Abstract
Facing the crucial issue of high cost in cellulase production from commercial celluloses, inexpensive lignocellulosic materials from agricultural wastes have been attractive. Therefore, several studies have focused on increasing the efficiency of cellulase production by potential microorganisms capable of secreting a high and diversified amount of enzymes using agricultural waste as valuable substrates. Especially, extremophilic bacteria play an important role in biorefinery due to their high value catalytic enzymes that are active even under harsh environmental conditions. Therefore, in this study, we aim to investigate the ability to produce cellulase from coconut-mesocarp of the potential bacterial strain FW2 that was isolated from kitchen food waste in South Korea. This strain was tolerant in a wide range of temperature (−6–75 °C, pH range (4.5–12)) and at high salt concentration up to 35% NaCl. The molecular weight of the purified cellulase produced from strain FW2 was estimated to be 55 kDa. Optimal conditions for the enzyme activity using commercial substrates were found to be 40–50 °C, pH 7.0–7.5, and 0–10% NaCl observed in 920 U/mL of CMCase, 1300 U/mL of Avicelase, and 150 U/mL of FPase. It was achieved in 650 U/mL, 720 U/mL, and 140 U/mL of CMCase, Avicelase, and FPase using coconut-mesocarp, respectively. The results revealed that enzyme production by strain FW2 may have significant commercial values for industry, argo-waste treatment, and other potential applications.
Keywords: coconut-mesocarp degradation, organic waste degrading bacteria, multiple enzyme-producing bacteria, extremophiles, cellulase-producing bacteria
1. Introduction
The crisis of energy, the combustion of petroleum-based fossil fuels, and the rapid increase in agricultural municipal cellulosic waste have warned us about the picture of living in the future. These issues have shifted global efforts to explore and utilize renewable resources for the production of green energy and eco-environmental waste treatment strategies. One of the most valuable sources is lignocellulosic biomass, which has been identified as a great potential for bioconversion to value-added bioproduct from lignocellulose fermentation [1,2,3]. Cellulase is the second most important enzyme, only behind amylase due to its environmentally friendly and economical biofuels development [4,5,6,7,8,9]. Since the 1960s, cellulase has been used increasingly in food, paper, pulp, textile industries, and pharmaceutical industries. However, there are limited studies that exploited the hydrolysis process of agricultural residues of lignocellulosic materials to produce high-value products with low cost [10,11,12]. Moreover, the problem with the biorefining process is that it is costly due to a lack of biocatalysts that are capable of withstanding a variety of environmental stressors such as low/high temperature, acidic/alkaline pH, high salinity, and expression of multi-enzyme complexes. The development of cellulase production from extremophilic bacterial candidates has attracted microbiologists due to their high active research area and their greater enzyme yield than fungi [13,14]. Since bacterial cellulase showed significant difference in stability, catalytic potential, cellulose degradation efficiency, and achieving tremendous benefit if biomass utilization, the investigation of new resources for isolating desirable catalytic potential cellulase has been attracting more attention [15,16].
The main members of cellulase include endoglucanase, exoglucanase or cellobiohydrolase, and β-glucosidase [17]. Bacillus sp., Clostridium sp., Cellulomonas, Thermomonospora, Ruminococcus, and Bacteroides are the well-known cellulase-producing bacterial species which have been isolated from various sources [18,19,20]. Among them, numerous members from the Bacillus genus are promising enzyme-producing candidates due to their capacity to produce and secrete large quantities of extracellular enzymes [21,22,23,24].
Moreover, cellulose synthesis and recycling accounted for a larger proportion of the carbon cycle, approximately 80% of the biosphere, and over 90% of marine environments at a lower temperature of 5 °C [25]. The cellulose degradation period was prolonged at both low and high temperatures because of its complex structure. The complete degradation of cellulose requires the activity of diverse enzymes, such as cellulases [26]. Therefore, to accelerate and enhance the cellulose degradation process, different cellulose-degrading bacteria forming a variety of low temperature habitats have recently been of interest for isolation [27].
Therefore, psychrozymes produced from indigenous psychrophilic microorganisms have been considered as excellent biocatalysts owing to their high catalytic activity at low temperature. It eliminates the need for a heating process to save energy and to become a cost-effective alternative in the industrial application.
Bacillus amyloliquefaciens DL-3, a hydrolyzing rice hull bacterial strain was isolated, identified, and first characterized the produced cellulase for the utilization of cellulosic biomass by Lee and co-authors [18]. Ye et al., optimized the fermentation conditions and properties of cellulase-producing bacterium B. amyloliquefaciens S1 for high efficiency production and other green products for geese products [28]. In another study, B. amyloliquefaciens-ASK11 was explored for the ability of cellulase production under high chromium stress [29].
Since large amount of coconut residues continue to increase, numerous previous studies provided the possibility to address this problem by investigating the bio-ethanol production from coconut mesocarp-based substrates [30,31,32]. Moreover, coconut mesocarp was found to be the natural renewable raw bioresource of carboxymethyl cellulose that was earlier considered as a better carbon source and a major factor responsible for maximum cellulase production [14,33]. Therefore, Dey et al. firstly studied the cellulase production from such highly abundant lignocellulosic bio-waste material by cellulase produced from a fungal strain Trichoderma reesei [34]. However, in this study, we continued to screen and characterize the cellulase enzymes produced from a bacterial strain B. amyloliquefaciens FW2 that was isolated and identified from the previous study [24]. This strain showed a high ability to degrade the coconut mesocarp. The optimal growth and enzyme production conditions of bacterial strain were also investigated.
2. Materials and Methods
2.1. Isolation of Cellulose—Degrading Bacteria
Following isolation steps from the previous study, the kitchen food waste collected from the Jowon Industry in South Korea was used as the source for the functional bacterial isolation [14,24]. Five grams of food waste was added to 50 mL of distilled water to make an original isolation solution. One milliliter of sample was then added to the culture medium including (g/mL): carboxymethylcellulose (CMC) 10; MgSO4, 0.024, NaCl 10, K2HPO4 0.3 and 10 mL of trace element mixture. The medium was adjusted to a pH of 7.0 at 25 °C. A vitamin solution of 10 mL and 10 mL of autoclaved soil extract (ASE) were added as the final step after autoclaving for 20 min (121 °C, 103 kPa). ASE was prepared by adding 100 g of soil into 1000 mL of distilled water and adjusted to pH 7 before autoclaving following Van Pham and Kim [35]. The vitamin solution contained (g/L): citric acid 0.02, folic acid 0.01, riboflavin 0.025, and para-amiobenzoic acid 0.01. The study targets to isolate the functional bacterial candidates that can be able to grow under extreme conditions. Therefore, these samples were incubated at different temperatures of −6 °C, 10 °C, 30 °C, 35 °C, 50 °C, 65 °C, 75 °C and 80 °C on a rotary shaker at 200 rpm (1 d interval for each temperature change). The bacterial culture in the medium (1 mL) was transferred to a fresh medium prepared as above and incubated at 30 °C for 5 d. Well-separated colonies were sub-cultured to obtain pure cultures, and subsequently examined for enzyme production and waste degradation ability in the subsequent steps [14].
2.2. Coconut Mesocarp Preparation
The coconut mesocarp was extracted from coconut fruit without liquid albumen using a stainless-steel knife and cut into smaller size. The raw material was dried at 105 °C in a forced-air oven to reduce the moisture content until it was less than 5% (w/w). Mesocarp material was then ground into powder, sieved with a 212-μm mesh, and stored inplastic zipper bags at 4 °C for future use.
2.3. Taxonomic Identification and Sequence Analysis
The bacterial strain FW2 was identified using the 16S rRNA gene by PCR amplification. Genomic DNA from the strain was extracted according to the manufacturer’s instructions using the InstaGene Matrix kit (Bio-Rad, Seoul, Korea). Following Frank et al., amplification of the 16S rRNA gene was then performed by PCR using primers (27F and 1492R) [36]. A multiscreen filter plate (Millipore Corp, Bedford, MA, USA) was used to purify the PCR products which were then sequenced using primers 518F (5′-CCA GCA GCC GCG GTA ATA CG-3′) and 800R (5′-TAC CAG GGT ATC TAA TCC-3′) with a PRISM BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA). This process was conducted at 95 °C for 5 min and then cooled on ice for 5 min and analyzed using an ABI Prism 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). Finally, the nearly full-length 16S rRNA sequence was assembled using SeqMan software (DNASTAR Inc., Madison, WI, USA). Sequence similarity was determined by comparison with the sequence available in the gen bank database using the EZBioCloud server [37].
Following the previous study, the FASTA sequences of related strains with strain FW2 obtained from the GenBank database were used in order to construct a phylogenetic tree [24]. The MEGA 7 program was used to align sequences and reconstruct the phylogenetic trees [38]. The best fit model used in this study for neighbor-joining (NJ) analysis was a Tamura 2-parameter model with gamma-distributed rates plus invariant site based on the minimum Bayesian information 140 criterion value (gamma parameter = 0.6 in this study) [39]. The reliability of the 141 phylogenetic trees was estimated by bootstrap values of 1500 replications [40].
2.4. Screening of Cellulase Production
To screen the extracellular enzyme production, bacterial strains were incubated in the basal medium with 1% KH2PO4, 0.25% Na2HPO4, 1% NaCl, 0.2% (NH4)2SO4, 0.005% MgSO4·7H2O, 0.005% CaCl2 added 1% of carboxymethylcellulose (CMC) 5 g or microcrystalline cellulose 5 g, and Yeast Extract 5 g. The growth and enzyme generation of strain FW2 were tested first at −6 °C and 80 °C following the previous study [14,24].
After 72 h of incubation, the plate was flooded with 1% iodine solution, and the clear zone around the colony indicated cellulose degradation by the produced bacterial enzyme.
2.5. Optimization of Enzyme Production
2.5.1. Optimization of Physio-Chemical Parameters
The enzyme production process was examined with different experimental conditions such as various pH (4–12.5), temperature (−6–80 °C), and NaCl (0–35%). Different pH was maintained using appropriate buffers: 0.1 M citrate buffer (pH 4–5), 0.2 M phosphate (pH 6–8) and phosphate–NaOH buffer (pH 8–12.5).
2.5.2. Effect of Carbon and Nitrogen Sources on Enzyme Production
Fermentation medium for strain FW2 was prepared in supplement of 1% (w/v) of each commercial cellulose substrate, including glucose, maltose, starch, dextrose, and lactose as various carbon sources. Other trials were added 1% (w/v) ammonium nitrate, potassium nitrate, yeast extract, casein, skim milk, and peptone as different nitrogen sources. The bacterial inoculated cultures were incubated at optimal temperature of 45 °C.
2.5.3. Enzyme Production Using Coconut Mesocarp Powder
Cellulase yield of strain FW2 was examined using a different concentration of coconut mesocarp powder ranging from 1% to 10% (1% interval, w/v) in a flask containing 1 L of base medium including 1% KH2PO4, 0.25% Na2HPO4, 1% NaCl, 0.2% (NH4)2SO4, 0.005% MgSO4·7H2O, 0.005% CaCl2, and 5 g of yeast extract as nitrogen source. The culture was incubated in a shaking incubator and maintained at 45 °C, 150 rpm and 10% of bacterial culture (v/v) during 7 days. The culture was then filtered and centrifuged at 10,000 rpm for 5 min at 4 °C. The produced enzyme in solution was collected and used for the enzyme assay. All experiments were carried out in triplicates.
2.5.4. Effect of Effects of Ions on Cellulase Activity
The effect of various metal ions on cellulase activity was determined by the presence of additives to 0.5 mL of crude enzyme preparation. The additives (NaCl, KCl, MgCl2, FeSO4, CaCl2, and ZnCl2) were used at final concentration of 5.0 mM. The reaction mixtures were incubated with the additives for 60 min at 37 °C at pH 5.0. Residual cellulase activity was measured by DNS (3,5-dinitrosalicylic acid) method [41]. The relative (%) value was calculated and compared with the control tube without any metal ion.
2.6. Cellulase Activity Assay
The bacterial strain was cultured in individual enrichment medium for cellulase fermentation, it contained (g/L): NaCl 5, peptone 5, yeast 10, CMC 5 (for CMCase), or microcrystalline cellulose (for Avicelase), and KH2PO4 1 at 45 °C and 150 rpm for 5 d.
The cellulase assay was performed by extracting the supernatant of the bacterial culture after centrifugation at 10,000 rpm at 4 °C. The reaction mixture contained 0.5 mL of different crude enzyme dilutions and 0.5 mL of 1% CMC as a substrate (in 0.1 M citrate buffer, pH 4.8). The mixture was incubated at 50 °C for 30 min, and the reaction was terminated by adding 3 mL of DNS solution, and the solution was boiled for exactly 5 min for color development. All samples were then cooled rapidly. The reduction in sugar was estimated spectrophotometrically at 540 nm following the method of Miller [41]. One unit of cellulase activity was defined as the amount of enzyme required to liberate 1 μmol of reducing sugars (measured as 2 mg of glucose) per milliliter per minute under assay conditions.
Assay of filter paper activity (FPase) was estimated using gravimetric determination. The bacterial culture media was composed of (g/L): KH2PO4, 0.5; MgSO4, 0.25; gelatin 2, Whatman filter paper 50 mg/20 mL/L of distilled water containing the target bacterial inocula. After 3 d of incubation, the cultures were centrifuged at 6000 rpm for 15 min at 4 °C. The collected pellets were used to estimate the constant weight after drying by comparing them with the trial without bacterial inocula. All experiments were performed in triplicates.
2.7. Purification of Cellulase Enzymes
The crude cellulase enzyme was extracted from the culture under optimal conditions and then precipitated overnight using ammonium sulphate at concentration of 40% and 80% saturation. The pellets were collected by centrifugation at 10,000 rpm, 4 °C for 10 min, and suspended in 50 mM phosphate buffer (pH 7) and dialyzed overnight. The enzyme sample was conducted with an anion exchange chromatography using a DEAE-Cellulose column equilibrated with phosphate buffer pH 8.0.
2.8. Molecular Weight Determination
The purity and molecular weight of the sample at each step was examined by Sodium Dodecyl Sulphate Poly Acrylamide Gel Electrophoresis (SDS-PAGE) using the method described by Laemmli on Mini Protean Tetra System (Bio Rad Laboratories, Inc., Hercules, CA, USA) [42]. The enzyme was separated on the 12 separating gel and 4% stacking gel. Electrophoresis was carried out for about 30 min at 200 V, and the protein bands were visualized with Comassie Brilliant Blue R-250 staining.
3. Results
3.1. Identification of Cellulase-Producing Bacterial Strain
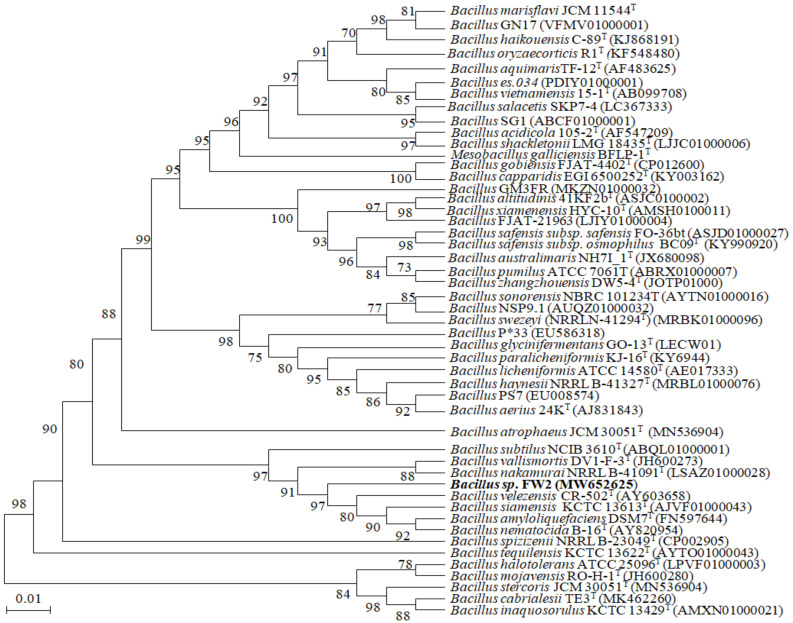
The phylogenetic bacterial strain FW2 in this study was identified by comparing the sequence of the amplified 16S rRNA gene against sequences deposited in the GenBank database with Accession No. MW652625. Based on the 16S rRNA sequences data, the strain FW2 had the highest homology with Bacillus amyloliquefaciens DSM 7T (99.86%) and pairwise similarity with other members shown in Table 1 and Figure 1 [24].
Table 1.
Related bacterial strains with B. amyloliquefaciens FW2 based on the similarity of the 16S rRNA sequences.
| Strain | Similarity (%) | Different Nucleotide/Comparison |
|---|---|---|
| Bacillus amyloliquefaciens DSM7T | 99.86 | 2/1472 |
| Bacillus siamensis KCTC 13613T | 99.86 | 2/1472 |
| Bacillus velezensis CR-502T | 99.86 | 2/1403 |
| Bacillus nematocida B-16T | 99.73 | 4/1470 |
| Bacillus subtilis NCIB 3610T | 99.59 | 6/1472 |
| Bacillus nakamurai NRRL B-41091T | 99.59 | 6/1472 |
| Bacillus cabrialesii TE3T | 99.52 | 7/1472 |
| Bacillus inaquosorum KCTC 13429T | 99.52 | 7/1472 |
| Bacillus stercoris JCM 30051T | 99.52 | 7/1472 |
| Bacillus vallismortis | 99.46 | 8/1472 |
Figure 1.
A detailed tree that displays the phylogeny of 16S rRNA gene sequences of strain FW2 and related Bacillus genus members. The tree was constructed using the neighbor-joining method. Bootstrapping was carried out with 1500 replicates.
3.2. Screening the Cellulase Production of Strain FW2
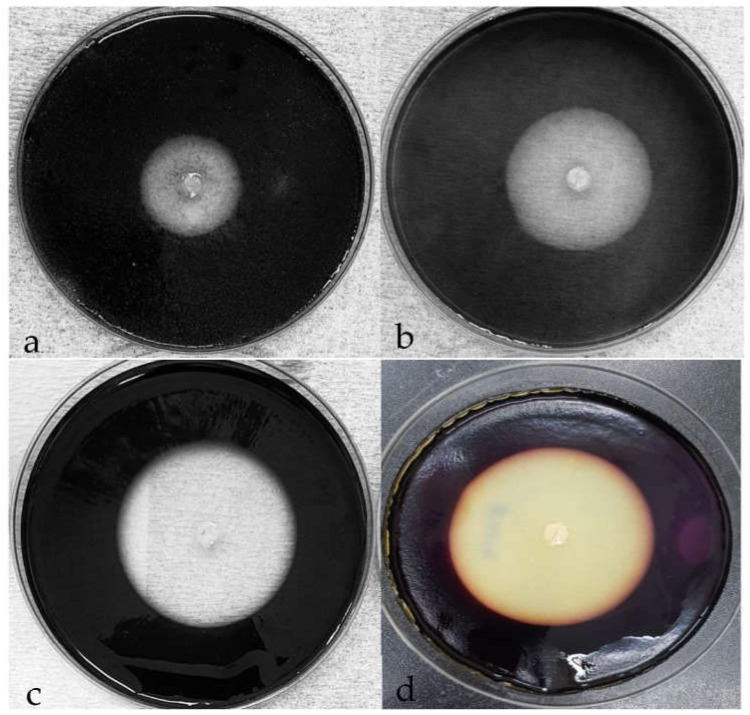
Enzyme activities were confirmed by clear zones after staining with iodine solution. Clearance > 1.0 cm was considered significant. Cellulases were observed even at pH 4.5 and 35% NaCl, and were weakly active at pH 4 and 40% NaCl. Cellulose and CMC degradation were weak at 80 °C. Optimal enzyme production profiles were obtained at pH 7–8, NaCl 0–10%, and temperature 40–45 °C (Figure 2). The result show that cellulases are strongly active in the pH range of 5–10.
Figure 2.
The growth and cellulose degradation of strain FW2 at 45 °C for 4 days: (a) at pH 12; (b) in the medium supplemented with 35% NaCl. The enzyme production under optimal conditions in 4-day incubation (pH 7–7.5; 45 °C): (c) cellulose degradation by CMCase; (d) cellulose degradation by Avicelase.
3.3. Effect of Culture Conditions on Cellulase Activity of Strain FW2
3.3.1. Effect of Physio-Chemical Parameters and Enzyme Production Stability
The largest sizes of the halo zone for cellulose and CMC degradation were 53 mm, and 48 mm, respectively, at 45 °C for 4 days (Figure 2c,d).
CMCase enzyme activity was determined from the clearance zone detected by iodine staining during the screening test on agar plates supplemented with 1% CMC, filter paper was added for total cellulase (FPase), and crystal cellulose was used for Avicelase at a wide range of parameters: −6 °C to 75 °C and weak at 80 °C, pH 4.5–12, and NaCl concentration at 0–35%. CMCase production increased from 35 °C and reached a halo zone peak of 48 mm measured at 40–45 °C, while Avicelase reached the highest production observed with 53 mm in diameter at 45 °C after 4 d of incubation, respectively.
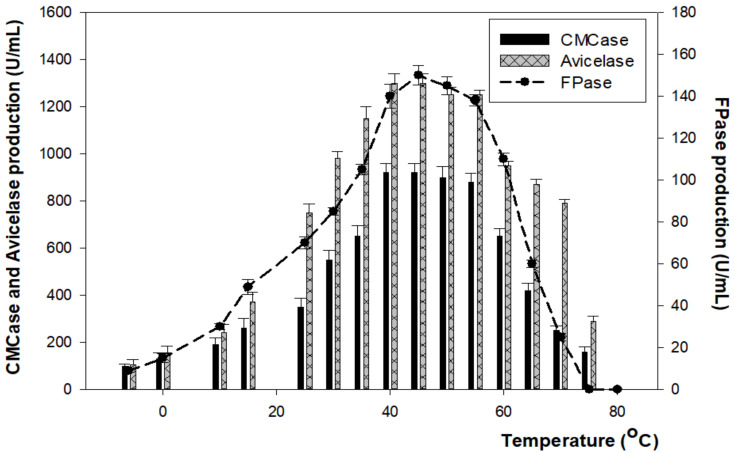
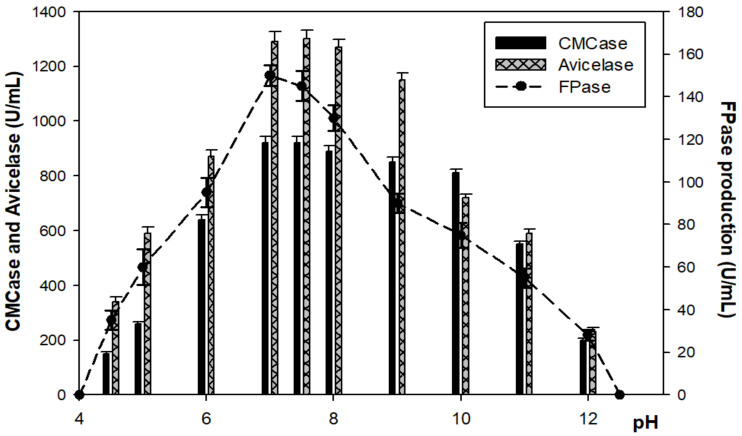
In the same pattern of CMCase, FPase and Avicelase enzymes showed the highest production at 54 h of incubation time with 920 U/mL, 150 U/mL, and 1300 U/mL at 40–45 °C and pH 7–7.5, respectively, before a gradual decrease at 60 °C (Figure 3 and Figure 4).
Figure 3.
The effect of temperature on enzyme production. Samples were incubated for 4 days at pH 7–7.5.
Figure 4.
The effect of pH on enzyme production. Samples were incubated for 4 days at 45 °C.
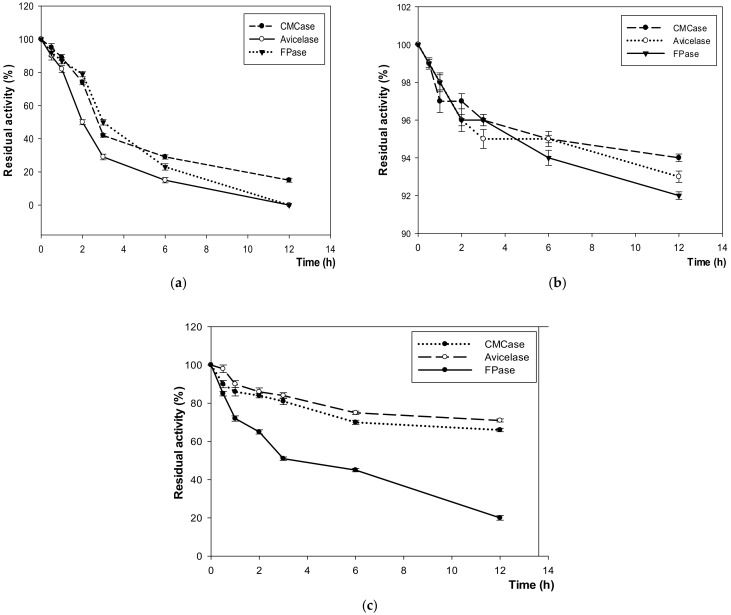
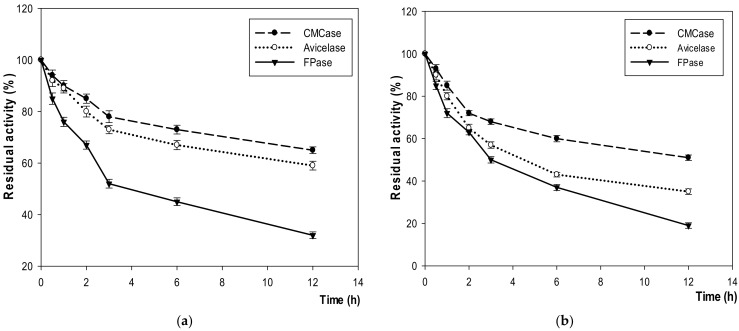
Especially, compared with other enzymes, this enzyme stability was high, retaining 90 at 45 °C and 71% at 75 °C after 12 h of incubation. Moreover, under acidic condition of pH 4.5 and alkaline pH 12, it was active with 59% and 35% after 12 h, respectively (Figure 5 and Figure 6). CMCase was stable at −6 °C with 74% after 2 h and retained only 15% after 12 h of incubation. However, its stability was observed to be 90% at 45 °C, 66% at 75 °C, 65% at pH 4.5, and 51% at pH 12 (Figure 6).
Figure 5.
The stability of enzymes at different extremes and optimal temperatures: (a) −6 °C; (b) 45 °C and (c) 75 °C.
Figure 6.
The stability of enzymes at (a) pH 4.5 and (b) pH 12.
3.3.2. Effect of Carbon and Nitrogen Sources on Enzyme Production
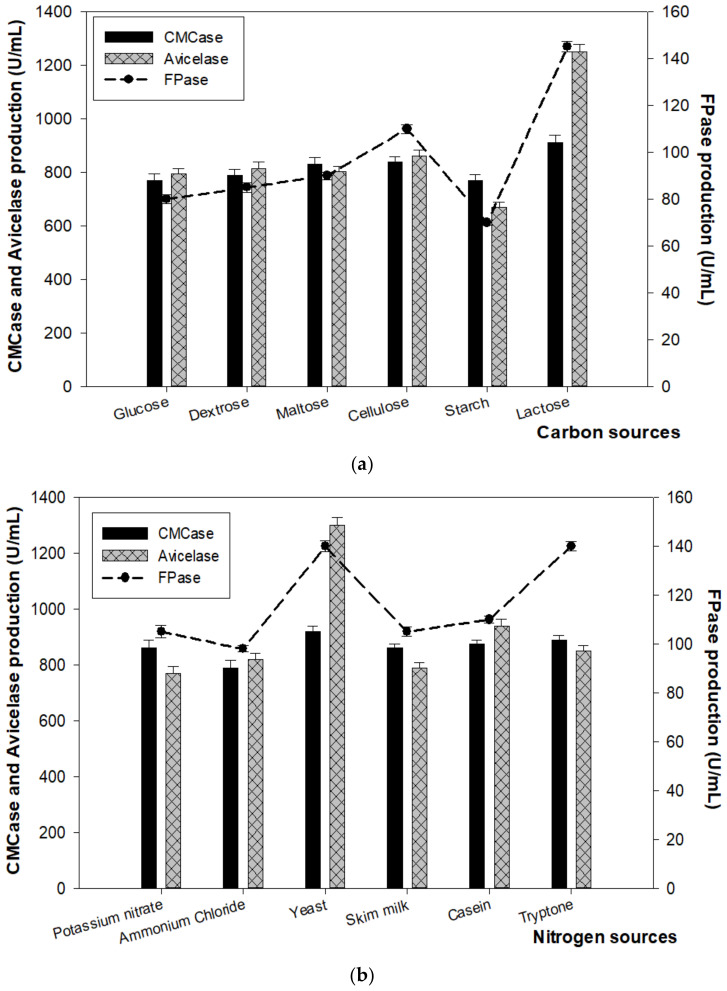
In the enzyme assays, the optimal fermentation parameters were determined. As shown in Figure 7a, glucose, dextrose, and maltose exhibited an effect on CMCase production at 795, 815, and 802 U/mL, respectively. It was revealed that lactose was the most effective, producing 910 U/mL and 1250 U/mL, while starch was a less suitable carbon source for cellulase production with only 770 U/mL and 670 U/mL of CMCase and Avicelase, respectively. The medium supplemented with yeast exhibited the highest enzyme production of 920 U/mL and 1300 U/mL, indicating that yeast was the best nitrogen source for CMCase and Avicelase production by strain FW2, respectively. These were followed by tryptone (890 U/mL), casein (875 U/mL), skim milk, and potassium nitrate in the same amount (860 U/mL), while minimum effect was exhibited by ammonium chloride (790 U/mL) for CMCase and Avicelase, yield was exhibited by potassium nitrate observed at 770 U/mL (Figure 7b).
Figure 7.
The effect of carbon sources (a) and nitrogen sources (b) on enzyme production. Samples were incubated at 45 °C and pH 7–7.5 for 4 days.
3.3.3. Effect of Coconut Mesocarp Substrate on Cellulase Production at Various Concentrations
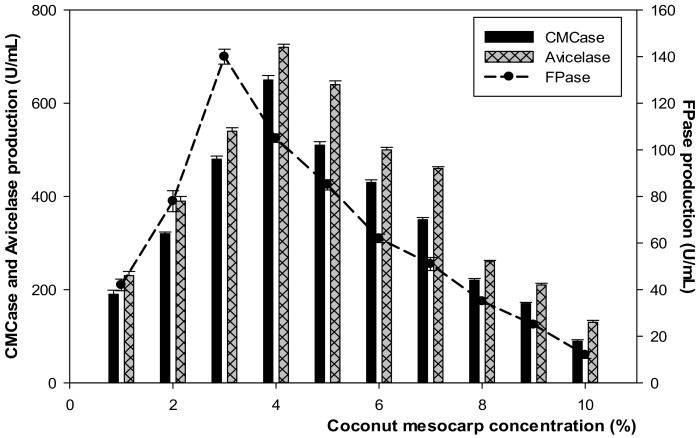
Figure 8 shows the effect of coconut mesocarp bio-base substrates on cellulase production. CMCase and Avicelase yielded the highest amounts observed at 650 U/mL and 720 U/mL at 4% of the substrate, respectively, whereas maximum FPase production was found at 3% of the substrate achieved in 140 U/mL.
Figure 8.
Production of cellulase enzymes at different concentrations of the coconut mesocarp without pretreatment. Samples were incubated at 45 °C, pH 7–7.5.
3.3.4. Effect of Metal Ions on Cellulase Activity
The result in Table 2 illustrates that the addition of Mg2+ and Ca2+ enhanced all types of cellulase enzymes. The presence of Mg2+ accelerated CMCase yield up to 138.5%, followed by FPase and Avicelase accounting for 130.5% and 120%, respectively. While Na+ had no effect on CMCase, both Na+ and K+ had a negligible effect on both FPase and Avicelase, accounting for around 95–99%. Fe2+ and Zn2+ had a negative effect on Avicelase by reducing it between 11% and 17%, on CMCase by between 4% and 15%, and on FPase by between 2% and 21%, respectively.
Table 2.
Effect of metal ions on cellulase activity of strain FW2.
| Metal Ions | Residual Activity (%) | ||
|---|---|---|---|
| FPase | CMCase | Avicelase | |
| Control | 100 | 100 | 100 |
| Na+ | 99 | 100 | 98 |
| K+ | 97 | 99 | 95 |
| Mg2+ | 130.5 | 138.5 | 120 |
| Fe2+ | 98 | 96 | 89 |
| Ca2+ | 115.8 | 122.5 | 108 |
| Zn2+ | 79 | 85 | 83 |
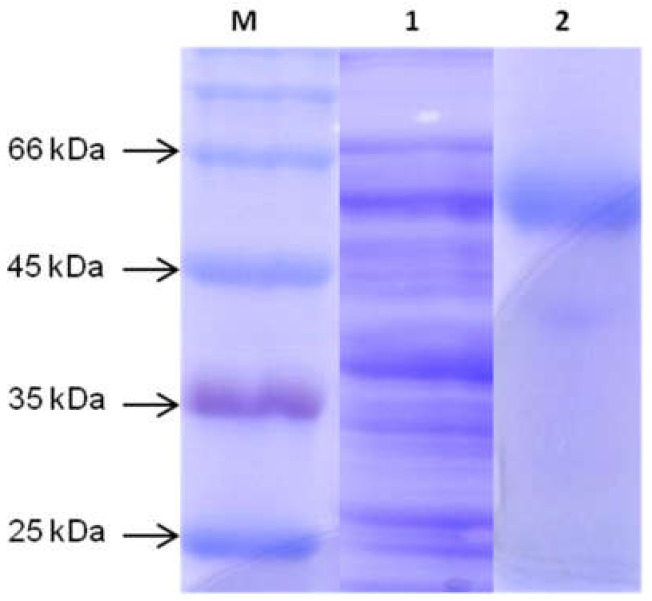
3.4. Determination of Molecular Weight of Extracted Cellulase
The homogenous enzyme preparation was obtained by SDS-PAGE analysis shown in Figure 9. Molecular weight mass of purified cellulase was estimated to be 55 kDa.
Figure 9.
SDS-Polyacrylamide gel electrophoresis of the purified cellulase produced from B. amyloliquefaciences FW2. (M) protein molecular makers; (1) supernatant culture broth; (2) purified enzyme.
4. Discussion
Following the previous study, Bacillus sp. FW2 was able to grow and produce enzyme under harsh conditions such as pH 4.5, pH 12, concentrations of NaCl up to 35% and withstand 75 °C. However, the related strains of strain FW2 grow within a limited temperature ranging from 15 to 45 °C [24,43]. In the other study, some halophilic bacterial strains were observed to yield cellulase at pH 6–12, temperature range of 30–90 °C, and up to 20% NaCl [44]. In this study, cellulase activity in the range of pH 5–10 by strain FW2 was consistent with the results of previous studies [45,46]. The optimal pH 7–7.5 for the bacterial strain FW2 was similar to the strain of B. amyloliquefaciens [18].
To date, there have been few studies on enzyme production at low temperatures. The cellulase produced by some psychrophilic microorganisms demonstrated optimal activity in the acidic to neutral pH range 4.5–7.0 [47,48]. However, other bacteria belonging to the genera Paenibacillus, Pseudoalteromonas, and Shewanella showed strong cellulase activity at neutral to alkaline pH [49,50]. Recently, thermostable enzyme production from bacteria has attracted considerable attention owing to its application in a wide range of fields. These thermo-enzymes are stable at high temperatures and active under other extreme conditions, such as varying pH values and salt concentrations [51,52,53,54]. However, these bacterial strains exhibited only one type of enzyme under thermophilic conditions. On the other hand, B. amyloliquefaciens DL-3, investigated in the previous study, showed high thermal stability at broad temperatures ranging from 40 to 80 °C [18].
Bioconversion of valuable products from lignocellulosic biomass requires combined pretreatment processes and catalytic degradation of that substrate. In such a way, plant dry materials in the form of lignocellulosic wastes were used to produce value-added products such as bio-ethanol, xylitol, and carboxylic acids [55,56,57,58]. However, the cellulase produced from various types of lignocellulosic materials was less observed using bacteria in previous studies in comparison with this study (Table 3).
Table 3.
Cellulase produced from various types of agricultural waste using bacterial strains in recent studies.
| Substrate | Bacterial Strains | Cellulase Production (U/mL) | Reference |
|---|---|---|---|
| Potato peel | Bacillus subtilis K-18 | CMCase (3.5) | [59] |
| Alkali-pretreated corn cob | Bacillus sp. BS-5 | CMCase (9.6), FPase (1.4) | [60] |
| Rice straw residues | Bacillus cereus RSI6 | CMCase (0.36) | [61] |
| Sugarcane bagasse | Paenibacillus polymyxa ND25 | CMCase (0.49) | [62] |
| Wheat bran | Bacillus sp. AO | CMCase (5.9), FPase (0.97) | [63] |
| Algal biomasses | Bacillus sp. TPF-1 | CMCase (9.12) | [64] |
| Corn husk | Sphingobacterium sp. ksn-11 | CMCase (3.55) | [65] |
| Coconut mesocarp | Bacillus sp. FW2 | CMCase (650), Avicelase (720) | This study |
There is no study on coconut mesocarp application of cellulase production by a fungal strain Trichoderma reesei besides the study of Dey et al. [34]. This study was the second in the research of bioconversion from coconut mesocarp, one of the prominent agricultural wastes in tropical countries using the high potential cellulose degrading Bacillus strain FW2 without any pretreatment. Moreover, coconut mesocarp is considered as a potential feed stock for production of value-added products including bioethanol, due to the presence of high content of cellulose (43.4%) and hemicellulose (19.9%) [66]. However, to achieve the high production efficiency of bio-products, the various pretreatments were carried out for breaking down the crystalline structure of lignocelluloses materials using physio-chemical techniques, such as liquid hot water [67] and sodium hydroxide treatment [10]. In the recent study, production of cellulase under optimized nutritional conditions was arrived at through the use of a response surface technique in Design Expert Software (version 8.0.4) [34].
Metal ions can form complexes in association with proteins and other molecules related to enzymes. They may act as donors or acceptors of the electron as structural regulators [68]. Mg2+ and Ca2+ were considered as the additives to enhance cellulase activity in many previous studies. The stimulatory effect of Mg2+ and Ca2+ on cellulase was also reported by Yoon et al., and Bakare et al. [69,70]. Fe2+ reduced FPase activity by 33.4% [71]. The negative effect of Fe2+ on cellulase was also reported by Yin et al. [72]. The production of cellulase was enhanced by the addition of NaCl and MgSO4 in another study [73].
The molecular mass of the purified cellulase produced from bacterial strain FW2 was estimated to be 55 kDa, which was found in the cellulase of Acinetobacter junii GAC 16.2, while it was 53 kDa of B. amyloliquefaciens DL-3 [18,74].
5. Conclusions
The isolate Bacillus amyloliquefaciens FW2 was investigated as a promising cellulase producer using various carbon and nitrogen sources. Moreover, this bacterial strain was able to degrade the coconut mesocarp that contains a high amount of lignin and hemicelluloses without pretreatment. Therefore, strain FW2 may be considered in the future as an effective degrader for more types of agricultural wastes. This study continues to explore the functional bacterial candidates that allow cost-effective production of cellulase and an ideal for a clean environment and biomass waste management. Further research should be carried out to optimize the fermentation conditions for the production of cellulase from this bacterial strain using coconut mesocarp in scale-up before application in a larger scale.
Author Contributions
Conceptualization: V.H.T.P.; methodology: V.H.T.P.; validation: V.H.T.P.; formal analysis and investigation: V.H.T.P.; resources: W.C.; writing—original draft preparation: V.H.T.P.; writing—review and editing: V.H.T.P., J.K. and W.C.; supervision: W.C. and S.C.; funding acquisition: J.S. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01529302)’, Rural Development Administration, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chukwuma O.B., Rafatullah M., Tajarudin H.A., Ismail N. A Review on bacterial contribution to lignocellulose breakdown into useful bio-products. Int. J. Environ. Res. Public Health. 2021;18:6001. doi: 10.3390/ijerph18116001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siqueira J.G.W., Cristine Rodrigues C., de Souza Vandenberghe L.P., Woiciechowski A.L., Soccol C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy. 2020;132:105419. doi: 10.1016/j.biombioe.2019.105419. [DOI] [Google Scholar]
- 3.Álvarez C., González A., Negro M.J., Ballesteros I., Oliva J.M., Sáez F. Optimized use of hemicellulose within a biorefinery for processing high value-added xylooligosaccharides. Ind. Crops Prod. 2017;99((Suppl. C)):41–48. doi: 10.1016/j.indcrop.2017.01.034. [DOI] [Google Scholar]
- 4.BCC Research The Global Biofuel Enzyme Market Value Is Expected to Reach Nearly $915 Million in 2017. [(accessed on 27 January 2022)]. Available online: https://www.bccresearch.com/pressroom/egy/global-biofuel-enzyme-market-value-expected-reach-nearly-$915-million-2017.
- 5.Grand View Research Biofuel Enzymes Market Report Biofuel Enzymes Market Analysis by Product (Amylases, Industrial Lipases), by Appli-Cation (Biodiesel, Starch/Corn Based Ethanol, Lignocellulosic Ethanol/Biofuels) and Segment Forecasts to 2020. [(accessed on 27 January 2022)]. Available online: https://www.grandviewresearch.com/industry-analysis/biofuel-enzymes-market.
- 6.Transparency Market Research Amylases Biofuel Enzymes Market—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2016–2024. [(accessed on 27 January 2022)]. Available online: https://www.transparencymarketresearch.com/amylases-biofuel-enzymes.html.
- 7.Chen M., Smith P.M. The U.S. cellulosic biofuels industry: Expert views on commercialization drivers and barriers. Biomass Bioenergy. 2017;102:52–61. doi: 10.1016/j.biombioe.2017.05.002. [DOI] [Google Scholar]
- 8.Neves P.V., Pitarelo A.P., Ramos L.P. Production of cellulosic ethanol from sugarcane bagasse by steam explosion: Effect of extractives content, acid catalysis and different fermentation technologies. Bioresour. Technol. 2016;208:184–194. doi: 10.1016/j.biortech.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 9.Phanchan N., Fiala K., Apirakakorn J. Isolation of cellulolytic Clostridia and their performance for one-step butanol production from sugarcane bagasse. Energy Proc. 2017;138:163–168. doi: 10.1016/j.egypro.2017.10.144. [DOI] [Google Scholar]
- 10.Kausar H., Ismail M.R., Saud H.M., Habib S.H., Othman R., Miah G. A novel lignocellulolytic bacterium for bioconversion of rice straw. Pak. J. Agric. Sci. 2016;53:523–533. [Google Scholar]
- 11.Magalhães B.L., Grassi M.C.B., Pereira G.A.G., Brocchi M. Improved n-butanol production from lignocellulosic hydrolysate by Clostridium strain and culture-medium optimization. Biomass Bioenergy. 2018;108:157–166. doi: 10.1016/j.biombioe.2017.10.044. [DOI] [Google Scholar]
- 12.Champreda V., Mhuantong W., Lekakarn H., Bunterngsook B., Kanokratana P., Zhao X.Q., Zhang F., Inoue H., Fujii T., Eurwilaichitr L. Designing cellulolytic enzyme systems for biorefinery: From nature to application. J. Biosci. Bioeng. 2019;128:637–654. doi: 10.1016/j.jbiosc.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Dumorné K., Córdova D.C., Astorga-Eló M., Renganathan P. Extremozymes: A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017;27:649–659. doi: 10.4014/jmb.1611.11006. [DOI] [PubMed] [Google Scholar]
- 14.Pham V.H.T., Ahn J.Y., Ro Y.H., Ravindran B., Kim J.S., Chang S.W., Shim J.H., Chung W.J. The efficiency of potential food waste-degrading bacteria under harsh conditions. J. Appl. Microbiol. 2022;132:340–350. doi: 10.1111/jam.15119. [DOI] [PubMed] [Google Scholar]
- 15.Jayasekara S., Ratnayake R. Cellulose. IntechOpen; London, UK: 2019. Microbial cellulase: An overview and applications. [Google Scholar]
- 16.Ejaz U., Sohail M., Ghanemi A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics. 2021;6:44. doi: 10.3390/biomimetics6030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behera B.C., Sethi B.K., Mishra R.R., Dutta S.K., Thatoi H.N. Microbial cellulases—Diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017;15:197–210. doi: 10.1016/j.jgeb.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanokratana P., Wongwilaiwalin S., Mhuantong W., Tangphatsornruang S., Eurwilaichitr L., Champreda V. Characterization of cellulolytic microbial consortium enriched on Napier grass using metagenomic approaches. J. Biosci. Bioeng. 2018;125:439–447. doi: 10.1016/j.jbiosc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Zhivin-Nissan O., Dassa B., Morag E., Kupervaser M., Levin Y., Bayer E.A. Unraveling essential cellulosomal components of the (Pseudo)Bacteroides cellulosolvens reveals an extensive reservoir of novel catalytic enzymes. Biotechnol. Biofuels Bioprod. 2019;12:115. doi: 10.1186/s13068-019-1447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham V.H.T., Kim J., Chang S., Chung W. Improvement of hydrogen production during anaerobic fermentation of food waste leachate by enriched bacterial culture using biochar as an additive. Microorganisms. 2021;9:2438. doi: 10.3390/microorganisms9122438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehghanikhah F., Shakarami J., Asoodeh A. Purification and Biochemical Characterization of Alkalophilic Cellulase from the Symbiotic Bacillus subtilis BC1 of the Leopard Moth, Zeuzera pyrina (L.) (Lepidoptera: Cossidae) Curr. Microbiol. 2020;77:1254–1261. doi: 10.1007/s00284-020-01938-z. [DOI] [PubMed] [Google Scholar]
- 22.Swathy R., Rambabu K., Banat F., Ho S.-H., Chu D.T., Show P.L. Production and optimization of high grade cellulase from waste date seeds by Cellulomonas uda NCIM 2353 for biohydrogen production. Int. J. Hydrogen Energy. 2020;45:22260–22270. doi: 10.1016/j.ijhydene.2019.06.171. [DOI] [Google Scholar]
- 23.Malik W.A., Khan H.M., Javed S. Bioprocess optimization for enhanced production of bacterial cellulase and hydrolysis of sugarcane bagasse. Bioenergy Res. 2021:1–14. doi: 10.1007/s12155-021-10259-3. [DOI] [Google Scholar]
- 24.Pham V.H.T., Kim J., Chang S.W., Chung W.J. Purification and characterization of strong simultaneous enzyme production of protease and amylase from an extremophile-Bacillus sp. FW2 and its possibility in food waste degradation. Fermentation. 2022;8:12. doi: 10.3390/fermentation8010012. [DOI] [Google Scholar]
- 25.Kuhad R.C., Gupta R., Ajay Singh A. Microbial cellulases and their industrial applications. Enzyme Res. 2011;2011:280696. doi: 10.4061/2011/280696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Mondéjar R., Zühlke D., Becher D., Riedel K., Baldrian P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016;6:25279. doi: 10.1038/srep25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng G., Yin T., Lu Z., yannick benz Boboua S., Li J., Zhou W. Degradation of rice straw at low temperature using a novel microbial consortium LTF-27 with efficient ability. Bioresour. Technol. 2020;304:123064. doi: 10.1016/j.biortech.2020.123064. [DOI] [PubMed] [Google Scholar]
- 28.Ye M., Sun L., Yang R., Wang Z., Qi K. The optimization of fermentation conditions for producing cellulase of Bacillus amyloliquefaciens and its application to goose feed. R. Soc. Open Sci. 2017;4:171012. doi: 10.1098/rsos.171012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aslam S., Hussain A., Qazi J.I. Production of Cellulase by Bacillus amyloliquefaciens-ASK11 under high chromium stress. Waste Biomass Valori. 2019;10:53–61. doi: 10.1007/s12649-017-0046-3. [DOI] [Google Scholar]
- 30.Soares J., Demeke M.M., Foulquié-Moreno M.R., de Velde M.V., Alex Verplaetse A., Ribeiro Fernandes A.A., Thevelein J.M., Bueno Fernandes P.M. Green coconut mesocarp pretreated by an alkaline process as raw material for bioethanol production. Bioresour. Technol. 2016;216:744–753. doi: 10.1016/j.biortech.2016.05.105. [DOI] [PubMed] [Google Scholar]
- 31.Cabral M.M.S., Abud A.K.S., Silva C.E.F., Almeida R.M.R.G. Bioethanol production from coconut husk fiber. Ciência Rural. 2016;46:1872–1877. doi: 10.1590/0103-8478cr20151331. [DOI] [Google Scholar]
- 32.Ebrahimi M., Caparanga A.R., Ordono E.E., Villaflores O.B. Evaluation of organosolv pretreatment on the enzymatic digestibility of coconut coir fibers and bioethanol production via simultaneous saccharification and fermentation. Renew. Energy. 2017;109:41–48. doi: 10.1016/j.renene.2017.03.011. [DOI] [Google Scholar]
- 33.Da Silva R.N., de Andrade Melo L.F., Finkler C.L.L. Optimization of the cultivation conditions of Bacillus licheniformis BCLLNF-01 for cellulase production. Biotechnol. Rep. 2021;29:e00599. doi: 10.1016/j.btre.2021.e00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dey P., Singh J., Scaria J., Anand A.P. Improved production of cellulase by Trichoderma reesei (MTCC 164) from coconut mesocarp-based lignocellulosic wastes under response surface-optimized condition. 3 Biotechnol. 2018;8:402. doi: 10.1007/s13205-018-1421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham V.H.T., Kim J. Bacillus thaonhiensis sp. nov., a new species, was isolated from the forest soil of Kyonggi University by using a modified culture method. Curr Microbiol. 2014;68:88–95. doi: 10.1007/s00284-014-0636-2. Erratum in: Curr. Microbiol. 2014, 69, 225. [DOI] [PubMed] [Google Scholar]
- 36.Frank J.A., Reich C.I., Sharma S., Weisbaum J.S., Wilson B.A., Olsen G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA Genes. Appl. Environ. Microbiol. 2008;74:2461–2470. doi: 10.1128/AEM.02272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon S.H., Ha S.M., Kwon S., Lim J., Kim Y., Seo H., Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nei M., Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press; New York, NY, USA: 2000. [Google Scholar]
- 40.Felsenstein J. Confidence limit on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 41.Miller G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 42.Laemmli U.K. Cleavage of structural proteins during assembly of head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 43.Borriss R., Chen X.H., Rueckert C., Blom J., Becker A., Baumgarth B., Fan B., Pukall R., Schumann P., Spröer C., et al. Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: A proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int. J. Syst. Evol. Microbiol. 2011;61:1786–1801. doi: 10.1099/ijs.0.023267-0. [DOI] [PubMed] [Google Scholar]
- 44.Rathakrishnan D., Gopalan A.K. Isolation and characterization of halophilic isolates from Indian salterns and their screening for production of hydrolytic enzymes. Environ. Chall. 2022;6:100426. doi: 10.1016/j.envc.2021.100426. [DOI] [Google Scholar]
- 45.Gozan M., Harahap A.F., Bakti C.P., Setyahadi S. Optimization of cellulase production by Bacillus sp. BPPT CC RK2 with pH and temperature variation using response surface methodology; Proceedings of the 3rd International Tropical Renewable Energy Conference “Sustainable Development of Tropical Renewable Energy”; Bali, Indonesia. 6–8 September 2018; p. 02051. [Google Scholar]
- 46.Islam F., Roy N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res. Notes. 2018;11:445. doi: 10.1186/s13104-018-3558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dar M.A., Dhole N.P., Xie R., Pawar K.D., Ullah K., Rahi P., Pandit R.S., Sun J. Valorization potential of a novel bacterial strain, Bacillus altitudinis RSP75, towards lignocellulose bioconversion: An Assessment of Symbiotic Bacteria from the Stored Grain Pest, Tribolium castaneum. Microorganisms. 2021;9:1952. doi: 10.3390/microorganisms9091952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Revin V., Liyaskina E., Nazarkina M., Bogatyreva A., Shchankin M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018;49:151–159. doi: 10.1016/j.bjm.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben Hmad I., Gargouri A. Neutral and alkaline cellulases: Production, engineering, and applications. J. Basic Microbiol. 2017;57:653–658. doi: 10.1002/jobm.201700111. [DOI] [PubMed] [Google Scholar]
- 50.Shajahan S., Ganesh Moorthy I., Sivakumar N., Selvakumar G. Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra, India. J. King Saud. Univ. Sci. 2017;29:302–310. doi: 10.1016/j.jksus.2016.08.001. [DOI] [Google Scholar]
- 51.Siu-Rodas Y., Calixto-Romo M.d.l.A., Guillén-Navarro K., Sánchez J.E., Zamora-Briseño J.A., Amaya-Delgado L. Bacillus subtilis with endocellulase and exocellulase activities isolated in the thermophilic phase from composting with coffee residues. Rev. Argent. Microbiol. 2018;50:234–243. doi: 10.1016/j.ram.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Zainudin M.H.M., Mustapha N.A., Hassan M.A. A highly thermostable crude endoglucanase produced by a newly isolated Thermobifida fusca strain UPMC 901. Sci. Rep. 2019;9:13526. doi: 10.1038/s41598-019-50126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta G.N., Srivastava S., Khare S.K., Prakash V. Extremophiles: An: Overview of Microorganism from Extreme Environment. Int. J. Agric. Environ. Biotechnol. 2014;7:371–380. doi: 10.5958/2230-732X.2014.00258.7. [DOI] [Google Scholar]
- 54.Kumar S., Dangi A.K., Shukla P., Baishya D., Khare S.K. Thermozymes: Adaptive strategies and tools for their biotechnological applications. Bioresour. Technol. 2019;278:372–382. doi: 10.1016/j.biortech.2019.01.088. [DOI] [PubMed] [Google Scholar]
- 55.Sajith S., Priji P., Sreedevi S., Benjamin S. An overview on fungal cellulases with an industrial perspective. J. Nutr. Food Sci. 2016;6:1–13. [Google Scholar]
- 56.Lee H., Lee Y.M., Heo Y.M., Lee J., Kim J.S., Kang K.Y., Kim J.J. Utilization of agricultural residues for enhancement of cellulolytic enzyme production and enzymatic saccharification by Trichoderma harzianum KUC1716. Ind. Crops Prod. 2017;109((Suppl. C)):185–191. doi: 10.1016/j.indcrop.2017.08.042. [DOI] [Google Scholar]
- 57.Kane S.D., French C.E. Characterisation of novel biomass degradation enzymes from the genome of Cellulomonas fimi. Enzyme Microb. Technol. 2018;113:9–17. doi: 10.1016/j.enzmictec.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanhueza C., Carvajal G., Soto-Aguilar J., Lienqueo M.E., Salazar O. The effect of a lytic polysaccharide monooxygenase and a xylanase from Gloeophyllum trabeum on the enzymatic hydrolysis of lignocellulosic residues using a commercial cellulase. Enzyme Microb. Technol. 2018;113:75–82. doi: 10.1016/j.enzmictec.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Irfan M., Mushtaq Q., Tabssum F., Shakir H.A., Qazi J.I. Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express. 2017;7:29. doi: 10.1186/s13568-017-0331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J., Gao Z., Wu B., He B. Lactose-inducted production of a complete lignocellulolytic enzyme system by a novel bacterium Bacillus sp. BS-5 and its application for saccharification of alkali-pretreated corn cob. Cellulose. 2017;24:2059–2070. doi: 10.1007/s10570-017-1247-4. [DOI] [Google Scholar]
- 61.Nevita T., Sharma G.D., Pandeya P. Differences in rice rhizosphere bacterial community structure by application of lignocellulolytic plant-probiotic bacteria with rapid composting traits. Ecol. Eng. 2018;120:209–221. doi: 10.1016/j.ecoleng.2018.06.007. [DOI] [Google Scholar]
- 62.Bohra V., Dafale N.A., Purohit H.J. Paenibacillus polymyxa ND25: Candidate genome for lignocellulosic biomass utilization. 3 Biotechnol. 2018;8:248. doi: 10.1007/s13205-018-1274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo H., Chen H., Fan L., Linklater A., Zheng B., Jiang D., Qin W. Enzymes produced by biomass-degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew. Energy. 2017;109:195–201. doi: 10.1016/j.renene.2017.03.025. [DOI] [Google Scholar]
- 64.Wu Y., Guo H., Zhang J., Chen X., Wu M., Qin W. Multiple applications of enzymes induced by algal biomasses from a new Bacillus isolate to saccharify algae and degrade chemical dyes. Waste Biomass Valor. 2019;10:2517–2526. doi: 10.1007/s12649-018-0277-y. [DOI] [Google Scholar]
- 65.Neelkant K.S., Shankar K., Jayalakshmi S.K., Sreeramulu K. Optimization of conditions for the production of lignocellulolytic enzymes by Sphingobacterium sp. ksn-11 utilizing agro-wastes under submerged condition. Prep. Biochem. Biotechnol. 2019;49:927–934. doi: 10.1080/10826068.2019.1643735. [DOI] [PubMed] [Google Scholar]
- 66.Miftahul Jannah A., Asip F. Bioethanol production from coconut fiber using alkaline pretreatment and acid hydrolysis method. Int. J. Adv. Sci. Eng. Inf. Technol. 2015;5:320. doi: 10.18517/ijaseit.5.5.570. [DOI] [Google Scholar]
- 67.Zhuang X., Wang W., Yu Q., Qi W., Wang Q., Tan X., Zhou G., Yuan Z. Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 2016;199:68–75. doi: 10.1016/j.biortech.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 68.Riordan J.F. The role of metals in enzyme activity. Ann. Clin. Lab. Sci. 1977;7:119–129. [PubMed] [Google Scholar]
- 69.Yoon S., Kim M.K., Hong J.S., Kim M.S. Production of polygalacturonase from Ganoderma lucidum. Korean J. Mycol. 1994;22:286–297. [Google Scholar]
- 70.Bakare M.K., Adewale I.O., Ajayi A., Shonukan O.O. Purification and characterization of cellulase from the wild-type and two improved mutants of Pseudomonas fluorescens. Afr. J. Biotechnol. 2005;4:898–904. [Google Scholar]
- 71.Moni Bhuyan P., Protim Sandilya S., Kumar Nath P., Gandotra S., Subramanian S., Kardong D., Kumar Gogoi D. Optimization and characterization of extracellular cellulase produced by Bacillus pumilus MGB05 isolated from midgut of muga silkworm (Antheraea assamensis Helfer) J. Asia-Pac. Entomol. 2018;21:1171–1181. doi: 10.1016/j.aspen.2018.08.004. [DOI] [Google Scholar]
- 72.Yin L.J., Lin H.H., Xiao Z.R. Purification and characterization of a cellulase from Bacillus subtilis YJ1.J. Mar. Sci. Technol. 2010;18:466–471. doi: 10.51400/2709-6998.1895. [DOI] [Google Scholar]
- 73.Singh J., Kaur P. Optimization of process parameters for cellulase production from Bacillus sp. JS14 in solid substrate fermentation using response surface methodology. Braz. Arch. Biol. Technol. 2012;55:505–512. doi: 10.1590/S1516-89132012000400004. [DOI] [Google Scholar]
- 74.Banerjee S., Maiti T.K., Roy R.N. Production, purification, and characterization of cellulase from Acinetobacter junii GAC 16.2, a novel cellulolytic gut isolate of Gryllotalpa africana, and its effects on cotton fiber and sawdust. Ann. Microbiol. 2020;70:28. doi: 10.1186/s13213-020-01569-6. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.