Abstract
Genetic studies have previously revealed that Cdc37p is required for the catalytic competence of v-Src in yeast. We have reasoned that temperature-sensitive mutants of Src family kinases might be more sensitive to the cellular level of p50Cdc37, the mammalian homolog of Cdc37p, than their wild-type counterpart, thus potentially providing a unique opportunity to elucidate the involvement of p50Cdc37 in the folding and stabilization of Src family kinases. A temperature-sensitive mutant of a constitutively active form of Hck (i.e., tsHck499F) was created by mutating two amino acids within the kinase domain of Hck499F. Significantly, overexpression of p50Cdc37 rescues the catalytic activity of tsHck499F at 33°C, while partially buffering it against inactivation at higher temperatures (e.g., 37 and 39°C). Hsp90 function is required for tsHck499F activity and its stabilization by p50Cdc37, but overexpression of Hsp90 is not sufficient to stabilize tsHck499F. Overexpression of p50Cdc37 promotes the association of tsHck499F with Hsp90, suggesting that the cellular level of p50Cdc37 might be the rate-limiting step in the association of tsHck499F with Hsp90. A truncation mutant of p50Cdc37 that cannot bind Hsp90 still has a limited capacity to rescue the catalytic activity of tsHck499F and promote its association with Hsp90. This is a particularly important observation, since it argues that rather than solely acting as a passive adapter protein to tether tsHck499F to Hsp90, p50Cdc37 may also act allosterically to enhance the association of tsHck499F with Hsp90. The findings presented here might also have implications for our understanding of the evolution of protein kinases and tumor development.
A wide range of cellular activities, including proliferation, differentiation, migration, and activation, are regulated by extracellular stimuli and a complex network of intracellular signal transduction pathways. Members of the Src family of protein tyrosine kinases are important components of many intracellular signal transduction pathways that regulate cell activity (27). The Src family kinases Lck and Lyn, for example, have been shown to play critical signal transducing roles in T lymphocytes (33) and B lymphocytes (18), respectively, whereas Src has been shown to play an essential signal transducing role in osteoclasts (44).
Src family kinases have a well-defined modular structure. Starting at their amino termini, they are characterized by a so-called unique domain, which is followed by Src homology 3 (SH3) and 2 (SH2) domains, a tyrosine kinase catalytic domain, and a conserved regulatory tyrosine residue (e.g., tyrosine-527 in Src) (2, 49). In addition to mediating the physical interaction of the kinases with cellular proteins (e.g., substrates), the SH3 and SH2 domains are also intimately involved in negatively regulating the catalytic activity of Src family kinases (2, 49). The binding of the phosphorylated regulatory tyrosine residue to the SH2 domain serves as the primary means by which the catalytic activity of the kinases is suppressed (2, 49). Accordingly, mutation of this tyrosine residue to phenylalanine leads to constitutive activation of the kinase (24). The SH3 domain also contributes to the repression of kinase activity by binding a sequence within the SH2-kinase domain linker region that assumes a left-handed polyproline type II helix (43).
Our interest lies with the Src family kinase Hck and its role in intracellular signal transduction. Hck is primarily expressed in hemopoietic cells of the B-lymphoid and myeloid lineages (19, 37, 57) and has been shown to play a role in regulating monocyte-macrophage cell adhesion and migration (5, 31, 41, 47). Additionally, Hck has also been implicated in the suppression of embryonic stem cell differentiation by leukemia inhibitory factor (8). Interestingly, two isoforms of Hck (p59Hck and p56Hck in murine cells) have been detected in a number of different mammalian cell types, and work from this laboratory has established that they arise from the utilization of alternative translational initiation codons within a single hck mRNA (26). Although specific functions have not been ascribed to the individual isoforms, it is intriguing that, at least in some cells, the two isoforms exhibit different subcellular localizations (26). Both isoforms associate with cellular membranes; however, a fraction of p59Hck is also found in the cytosol (26).
To fulfill their signal transducing function, protein kinases, such as Hck, must first be folded into a catalytically competent conformation and then maintained in this form. Both pharmacological and genetic approaches have been utilized to investigate the relationship between chaperone machinery containing the 90-kDa heat shock protein Hsp90 and the biogenesis of catalytically active Src family kinases (15, 16, 52, 55, 56). These studies have revealed that not only is Hsp90 function required for the de novo folding of Src family kinases into a catalytically active conformation, but it might also be required for maintaining them in their active conformation (15).
Similarly, Cdc37p, the yeast homolog of the mammalian Hsp90-binding protein p50Cdc37 (51), has been shown genetically to be required for v-Src to achieve a catalytically active conformation in Saccharomyces cerevisiae (7). The CDC37 gene was first identified in a mutant strain of S. cerevisiae with a G1 cell cycle arrest phenotype (38). Subsequent analysis revealed that the function of several protein kinases (e.g., Cdc28 and MPS1 kinase) is impaired in yeast cdc37 mutants (11, 42), while mutations in the Drosophila homolog compromise signaling by the sevenless receptor tyrosine kinase (6). Mammalian p50Cdc37 has approximately 45 and 20% sequence identity to Drosophila CDC37 and S. cerevisiae Cdc37p, respectively (20). Significantly, coexpression of p50Cdc37 with the protein serine/threonine kinase Cdk4 (46) or Raf (12) is sufficient to facilitate their association with Hsp90 in cells. In contrast, overexpression of a carboxy-terminal truncation mutant of p50Cdc37 that is unable to bind Hsp90 perturbs the association of Raf with Hsp90 (12). Taken together, these observations have led to the proposal that p50Cdc37 acts as a kinase-targeting subunit of Hsp90 to facilitate the recruitment of Hsp90 to protein kinases (12, 46). Notably, when Hsp90 activity is compromised in S. cerevisiae, the catalytic activity of v-Src can be rescued by overexpression of yeast Cdc37p (23). However, the finding that Cdc37p possesses in vitro chaperone-like activity, at least towards some proteins (23), raises the intriguing possibility that in addition to promoting the recruitment of Hsp90 to client protein kinases, p50Cdc37 may also act as a kinase chaperone in its own right.
It has previously been reported that temperature-sensitive mutants of v-Src show higher levels of associated p50Cdc37 (known at that time simply as p50) than does wild-type v-Src (3, 4). Given this finding, we have reasoned that temperature-sensitive mutants of Src family kinases might be more sensitive to the cellular level of p50Cdc37 than their parental counterpart and may thus represent a unique opportunity to study the involvement of p50Cdc37 in the folding and stabilization of Src family kinases in mammalian cells. Reported here is the creation of a temperature-sensitive mutant of a constitutively active form of Hck (i.e., tsHck499F). Significantly, the catalytic activity of this mutant is markedly enhanced by the overexpression of p50Cdc37. This mutant has allowed us to explore the functional role of p50Cdc37 in promoting the folding and stabilization of Hck499F into a catalytically active conformation in mammalian cells.
MATERIALS AND METHODS
Reagents.
Cell culture medium and supplements were from Life Technologies, Inc. Fetal calf serum (FCS) was from CSL, Ltd. (Melbourne, Australia). A rabbit anti-Hck polyclonal antibody (1077) was a generous gift from Clifford Lowell (University of California, San Francisco), while a rat anti-murine Hck monoclonal antibody (H34) was developed in this laboratory. The anti-phosphotyrosine monoclonal antibody (4G10) was from Upstate Biotechnology, Inc. The anti-paxillin monoclonal antibody and anti-rat immunoglobulin G (IgG) beads were from Zymed, Inc. The anti-Flag (M2) monoclonal antibody and anti-Flag (M2) beads were obtained from Sigma. The rabbit anti-Hsp90 antiserum “84/86” was a generous gift from Stephen Ullrich (50) (National Cancer Institute, National Institutes of Health). Polyclonal antibodies to human p50Cdc37 were developed by immunizing mice with recombinant human p50Cdc37. Protein A-Sepharose and enhanced chemiluminescence (ECL) reagents were from Amersham Pharmacia Biotech. [γ-32P]ATP (3,000 Ci/mmol) was obtained from Bresatec, Ltd. (Adelaide, Australia). Pfu DNA polymerase was obtained from Stratagene. The FuGENE-6 transfection reagent was from Roche. Cycloheximide was purchased from Sigma-Aldrich. All other reagents were of the highest grade available.
Plasmid construction.
The mammalian expression vector pCDM8 Hck499F was a generous gift of Margaret L. Hibbs (Ludwig Institute for Cancer Research, Melbourne, Australia). Plasmid pCDM8 tsHck499F (in which isoleucine-433 is mutated to methionine and proline-475 is mutated to serine) was created by performing sequential rounds of oligonucleotide-mediated site-directed mutagenesis on pCDM8 Hck499F. The cDNA inserts from pCDM8 Hck499F and pCDM8 tsHck499F were excised with XbaI and subcloned into the XbaI sites of pEF-BOS (32) to create pEF-Hck499F and pEF-tsHck499F, respectively. DNA encoding human paxillin was generated by PCR using Pfu DNA polymerase with plasmid pBabePuro-paxillin (30) (a generous gift of Hisataka Sabe, Institute for Virus Research, Kyoto, Japan) as the template for PCR. The PCR product generated was subcloned into the XbaI sites of pEF-BOS to create pEF-paxillin. Expression constructs encoding epitope-tagged versions of either full-length human p50Cdc37 or p50Cdc37ΔCT (i.e., amino acids 1 to 164 of full-length p50Cdc37) were created by PCR using Pfu polymerase, with plasmid pT7T3D-p50Cdc37 (which encodes human p50Cdc37 and was purchased from the IMAGE EST Consortium) as the template. The PCR products were cut with MluI and subcloned into the corresponding site in the pEF-Flag vector (a generous gift of Douglas Hilton, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia). The sense PCR primer was designed such that when the p50Cdc37-encoding PCR products are subcloned into pEF-Flag, they are introduced in frame into a sequence encoding the Flag epitope. Consequently, when the p50Cdc37 proteins are expressed from this vector, they carry an amino-terminal Flag epitope. The expression vector pEF-Hsp90α was created by subcloning the cDNA insert from pGEM-Hsp90α (a generous gift of David Toft, Mayo Graduate School, Rochester, N.Y.) into pEF-BOS. The fidelity of all constructs was confirmed by restriction mapping and/or automated DNA sequencing.
Cell culture and transient transfection.
Human 293T cells were maintained in RPMI medium supplemented with 10% FCS and grown at 37°C in a humidified atmosphere of 5% CO2. The cells were transfected for 4 h with a total of 12 to 15 μg of plasmid using polyethylenimine (1) or the FuGENE-6 transfection reagent. The cells were incubated overnight at 37°C and then at the required temperature (33, 37, or 39°C) for the times indicated in the figure legends.
Cell lysis.
Cells were lysed directly in tissue culture dishes with either NP-40 lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol [DTT], 1% Nonidet P-40, 10% glycerol, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 1 mM Pefabloc, 10 μg of leupeptin/ml, 100 U of aprotinin/ml) or p50Cdc37 lysis buffer (20 mM HEPES [pH 7.4], 100 mM NaCl, 2 mM EGTA, 1 mM DTT, 0.5% Nonidet P-40, 10% glycerol, 1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 1 mM Pefabloc, 10 μg of leupeptin/ml, 100 U of aprotinin/ml) for 30 min on ice. Lysates were clarified by centrifugation at 13,000 × g for 10 min at 4°C, and then protein concentrations were measured with a Bio-Rad protein assay kit.
Western blotting and immunoprecipitation.
Western blotting of whole-cell lysates was performed by standard techniques. Hck was immunoprecipitated from aliquots of whole-cell lysates using either a rabbit polyclonal (1077) or a rat monoclonal anti-Hck (M34) antibody. Flag-tagged p50Cdc37 proteins were immunoprecipitated by employing anti-Flag beads. In both cases the immunoprecipitates were washed four times with lysis buffer prior to fractionation on sodium dodecyl sulfate (SDS)-polyacrylamide gels and Western blotting with the appropriate antibody. Immune complexes were visualized by ECL and exposure to Fuji X-ray film. The results were digitized using a Computing Densitometer, quantified using the ImageQuant program, version 4.2, and then converted to TIF files using the program Convert 16 to 8, version 1.5a (all from Molecular Dynamics).
Hck kinase assays.
Anti-Hck immunoprecipitates were incubated at room temperature for 5 min in 30 μl of kinase buffer (20 mM HEPES [pH 7.4], 10 mM MnCl2, 0.1% NP-40, and 0.1 mM sodium orthovanadate) containing 10 μCi of [γ-32P]ATP. Reactions were terminated by the addition of an equal volume of 2× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer and heating for 5 min at 95°C. Phosphorylated proteins were analyzed by SDS-PAGE, followed by exposure to a PhosphorImager screen (Molecular Dynamics).
RESULTS
Mutation of isoleucine-433 and proline-475 converts Hck499F into a temperature-sensitive kinase.
Genetic and biochemical dissection of the temperature-sensitive Rous Sarcoma virus (RSV) variant NY72-4 has previously revealed that two specific mutations (valine-461 to methionine and proline-503 to serine) within the catalytic domain of v-Src are responsible for the temperature-sensitive phenotype of the kinase (10, 29). An alignment of the amino acid sequences of the carboxy-terminal domain of Hck (amino acids 397 to 503) with the corresponding region of v-Src from the Schmidt-Ruppin, subgroup A strain of RSV (SRA-RSV) and NY72-4 reveals that in Hck an isoleucine residue is in a position corresponding to valine-461 in v-Src from SRA-RSV, while the proline is conserved between the two kinases (Fig. 1). The crystal structures of Hck (40, 43) and Src (53, 54) reveal that isoleucine-433 and proline-475 in Hck superimpose with valine-461 and proline-503 in Src.
FIG. 1.
Sequence alignment of Hck with v-Src and ts v-Src. Shown is a sequence alignment of the C-terminal portions of the kinase domains of mHck (murine Hck), v-Src, and ts v-Src. Alpha-helical secondary-structure elements from the crystal structure of Hck are shaded. Isoleucine-433 in Hck, valine-461 in v-Src, and methionine-461 in ts v-Src are boxed, as are proline-475 in Hck, proline-503 in v-Src, and serine-503 in ts v-Src.
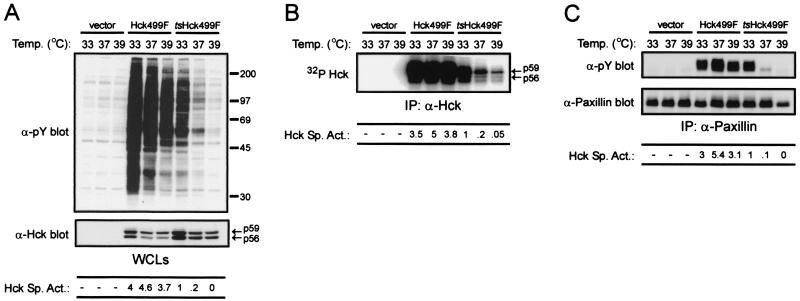
To determine if a constitutively active form of Hck (i.e., Hck499F) could be converted into a temperature-sensitive kinase analogous to v-Src from the NY72-4 variant of RSV, isoleucine-433 and proline-475 within the kinase domain of Hck499F were mutated to methionine and serine, respectively. The temperature sensitivity of the resulting mutant kinase (i.e., tsHck499F) was then compared to that of Hck499F by transiently expressing the kinases in human 293T cells (Fig. 2). Both in vivo and in vitro assays were used to assess the relative catalytic activity of the two kinases. Phosphorylation of either endogenous cellular proteins or cotransfected paxillin was used as an in vivo measure of kinase activity, whereas the ability of the kinases to autophosphorylate in the presence of [γ-32P]ATP was used as an in vitro measure of their catalytic activity. The relative specific activities of Hck499F and tsHck499F were determined by quantifying the tyrosine phosphorylation of endogenous cellular proteins and cotransfected paxillin, or their autophosphorylation, and the expression level of the kinases. The specific activity of tsHck499F was given an arbitrary value of 1. As shown in Fig. 2A, the ability of tsHck499F to phosphorylate endogenous cellular proteins was found to be significantly perturbed, in comparison to that of Hck499F, when the transfected cells were incubated at progressively higher temperatures (e.g., 33, 37, and 39°C). This decrease in the in vivo activity of tsHck499F upon incubation of the cells at 37 or 39°C was also reflected in the decreased ability of the kinase to autophosphorylate in vitro (Fig. 2B). Similarly, the ability of tsHck499F to phosphorylate cotransfected paxillin was severely compromised upon incubation of the transfected 293T cells at 37 or 39°C (Fig. 2C). In contrast, the catalytic activity of Hck499F was only modestly reduced upon incubation of the cells at 39°C (Fig. 2). Western blotting of whole-cell lysates of the transfected cells with an anti-Hck monoclonal antibody revealed a small decrease (approximately twofold) in the expression level of tsHck499F at 37 and 39°C compared to that at 33°C (Fig. 2A). Although this accounts in part for the decrease in tsHck499F activity, incubation of the cells at 37 and 39°C also resulted in a profound decrease (at least 20-fold) in the specific activity of tsHck499F (Fig. 2). It should be noted that even at 33°C, tsHck499F is approximately fourfold less active than Hck499F (Fig. 2A). Experiments in which the transfected cells were shifted from 39 to 33°C for various periods of time revealed that the recovery of tsHck499F activity is a relatively slow process. A small increase in tsHck499F activity was observed within 1 h of shifting the cells to 33°C; tsHck499F activity increased further by 6 h, reaching a maximum after 24 to 48 h (data not shown).
FIG. 2.
Conversion of Hck into a temperature-sensitive kinase. (A) 293T cells transiently expressing Hck499F or tsHck499F were incubated at the indicated temperature for 48 h and then lysed with NP-40 lysis buffer. Aliquots of the whole-cell lysates (WCLs) were sequentially Western blotted with anti-phosphotyrosine (α-pY) and Hck (α-Hck) monoclonal antibodies. The positions of molecular weight markers (in thousands) are shown on the right. The relative specific activities (Hck Sp. Act.) of Hck499F and tsHck499F are shown at the bottom. The specific activity of tsHck499F was given an arbitrary value of 1.0. (B) Hck was immunoprecipitated (IP) from aliquots of the WCLs and subjected to an in vitro autophosphorylation reaction. (C) 293T cells transiently expressing paxillin alone, or together with either Hck499F or tsHck499F, were incubated at the indicated temperature for 48 h and then lysed with NP-40 lysis buffer. Paxillin was immunoprecipitated from aliquots of the WCLs and subjected to Western blotting with anti-pY and anti-paxillin monoclonal antibodies.
Overexpression of p50Cdc37 can rescue the catalytic activity of tsHck499F at 33°C.
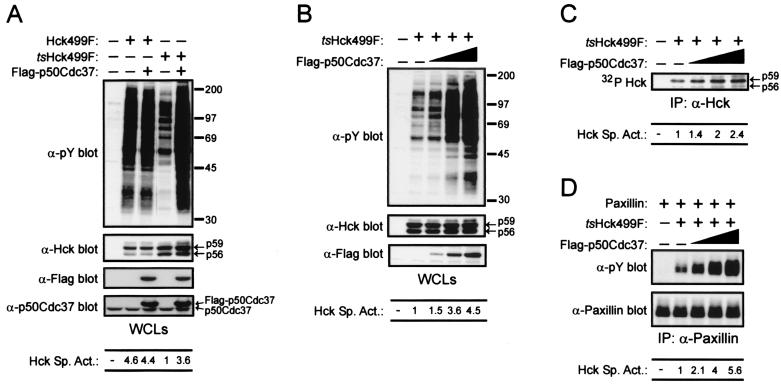
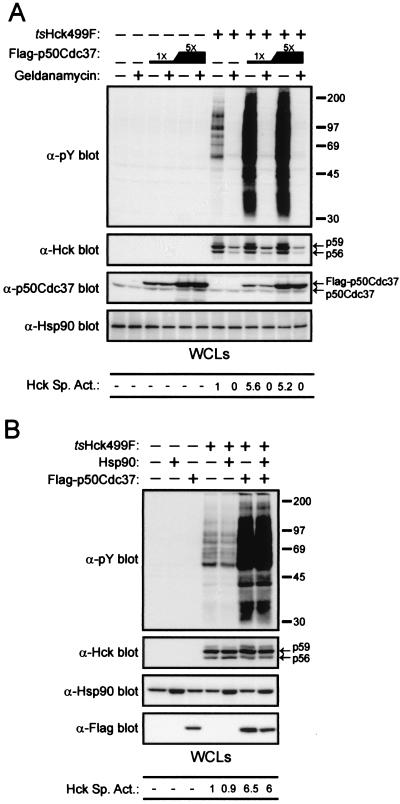
Given that the kinase domain of tsHck499F is likely to be thermodynamically less stable than that of Hck499F, we were curious to determine if the temperature-sensitive properties of tsHck499F could be suppressed by overexpression of p50Cdc37. In order to discriminate between endogenous and transfected p50Cdc37, a p50Cdc37 expression vector was constructed such that the expressed protein contains the Flag epitope at its amino terminus. As judged by tyrosine phosphorylation of endogenous cellular proteins, the specific activity of tsHck499F at 33°C is markedly enhanced (approximately 3.5-fold) by the overexpression of Flag-p50Cdc37 (Fig. 3A). In contrast, no increase in the specific activity of Hck499F occurred upon its coexpression with Flag-p50Cdc37 (Fig. 3A and data not shown). Western blotting of whole-cell lysates of the transfected cells with an anti-Hck monoclonal antibody revealed a small increase (approximately 1.5-fold) in the expression of tsHck499F when it was simultaneously coexpressed with Flag-p50Cdc37 (Fig. 3A). No increase in the expression of Hck499F was observed (Fig. 3A). Interestingly, the anti-Hck monoclonal antibody detected the presence of three immunoreactive species in the whole-cell lysates (Fig. 3A). The two faster-migrating species represent the p56 and p59 isoforms of Hck499F, while the slower-migrating species most likely represents a hyperphosphorylated form of p59 Hck499F, since this species is not observed when a kinase-inactive form of Hck499F is expressed in 293T cells (data not shown). Significantly, coexpression of tsHck499F with Flag-p50Cdc37 resulted in a two- to threefold increase in the abundance of the slower-migrating kinase species (Fig. 3A and B). These findings suggest that while an increase in the expression of tsHck499F contributes to a limited extent to the increase in tsHck499F activity upon overexpression of Flag-p50Cdc37, the increase is primarily the result of an increase (approximately fourfold) in the specific activity of tsHck499F. Western blotting with an anti-p50Cdc37 antibody revealed that Flag-p50Cdc37 was present in the whole-cell lysates at levels three- to fourfold greater than that of endogenous p50Cdc37 (Fig. 3A). However, given that the efficiency of transfection achieved in these experiments was estimated to have been approximately 20 to 25% (data not shown), the successfully transfected cells would have contained Flag-p50Cdc37 at levels approximately 15-fold greater than that of endogenous p50Cdc37. The ability of Flag-p50Cdc37 to enhance the catalytic activity of tsHck499F, as determined by both in vivo and in vitro assays, was found to correlate with the level of overexpression of Flag-p50Cdc37 (Fig. 3B, C, and D).
FIG. 3.
p50Cdc37 rescues the catalytic activity of tsHck499F at 33°C. (A) 293T cells transiently expressing Hck499F or tsHck499F alone or together with Flag-p50Cdc37 were incubated at 33°C for 48 h and then lysed with NP-40 lysis buffer. Aliquots of the whole-cell lysates (WCLs) were then sequentially Western blotted with anti-phosphotyrosine (α-pY), anti-Hck, anti-Flag, and anti-p50Cdc37 antibodies. (B) 293T cells transiently expressing tsHck499F and increasing (fivefold) amounts of Flag-p50Cdc37 were incubated at 33°C for 48 h and then lysed with NP-40 lysis buffer. Aliquots of the WCLs were then sequentially Western blotted with anti-pY, anti-Hck, and anti-Flag monoclonal antibodies. (C) Hck was immunoprecipitated (IP) from aliquots of the WCLs shown in panel B and subjected to an in vitro autophosphorylation reaction. (D) 293T cells transiently coexpressing paxillin and tsHck499F, together with increasing amounts of Flag-p50Cdc37, were incubated at 33°C for 48 h and then lysed with NP-40 lysis buffer. Paxillin was immunoprecipitated from aliquots of the WCLs and subjected to Western blotting with anti-pY and anti-paxillin monoclonal antibodies.
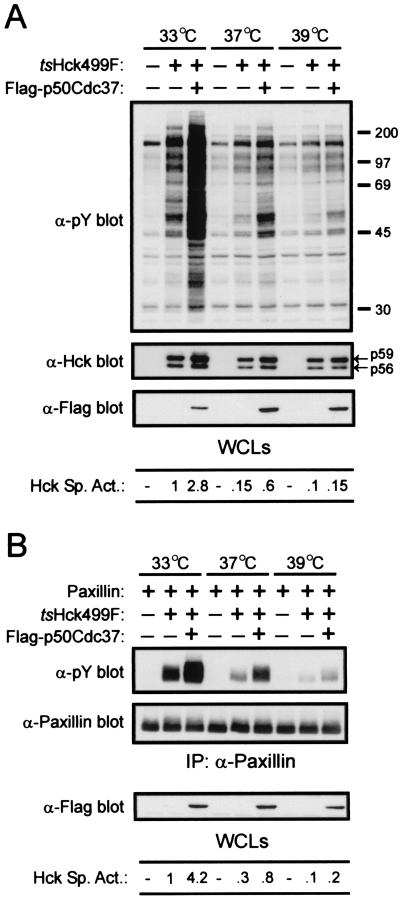
Overexpression of p50Cdc37 partially rescues the catalytic activity of tsHck499F at higher temperatures.
Since overexpression of Flag-p50Cdc37 was found to markedly enhance the specific activity of tsHck499F at 33°C, we wanted to determine if overexpression of Flag-p50Cdc37 could similarly rescue the catalytic activity of tsHck499F at 37 or 39°C, temperatures at which the kinase is weakly active and inactive, respectively. As shown in Fig. 4A, coexpression of Flag-p50Cdc37 with tsHck499F can partially rescue the catalytic activity of the kinase at both 37 and 39°C, albeit to a lesser extent than is seen at 33°C (Fig. 4A). In particular, when tsHck499F was coexpressed with Flag-p50Cdc37 at 37°C, its specific activity, as judged by the tyrosine phosphorylation of endogenous cellular proteins, was similar to that of tsHck499F expressed at 33°C in the absence of Flag-p50Cdc37 (Fig. 4A). At 39°C, Flag-p50Cdc37 restored the specific activity of tsHck499F to a level comparable to that exhibited by the kinase at 37°C in the absence of coexpressed Flag-p50Cdc37 (Fig. 4A). Similar results were obtained when the ability of tsHck499F to phosphorylate cotransfected paxillin (Fig. 4B) or autophosphorylate in vitro (data not shown) was used as a measure of its catalytic activity.
FIG. 4.
p50Cdc37 partially rescues the catalytic activity of tsHck499F at 37 and 39°C. (A) 293T cells transiently expressing tsHck499F alone or together with Flag-p50Cdc37 were incubated at the indicated temperatures for 48 h and then lysed with NP-40 lysis buffer. Aliquots of the whole-cell lysates (WCLs) were then sequentially Western blotted with anti-phosphotyrosine (α-pY), anti-Hck, and anti-Flag antibodies. (B) 293T cells transiently expressing paxillin either alone or together with tsHck499F and Flag-p50Cdc37 were incubated at the indicated temperature for 48 h and then lysed with NP-40 lysis buffer. Paxillin was immunoprecipitated (IP) from aliquots of the WCLs and subjected to Western blotting with anti-pY and anti-paxillin monoclonal antibodies. The WCLs were subjected to Western blotting with an anti-Flag monoclonal antibody.
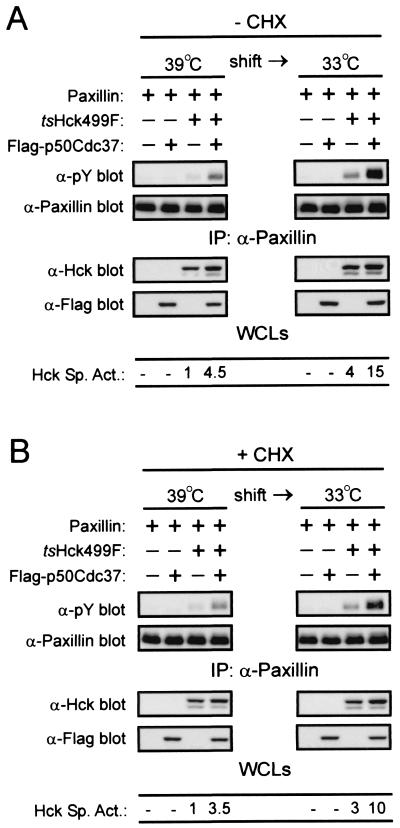
p50Cdc37 can act posttranslationally to enhance the specific activity of tsHck499F.
The increase in the specific activity of tsHck499F that occurs upon coexpression with Flag-p50Cdc37 could potentially be due to Flag-p50Cdc37 promoting the folding of nascent and/or mature tsHck499F into a more stable and catalytically active conformation. To specifically test if mature tsHck499F can refold into an active conformation, and if this process is enhanced by Flag-p50Cdc37, 293T cells that had been transfected with the tsHck499F expression vector were incubated at 39°C for 48 h. Such treatment should lead to the accumulation of mature but catalytically inactive tsHck499F. The cells were subsequently treated with the protein synthesis inhibitor cycloheximide for 90 min to inhibit the synthesis of nascent tsHck499F and then were either maintained at 39°C or shifted to 33°C for 6 h. Tyrosine phosphorylation of cotransfected paxillin was used as a measure of the specific activity of tsHck499F in these assays. Irrespective of whether the cells had been pretreated with cycloheximide, a three- to fourfold increase in the specific activity of tsHck499F occurred upon shifting of the cells from 39 to 33°C (Fig. 5). Although the specific activity of tsHck499F in cells overexpressing Flag-p50Cdc37 at 39°C was 3.5-fold higher than that in cells expressing the kinase alone, the increase in the specific activity of tsHck499F upon shifting of the cells to 33°C was also greater (approximately 3-fold) in cells overexpressing Flag-p50Cdc37 (Fig. 5B). It should be noted that in the absence of cycloheximide pretreatment, a small increase (approximately 1.5-fold) in the expression of tsHck499F was observed upon shifting of the cells to 33°C (Fig. 5A), whereas no such increase was observed when the cells were pretreated with cycloheximide prior to shifting to 33°C (Fig. 5B).
FIG. 5.
Posttranslational stabilization of tsHck499F by p50Cdc37. 293T cells transiently expressing paxillin alone, or together with tsHck499F and/or Flag-p50Cdc37, were incubated at 39°C for 48 h and then treated with either ethanol vehicle (−CHX) (A) or 100 μg of cycloheximide/ml (+CHX) (B) for 90 min. The cells were then either left at 39°C or shifted to 33°C for 6 h prior to lysing with NP-40 lysis buffer. Paxillin was immunoprecipitated (IP) from aliquots of the whole-cell lysates (WCLs) and subjected to Western blotting with anti-phosphotyrosine (α-pY) and anti-paxillin monoclonal antibodies. The WCLs were subjected to Western blotting with anti-Hck and anti-Flag antibodies.
p50Cdc37 acts in concert with Hsp90 to rescue the catalytic activity of tsHck499F.
To test if Hsp90 function is required for the stabilization of tsHck499F by Flag-p50Cdc37, transfected 293T cells were treated for 4 h with the pharmacological Hsp90 inhibitor geldanamycin (45, 52). In this experiment Flag-p50Cdc37 was expressed to different extents, the lower level of Flag-p50Cdc37 expression being sufficient to maximally enhance the specific activity of tsHck499F (Fig. 6A). As shown in Fig. 6A, the catalytic activity of tsHck499F was totally inhibited by geldanamycin. Notably, overexpression of Flag-p50Cdc37 was unable to rescue the catalytic activity of tsHck499F (Fig. 6A). Western blotting of whole-cell lysates of the transfected 293T cells with an anti-Hck antibody revealed that geldanamycin had a detrimental effect (approximately threefold reduction) on the expression level of tsHck499F that was not alleviated by the overexpression of Flag-p50Cdc37 (Fig. 6A). Geldanamycin had no effect on the expression level of Flag-p50Cdc37, endogenous p50Cdc37, or Hsp90 (Fig. 6A).
FIG. 6.
Hsp90 function is required for tsHck499F activity. (A) 293T cells expressing tsHck499F and/or Flag-p50Cdc37 were treated for 4 h at 33°C with 2.5 μM geldanamycin and then lysed with NP-40 lysis buffer. The whole-cell lysates (WCLs) were subjected to Western blotting with anti-phosphotyrosine (α-pY), anti-Hck, anti-Flag, and anti-Hsp90 antibodies. (B) 293T cells expressing tsHck499F alone or together with either Hsp90 or Flag-p50Cdc37, or both Hsp90 and Flag-p50Cdc37, were incubated at 33°C for 48 h and then lysed with NP-40 lysis buffer. The WCLs were then subjected to Western blotting with anti-pY, anti-Hck, anti-Hsp90, and anti-Flag antibodies.
In view of these findings, we next sought to ascertain if overexpression of Hsp90 alone could suppress the temperature-sensitive properties of tsHck499F. Although transfected Hsp90 was expressed at levels approximately fivefold greater than those of endogenous Hsp90, no enhancement in the specific activity of tsHck499F was detected (Fig. 6B). Additionally, the simultaneous overexpression of both Hsp90 and Flag-p50Cdc37 did not enhance the specific activity of tsHck499F above that seen upon overexpression of Flag-p50Cdc37 alone (Fig. 6B). Overexpression of Hsp90 had no effect on the expression level of Flag-p50Cdc37 (Fig. 6B) or endogenous p50Cdc37 (data not shown).
p50Cdc37 promotes the association of Hsp90 with tsHck499F.
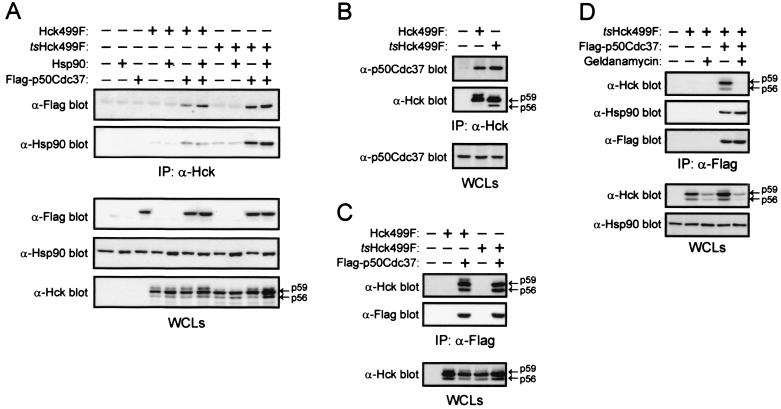
Given that p50Cdc37 has previously been shown to promote the association of Cdk4 and Raf with Hsp90 (12, 46), we were curious to determine if Flag-p50Cdc37 may stabilize the catalytic activity of tsHck499F by promoting its association with Hsp90. Western blotting of anti-Hck immunoprecipitates derived from whole-cell lysates of transfected cells with an anti-Flag monoclonal antibody revealed that the levels of Flag-p50Cdc37 associated with tsHck499F are slightly higher (1.7-fold) than those associated with Hck499F (Fig. 7A). The faint band seen in all 11 lanes of the anti-Flag Western blot shown in Fig. 7A is the heavy chain of the rat anti-Hck monoclonal antibody used in the immunoprecipitation reactions. No significant difference between the levels of endogenous p50Cdc37 associated with Hck499F and tsHck499F was observed (Fig. 7B). Likewise, in reciprocal immunoprecipitation reactions, both Hck499F and tsHck499F associated with Flag-p50Cdc37 to similar extents (Fig. 7C). The association of Flag-p50Cdc37 (or endogenous p50Cdc37) with both Hck499F and tsHck499F appears to be specific, since no Flag-p50Cdc37 (or p50Cdc37) was detected in anti-Hck immunoprecipitates derived from cells that had been transfected with the Flag-p50Cdc37 expression vector (or empty vector) alone (Fig. 7A and B). Similarly, neither Hck499F nor tsHck499F was detected in anti-Flag immunoprecipitates derived from cells transfected with the Hck499F or tsHck499F expression vector alone (Fig. 7C).
FIG. 7.
p50Cdc37 enhances the association of endogenous Hsp90 with tsHck499F. 293T cells expressing tsHck499F alone or together with either Hsp90 or Flag-p50Cdc37, or both Hsp90 and Flag-p50Cdc37, were incubated at 33°C for 24 h and then lysed with p50Cdc37 lysis buffer. (A) Anti-Hck immunoprecipitates (IP) derived from aliquots of whole-cell lysates (WCLs) were subjected to Western blotting with anti-Flag and anti-Hsp90 antibodies. The WCLs were subjected to Western blotting with anti-Flag, anti-Hsp90, and anti-Hck antibodies. (B) Anti-Hck immunoprecipitates derived from aliquots of the WCLs were subjected to Western blotting with anti-p50Cdc37 and anti-Hck antibodies. The WCLs were subjected to Western blotting with anti-p50Cdc37 antibodies. (C) Anti-Flag immunoprecipitates derived from aliquots of the WCLs were subjected to Western blotting with anti-Hck and anti-Flag antibodies. The WCLs were subjected to Western blotting with anti-Hck antibodies. (D) Transfected 293T cells were treated for 4 h at 33°C with 2.5 μM geldanamycin and then lysed with p50Cdc37 lysis buffer. Anti-Flag immunoprecipitates derived from aliquots of the WCLs were subjected to Western blotting with anti-Hck, anti-Hsp90, and anti-Flag antibodies. The WCLs were subjected to Western blotting with anti-Hck and anti-Hsp90 antibodies.
Western blotting of the anti-Hck immunoprecipitates in Fig. 7A revealed low levels of endogenous Hsp90 associated with Hck499F in 293T cells; slightly higher levels (1.5-fold) were found to be associated with tsHck499F (Fig. 7A). Notably, in comparison to that associated with Hck499F, the level of Hsp90 associated with tsHck499F was markedly enhanced (7.5-fold versus 2.5-fold) by the overexpression of Flag-p50Cdc37 (Fig. 7A). Significantly, no increase in the association of Hsp90 with either Hck499F or tsHck499F was observed upon overexpression of Hsp90 (Fig. 7A). Furthermore, simultaneous overexpression of both Hsp90 and Flag-p50Cdc37 did not increase the association of Hsp90 with tsHck499F above that seen upon overexpression of Flag-p50Cdc37 alone (Fig. 7A). Interestingly, geldanamycin completely inhibited the coimmunoprecipitation of tsHck499F with Flag-p50Cdc37, whereas the coimmunoprecipitation of endogenous Hsp90 with Flag-p50Cdc37 was unaffected (Fig. 7D).
To investigate whether the kinase domain of tsHck499F (and Hck499F) mediates its association with p50Cdc37, an Hck truncation mutant lacking a kinase domain was coexpressed with Flag-p50Cdc37 in 293T cells. Although the truncation mutant was expressed at levels equivalent to those of tsHck499F, coimmunoprecipitation with Flag-p50Cdc37 was not observed (data not shown). Such a finding is consistent with the notion that the kinase domain of tsHck499F mediates its association with p50Cdc37.
Overexpression of p50Cdc37ΔCT partially rescues the catalytic activity of tsHck499F.
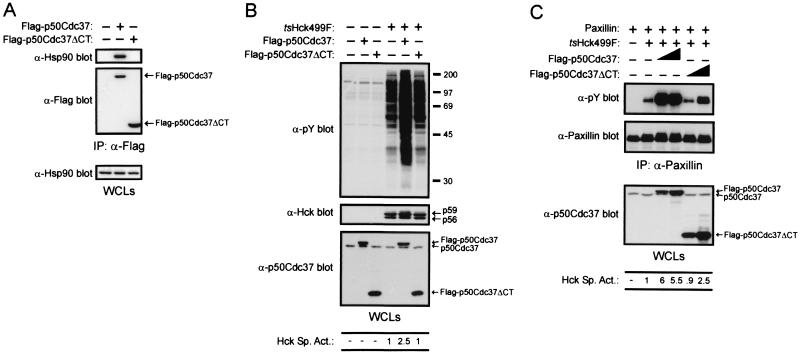
Grammatikakis et al. (12) have recently reported that deletion of the carboxy-terminal half of p50Cdc37 yields a protein (p50Cdc37ΔCT) that is unable to bind Hsp90 but can act in a dominant-negative fashion, with respect to endogenous p50Cdc37, to perturb the recruitment of Hsp90 to Raf. We have likewise found that when expressed in 293T cells, Flag-p50Cdc37ΔCT is unable to bind Hsp90 (Fig. 8A). If Flag-p50Cdc37 enhances the catalytic activity of tsHck499F by simply recruiting Hsp90 to the kinase, it would be expected that coexpression of p50Cdc37ΔCT with tsHck499F would have a detrimental effect on its kinase activity. Although Flag-p50Cdc37ΔCT was expressed at levels at least 20-fold higher than that of endogenous p50Cdc37, it had no discernibly deleterious effect on the specific activity of tsHck499F (Fig. 8B and C). In fact, an increase (2.5-fold) in the specific activity of tsHck499F was observed when the truncation mutant was expressed at levels approximately 100-fold greater than that of endogenous p50Cdc37 (Fig. 8C, last lane). Even when expressed at this level, the Flag-p50Cdc37ΔCT mutant was still less effective (2.5- versus 6-fold) than its full-length counterpart in enhancing the specific activity of tsHck499F (Fig. 8C). It has only been possible to express a mutant form of Flag-p50Cdc37 lacking the amino-terminal half of the protein (i.e., Flag-p50Cdc37ΔNT) at levels ≤5% of that for full-length Flag-p50Cdc37 (data not shown). Accordingly, we have not attempted to ascertain what effect, if any, overexpression of this mutant has on the activity of tsHck499F.
FIG. 8.
p50Cdc37ΔCT partially enhances the catalytic activity of tsHck499F. (A) 293T cells expressing either Flag-p50Cdc37 or Flag-p50Cdc37ΔCT were incubated at 33°C for 48 h and then lysed with p50Cdc37 lysis buffer. Anti-Flag immunoprecipitates (IP) derived from whole-cell lysates (WCLs) were subjected to Western blotting with anti-Hsp90 and anti-Flag antibodies. The WCLs were subjected to Western blotting with an anti-Hsp90 antibody. (B) 293T cells expressing tsHck499F alone or tsHck499F together with either Flag-p50Cdc37 or Flag-p50Cdc37ΔCT were incubated at 33°C for 48 h and then lysed with NP-40 lysis buffer. WCLs were subjected to Western blotting with anti-phosphotyrosine (α-pY), anti-Hck, and anti-p50Cdc37 antibodies. The positions of Flag-p50Cdc37, Flag-p50Cdc37ΔCT, and endogenous p50Cdc37 are shown on the right. (C) 293T cells transiently expressing paxillin either alone or together with tsHck499F and Flag-p50Cdc37, or tsHck499F and Flag-p50Cdc37ΔCT, were incubated at 39°C for 48 h and then lysed with NP-40 lysis buffer. Paxillin was immunoprecipitated from aliquots of the WCLs and subjected to Western blotting with anti-pY and anti-paxillin monoclonal antibodies. The WCLs were subjected to Western blotting with anti-p50Cdc37 antibodies.
p50Cdc37ΔCT has a limited capacity to promote the association of Hsp90 with tsHck499F.
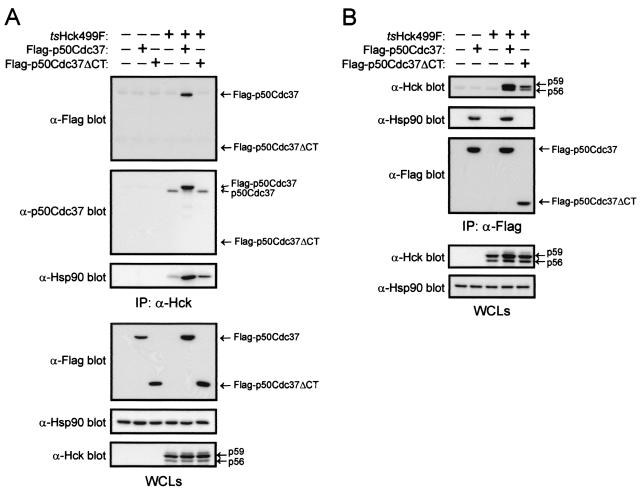
As a result of the above findings, we wanted firstly to establish if Flag-p50Cdc37ΔCT is able to associate with tsHck499F in 293T cells and secondly to determine if it can influence the association of endogenous p50Cdc37 and Hsp90 with tsHck499F. We were unable to detect the presence of Flag-p50Cdc37ΔCT in anti-Hck immunoprecipitates derived from cells expressing both tsHck499F and Flag-p50Cdc37ΔCT (Fig. 9A). However, tsHck499F was detected in anti-Flag immunoprecipitates of the same whole-cell lysates, although the association of tsHck499F with Flag-p50Cdc37ΔCT was approximately threefold less than that observed with full-length Flag-p50Cdc37 (Fig. 9B). These findings suggest that the affinity of the interaction between tsHck499F and Flag-p50Cdc37ΔCT is considerably lower than that of the interaction between tsHck499F and full-length Flag-p50Cdc37. To determine if overexpression of Flag-p50Cdc37 and Flag-p50Cdc37ΔCT impacts on the association of endogenous p50Cdc37 with tsHck499F, the anti-Hck immunoprecipitates were Western blotted with an anti-p50Cdc37 polyclonal antibody. Significantly, overexpression of full-length Flag-p50Cdc37, but not of Flag-p50Cdc37ΔCT, dramatically reduced (ninefold) the level of endogenous p50Cdc37 associated with tsHck499F (Fig. 9A). This finding indicates that unlike Flag-p50Cdc37ΔCT, Flag-p50Cdc37 can effectively compete with endogenous p50Cdc37 for binding to tsHck499F. The anti-Hck immunoprecipitates were also subjected to Western blotting with an anti-Hsp90 polyclonal antibody to establish if overexpression of Flag-p50Cdc37 or Flag-p50Cdc37ΔCT affects the association of endogenous Hsp90 with tsHck499F. As shown in Fig. 9A, overexpression of full-length Flag-p50Cdc37 markedly enhanced (eightfold) the association of Hsp90 with tsHck499F. Intriguingly, overexpression of Flag-p50Cdc37ΔCT was also found to enhance (approximately 2.5-fold) the association of endogenous Hsp90 with tsHck499F (Fig. 9A).
FIG. 9.
p50Cdc37ΔCT enhances the association of Hsp90 with tsHck499F. 293T cells expressing tsHck499F alone or together with Flag-p50Cdc37 or Flag-p50Cdc37ΔCT were incubated at 33°C for 48 h and then lysed with p50Cdc37 lysis buffer. (A) Anti-Hck immunoprecipitates (IP) derived from aliquots of the whole-cell lysates (WCLs) were subjected to Western blotting with anti-Flag, anti-p50Cdc37, and anti-Hsp90 antibodies. The WCLs were subjected to Western blotting with anti-Flag, anti-Hsp90, and anti-Hck antibodies. (B) Anti-Flag immunoprecipitates derived from the WCLs were subjected to Western blotting with anti-Hck, anti-Hsp90, and anti-Flag antibodies. The WCLs were subjected to Western blotting with anti-Hck and anti-Hsp90 antibodies.
DISCUSSION
We have succeeded in generating a temperature-sensitive mutant of a constitutively active form of Hck by introducing into its kinase domain two mutations (isoleucine-433 to methionine and proline-475 to serine) that have previously been shown to be sufficient to bestow upon v-Src a temperature-sensitive phenotype (10, 29). The crystal structure of Hck (40, 43) reveals that both isoleucine-433 and proline-475 are situated in loop regions with isoleucine-433 located between α-helices F and G and proline-475 between α-helices H and I (Fig. 10A). These two amino acids are situated on opposite faces of the bottom lobe of the kinase domain, with a C-alpha distance of more than 23 Å between the two residues. Significantly, these residues are situated away from the active site of the enzyme; the side chain of isoleucine-433 is at least 9 Å from the γ-phosphate of the ATP analog in the Hck crystal structure (40, 43). Furthermore, neither of these residues is in contact with the activation loop or in close proximity to the SH2 and SH3 domains.
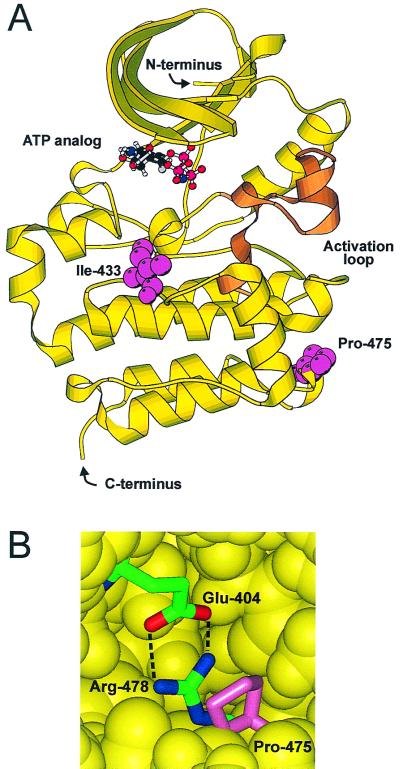
FIG. 10.
Structure of the kinase domain of Hck. (A) Schematic of the kinase domain of human Hck (40, 43), showing the activation loop (brown), ATP analog, and Ile-433 and Pro-475 (pink) (prepared using MOLSCRIPT [25]). (B) Schematic showing the salt bridge formed between Glu-404 (red) and Arg-478 (blue) in Hck. Pro-475 is shown in pink. The surrounding protein is represented by a yellow van der Waals surface.
Mutation of isoleucine-433 to methionine alone makes only a minor contribution to the temperature-sensitive properties of tsHck499F (data not shown). The absence of the C beta-methyl group as a result of the isoleucine (or valine)-to-methionine mutation leaves a small hydrophobic pocket that would cause a slight disruption to the tight hydrophobic packing within this region. The presence of this pocket would also allow some increased side chain movement in response to an increase in temperature, potentially contributing to the temperature-sensitive phenotype of tsHck499F. The role of proline-475 in the Hck structure, on the other hand, is twofold. Firstly, it stabilizes the structurally conserved loop between the H and I helices. Secondly, it caps the salt bridge formed between the conserved glutamic acid residue (Glu-404) within the alanine-proline-glutamic acid (APE) motif and the conserved arginine residue (Arg-478) within the proline-glutamic acid-glutamic acid-arginine (PEER) motif (Fig. 10B). The Hck structure has the proline ring packed against the side chain of the arginine residue, effectively holding it in the position required for the buried salt bridge. Replacement of the proline residue with serine is likely to increase the accessibility of this region to solvents. The associated increase in the local dielectric constant in the region surrounding the glutamic acid-arginine salt bridge would decrease the strength of this interaction. The combined effect of these two mutations, situated on opposite faces of the kinase domain, is thus to act in concert to destabilize the structure of the C-terminal lobe of the kinase domain at elevated temperatures, and hence to reduce the catalytic activity of tsHck499F.
Sequence alignment of all nine Src family kinases reveals that the isoleucine-433 in Hck is conserved in Lyn and Lck, whereas a valine residue is found in an equivalent position in Fyn, Yes, Fgr, Blk, Yrk, and Src (14). The proline-475 in Hck is conserved among all Src family kinases, with the exception of Lyn, where an alanine is found in this position (14). Interestingly, Hurley et al. (21) have created a temperature-sensitive mutant of an activated form of Lck by mutating the equivalent isoleucine and proline residues within its kinase domain. Thus, on the basis of both our findings and those of Hurley et al. (21), it seems likely that temperature-sensitive mutants of all Src family kinases could be made similarly. Given that Lyn contains an alanine residue in a position equivalent to proline-475 in Hck, it will be interesting to determine if mutation of the isoleucine residue alone is sufficient to convert Lyn into a temperature-sensitive kinase, or if wild-type Lyn is intrinsically more temperature sensitive than other Src family kinases.
We have reasoned that a temperature-sensitive mutant of Hck499F might be more dependent on p50Cdc37 function for its catalytic activity than Hck499F and thus may provide a unique opportunity to explore the functional role of p50Cdc37 in the folding and stabilization of Src family kinases in mammalian cells. Since no pharmacological inhibitors of p50Cdc37 have been described, the dependence of tsHck499F on p50Cdc37 function for catalytic activity was evaluated by coexpressing the kinase with an epitope-tagged form of p50Cdc37 (i.e., Flag-p50Cdc37) in 293T cells. Significantly, increasing the expression level of Flag-p50Cdc37 resulted in a corresponding increase in the catalytic activity of tsHck499F. The higher catalytic activity is primarily a consequence of an increase in the specific activity of tsHck499F, although a modest increase (up to 1.5-fold) in the expression of tsHck499F also contributes to the higher kinase activity. Hence p50Cdc37 appears to be capable of maintaining the kinase domain of tsHck499F in a relatively stable and catalytically active conformation, thereby buffering, to some extent, the detrimental effect of increasing temperature on the structure of tsHck499F. Additionally, p50Cdc37 can act posttranslationally to promote the refolding of mature but inactive tsHck499F into a catalytically active conformation. This conclusion, however, does not exclude the possibility that p50Cdc37 may also act cotranslationally to enhance the correct folding of nascent tsHck499F. Indeed, previous studies investigating the association of v-Src with Hsp90 and p50Cdc37 during cellular transformation established that newly synthesized, rather than mature, molecules of v-Src are in complex with Hsp90 and p50Cdc37 (3, 4). Notably, Farrell and Morgan (9) have recently reported that loss of Cdc37p function during but not after translation results in the destabilization of the serine/threonine kinases Cdc28 and Cak1 in S. cerevisiae.
Of direct relevance to our findings is a recent report by Matsuda et al. (28) describing the ability of p50Cdc37 to restore the expression of a temperature-sensitive kinase domain mutant of the tyrosine kinase ZAP70. Although Matsuda et al. (28) did not indicate if the recovered ZAP70 protein was catalytically active, the findings nonetheless underscore the importance of p50Cdc37 for protein kinases to achieve and maintain a stable conformation. The stabilization of the temperature-sensitive mutants of ZAP70 and Hck499F by the overexpression of p50Cdc37 suggests that the level of endogenous p50Cdc37 in cells might be a rate-limiting factor in the folding of these mutant kinases into stable and catalytically active conformations. The fact that the level of endogenous p50Cdc37 in 293T cells does not increase in response to the overexpression of tsHck499F raises the intriguing possibility that under some circumstances p50Cdc37 may also represent the rate-limiting factor in the folding of nonmutant forms of protein kinases into catalytically active conformations. If true, this would provide another mechanism, in addition to subunit association, phosphorylation, and degradation, by which the cell could potentially regulate the activity of protein kinases.
Precisely how p50Cdc37 promotes the folding of the kinase domain of tsHck499F into a catalytically active conformation is still to be determined. Stepanova et al. (46) and Grammatikakis et al. (12) have proposed that p50Cdc37 stabilizes Cdk4 and Raf, respectively, by acting as a kinase-targeting subunit of Hsp90 to promote their association with Hsp90. Significantly, we have found that overexpression of p50Cdc37 markedly enhances the association of endogenous Hsp90 with tsHck499F. Thus, the stabilization of tsHck499F by p50Cdc37 is likely to be a consequence of p50Cdc37 promoting the association of tsHck499F with Hsp90. Indeed, Hsp90 function is required for the stabilization of tsHck499F activity by p50Cdc37, although overexpression of Hsp90 alone is not sufficient to enhance the catalytic activity of tsHck499F or to lead to higher levels of Hsp90 being associated with tsHck499F. Consequently, the recruitment of tsHck499F to Hsp90 by p50Cdc37 might represent the rate-limiting step in the folding of tsHck499F into a catalytically active conformation.
It is worth noting, however, that Kimura et al. (23) have previously reported that the overexpression of Cdc37p can rescue the catalytic activity of v-Src in a strain of S. cerevisiae with compromised Hsp90 activity, suggesting that Cdc37p can act independently of Hsp90 to promote the folding of v-Src. Although this finding initially seems at odds with our own observations, the two findings can be reconciled. The sole source of Hsp90 activity in the strain of S. cerevisiae employed by Kimura et al. (23) is provided by the temperature-sensitive Hsp90 mutant Hsp82G170D. This Hsp90 mutant exhibits almost wild-type activity when the cells are grown at 25°C but displays significantly reduced activity at 34°C (34). Nonetheless, this reduced level of Hsp90 activity at 34°C could be sufficient to facilitate the folding of v-Src under circumstances where the overexpression of Cdc37p increases the efficiency with which v-Src is targeted to Hsp90.
To investigate the mechanism underlying the recruitment of Hsp90 to tsHck499F, we coexpressed tsHck499F with p50Cdc37ΔCT. This mutant of p50Cdc37 has previously been shown by Grammatikakis et al. (12) to bind Raf but not Hsp90. Moreover, the fact that this mutant could act in a dominant-negative fashion to perturb the recruitment of Hsp90 to Raf led Grammatikakis et al. (12) to propose that p50Cdc37 promotes the association of Hsp90 with Raf by acting as an adapter protein to physically tether Hsp90 to Raf. We have found that expression of p50Cdc37ΔCT at levels approximately 20-fold greater than that of endogenous p50Cdc37 has no discernible detrimental effect on the catalytic activity of tsHck499F. The failure of p50Cdc37ΔCT to perturb the catalytic competence of tsHck499F could potentially be explained by the fact that its ability to bind tsHck499F is considerably less than that of full-length p50Cdc37, and thus it is unable to effectively compete with endogenous p50Cdc37 for binding tsHck499F. Intriguingly, though, the catalytic activity of tsHck499F is enhanced when the truncation mutant is expressed at sufficiently high levels (e.g., 100-fold above that of endogenous p50Cdc37). Additionally, overexpression of p50Cdc37ΔCT promotes, rather than perturbs, the association of endogenous Hsp90 with tsHck499F, albeit not to the same extent as full-length p50Cdc37. Thus, even though p50Cdc37ΔCT has a diminished capacity to bind tsHck499F and is unable to bind Hsp90, it still has a limited ability to promote the association of tsHck499F with Hsp90. This is a particularly important finding, since it argues that rather than solely acting as a passive adapter protein to tether Hsp90 to tsHck499F, p50Cdc37 may also act allosterically to increase the affinity of tsHck499F for Hsp90. It will be important to establish if p50Cdc37ΔCT perturbs or promotes the association of Hsp90 with other protein kinases. It is worth noting that yeast Cdc37p has been shown to possess a chaperone-like activity toward some proteins (e.g., β-galactosidase and firefly luciferase) (23) and that the region of greatest sequence conservation between yeast Cdc37p and mammalian p50Cdc37 is found within the amino-terminal portions of the proteins (20). Establishing if mammalian p50Cdc37 also possesses chaperone-like activity and, if so, if this activity resides within either the amino- or carboxy-terminal half of the protein should shed further light on how p50Cdc37 promotes the association of Hsp90 with client protein kinases.
Intriguingly, the pharmacological Hsp90 inhibitor geldanamycin disrupts the association of tsHck499F with p50Cdc37 but not that between Hsp90 and p50Cdc37. Geldanamycin inhibits Hsp90 by competitively binding to its nucleotide-binding site, thereby locking Hsp90 into an inactive conformation (13, 17, 35, 36, 45, 48). Thus, the ability of geldanamycin to inhibit the association of tsHck499F with p50Cdc37 implies that the protein kinase binding activity of p50Cdc37 is regulated, at least in part, by the conformational status of Hsp90. Consequently, p50Cdc37 and Hsp90 might interdependently regulate the stable formation of a heterotrimeric complex consisting of the client protein kinase (e.g., tsHck499F), p50Cdc37, and Hsp90.
The findings presented here may also have important implications for our understanding of the evolution of protein kinases. An elegant genetic study by Rutherford and Lindquist (39) has revealed that although widespread genetic variations affecting morphogenic pathways exist in Drosophila, they are usually silent as a result of buffering by Hsp90. However, when Hsp90 function is compromised (e.g., following heat- or chemical-induced protein damage), these cryptic genetic variations can become expressed and potentially subjected to the forces of natural selection, leading Rutherford and Lindquist to propose that Hsp90 might act as a capacitor for morphological evolution (39). In view of the intimate relationship between Hsp90 and p50Cdc37, it is feasible that under some circumstances p50Cdc37 may act as a capacitor for the evolution of protein kinases. Our demonstration that p50Cdc37 can buffer, to some extent, the deleterious effect of mutations on the catalytic activity of Hck499F provides support for this proposal.
This ability of p50Cdc37 to buffer the detrimental effect of mutations on protein kinases could potentially influence the proliferation and survival of cells, particularly tumor cells. Since tumor cells have a higher mutation rate than normal somatic cells (22), it would be expected that mutations in protein kinase-encoding genes would occur at an accelerated frequency in tumor cells. In the main, these mutations would be expected to compromise the activity of the protein kinase, and possibly the growth and survival of the tumor cell. However, overexpression of endogenous p50Cdc37 (e.g., as a consequence of amplification or rearrangement of the CDC37 gene) might buffer the harmful effect of these mutations on protein kinases and thus support protein kinase-dependent tumor cell growth. Overexpression of p50Cdc37 may also mask changes in other properties of protein kinases, including regulation and substrate specificity. These altered properties could become revealed if p50Cdc37 function were to be compromised subsequently (e.g., by mutations or deletions in the CDC37 gene) and potentially bestow upon the tumor cell a growth or survival advantage over other cells. Thus, p50Cdc37 may represent an attractive target for the development of new antitumor drugs.
ACKNOWLEDGMENTS
We thank Margaret Hibbs, Douglas Hilton, Clifford Lowell, Hisataka Sabe, David Toft, and Stephen Ullrich for gifts of various reagents. We also thank Antony Burgess for critical comments on the manuscript.
This work was supported in part by grants from the National Health and Medical Research Council of Australia (to G.S. and A.R.D.), the Oklahoma Center for the Advancement of Science and Technology (HN6-018, to S.D.H.), the National Institutes for Health (GM51608, to R.L.M.), and the Oklahoma Agricultural Experiment Station (project 1975, to R.L.M.).
REFERENCES
- 1.Boussif O, Lezoualc'h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown M T, Cooper J A. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 3.Brugge J, Yonemoto W, Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983;3:9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- 5.Chiaradonna F, Fontana L, Iavarone C, Carriero M V, Scholz G, Barone M V, Stoppelli M P. Urokinase receptor-dependent and -independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. EMBO J. 1999;18:3013–3023. doi: 10.1093/emboj/18.11.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutforth T, Rubin G M. Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell. 1994;77:1027–1036. doi: 10.1016/0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 7.Dey B, Lightbody J J, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst M, Gearing D P, Dunn A R. Functional and biochemical association of Hck with the LIF/IL-6 receptor signal transducing subunit gp130 in embryonic stem cells. EMBO J. 1994;13:1574–1584. doi: 10.1002/j.1460-2075.1994.tb06420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrell A, Morgan D O. Cdc37 promotes the stability of protein kinases cdc28 and cak1. Mol Cell Biol. 2000;20:749–754. doi: 10.1128/mcb.20.3.749-754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber E A, Mayer B J, Jove R, Hanafusa H. Analysis of p60v-src mutants carrying lesions involved in temperature sensitivity. J Virol. 1987;61:354–360. doi: 10.1128/jvi.61.2.354-360.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber M R, Farrell A, Deshaies R J, Herskowitz I, Morgan D O. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grammatikakis N, Lin J H, Grammatikakis A, Tsichlis P N, Cochran B H. p50Cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grenert J P, Sullivan W P, Fadden P, Haystead T A J, Clark J, Mimnaugh E, Krutzsch H, Ochel H J, Schulte T W, Sausville E, Neckers L M, Toft D O. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J Biol Chem. 1997;272:23843–23850. doi: 10.1074/jbc.272.38.23843. [DOI] [PubMed] [Google Scholar]
- 14.Hanks S K, Quinn A M, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 15.Hartson S D, Barrett D J, Burn P, Matts R L. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry. 1996;35:13451–13459. doi: 10.1021/bi961332c. [DOI] [PubMed] [Google Scholar]
- 16.Hartson S D, Ottinger E A, Huang W, Barany G, Burn P, Matts R L. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J Biol Chem. 1998;273:8475–8482. doi: 10.1074/jbc.273.14.8475. [DOI] [PubMed] [Google Scholar]
- 17.Hartson S D, Thulasiraman V, Huang W, Whitesell L, Matts R L. Molybdate inhibits hsp90, induces structural changes in its C-terminal domain, and alters its interactions with substrates. Biochemistry. 1999;38:3837–3849. doi: 10.1021/bi983027s. [DOI] [PubMed] [Google Scholar]
- 18.Hibbs M L, Tarlinton D M, Armes J, Grail D, Hodgson G, Maglitto R, Stacker S A, Dunn A R. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 19.Holtzman D A, Cook W D, Dunn A R. Isolation and sequence of a cDNA corresponding to a src-related gene expressed in murine hemopoietic cells. Proc Natl Acad Sci USA. 1987;84:8325–8329. doi: 10.1073/pnas.84.23.8325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter T, Poon R Y C. Cdc37: a protein kinase chaperone? Trends Cell Biol. 2000;7:157–161. doi: 10.1016/S0962-8924(97)01027-1. [DOI] [PubMed] [Google Scholar]
- 21.Hurley T R, Amrein K E, Sefton B M. Creation and characterization of temperature-sensitive mutants of the lck tyrosine protein kinase. J Virol. 1992;66:7406–7413. doi: 10.1128/jvi.66.12.7406-7413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson A L, Loeb L A. On the origin of multiple mutations in human cancers. Semin Cancer Biol. 1998;8:421–429. doi: 10.1006/scbi.1998.0113. [DOI] [PubMed] [Google Scholar]
- 23.Kimura Y, Rutherford S L, Miyata Y, Yahara I, Freeman B C, Yue L, Morimoto R I, Lindquist S. Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev. 1997;11:1775–1785. doi: 10.1101/gad.11.14.1775. [DOI] [PubMed] [Google Scholar]
- 24.Kmiecik T E, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell. 1987;49:65–73. doi: 10.1016/0092-8674(87)90756-2. [DOI] [PubMed] [Google Scholar]
- 25.Kraulis P J. A program to produce both detailed and schematic plots of protein structures. J Appl Crystallogr. 1991;24:946–950. [Google Scholar]
- 26.Lock P, Ralph S, Stanley E, Boulet I, Ramsay R, Dunn A R. Two isoforms of murine hck, generated by utilization of alternative translational initiation codons, exhibit different patterns of subcellular localization. Mol Cell Biol. 1991;11:4363–4370. doi: 10.1128/mcb.11.9.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowell C A, Soriano P. Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 28.Matsuda S, Suzuki-Fujimoto T, Minowa A, Ueno H, Katamura K, Koyasu S. Temperature-sensitive ZAP70 mutants degrading through a proteasome-independent pathway. Restoration of a kinase domain mutant by Cdc37. J Biol Chem. 1999;274:34515–34518. doi: 10.1074/jbc.274.49.34515. [DOI] [PubMed] [Google Scholar]
- 29.Mayer B J, Jove R, Krane J F, Poirier F, Calothy G, Hanafusa H. Genetic lesions involved in temperature sensitivity of the src gene products of four Rous sarcoma virus mutants. J Virol. 1986;60:858–867. doi: 10.1128/jvi.60.3.858-867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazaki Y, Hashimoto S, Sabe H. Monocyte cells and cancer cells express novel paxillin isoforms with different binding properties to focal adhesion proteins. J Biol Chem. 1997;272:7437–7444. doi: 10.1074/jbc.272.11.7437. [DOI] [PubMed] [Google Scholar]
- 31.Meng F, Lowell C A. A beta 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina T J, Kishihara K, Siderovski D P, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige C J, Hartmann K U, Veillette A. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 34.Nathan D F, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaretou B, Prodromou C, Roe S M, O'Brien R, Ladbury J E, Piper P W, Pearl L H. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prodromou C, Roe S M, O'Brien R, Ladbury J E, Piper P W, Pearl L H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 37.Quintrell N, Lebo R, Varmus H, Bishop J M, Pettenati M J, Le Beau M M, Diaz M O, Rowley J D. Identification of a human gene (HCK) that encodes a protein-tyrosine kinase and is expressed in hemopoietic cells. Mol Cell Biol. 1987;7:2267–2275. doi: 10.1128/mcb.7.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed S I. The selection of S. cerevisiae mutants defective in the start event of cell division. Genetics. 1980;95:561–577. doi: 10.1093/genetics/95.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutherford S L, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 40.Schindler T, Sicheri F, Pico A, Gazit A, Levitzki A, Kuriyan J. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol Cell. 1999;3:639–648. doi: 10.1016/s1097-2765(00)80357-3. [DOI] [PubMed] [Google Scholar]
- 41.Scholz G, Cartledge K, Dunn A R. Hck enhances the adherence of lipopolysaccharide-stimulated macrophages via Cbl and phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:14615–14623. doi: 10.1074/jbc.275.19.14615. [DOI] [PubMed] [Google Scholar]
- 42.Schutz A R, Giddings T H, Jr, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sicheri F, Moarefi I, Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 44.Soriano P, Montogomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 45.Stebbins C E, Russo A A, Schneider C, Rosen N, Hartl F U, Pavletich N P. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 46.Stepanova L, Leng X, Parker S B, Harper J W. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 47.Suen P W, Ilic D, Caveggion E, Berton G, Damsky C H, Lowell C A. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J Cell Sci. 1999;112:4067–4078. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan W, Stensgard B, Caucutt G, Bartha B, McMahon N, Alnemri E S, Litwack G, Toft D. Nucleotides and two functional states of hsp90. J Biol Chem. 1997;272:8007–8012. doi: 10.1074/jbc.272.12.8007. [DOI] [PubMed] [Google Scholar]
- 49.Superti-Furga G, Courtneidge S A. Structure-function relationships in Src family and related protein tyrosine kinases. Bioessays. 1995;17:321–330. doi: 10.1002/bies.950170408. [DOI] [PubMed] [Google Scholar]
- 50.Ullrich S J, Robinson E A, Law L W, Willingham M, Appella E. A mouse tumor-specific transplantation antigen is a heat shock-related protein. Proc Natl Acad Sci USA. 1986;83:3121–3125. doi: 10.1073/pnas.83.10.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitelaw M L, Hutchison K, Perdew G H. A 50-kDa cytosolic protein complexed with the 90-kDa heat shock protein (hsp90) is the same protein complexed with pp60v-src hsp90 in cells transformed by the Rous sarcoma virus. J Biol Chem. 1991;266:16436–16440. [PubMed] [Google Scholar]
- 52.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu W, Doshi A, Lei M, Eck M J, Harrison S C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 54.Xu W, Harrison S C, Eck M J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 55.Xu Y, Lindquist S. Heat-shock protein hsp90 governs the activity of pp60v-src kinase. Proc Natl Acad Sci USA. 1993;90:7074–7078. doi: 10.1073/pnas.90.15.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y, Singer M A, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ziegler S F, Marth J D, Lewis D B, Perlmutter R M. Novel protein-tyrosine kinase gene (hck) preferentially expressed in cells of hematopoietic origin. Mol Cell Biol. 1987;7:2276–2285. doi: 10.1128/mcb.7.6.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]