Abstract
Polymers that can change their properties in response to an external or internal stimulus have become an interesting platform for drug delivery systems. Polymeric nanoparticles can be used to decrease the toxicity of drugs, improve the circulation of hydrophobic drugs, and increase a drug’s efficacy. Furthermore, polymers that are sensitive to specific stimuli can be used to achieve controlled release of drugs into specific areas of the body. This review discusses the different stimuli that can be used for controlled drug delivery based on internal and external stimuli. Internal stimuli have been defined as events that evoke changes in different characteristics, inside the body, such as changes in pH, redox potential, and temperature. External stimuli have been defined as the use of an external source such as light and ultrasound to implement such changes. Special attention has been paid to the particular chemical structures that need to be incorporated into polymers to achieve the desired stimuli response. A current trend in this field is the incorporation of several stimuli in a single polymer to achieve higher specificity. Therefore, to access the most recent advances in stimuli-responsive polymers, the focus of this review is to combine several stimuli. The combination of different stimuli is discussed along with the chemical structures that can produce it.
Keywords: stimuli-responsive, drug delivery, polymer particles
1. Introduction
Controlled release of drugs is a growing field with many challenges to overcome. Many drugs are hydrophobic, which limits their bioavailability. Other drugs, such as chemotherapy drugs, are very toxic and ideally should only be released once, at the tumor site. Polymeric nanoparticles have been extensively studied as a platform for specific and controlled drug delivery, and can potentially solve these problems. Polymeric nanoparticles for drug delivery have been proven to increase the circulation time, enhance drug accumulation at the tumor site in cancer therapies, reduce the side effects of drugs, and improve tolerance [1]. Biocompatibility and biodegradability are two other factors that make polymers so favorable [2]. Many polymers have been extensively used in the field of drug delivery [3,4]. The most commonly used biodegradable polymers are poly(lactic-co-glycolic) acid (PLGA) and poly (ε-caprolactone) (PCL). Whereas the most common non-biodegradable polymers are poly (methyl methacrylate) and polyacrylate [4].
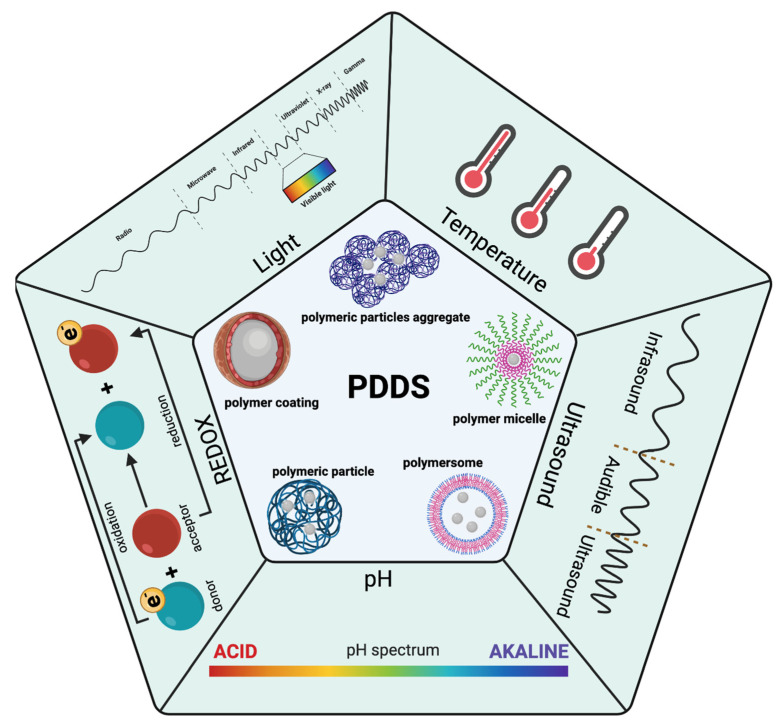
External or internal stimuli can trigger the controlled release of drugs. Internal stimuli can be considered thermal, pH, and redox potential, while external stimuli consist of light and ultrasound as represented in Figure 1. Moreover, dual-responsive polymers enable drug delivery methods and therapeutic efficacy to be fine-tuned. Previous reviews have investigated stimuli-responsive polymers and their applications in drug delivery; however, the field has been developing quickly and there have been many advances in recent years. Multiple stimuli polymers have emerged as the new trend to achieve finer control of the release of drugs and avoid side effects [5,6,7,8]. While there are reviews focusing on the use of stimuli-responsive polymers for targeting or imaging purposes [9,10,11,12,13], this review will specifically summarize the progress in stimuli-responsive polymers as particles for controlled drug release, with a focus on the recent advances in the field. As there are already several recent reviews discussing the role of hydrogels in drug delivery [14,15,16,17,18,19], including the use of nanogels as particles for drug delivery, we will not include hydrogel polymer particles in this discussion. Instead, we will discuss the attributes that make a polymer responsive to stimuli, how they are used as drug delivery particles for controlled drug release, and possible future uses.
Figure 1.
A schematic representation of stimuli that can trigger drug release using particle drug delivery systems (PDDS) based on polymers. Created in Biorender.com, accessed date (2 September 2022).
2. Single Stimuli
Stimuli-responsive polymer particles have become important in the field of drug delivery due to the potential for controlled release. Several stimuli can be used for this purpose. Table 1 presents a brief summary of the different stimuli that we will discuss in this review with some examples of the active parts needed within a polymer to achieve the desired stimuli response. Further discussion will be provided in the following sections for each stimulus.
Table 1.
Single stimuli-responsive polymers.
| Stimuli | Active Part | Examples | Ref. |
|---|---|---|---|
| pH | Cleavable bonds | Imine bond: HA-mPEG hyaluronic acid-methoxy Poly(ethylene-glycol) amine (Di)methyl maleate bond: PDLLA-PEG Poly(D,L-lactide)-Poly(ethylene-glycol) |
[20,21] |
| Redox potential | Disulfide bond | MPEG-P(BHD-SS)-MPEG Poly(ethylene-glycol)-b-polycarbonate-Poly(ethylene-glycol) |
[22] |
| Temperature | Lower critical solution (LCST) Upper critical solution (UCST) |
LCST: PNIPAM Poly-N-isopropylacrylamide UCST: iMAPA Insoluble multi-L-arginyl-poly-L-aspartic |
[23,24] |
| Light | Photo-triggered groups | Polydopamine | [25,26] |
| Ultrasound | Disulfide bond Particles aggregates |
PLA-S-S-PEG Poly(L-lactide)-S-S-Poly(ethylene-glycol) PLGA aggregates Poly(lactic-co-glycolic acid) |
[27,28] |
| Magnetism | Incorporation of magnetic particles | Iron nanoparticles | [29,30] |
| Shear stress | Flexible particles, generally hydrogels | ADEN/THYM polymersomes Adenine/thymine functionalized block co polymers |
[31] |
2.1. Internal Stimuli
2.1.1. pH-Responsive
It is well known that different parts of the body have different pH values, especially in the gastrointestinal tract (GI), where the pH gradient varies dramatically [32]. However, the pH gradient is not just limited to the GI tract; different pH’s exist inside the cell itself. For instance, lysosomes have a pH of 4.5–5, endosomes 5.5–6, Golgi apparatus 6.4, and cytosol 7.4 [33]. One of the most important differences in pH can be observed between tumors (pH 6.5–6.8) and normal tissue (pH 7.4) [34]. This change in pH is due to a phenomenon known as the Warburg effect [35,36]. In this phenomenon a discrepancy in pH between healthy tissue and cancerous tissue is observed due to the rapid proliferation of cancer cells which decreases the blood supply, limiting the supply of oxygen and nutrients. The limited oxygen decreases the process of phosphorylation by the cells, forcing cells to take energy from glycolysis producing lactic acid, thereby decreasing the pH of that area. Based on the Warburg effect many studies have focused on polymeric nanoparticles sensitive to pH [35,36,37,38,39].
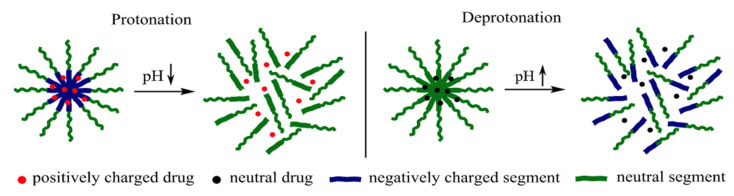
Drug-loaded polymeric nanoparticles with pH-sensitive functional groups can alter their density of charges in response to a variation in pH. This mechanism is based on the hydrophobicity of the nanoparticles as a result of protonation or deprotonation [40]. For example, co-polymer micelles can release a drug in response to pH changes as we can see in Figure 2 [41,42,43]. Palanikumar et al. synthesized polymeric nanoparticles with a functionalized membrane of acid-triggered peptide (ATRAM) [44]. ATRAM peptide has a pKa of 6.5, which gives it a high specificity for use in the acidic microenvironment of tumors.
Figure 2.
A schematic illustration of drug release from a polymer micelle. Protonation (left) or deprotonation (right) destroys the polymer micelle [43]. Reprinted from Saudi Pharmaceutical Journal, 28, M. Alsehli, Polymeric Nanocarriers as Stimuli-Responsive Systems for Targeted Tumor (Cancer) Therapy: Recent Advances in Drug Delivery, 255–265, Copyright (2020), with permission from Elsevier.
Another approach that can be used to make particles pH-responsive is the incorporation of cleavable bonds. The most important cleavable bonds are imine, hydrazone, hydrazide, oxime, and (di)methyl maleate. Table 2 shows a list of these cleavable bonds that can be incorporated into polymers with the relevant pH for drug delivery [45,46].
Table 2.
Cleavable pH-responsive bonds.
| Cleavable Bond | pH |
|---|---|
| Imine | <5–7 |
| Hydrazone | <5 |
| Hydrazide | <5 |
| Oxime | <5 |
| (di)Methyl maleate | <6.8 |
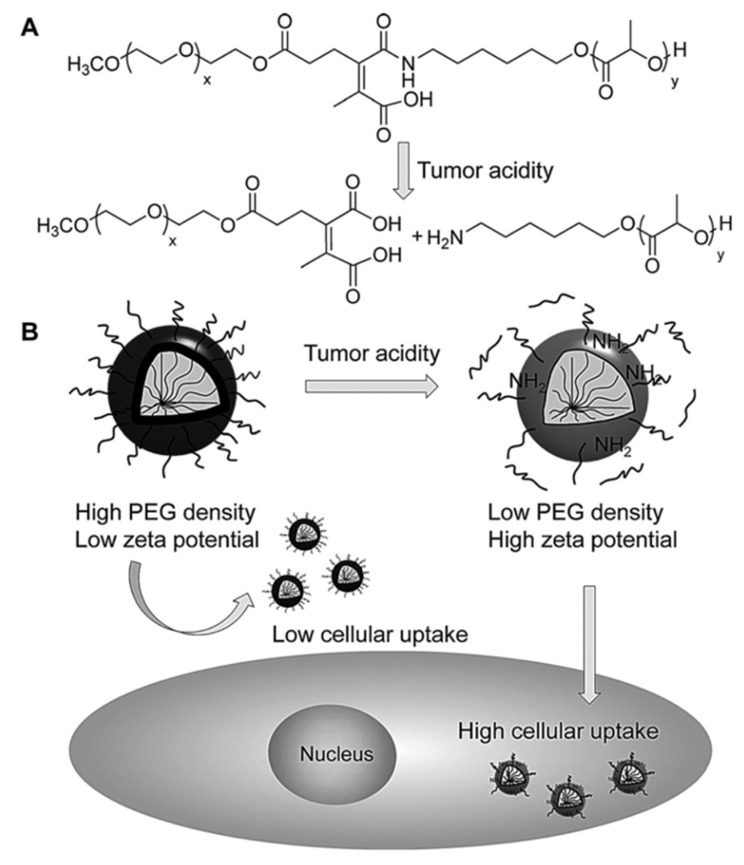
For instance, poly(L-histidine)-b-poly(ethylene glycol) (PH-PEG) combined with poly(L-lactic acid)-b-poly(ethylene glycol) (PLA-PEG) has been studied for tumor targeting [47]. The advantage of this system is the sharp transition between a stable and an unstable drug delivery system. It is non-ionized and hydrophobic at pH 7.4, but ionized and hydrophilic at pH 6.6 [48]. Zhang et al. produced a nano-carrier that is pH-responsive by using an imine bond [20]. In another example of pH-responsive polymers, Sun et al. synthesized polymeric nanoparticles of poly(D, L-lactide) (PDLLA) and poly(ethylene glycol) (PEG) which were linked by a (di)methyl maleate group [21]. In a weak acidic environment, the PEG dissolves, promoting endocytosis of the particles and the release of the drug [21]. The acidic pH at the tumor site triggers the cleavable bond, decreasing the PEG density and increasing the uptake of the particles by the cells (Figure 3).
Figure 3.
(A) Chemical structure and cleavable bond in acidic conditions. (B) A schematic illustration of the uptake mechanism of particles based on pH [21]. Reproduced with permission from C. Y. Sun et al., Angewandte Chemie—International Edition, published by John Wiley and Sons, Copyright 2016.
2.1.2. Redox Potential-Responsive
Drug delivery systems for cancer and gene therapy are advantageous when they degrade directly in the nucleus and the cytosol of the cell while maintaining their stability in the extracellular environment [49]. Many redox processes occur in the intracellular environment, such as NADP+/NADPH, O2/O2−, and glutathione (GSH) [50]. Specifically, GSH has attracted interest in the drug delivery field. GSH’s chemical name is γ-L-glutamyl-L-cysteinyl-glycine, and it is a peptide composed of glycine, cysteine, and L-glutamic acid [51]. GSH’s concentration is used in drug delivery due to the abrupt concentration change between the intracellular (1–10 mM) and the extracellular environment (1–10 μM) [52,53,54] (Table 3). Nevertheless, GSH concentration in tumor tissue has been found to be four-fold higher than healthy tissue in mice, making GSH level a good trigger for drug delivery systems [55,56]. However, the cancer environment changes between different types of cancer. For example, in brain tumors, GSH concentration has been found to be between 0.5–3 mM [57]. Gamcsik et al., categorized many different cancer tissues and the difference in GSH levels compared to healthy tissue [58]. However, due to the high variability between the studies, the numbers have not been included in Table 3. Nevertheless, there is a general trend towards using increased levels of GSH in cancer tissue as a trigger for drug delivery systems.
Table 3.
GSH level for different cellular environments.
| Environment | GSH Level |
|---|---|
| Intracellular | 1–10 mM |
| Extracellular | 1–10 μM |
| Brain Cancer | 0.5–3 mM |
The design of drug delivery systems sensitive to redox potential can be very versatile, and the use of polymers for these kinds of conformations is very popular [59]. One technique used to create degradable polymeric micelles involves using amphiphilic copolymers with a disulfide bond connecting the two blocks [60,61,62]. In a study by Sun et al., polymer micelles were used to deliver doxorubicin. Micelles were synthesized by using a graft copolymer of poly(acrylic acid)-g-poly(ethylene glycol) (PAA-g-PEG) which contains a disulfide bond [63]. By adding this disulfide bond micelles remained assembled until they found reductive conditions that could break the bond. Another approach for GSH-responsive particles is the incorporation of a GSH-responsive crosslinking agent in the core or the shell of the micelle [64]. The mechanism of how these polymeric micelles disassemble is based on the reduction of the disulfide bond in the polymer by the interaction with GSH, Figure 4 [65]. The micelle destabilization can shift the hydrophobic/hydrophilic balance promoting the fragmentation of the polymer into monomers, releasing the drug [66].
Figure 4.
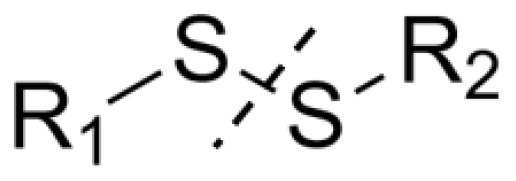
Disulfide bond responsive to redox potential (GSH).
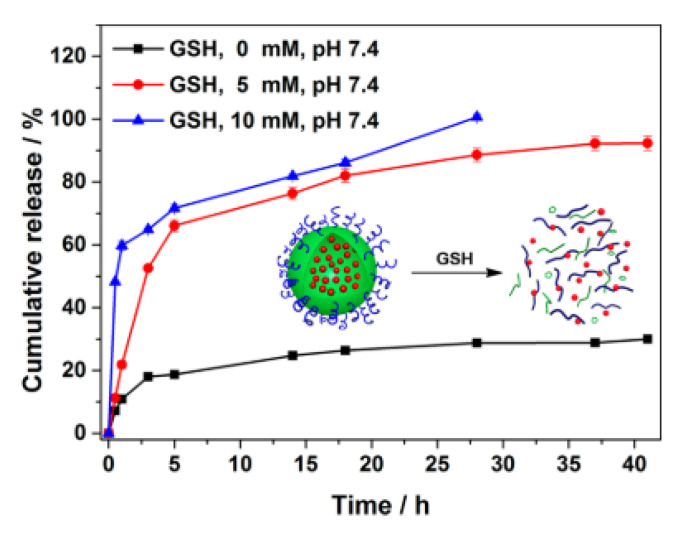
Xu et al. synthesized a triblock copolymer, with a disulfide bond [22]. Doxorubicin (an anticancer drug) was encapsulated in the polymeric system. The GSH concentration gradient was used as a delivery trigger to achieve specificity for cancer cells. By combining the enhanced permeability and retention effect (EPR) with the GSH gradient, particles delivered the drug to cancer cells. Figure 5 shows how the increase of GSH triggers the release of doxorubicin from the particles. The highest concentration of GSH achieved the fastest release of the drug.
Figure 5.
Doxorubicin release from polymeric particles at different concentrations of GSH, at the same temperature [22]. Reprinted with permission from Xu et al. ACS Biomaterials Science and Engineering 2015, 1 (7), 585–592. Copyright 2015 American Chemical Society.
2.1.3. Thermo-Responsive
Temperature is one of the most investigated triggers for stimuli-responsive drug delivery systems. The temperature stimulus can be internal or external. Several studies have highlighted an increase in temperature at pathological sites and tumors because of the abnormal blood flow, a high rate of cell proliferation, and metabolic activity. These temperature differences between healthy and tumorous tissue can be used as a trigger for drug delivery systems [47]. External temperature can also be applied to activate the delivery of a drug. For instance, hyperthermia can be used as a cancer treatment where the temperature increases to 45 °C at the tumor site, damaging and killing cancer cells [67].
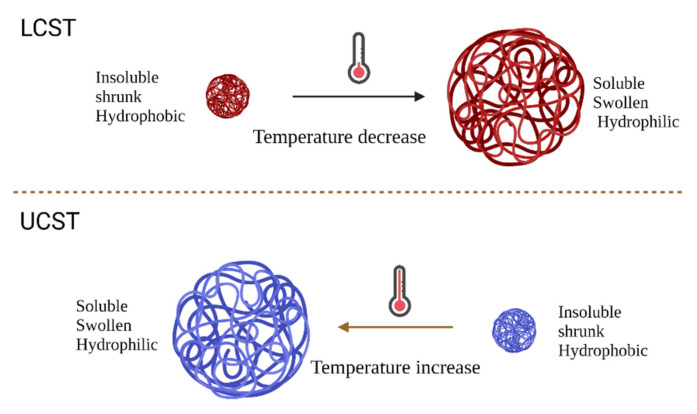
Many different materials can be used that are temperature-responsive. However, temperature-sensitive polymers are one of the most well-known materials. These polymers change their structure from a shrunken to a swollen form, in response to temperature change. The characterization of these polymers is made by the upper critical solution temperature (UCST) or the lower critical solution temperature (LCST) [59,68,69,70]. The change in the polymer conformation is activated by reaching one of those temperatures, leading to either swelling or shrinking as shown in Figure 6.
Figure 6.
A schematic representation of LCST and UCST concepts and the polymer properties. Created in Biorender.com, accessed date (9 February 2022).
The first polymer studied of this kind was poly(N-isopropyl acrylamide) (PNIPAM) Figure 7. This polymer attracted the attention of researchers due to its biocompatibility and corresponding LCST of around 32–33 °C in water, which is close to the temperature of the human body [71,72]. The LCST of the polymer can be changed by shifting the hydrophilic/hydrophobic balance by coupling it with another polymer. It has been proven that hydrophilic compounds make the LCST behavior of the polymer disappear; therefore, by changing the ratio of hydrophilic compounds the LCST can be shifted [73]. If the comonomer used is hydrophobic, it increases the LCST. If the comonomer is hydrophilic, the LCST will decrease [73,74].
Figure 7.
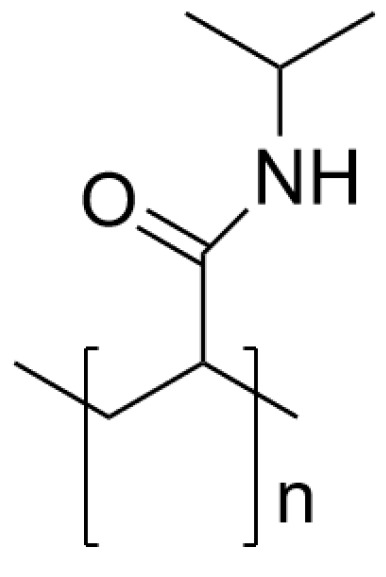
Chemical structure of PNIPAM.
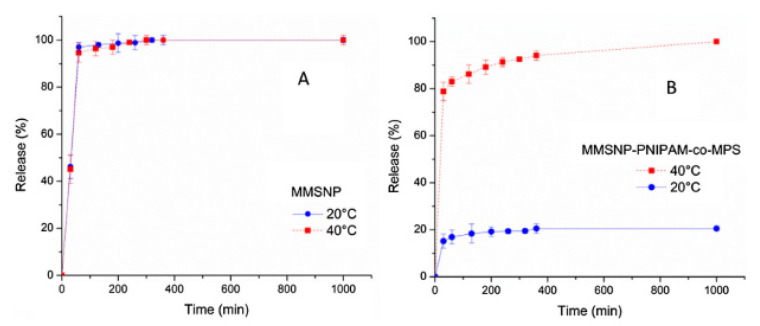
Polymers responsive to temperature have emerged in biomedicine, as a potential targeted drug delivery system. Peralta et al. synthesized a temperature-responsive nanocarrier to deliver magnetic mesoporous silica nanoparticles, based on PNIPAM [75]. Nanosilica is a porous material that can be used to deliver drugs (in this case ibuprofen), and the combination with PNIPAM on the surface of the particles prevents the release of the drug at low temperatures (Figure 8). The drug release from the particles was tested at two different temperatures, 20 °C, and 40 °C, without the grafted polymer on the surface (Figure 8A) and with the grafted polymer on the surface (Figure 8B). When the polymer was not used, the drug was released immediately, with no difference between the temperatures; however, by grafting PNIPAM to the surface of the particles the release increased from 20% at a temperature of 20 °C to 80% at a temperature of 40 °C initially, and at 40 °C a final release of almost 100% of the drug was achieved.
Figure 8.
Ibuprofen release curves (A) nanosilica particles. (B) PNIPAM grafted nanosilica particles at 20 °C (blue) and 40 °C (red) [75]. Reprinted from Journal of Colloid and Interface Science, 544, M. E. Peralta et al., Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery, 198–205, Copyright (2019), with permission from Elsevier.
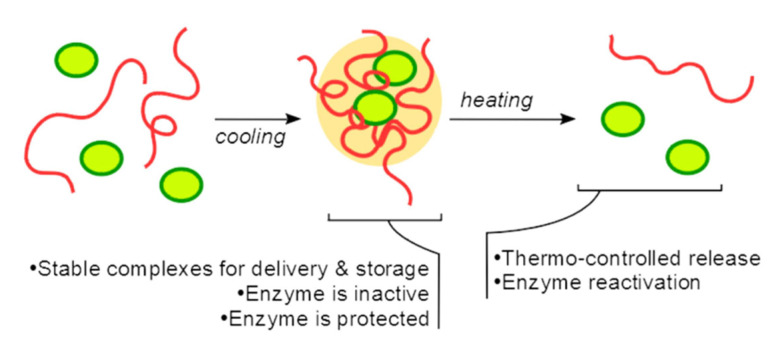
LCST polymers are the dominant temperature-responsive polymers in drug delivery applications; however, UCST polymers have been gaining more importance in recent years [23,76]. Compared to commonly used LCST polymers, there are fewer polymers that exhibit a UCST response [77]. Lin et al. synthesized a thermo-sensitive nanocarrier based on a UCST response for doxorubicin using the insoluble fraction of multi-L-arginyl-poly-L-aspartate (iMAPA) [78]. Additionally, iMAPA was crosslinked with hyaluronic acid (HA) to achieve selectivity to the receptors of malignant tissue. iMAPA-HA exhibits a phase transition in aqueous solutions becoming soluble at high temperatures with a UCST response. Semenyuk et al. proposed the use of poly(N-acryloyl glycinamide) (PNAGA), a UCST-responsive polymer soluble at high temperatures [79]. Figure 9 describes the technology proposed to deliver an enzyme based on a UCST thermo responsive polymer.
Figure 9.
A schematic representation of an enzyme delivery system based on PNAGA [79]. Reproduced with permission from P. I. Semenyuk et al., Polymers; published by MDPI, 2021.
2.2. External Stimuli
2.2.1. Light-Responsive
Drug carriers that are responsive to light are attractive for drug delivery as the spatiotemporal release of the encapsulated material can be controlled. Many physical and chemical processes can be triggered by the radiation of a specific wavelength. Functional groups sensitive to this kind of interaction have the ability to break cleavage bonds, switch the electrostatic charge, or change the chemical conformation from cis to trans [80]. Polymers incorporating these functional groups can be used as light-responsive drug delivery systems [33,81,82].
The safe use of light in medicine is conditional on the wavelength of the light itself. Certain wavelengths can go deeper into the body but damage healthy tissue at the same time. Therefore, the use of far-UV light (a wavelength shorter than 200 nm) should be excluded from these treatments due to its potential hazard. Long-UV lasers (200–400 nm), however, can leave both the drug and tissue intact while releasing the drug from the polymer [83,84]. Visible light (400–700 nm) can also be used as a trigger, but these wavelengths are only suitable for topical treatments due to their limited penetration depth [85]. Finally, NIR radiation (750–1000 nm) has the advantage of penetrating deeper into the tissue and being benign [86].
UV and NIR light are, therefore, the most suitable wavelengths for light-responsive drug delivery particles. An example of a drug delivery system using polymers sensitive to both UV and NIR is the research by Liu et al. which used polymer micelles for the encapsulation of a drug [87]. In this study, dextran was combined with 2-diazo-1,2-naphthoquinone (DNQ) which is a photo-triggered group activated by interaction with UV light. However, in this study, they proved that the DNQ group can also be triggered by using NIR light which is safer than UV. When the radiation is applied, the DNQ changes charge, resulting in a change of the polymer from amphiphilic to hydrophilic, allowing delivery of the drug [87].
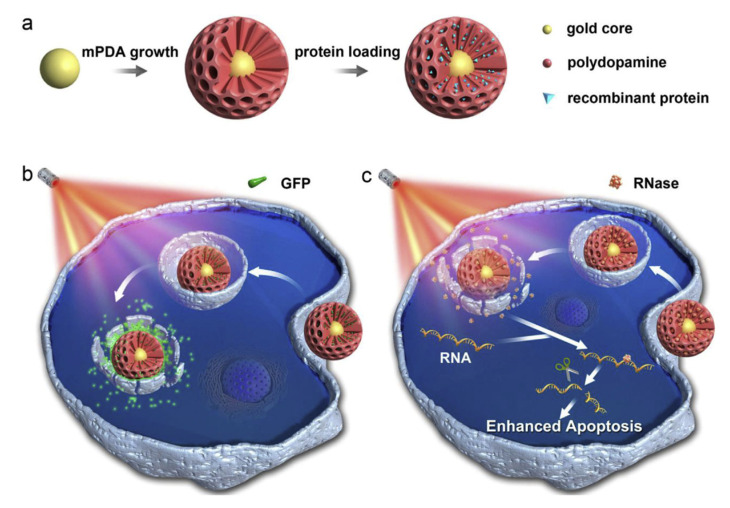
Polydopamine (PDA) is a biopolymer used for drug delivery due to its biocompatibility, easy polymerization on the surface of particles, and its NIR-sensitive properties [88,89,90,91]. PDA exhibits a strong NIR absorption, which allows for the controlled release of encapsulated particles when irradiated with a laser at 750–1000 nm [25,26]. Wu et al. delivered proteins by attaching proteins in a mesoporous PDA delivery system [92]. By applying NIR to the system, two different types of proteins were released (Figure 10).
Figure 10.
A schematic representation of (a) protein loading into mPDA matrix, (b) release of GFP protein based on NIR, and (c) release of RNase based on NIR [92]. Reprinted from Biomaterials, 238, D. Wu et al., Mesoporous Polydopamine with Built-in Plasmonic Core: Traceable and NIR Triggered Delivery of Functional Proteins, 119847, Copyright (2020), with permission from Elsevier.
2.2.2. Ultrasound-Responsive
Ultrasound is high-frequency sound waves (greater than 20 kHz) produced by mechanical oscillations. Ultrasound has been used in medical applications frequently because it is a non-invasive technique that can penetrate centimeters deep into the tissue. It also has the ability to focus on a single point with high intensity. Therefore, the use of ultrasound-responsive polymers for drug delivery has recently been of interest to researchers [93].
High intensity focused ultrasounds (HIFU) can focus on a very small area; therefore, historically it has been used as a tumor treatment. Nevertheless, nanocarriers based on polymers responsive to ultrasound are beginning to be developed [94,95]. Disulfide bonds (S-S) are mechano-labile weak bonds that respond rapidly to HIFU, improving the ultrasound response of polymeric nanocarriers [96,97]. For example, in the research by Li et al., a block copolymer of polylactic acid (PLA) and polyethylene glycol (PEG) with a disulfide bond (PLA-S-S-PEG) was synthesized for drug delivery purposes [27]. Nanoparticles were then obtained by self-assembly of the copolymer, including a central disulfide linkage to promote sensitivity to HIFU.
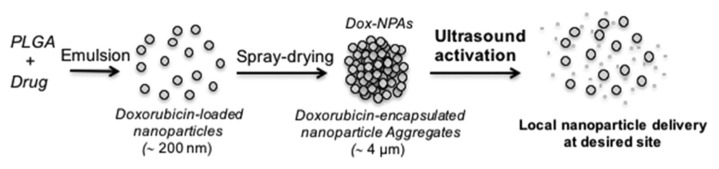
Papa et al. produced nanoparticle aggregates based on polylactic-co-glycolic acid (PLGA), to carry doxorubicin [28]. The use of ultrasound triggers the separation of the aggregates, releasing the drug into the desired area of the body (Figure 11). For the sonication process, the particles were exposed to ultrasound for 3 min with an intensity of 2.2 Watt/cm2. After the sonication process, size and aggregate distribution were characterized using diffraction light scattering and scanning electron microscopy. After applying ultrasound, the particles disintegrated into either single particles or smaller aggregates.
Figure 11.
A schematic representation of PLGA carriers with ultrasound release [28]. Reprinted from Biomaterials, 139, A-L. Papa et al., Ultrasound-Sensitive Nanoparticle Aggregates for Targeted Drug Delivery, 187–194, Copyright (2017), with permission from Elsevier.
2.2.3. Others
The previously mentioned single stimuli are those most commonly used in polymers for drug delivery; however, other stimuli can be implemented such as magnetism and shear force.
Magnetism could be used as an external stimuli. A magnetic PDDS has the capability of targeting a disease site and releasing the drug when a magnetic field is applied. Several studies have combined metallic nanoparticles with polymers for this purpose. The use of a polymer helps with the compatibility of the particles, can incorporate an active target, and increase the circulation time [29,30,98,99,100]. However, the magnetic properties of these systems is achieved by the metallic nanoparticles, such as iron as was used in the studies by Cao et al. [29] and García-García et al. [30] They both used polymers as a coating on the iron nanoparticles to achieve better biocompatibility and targeting.
Shear stress is a type of mechanical force that is interesting as a target for PDDS because it is associated with blood flow. Shear stress is commonly used as a diagnostic tool for cardiovascular diseases. Normal shear stress in arteries is 10–70 dyn cm−2 and 1–6 dyn cm−2 in veins, while for cardiovascular pathologies or hemorrhages it increases up to 100 dyn cm−2 [101]. Therefore, this difference can be used as an internal stimulus for PDDS. Some micelles and polymersomes have been studied due to their ability to deform their shape and release the drug under specific shear conditions. Rifaie-Graham et al. synthesized polymersomes that change shape with shear stress, thereby releasing the cargo in high shear stress conditions [31]. Shen et al. prepared micelles that are responsive to ROS production and shear stress to treat atherosclerosis, which is a type of cardiovascular disease [102]. However, most research on shear stress-responsive polymers has focused on hydrogel nanoparticles because of their flexibility [101].
3. Combination of Various Stimuli for Polymers
Based on the type of environment and the response needed, different multiple-response polymers can be synthesized: pH/temperature, pH/redox, temperature/redox, enzyme/pH, temperature/light [103], light/redox, double pH, and temperature/pH/redox (Table 4).
Table 4.
A summary of recent multiple responsive polymers used for particle drug delivery systems (PDDS).
| Polymer | Stimuli | Description | Ref. |
|---|---|---|---|
| PMAEFc-ONB-PDMAEMA poly(2-methacryloyloxyethyl ferrocenecarboxylate)-(5-propargylether-2-nitrobenzyl bromoisobutyrate)-poly(di-methylaminoethyl methacrylate) |
Light pH Temperature Redox: -oxidative -reduction |
pH-responsive and LCST: PDMAEMA. Oxidation/reduction: ferrocenyl groups. Light responsive: o-nitrobenzyl methyl esters. |
[104] |
| Poly[HBCEEM-b-(NIPA-r-PEGMA)] (PHNP) 2-(2-((4-(hexyloxy)benzyloxy)carbonyl)ethylthio)ethyl acrylate, N-isopropyl acrylamide, poly(ethylene glycol methyl ether acrylate) |
pH Temperature Redox |
pH-responsive: HBCEEA. Disulfide bond (S-S): redox responsive. Temperature-responsive: NIPA and PEGMA. |
[105] |
| Fc-DEAE-AM poly(2-(3-(N-(2-(diethylamino)ethyl)acrylamido)-propanoyloxy)ethyl ferrocenecarboxylate) |
Redox pH CO2 |
Redox-responsive: Fc. pH-responsive and CO2: DEAE. |
[106] |
| PDA Polydopamine |
Light pH Redox (if S-S) |
NIR-responsive and pH: dopamine. Redo-responsive: incorporation of disulfide bond (S-S). |
[25,88,89,92,107,108] |
| P(MEO2MA-co-OEGMA)-b-P(MAA-co-SPMA) Poly(2-(2-methoxyethoxy)ethylmethacrylate-co-oligo(ethylene glycol) methacrylate)-block-poly(methacrcid-co-spiropyran methacrylate) |
pH Light Temperature |
UV light-responsive: SP-MC. pH-responsive: P(MAA-co-SPMA). LCST: change based on monomer ratio. |
[109] |
| PSB-block-P(NIPAM-A)) poly(sulfobetaine)-b-poly(N-isopropylacrylamide-co-dopamine methacrylamide) iMAPA-HA insoluble Multi-L-arginyl-poly-L-aspartate- hyaluronic acid 700DX-P(NIPAAm/AIPAAm-PMM) poly(N-isopropylacrylamide) -2-aminoisoprpylacrylamide-2-propionic-3-methyl-maleic PAA@PHEMA poly(acrylic acid)-poly(2-hydroxyethylmethacrylate) PBM-b-ND poly(butyl methacrylate)-b-poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) PMAA-b-PNIPAM poly(methacrylic acid)-b-poly(N-isopropylacrylamid poly(NIPAM-co-GMA) poly(N-isopropylacrylamid)-co-glycidyl methacrylate |
Temperature pH |
Thermo-responsive (LCST): NIPAM. Thermo-responsive (UCST): iMAPA, combined with pH-responsive block: -poly(acrylic acid) PAA, -metal–catecholate, -iMAPA, -N-alkyl groups, -PDPA, -hydrazine units. |
[78,110,111,112,113,114,115] |
| HA-VE and PBAEss hyaluronic acid-vitamine E poly(β-amino ester) mPEG-P(TPE-co-AEMA) poly ethylene glycol-poly(tetrapheny-lethene-co-2-azepane ethyl methacrylate) HA-SH-CS thiol-hyaluronic acid-chitosan PPZ Polyphosphazene PEG modified trimethyl chitosan Polyethylene glycol-trimethyl chitosan PAE(-ss-mPEG)-g-Chol poly(-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol PEG-SS-CPT Polyethylene glycol- disulfide bond- camptothecin |
pH Redox |
Redox-responsive: disulfide bond (S-S). pH-responsive segments: -poly(β-amino ester), -(PAEMA): pH > 6.8 hydrophobic, pH < 6.8 hydrophilic, -polyelectrolyte complexes, -cross-linked polyphosphazene, -trimethyl chitosan, -copolymer poly(-amino ester)-g-poly(ethylene glycol) methyl ether-cholesterol. |
[116,117,118,119,120,121,122] |
| PEG-PEI-GEM polyethylenimine-graft-poly(ethylene glycol)- gemcitabine |
pH Light |
Light-responsive: photo-cleavable-o-nitrobenzyl, with a linker of thermosensitive: PEG–PEI. |
[123] |
| PEO-PEtG-PEO Poly(ethyl glyoxylate)-Poly(ethylene oxide) |
Light (UV) Redox |
Redox-responsive: disulfide bond (S-S). Light-responsive: o-nitrobenzyl moiety. |
[124] |
| BU-PPG Uraciland-oligomeric polypropylene glycol |
Temperature Light |
Light-responsive: uracil. Thermoresponsive: oligomeric PPG. |
[103] |
| pDHPMA-DOX poly[N-(1, 3-dihydroxypropyl) methacrylamide]-doxorubicin |
pH Enzyme |
Enzyme-responsive: Gly–Phe–Leu–Gly (GFLG), with a linker of pH-responsive: hydrazone bond. |
[125] |
Dual-responsive nanoparticles or micelles are synthesized by means of a block copolymer [125,126,127]. Block copolymers function similarly to surfactants or dispersants. These are molecules with short chains or hydrophilic and hydrophobic components, that form micelles with a hydrophobic core and a hydrophilic outer shell. In solution, block copolymers exist as individual polymer chains. However, once the critical micelle concentration (CMC) is reached, they start to form micelles [128]. In some cases, block copolymers can also form nanoparticles through kinetically controlling factors such as temperature, solvent contents, and pH. For nanoparticles to form, the CMC should be <10−3 wt% with a free energy change greater than 5 kT [129].
3.1. pH/Temperature-Responsive Polymers
pH/thermo-responsive polymers are the most widely studied dual-responsive polymers [78,110,111,112,128]. Similar to the individual pH-responsive polymers and temperature-responsive polymers, these dual-responsive polymers allow for a much more specific and targeted environment to activate the polymer. Often, dual-responsive polymers will be formulated by conjugating a pH-sensitive polymer to a thermo-sensitive polymer [130]. However, some have used a mixture of the two different classes of sensitive polymers [131]. The most common building block for thermo-responsiveness is poly(N-isopropylacrylamide) (PNIPAAm) [132]. This particular polymer can go from a water-soluble state to a water-insoluble state through an LCST transition. The building blocks for pH-responsiveness are often polymers such as weak acids, acrylic acids, poly[2-(diisopropylamine)ethyl methacrylate] (PDPA), and chitosan [113,131,133,134]. Once mixed, they follow the same process of a normal block copolymer to create micelles or nanoparticles.
pH and temperature-responsive polymers are frequently proposed for potential cancer therapies since the tumor environment has an increased temperature and a decreased pH [135,136,137]. In the research of Zhang et al., the effectiveness of nanoparticles made from a block copolymer of thermo-responsive hydrophilic poly(N-isopropylacrylamide-co-acrylic acid) [P(NIPAM-co-AAc)] and a hydrophobic polycaprolactone (PCL), was explored [134]. [P(NIPAM-co-AAc)] is a common polymer used for thermo-sensitive applications and PCL was chosen for its good drug encapsulation properties. This study showed that the nanoparticles released the encapsulated drug much faster at higher temperature and lower pH conditions, as are commonly seen in the tumor environment [134].
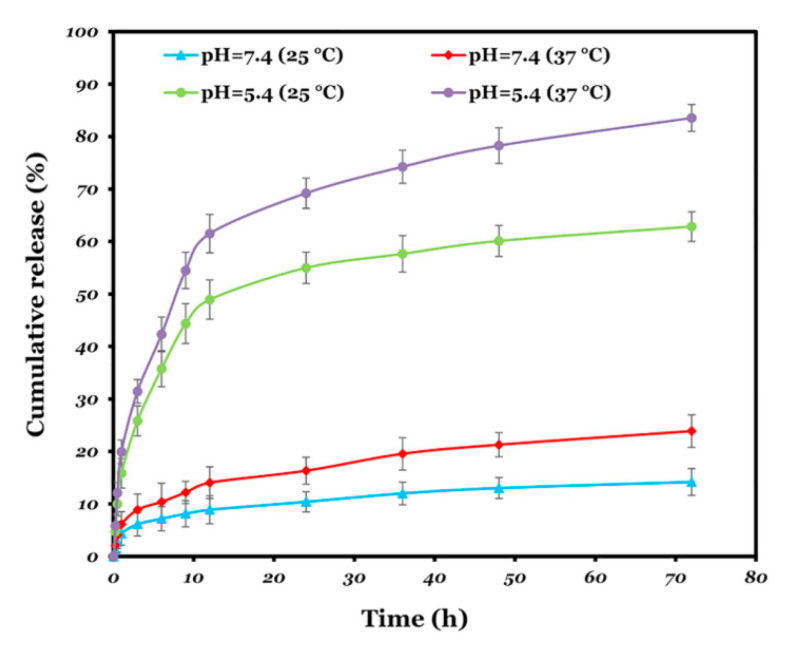
Zheng et al. created nanoparticles using another pH and thermo-responsive copolymer consisting of poly(methacrylic acid) (PMAA) and poly(N-isopropylacrylamide) (PNIPAM) [115]. PMAA is sensitive to pH while PNIPAM is sensitive to temperature. These nanoparticles were loaded with doxorubicin (DOX), a common chemotherapy drug. Through experimentation, it was found that these particles released the DOX in acidic environments. This phenomenon was due to the electrostatic attraction between DOX (positively charged) and the polymer (negatively charged). This interaction prevented the release at neutral pH. However, the protonation of the carboxylic groups of the polymer at acidic pH weakened the interaction between DOX and the polymer, allowing for the release of the drug. The release rate was observed to be even faster when the temperature was increased above the LCST. Pourjavadi et al. used N-isopropylacrylamide (NIPAM) co-polymerized with glycidyl methacrylate (GMA), a common monomer that contains an epoxy ring, to form a copolymer of poly(NIPAM-co-GMA) (PNG) [114]. This combination of polymers provides a pH and thermo-sensitive release (Figure 12). The decrease of the pH combined with the increase in temperature to a physiological level produces a higher cumulative release, which is selective for body temperature and the pH of the endosomes.
Figure 12.
The cumulative release of doxorubicin: blue: pH 7.4 and 25 °C, green: pH 5.4 and 25 °C, red: pH 7.4 and 37 °C, and purple: pH 5.4 and 37 °C [114]. Reprinted from Materials Science and Engineering C, 108, A. Pourjavadi et al., pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery, 110418, Copyright (2020), with permission from Elsevier.
3.2. pH/Redox-Responsive Polymers
Because redox reactions and differences in pH occur naturally in the body, these two stimuli are very appealing for drug delivery applications [133]. These types of polymers have been created for a myriad of applications, such as enhancing drug delivery and tumor cell uptake, creating a faster drug release rate within the cytoplasm and nucleus, and further stabilizing the stability of nanoparticles in vivo [116,133].
Bahadur et al. conjugated polyethylene glycol and cyclo(Arg-Gly-Asp-d-Phe-Cys) (cRGD) peptide to poly(2-(pyridin-2-yldisulfanyl)ethyl acrylate) (PDS) to create an RPDSG polymer [138]. Nanoparticles were created with this copolymer and DOX was encapsulated inside the nanoparticles. To induce a redox reaction, varying amounts of GSH were used in the experiment. It was found that the concentration of GSH within the extracellular fluid is less than 0.01 mM and is 1–11 mM intracellularly. After experimentation in different pH values and with different concentrations of GSH, the DOX release rate was found to be much slower at higher pH values. Under acidic conditions, the ester bonds of PDSG can be hydrolyzed to produce a faster release rate than at neutral pH, and therefore a faster release rate was achieved at pH 5.5 than at pH 7.4. Moreover, the amount of DOX released was observed to increase with a higher concentration of GSH. Mahmoud et al. took advantage of the characteristic inflammation caused by infections, cancer, or other diseases as inflamed tissues have a decreased pH as well as having reactive oxygen species present [139]. Mahmoud et al. synthesized polymeric nanoparticles that incorporate a thioether moiety into the polymer backbone [139]. In this study, they created environments that simulated healthy tissue and infected tissue with differences in pH and redox potential. It was found that the particles subjected to a pH of 5 in the presence of H2O2 were the only ones to disperse and degrade.
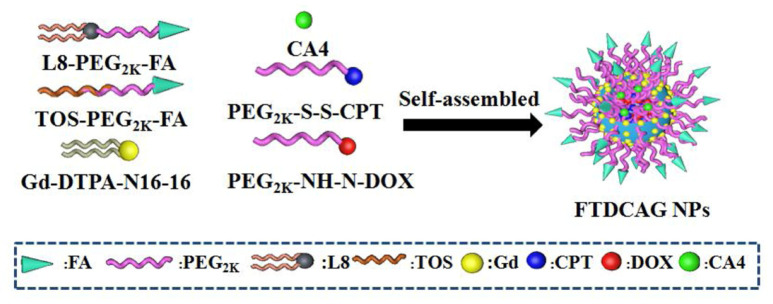
In recent years, the combination of GSH concentration and pH has gained importance in the drug delivery field [117,118,119,120,122,140]. Wang et al. created dual-responsive polymeric nanoparticles based on pH and GSH concentration to deliver multiple drugs in cancerous environments [121]. The disulfide bond connecting poly(ethylene glycol) (PEG) and camptothecin (CPT), a chemotherapeutic drug, allows for the release at high concentrations of GSH (Figure 13), while the NH-N bond between PEG and doxorubicin (DOX), another chemotherapeutic drug, allows for the breaking of the hydrazine bond in acidic environments.
Figure 13.
A schematic illustration of the configuration of the nanocarrier, based on double sensitive polymers with NH-H bonds and S-S bonds [121]. Reprinted from Colloids and Surfaces B: Biointerfaces, 205, N. Wang et al., A Traceable, GSH/PH Dual-Responsive Nanoparticles with Spatiotemporally Controlled Multiple Drugs Release Ability to Enhance Antitumor Efficacy, 111866, Copyright (2021), with permission from Elsevier.
3.3. Double-pH-Responsive Polymers
Not only can polymers be made with responses to different stimuli, but they can also be fabricated to respond to the same stimuli but at different values. Polymers like this respond to stimuli similar to an “AND” logic gate [141]. The second event will only occur once the first event has happened. Double pH-responsive polymers are an example of this type of technology. A polymer capable of responding to two different pH values, PPC-Hyd-DOX-DA, was synthesized by Du et al. and made into DOX encapsulated nanoparticles [142]. This nanoparticle changes its surface charge from negative to positive when exposed to the pH of a tumor environment (~6.8). This change in surface charge encourages cellular internalization by the tumor cells. Once inside the endosome, the pH (~5.0) triggers DOX release within the cell [142]. This technique helps ensure that drugs targeted for tumors are specifically within the site before subsequent release.
Another example of a dual pH-responsive polymer is poly([2,2′-(propane-2,2-diylbis(oxy))bis(ethane-2,1-diyl) diacrylate]-co-[hexane-1,6-diyl diacrylate]-4,4′-trimethylene dipiperidine), (poly-β-aminoester ketal) [141]. When the pH is decreased, the tertiary amines in the backbone of this polymer are protonated, switching the polymer from hydrophobic to hydrophilic. This then leads to an increase in water uptake which causes bulk dissolution, which then triggers ketal hydrolysis causing surface degradation. These particles are stable at physiological pH but degrade at a pH of 5, subsequently releasing the contents of the nanoparticle.
3.4. Multiple-Stimuli-Responsive Polymers
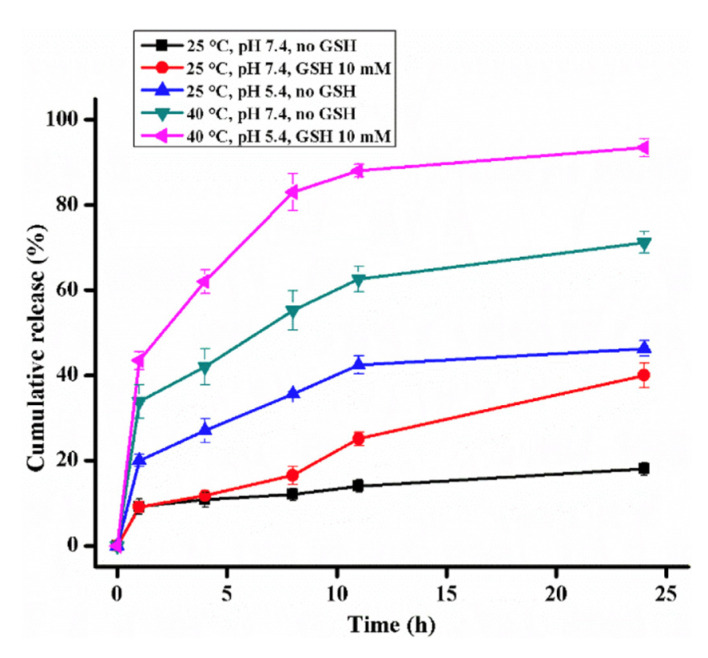
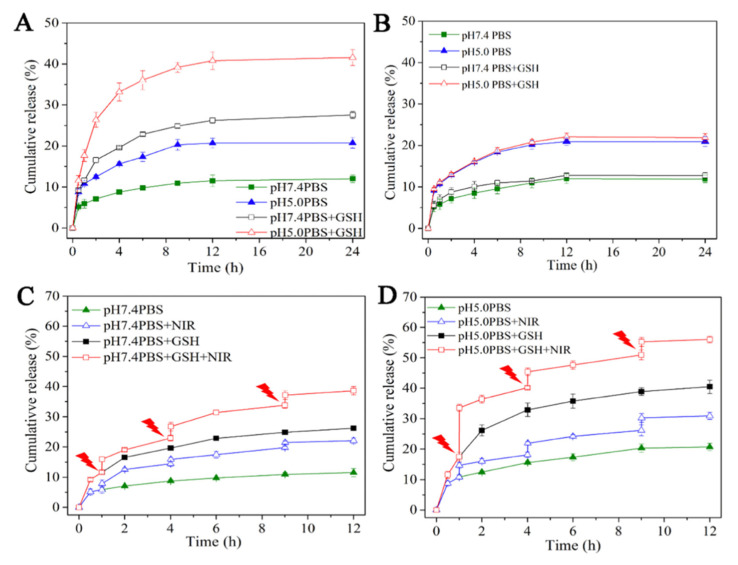
There has been a trend in recent years to incorporate the potential for many stimuli to trigger the drug release by a carrier to a specific disease site [90,106,107,109,123,124]. Poddar et al. synthesized a triple-stimuli-responsive polymer to achieve the release of a drug under the conditions of pH 5, 40 °C, and GSH ≥ 10 mM [106]. In this study, they synthesized two different polymers, 2-(2-((4-(hexyloxy)benzyloxy)carbonyl)ethylthio)ethyl acrylate (HBCEEA), which is sensitive to pH, and the copolymer of N-isopropyl acrylamide (NIPA) and poly(ethylene glycol methyl ether acrylate) (PEGMA), which is sensitive to temperature and redox potential. The combination of these polymers creates the triple-responsive polymer poly[HBCEEM-b-(NIPA-r-PEGMA)] (PHNP) [105]. The drug release from the polymer is much faster in the presence of all three stimuli, as shown in Figure 14.
Figure 14.
The cumulative release of doxorubicin under different conditions [105]. Reprinted from Reactive and Functional Polymers, 154, P. Poddar et al., Synthesis of a New Triple-Responsive Biocompatible Block Copolymer: Self-Assembled Nanoparticles as Potent Anticancer Drug Delivery Vehicle, 104679, Copyright (2020), with permission from Elsevier.
Lei et al. used mesoporous silica as the nanocarrier for doxorubicin and coated the particles with polydopamine [108]. As previously discussed, polydopamine (PDA) is highly sensitive to NIR, and with the incorporation of a disulfide bond, the particles became responsive to pH and GSH as well, achieving a multi-stimuli-responsive drug carrier. As shown in Figure 15A, mesoporous silica-disulfide bond-polydopamine (MSN-SS-PDA) and in Figure 15B, mesoporous silica-polydopamine (MSN-PDA), the incorporation of a disulfide bond increases the release rate when exposed to a low pH and high GSH. Moreover, when combined with NIR the highest cumulative release rate is observed at acidic pH combined with GSH (Figure 15D) as opposed to the neutral pH with GSH (Figure 15C), proving the multi-stimuli nature of the particles [108]. The use of an acidic pH degrades the polydopamine that coats the silica particles, allowing for a faster release of the drug.
Figure 15.
The cumulative release (A) MSN-PDA, (B) MSN-S-S-PDA, (C) MSN-S-S-PDA NIR pH 7.4, and (D) MSN-S-S-PDA NIR pH 5 [108]. Reprinted from Materials Science and Engineering C, 105, W. Lei et al., Polydopamine-Coated Mesoporous Silica Nanoparticles for Multi-Responsive Drug Delivery and Combined Chemo-Photothermal Therapy, 110103, Copyright (2019), with permission from Elsevier.
Furthermore, Zhang et al. synthesized a quintuple-stimuli-responsive nanocarrier based on the self-assembly of an amphiphilic diblock copolymer [104]. The conjugation of poly(2-methacry-loyloxyethyl ferrocenecarboxylate)-(5-propargylether-2-nitrobenzylbromoisobutyrate)-poly(dimethylaminoethyl methacrylate) (PMAEFc-ONB-PDMAEM), allows for the release of the drug based on temperature, pH, light, oxidation, and reduction.
4. Conclusions and Future Research
Stimuli-responsive polymer particles have become a trend in the drug delivery field due to the potential to trigger the release of drugs at specific sites, owing to changes in the environment. Specifically, in cancer research, stimuli-responsive polymer particles have become important because of the great divergence between the environment of healthy tissue and cancer tissue. In this review, many of the different stimuli that can be used to trigger the release of drugs have been studied and discussed. The current trend in stimuli-responsive PDDSs is to combine two or more stimuli. We explored the recent combinations that have been studied such as pH/temperature, pH/redox, light/pH, etc., as summarized in Table 4. Taking a more synergistic approach to the use of polymers in PDDSs, a combination of different stimuli would increase the specificity of delivery and maximize the dosage release at the tumor site.
Currently, only simple polymeric drug delivery systems, such as PLGA particles, are available commercially. There is a significant opportunity in the market for more complex drug delivery systems such as those using responsive polymers. A few startup companies exist that are exploring the potential of stimuli-responsive particles for drug delivery applications; however, there are many challenges to overcome in taking these products to market. For instance, although using stimuli-responsive polymer particles has many advantages for drug delivery, not many have been tested in vivo. In fact, the combination of multiple stimuli in particles has not been tested in clinical trials at all, and only a few have been used in animal studies [102,143,144]. The increase in the complexity of multiple stimuli particles creates a significant hurdle in terms of the practical application of these particles in animal studies and eventually clinical trials. In addition, the stringent requirements as to reproducibility of particles in drug delivery systems will require fastidious production methods. Therefore, more research is needed to address the complexity in producing multiple stimuli particles, as this complexity hinders the commercial application of these types of particle systems.
Author Contributions
Writing original draft preparation A.L.R., writing double stimuli section A.R., review editing and supervision K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maeda H., Nakamura H., Fang J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Dang W., Daviau T., Brem H. Morphological Characterization of Polyanhydride Biodegradable Implant Gliadel during in Vitro and in Vivo Erosion Using Scanning Electron Microscopy. Pharm. Res. 1996;13:683–684. doi: 10.1023/A:1016035229961. [DOI] [PubMed] [Google Scholar]
- 3.Sung Y.K., Kim S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020;24:12. doi: 10.1186/s40824-020-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghasemiyeh P., Mohammadi-Samani S. Polymers Blending as Release Modulating Tool in Drug Delivery. Front. Mater. 2021;8:752813. doi: 10.3389/fmats.2021.752813. [DOI] [Google Scholar]
- 5.Fu X., Hosta-Rigau L., Chandrawati R., Cui J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem. 2018;4:2084–2107. doi: 10.1016/j.chempr.2018.07.002. [DOI] [Google Scholar]
- 6.Jia R., Teng L., Gao L., Su T., Fu L., Qiu Z., Bi Y. Advances in multiple stimuli-responsive drug-delivery systems for cancer therapy. Int. J. Nanomed. 2021;16:1525–1551. doi: 10.2147/IJN.S293427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatai J., Hirschhäuser C., Niemeyer J., Schmuck C. Multi-Stimuli-Responsive Supramolecular Polymers Based on Noncovalent and Dynamic Covalent Bonds. ACS Appl. Mater. Interfaces. 2020;12:2107–2115. doi: 10.1021/acsami.9b19279. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L., Gray D.M., Niezabitowska E., McDonald T.O. Multi-stimuli-responsive aggregation of nanoparticles driven by the manipulation of colloidal stability. Nanoscale. 2021;13:7879–7896. doi: 10.1039/D1NR01190A. [DOI] [PubMed] [Google Scholar]
- 9.Das S.S., Bharadwaj P., Bilal M., Barani M., Rahdar A., Taboada P., Bungau S., Kyzas G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers. 2020;12:1397. doi: 10.3390/polym12061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hershberger K.K., Gauger A.J., Bronstein L.M. Utilizing Stimuli Responsive Linkages to Engineer and Enhance Polymer Nanoparticle-Based Drug Delivery Platforms. ACS Appl. Bio Mater. 2021;4:4720–4736. doi: 10.1021/acsabm.1c00351. [DOI] [PubMed] [Google Scholar]
- 11.Liu G., Lovell J.F., Zhang L., Zhang Y. Stimulus-Responsive Nanomedicines for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2020;21:6380. doi: 10.3390/ijms21176380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sawy H.S., Al-Abd A.M., Ahmed T.A., El-Say K.M., Torchilin V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano. 2018;12:10636–10664. doi: 10.1021/acsnano.8b06104. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym.-Plast. Technol. Mater. 2021:1996–2024. doi: 10.1080/25740881.2021.1939715. [DOI] [Google Scholar]
- 14.Jiang Z., Chen J., Cui L., Zhuang X., Ding J., Chen X. Advances in Stimuli-Responsive Polypeptide Nanogels. Small Methods. 2018;3:1700307. doi: 10.1002/smtd.201700307. [DOI] [Google Scholar]
- 15.Hajebi S., Rabiee N., Bagherzadeh M., Ahmadi S., Rabiee M., Roghani-Mamaqani H., Tahriri M., Tayebi L., Hamblin M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019;17:1–18. doi: 10.1016/j.actbio.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suhail M., Rosenholm J.M., Minhas M.U., Badshah S.F., Naeem A., Khan K.U., Fahad M. Nanogels as Drug-Delivery Systems: A Comprehensive Overview. Ther. Deliv. 2019;10:697–717. doi: 10.4155/tde-2019-0010. [DOI] [PubMed] [Google Scholar]
- 17.Alami-Milani M., Zakeri-Milani P., Valizadeh H., Salehi R., Salatin S., Naderinia A., Jelvehgari M. Novel Pentablock Copolymers as Thermosensitive Self-Assembling Micelles for Ocular Drug Delivery. Adv. Pharm. Bull. 2017;7:11–20. doi: 10.15171/apb.2017.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicario-De-la-torre M., Forcada J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels. 2017;3:16. doi: 10.3390/gels3020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vázquez-González M., Willner I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem.-Int. Ed. 2020;36:15342–15377. doi: 10.1002/anie.201907670. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X., Zhao M., Cao N., Qin W., Zhao M., Wu J., Lin D. Construction of a Tumor Microenvironment PH-Responsive Cleavable PEGylated Hyaluronic Acid Nano-Drug Delivery System for Colorectal Cancer Treatment. Biomater. Sci. 2020;8:1885–1896. doi: 10.1039/C9BM01927H. [DOI] [PubMed] [Google Scholar]
- 21.Sun C.Y., Liu Y., Du J.Z., Cao Z.T., Xu C.F., Wang J. Facile Generation of Tumor-PH-Labile Linkage-Bridged Block Copolymers for Chemotherapeutic Delivery. Angew. Chem.-Int. Ed. 2016;55:1010–1014. doi: 10.1002/anie.201509507. [DOI] [PubMed] [Google Scholar]
- 22.Xu Z., Liu S., Kang Y., Wang M. Glutathione-Responsive Polymeric Micelles Formed by a Biodegradable Amphiphilic Triblock Copolymer for Anticancer Drug Delivery and Controlled Release. ACS Biomater. Sci. Eng. 2015;1:585–592. doi: 10.1021/acsbiomaterials.5b00119. [DOI] [PubMed] [Google Scholar]
- 23.Tseng W.C., Fang T.Y., Lin Y.C., Huang S.J., Huang Y.H. Reversible Self-Assembly Nanovesicle of UCST Response Prepared with Multi-L-Arginyl-Poly-L-Aspartate Conjugated with Polyethylene Glycol. Biomacromolecules. 2018;19:4585–4592. doi: 10.1021/acs.biomac.8b01274. [DOI] [PubMed] [Google Scholar]
- 24.Conzatti G., Cavalie S., Combes C., Torrisani J., Carrere N., Tourrette A. PNIPAM grafted surfaces through ATRP and RAFT polymerization: Chemistry and bioadhesion. Colloids Surf. B: Biointerfaces. 2017;151:143–155. doi: 10.1016/j.colsurfb.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y., Zhang M., Lin Z., Shi X. Fast Near-Infrared Light Responsive Shape Memory Composites: Polydopamine Nanospheres Hybrid Polynorbornene. Polymer. 2020;206:122898. doi: 10.1016/j.polymer.2020.122898. [DOI] [Google Scholar]
- 26.Li Y., Wang X., Yang D., Hu P., Gao L., Chen D., Qiao Y., Wu Y., Jiang X., Li G. Polydopamine-Coated Gold Nanostars for near-Infrared Cancer Photothermal Therapy by Multiple Pathways. J. Mater. Sci. 2019;54:12036–12048. doi: 10.1007/s10853-019-03774-4. [DOI] [Google Scholar]
- 27.Li Y., Tong R., Xia H., Zhang H., Xuan J. High Intensity Focused Ultrasound and Redox Dual Responsive Polymer Micelles. Chem. Commun. 2010;46:7739–7741. doi: 10.1039/c0cc02628j. [DOI] [PubMed] [Google Scholar]
- 28.Papa A.-L., Korin N., Kanapathipillai M., Mammoto A., Mammoto T., Jiang A., Mannix R., Uzun O., Johnson C., Bhatta D., et al. Ultrasound-Sensitive Nanoparticle Aggregates for Targeted Drug Delivery. Biomaterials. 2017;139:187–194. doi: 10.1016/j.biomaterials.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y., Cheng Y., Zhao G. Near-Infrared Light-, Magneto-, and pH-Responsive GO-Fe3O4/Poly(N-isopropylacrylamide)/alginate Nanocomposite Hydrogel Microcapsules for Controlled Drug Release. Langmuir. 2021;37:5522–5530. doi: 10.1021/acs.langmuir.1c00207. [DOI] [PubMed] [Google Scholar]
- 30.García-García G., Fernández-álvarez F., Cabeza L., Delgado Á.V., Melguizo C., Prados J.C., Arias J.L. Gemcitabine-loaded magnetically responsive poly(ε-caprolactone) nanoparticles against breast cancer. Polymers. 2020;12:2790. doi: 10.3390/polym12122790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rifaie-Graham O., Galensowske N.F.B., Dean C., Pollard J., Balog S., Gouveia M.G., Chami M., Vian A., Amstad E., Lattuada M., et al. Shear Stress-Responsive Polymersome Nanoreactors Inspired by the Marine Bioluminescence of Dinoflagellates. Angew. Chem.-Int. Ed. 2021;60:904–909. doi: 10.1002/anie.202010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vertzoni M., Augustijns P., Grimm M., Koziolek M., Lemmens G., Parrott N., Pentafragka C., Reppas C., Rubbens J., Van Den Abeele J., et al. Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: An UNGAP review. Eur. J. Pharm. Sci. 2019;134:153–175. doi: 10.1016/j.ejps.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Lorenzo C., Concheiro A. In: From Drug Dosage Forms to Intelligent Drug-Delivery Systems: A Change of Paradigm. Alvarez-Lorenzo A., Concheiro A., editors. Volume 1 Royal Society of Chemistry; London, UK: 2013. RSC Smart Materials No. 2 Smart Materials for Drug Delivery: Volume 1. [Google Scholar]
- 34.Ojugo A.S.E., Mcsheehy P.M.J., Mcintyre D.J.O., Mccoy C., Stubbs M., Leach M.O., Judson I.R., Grif J.R. Measurement of the Extracellular PH of Solid Tumours in Mice by Magnetic Resonance Spectroscopy: A Comparison of Exogenous 19 F and 31 P Probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(SICI)1099-1492(199912)12:8<495::AID-NBM594>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Kost J., Langer R. Responsive Polymeric Delivery Systems. Adv. Drug Deliv. Rev. 2012;64:327–341. doi: 10.1016/j.addr.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Nasab N.A., Kumleh H.H., Beygzadeh M., Teimourian S., Kazemzad M. Delivery of Curcumin by a PH-Responsive Chitosan Mesoporous Silica Nanoparticles for Cancer Treatment. Artif. Cells Nanomed. Biotechnol. 2018;46:78–85. doi: 10.1080/21691401.2017.1290648. [DOI] [PubMed] [Google Scholar]
- 37.Hu L., Xiong C., Wei G., Yu Y., Li S., Xiong X., Zou J.-J., Tian J. Stimuli-Responsive Charge-Reversal MOF@polymer Hybrid Nanocomposites for Enhanced Co-Delivery of Chemotherapeutics towards Combination Therapy of Multidrug-Resistant Cancer. J. Colloid Interface Sci. 2022;608:1882–1893. doi: 10.1016/j.jcis.2021.10.070. [DOI] [PubMed] [Google Scholar]
- 38.Saw P.E., Yao H., Lin C., Tao W., Farokhzad O.C., Xu X. Stimuli-Responsive Polymer-Prodrug Hybrid Nanoplatform for Multistage SiRNA Delivery and Combination Cancer Therapy. Nano Lett. 2019;19:5967–5974. doi: 10.1021/acs.nanolett.9b01660. [DOI] [PubMed] [Google Scholar]
- 39.Elbaz N.M., Owen A., Rannard S., McDonald T.O. Controlled Synthesis of Calcium Carbonate Nanoparticles and Stimuli-Responsive Multi-Layered Nanocapsules for Oral Drug Delivery. Int. J. Pharm. 2020;574:118866. doi: 10.1016/j.ijpharm.2019.118866. [DOI] [PubMed] [Google Scholar]
- 40.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy Angewandte. Nanomedicine. 2014;53:2–47. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 41.Felber A.E., Dufresne M.H., Leroux J.C. PH-Sensitive Vesicles, Polymeric Micelles, and Nanospheres Prepared with Polycarboxylates. Adv. Drug Deliv. Rev. 2012;64:979–992. doi: 10.1016/j.addr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Bae Y., Fukushima S., Harada A., Kataoka K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles That Are Responsive to Intracellular PH Change. Angew. Chem.-Int. Ed. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 43.Alsehli M. Polymeric Nanocarriers as Stimuli-Responsive Systems for Targeted Tumor (Cancer) Therapy: Recent Advances in Drug Delivery. Saudi Pharm. J. 2020;28:255–265. doi: 10.1016/j.jsps.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palanikumar L., Al-Hosani S., Kalmouni M., Nguyen V.P., Ali L., Pasricha R., Barrera F.N., Magzoub M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020;3:1–17. doi: 10.1038/s42003-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleige E., Quadir M.A., Haag R. Stimuli-Responsive Polymeric Nanocarriers for the Controlled Transport of Active Compounds: Concepts and Applications. Adv. Drug Deliv. Rev. 2012;64:866–884. doi: 10.1016/j.addr.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Tao Y., Liu S., Zhang Y., Chi Z., Xu J. A PH-Responsive Polymer Based on Dynamic Imine Bonds as a Drug Delivery Material with Pseudo Target Release Behavior. Polym. Chem. 2018;9:878–884. doi: 10.1039/C7PY02108A. [DOI] [Google Scholar]
- 47.Schmaljohann D. Thermo- and PH-Responsive Polymers in Drug Delivery ☆. Adv. Drug Deliv. Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 48.Lee E.S., Na K., Bae Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/S0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 49.Meng F., Cheng R., Deng C., Zhong Z. Intracellular Drug Release Nanosystems. Mater. Today. 2012;15:436–442. doi: 10.1016/S1369-7021(12)70195-5. [DOI] [Google Scholar]
- 50.Quinn J.F., Whittaker M.R., Davis T.P. Glutathione Responsive Polymers and Their Application in Drug Delivery Systems. Polym. Chem. 2017;8:97–126. doi: 10.1039/C6PY01365A. [DOI] [Google Scholar]
- 51.Li W., Li M., Qi J. Nano-Drug Design Based on the Physiological Properties of Glutathione. Molecules. 2021;26:5567. doi: 10.3390/molecules26185567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith C.V., Jones D.P., Guenthner T.M., Lash L.H., Lauterburg B.H. Compartmentation of Glutathione: Implications for the Study of Toxicity and Disease. Toxicol. Appl. Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 53.Stratford I.J., Adams G.E., Bremner J.C.M., Cole S., Edwards H.S., Robertson N., Wood P.J. Manipulation and Exploitation of the Tumour Environment for Therapeutic Benefit. Int. J. Radiat. Biol. 1994;65:85–94. doi: 10.1080/09553009414550121. [DOI] [PubMed] [Google Scholar]
- 54.Montero D., Tachibana C., Rahr Winther J., Appenzeller-Herzog C. Intracellular Glutathione Pools Are Heterogeneously Concentrated. Redox Biol. 2013;1:508–513. doi: 10.1016/j.redox.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuppusamy P., Li H., Ilangovan G., Cardounel A.J., Zweier J.L., Yamada K., Krishna M.C., Mitchell J.B. Noninvasive Imaging of Tumor Redox Status and Its Modification by Tissue Glutathione Levels. Cancer Res. 2002;62:307–312. [PubMed] [Google Scholar]
- 56.Huo M., Yuan J., Tao L., Wei Y. Redox-Responsive Polymers for Drug Delivery: From Molecular Design to Applications. Polym. Chem. 2014;5:1519–1528. doi: 10.1039/C3PY01192E. [DOI] [Google Scholar]
- 57.Wright A.J., Fellows G.A., Griffiths J.R., Wilson M., Bell B.A., Howe F.A. Ex-vivo HRMAS of adult brain tumours: Metabolite quantification and assignment of tumour biomarkers. Mol. Cancer. 2010;9:66. doi: 10.1186/1476-4598-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gamcsik M.P., Kasibhatla M.S., Teeter S.D., Colvin O.M. Glutathione levels in human tumors. Biomarkers. 2012;17:671–691. doi: 10.3109/1354750X.2012.715672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karimi M., Ghasemi A., Zangabad P.S., Rahighi R., Basri S.M.M., Mirshekari H., Amiri M., Pishabad Z.S., Aslani A., Bozorgomid M., et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems. Chem. Soc. Rev. 2016;45:1457–1501. doi: 10.1039/C5CS00798D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y., Yan X., Yuan T., Liang J., Fan Y., Gu Z., Zhang X. Disassemblable Micelles Based on Reduction-Degradable Amphiphilic Graft Copolymers for Intracellular Delivery of Doxorubicin. Biomaterials. 2010;31:7124–7131. doi: 10.1016/j.biomaterials.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y., Wang F., Sun T., Wang J. Redox-Responsive Nanoparticles from the Single Disulfide Bond-Bridged Block Copolymer as Drug Carriers for Overcoming Multidrug Resistance in Cancer Cells. Bioconjugate Chem. 2011;22:1939–1945. doi: 10.1021/bc200139n. [DOI] [PubMed] [Google Scholar]
- 62.Breitenbach B.B., Steiert E., Konhäuser M., Vogt L.M., Wang Y., Parekh S.H., Wich P.R. Double Stimuli-Responsive Polysaccharide Block Copolymers as Green Macrosurfactants for near-Infrared Photodynamic Therapy. Soft Matter. 2019;15:1423–1434. doi: 10.1039/C8SM02204F. [DOI] [PubMed] [Google Scholar]
- 63.Sun H., Guo B., Li X., Cheng R., Meng F., Liu H., Meng F., Zhong Z. Shell-Sheddable Micelles Based on Dextran-SS-Poly(ε-Caprolactone) Diblock Copolymer for Efficient Intracellular Release of Doxorubicin. Biomacromolecules. 2010;11:848–854. doi: 10.1021/bm1001069. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Xiao K., Luo J., Xiao W., Lee J.S., Gonik A.M., Kato J., Dong T.A., Lam K.S. Biomaterials Well-defined, Reversible Disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011;32:6633–6645. doi: 10.1016/j.biomaterials.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y., Lokitz B.S., Armes S.P., McCormick C.L. Synthesis of Reversible Shell Cross-Linked Micelles for Controlled Release of Bioactive Agents. Macromolecules. 2006;39:2726–2728. doi: 10.1021/ma0604035. [DOI] [Google Scholar]
- 66.Takeoka Y., Aoki T., Sanui K., Ogata N., Yokoyama M., Okano T., Sakurai Y., Watanabe M. Electrochemical Control of Drug Release from Redox-Active Micelles. J. Control. Release. 1995;33:79–87. doi: 10.1016/0168-3659(94)00078-9. [DOI] [Google Scholar]
- 67.Hyperthermia in Cancer Treatment-National Cancer Institute. [(accessed on 25 March 2019)]; Available online: https://www.cancer.gov/about-cancer/treatment/types/surgery/hyperthermia-fact-sheet.
- 68.Kujawa P., Winnik F.M. Volumetric Studies of Aqueous Polymer Solutions Using Pressure Perturbation Calorimetry: A New Look at the Temperature-Induced Phase Transition of Poly(N-Isopropylacrylamide) in Water and D2O. Macromolecules. 2001;34:4130–4135. doi: 10.1021/ma002082h. [DOI] [Google Scholar]
- 69.Cummings C., Murata H., Koepsel R., Russell A.J. Dramatically Increased PH and Temperature Stability of Chymotrypsin Using Dual Block Polymer-Based Protein Engineering. Biomacromolecules. 2014;15:763–771. doi: 10.1021/bm401575k. [DOI] [PubMed] [Google Scholar]
- 70.Kotsuchibashi Y. Recent Advances in Multi-Temperature-Responsive Polymeric Materials. Polym. J. 2020;52:681–689. doi: 10.1038/s41428-020-0330-0. [DOI] [Google Scholar]
- 71.Lee J., Ku K.H., Kim M., Shin J.M., Han J., Park C.H., Yi G.R., Jang S.G., Kim B.J. Stimuli-Responsive, Shape-Transforming Nanostructured Particles. Adv. Mater. 2017;29:1700608. doi: 10.1002/adma.201700608. [DOI] [PubMed] [Google Scholar]
- 72.Lu Z., Zhang Z., Tang Y. Conjugated Polymers-Based Thermal-Responsive Nanoparticles for Controlled Drug Delivery, Tracking, and Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Bio Mater. 2019;2:4485–4492. doi: 10.1021/acsabm.9b00640. [DOI] [PubMed] [Google Scholar]
- 73.Kuckling D., Adler H.-J.P., Arndt K.-F., Ling L., Habicher W.D. Temperature and pH dependent solubility of novel poly(N-Isopropylacrylamide) copolymers. Macromol. Chem. Phys. 2000;201:273–280. doi: 10.1002/(SICI)1521-3935(20000201)201:2<273::AID-MACP273>3.0.CO;2-E. [DOI] [Google Scholar]
- 74.Principi T., Goh C.C.E., Liu R.C.W., Winnik F.M. Solution Properties of Hydrophobically Modified Copolymers of N-Isopropylacrylamide and N-Glycine Acrylamide: A Study by Microcalorimetry and Fluorescence Spectroscopy. Macromolecules. 2000;33:2958–2966. doi: 10.1021/ma9919054. [DOI] [Google Scholar]
- 75.Peralta M.E., Jadhav S.A., Magnacca G., Scalarone D., Mártire D.O., Parolo M.E., Carlos L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019;544:198–205. doi: 10.1016/j.jcis.2019.02.086. [DOI] [PubMed] [Google Scholar]
- 76.Li W., Huang L., Ying X., Jian Y., Hong Y., Hu F., Du Y. Antitumor Drug Delivery Modulated by a Polymeric Micelle with an Upper Critical Solution Temperature. Angew. Chem.-Int. Ed. 2015;54:3126–3131. doi: 10.1002/anie.201411524. [DOI] [PubMed] [Google Scholar]
- 77.Li L., Kiick K.L. Resilin-Based Materials for Biomedical Applications. ACS Macro Letters. 2013;2:635–640. doi: 10.1021/mz4002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Y.C., Fang T.Y., Kao H.Y., Tseng W.C. Nanoassembly of UCST Polypeptide for NIR-Modulated Drug Release. Biochem. Eng. J. 2021;176:108194. doi: 10.1016/j.bej.2021.108194. [DOI] [Google Scholar]
- 79.Semenyuk P.I., Kurochkina L.P., Mäkinen L., Muronetz V.I., Hietala S. Thermocontrolled Reversible Enzyme Complexation-Inactivation-Protection by Poly(N-acryloyl glycinamide) Polymers. 2021;13:3601. doi: 10.3390/polym13203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dugave C., Demange L. Cis−Trans Isomerization of Organic Molecules and Biomolecules: Implications and Applications. Chem. Rev. 2003;103:2475–2532. doi: 10.1021/cr0104375. [DOI] [PubMed] [Google Scholar]
- 81.Kamaly N., Yameen B., Wu J., Farokhzad O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016;116:2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang L., Fang T., Liu X., Ni Y., Lu C., Xu Z. Precise stimulation of near-infrared light responsive shape-memory polymer composites using upconversion particles with photothermal capability. Compos. Sci. Technol. 2017;152:190–197. doi: 10.1016/j.compscitech.2017.09.021. [DOI] [Google Scholar]
- 83.Bisby R.H., Mead C., Morgan C.G. Wavelength-Programmed Solute Release from Photosensitive Liposomes. Biochem. Biophys. Res. Commun. 2000;276:169–173. doi: 10.1006/bbrc.2000.3456. [DOI] [PubMed] [Google Scholar]
- 84.Nagasaki T., Shinkai S. The concept of molecular machinery is useful for design of stimuli-responsive gene delivery systems in the mammalian cell. J. Incl. Phenom. Macrocycl. Chem. 2007;58:205–219. doi: 10.1007/s10847-007-9303-6. [DOI] [Google Scholar]
- 85.Mahmoud B.H., Hexsel C.L., Hamzavi I.H., Lim H.W. Effects of Visible Light on the Skin. Photochem. Photobiol. 2008;84:450–462. doi: 10.1111/j.1751-1097.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 86.Yi Q., Sukhorukov G.B. UV Light Stimulated Encapsulation and Release by Polyelectrolyte Microcapsules. Adv. Colloid Interface Sci. 2014;207:280–289. doi: 10.1016/j.cis.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 87.Liu G.Y., Chen C.J., Li D.D., Wang S.S., Ji J. Near-Infrared Light-Sensitive Micelles for Enhanced Intracellular Drug Delivery. J. Mater. Chem. 2012;22:16865–16871. doi: 10.1039/c2jm00045h. [DOI] [Google Scholar]
- 88.Lin L.S., Cong Z.X., Cao J.B., Ke K.M., Peng Q.L., Gao J., Yang H.H., Liu G., Chen X. Multifunctional Fe3O4@polydopamine Core-Shell Nanocomposites for Intracellular MRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano. 2014;8:3876–3883. doi: 10.1021/nn500722y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han L., Liu M., Yan B., Li Y.S., Lan J., Shi L., Ran R. Polydopamine/Polystyrene Nanocomposite Double-Layer Strain Sensor Hydrogel with Mechanical, Self-Healing, Adhesive and Conductive Properties. Mater. Sci. Eng. C. 2020;109:110567. doi: 10.1016/j.msec.2019.110567. [DOI] [PubMed] [Google Scholar]
- 90.Xu C., Gao F., Wu J., Niu S., Li F., Jin L., Shi Q., Du L. Biodegradable Nanotheranostics with Hyperthermia-Induced Bubble Ability for Ultrasound Imaging–Guided Chemo-Photothermal Therapy. Int. J. Nanomed. 2019;14:7141–7153. doi: 10.2147/IJN.S213518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun X., Meng Z., Yu Q., Wang X., Zhao Z. Engineering PDA-Coated CM-CS Nanoparticles for Photothermo-Chemotherapy of Osteosarcoma and Bone Regeneration. Biochem. Eng. J. 2021;175 doi: 10.1016/j.bej.2021.108138. [DOI] [Google Scholar]
- 92.Wu D., Zhou J., Chen X., Chen Y., Hou S., Qian H., Zhang L., Tang G., Chen Z., Ping Y., et al. Mesoporous Polydopamine with Built-in Plasmonic Core: Traceable and NIR Triggered Delivery of Functional Proteins. Biomaterials. 2020;238:119847. doi: 10.1016/j.biomaterials.2020.119847. [DOI] [PubMed] [Google Scholar]
- 93.Husseini G.A., Pitt W.G. Ultrasonic-Activated Micellar Drug Delivery for Cancer Treatment. J. Pharm. Sci. 2007;98:795–811. doi: 10.1002/jps.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei P., Sun M., Yang B., Xiao J., Du J. Ultrasound-Responsive Polymersomes Capable of Endosomal Escape for Efficient Cancer Therapy. J. Control. Release. 2020;322:81–94. doi: 10.1016/j.jconrel.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 95.Yang B., Du J.Z. Ultrasound-Responsive Homopolymer Nanoparticles. Chin. J. Polym. Sci. (Engl. Ed.) 2020;38:349–356. doi: 10.1007/s10118-020-2345-6. [DOI] [Google Scholar]
- 96.Shi Z., Wu J., Song Q., Göstl R., Herrmann A. Toward Drug Release Using Polymer Mechanochemical Disulfide Scission. J. Am. Chem. Soc. 2020;142:14725–14732. doi: 10.1021/jacs.0c07077. [DOI] [PubMed] [Google Scholar]
- 97.Lin Y.K., Fang J.Y., Wang S.W., Lee R.S. Synthesis and characterization of triple-responsive PNiPAAm-S-S-P(αN3CL-g-alkyne) copolymers bearing cholesterol and fluorescence monitor. React. Funct. Polym. 2018;130:29–42. doi: 10.1016/j.reactfunctpolym.2018.05.008. [DOI] [Google Scholar]
- 98.Sharifianjazi F., Irani M., Esmaeilkhanian A., Bazli L., Asl M.S., Jang H.W., Kim S.Y., Ramakrishna S., Shokouhimehr M., Varma R.S. Polymer incorporated magnetic nanoparticles: Applications for magnetoresponsive targeted drug delivery. Mater. Sci. Eng. B. 2021;272:115358. doi: 10.1016/j.mseb.2021.115358. [DOI] [Google Scholar]
- 99.Beagan A.M., Alghamdi A.A., Lahmadi S.S., Halwani M.A., Almeataq M.S., Alhazaa A.N., Alotaibi K.M., Alswieleh A.M. Folic acid-terminated poly(2-diethyl amino ethyl methacrylate) brush-gated magnetic mesoporous nanoparticles as a smart drug delivery system. Polymers. 2021;13:59. doi: 10.3390/polym13010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Asgari M., Soleymani M., Miri T., Barati A. Design of thermosensitive polymer-coated magnetic mesoporous silica nanocomposites with a core-shell-shell structure as a magnetic/temperature dual-responsive drug delivery vehicle. Polym. Adv. Technol. 2021;32:4101–4109. doi: 10.1002/pat.5417. [DOI] [Google Scholar]
- 101.Wang Y., Pisapati A.V., Zhang X.F., Cheng X. Recent Developments in Nanomaterial-Based Shear-Sensitive Drug Delivery Systems. Adv. Healthc. Mater. 2021;10:2002196. doi: 10.1002/adhm.202002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shen M., Li H., Yao S., Wu X., Liu S., Yang Q., Zhang Y., Du J., Qi S., Li Y. Shear stress and ROS-responsive biomimetic micelles for atherosclerosis via ROS consumption. Mater. Sci. Eng. C. 2021;126:112164. doi: 10.1016/j.msec.2021.112164. [DOI] [PubMed] [Google Scholar]
- 103.Gebeyehu B.T., Huang S.-Y., Lee A.-W., Chen J.-K., Lai J.-Y., Lee D.-J., Cheng C.-C. Dual Stimuli-Responsive Nucleobase-Functionalized Polymeric Systems as Efficient Tools for Manipulating Micellar Self-Assembly Behavior. Macromolecules. 2018;51:1189–1197. doi: 10.1021/acs.macromol.7b02637. [DOI] [Google Scholar]
- 104.Zhang K., Liu J., Guo Y., Li Y., Ma X., Lei Z. Synthesis of Temperature, PH, Light and Dual-Redox Quintuple-Stimuli-Responsive Shell-Crosslinked Polymeric Nanoparticles for Controlled Release. Mater. Sci. Eng. C. 2018;87:1–9. doi: 10.1016/j.msec.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 105.Poddar P., Maity P., Maiti S., Sahoo S., Dhara S., Dhara D. Synthesis of a New Triple-Responsive Biocompatible Block Copolymer: Self-Assembled Nanoparticles as Potent Anticancer Drug Delivery Vehicle. React. Funct. Polym. 2020;154:104679. doi: 10.1016/j.reactfunctpolym.2020.104679. [DOI] [Google Scholar]
- 106.Jiang X., Li R., Feng C., Lu G., Huang X. Triple-Stimuli-Responsive Ferrocene-Containing Homopolymers by RAFT Polymerization. Polym. Chem. 2017;8:2773–2784. doi: 10.1039/C7PY00091J. [DOI] [Google Scholar]
- 107.Lin X., Song X., Zhang Y., Cao Y., Xue Y., Wu F., Yu F., Wu M., Zhu X. Multifunctional Theranostic Nanosystems Enabling Photothermal-Chemo Combination Therapy of Triple-Stimuli-Responsive Drug Release with Magnetic Resonance Imaging. Biomater. Sci. 2020;8:1875–1884. doi: 10.1039/C9BM01482A. [DOI] [PubMed] [Google Scholar]
- 108.Lei W., Sun C., Jiang T., Gao Y., Yang Y., Zhao Q., Wang S. Polydopamine-Coated Mesoporous Silica Nanoparticles for Multi-Responsive Drug Delivery and Combined Chemo-Photothermal Therapy. Mater. Sci. Eng. C. 2019;105:110103. doi: 10.1016/j.msec.2019.110103. [DOI] [PubMed] [Google Scholar]
- 109.Jiang M., Gao X., Zhao N., Cheng X., Yuan W. Amphiphilic Copolymers with Light-PH-Temperature Triple Stimuli-Responses: Preparation, Self-Assembly and Controlled Drug Release. Mater. Lett. 2021;284:129008. doi: 10.1016/j.matlet.2020.129008. [DOI] [Google Scholar]
- 110.Ganguly R., Saha P., Banerjee S.L., Pich A., Singha N.K. Stimuli-Responsive Block Copolymer Micelles Based on Mussel-Inspired Metal-Coordinated Supramolecular Networks. Macromol. Rapid Commun. 2021;42:2100312. doi: 10.1002/marc.202100312. [DOI] [PubMed] [Google Scholar]
- 111.Muttaqien S.E., Nomoto T., Dou X., Takemoto H., Matsui M., Nishiyama N. Photodynamic Therapy Using LCST Polymers Exerting PH-Responsive Isothermal Phase Transition. J. Control. Release. 2020;328:608–616. doi: 10.1016/j.jconrel.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 112.Nikravan G., Haddadi-Asl V., Salami-Kalajahi M. Synthesis of Dual Temperature–and PH-Responsive Yolk-Shell Nanoparticles by Conventional Etching and New Deswelling Approaches: DOX Release Behavior. Colloids Surf. B Biointerfaces. 2018;165:1–8. doi: 10.1016/j.colsurfb.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 113.Hiruta Y., Kanda Y., Katsuyama N., Kanazawa H. Dual Temperature-and PH-Responsive Polymeric Micelle for Selective and Efficient Two-Step Doxorubicin Delivery. RSC Adv. 2017;7:29540–29549. doi: 10.1039/C7RA03579A. [DOI] [Google Scholar]
- 114.Pourjavadi A., Kohestanian M., Streb C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C. 2020;108:110418. doi: 10.1016/j.msec.2019.110418. [DOI] [PubMed] [Google Scholar]
- 115.Zheng Y., Wang L., Lu L., Wang Q., Benicewicz B.C. PH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega. 2017;2:3399–3405. doi: 10.1021/acsomega.7b00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi J., Ren Y., Ma J., Luo X., Li J., Wu Y., Gu H., Fu C., Cao Z., Zhang J. Novel CD44-Targeting and PH/Redox-Dual-Stimuli-Responsive Core–Shell Nanoparticles Loading Triptolide Combats Breast Cancer Growth and Lung Metastasis. J. Nanobiotechnol. 2021;19 doi: 10.1186/s12951-021-00934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhuang W., Xu Y., Li G., Hu J., Ma B., Yu T., Su X., Wang Y. Redox and Ph Dual-Responsive Polymeric Micelle with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. ACS Appl. Mater. Interfaces. 2018;10:18489–18498. doi: 10.1021/acsami.8b02890. [DOI] [PubMed] [Google Scholar]
- 118.Xia D., Wang F., Pan S., Yuan S., Liu Y., Xu Y. Redox/PH-Responsive Biodegradable Thiol-Hyaluronic Acid/Chitosan Charge-Reversal Nanocarriers for Triggered Drug Release. Polymers. 2021;13:3785. doi: 10.3390/polym13213785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y., Liu C., Tang C., Yin C. Dual Stimulus-Responsive Chitosan-Based Nanoparticles Co-Delivering Doxorubicin and Quercetin for Cancer Therapy. Mater. Lett. 2021;305:130826. doi: 10.1016/j.matlet.2021.130826. [DOI] [Google Scholar]
- 120.Liu J., Li J., Liu T. Fabrication of Mixed Polymeric Micelles Based on Stimuli-Responsive Amphiphilic Copolymers for Drug Delivery and Controlled Release. Nano. 2020;15 doi: 10.1142/S179329202050040X. [DOI] [Google Scholar]
- 121.Wang N., Liu C., Yao W., Zhou H., Yu S., Chen H., Qiao W. A Traceable, GSH/PH Dual-Responsive Nanoparticles with Spatiotemporally Controlled Multiple Drugs Release Ability to Enhance Antitumor Efficacy. Colloids Surf. B Biointerfaces. 2021;205:111866. doi: 10.1016/j.colsurfb.2021.111866. [DOI] [PubMed] [Google Scholar]
- 122.Jing X., Zhi Z., Jin L., Wang F., Wu Y., Wang D., Yan K., Shao Y., Meng L. pH/Redox Dual-Stimuli-Responsive Cross-Linked Polyphosphazene Nanoparticles for Multimodal Imaging-Guided Chemo-Photodynamic Therapy. Nanoscale. 2019;11:9457–9467. doi: 10.1039/C9NR01194C. [DOI] [PubMed] [Google Scholar]
- 123.Panda S., Bhol C.S., Bhutia S.K., Mohapatra S. PEG-PEI-Modified Gated N-Doped Mesoporous Carbon Nanospheres for pH/NIR Light-Triggered Drug Release and Cancer Phototherapy. J. Mater. Chem. B. 2021;9:3666–3676. doi: 10.1039/D1TB00362C. [DOI] [PubMed] [Google Scholar]
- 124.Fan B., Gillies E.R. Poly(Ethyl Glyoxylate)-Poly(Ethylene Oxide) Nanoparticles: Stimuli-Responsive Drug Release via End-to-End Polyglyoxylate Depolymerization. Mol. Pharm. 2017;14:2548–2559. doi: 10.1021/acs.molpharmaceut.7b00030. [DOI] [PubMed] [Google Scholar]
- 125.Chen K., Cai H., Zhang H., Zhu H., Gu Z., Gong Q., Luo K. Stimuli-Responsive Polymer-Doxorubicin Conjugate: Antitumor Mechanism and Potential as Nano-Prodrug. Acta Biomater. 2019;84:339–355. doi: 10.1016/j.actbio.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 126.Cho H.K., Cheong I.W., Lee J.M., Kim J.H. Polymeric Nanoparticles, Micelles and Polymersomes from Amphiphilic Block Copolymer. Korean J. Chem. Eng. 2010;27:731–740. doi: 10.1007/s11814-010-0216-5. [DOI] [Google Scholar]
- 127.Zhang D., Li J., Xie H., Zhu A., Xu Y., Zeng B., Luo W., Dai L. Polyion Complex Micelles Formed by Azobenzene-Based Polymer with Multi-Responsive Properties. J. Appl. Polym. Sci. 2021;138 doi: 10.1002/app.50580. [DOI] [Google Scholar]
- 128.Kalhapure R.S., Renukuntla J. Thermo- and PH Dual Responsive Polymeric Micelles and Nanoparticles. Chem.-Biol. Interact. 2018;295:20–37. doi: 10.1016/j.cbi.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 129.Johnson B.K., Prud’homme R.K. Mechanism for Rapid Self-Assembly of Block Copolymer Nanoparticles. Phys. Rev. Lett. 2003;91:1–4. doi: 10.1103/PhysRevLett.91.118302. [DOI] [PubMed] [Google Scholar]
- 130.Almeida H., Amaral M.H., Lobão P. Temperature and PH Stimuli-Responsive Polymers and Their Applications in Controlled and Selfregulated Drug Delivery. J. Appl. Pharm. Sci. 2012;2:01–10. doi: 10.7324/JAPS.2012.2609. [DOI] [Google Scholar]
- 131.Chuang C.-Y., Don T.-M., Chiu W.-Y. Synthesis and Properties of Chitosan-Based Thermo- and PH-Responsive Nanoparticles and Application in Drug Release. J. Polym. Sci. 2009;47:2798–2810. doi: 10.1002/pola.23369. [DOI] [Google Scholar]
- 132.Heskins M., Guillet J.E. Solution Properties of Poly(N-Isopropylacrylamide) J. Macromol. Sci. Part A-Chem. 1968;2:1441–1455. doi: 10.1080/10601326808051910. [DOI] [Google Scholar]
- 133.Cheng R., Meng F., Deng C., Klok H., Zhong Z. Biomaterials Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Programmed Site-Speci Fi c Drug Delivery. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]
- 134.Zhang L., Guo R., Yang M., Jiang X., Liu B. Thermo and PH Dual-Responsive Nanoparticles for Anti-Cancer Drug Delivery. Adv. Mater. 2007;19:2988–2992. doi: 10.1002/adma.200601817. [DOI] [Google Scholar]
- 135.Kuo C.Y., Liu T.Y., Hardiansyah A., Lee C.F., Wang M.S., Chiu W.Y. Self-Assembly Behaviors of Thermal- and PH- Sensitive Magnetic Nanocarriers for Stimuli-Triggered Release. Nanoscale Res. Lett. 2014;9 doi: 10.1186/1556-276X-9-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gerweck L.E., Seetharaman K. Cellular pH Gradient in Tumor versus Normal Tissue: Potential Exploitation for the Treatment of Cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 137.Stefanadis C., Chrysochoou C., Markou D., Petraki K., Panagiotakos D.B., Fasoulakis C., Kyriakidis A., Papadimitriou C., Toutouzas P.K. Increased Temperature of Malignant Urinary Bladder Tumors in Vivo: The Application of a New Method Based on a Catheter Technique. J. Clin. Oncol. 2001;19:676–681. doi: 10.1200/JCO.2001.19.3.676. [DOI] [PubMed] [Google Scholar]
- 138.Remant B., Thapa B., Xu P. pH and Redox Dual Responsive Nanoparticle for Nuclear Targeted Drug Delivery. Mol. Pharm. 2012;9:2719–2729. doi: 10.1021/mp300274g. [DOI] [PubMed] [Google Scholar]
- 139.Mahmoud E.A., Sankaranarayanan J., Morachis J.M., Kim G., Almutairi A. Inflammation Responsive Logic Gate Nanoparticles for the Delivery of Proteins. Bioconjugate Chem. 2011;22:1416–1421. doi: 10.1021/bc200141h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santra S., Sk M.A., Mondal A., Molla M.R. Self-Immolative Polyurethane-Based Nanoassemblies: Surface Charge Modulation at Tumor-Relevant PH and Redox-Responsive Guest Release. Langmuir. 2020;36:8282–8289. doi: 10.1021/acs.langmuir.0c01474. [DOI] [PubMed] [Google Scholar]
- 141.Sankaranarayanan J., Mahmoud E.A., Kim G., Morachis M., Almutairi A. Multiresponse Strategies to Modulate Burst Degradation and Release from Nanoparticles. ACS Nano. 2010;4:5930–5936. doi: 10.1021/nn100968e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Du J.Z., Du X.J., Mao C.Q., Wang J. Tailor-Made Dual pH-Sensitive Polymer-Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 2011;133:17560–17563. doi: 10.1021/ja207150n. [DOI] [PubMed] [Google Scholar]
- 143.Zhang F., Ni Q., Jacobson O., Cheng S., Liao A., Wang Z., He Z., Yu G., Song J., Ma Y., et al. Polymeric Nanoparticles with a Glutathione-Sensitive Heterodimeric Multifunctional Prodrug for In Vivo Drug Monitoring and Synergistic Cancer Therapy. Angew. Chem.-Int. Ed. 2018;57:7066–7070. doi: 10.1002/anie.201801984. [DOI] [PubMed] [Google Scholar]
- 144.Ao L., Wu C., Liu K., Wang W., Fang L., Huang L., Su W. Polydopamine-Derivated Hierarchical Nanoplatforms for Efficient Dual-Modal Imaging-Guided Combination in Vivo Cancer Therapy. ACS Appl. Mater. Interfaces. 2018;10:12544–12552. doi: 10.1021/acsami.8b02973. [DOI] [PubMed] [Google Scholar]