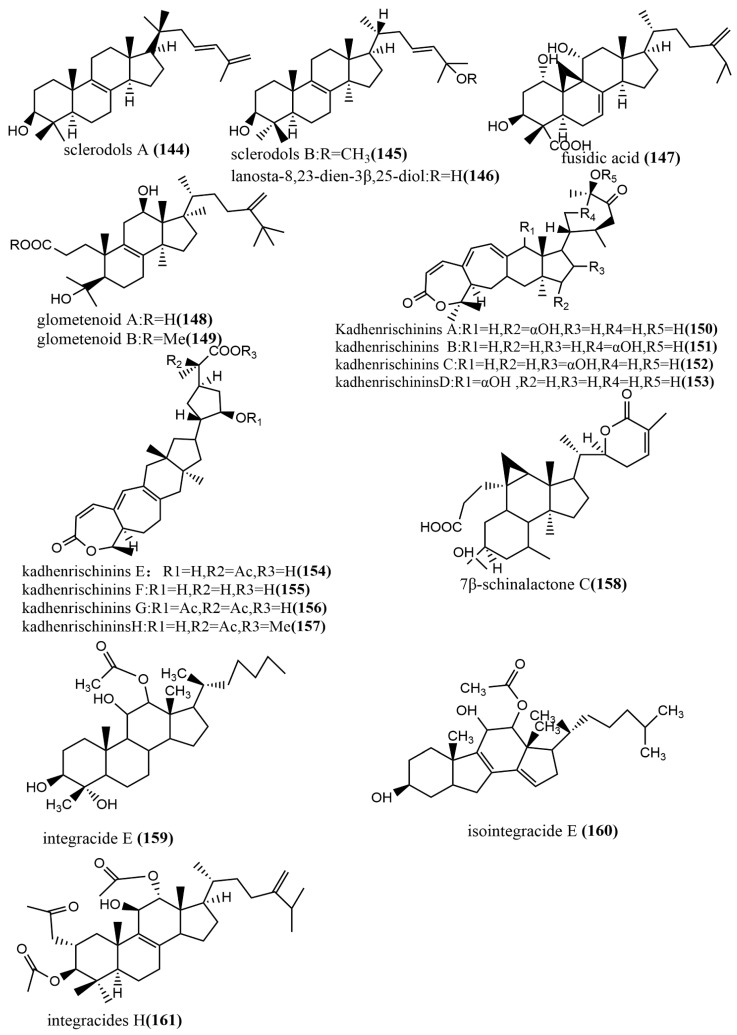
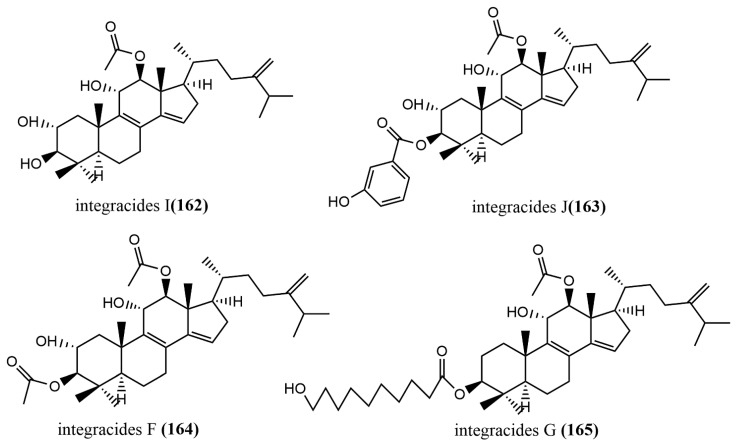
The 24-homo-30-nor-cycloartane triterpenoid 154 (Figure 11) was isolated from the endophytic fungus Mycoleptodiscus indicus FT1137. Compound 154 showed no activity against the human ovarian cancer cell line A2780 at a concentration of 20 μg/mL [83]. Three Lanostane-type triterpenes—sclerodols A–B 144–145 (Figure 11) and lanosta-8,23-dien-3β,25-diol 146 (Figure 11)—were obtained from Eucalyptus grandis cultures derived from the endophytic fungus Scleroderma UFSMSc1, and the antifungal activities of compounds 144–146 against Candida albicans and Candida parapsolosis were evaluated by the agar diffusion method. Compounds 144–146 showed moderate to weak antifungal activities, with MIC values ranging from 12.5 to 50 μg/mL. The antifungal effects of these compounds against C. albicans were associated with the inhibition of the selenocysteine methyltransferase (SMT) activity [84]. Fusidic acid 147 (Figure 11) was obtained from the cultures of the endophytic fungus Acremonium pilosum F47, isolated from the stem of Mahonia fortunei using the bioactivity-guided assay, and the antimicrobial activities of compound 147 against four human pathogens were tested (S. aureus ATCC 6538, B. subtilis ATCC 9372, P. aeruginosa ATCC 27853, and E. coli ATCC 25922) and evaluated. Compound 147 showed effective antimicrobial activities against S. aureus ATCC 6538 and B. subtilis ATCC 9372. The acetylation of the C-16 hydroxyl group of compound 147 was essential for antimicrobial action [85]. Two new ring A-cleaved lanostane-type triterpenoids, glometenoid A–B 148–149 (Figure 11), were obtained from the ethyl acetate extract of the mason pine endophytic fungus Glomerella sp. F00244. The cytotoxic activity of compounds 148–149 against the human ovarian cancer cell line HeLa was tested using the MTT assay. Compound 148 showed weak cytotoxic activity at a concentration of 10 μM with 21% inhibition [83]. Nine highly oxygenated schitriterpenoids—kadhenrischinins A–H 150–157 (Figure 11) and 7β-schinalactone C 158 (Figure 11)—were isolated from Penicillium sp. SWUKD4.1850, and compounds 154–157 contained a unique 3-one-2-oxabicyclo [1,2,3]-octane motif. All compounds were tested for their cytotoxic activities against the HepG2 tumor cell lines using the MTT assay, and these compounds showed weak cytotoxic activities, with IC50 values ranging from 14.3 to 40 μM [86]. Two tetracyclic triterpenoids—integracide E 159 (Figure 11) and isointegracide E 160 (Figure 11)—were isolated from the mycelia of Hypoxylon sp. 6269. Compound 159 showed weak inhibition activity against the HIV-1 integrase, with an IC50 value of 31.63 μM [87]. The tetracyclic triterpenoids, integracides H–J 161–163 (Figure 11), were obtained from the endophytic fungus Fusarium sp., which was isolated from the roots of Mentha longifolia L. (Labiatae) and were evaluated for antileishmanial activity against L. donovani promastigotes. Compound 161 showed significant antileishmanial activity, with an IC50 value of 4.75 μM, exceeding the positive control Pentamidine (IC50 value of 6.35 μM) [88]. The tetracyclic triterpenoids, integracides F–G 164–165 (Figure 11), were obtained from the endophytic fungus Fusarium sp. of Mentha longifolia L. (Labiatae). Compounds 164–165 were evaluated for their antileishmanial and cytotoxic activities to BT-549 and SKOV-3 cells and Leishmania donovani promastigotes. Compounds 164–165 showed significant cytotoxic activities against SKOV-3 and BT-549 cell lines, with IC50 values ranging from 0.16 to 1.97 μg/mL and 0.12 to 1.76 μg/mL, respectively. (The IC50 value of the positive control Pentamidine was 2.1 μg/mL.) Compounds 164–165 showed potent antileishmanial activities against L. donovani promastigotes, with IC50 values of 3.74 μg/mL and 2.53 μg/mL, respectively [89].
Figure 11.
Chemical structures of terpenoids.
2.3.4. Meroterpenoids
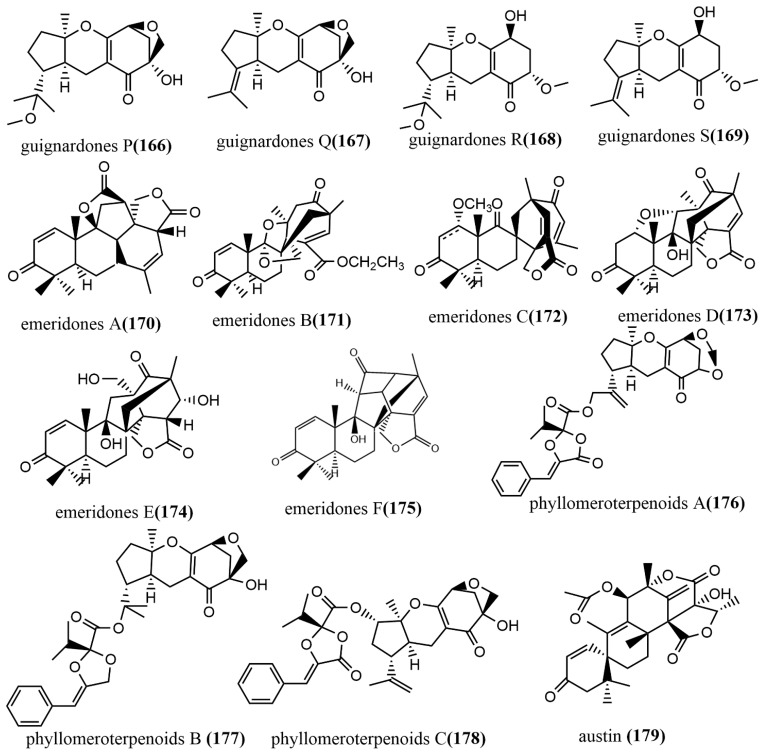
Guignardones P–S 166–169 (Figure 12) were obtained from Guignardia mangiferae A348 cultures, and the cytotoxic activities of compounds 166–169 against three human cancer cell lines (SF-268, MCF-7, and NCI-H460) were tested using an MTT assay. Compounds 167 and 169 only showed weak cytotoxic activities against MCF-7 cell lines, with IC50 values ranging from 83.7 to 92.1 µM [90]. Six 3, 5-demethylorsellinic acid-based meroterpenoids emeridones A–F 170–175 (Figure 12) were isolated from Emericella sp. TJ29 cultures. Compound 171 possessed a 2,6 dioxabicyclo [2.2.1] heptane and a spiro [bicycle [3.2.2] nonane-2,1′-cyclohexane] moiety. The cytotoxic activities of all compounds against five human cancer cell lines (HL-60, SMMC7721, A549, MCF-7, and SW-480) were tested using the MTT assay, and compounds 172, 173, and 175 showed moderate cytotoxic activities against all tested cell lines, with IC50 values ranging from 8.19 to 18.8 µM [91]. Phyllomeroterpenoids A–C 176–178 (Figure 12) were isolated from the crude extract of Phyllosticta sp. J13-2-12Y fermentation broth. Compounds 176–178 showed moderate antimicrobial activities against Staphylococcus aureus 209P, Candida aureus 209P, and Candida albicans FIM709, with MIC values ranging from 32 to 128 μg/mL [92]. Austin 179 (Figure 12) was obtained from the ethyl acetate extract of Talaromyces purpurogenus H4 and Phanerochaete sp. H2 co-cultures, which showed moderate trypanocidal activity against T. cruzi at a concentration of 100 μg/mL, with an IC50 value of 36.6 µM. Notably, neither of the two endophytic fungi produced compound 179 when cultured separately under similar conditions [93].
Figure 12.
Chemical structures of Meroterpenoids.
To sum up, Meroterpenoids and their derivatives, which are mainly known for their antifungal properties in most plants species, have been found in endophytic fungi. However, recent studies have also reported anti-oxidative, anti-inflammatory, and anti-cancer activities from these compounds. Therefore, these microorganisms can be used in the development of drugs candidates for human, animal, and other agricultural activities.
2.4. Lactones
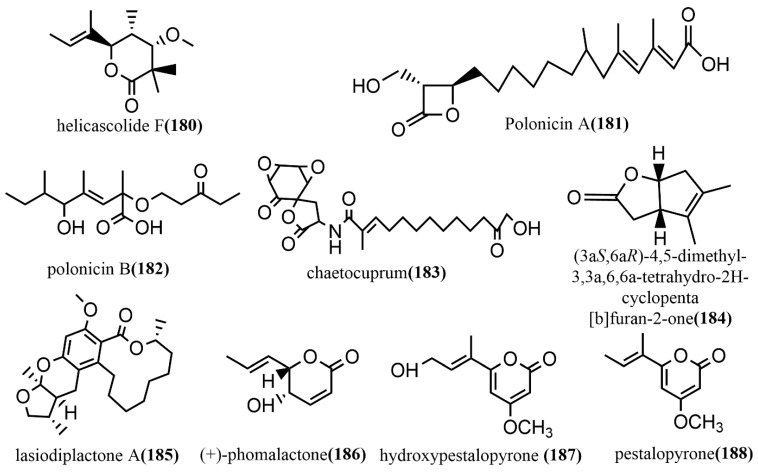
Helicascolide F 180 (Figure 13) was obtained from Talaromyces assiutensis JTY2 isolated from Ceriops tagal leaves. The cytotoxic activities of compound 180 against three human cancer cell lines (HeLa, MCF-7, and A549) were tested using an MTT assay, in which compound 180 showed a moderate cytotoxic effect on all tested cell lines, with an IC50 value range of 14.1–38.6 μM [94]. Two β-lactones, polonicin A–B 181–182 (Figure 13), were obtained from the brown rice culture of the endophytic fungus Penicillium polonicum in the fruit of Camptotheca acuminata. Compound 181 showed effective glucose uptake activity at a concentration of 30 μg/mL on rat skeletal myoblast cell line L6, which enhanced 1.8-fold compared to that of the control. Compound 182 was used to assess its effect on GLUT4 translocation by using the fluorescent protein, IRAP-mOrange, which is stably expressed in L6 cells. It showed a 2.1-fold increase in fluorescence intensity on L6 cell membranes compared to the untreated controls [95]. The spirodilactone compound chaetocuprum 183 (Figure 13) was obtained from cultures of the endophytic fungus Chaetomium cupreum of wild Anemopsis californica from New Mexico, U.S.A. Compound 183 showed a weak antimicrobial activity against S. aureus, with an MIC value of 50 μg/mL [96]. A phytotoxic bicyclic lactone, (3aS,6aR)-4,5-dimethyl-3,3a,6,6a-tetrahydro-2H-cyclopenta [b] furan-2-one 184 (Figure 13), was obtained from the fermentation broth of Xylaria curta 92092022. Compound 184 contained a rare 5/5 rings-fusion system and was tested for antimicrobial activities against four pathogens (Pseudomonas aeruginosa ATCC 15442, Staphylococcus aureus NBRC 13276, Aspergillus clavatus F318a, and Candida albicans ATCC 2019) and the phytotoxicity against lettuce seedlings. Compound 184 showed moderate antimicrobial activities against Pseudomonas aeruginosa ATCC 15442 and Staphylococcus aureus NBRC 13276 at a concentration of 100 μg/disk, with inhibitory zone diameters of 13 mm and 12 mm, respectively. At the concentration of 25 μg mL −1, compound 184 showed 50% inhibition on lettuce roots with a root length of 1.6 ± 0.3 cm (3.2 ± 0.5 cm for the control). At a concentration of 200 μg mL −1, compound 184 strongly inhibited lettuce seed germination, with 90% inhibition [97]. Lasiodiplactone A 185 (Figure 13) was obtained from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1 and contained a unique tetracyclic system (12/6/6/5) of RAL 12 (12-membered β-resorcylic acid lactone) with a pyran ring and a furan ring. Compound 185 showed significant anti-inflammatory activity by inhibiting the LPS-induced NO production in RAW 264.7 cells, with an IC50 value of 23.5 μM, which was stronger than the positive control indomethacin (IC50 = 26.3 μM). Additionally, compound 185 showed potent α-glucosidase inhibition activity, with an IC50 value of 29.4 μM, which was superior to the commonly used clinical drug acarbose (IC50 = 36.7 μM) [98]. (+)-phomalactone 186 (Figure 13), hydroxypestalopyrone 187 (Figure 13), and pestalopyrone 188 (Figure 13) were isolated from the endophytic fungus Aspergillus pseudonomiae J1 cultures and evaluated for in vitro anti-trypanosomal activity against the Trypanosoma cruzi Y strain using an anti-epimastigote assay. Compounds 186–188 showed moderate to weak anti-trypanosomal activities, with IC50 values of 0.86 μM, 88.33 μM, and 580.19 μM, respectively [99].
Figure 13.
Chemical structures of Lactones.
In summary, this review reported that fungal endophytes could produce Lactones and their derivatives through their metabolic activities. In addition, these compounds possessed biological activities, such as antimicrobial, anti-cancer, allelopathic, and anti-inflammatory; thus, fungal endophytes that produce these compounds may be utilized in the pharmacological setup as alternatives to plant-derived compounds.
2.5. Anthraquinones, Quinones, and Related Glycosides
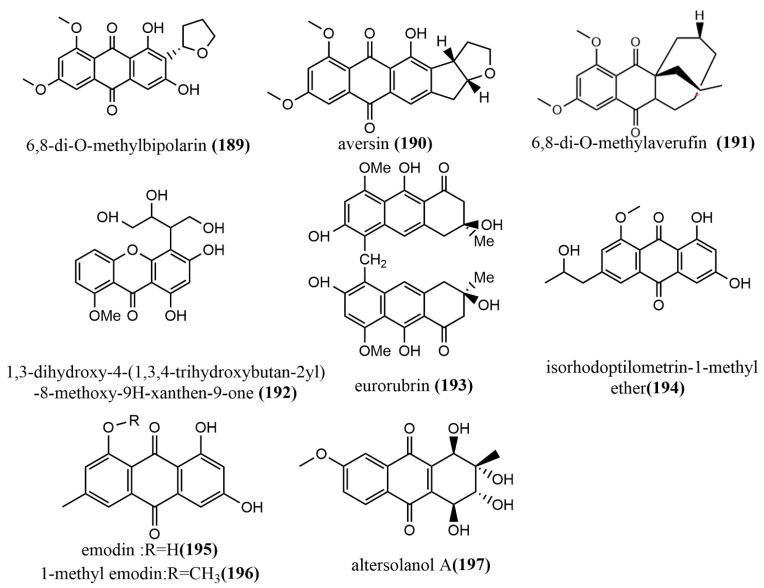
6,8-di-O-methylbipolarin 189 (Figure 14), aversin 190 (Figure 14), and 6,8-di-O-methylaverufin 191 (Figure 14) were obtained from rice cultures of the marine red algae endophytic fungus Acremonium vitellinum from Qingdao, China. Compounds 189–191 showed moderate insecticidal activities against the third-instar larvae of Helicoverpa armigera, with LC50 values of 0.72 mg/mL, 0.78 mg/mL, and 0.87 mg/mL, respectively. (The LC50 value for the positive control, matrine, was 0.29 mg/mL.) Additionally, the molecular mechanism of the insecticidal activity of compound 191 was investigated based on transcriptome sequencing. The identification of 5,732 differentially expressed genes was performed, of which 2,904 genes were downregulated and 2,828 genes were upregulated. The upregulated genes were primarily involved in cell autophagy, apoptosis, DNA mismatch repair, and replication [100]. A new quinone, identified as 1,3-dihydroxy-4-(1,3,4-trihydroxybutan-2-yl)-8-methoxy-9H-xanthen-9-one 192 (Figure 14), was obtained from Phomopsis sp. isolated from the rhizome of Paris polyphyllavar. in Yunnan, China. Compound 192 showed significant cytotoxic activities against A549 and PC3 cell lines, with IC50 values of 5.8 μM and 3.6 μM, respectively [101]. The anthraquinone derivative eurorubrin 193 (Figure 14) was obtained from the ethyl acetate extract of the endophytic fungus Eurotium cristatum EN-220 of the seaweed Sargassum thunbergii and tested for its antimicrobial activities against three tested pathogens (E. coli, Physalospora obtuse, and Valsa mali), including its fatal activity against brine shrimp larvae. Compound 193 only showed a weak antimicrobial activity against E. coli, with an MIC value of 64 μg/mL. At the concentration of 10 μg/mL, compound 193 showed moderate fatal activity against brine shrimp larvae, with a fatality rate of 41.4% [102]. Isorhodoptilometrin-1-methyl ether 194 (Figure 14), emodin 195 (Figure 14), and 1-methyl emodin 196 (Figure 14) were obtained from cultures of the endophytic fungus Aspergillus versicolor of the red seaweed Halimeda opuntia. Compounds 194–196 were evaluated for their inhibiting activities against the hepatitis C virus NS3/4A protease, where Compounds 195–196 showed weak inhibition activities, with IC50 values ranging from 22.5 to 40.2 μg/mL [103]. The quinone altersolanol A 197 (Figure 14) was isolated from the endophytic fungus Stemphylium globuliferum of the medicinal plant Mentha pulegium (Lamiaceae). Compound 197 inhibited the proliferation of K562 and A549 cells in a time-dependent, dose-dependent manner and caused apoptosis by cleaving Caspase-3 and Caspase-9 and decreasing anti-apoptotic protein expression [104].
Figure 14.
Chemical structure of anthraquinones, quinones, and related glycosides.
Anthraquinones, quinones, and related glycosides are known for their anti-viral and anti-apoptotic activity both in vitro and in vivo. Interestingly, these compounds have been identified and isolated from fungal endophytes by various studies and have similarly shown anti-viral and anti-apoptotic activities. Thus, endophytes that produce these compounds may serve as cheap and environmentally friendly alternative sources for the development of antimicrobial drugs instead to plant sources.
2.6. Steroids
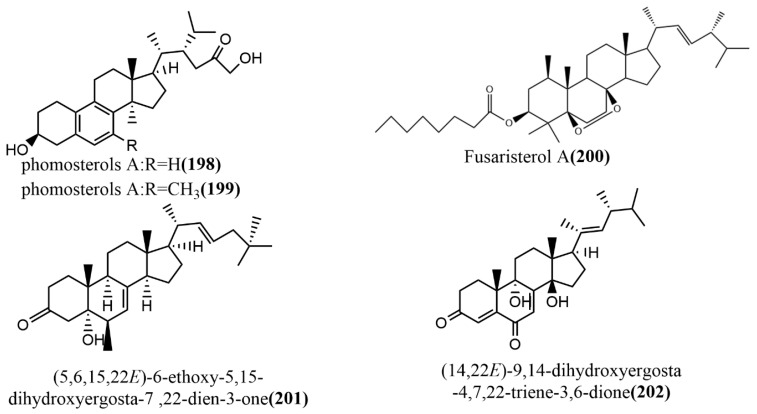
Phomosterols A–B 198–199 (Figure 15) were isolated from the endophytic fungus Phoma sp. SYSU-SK-7 of mangrove plants. Compounds 198–199 had an unusual aromatic B ring skeleton and showed significant inhibition activities against LPS-induced NO production in RAW 264.7 cells, with IC50 values of 13.5 μM and 25.0 μM, respectively. Additionally, compounds 198–199 showed potent α-glucosidase inhibition activities with IC50 values of 51.2 μM and 46.8 μM, respectively, exceeding the positive control 1-deoxynojirimycin (IC50 value of 62.8 μM) [105]. The ergosterol derivative fusaristerol A 200 (Figure 15) was obtained from the endophytic fungus Fusarium sp., which was isolated from the root of Mentha longifolia L. This compound showed significant antimicrobial activity against Candida albicans, with an MIC value of 8.3 μg/disc. Additionally, compound 200 showed moderate cytotoxic activity against human colorectal cancer cell line HCT 116, with an IC50 value of 0.21 μΜ, compared to the positive control adriamycin (IC50 value of 0.06 μΜ) [106]. (5,6,15,22E)-6-ethoxy-5,15-dihydroxyergosta-7,22-dien-3-one 201 (Figure 15) and (14,22E)-9,14-dihydroxyergosta-4,7,22-triene-3,6-dione 202 (Figure 15) were isolated from the endophytic fungus Phomopsis sp. of Aconitum carmichaeli in Yunnan, China. Compounds 201–202 were analyzed against six tested pathogenic fungi (Candida albicans, Aspergillus niger, Fusarium avenaceum, Pyricularia oryzae, Hormodendrum compactum, and Trichophyton gypseum) using a broth microdilution assay. Compounds 201–202 showed weak antifungal activities against C. albicans and F. avenaceum, with MIC values ranging from 64 to 128 μg/mL [107].
Figure 15.
Chemical structures of steroids.
To summarize, endophytic fungi are alternative sources of steroids and their derivatives; thus, they may be harnessed for the production of various drugs since they have shown antimicrobial and anticancer activity in previous studies.
2.7. Other Types of Compounds
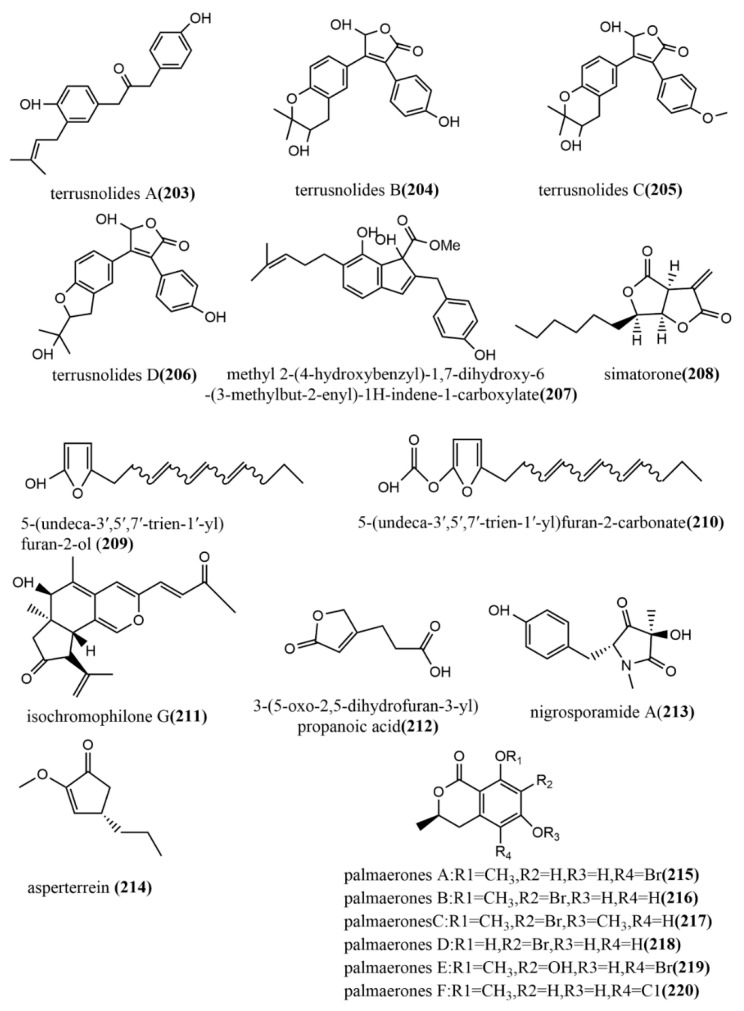
Four lignans, terrusnolides A–D 203–206 (Figure 16), were obtained from the endophytic fungus Aspergillus sp. isolated from the root of Tripterygium wilfordii. Compounds 203–206 showed significant inhibition of LPS-induced IL-1β, TNF-α, and NO production in RAW264.7 cells, with IC50 values ranging from 16.21 to 35.23 μΜ, 19.83 to 42.57 μΜ, and 16.78 to 38.15 μΜ, respectively, which were comparable to the positive control indomethacin (IC50 value of 15.67–21.34 μΜ) [108]. The indene derivative methyl 2-(4-hydroxybenzyl)-1,7-dihydroxy-6-(3-methylbut-2-enyl)-1H-indene-carboxylate 207 (Figure 16) obtained from the endophytic fungus Aspergillus flavipes Y-62 isolated from Suaeda glauca Bunge in Zhoushan, Zhejiang, China, showed weak antimicrobial activities against Pseudomonas aeruginosa, Klebsiella pneumonia, and Staphylococcus aureus, with MIC values ranging from 32 to 128 μg/mL [109]. The polychlorinated triphenyl diether simatorone 208 (Figure 16) was isolated from Microsphaeropsis sp. cultures, and its antimicrobial activities against three pathogens (Escherichia coli, Bacillus megaterium, and Microbotryum violaceum) were evaluated using an agar diffusion assay. Compound 208 showed effective antimicrobial activities against B. megaterium and E. coli with inhibitory zone diameters of 14 mm and 18 mm, respectively [110]. Two alkylated furan derivatives—5-(undeca-3′,5′,7′-trien-1′-yl) furan-2-ol 209 (Figure 16) and 5-(undeca-3′,5′,7′-trien-1′-yl) furan-2-carbonate 210 (Figure 16)—were obtained from the methanol extract of the endophytic fungus Emericella sp. XL029 isolated from Panax notoginseng leaves in Hebei, China. Compounds 209–210 both showed potent antifungal activities against six tested plant pathogenic fungi (Rhizoctorzia solani, Verticillium dahliae Kleb, Helminthosporium maydis, Fusarium oxysporum, Fusarium tricinctum, and Botryosphaeria dothidea), with MIC values ranging from 25 to 3.1 μg/mL [111]. The new azaphilone, isochromophilone G 211 (Figure 16), was obtained from the endophytic fungus Diaporthe perseae sp. isolated from Pongamia pinnata (L.) Pierre. Compound 211 showed significant DPPH and ABTS radical scavenging activities, with IC50 values of 7.3 μmol/mL and 1.6 μmol/mL, respectively [112]. The furan derivative, 3-(5-oxo-2,5-dihydrofuran-3-yl) propanoic acid 212 (Figure 16), was obtained from the endophytic fungus Aspergillus tubingensis DS37 isolated from Decaisnea insignis (Griff.) Hook & Thomson, and showed significant inhibition activities against Fusarium graminearum and Streptococcus lactis, with MIC values of 16 μg/mL and 32 μg/mL, respectively [113]. The pyrrolidinone derivative, nigrosporamide A 213 (Figure 16), was isolated from the endophytic fungus Nigrospora sphaerica ZMT05 of Oxya chinensis Thunberg and showed a three-fold higher α-glucosidase inhibition activity than the positive control acarbose (IC50 value of 446.7 µM) with an IC50 value of 120.3 µM. Compound 213 has the potential to be a lead compound for the development of α-glucosidase inhibitors [114]. The production of the terrein derivative asperterrein 214 (Figure 16) was induced by co-culturing endophytic fungi Aspergillus terreus EN-539 and Paecilomyces lilacinus EN-531 of the marine red alga Laurencia okamurai. Compound 214 showed weak antimicrobial activities against Physalospora piricola and Staphylococcus aureus, with MIC values ranging from 32 to 64 μg/mL. Additionally, compound 214 was not detected in the sterile cultures of the two fungi alone [115]. The endophytic fungus Lachnum palmae of Przewalskia tangutica was isolated to halogenated dihydroisocoumarins palmaerones A–F 215–220 (Figure 16) under the guidance of UPLC-ESIMS. The antimicrobial activities of all compounds against five tested pathogens (Cryptococcus neoformans, Penicillium sp., Candida albicans, Bacillus subtilis, and Staphylococcus aureus) were evaluated using the broth microdilution method. Compounds 215–220 showed potent to weak antimicrobial activities against all tested pathogens, with MIC values ranging from 10 to 55 μg/mL. Additionally, compounds 215 and 219 showed moderate inhibition of LPS-induced NO production in RAW264.7 macrophages, with IC50 values of 26.3 μM and 38.7 μM, respectively [116].
Figure 16.
Chemical structures of other new compounds.
Over the past few years, plants have been a major source of numerous compounds that possess biological activities; however, this review revealed that most of these compounds were also produced by various endophytes, especially fungi. Therefore, the isolation and development of these compounds as novel drug candidates would be of great importance to the pharmacological industry since endophytes are easy to manage, keep, and work with compared with plants. Thus, we conclude that endophytic fungi may serve as alternative sources of bioactive compounds of pharmacological interest.
All the information about the new compounds have been summarized below in Table 2.
Table 2.
Brief summary of new compounds.
| Compound | Molecular Formula | Color and Morphology | Endophytic Fungus | Host Plant | Site and Nation | Pharmacological Activity | Ref. |
|---|---|---|---|---|---|---|---|
| Polyketides Chromones | |||||||
| 1 | C12H13O6 | colorless powder | Botryosphaeria ramosa L29 | leaf of Myoporum bontioides | Leizhou Peninsula, China | Displayed acceptable antimicrobial activities against Fusarium oxysporum | [36] |
| 2 | C14H15O6 | white powder | |||||
| 3 | C11H11O5 | ||||||
| 4 | C17H19N3O3S2 | yellow crystals | Phaeosphaeria fuckelii | Phlomis umbrosa | Mount Hua, China | Mushroom tyrosinase inhibitory activity | [37] |
| 5 | C15H16O7S | yellow powder | Chaetomium seminudum | Showed antifungal activity (5–6); Exhibited radical scavenging activity against DPPH; Showed significant antioxidant activity ((5) | [38] | ||
| 6 | C16H18O7S | ||||||
| 7 | C16H24O5 | colorless oil | Pestalotiopsis fici W106-1 | Camellia sinensis | Hangzhou, China | Displayed inhibitory effects on HIV-1 replication in C8166 cells ((7–8); Showed low to moderate cytotoxic activity (9–10); Displayed significant antifungal activity (9) | [39] |
| 8 | |||||||
| 9 | C32H54O6 | ||||||
| 10 | C32H54O6Na | ||||||
| Polyketides α-pyrones | |||||||
| 11 | C11H14O4 | colorless crystals | Neurospora udagawae | shoot of Quercus macranthera | Exhibited moderate antifungal (vs. Rhodoturula glutinis) activity and cytotoxicity against KB3.1 cells (12) | [40] | |
| 12 | C10H10O4 | colorless oil | |||||
| 13 | C28H42O4 | colorless amorphous powder | Aspergillus niger MA-132 | Avicennia marina | Hainan Province, China | Showed potent antifungal and cytotoxic activities | [41] |
| 14 | |||||||
| 15 | C14H22O5 | yellow oil | Pestalotiopsis fici | branches of Camellia sinensis (Theaceae) | Hangzhou, China | Displayed significant antifungal against Gibberella zeae | [42] |
| 17 | C10H14O4 | yellow oil | Aspergillus oryzae | Paris polyphylla var. yunnanensis | Dali, Yunnan Province, China | The biological activities of compounds 17–18 were not tested | [43] |
| 18 | |||||||
| 19 | C11H16O4 | yellow gum | Penicillium herque | Cordyceps sinensis | Xiahe, China | Weak cytotoxic activity | [44] |
| 20 | C12H16O5 | ||||||
| 21 | |||||||
| Polyketides: Other polyketides | |||||||
| 22 | C22H35ClO7 | white powder | Phoma sp. NTOU4195 | Pterocladiella capillacea | Taiwan, China | Showed potent anti-angiogenic activity (22); Exhibited inhibition of nitric oxide production in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells (24) | [45] |
| 23 | |||||||
| 24 | |||||||
| 25 | |||||||
| 26 | |||||||
| 27 | C22H34O6 | ||||||
| 28 | C22H26NO8 | ||||||
| 29 | C24H35NO5 | colorless oil | Simplicillium subtropicum SPC3 | fresh bark of Duguetia staudtii | Cameroon | Weak cytotoxic activity | [46] |
| 30 | C24H35NO6 | ||||||
| 31 | C12H20O3 | colorless oil | Cladosporium cladosporioides MA-299 | leaves of the mangrove plant Bruguiera gymnorrhiza | Hainan Island, China | Showed potent antimicrobial((vs. Escherichia coli and Staphylococcus aureus) activity and moderate inhibition activity against acetylcholinesterase (33) | [47] |
| 32 | |||||||
| 33 | C12H22O4 | pale yellow powder | |||||
| 34 | C14H24O5 | pale yellow oil | |||||
| 35 | C12H20O4 | colorless crystals | |||||
| 36 | C14H20O5 | colorless powder | Aspergillus fumigatiaffnis | Tribulus terestris | Weak antimicrobial activities | [48] | |
| 37 | C14H12O6Na | white amorphous solid | Alternariaalternata ZHJG5 | leaf of Cercis hinensis | Nanjing, China | Exhibited potent antimicrobial activity; Showed significant protective effect against the bacterial blight of rice (37) | [49] |
| 38 | C14H12O5Na | white powder | |||||
| 39 | C14H12O5Na | ||||||
| 40 | C29H22O12Na | ||||||
| 41 | C24H27NO5 | brown solid | Peyronellaea sp. FT431 | healthy leaf of a Hawaiian indigenous plant, Verbena sp. | Lyon, France | Showed weak to moderate cytotoxic activity (41–42) | [50] |
| 42 | C24H26O7 | ||||||
| 43 | C24H26O7 | ||||||
| 44 | C18H20O5 | ||||||
| 45 | C29H22O9 | red wine colored lump crystal | Alternaria sp. MG1 | Vitis quinquangularis | Showed weak cytotoxicity | [51] | |
| Alkaloids Cytochalasin | |||||||
| 46 | C22H32N2O5 | white amorphous solid | Phomopsis sp. sh917 | Fresh stems of I. eriocalyx var. laxiflora | Kunming, China | Significant inhibitory activity against NO production in LPS-induced RAW264.7 cells (46) | [52] |
| 47 | C22H33NO4 | ||||||
| 48 | |||||||
| 49 | C32H40N2O6 | colorless amorphous powder | Chaetomium globosum TW1-1 | Armadillidium vulgare | Hubei Province, China | Showed potential cytotoxic activities against cancer cell lines (HL-60, A-549, SMMC-7721, MCF-7, and SW-480) | [53] |
| 50 | C32H38N2O6 | ||||||
| 51 | |||||||
| 52 | C32H40N2O6 | ||||||
| 53 | C32H38N2O5 | white amorphous powder | |||||
| 54 | C32H83N2O6 | ||||||
| 55 | C32H36N2O4 | ||||||
| 56 | colorless amorphous powder | ||||||
| 57 | C34H42N2O7Na | ||||||
| 58 | C28H37NO3 | white amorphous solid | Diaporthe sp. SC-J0138 | Cyclosorus parasiticus (Thelypteridaceae) | Guangdong Province, China | Showed significant cytotoxic activities against four human cancer cell lines (A549, HeLa, HepG2, and MCF-7) (58); Exhibited selective cytotoxic activity (59–62) | [54] |
| 59 | C28H37NO | ||||||
| 60 | |||||||
| 61 | C28H37NO4 | ||||||
| 62 | |||||||
| 63 | C25H37NO4 | colorless crystal | Cytospora chrysosperma HYQZ-931 | Hippophae rhamnoides | Exhibited significant antibacterial activity (63,65) | [55] | |
| 64 | white amorphous powder | ||||||
| 65 | C26H41NO5 | ||||||
| Alkaloids Indole alkaloids | |||||||
| 66 | C26H28N3O4 | brilliant yellowish powder | Aspergillus sp. YJ191021 | Zhejiang Province, China | Exhibited moderate antibacterial activity (66); Displayed notable anti-inflammatory; Exhibited notable cytotoxicity (66–69) | [56] | |
| 67 | C26H29N3O5 | white powder | |||||
| 68 | C27H31N3O6Na | ||||||
| 69 | C27H31N3O6 | ||||||
| 70 | C28H31N3O6 | ||||||
| 71 | C26H31N3O6 | ||||||
| 72 | C30H33NO7 | white amorphous powder | Penicillium chrysogenum XNM-12 | Leathesia nana | Shandong Province, China | Exhibited moderate antibacterial effects against Ralstonia solanacearum | [57] |
| 73 | C23H38N1NaO3 | amorphous powder | Hypomontagnella monticulosa Zg15SU | fresh rhizome of Zingiber griffithii Baker | Indonesia | Showed potent cytotoxic activity | [58] |
| 74 | C20H22N2NaO4 | yellowish powder | Aspergillus lentulus DTO 327G5 | Caenagrion | Shanghai, China | Displayed weak to moderate antibacterial activity | [59] |
| 75 | C19H21O4N2 | white powder | |||||
| 76 | C24H25N3NaO3 | ||||||
| Alkaloids Diketopiperazine derivatives | |||||||
| 77 | C20H27N3O3S2Na | white solid powder | Phaeosphaeria fuckelii | Phlomis umbrosa | Mount Hua, China | Showed strong inhibitory effects on mushroom tyrosinase | [60] |
| 78 | C26H29N3O5 | colorless oil | Paecilomyces variotii EN-291 | Grateloupia turuturu | Qingdao Province, China | Exhibited potent antifungal effects | [61] |
| 79 | C22H23N3O4 | ||||||
| 80 | C21H25O3N3 | yellow powder | Aspergillus sp. 16-5c | leaf of S. apetala | Hainan Island, China | Showed potent to moderate α-glucosidase inhibitory activity (80–81) | [62] |
| 81 | C21H23O4N3 | white powder | |||||
| 82 | C21H23O3N3 | yellow powder | |||||
| 83 | C21H25O3N3 | ||||||
| 84 | C22H27O3N3 | ||||||
| 85 | C18H15O4N3 | white powder | |||||
| 86 | C19H24N2O6S | colorless crystals | Penicillium brocae MA-231 | Avicennia marina | Displayed moderate to high activities against Staphylococcus aureus | [63] | |
| 87 | C19H22N2O5S | yellowish solid | |||||
| 88 | C20H26N2O6S2 | colorless crystals | |||||
| 89 | C20H26N2O6S2 | colorless solid | |||||
| 90 | C20H24N2O6S2 | ||||||
| 91 | C19H18N2O4S | colorless crystals | Penicillium brocae MA-231 | Showed moderate antimicrobial activities against S. aureus and Aeromonas hydrophilia | [64] | ||
| 92 | C19H16N2O4S | ||||||
| 93 | C18H14N2O4 | ||||||
| Alkaloids: Other types of alkaloids | |||||||
| 94 | C21H16N4O2 | colorless needles | Penicillium vinaceum (X17) | corm of Crocus sativus | Shanghai, China | Showed weak cytotoxic activities against three human tumor cell lines (A549, LOVO, and MCF-7) | [65] |
| 95 | C14H16Br2N2O5 | colorless amorphous powder | Acanthodendrilla sp. | Thailand | Exhibited efficient and selective cytotoxic activities against two human tumor cell lines (H292 and HaCaT) | [66] | |
| 96 | |||||||
| 97 | C19H20NO3 | colorless needles crystal | Penicillium sumatrense GZWMJZ-313 | leaf of Garcinia multiflora | Guizhou, China | Showed moderate to weak antimicrobial activities against Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli | [67] |
| 98 | C20H24NO3 | white powder | |||||
| 99 | C20H21NO3 | ||||||
| 100 | C25H29O5N | white powder | Diaporthe sp. SYSUHQ3 | fresh branch of the mangrove plant Excoecaria agallocha | Showed potent inhibition activity against Mycobacterium tuberculosis protein-tyrosine phosphatase B | [68] | |
| 101 | C25H29O5N | ||||||
| Terpenoids Sesquiterpenoids and their derivatives | |||||||
| 102 | C15H22O3Na | colorless oil | Rhizopycnis vagum Nitaf22 | Nicotiana tabacum | Exhibited high selective cytotoxicity against NCI-H1650 and BGC823 cell lines (115); Showed strong phytotoxic activities against radicle growth in rice seedlings (106–107, 113–114) | [70] | |
| 103 | C15H23O3 | ||||||
| 104 | C15H22O4Na | colorless amorphous solid | |||||
| 105 | C15H20O3Na | colorless oil | |||||
| 106 | C15H24O3Na | ||||||
| 107 | C15H22NaO3 | ||||||
| 108 | C15H21O4 | ||||||
| 109 | C15H25O4 | ||||||
| 110 | C15H22O3Na | ||||||
| 111 | C15H19O5 | colorless amorphous solid | |||||
| 112 | C15H13O5 | brown amorphous solid |
Rhizopycnis vagum Nitaf22
Rhizopycnis vagum Nitaf22 |
Nicotiana tabacum
Nicotiana tabacum |
Exhibited high selective cytotoxicity against NCI-H1650 and BGC823 cell lines (115); Showed strong phytotoxic activities against radicle growth in rice seedlings (106–107, 113–114) | [70] | |
| 113 | C15H14O4Na | yellowish oil | |||||
| 114 | C17H18NO4 | greenish-yellow amorphous solid | |||||
| 115 | C14H15NO4 | light-yellowish amorphous solid | |||||
| 116 | C15H22O3 | colorless oil | Trichoderma sp. PR-35 | healthy stem of Paeonia delavayi | Yunnan Province, China | Showed moderate to weak antimicrobial activities against Escherichia coli and Shigella sonnei | [69] |
| 117 | C15H26O2 | ||||||
| 118 | C15H28O3Na | colorless oil | Fusarium proliferatum AF-04 | Lanzhou, China | Displayed weak antimicrobial against Bacillus subtilis, Clostridium perfringens, E. coli, and MRSA | [71] | |
| 119 | C15H26O2Na | ||||||
| 120 | C15 H 20 O5 | colorless oil | Aspergillus sydowii EN-434 | Symphyocladia latiuscula | Qingdao Province, China | Exhibited radical scavenging activity against DPPH | [72] |
| 121 | C25H37O4 | amorphous powder | Ulocladium sp. | Yunnan Province, China | Showed moderate antimicrobial activities against B. subtilis and multi-drug-resistant S. aureus (121–125); Exhibited high selective cytotoxicity against the HepG2 cell line (125) | [73] | |
| 122 | |||||||
| 123 | C26H38O4Na | ||||||
| 124 | C26H40O5Na | ||||||
| 125 | C25H34O3Na | ||||||
| 126 | C15H26O3 | colorless crystals | Trichoderma virens QA-8 | fresh inner root tissue of the grown medicinal herb Artemisia argyi H. Lév. and Vaniot | Hubei Province, China | Showed significant antimicrobial activities against E. coli | [74] |
| 127 | amorphous powder | ||||||
| 128 | colorless oil | ||||||
| 129 | C15H24O2 | ||||||
| 130 | C15H26O3 | colorless waxy solid | |||||
| Terpenoids Diterpenoids | |||||||
| 131 | C20H32O6Na | colorless oil | Diaporthe sp. QYM12 | healthy leaves of Kandelia candel | Hainan Province, China | Showed significant anti-inflammatory effects through the inhibition of NO production | [75] |
| 132 | C21H28O6 | colorless crystals | Phomopsis sp. S12 | seed of Illigera rhodantha | Showed excellent inhibitory effects on the production of IL-1β and IL-18; Effects on the NF-κB signaling pathway | [76] | |
| 133 | C20H26O5 | colorless needle crystal | Phomopsis sp. S12 | seed of Illigera rhodantha | Exhibited anti-inflammatory activity against the production of IL-1b and IL-6 induced by lipopolysaccharide (LPS) in macrophages | [77] | |
| 134 | C20H28O4 | colorless oil | |||||
| 135 | C20H26O6 | ||||||
| 136 | C16H20O5 | colorless needles | Botryosphaeria sp. P483 | Chinese Herbal Medicine Huperzia serrata | Kunming, China | Showed effective antifungal antifungal activities against Gaeumannomyces graminis, Fusarium solani, and Pyricularia oryza (136); Showed weak nematicidal activities | [78] |
| 137 | C16H20O6 | colorless solid | |||||
| 138 | C20H28O6 | white amorphous solid | Smardaea sp. AZ0432 | photosynthetic tissue of the moss Ceratodon purpureus | Chiricahua Mountains of southeastern Arizona, USA | Exhibited selective cytotoxicity | [79] |
| 139 | C20H16O5 | yellowish needles | Pestalotiopsis adusta | Fresh, healthy stems of Clerodendrum canescens | Yandang, Zhejiang Province, China | Demonstrated cytotoxic activities against the HL-60 tumor cell line | [80] |
| 140 | C20H34O2 | colorless needles | Trichoderma atroviride S361 | Cephalotaxus fortunei | Jiande, Zhejiang, China | Bioactivity tests were not performed | [81] |
| 141 | C16H16O5 | colorless needles | Aspergillus wentii EN-48 | unidentified marine brown algal species of the genus Sargassum | Qingdao Province, China | Showed moderate cytotoxic activities against seven human tumor cell lines (NCI-H460, MDA-MB-231, HeLa, MCF-7, SMMC-7721, HepG2, and SW1990) | [82] |
| 142 | C16H16O5 | ||||||
| 143 | C16H24O5 | ||||||
| Terpenoids Triterpenoids | |||||||
| 144 | C30H48O | colorless solid | Scleroderma UFSMSc1 | Eucalyptus grandis | Showed moderate to weak antifungal activities against Candida albicans and Candida parapsolosis | [84] | |
| 145 | |||||||
| 146 | |||||||
| 147 | C29H46O5 | white powder | Acremonium pilosum F47 | pedicel of the Chinese medicinal plant Mahonia fortunei | Qingdao Province, China | Displayed effective antimicrobial activities against S. aureus and B. subtili | [85] |
| 148 | C30H50O6 | yellow amorphous powder | Glomerella sp. F00244 | stem of mason pine | Fujian Province, China | Showed weak cytotoxic activity (148) | [83] |
| 149 | C31H52O6 | white amorphous powder | |||||
| 150 | C30H40O6 | yellowish needle crystals | Penicillium sp. SWUKD4.1850 | healthy branches of Kadsura angustifolia | Yunnan Province, China | Exhibited moderate in vitro cytotoxic activities | [86] |
| 151 | C30H40O6 | white needle crystals | |||||
| 152 | C30H40O6 | white amorphous solid | |||||
| 153 | C30H41O6 | ||||||
| 154 | C32H44O7 | white amorphous powder | |||||
| 155 | C30H42O6 | white powder | |||||
| 156 | C34H46O8 | yellow amorphous solid | |||||
| 157 | C31H44O6 | ||||||
| 158 | C30H46O6 | white amorphous powder | |||||
| 159 | C32H50O5 | white amorphous powder | Hypoxylon sp. 6269 | Artemisia annua | Weak inhibition activity against the HIV-1 integrase (159) | [87] | |
| 160 | C29H44O4 | ||||||
| 161 | C36H55O7 | white amorphous powder |
Fusarium sp. | roots of Mentha longifolia | Saudi Arabia | Showed significant antileishmanial activity (161) | [88] |
| 162 | C32H51O5 | ||||||
| 163 | C39H55O7 | ||||||
| 164 | C34H53O6 | colorless powder | Fusarium sp. | roots of Mentha longifolia | Saudi Arabia | Displayed potent cytotoxic activity towards BT-549 and SKOV-3; Showed potent antileishmanial activities against L. donovani promastigotes | [89] |
| 165 | C42H68O7 | white amorphous powder | |||||
| Terpenoids Meroterpenoids | |||||||
| 166 | C18H26O5 | colorless crystal | Guignardia mangiferae A348 | Medicinal Plant Smilax glabra | Luofu Mountain Natural Reservation, Guangdong Province, China | Showed weak cytotoxic activities against MCF-7 cell lines(167,169) | [90] |
| 167 | C17H22O4 | ||||||
| 168 | C18H28O5 | white powder | |||||
| 169 | C17H24O4 | ||||||
| 170 | C25H30O5 | colorless amorphous powder | Emericella sp. TJ29 | root of the plant Hypericum perforatum | the Shennongjia areas of Hubei Province, China | Showed moderate cytotoxic activities against five human tumor cell lines (HL-60, SMMC7721, A549, MCF-7, and SW-480) (172, 173, 175) | [91] |
| 171 | C27H34O6 | white powder | |||||
| 172 | C26H32O6 | colorless crystals | |||||
| 173 | C25H30O6 | colorless crystals | |||||
| 174 | C25H32O6 | white powder | |||||
| 175 | C25H28O6 | colorless crystals | |||||
| 176 | C31H35O9 | yellowish oil | Phyllosticta sp. J13-2-12Y | leaf of Acorus tatarinowii | Guangxi Province, China | Exhibited moderate antimicrobial activities against Staphylococcus aureus 209P, Candida aureus 209P, and Candida albicans FIM709 | [92] |
| 177 | C31H37O9 | ||||||
| 178 | C31H34O9 | ||||||
| 179 | C27H32O9 | white powder | Co-culture Talaromyces purpurogenus H4 and Phanerochaete sp. H2 | Handroanthus impetiginosus | Alfenas, Minas Gerais, Brazil. | Showed moderate trypanocidal activity against T. cruzi | [93] |
| Lactones | |||||||
| 180 | C13H22O3 | colorless gum | Talaromyces assiutensis JTY2 | leaf of Ceriops tagal | South China Sea, China | Showed moderate cytotoxic activities against three human cancer cell lines (HeLa, MCF-7, and A549) | [94] |
| 181 | C21H34O5 | yellow oil | Penicillum polonicum | fruits of Camptotheca acuminata Decne | Wuhan, China | Showed effective glucose uptake activity on rat skeletal muscle myoblast L6 (181); Significantly promoted GLUT4 translocation in L6 cells | [95] |
| 182 | C16H28O5 | light red oil | |||||
| 183 | C24H33NO8 | colorless crystal | Chaetomium cupreum | Anemopsis californica | New Mexico, U.S.A. | Showed weak antimicrobial activity against S. aureus | [96] |
| 184 | Xylaria curta 92092022 | Taiwan, China | Showed moderate antimicrobial activities against Pseudomonas aeruginosa and Staphylococcus aureus; Displayed strongly inhibited lettuce seed germination | [97] | |||
| 185 | C24H34O5 | white powder | Lasiodiplodia theobromae ZJ-HQ1 | healthy leaves of the marine mangrove Acanthus ilicifolius | South China Sea, China | Exhibited inhibitory effects on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells; Showed moderate inhibitory activity against α-glucosidase | [98] |
| 186 | C8H10O3 | Aspergillus pseudonomiae J1 | Euphorbia umbellata (Pax) Bruyns (Euphorbiaceae) | Bahia, Brazil | Showed moderate to weak anti-trypanosomal activity | [99] |
|
| 187 | C10H12O4 | ||||||
| 188 | C10H12O3 | ||||||
| Anthraquinones, quinones, and related glycosides | |||||||
| 189 | C20H19O7 | Brilliant yellowish oil | Acremonium vitellinum | Acanthus ilicifolius Linn | Qingdao Province, China | Showed moderate insecticidal activities against the third-instar larvae of Helicoverpa ar-migera | [100] |
| 190 | C20H16O7 | yellow solid | |||||
| 191 | C22H21O7 | ||||||
| 192 | C18H18O8Na | yellow amorphous powder | Phomopsis sp. | Paris polyphyllavar | Yunnan Province, China | Showed significant cytotoxic activities against A549 and PC3 cell lines | [101] |
| 193 | C21H20O10 | red amorphous powder | Eurotium cristatum EN-220 | Sargassum thunbergii | Qingdao Province, China | Showed weak antimicrobial activity against E. coli only; Showed moderate fatal activity against brine shrimp larvae | [102] |
| 194 | C18H15O6 | orange yellow powder | Aspergillus versicolor | Halimeda opuntia | South Sinai, Egypt | Weak inhibitory activity against hepatitis C virus NS3/4A protease | [103] |
| 195 | C12H11O4 | red powder | |||||
| 196 | C16H11O5 | orange powder | |||||
| 197 | C16H21O7 | red powder | Stemphylium globuliferum | healthy stems of Mentha pulegium | Beni Mellal, Morocco | Showed significant inhibition of proliferation of K562 and A549 cells | [104] |
| Steroids | |||||||
| 198 | C27H40O3 | white crystals | Phoma sp. SYSU-SK-7 | Guangdong Province, China | Exhibited inhibitory effects on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells; Showed moderate inhibitory activity against α-glucosidase | [105] | |
| 199 | C28H41O3 | white solid | |||||
| 200 | C38H64O4 | white amorphous powder | Fusarium sp. | Mentha longifolia | Egypt | Showed moderate cytotoxic activity against human colorectal cancer cell line HCT 116 | [106] |
| 201 | C28H40O4 | Phomopsis sp. | Aconitum carmichaeli | Huize County, Yunnan Province, China | Showed weak antifungal activities against C. albicans and F. avenaceum | [107] | |
| 202 | C30H48O4 | ||||||
| Other types of compounds | |||||||
| 203 | C20H22O3 | yellow oil | Aspergillus sp. | root of Tripterygium wilfordii | Wuhan, China | Showed significant inhibition of LPS-induced IL-1β, TNF-α, and NO production in RAW264.7 cells | [108] |
| 204 | C24H26O6 | ||||||
| 205 | C24H26O6 | colorless oil | |||||
| 206 | C23H24O6 | ||||||
| 207 | C23H24O5 | brown powder | Aspergillus flavipes Y-62 | stems of plant Suaeda glauca Bunge | Zhoushan coast, Zhejiang province, China | Exhibited antimicrobial activities against the Gram-negative pathogens Pseudomonas aeruginosa and Klebsiella pneumoniae | [109] |
| 208 | C23H21O5 | white powder | Microsphaeropsis sp. | Showed effective antimicrobial activities against B. megaterium and E. coli | [110] | ||
| 209 | C15H20O2 | brown amorphous powder | Emericella sp. XL029 | leaf of Panax notoginseng | Shijiazhuang, Hebei province, China | Showed potent antifungal activities against six tested plant pathogenic fungi (Rhi-zoctorzia solani, Verticillium dahliae Kleb, Helminthosporium maydis, Fusarium oxysporum, Fusarium tricinctum, and Botryosphaeria dothidea) | [111] |
| 210 | C16H20O4 | ||||||
| 211 | C18H18O6Cl | yellow powder | Diaporthe perseae sp. | stem of Chinese mangrove Pongamia pinnata | Hainan city, China | Showed significant DPPH and ABTS radical scavenging activities | [112] |
| 212 | C7H7O4 | colorless flake crystal | Aspergillus tubingensis DS37 | Decaisnea insignis (Griff.) Hook. f. and Thomson | Showed significant inhibition activities against Fusarium graminearum and Streptococcus lactis | [113] | |
| 213 | C13H15NO4Na | amorphous powder | Nigrospora sphaerica ZMT05 | Oxya chinensis Thunber | Guangdong Province, China. | Showed significant α-glucosidase inhibitory activity | [114] |
| 214 | C9H14O2 | colorless oil | Co-culture Aspergillus terreus EN-539 & Paecilomyces lilacinus EN-531 | Laurencia okamurai | Qingdao, China | Showed weak antimicrobial activities against Physalospora piricola and Staphylococcus aureus | [115] |
| 215 | C11H11BrO4 | white amorphous powder | Lachnum palmae | Przewalskia tangutica | Exhibited potent to weak antimicrobial activities against Cryptococcus neoformans, Penicillium sp., Candida albicans, Bacillus subtilis, and Staphy-lococcus aureus (215–220); Showed moderate inhibitory effects on NO production in LPS-induced RAW 264.7 cells (215,219) | [116] | |
| 216 | |||||||
| 217 | C12H13BrO4 | ||||||
| 218 | C10H9BrO4 | ||||||
| 219 | C11H11BrO5 | ||||||
| 220 | C11H11ClO4 | ||||||
3. Future Prospects and Challenges of Using Endophytic Fungi as an Alternative Source of Plant Bioactive Compounds
Endophytic fungi are hidden and subtle dwellers in several plant tissues and intercellular spaces and can produce diverse chemical structures and efficient, low-toxic new secondary metabolites that were initially thought to be produced by the host plants. The current reports on the biosynthesis of plant metabolites by endophytic fungi, in conjunction with recent research advances in fermentation culture, extraction, isolation, and structure identification techniques, permit us to rapidly uncover new valuable compounds. Generally, fungi are chemically diverse, easily cultured, and biologically active modalities that have great flexibility to be regulated by adding precursors, elicitors, and specific enzymes to effectively increase the quantity and yield of bioactive compounds. Table 3 represents the culture conditions and specific bioactive secondary metabolites and yields produced by various endophytic fungi. Endophytic fungi can convert active compounds of the host plant into more potent derivatives. This makes endophytic fungi an alternative and sustainable source of plant bioactive compounds [117,118]. The search for new compounds in endophytic fungi requires specific theories and ingenious bioprospecting strategies. Along with the continuously growing literature reports, the most promising host plants can be selected. It includes the selection of (A) plants from special habitats or growing in biodiversity-rich areas, including mangrove plants in tropical marine intertidal zones, and (B) medicinal and indigenous plants with ethnopharmacological uses, including Camptotheca acuminata and Ageratina adenophora. These selection criteria provide a reference for the current and future screening of host plants for endophytic fungi with new bioactive compounds [119,120]. This review has summarized 220 new compounds obtained between 2011 and 2021 from endophytic fungi using different culture methods, including the common culture, co-culture with bacteria or other fungi, and the addition of metal ions. These new compounds have unique molecular structures, and these rare structures allow these compounds to possess diverse biological activities, including significant antimicrobial and cytotoxic activities and α-glucosidase inhibition. These compounds have the potential to be modified as pro-drug molecules or directly developed as drugs for treating certain diseases. However, most of the current studies on the activity of new compounds with endophytic fungal sources are limited to in vitro studies; therefore, animal experiments and human intervention clinical trials are needed to further investigate the in vivo activities and mechanisms of action of the new compounds.
Table 3.
Culture conditions and yields of bioactive secondary metabolites produced by endophytic fungi.
| No. | Endophytic Fungus | Host Plant | Culture Conditions | Secondary Metabolites | Yield | Ref. |
|---|---|---|---|---|---|---|
| 1 | Hansfordia biophila | Hedychium acuminatum Roscoe | Inoculated in potato glucose broth (PDB) medium and shaken at 120 rpm at 25 °C for 7 days. | Tannin | 41.6 μm·mL−1 | [121] |
| 2 | Aspergillus terreus | Ficus elastica | Inoculated into PDB medium and incubated at 30 °C for 20 days on a rotatory shaker incubator at 140 rpm. | Camptothecin | 320 μg/L | [122] |
| 3 | Guignardia mangiferae HAA11 | Taxus x media | Inoculated into (PDB) medium and incubated at 200 rpm at 28 °C for 5 days. | Paclitaxel | 720 ng/L | [123] |
| 4 | Papulasora sp.S6 | Phellodendron amurense Rupr | Mutagenesis by UV, X-ray rays, and NaNO2, inoculated in PDB medium, and shaken at 100 rpm at 28 °C for 7 days. | Berberine | 12.28 mg/L | [124] |
| 5 | Actinoplanes teichomyceticus | Improvement of the output of teicoplanin by genome shuffling; Inoculated teicoplanin medium and cultured at 28 °C for 15–20 days. | Teicoplanin | 3016 μm·mL−1 | [125] | |
| 6 | Phialocephala fortinii | Podophyllum peltatum | Inoculated in Sabouraud’s dextrose agar (SDA) and cultured at 23 °C for 4–6 weeks. | Podophyllotoxin | 189 µg/L | [126] |
| 7 |
Entrophospora infrequens
RJMEF001 |
Nothapodytes foetida | Inoculated into wheat bran containing Sabouraud’s broth, and incubation was carried out at 28 ± 2 °C for 28 days. |
Camptothecin | 503 ± 25 μg/100 g dry cell mass (in Sabouraud broth) | [127] |
| 8 |
Epicoccum nigrum SZMC 23769 |
Hypericum perforatum | Fungal isolates were grown in potato dextrose broth (PDB) for 7 days at 25 °C. | Hypericin, Emodin | 117.1 μg/mL, 87.7 μg/mL | [128] |
Unfortunately, endophytic fungi as new sources of bioactive secondary metabolites encounter various limitations, including the attenuated yield of secondary metabolites due to long-term storage and repeated passages under laboratory culture conditions, silencing of biosynthetic gene clusters or low level of expression (activation of gene clusters depends on environmental factors). Thus, the ability of endophytic fungi to produce new compounds of interest has been underestimated [129]. The expression could be upregulated by physicochemical and genetic manipulation techniques to increase the production of specific metabolites in endophytic fungi and to produce analogs of new active secondary metabolites. Methods including the OSMAC strategy (activation of silent biosynthetic gene clusters mediated by changes in medium composition, temperature, and aeration efficiency to produce desired metabolites), co-culture (mimicking natural ecosystems and triggering silent gene clusters to promote metabolite secretion and enhance bioactive metabolite production by microbial interaction-induced stress responses), and chemical epigenetic modification methods have been used to isolate new compounds. It was found that the addition of micromolar or even nanomolar small-molecule chemicals to cultures inhibits or activates relevant enzymes and remodels the fungal epigenome to increase the diversity of its secondary metabolites, including DNA methyltransferases (DNMTs) and histone deacetylase inhibitors (HDACs) [130,131]. The addition of epigenetic modifiers (5 μM SAHA and 10 μM AZA) to the endophytic fungus Xylaria psidii isolated from leaves of Vitis vinifera showed elevated resveratrol concentrations of 52.32 μg/mL and 48.94 μg/mL, respectively, by HPLC analysis (control concentration was 35.43 μg/mL). The treatments with 5 μM SAHA and 10 μM AZA showed stronger antioxidant activity with 30.92% and 33.82% DPPH radical scavenging, respectively, compared to the wild strain (19.26%) [132]. Unlike the chemical epigenetic modification methods reported, introducing exogenous substances as precursors into the cultures, including methyl jasmonate, causes the production of new compounds containing their structural units [133]. However, the addition of host plant components to the culture to induce the production of new compounds has rarely been reported. Additionally, it is necessary to elucidate the pathways by which endophytic fungi biosynthesize secondary metabolites, including the enzymes and genes involved via “omics” techniques—genomics, transcriptomics, and metabolomics—in regulating and manipulating the biosynthetic process to increase the number of new compounds [134].
4. Conclusions
Pharmaceutical chemists are turning their focus on the development of safe, efficient, and low-toxic new drugs from natural sources. Endophytic fungi may serve as renewable sources of novel bioactive compounds with pharmacological activities, as the number of new compounds to be isolated in the future tends to increase exponentially and rapidly. In addition, numerous studies have also reported that these bioactive compounds isolated from the endophytic fungi are also present in plants and have similar biological activities as the compounds from plant sources. Therefore, we conclude that endophytic fungi may be the best alternative for harnessing pharmacological bioactive compounds for the development of drugs for both human and animal use. Hence, there is a need for the identification of more compounds with pharmacological activity from endophytic fungi and elucidate their mechanisms of action through biological, pharmacodynamic, biochemical, bioinformatics, and pre-clinical approaches.
Author Contributions
J.W. (Juan Wen) and S.K.O. drafted the manuscript; S.K.O., S.W., Y.R. and J.W. (Juan Wen) generated the figures and tables; S.K.O., Y.H., J.W. (Jianchen Wang) and L.X. discussed literatures; J.W. (Juan Wen) designed the work, drafted and revised the manuscript, Y.H. supervised and funded the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Sichuan Province Science and Technology Support Program (Grant No. 2020YFS0337).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hyde K.D., Xu J.C., Rapior S., Jeewon R., Lumyong S., Niego A.G.T., Abeywickrama P.D., Aluthmuhandiram J.V.S., Brahamanage R.S., Brooks S. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019;97:1–136. doi: 10.1007/s13225-019-00430-9. [DOI] [Google Scholar]
- 2.Patchett A., Newman J.A. Comparison of Plant Metabolites in Root Exudates of Lolium perenne Infected with Different Strains of the Fungal Endophyte Epichlo festucae var. lolii. J. Fungi. 2021;7:148. doi: 10.3390/jof7020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odelade K.A., Babalola O.O. Bacteria, fungi and archaea domains in rhizospheric soil and their effects in enhancing agricultural productivity. Int. J. Environ. Res. Public Health. 2019;16:3873. doi: 10.3390/ijerph16203873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igiehon N.O., Babalola O.O., Cheseto X., Torto B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021;242:126640. doi: 10.1016/j.micres.2020.126640. [DOI] [PubMed] [Google Scholar]
- 5.Jin J., Zhao Q., Zhang X.M., Li W.J. Research progress on bioactive products from endophytes. J. Microbiol. 2018;38:103–113. doi: 10.3969/j.issn.1005-7021.2018.03.17. [DOI] [Google Scholar]
- 6.Deshmukh S.K., Gupta M.K., Prakash V., Saxena S. Endophytic fungi: A Source of Potential Antifungal Compounds. J. Fungi. 2018;4:77. doi: 10.3390/jof4030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kouipou R.M.T., Boyom F.F. Endophytic fungi from Terminalia species: A comprehensive review. J. Fungi. 2019;5:43. doi: 10.3390/jof5020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popli D., Anila V., Subramanyama A.B., Namratha M.N., Ranjitha V.R., Raoa S.N., Ravishankar V., Govindappaa M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif. Cell Nanomed. Biotechnol. 2018;46:676–683. doi: 10.1080/21691401.2018.1434188. [DOI] [PubMed] [Google Scholar]
- 9.Zheng R.H., Li S.J., Zhang X., Zhao C.Q. Biological activities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021;22:959. doi: 10.3390/ijms22020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rustamova N., Bozorov K., Efferth T., Yili A. Novel secondary metabolites from endophytic fungi: Synthesis and biological properties. Phytochem. Rev. 2020;19:425–448. doi: 10.1007/s11101-020-09672-x. [DOI] [Google Scholar]
- 11.Ding W.J., Wang S.S., Ren J.Q., Li G., Zhan J.P. Progress on plant endophyte. Curr. Biotechnol. 2015;5:425–428. doi: 10.3969/j.issn.2095~2341.2015.06.04. [DOI] [Google Scholar]
- 12.Jin L.R., Yang L., Li W.J., Xu D., Yang N.N., Li G.Q., Wan P. Diversity and Biocontrol Potential of Culturable Endophytic Fungi in Cotton. Front. Microbiol. 2021;12:698930. doi: 10.3389/fmicb.2021.698930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S.S., Liu J.M., Sun J., Huang Y.T., Jin N., Li M.M., Liang Y.T., Fan B., Wang F.Z. Analysis of Endophytic Bacterial Diversity From Different Dendrobium Stems and Discovery of an Endophyte Produced Dendrobine-Type Sesquiterpenoid Alkaloids. Front. Microbiol. 2020;12:775665. doi: 10.3389/fmicb.2021.775665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharwar R.N., Mishra A., Stierle A., Kharwar R.N., Gond S.K., Stierle D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011;28:1208–1228. doi: 10.1039/c1np00008j. [DOI] [PubMed] [Google Scholar]
- 15.Hawksworth D.L. The fungal dimension of biodiversity: Magnitude, significance, and conservation. Mycol. Res. 1991;95:641–655. doi: 10.1016/S0953-7562(09)80810-1. [DOI] [Google Scholar]
- 16.Wu B., Hussain M., Zhang W.W., Stadler M., Liu X.Z., Xiang M.C. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology. 2019;10:127–140. doi: 10.1080/21501203.2019.1614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogl A. Mehl und die andiron mehlprodukt der cerealien und leguminosen. Nahrunsm. Unters. Hug. Waren. 1898;12:25–29. [Google Scholar]
- 18.Stierle A., Strobel G., Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 19.Cruz J.S., Silva C.A., Hamerski L. Natural Products from Endophytic Fungi associated with Rubiaceae Species. J. Fungi. 2020;6:128. doi: 10.3390/jof6030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gokhale M., Gupta D., Gupta U., Faraz R., Sandhu S.S. Patents on endophytic fungi. Curr. Med. Chem. 2017;11:120–140. doi: 10.2174/1872208311666170215151834. [DOI] [PubMed] [Google Scholar]
- 21.Oberhofer M., Wackerlig J., Zehl M., Büyük H., Cao J.J., Urban E., Zotchev S.B. Endophytic Akanthomyces sp. LN303 from edelweiss produces emestrin and two new 2-hydroxy-4 pyridone alkaloids. ACS Omega. 2021;6:2184–2191. doi: 10.1021/acsomega.0c05472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao Z.L., Zhang W.H., Wu C.Y., Feng H., Peng Y.H., Shahid H., Cui Z.N., Ding P., Shan T.J. Diversity and antibacterial activity of fungal endophytes from Eucalyptus exserta. BMC Microbiol. 2021;21:155. doi: 10.1186/s12866-021-02229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira C.B., Oliveira D.M., Hughes A.F., Kohlhoff M., Vieira1 M.L. Endophytic fungal compounds active against Cryptococcus neoformans and C. gattii. J. Antibiot. 2015;68:436–444. doi: 10.1038/ja.2015.11. [DOI] [PubMed] [Google Scholar]
- 24.Abdou R., Shabana S., Rateb M.E. Terezine E, bioactive prenylated tryptophan analogue from an endophyte of Centaurea stoebe. Nat. Prod. Res. 2020;34:503–510. doi: 10.1080/14786419.2018.1489393. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho C.R.D., Vieira M.D.L.A., Cantrell C.L., Wedge D.E., Alves T.M., Zani C.L., Pimenta R.S., Rosaa L.H. Biological activities of ophiobolin k and 6-epi-ophiobolin k produced by the endophytic fungus Aspergillus calidoustus. Nat. Prod. Res. 2016;30:478–481. doi: 10.1080/14786419.2015.1022777. [DOI] [PubMed] [Google Scholar]
- 26.Jouda J.B., Tamokou J., Mbazoa C.D., Douala-Meli C., Sarkar P., Bag P.K., Wandji J. Antibacterial and cytotoxic cytochalasins from the endophytic fungus Phomopsis sp. harbored in garcinia kola (heckel) nut. BMC Complement. Altern. Med. 2016;16:462. doi: 10.1186/s12906-016-1454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sana T., Siddiqui B.S., Shahzad S., Farooq A.D., Siddiqui F., Sattar S., Begumet S. Antiproliferative activity and characterization of metabolites of Aspergillus nidulans: An endophytic fungus from Nyctanthes arbor-tristis linn. against three human cancer cell lines. Med. Chem. 2019;15:352–359. doi: 10.2174/1573406414666180828124252. [DOI] [PubMed] [Google Scholar]
- 28.Sahar L., Doustmorad Z. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front. Microbiol. 2018;9:1484. doi: 10.3389/fmicb.2018.01484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhakshinamoorthy M., Ponnusamy S.K., Kannaian U.P., Srinivasan B., Shankar S.N., Packiam K.K. Plant-microbe interactions implicated in the production of camptothecin—An anticancer biometabolite from Phyllosticta elongata MH458897 a novel endophytic strain isolated from medicinal plant of Western Ghats of India. Environ. Res. 2021;201:111564. doi: 10.1016/j.envres.2021.111564. [DOI] [PubMed] [Google Scholar]
- 30.Han W.X., Li W.Z., Li X.F., Zhang H., Yang S.Z., Du J.F., Jing H., Cheng C.B. Isolation and identification of endophytic fungus producing Huperzine A from Huperzia serrata. Microbiol. China. 2017;44:2153–2160. doi: 10.13344/j.microbiol.china.170160. [DOI] [Google Scholar]
- 31.Khan N., Afroz F., Begum M.N., Rony S.R., Sharmin S., Moni F., Hasan C.M., Shaha K., Sohrab M.H. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018;5:970–976. doi: 10.1016/j.toxrep.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazlan N.W., Tate R., Yusoff Y.M., Clements C., Edrada-Ebel R. Metabolomics-guided isolation of anti-trypanosomal compounds from endophytic fungi of the mangrove plant Avicennia lanata. Curr. Med. Chem. 2020;27:1815–1835. doi: 10.2174/0929867326666190704130105. [DOI] [PubMed] [Google Scholar]
- 33.Okoye F.B., Lu S., Nworu C.S., Esimone C.O., Proksch P., Chadlic A., Debbaba A. Depsidone and diaryl ether derivatives from the fun gus Corynespora cassiicola, an endophyte of Gongronema latifolium. Tetrahedron Lett. 2013;54:4210–4214. doi: 10.1016/j.tetlet.2013.05.117. [DOI] [Google Scholar]
- 34.Liu G.R., Huo R.Y., Zhai Y.N., Liu L. New bioactive sesquiterpeniods from the plant endophytic fungus Pestalotiopsis theae. Front. Microbiol. 2021;12:641504. doi: 10.3389/fmicb.2021.641504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seetharaman P., Gnanasekar S., Chandrasekaran R., Chandrakasan G., Kadarkarai M., Sivaperumal S. Isolation and characterization of anticancer flavone chrysin (5,7-dihydroxy flavone)-producing endophytic fungi from Passiflora incarnata L. leaves. Ann. Microbiol. 2017;67:321–331. doi: 10.1007/s13213-017-1263-5. [DOI] [Google Scholar]
- 36.Hu Z.B., Wu Z.H., Su Q.H., Li M.Z., Wu S.S., Meng R.Q., Ding W.J., Li C.Y. Metabolites with phytopathogenic fungi inhibitory activities from the mangrove endophytic fungus Botryosphaeria ramose. Bioorg. Chem. 2020;104:104300. doi: 10.1016/j.bioorg.2020.104300. [DOI] [PubMed] [Google Scholar]
- 37.Hashad N., Ibrahim R., Mady M., Abdel-Aziz M.S., Moharram F.A. Review: Bioactive metabolites and host-specific toxins from endophytic fungi, alternaria alternate. Vietnam. J. Chem. 2021;59:733–759. doi: 10.1002/vjch.202100099. [DOI] [Google Scholar]
- 38.Li H., Tian J.M., Tang H.Y., Pan S.Y., Zhang A.L., Gao J.M. Chaetosemins A–E, new chromones isolated from an Ascomycete Chaetomium seminudum and their biological activities. RSC Adv. 2015;37:29185–29192. doi: 10.1039/C5RA00525F. [DOI] [Google Scholar]
- 39.Liu S.C., Liu L. Isoprenylated chromones from the plant endophytic fungus Pestalotiopsis fici. Mycosystema. 2011;29:585–587. doi: 10.1021/np900308s. [DOI] [Google Scholar]
- 40.Apgma B., Ajcc A., Anb C., Ma C., Ba C., Laep A. Tetrasubstituted α-pyrone derivatives from the endophytic fungus, Neurospora udagawae. Phytochem. Lett. 2020;35:147–151. doi: 10.1016/j.phytol.2019.11.010. [DOI] [Google Scholar]
- 41.Dong L., Li X.M., Li C.S., Wang B.G. Nigerasterols A and B, Antiproliferative Sterols from the Mangrove-Derived Endophytic Fungus Aspergillus niger MA-132. Helv. Chim. Acta. 2013;96:1055–1061. doi: 10.1002/hlca.201200332. [DOI] [Google Scholar]
- 42.Liu S., Liu X., Guo L., Che Y.S., Liu L. 2H-Pyran-2-one and 2H-Furan-2-one Derivatives from the plant Endophytic Fungus Pestalotiopsis fici. Chem. Biodivers. 2013;10:2007–2013. doi: 10.1002/cbdv.201200361. [DOI] [PubMed] [Google Scholar]
- 43.Yu H.M., Hou Y.T., Zhu L.J., Chen Y.J., Dong M., Zhou M., Shen D., Chen M.H. Two new α-pyronoids from endophytic fungus Aspergillus oryzae derived from Paris polyphylla var. yunnanensis. Chin. Tradit. Herb. Drugs. 2020;51:4891–4895. doi: 10.7501/j.issn.0253-2670.2020.19.006. [DOI] [Google Scholar]
- 44.Guo D.L., Qiu L., Feng D., He X., Li X.H., Cao Z.X., Gu Y.C., Mei L., Deng F., Deng Y. Three new α-pyrone derivatives induced by chemical epigenetic manipulation of Penicillium herquei, an endophytic fungus isolated from Cordyceps sinensis. Nat. Prod. Res. 2020;34:958–964. doi: 10.1080/14786419.2018.1544974. [DOI] [PubMed] [Google Scholar]
- 45.Lee M.S., Wang S.W., Wang G.J., Pang K.L., Lee C.K., Kuo Y.H., Cha H.Y., Lin R.K., Lee T.H. Angiogenesis Inhibitors and Anti-Inflammatory Agents from Phoma sp. NTOU4195. J. Nat. Prod. 2016;79:2983–2990. doi: 10.1021/acs.jnatprod.6b00407. [DOI] [PubMed] [Google Scholar]
- 46.Anoumedem G., Mountessou B., Kouam S.F., Narmani A., Surup F. Simplicilones A and B Isolated from the Endophytic Fungus Simplicillium subtropicum SPC3. Antibiotics. 2020;9:753. doi: 10.3390/antibiotics9110753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F.Z., Li X.M., Li X., Yang S.Q., Meng L.H., Wang B.G. Polyketides from the Mangrove-Derived Endophytic Fungus Cladosporium cladosporioides. Mar. Drugs. 2019;17:296. doi: 10.3390/md17050296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ola A.R., Tawo B.D., Belli H.L., Proksch P., Tommy D., Hakim E.H. A new antibacterial polyketide from the endophytic fungi Aspergillus fumigatiaffinis. Nat. Prod. Commun. 2018;13:1573–1574. doi: 10.1177/1934578X1801301202. [DOI] [Google Scholar]
- 49.Zhao S.S., Wang B., Tian K.L., Ji W.X., Zhang T.Y., Ping C., Yan W., Ye Y.H. Novel metabolites from the Cercis chinensis derived endophytic fungus Alternaria alternata ZHJG5 and their antibacterial activities. Pest. Manag. Sci. 2021;77:2264–2271. doi: 10.1002/ps.6251. [DOI] [PubMed] [Google Scholar]
- 50.Li C.S., Sarotti A.M., Wu X.H., Yang B.J., Turkson J., Chen Y.F., Liu Q.S., Cao S.G. An Unusual Benzoisoquinoline-9-one Derivative and Other Related Compounds with Antiproliferative Activity from Hawaiian Endophytic Fungus Peyronellaea sp. FT431. Molecules. 2019;24:196. doi: 10.3390/molecules24010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J.C., Hou Y., Xu Q.H., Jin X.J., Chen Y.X., Fang J.G., Hu B.R., Wu Q.X. (±)-Alternamgin, a Pair of Enantiomeric Polyketides, from the Endophytic Fungi Alternaria sp. MG1. Org. Lett. 2019;21:1551–1554. doi: 10.1021/acs.orglett.9b00475. [DOI] [PubMed] [Google Scholar]
- 52.Tang J.W., Hu K., Su X.Z., Li X.N., Puno P.T. Phomopsisins A-C: Three new cytochalasans from the plant endophytic fungus Phomopsis sp. sh917. Tetrahedron. 2020;76:131475. doi: 10.1016/j.tet.2020.131475. [DOI] [Google Scholar]
- 53.Chen C.M., Tong Q.Y., Zhu H.C., Tan D.D., Zhang J.W., Xue Y.B., Yao G.M., Luo Z.W., Wang J.P., Wang Y.Y. Nine new cytochalasan alkaloids from Chaetomium globosum TW1-1 (Ascomycota, Sordariales) Sci. Rep. 2016;6:18711. doi: 10.1038/srep18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X.L., Wu P., Xue J.H., Li H.X., Wei X.Y. Cytochalasans from endophytic fungus Diaporthe sp. SC-J0138. Fitoterapia. 2020;145:104611. doi: 10.1016/j.fitote.2020.104611. [DOI] [PubMed] [Google Scholar]
- 55.Mou Q.L., Yang S.X., Xiang T., Liu W.W., Yang J., Guo L.P., Wang W.J., Yang X.L. New cytochalasan alkaloids and cyclobutane dimer from an endophytic fungus Cytospora chrysosperma in Hippophae rhamnoides and their antimicrobial activities. Tetrahedron Lett. 2021;87:153207. doi: 10.1016/j.tetlet.2021.153207. [DOI] [Google Scholar]
- 56.Yang J., Gong L.Z., Guo M.M., Jiang Y., Ding Y., Wang Z.J., Xin X.J., An F.L. Bioactive Indole Diketopiperazine Alkaloids from the Marine Endophytic Fungus Aspergillus sp. YJ191021. Mar. Drugs. 2021;19:157. doi: 10.3390/md19030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu K., Wei X.L., Xue L., Zhang Z.F., Zhang P. Antimicrobial Meroterpenoids and Erythritol Derivatives Isolated from the Marine-Algal-Derived Endophytic Fungus Penicillium chrysogenum XNM-12. Mar. Drugs. 2020;18:578. doi: 10.3390/md18110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutfia L., Munir E., Yurnaliza Y., Basyuni M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber griffithii Baker. Heliyon. 2021;7:e06292. doi: 10.1016/j.heliyon.2021.e06292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Z.J., Jiang Y., Xin X.J., An F.L. Bioactive indole alkaloids from insect derived endophytic Aspergillus lentulus. Fitoterapia. 2021;153:104973. doi: 10.1016/j.fitote.2021.104973. [DOI] [PubMed] [Google Scholar]
- 60.Zhai Y.J., Huo G.M., Zhang Q., Li D., Wang D.C., Qi J.Z., Gao J.M. Phaeosphaones: Tyrosinase Inhibitory Thiodiketopiperazines from an Endophytic Phaeosphaeria fuckelii. J. Nat. Prod. 2020;83:1592–1597. doi: 10.1021/acs.jnatprod.0c00046. [DOI] [PubMed] [Google Scholar]
- 61.Zhang P., Li X.M., Wang J.N. Oxepine-Containing Diketopiperazine Alkaloids from the Algal-Derived Endophytic Fungus Paecilomyces variotii EN-291. Helv. Chim. Acta. 2015;98:800–804. doi: 10.1002/hlca.201400328. [DOI] [Google Scholar]
- 62.Ye G.T., Huang C.Y., Li J.L., Chen T., Tang J., Liu W.B., Long Y.H. Isolation, Structural Characterization and Antidiabetic Activity of New Diketopiperazine Alkaloids from Mangrove Endophytic Fungus Aspergillus sp. 16-5c. Mar. Drugs. 2021;19:402. doi: 10.3390/md19070402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meng L.H., Peng Z., Li X.M., Wang B.G. Penicibrocazines A–E, Five New Sulfide Diketopiperazines from the Marine-Derived Endophytic Fungus Penicillium brocae. Mar. Drugs. 2015;13:276–287. doi: 10.3390/md13010276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng L.H., Wang C.Y., Mándi A., Li X.M., Hu X.Y., Kassack M.U. Three Diketopiperazine Alkaloids with Spirocyclic Skeletons and One Bisthiodiketopiperazine Derivative from the Mangrove-Derived Endophytic Fungus Penicillium brocae MA-231. Org. Lett. 2016;18:5304–5307. doi: 10.1021/acs.orglett.6b02620. [DOI] [PubMed] [Google Scholar]
- 65.Zheng C.J., Li L., Zou J.P., Han T., Qin L.P. Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm. Biol. 2012;50:129–133. doi: 10.3109/13880209.2011.569726. [DOI] [PubMed] [Google Scholar]
- 66.Sirimangkalakitti N., Yokoya M., Chamni S., Chanvorachote P., Plubrukrn A., Saito N., Suwanborirux K. Synthesis and Absolute Configuration of Acanthodendrilline, a New Cytotoxic Bromotyrosine Alkaloid from the Thai Marine Sponge Acanthodendrilla sp. Chem. Pharm. Bull. 2016;64:258–262. doi: 10.1248/cpb.c15-00901. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y.C., Wang L.P., Zhu G.L., Zuo M.X., Gong Q.Y., He W.W., Li M.P., Yuan C.M., Hao X.J., Zhu W.M. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019;30:431–434. doi: 10.1016/j.cclet.2018.08.015. [DOI] [Google Scholar]
- 68.Cui H., Lin Y., Luo M.C., Lu Y.J., Huang X.S., She Z.G. Diaporisoindoles A−C: Three Isoprenylisoindole Alkaloid Derivatives from the Mangrove Endophytic Fungus Diaporthe sp. SYSU-HQ3. Org. Lett. 2017;19:5621–5624. doi: 10.1021/acs.orglett.7b02748. [DOI] [PubMed] [Google Scholar]
- 69.Wu S.H., Zhao L.X., Chen Y.W., Huang R., Miao C.P., Wang J. Sesquiterpenoids from the Endophytic Fungus Trichoderma sp. PR-35 of Paeonia delavayi. Chem. Biodivers. 2011;9:1717–1723. doi: 10.1002/cbdv.201000236. [DOI] [PubMed] [Google Scholar]
- 70.Wang A., Yin R.Y., Zhou Z.Y., Gu G., Dai J.G., Lai D.W., Zhou L.G. Eremophilane-Type Sesquiterpenoids from the Endophytic Fungus Rhizopycnis vagum and Their Antibacterial, Cytotoxic, and Phytotoxic Activities. Front. Chem. 2020;26:596889. doi: 10.3389/fchem.2020.596889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang C.X., Li J., Zhang J.M., Jin X.J., Yu B., Fang J.G., Wu Q.X. Isolation, Identification, and Activity Evaluation of Chemical Constituents from Soil Fungus Fusarium avenaceum SF-1502 and Endophytic Fungus Fusarium proliferatum AF-04. J. Agric. Food Chem. 2019;67:839–1846. doi: 10.1021/acs.jafc.8b05576. [DOI] [PubMed] [Google Scholar]
- 72.Hu X.Y., Li X.M., Meng L.H., Wang B.G. Antioxidant bisabolane-type sesquiterpenoids from algal-derived fungus Aspergillus sydowii EN-434. J. Oceanol. Limnol. 2020;38:1532–1536. doi: 10.1007/s00343-020-0049-y. [DOI] [Google Scholar]
- 73.Wang Q.X., Bao L., Yang X.L., Liu D.L., Guo H., Dai H.Q., Song F.H., Zhang L.X., Guo L.D., Li S.J., et al. Ophiobolins P–T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia. 2013;90:220–227. doi: 10.1016/j.fitote.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 74.Shi X.S., Song Y.P., Meng L.H., Yang S.Q., Li X.M. Isolation and Characterization of Antibacterial Carotane Sesquiterpenes from Artemisia argyi Associated Endophytic Trichoderma virens QA-8. Antibiotics. 2021;10:213. doi: 10.3390/antibiotics10020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen Y., Zou G., Yang W.C., Zhao Y.Y., Tan Q., Chen L., Wang J.M., Ma C.Y., Kang W.Y., She Z.G. Metabolites with Anti-Inflammatory Activity from the Mangrove Endophytic Fungus Diaporthe sp. QYM12. Mar. Drugs. 2021;19:56. doi: 10.3390/md19020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan M.M., Xiang G., Chen J.W., Gao J., Xue W.X., Wang Y.X., Li W.H., Zhou L., Jiao R.H., Shen Y., et al. Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF-κB and NLRP3 inflammasome activation. Int. Immunopharmacol. 2020;80:106144. doi: 10.1016/j.intimp.2019.106144. [DOI] [PubMed] [Google Scholar]
- 77.Xu K., Zhang X., Chen J.W., Shen Y., Jiang N., Tan R.X., Jiao R.H., Ge H.M. Anti-inflammatory diterpenoids from an endophytic fungus Phomopsis sp. S12. Tetrahedron Lett. 2019;60:151045. doi: 10.1016/j.tetlet.2019.151045. [DOI] [Google Scholar]
- 78.Chen Y.M., Yang Y.H., Li X.N., Zou C., Zhao P.J. Diterpenoids from the Endophytic Fungus Botryosphaeria sp. P483 of the Chinese Herbal Medicine Huperzia serrata. Molecules. 2015;20:16924–16932. doi: 10.3390/molecules200916924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X.N., Bashyal B.P., Wijeratne E.M., Ren J.M., Liu M.X., Gunatilaka M.K. Smardaesidins A-G, Isopimarane and 20-Nor-isopimarane Diterpenoids from Smardaea sp., a Fungal Endophyte of the Moss Ceratodon purpureus. J. Nat. Prod. 2011;74:2052–2061. doi: 10.1021/np2000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu M.F., Jia O.Y., Wang S.J., Zhu Q. A new bioactive diterpenoid from pestalotiopsis adusta, an endophytic fungus from Clerodendrum canescens. Nat. Prod. Res. 2016;30:2642–2647. doi: 10.1080/14786419.2016.1138297. [DOI] [PubMed] [Google Scholar]
- 81.Sun P.X., Zheng C.J., Li W.C., Jin G.L., Huang F., Qin L.P. Trichodermanin A, a novel diterpenoid from endophytic fungus culture. J. Nat. Med. 2011;65:381–384. doi: 10.1007/s11418-010-0499-1. [DOI] [PubMed] [Google Scholar]
- 82.Sun H.F., Li X.M., Meng L., Cui C.M., Gao S.S., Li C.S., Huang C.G., Wang B.G. Asperolides A−C, Tetranorlabdane Diterpenoids from the Marine Alga-Derived Endophytic Fungus Aspergillus wentii EN-48. J. Nat. Med. 2012;75:148–152. doi: 10.1021/np2006742. [DOI] [PubMed] [Google Scholar]
- 83.Guo K., Fang H.Q., Gui F., Wang Y.Z., Xu Q.Y., Deng X.M. Two New Ring A-Cleaved Lanostane-Type Triterpenoids and Four Known Steroids Isolated from Endophytic Fungus Glomerella sp. F00244. Helv. Chim. Acta. 2016;99:601–607. doi: 10.1002/hlca.201600039. [DOI] [Google Scholar]
- 84.Morandini L., Neto A.T., Pedroso M., Antoniolli Z.I., Burrow R., Morel A.F. Lanostane-type triterpenes from the fungal endophyte Scleroderma UFSMSc1 (Persoon) Fries. Bioorg. Med. Chem. Lett. 2016;47:1173–1176. doi: 10.1016/j.bmcl.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 85.Tian C., Gao H., Peng X.P., Li G., Lou H.X. Fusidic acid derivatives from the endophytic fungus Acremonium pilosum F47. J. Asian Nat. Prod. Res. 2021;23:1148–1155. doi: 10.1080/10286020.2020.1866559. [DOI] [PubMed] [Google Scholar]
- 86.Qin D., Shen W.Y., Wang Y.Q., Han M.J., Chai F.N., Duan X.X., Yan X., Guo J.L., Gao T.C., Zuo S.H., et al. Enhanced production of unusual triterpenoids from Kadsura angustifolia fermented by a symbiont endophytic fungus, Penicillium sp. SWUKD4.1850. Phytochemistry. 2019;158:56–66. doi: 10.1016/j.phytochem.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Liang H.Q., Zhang D.W., Guo S.X., Jie Y. Two new tetracyclic triterpenoids from the endophytic fungus Hypoxylon sp. 6269. J. Asian Nat. Prod. Res. 2018;20:1485662. doi: 10.1080/10286020.2018.1485662. [DOI] [PubMed] [Google Scholar]
- 88.Ibrahim R.M., Abdallah H.M., Mohamed G.A., Ross S.A. Integracides H-J: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia. 2016;112:161–167. doi: 10.1016/j.fitote.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Ibrahim R.M., Abdallah H.M., Mohamed G.A., Ross S.A. Integracides F and G: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Phytochem. Lett. 2016;15:125–130. doi: 10.1016/j.phytol.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Sun Z.H., Liang F.L., Wu W., Chen Y.C., Pan Q.L., Li H.H., Ye W., Liu H.X., Li S.N., Tan G.H., et al. Guignardones P-S, New Meroterpenoids from the Endophytic Fungus Guignardia mangi ferae A348 Derived from the Medicinal Plant Smilax glabra. Molecules. 2015;20:22900–22907. doi: 10.3390/molecules201219890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q., Chen C.M., Cheng L., Wei M.S., Dai C., He Y., Gong J.J., Zhu R.Q., Li X.N., Liu J.J., et al. Emeridones A−F, a Series of 3,5-Demethylorsellinic Acid-Based Meroterpenoids with Rearranged Skeletons from an Endophytic Fungus Emericella sp. TJ29. J. Org. Chem. 2019;84:1534–1541. doi: 10.1021/acs.joc.8b02830. [DOI] [PubMed] [Google Scholar]
- 92.Yang H.G., Zhao H., Li J.J., Chen S.M., Mou L.M., Zou J., Chen G.D., Qin S.Y., Wang C.X., Hu D., et al. Phyllomeroterpenoids A-C, Multi-biosynthetic Pathway Derived Meroterpenoids from the TCM Endophytic Fungus Phyllosticta sp. and their Antimicrobial Activities. Sci. Rep. 2017;7:12925. doi: 10.1038/s41598-017-13407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nascimento J., Silva F.M., Magallanes-Noguera C.A., Kurina-Sanz M., Silva E. Natural trypanocidal product produced by endophytic fungi through co-culturing. Folia Microbiol. 2020;65:323–328. doi: 10.1007/s12223-019-00727-x. [DOI] [PubMed] [Google Scholar]
- 94.Li Y.L., Yi J.L., Cai J., Zhou X.M., Chen L., Zhuo X., Lai X.Y. Two new bioactive secondary metabolites from the endophytic fungus Talaromyces assiutensis JTY2. Nat. Prod. Res. 2021;17:1–6. doi: 10.1080/14786419.2021.1881961. [DOI] [PubMed] [Google Scholar]
- 95.Wen Y.Z., Lv Y.B., Hao J., Chen H., Yang X., Huang Y., Liu C., Huang H.Q., Ma Y.R., Yang X.Z. Two new compounds of Penicillium polonicum, an endophytic fungus from Camptotheca acuminata Decne. Nat. Prod. Res. 2019;34:1879–1883. doi: 10.1080/14786419.2019.1569003. [DOI] [PubMed] [Google Scholar]
- 96.Bussey R.O., Kaur A., Todd D.A., Egan J.M., El-Elimat T., Graf T.N., Raja H.A., Oberlies N.H., Cech N.J. Comparison of the chemistry and diversity of endophytes isolated from wild-harvested and greenhouse-cultivated yerba mansa (Anemopsis californica) Phytochem. Lett. 2015;11:202–208. doi: 10.1016/j.phytol.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tchoukoua A., Ota T., Akanuma R., Ju Y.M., Supratman U., Murayama T., Koseki T., Shiono Y. A phytotoxic bicyclic lactone and other compounds from endophyte Xylaria curta. Nat. Prod. Res. 2017;31:2113–2118. doi: 10.1080/14786419.2016.1277352. [DOI] [PubMed] [Google Scholar]
- 98.Chen S.H., Liu Z.M., Liu H.J., Long Y.H., Chen D.N., Lu Y.J., She Z.G. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. Org. Biomol. Chem. 2017;15:6338–6341. doi: 10.1039/C7OB01657C. [DOI] [PubMed] [Google Scholar]
- 99.Gusmao A.S., Abreu L.S., Tavares J.F., Freitas H.F., Pita S., Santos E., Caldas I., Vieira A.A., Silva E.O. Computer-guided trypanocidal activity of natural lactones produced by endophytic fungus of Euphorbia umbellata. Chem. Biodivers. 2021;18:e2100493. doi: 10.1002/cbdv.202100493. [DOI] [PubMed] [Google Scholar]
- 100.Yuan X.L., Wang X.F., Xu K., Li W., Chen D., Zhang P. Characterization of a New Insecticidal Anthraquinone Derivative from an Endophyte of Acremonium vitellinum against Helicoverpa armigera. J. Agric. Food Chem. 2020;68:11480–11487. doi: 10.1021/acs.jafc.0c05680. [DOI] [PubMed] [Google Scholar]
- 101.Yang Y.C., Yang H.Y., Li Y., Ye Y.Q., Hu Q.F., Gao X.M., Du G.A. New Xanthone from the Fermentation Products of Endophytic Fungus of Phomopsis Species. Asian J. Chem. 2014;26:4591–4593. doi: 10.14233/ajchem.2014.16127. [DOI] [Google Scholar]
- 102.Du F.Y., Li X.M., Song J.Y., Li C.S., Wang B.G. Anthraquinone Derivatives and an Orsellinic Acid Ester from the Marine Alga-Derived Endophytic Fungus Eurotium cristatum EN-220. Helv. Chim. Acta. 2014;97:973–978. doi: 10.1002/hlca.201300358. [DOI] [Google Scholar]
- 103.Hawas U.W., El-Beih A.A., El-Halawany A.M. Bioactive Anthraquinones from Endophytic Fungus Aspergillus versicolor Isolated from Red Sea Algae. Arch. Pharm. Res. 2012;35:1749–1756. doi: 10.1007/s12272-012-1006-x. [DOI] [PubMed] [Google Scholar]
- 104.Teiten M.H., Mack F., Debbab A., Aly A.H., Dicato M., Proksch P., Diederich M. Anticancer effect of altersolanol A, a metabolite produced by the endophytic fungus Stemphylium globuliferum, mediated by its proapoptotic and antiinvasive potential via the inhibition of NF-kB activity. Bioorg. Med. Chem. 2013;21:3850–3858. doi: 10.1016/j.bmc.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 105.Chen Y., Yang W.C., Zou G., Yan Z.Y., Qiu P., Long Z.G., She Z.G. Metabolites with anti-inflammatory and α-glucosidase inhibitory activities from the mangrove endophytic fungus Phoma sp. SYSU-SK-7. Tetrahedron Lett. 2020;61:152578. doi: 10.1016/j.tetlet.2020.152578. [DOI] [Google Scholar]
- 106.Ibrahim S., Mohamed G.A., Haidari R., El-Kholy A., Zayed M.F. Fusaristerol A: A New Cytotoxic and Antifungal Ergosterol Fatty Acid Ester from the Endophytic Fungus Fusarium sp. Associated with Mentha longifolia Roots. Pharmacogn. Mag. 2018;56:308–311. doi: 10.4103/pm.pm_113_18. [DOI] [Google Scholar]
- 107.Wu S.H., Huang R., Miao C.P., Chen Y.W., Chen Y.W. Two New Steroids from an Endophytic Fungus Phomopsis sp. Chem. Biodivers. 2013;10:1276–1283. doi: 10.1002/cbdv.201200415. [DOI] [PubMed] [Google Scholar]
- 108.Qi C.X., Gao W.X., Wang J.P., Liu M.T., Zhang J.W., Chen C.M., Hu Z.X., Xue Y.B., Li D.X., Zhang Y.H. Errusnolides A-D, new butenolides with anti-inflammatory activities from an endophytic Aspergillus from Tripterygium wilfordii. Fitoterapia. 2018;130:134–139. doi: 10.1016/j.fitote.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 109.Akhter N., Pan C.Q., Liu Y.Q., Shi Y.T., Wu B. Isolation and structure determination of a new indene derivative from endophytic fungus Aspergillus flavipes Y-62. Nat. Prod. Res. 2019;33:2939–2944. doi: 10.1080/14786419.2018.1510399. [DOI] [PubMed] [Google Scholar]
- 110.Hussain H., Root N., Jabeen F., Al-Harrasi A., Ahmad M., Mabood F. Microsphaerol and Seimatorone: Two New Compounds Isolated from the Endophytic Fungi, Microsphaeropsis sp. and Seimatosporium sp. Chem. Biodivers. 2015;46:289–294. doi: 10.1002/cbdv.201400098. [DOI] [PubMed] [Google Scholar]
- 111.Wu X., Pang X.J., Xu L.L., Zhao T., Long X.Y., Zhang Q.Y., Qin H.L., Yang D.F., Yang X.L. Two new alkylated furan derivatives with antifungal and antibacterial activities from the plant endophytic fungus Emericella sp. XL029. Nat. Prod. Res. 2017;32:2625–2631. doi: 10.1080/14786419.2017.1374269. [DOI] [PubMed] [Google Scholar]
- 112.Niaz S.I., Khan D., Naz R., Safdar K., Zain S., Abidin U., Khan I.U., Gul R., Khan W.U., Aman M., et al. Antimicrobial and antioxidant chlorinated azaphilones from mangrove Diaporthe perseae sp. isolated from the stem of Chinese mangrove Pongamia pinnata. J. Asian Nat. Prod. Res. 2020;23:1077–1084. doi: 10.1080/10286020.2020.1835872. [DOI] [PubMed] [Google Scholar]
- 113.Yang X.X., Wang N.N., Kang Y.F., Ma Y.M. New furan derivative from an endophytic Aspergillus tubingensis of Decaisnea insignis (Griff.) Hook.f. & Thomson. Nat. Prod. Res. 2019;33:2777–2783. doi: 10.1080/14786419.2018.1501687. [DOI] [PubMed] [Google Scholar]
- 114.Zhu X.W., Chen J.Q., Zhu S.R., He Y.Y., Ding W.J., Li C.Y. Two new compounds from Nigrospora sphaerica ZMT05, a fungus derivated from Oxya chinensis Thunber. Nat. Prod. Res. 2018;32:2375–2381. doi: 10.1080/14786419.2017.1413566. [DOI] [PubMed] [Google Scholar]
- 115.Li H.L., Li X.M., Yang S.Q., Cao J., Li Y.H., Wang B.G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2020;73:108–111. doi: 10.1038/s41429-019-0242-4. [DOI] [PubMed] [Google Scholar]
- 116.Zhao M., Yuan L.Y., Guo D.L., Ye Y., Da-Wa Z.M., Wang X.L., Ma F.W., Chen L. Bioactive halogenated dihydroisocoumarins produced by the endophytic fungus Lachnum palmae isolated from Przewalskia tangutica. Phytochemistry. 2018;148:97–103. doi: 10.1016/j.phytochem.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 117.Singh A., Singh D.K., Kharwar R.N., White J.F., Gond S.K. Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges. Microorganisms. 2021;9:197. doi: 10.3390/microorganisms9010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chandra S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012;95:47–59. doi: 10.1007/s00253-012-4128-7. [DOI] [PubMed] [Google Scholar]
- 119.Yu H.S., Zhang L., Li L., Zheng C.J., Guo L., Li W.C., Sun P.X., Qin L.P. Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol. Res. 2010;165:437–449. doi: 10.1016/j.micres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 120.Strobel G. The Emergence of Endophytic Microbes and Their Biological Promise. J. Fungi. 2018;4:57. doi: 10.3390/jof4020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hastuti U.S., Asna P.M.A., Rahmawati D. Histologic observation, identification, and secondary metabolites analysis of endophytic fungi isolated from a medicinal plant, hedychium acuminatum Roscoe. AIP Conf. Proc. 2018;2002:020070. doi: 10.1063/1.5050166. [DOI] [Google Scholar]
- 122.El-Sayed A., Khalaf S.A., Azez H.A., Hussein H.A., El-Baz A.F. Production, bioprocess optimization and anticancer activity of camptothecin from Aspergillus terreus and Aspergillus flavus, endophytes of ficus elastica. Process Biochem. 2021;107:59–73. doi: 10.1016/j.procbio.2021.05.007. [DOI] [Google Scholar]
- 123.Xiong Z.Q., Yang Y.Y., Zhao N., Wang Y. Diversity of endophytic fungi and screening of fungal paclitaxel producer from anglojap yew, Taxus x media. BMC Microbiol. 2013;13:71. doi: 10.1186/1471-2180-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao Y., Yin H., Sun Y., Zhang Z., Cui Y. Mutagenesis of a berberine-producing endophytic fungus. J. Fungal Res. 2008;6:216–225. [Google Scholar]
- 125.Xu B., Wang M.R., Xia Y., Yang K., Zhang C.Y. Improvement of the output of teicoplanin by genome shuffling. Chin. J. Antibiot. 2006;31:237–242. doi: 10.1360/yc-006-1627. [DOI] [Google Scholar]
- 126.Eyberger A.L., Dondapati R., Porter J.R. Endophyte Fungal Isolates from Podophyllum peltatum Produce Podophyllotoxin. J. Nat. Prod. 2006;68:1121–1124. doi: 10.1021/np060174f. [DOI] [PubMed] [Google Scholar]
- 127.Venugopalan A., Srivastava S. Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv. 2015;33:873–887. doi: 10.1016/j.biotechadv.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Vigneshwari A., Rakk D., Németh A., Kocsubé S. Host metabolite producing endophytic fungi isolated from Hypericum perforatum. PLoS ONE. 2019;14:e0217060. doi: 10.1371/journal.pone.0217060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta S., Chaturvedi P., Kulkarni M.G., Staden J.V. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020;39:107462. doi: 10.1016/j.biotechadv.2019.107462. [DOI] [PubMed] [Google Scholar]
- 130.Wei Q., Bai J., Yan D.J., Bao X.Q., Li W.T., Hu Y.C. Genome mining combined metabolic shunting and osmac strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B. 2021;11:572–587. doi: 10.1016/j.apsb.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang L.H., Niaz S.I., Khan D., Wang Z., Zhu Y.H., Zhou H.Y., Lin Y.C., Li J., Liu L. Induction of Diverse Bioactive Secondary Metabolites from the Mangrove Endophytic Fungus Trichoderma sp. (Strain 307) by Co-Cultivation with Acinetobacter johnsonii (Strain B2) Mar. Drugs. 2017;15:35. doi: 10.3390/md15020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vagish D., Shreya K., Sanjai S. Isolation and enhancement of resveratrol production in Xylaria psidii by exploring the phenomenon of epigenetics: Using DNA methyltransferases and Histone deacetylase as epigenetic modifiers. Mol. Biol. Rep. 2019;46:4123–4137. doi: 10.1007/s11033-019-04862-z. [DOI] [PubMed] [Google Scholar]
- 133.Pu X., Qu X.X., Chen F., Bao J.K., Zhang G.L., Luo Y.G. Camptothecin-producing endophytic fungus Trichoderma atroviride ly357: Isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol. 2013;97:9365–9375. doi: 10.1007/s00253-013-5163-8. [DOI] [PubMed] [Google Scholar]
- 134.Pelo S.P., Adebo O.A., Green E. Chemotaxonomic profiling of fungal endophytes of Solanum mauritianum (alien weed) using gas chromatography high resolution time-of-flight mass spectrometry (GC-HRTOF-MS) Metabolomics. 2021;17:43. doi: 10.1007/s11306-021-01790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.