Abstract
Acid sphingomyelinase deficiency (ASMD) is a rare inherited lipid storage disorder caused by a deficiency in lysosomal enzyme acid sphingomyelinase which results in the accumulation of sphingomyelin, predominantly within cells of the reticuloendothelial system located in numerous organs, such as the liver, spleen, lungs, and central nervous system. Although all patients with ASMD share the same basic metabolic defect, a wide spectrum of clinical presentations and outcomes are observed, contributing to treatment challenges. While infantile neurovisceral ASMD (also known as Niemann–Pick disease type A) is rapidly progressive and fatal in early childhood, and the more slowly progressive chronic neurovisceral (type A/B) and chronic visceral (type B) forms have varying clinical phenotypes and life expectancy. The prognosis of visceral ASMD is mainly determined by the association of hepatosplenomegaly with secondary thrombocytopenia and lung disease. Early diagnosis and appropriate management are essential to reduce the risk of complications and mortality. The accessibility of the new enzyme replacement therapy olipudase alfa, a recombinant human ASM, has been expedited for clinical use based on positive clinical data in children and adult patients, such as improved respiratory status and reduced spleen volume. The aim of this article is to share the authors experience on monitoring ASMD patients and stratifying the severity of the disease to aid in care decisions.
Keywords: acid sphingomyelinase deficiency, ceramide, enzyme replacement therapy, morbidity, mortality, Niemann–Pick disease, olipudase alfa, recombinant human acid sphingomyelinase, sphingomyelin
1. Introduction
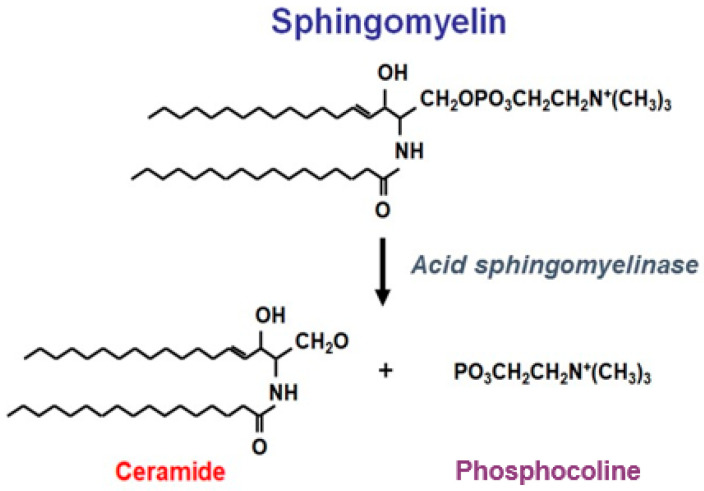
Acid sphingomyelinase deficiency (ASMD) is an inborn error of metabolism that leads to the accumulation of sphingomyelin in cells and tissues causing the clinical condition also known as Niemann–Pick disease type A, A/B and B (NPD) [1]. In ASMD, the enzymatic deficiency of the lysosomal acid sphingomyelinase (ASM), is caused by pathogenic variants of the sphingomyelin phosphodiesterase 1 gene (SMPD1; EC 3.1.4.12). Sphingomyelin is a major structural component of all plasma membranes. Cellular physiological function requires ASM to catalyze the hydrolysis of sphingomyelin to ceramide and phosphocholine (Figure 1). ASMD results in the progressive accumulation of sphingomyelin within all cells, but mainly in reticuloendothelial cells and so in spleen, liver, lung, bone marrow and lymph nodes, but also in neurons in neurovisceral forms. Sphingomyelinase enzyme activity generates ceramides that are bioactive sphingolipids that play a major role in inflammation. Ceramides act as second messengers transducing pro-inflammatory signals such as tumour necrosis factor-alpha [2,3]. Ceramides activate NFkB that in turn encodes for pro-inflammatory cytokines, such as interleukin (IL)-1 beta and IL-6 [4].
Figure 1.
Enzymatic action of acid sphingomyelinase (ASM). Normal physiological function requires ASM to catalyse the hydrolysis of sphingomyelin to ceramide and phosphocoline. Ceramides have a proinflammatory effect as they are bioactive sphingolipids that act as second messengers which transduce pro-inflammatory signals.
Numerous variants of the SMPD1 gene, along with variability in residual ASM activity and other genetic/epigenetic factors, result in a spectrum of ASMD disease severity from a uniformly fatal form with death occurring by 3–4 years of age (ASMD type A or infantile neurovisceral ASMD previously known as NPD A) to chronic forms characterised by visceral (ASMD type B or chronic visceral ASMD, previously NPD B) and neurovisceral disease (ASMD type A/B chronic neurovisceral ASMD, previously NPD A/B) (Table 1) [5,6,7]. Of note, while Niemann–Pick disease type C and ASMD share several phenotypic features, they represent two distinct disease entities [8]. Reliable estimates of the birth prevalence of ASMD are currently lacking [9]; however, an estimated birth prevalence of 0.4–0.6 per 100,000 births has been reported [10]. In France, the birth prevalence of the chronic visceral, non-neurological form is 1/230,000 births and is recognised as being an underdiagnosed disease (clinical experience suggests 150 patients diagnosed in France over the last 30 years) [11].
Table 1.
Nomenclature for ASMD, adapted from [7].
| Historical Classification | Recommended Nomenclature |
|---|---|
| Niemann–Pick disease type A (NPD A) |
Infantile neurovisceral ASMD (ASMD Type A) |
| Intermediate or variant phenotype (NPD A/B) |
Chronic neurovisceral ASMD (ASMD Type A/B) |
| Niemann–Pick disease type B (NPD B) |
Chronic visceral ASMD (ASMD Type B) |
ASMD, acid sphingomyelinase deficiency; NPD, Niemann–Pick disease.
The clinical phenotype and life expectancy of patients with ASMD type B appears to vary, with many adults reaching a normal lifespan, while others die prematurely from ASMD-related complications, such as respiratory insufficiency and liver disease [1,5,6,7,12]. Most patients with ASMD type B have interstitial lung disease with progressive impairment of pulmonary function, hepatosplenomegaly, haematological abnormalities (such as anaemia and thrombocytopenia) with bleeding, and an atherogenic lipid profile [7,9]. Diffuse interstitial lung disease with ground glass opacity, interlobular septal thickening, and intralobular lines, is common in ASMD type B [13]; however, symptoms vary from normal function through to respiratory failure [1,14]. Other common manifestations include liver disease (from persistent elevated transaminases [5] to cirrhosis [15] with possible liver failure [9]), heart disease (cardiac hypertrophy, valve regurgitation, conduction abnormalities, early myocardial infarction) [14], skeletal abnormalities, failure to thrive, and growth deficits in children which can persist into adulthood [7,16,17].
Data remain limited regarding predictors of disease-related morbidity, healthcare use, and lifestyle impact in patients with chronic ASMD; however, the physical, emotional, financial, and psychosocial burden of illness in patients with ASMD type B and ASMD type A/B is substantial [1,9,18,19]. McGovern et al. (2021) reported predictive factors of mortality that include both total splenectomy and spleen volume ≥15 multiples of normal (MN) at baseline [1]. Indeed, in an 11-year prospective natural history study of children and adults with ASMD, patients with a history of either severe splenomegaly or prior splenectomy had 10 times increased the likelihood of dying during follow up compared with those patients with moderate splenomegaly or intact spleens. On the contrary, liver volume of ≥2.5 MN was not shown to be a predictor of mortality. Interstitial lung disease was reported to worsen gradually with a mean diffusion capacity of carbon monoxide (DLCO) decreasing below 50% of the predicted value in adult patients. Atherogenic lipid profiles typically worsen with age in patients with ASMD type B [5], and lipid abnormalities may be associated with early coronary artery disease [20].
Due to the rarity and heterogeneity of the disease, there is a lack of robust quantitative data regarding the impact of the disease on patients’ and caregivers’ quality of life (QoL), compounded by the lack of adequate disease-specific instruments to measure QoL [9].
2. Clinical Study Evidence for the Use of Olipudase Alfa in the Non-Neurologic Manifestations of ASMD
Knockout mouse models have demonstrated that enzyme replacement therapy (ERT) is likely to be a useful therapeutic approach for patients with non-neurological ASMD, with a strong response to ERT in the major organs involved in the disease (liver, spleen, and lung) [21]. For the neurological manifestation of ASMD, ERT is unlikely to evoke any therapeutic response in the central nervous system since it does not cross the blood–brain barrier. Importantly, progressive dose escalation appeared essential to prevent the possibility of a deadly cytokine storm due to the abrupt release of high levels of ceramide [22].
Olipudase alfa is currently being investigated as an ERT for the treatment of the non-neurological manifestations of ASMD [21,23]. Of note, olipudase alfa is the first and only investigational ERT in late-stage development for ASMD. Olipudase alfa acts by targeting the underlying metabolic defect by supplementing the deficient enzyme activity [24].
A Phase 1A study assessed the infusion of a single dose (0.03–1.0 mg/kg) of olipudase alfa in 11 patients with ASMD type B [25]; a maximum starting dose of 0.6 mg/kg was identified which supported a dose-escalation strategy for the gradual clearance of accumulated sphingomyelin which was applied in a Phase 1B study. Of note, the aim of the dose-escalation process of olipudase alfa is gradual sphingomyelin debulking to prevent high ceramide release (Figure 1). Blood ceramide levels increased up to five-fold (both dose- and time-dependent), beginning 2–6 h after infusion, peaking between 24 and 48 h and returning to pre-infusion levels by Day 14. This led to systemic inflammation illustrated by high levels of C-reactive protein and bilirubin reported in patients who received the highest dosages of olipudase alfa (i.e., 0.6 and 1.0 mg/kg). No serious adverse drug reactions occurred during the study.
A Phase 1B study assessed safety and tolerability of olipudase alfa in five patients with ASMD type B reported no death, serious or severe adverse events (AEs), or AEs leading to discontinuation over the 26-week study period [26]; most AEs were mild (97%) and resolved without sequelae and all patients were successfully escalated to the maximum study dose of 3.0 mg/kg. A positive therapeutic response was also observed in liver sphingomyelin content, spleen and liver volumes, along with improvements in infiltrative lung disease, lipid profiles, platelet counts, and QoL. A sustained safety profile and continued improvements in clinically relevant parameters following up to 42 months of treatment in these patients has been reported [26,27,28].
A long-term safety study (NCT02004704) has demonstrated that olipudase alfa provides improvements in lipid profiles up to 42 months [28,29,30]; these include progressive reductions from baseline in pro-atherogenic lipid profiles (total cholesterol, low-density lipoprotein cholesterol [LDL-C], very low-density lipoprotein cholesterol, triglycerides) and progressive increases in anti-atherogenic markers (high-density lipoprotein cholesterol and apolipoprotein A-I).
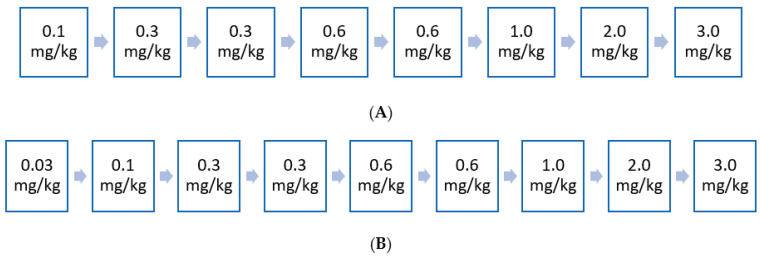
Two separate clinical studies evaluating olipudase alfa for the treatment of chronic ASMD in adult and paediatric patients have also demonstrated positive results [27,31]. The Phase I/II open label, ascending dose, multicentre ASCEND-Peds study (NCT02292654) evaluated the safety, tolerability, and pharmacokinetics of olipudase alfa in 20 paediatric patients with chronic visceral ASMD over a 64-week study period [27]. A clinically meaningful improvement in pulmonary function assessed by the DLco and reduction in spleen size were reported—after 52 weeks of treatment, mean spleen volume decrease was shown to be 49%, while the nine patients who were able to perform the DLco test showed a mean increase of 33%. Most AEs were mild to moderate in severity, and no permanent treatment discontinuations were reported. The ongoing Phase II/III randomised, double-blind, placebo-controlled ASCEND (adults) study (NCT02004691) evaluated the efficacy, safety, and pharmacokinetics/pharmacodynamics of olipudase alfa in 36 adults with chronic visceral ASMD over a 52-week primary analysis period, which is now being followed by an extension period (all treated patients) of up to 4 years [31]. Patients received either olipudase alfa intravenous infusion or placebo every two weeks at an escalating dose from 0.1 mg/kg up to 3 mg/kg administered every two weeks. Figure 2 shows the dose-escalation strategy used in the ASCEND (adults) and ASCEND-Peds studies. The ASCEND (adults) study assessed two independent primary efficacy endpoints: DLco and spleen volume to reflect the separate critical manifestations of chronic visceral ASMD, with the trial outcome being deemed positive if one of these endpoints was met. Treatment outcomes favoured olipudase alfa (vs placebo) at Week 52 for spleen volume, percentage predicted DLco, liver volume and platelet levels. Overall, spleen volume was significantly decreased by 39.5% in the olipudase alfa arm compared with a 0.5% increase in the placebo arm (40% difference; p < 0.0001). In addition, percent change from baseline in percentage predicted DLco was +22% for the olipudase alfa arm at Week 52 compared with +3% for the placebo arm (19% difference; p = 0.0004). In addition, all treatment-related AEs were mild-to-moderate in severity and no patients discontinued due to AEs [19].
Figure 2.
(A) Dose escalation regimen for adult patients administered every 2 weeks. Dose-escalation strategy for olipudase alfa after the Phase 1B study [31]. The aim of the dose-escalation process of olipudase alfa is gradual sphingomyelin debulking to prevent high ceramide release. BMI, body mass index. (B) Dose escalation regimen for pediatric patients administered every 2 weeks. Dose escalation regimen of olipudase alfa phase I/II ASCEND PEDS [27].
In 2015, the US Food and Drug Administration granted a breakthrough therapy designation to olipudase alfa based on the Phase 1b study data, an approach used to expedite the development and review of drugs intended to treat serious or life-threatening diseases and conditions [32]. Similarly, in 2016, olipudase alfa has gained orphan designation and ‘PRIority Medicines’ (PRIME) designation by the European Medicines Agency, along with being awarded the SAKIGAKE designation in Japan, in order to expedite the regulatory process [33].
3. Materials and Methods
Four expert meetings took place in Paris from 2019 to 2021, followed by several teleconferences, to gather expert opinion of French physicians with regards to the monitoring and severity stratification of children and adult patients with ASMD types B and A/B. All authors participated in the meetings apart from W.M. W.M., O.L., and A.B. have coordinated the process of early access to treatment in French ASMD patients. All co-authors have worked on this process.
4. French Expert Opinion: Monitoring of ASMD and Patient Severity Stratification
4.1. General Care of Patients with ASMD Type B Forms
Treatment goals for patients with chronic visceral ASMD should focus on reducing spleen and liver volumes, limiting hepatic fibrosis, decreasing cytopenia, improving liver function tests, and respiratory status in order to decrease morbidity and mortality [12]. However, the current management of ASMD type B has been limited to supportive care and palliation [9,13,34].
Regardless of aetiology, any respiratory failure should be treated with oxygen and respiratory rehabilitation [9,35,36]. Patients should also be encouraged to stop or avoid smoking (including second-hand exposure), to reduce the risk of lung and coronary disease, as well as cancer [13,37]. There is no specific recommendation but standard lipid-lowering agents, such as statins or ezetimibe, have shown benefits and are classically proposed to manage blood lipid abnormalities [9,13,38].
While there is no available treatment shown to improve bone density in patients with ASMD type B, the use of regular calcium and vitamin D supplementation to support healthy bone function is recommended [9]; of note, the use of bisphosphonates should be avoided given their potent inhibition of ASM [39].
Due to the low platelet count and splenomegaly, contact sports (particularly those with a risk of abdominal impact) should be avoided [9]. Splenectomy should be avoided as it has been demonstrated to increase the risk of lung disease and overall mortality [5].
Optimised immunisation in ASMD patient population is challenging because of a data gap in vaccine trials. Vaccinations against pneumococci, Haemophilus influenzae type B, meningococci, and influenza virus are recommended for patients with anatomic or functional asplenia [9,13,40]. While the use of the meningitis vaccine is mandatory in paediatric patients, its use in adult patients is also mandatory in patients with anatomic or functional asplenia [41]. In adult patients with platelets >30 G/L, vaccination against COVID-19 should now empirically be offered, as with all patients with a higher risk of severe COVID-19 due to chronic lung and/or liver disease [42].
4.2. Monitoring of Patients with ASMD Type B Forms
Scheduled patient monitoring remains essential to manage symptoms as early as possible, in order to minimise the risk of poor clinical outcomes. Based on our experience, a summary of key monitoring parameters along with the frequency of assessment for patients with ASMD type B are shown in Table 2 (pulmonary, hepato-splenic, and haematological assessments) and Table 3 (summary of all monitoring parameters). It is recommended that patient monitoring would be undertaken by a multidisciplinary team in an experienced centre (referral or competence centre in France). As with other diseases, the frequency of monitoring must be determined by patient severity and assessment results, as well as the progression of the disease.
Table 2.
Key monitoring parameters and frequency of assessments for patients with ASMD type B forms.
| Exam | Baseline Visit | Follow-Up Visit in the First 6 Months | Follow-Up Visit Every 3 Months for the First Year | Follow-Up Visit Every Year |
|---|---|---|---|---|
| Pulmonary assessment | ||||
| Respiratory functional exploration (DLco—not before 6 years) | X | X | X | |
| CT: thoracic | X | X a | ||
|
Hepato-splenic
assessment |
||||
| Splenic and hepatic volume (ultrasound +/− abdominal MRI in adults; ultrasound in children) | X | X | X | |
| Portal hypertension (abdominal ultrasound with Doppler) | X | X | X | |
| Transaminases, alkaline phosphatase, bilirubin |
X | X | X | |
|
Haematological
assessment |
||||
| CBC, platelets, haemostasis assessment (PT, factor V, fibrin, APTT, ferritin) | X | X | X | |
| Lipid blood-test including total, LDL and HDL cholesterol and triglycerides |
X | X | X |
a If normal, then every 5 years. APTT, activated partial thromboplastin time; CBC, complete blood count; CT, computed tomography; DLco, diffusing capacity of the lungs for carbon monoxide; HDL, high density lipoprotein, LDL, low density lipoprotein; MRI, magnetic resonance imaging; PT, prothrombin time.
Table 3.
Key monitoring parameters and frequency of assessment for patients with ASMD type B forms (NPD B).
| Test | Monitoring Frequency | Additional Points |
|---|---|---|
| Lung assessment | ||
| PFT | PFT possible in children from 5–6 years Need to monitor DLco (annually or at clinician’s discretion) following olipudase alfa treatment initiation |
|
| Blood gases | - | Non-predictive test Not routinely recommended, especially in children, and depending on symptomatology in adults |
| CT scan | At baseline visit and at follow-up visit annually, unless normal (then assess every 5 years) | Radiation examination in a population at risk of cancer In paediatric patients <4–5 years, sedation or general anaesthesia is required (depending on the child’s behaviour) |
|
Hepato-splenic
assessment |
||
| Liver blood tests | At baseline visit, at follow-up visit in first 6 months, and at follow-up visit annually | Assess GGT, transaminases, ALP, bilirubin, PT, CRP, Factor V |
| Abdominal ultrasound with doppler | At baseline visit, at follow-up visit in first 6 months, and at follow-up visit annually | Early detection of steatosis, PHT, cirrhosis or nodule Possible in children < 5 years of age |
| Abdominal MRI (adult patients) | At initial assessment and then every 2 years | Higher sensitivity for nodule detection Possible in children > 5 years of age |
| Fibroscan | - | Not recommended as not validated for ASMD |
| Liver biopsy | To consider if hepatocarcinoma is suspected | No correlation with biological tests (transaminases usually < 5 N). Characterisation of nodule may require alpha-foetoprotein, liver ultrasound, and MRI |
| Alpha-fetoprotein | - | No recommendation for systematic assessment |
|
Haematological
assessment |
||
| CBC, platelets, haemostasis test (PT, Factor V, fibrin, aPTT), ferritin | Assessment at baseline visit, at follow-up visit every 3 months during first year, and at follow-up visit annually | - |
| Protein electrophoresis | At initial assessment and then annually | Risk of hyper or hypogammaglobulinemia and risk of MGUS No need to perform any immunoelectrophoresis |
| Serum albumin | At initial assessment then every 2–3 years if the initial assay is normal | - |
| Bone assessment and growth evaluation | ||
| Growth curve | Assessment at baseline visit and at follow-up visit every 6 months | - |
| Phosphocalcium balance | Assessment at baseline visit and at follow-up visit every 6 months | Assessment to include blood calcium and phosphorus, vitamin D, creatinine, proteinuria, urine calcium and sodium, and creatininuria Patients are at risk of cholestasis |
| Absorptiometry | Assessment at baseline visit and then every 5 years if normal or every 3 years if anormal | Possible in children aged from 5–6 years old |
| Resorption markers | Optional | To be performed if osteoporosis is known |
| Cardiovascular assessment and lipid profile | ||
| Echocardiography | At initial assessment and then every 2 years | |
| Coronary computed tomographic angiography | Discuss coronary computed tomographic angiography depending on lipid profile and other cardiovascular risk factors | No monitoring data in ASMD type B disease |
| Lipid profile | Assessment at baseline visit, at follow-up visit every 3 months during first year, and at follow-up visit annually | Unproven benefit of statins in primary prevention |
| Neurological assessment a | ||
| Peripheral | ||
| Clinical examination | Monitoring frequency: at initial assessment and then annually | |
| EMG | Monitoring frequency: to be considered if there are clinical call points | |
| Central nervous system | ||
| Brain MRI | Monitoring frequency in adults: to be considered at initial assessment according to the clinical context and the neuropsychological tests Monitoring frequency in children: to be carried out at initial assessment if the diagnosis is made at a very early stage of childhood |
|
| Ophthalmic assessment | ||
| Ocular fundus | At initial assessment | The macular cherry-red spot is present in all patients with form A, and has been reported in one third of patients with form B [20] |
| Visual acuity | At initial assessment | Loss of visual acuity in infantile type (no effect in chronic visceral ASMD) |
| Other | ||
| Dermatological examination | At initial assessment and then annually | Necessary in order to assess the presence of lymphoedema (eyelid infiltration) |
| TSH assay (in combination with anti-TPO antibodies) | At initial assessment and then annually | Necessary for the investigation of thyroid autoimmunity |
| Biomarkers | ||
| Chitotriosidase | At initial assessment, follow-up every 3 months for the first year, and then every year | Chitotriosidase activity is absent in 6–8% of individuals in the general population |
| Specialised assays, contact reference laboratories | ||
| Lysosphingomyelin + Lysosphingomyelin isoform 509 |
At initial assessment, follow-up every 3 months for the first year, and then every year | Specialised assays, contact reference laboratories |
a Note: The clinical dichotomy between forms A and B occurs very early before the age of 1 year (around 3–6 months). For form A/B, the age of onset of neurological signs is highly variable (delayed acquisition at a variable age, possible after the age of 3 years). aPTT, activated partial thromboplastin time; CBC, complete blood count; CRP, C-reactive protein; CT, computerised tomography; DLco, diffusing capacity of the lungs for carbon monoxide; EMG, electromyography; GGT, gamma-glutamyl transferase; MGUS, monoclonal gammopathy of unknown significance; MRI, magnetic resonance imaging; PFT, pulmonary function test; PHT, portal hypertension; PT, prothrombin time; TSH, thyroid-stimulating hormone; TPO, thyroid peroxidase.
Given the future availability of ERT, there is a need for biomarkers that predict or reflect disease progression [17]; suitable markers need to be validated for their use as surrogate markers of clinically relevant endpoints. Eskes et al. (2020) highlighted that the best evaluated potential biomarkers for ASMD are DLco, spleen volume, platelet count, LDL-C, liver fibrosis (measured with fibroscan) and lysosphingomyelin [17]. Our opinion is that the biomarkers chitotriosidase, lysosphingomyelin, and lysosphingomyelin 509 should be assessed at baseline, with follow up every 3 or 6 months for the first year, and then every year (Table 3).
Given that ASMD is an autosomal recessive inherited condition, carrier testing for at-risk relatives, prenatal testing for a pregnancy at increased risk, and preimplantation genetic testing are all possible, following the identification of a SMPD1 pathogenic variant in an affected family member [43,44]. In general, there is no specific complication during pregnancy in patients with ASMD type B; although, episodes of bleeding have been reported. ASMD type A is the most severe form of ASMD with rapidly progressive neurovisceral manifestations and fatal issues with multisystem involvement. For ASMD type A, prenatal diagnosis is routinely accomplished by mutational analysis when an index case is identified in the family [43].
4.3. Patients with ASMD: Stratification by Disease Severity
In France, the exceptional use of proprietary medicinal products which do not have a marketing authorisation and are not the subject of a clinical trial can occur under the following three conditions: (1) specialties are intended to treat, prevent, or diagnose serious or rare diseases; (2) there is no appropriate treatment available on the market; and (3) their efficacy and safety of use are presumed in the state of scientific knowledge and the implementation of the treatment cannot be postponed [45]. This exceptional so-called ‘Temporary Use Authorisation’ (TUA) is delineated by specific criteria that have to be defined according to the results of the different trials and the criteria of the expected future marketing authorisation.
Where treatment is warranted based on disease severity, early access to olipudase alfa can be possible based on the following conditions: (1) the patient or person of trust must be informed of the exceptional and critical nature of this requirement; (2) specialties are intended to treat, prevent, or diagnose serious or rare diseases; (3) there is no appropriate treatment available on the market; (4) the patient cannot be included in a clinical trial; and (5) the effectiveness and safety of use of the prescribed treatment are not only presumed in the state of scientific knowledge and the implementation of the processing cannot be postponed.
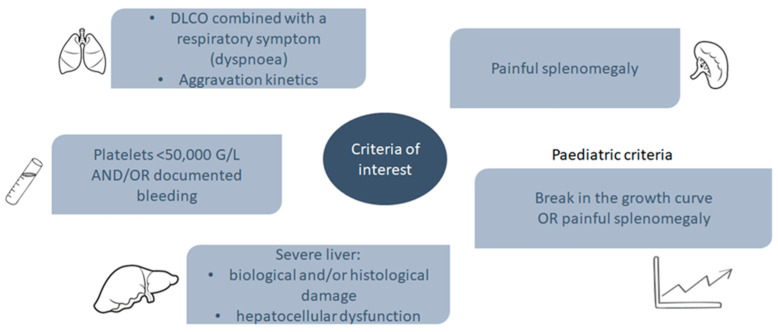
In order to define the access criteria to the TUA procedure, the Therapeutic Evaluation Committee for Visceral Lipidoses (CETLv) in France convened a disease severity classification of patients with ASMD type B or A/B, i.e., via the Brassier–Lidove classification (Table 4). Worsening dyspnoea, low platelets <50 G/L and/or recurrent bleeding, biological and/or histological liver damage, and painful splenomegaly are key features of severe disease. Important clinical criteria in paediatric patients include a break in the growth curve or painful splenomegaly (Figure 3).
Table 4.
Patients with chronic ASMD grouped among disease severity (CETLv classification).
| Group | Definition |
|---|---|
| Group outside the early access spectrum application | ASMD types B and A/B (NPD B or A/B) with a short-term vital prognosis (e.g., cancer) |
| Group 1: Patients with severe organ involvement | DLco a <50% and/ or dyspnoea Platelets <50 G/L ± recurrent bleeding or bruising Abdominal pain Urgent need to start treatment Break in growth curve (≥2 standard deviation) |
| Group 2: Patients in therapeutic trials | Patient continues to receive treatment as part of clinical trial or extension study |
| Group 3: Patients with moderate organ involvement | 50% < DLco < 70% 50 G/L < Platelets < 100 G/L without bleeding or bruising Failure to thrive/break in growth curve (>1 standard deviation) |
| Group 4: Patients with mild organ involvement | DLco > 70% Platelets > 100 G/L Abnormal thoracic imagery and/or hepatosplenomegaly |
| Group 5: Patients with no symptoms | DLCO > 70% Platelets > 100 G/L No symptom Normal thoracic imagery No hepato-splenomegaly |
ASMD, acid sphingomyelinase deficiency; DLco, diffusing capacity of the lungs for carbon monoxide; NPD, Niemann–Pick disease. a DLco not available for patients aged <6 years old.
Figure 3.
Clinical criteria of interest for patients with ASMD type B or A/B. ASMD, acid sphingomyelinase deficiency; DLco, diffusing capacity of the lungs for carbon monoxide.
During nominative early access to olipudase alfa the Brassier–Lidove classification (CETLv classification) (Table 4) has been applied. Of 29 adult patients from the Croix Saint-Simon Hospital (Paris, France), two were deemed to be outside the early access application because of a limited life expectancy, two were in CETLv Group 1 and were too severely affected to participate in ASCEND trial (Table 5) (one patient with symptomatic thrombocytopenia <40 G/L and one patient with severe pulmonary failure awaiting lung transplantation), two were in CETLv Group 2 (both patients were participating in the ASCEND study), eight patients were in CETLv Group 3 (including two patients receiving psychotropic drugs), ten patients were in CETLv Group 4, and five patients in CETLv Group 5. Of this patient cohort, 10/29 (34.5%) patients were deemed to be eligible for olipudase alfa via ATU according to the CETLv classification. In the Referral Center for Inherited Diseases of Metabolism at the Hospices Civils of Lyon (Lyon, France), there have been two children treated since 2015 and 2018 as part of the pediatric clinical trial and two children treated as part of a nominative ATU (Group 1). Two adults have also been treated in the ATU for severe lung damage (Group 1).
Table 5.
Criteria in ASCEND (adults and peds) study for use of olipudase alfa.
| Criteria | ASCEND (Adults) | ASCEND Peds |
|---|---|---|
| Lung | Inclusion criteria:
|
Exclusion criteria:
|
| Spleen | Inclusion criteria:
|
Inclusion criteria:
|
| Liver | Exclusion criteria:
|
Exclusion criteria:
|
| Blood | Exclusion criteria:
|
Exclusion criteria:
|
| Bone/growth retardationBone damageDelayed growth |
|
|
| Central nervous system |
|
Exclusion criteria:
|
| Cardiovascular | Exclusion criteria:
|
Exclusion criteria:
|
| Pain/fatigue | Inclusion criteria:
|
|
ALT, alanine aminotransferase; AST, aspartate transaminase; DLco, diffusing capacity of the lung; INR, international normalised ratio; MN, multiples of normal; MRI, magnetic resonance imaging. SRS (Splenomegaly Related Symptom) score: a score composed of 5 items (abdominal pain, abdominal discomfort, early satiety, self-image, ability to stoop). This score is derived from the Myeloproliferative Syndrome Assessment Score (MF-SAF) (JAKARTA, NCT# 01437787). The questions assess the effect over the last 24 h of the splenomegaly in patients with NPB disease.
5. Early Access Experience to ERT in France
Prior to full marketing authorisation, as of 14 October 2021, compassionate use of olipudase alfa was initiated in France in 4 nominative pediatric patients and 19 adult patients. A total of 14 physicians originating from 12 different cities (Lyon, Clermont-Ferrand, Bordeaux, Angers, Paris, Marseille, Quimper, Clichy, Strasbourg, Lille, Toulouse, and Caen) are currently treating ASMD patients, at the rate of about 1 to 5 patients per center. Of note, 16 out of these 23 patients were initiated with olipudase alfa by the co-authors of this work. Dose escalation (Figure 2) was conscientiously followed.
6. Discussion
In ASMD, as the lysosomal sphingomyelinase enzymatic defect is expressed ubiquitously throughout the organs, improvement in one tissue may be considered as representative of an overall improvement in non-neurological clinical status.
Available evidence from the natural history of ASMD and observational studies support the use of DLco and splenomegaly as clinically meaningful endpoints that assess disease burden for patients. Worsening DLco is a strong indicator of progressive lung disease in chronic visceral ASMD, contributing to increased disease burden. Likewise, spleen volume may be considered as a surrogate marker for bleeding risk and liver disease. Pneumonia/respiratory failure, and/or liver failure, are often the causes of death [9].
The main clinical criteria that were considered in France to prioritise early access to treatment with olipudase alfa in patients with ASMD type B or type A/B are low DLco with worsening dyspnoea, thrombocytopenia <50 G/L or recurrent bleeding, biological and/or histological liver damage, and painful splenomegaly (Figure 3), and in the case of paediatric patients a break in the growth curve or painful splenomegaly should be considered (Figure 3). Thus, a complete evaluation of the patient prior treatment initiation is mandatory in order to assess the potential benefit of olipudase alfa on the spleen volume, respiratory function, and platelet count, as well as any possible issues related to tolerance and comorbidities, such as tobacco use. In addition, as lung, spleen, liver, platelet status, and growth curve can worsen, a regular follow up with clinical, biological, and morphological of pauci/asymptomatic patients must be performed. The CETLv stratification of severity may aid the physician in health care decisions.
Data from the ASCEND (adult) and ASCEND-Peds (paediatric) studies have demonstrated that olipudase alfa can improve respiratory status and reduce spleen volume in patients with visceral ASMD (Table 5) [27,31]. In addition, the clinical relevance of the endpoints used in these studies is well documented [46]. Of note, patients included in the ASCEND (adult) and ASCEND-Peds studies did not exhibit the full spectrum of severity in patients with ASMD, excluding asymptomatic individuals and those with very severe disease. After dose escalation, steady-state dose infusions, and record of adequate tolerance to olipudase alfa, home therapy with a multidisciplinary team and regular follow-up should be considered. An ‘emergency card’ delivered to the patient could be helpful to improve the communication between the patient, and home and hospital caregivers in patients in home infusion.
The present early experience can be used as the basis for further clinical evaluations.
7. Conclusions
The management of chronic visceral and ASMD type B and A/B has traditionally been limited to supportive care and palliation. Clinical studies in patients with chronic ASMD have demonstrated positive results with the novel ERT olipudase alfa, comprising improvement in respiratory status and spleen volume reduction, as well as improvement in other clinical manifestations. In France, low DLco with worsening dyspnoea, thrombocytopenia <50 G/L or recurrent bleeding, biological and/or histological liver damage, and painful splenomegaly have been used to prioritise early compassionate use of olipudase alfa in nominative paediatric and adult patients with ASMD. Of note, the use of any decision-making process/stratification for patients with ASMD need to be confirmed by ‘real-life’ evidence. In addition, scheduled follow up of treated patients outside clinical trials is mandatory to assess the effect of olipudase alfa in those individuals with chronic ASMD. Regular follow up must be performed in untreated patients with ASMD. Asymptomatic patients should be evaluated by experts on a ‘case-by-case’ basis to ensure opportune early treatment initiation.
Acknowledgments
Third-party medical writing support was provided by Matthew Joynson of Springer Healthcare Ltd. We would like to acknowledge M.T. Vanier, T. Levade, and R. Froissart who have performed the majority of diagnoses of ASMD in France over the last 30 years. In addition, we would like to thank patients and families, French patient association Vaincre les Maladies Lysosomales (VML, www.vml-asso.org, accessed on 30 November 2021), the Reference Center for Lysosomal Diseases (CRML), the members of the CETLv, the co-workers of the Groupe collaboratif français, and other collaborators (physicians, biochemists, geneticists, pharmacists, and other supporting disciplines).
Author Contributions
The study was conceived and designed by W.M., O.L. and A.B. All authors were responsible for the acquisition of data. Analysis of data, drafting, and writing of the manuscript was undertaken by W.M., O.L. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Third-party medical writing support for this manuscript was funded by Sanofi Genzyme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Wladimir Mauhin declares honoraria and travel hospitality from Sanofi-Genzyme, Shire-Takeda, Chiesi and Amicus Therapeutics. Raphaël Borie received honoraria from Sanofi Genzyme, Roche and Boehringer. Florence Dalbies has received travel grants and speaker honoraria from Sanofi Genzyme. Claire Douillard has received travel grants honoraria from Sanofi Genzyme, Biomarin, Ultragenyx, Shire. Nathalie Guffon is involved as a Principal Investigator into ASCEND Peds clinical trials a Sanofi Genzyme sponsored trial and received some grants/fees from Sanofi Genzyme, Ultragenyx, Takeda, BioMarin. Christian Lavigne has received travel grants honoraria and speaker honoraria from Sanofi Genzyme, Swedish Orphan Biovitrum, Ultragenyx, Shire and Biomarin. Olivier Lidove is principal investigator in ASCEND, a Sanofi-Genzyme sponsored trial, and has received travel grants and speaker honoraria from Amicus and Sanofi Genzyme. Anaïs Brassier has received travel grants and speaker honoraria from Sanofi Genzyme, Shire HGT, Orchard therapeutics, Biomarin, and Alexion.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGovern M.M., Wasserstein M.P., Bembi B., Giugliani R., Mengel K.E., Vanier M.T., Zhang Q., Peterschmitt M.J. Prospective study of the natural history of chronic acid sphingomyelinase deficiency in children and adults: Eleven years of observation. Orphanet J. Rare Dis. 2021;16:212. doi: 10.1186/s13023-021-01842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathias S., Dressler K.A., Kolesnick R.N. Characterization of a ceramide-activated protein kinase: Stimulation by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albeituni S., Stiban J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv. Exp. Med. Biol. 2019;1161:169–191. doi: 10.1007/978-3-030-21735-8_15. [DOI] [PubMed] [Google Scholar]
- 4.Schütze S., Potthoff K., Machleidt T., Berkovic D., Wiegmann K., Krönke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-O. [DOI] [PubMed] [Google Scholar]
- 5.Wasserstein M.P., Desnick R.J., Schuchman E.H., Hossain S., Wallenstein S., Lamm C., McGovern M.M. The natural history of type B Niemann-Pick disease: Results from a 10-year longitudinal study. Pediatrics. 2004;114:e672–e677. doi: 10.1542/peds.2004-0887. [DOI] [PubMed] [Google Scholar]
- 6.McGovern M.M., Aron A., Brodie S.E., Desnick R.J., Wasserstein M.P. Natural history of Type A Niemann-Pick disease: Possible endpoints for therapeutic trials. Neurology. 2006;66:228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 7.McGovern M.M., Dionisi-Vici C., Giugliani R., Hwu P., Lidove O., Lukacs Z., Mengel K.E., Mistry P.K., Schuchman E.H., Wasserstein M.P. Consensus recommendation for a diagnostic guideline for acid sphingomyelinase deficiency. Genet. Med. 2017;19:967–974. doi: 10.1038/gim.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deodato F., Boenzi S., Taurisano R., Semeraro M., Sacchetti E., Carrozzo R., Dionisi-Vici C. The impact of biomarkers analysis in the diagnosis of Niemann-Pick C disease and acid sphingomyelinase deficiency. Clin. Chim. Acta. 2018;486:387–394. doi: 10.1016/j.cca.2018.08.039. [DOI] [PubMed] [Google Scholar]
- 9.McGovern M.M., Avetisyan R., Sanson B.J., Lidove O. Disease manifestations and burden of illness in patients with acid sphingomyelinase deficiency (ASMD) Orphanet J. Rare Dis. 2017;12:41. doi: 10.1186/s13023-017-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingma S.D., Bodamer O.A., Wijburg F.A. Epidemiology and diagnosis of lysosomal storage disorders; challenges of screening. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:145–157. doi: 10.1016/j.beem.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Lidove O., Belmatoug N., Froissart R., Lavigne C., Durieu I., Mazodier K., Serratrice C., Douillard C., Goizet C., Cathebras P., et al. Acid sphingomyelinase deficiency (Niemann-Pick B disease) in adulthood: A retrospective multicentre study of 28 adult patients. Rev. Med. Interne. 2017;38:291–299. doi: 10.1016/j.revmed.2016.10.387. [DOI] [PubMed] [Google Scholar]
- 12.Cassiman D., Packman S., Bembi B., Ben Turkia H., Al-Sayed M., Schiff M., Imrie J., Mabe P., Takahashi T., Mengel K.E., et al. Cause of death in patients with chronic visceral and chronic neurovisceral acid sphingomyelinase deficiency (Niemann-Pick disease type B and B variant): Literature review and report of new cases. Mol. Genet. Metab. 2016;118:206–213. doi: 10.1016/j.ymgme.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Borie R., Crestani B., Guyard A., Lidove O. Interstitial lung disease in lysosomal storage disorders. Eur. Respir. Rev. 2021;30:200363. doi: 10.1183/16000617.0363-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern M.M., Wasserstein M.P., Giugliani R., Bembi B., Vanier M.T., Mengel E., Brodie S.E., Mendelson D., Skloot G., Desnick R.J., et al. A prospective, cross-sectional survey study of the natural history of Niemann-Pick disease type B. Pediatrics. 2008;122:e341–e349. doi: 10.1542/peds.2007-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuchman E.H., Desnick R.J. Types A and B Niemann-Pick disease. Mol. Genet. Metab. 2017;120:27–33. doi: 10.1016/j.ymgme.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langeveld M., Hollak C.E.M. Bone health in patients with inborn errors of metabolism. Rev. Endocr. Metab. Disord. 2018;19:81–92. doi: 10.1007/s11154-018-9460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskes E.C.B., Sjourke B., Vaz F.M., Goorden S.M., van Kuilenburg A.B., Aerts J., Hollak C.E. Biochemical and imaging parameters in acid sphingomyelinase deficiency: Potential utility as biomarkers. Mol. Genet. Metab. 2020;130:16–26. doi: 10.1016/j.ymgme.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 18.McGovern M.M., Lippa N., Bagiella E., Schuchman E.H., Desnick R.J., Wasserstein M.P. Morbidity and mortality in type B Niemann-Pick disease. Genet. Med. 2013;15:618–623. doi: 10.1038/gim.2013.4. [DOI] [PubMed] [Google Scholar]
- 19.Pokrzywinski R., Hareendran A., Nalysnyk L., Cowie S., Crowe J., Hopkin J., Joshi D., Pulikottil-Jacob R. Impact and burden of acid sphingomyelinase deficiency from a patient and caregiver perspective. Sci. Rep. 2021;11:20972. doi: 10.1038/s41598-021-99921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGovern M.M., Pohl-Worgall T., Deckelbaum R.J., Simpson W., Mendelson D., Desnick R.J., Schuchman E.H., Wasserstein M.P. Lipid abnormalities in children with types A and B Niemann Pick disease. J. Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Miranda S.R., He X., Simonaro C.M., Gatt S., Dagan A., Desnick R.J., Schuchman E.H. Infusion of recombinant human acid sphingomyelinase into niemann-pick disease mice leads to visceral, but not neurological, correction of the pathophysiology. FASEB J. 2000;14:1988–1995. doi: 10.1096/fj.00-0014com. [DOI] [PubMed] [Google Scholar]
- 22.Murray J.M., Thompson A.M., Vitsky A., Hawes M., Chuang W.L., Pacheco J., Wilson S., McPherson J.M., Thurberg B.L., Karey K.P., et al. Nonclinical safety assessment of recombinant human acid sphingomyelinase (rhASM) for the treatment of acid sphingomyelinase deficiency: The utility of animal models of disease in the toxicological evaluation of potential therapeutics. Mol. Genet. Metab. 2015;114:217–225. doi: 10.1016/j.ymgme.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Wasserstein M.P., Jones S.A., Soran H., Diaz G.A., Lippa N., Thurberg B.L., Culm-Merdek K., Shamiyeh E., Inguilizian H., Cox G.F., et al. Successful within-patient dose escalation of olipudase alfa in acid sphingomyelinase deficiency. Mol. Genet. Metab. 2015;116:88–97. doi: 10.1016/j.ymgme.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wasserstein M., Arash-Kaps L., Barbato A., Gallagher R.C., Giugliani R., Guelbert N.B., Hollak C., Ikezoe T., Lachmann R., Lidove O., et al. Adults with chronic acid sphingomyelinase deficiency show significant visceral, pulmonary, and hematologic improvements after enzyme replacement therapy with olipudase alfa: 1-year results of the ASCEND placebo-controlled trial. Mol. Genet. Metab. 2021;132:S110. doi: 10.1016/j.ymgme.2020.12.271. [DOI] [Google Scholar]
- 25.McGovern M.M., Wasserstein M.P., Kirmse B., Duvall W.L., Schiano T., Thurberg B.L., Richards S., Cox G.F. Novel first-dose adverse drug reactions during a phase I trial of olipudase alfa (recombinant human acid sphingomyelinase) in adults with Niemann–Pick disease type B (acid sphingomyelinase deficiency) Genet. Med. 2016;18:34–40. doi: 10.1038/gim.2015.24. [DOI] [PubMed] [Google Scholar]
- 26.Wasserstein M.P., Diaz G.A., Lachmann R.H., Jouvin M.-H., Nandy I., Ji A.J., Puga A.C. Olipudase alfa for treatment of acid sphingomyelinase deficiency (ASMD): Safety and efficacy in adults treated for 30 months. J. Inherit. Metab. Dis. 2018;41:829–838. doi: 10.1007/s10545-017-0123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz G.A., Jones S.A., Scarpa M., Mengel K.E., Giugliani R., Guffon N., Batsu I., Fraser P.A., Li J., Zhang Q., et al. One-year results of a clinical trial of olipudase alfa enzyme replacement therapy in pediatric patients with acid sphingomyelinase deficiency. Genet. Med. 2021;23:1543–1550. doi: 10.1038/s41436-021-01156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thurberg B.L., Wasserstein M.P., Jones S.A., Schiano T.D., Cox G.F., Puga A.C. Clearance of hepatic sphingomyelin by olipudase alfa is associated with improvement in lipid profiles in acid sphingomyelinase deficiency. Am. J. Surg. Pathol. 2016;40:1232–1242. doi: 10.1097/PAS.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurberg B.L., Diaz G.A., Lachmann R.H., Schiano T., Wasserstein M.P., Ji A.J., Zaher A., Peterschmitt M.J. Long-term efficacy of olipudase alfa in adults with acid sphingomyelinase deficiency (ASMD): Further clearance of hepatic sphingomyelin is associated with additional improvements in pro- and anti-atherogenic lipid profiles after 42 months of treatment. Mol. Genet. Metab. 2020;131:245–252. doi: 10.1016/j.ymgme.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Garside B., Ho J.H., Kwok S., Liu Y., Dhage S., Donn R., Iqbal Z., Jones S.A., Soran H. Changes in PCSK 9 and apolipoprotein B100 in Niemann-Pick disease after enzyme replacement therapy with olipudase alfa. Orphanet J. Rare Dis. 2021;16:107. doi: 10.1186/s13023-021-01739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasserstein M., Arash-Kaps L., Barbato A., Gallagher R., Giugliani R., Guelbert N., Hollak C., Ikezoe T., Lachmann R., Lidove O., et al. One-year results of the placebo-controlled ASCEND trial of olipudase alfa enzyme replacement therapy in adults with chronic acid sphingomyelinase deficiency [OP093] Mol. Genet. Metab. 2021;132:S64–S65. doi: 10.1016/S1096-7192(21)00178-5. [DOI] [PubMed] [Google Scholar]
- 32.Sanofi-Genzyme Press Release. Sanofi: FDA Grants Breakthrough Therapy Designation for Genzyme’s Olipudase Alfa. [(accessed on 22 September 2021)]. Available online: https://www.sanofi.com/en/media-room/press-releases/2015/2015-06-04-07-00-00.
- 33.European Medicines Agency EU/3/01/056: Orphan Designation for the Treatment of Niemann-Pick Disease. [(accessed on 21 September 2021)]. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu301056.
- 34.Vanier M.T. Niemann-Pick diseases. Handb. Clin. Neurol. 2013;113:1717–1721. doi: 10.1016/B978-0-444-59565-2.00041-1. [DOI] [PubMed] [Google Scholar]
- 35.Bell E.C., Cox N.S., Goh N., Glaspole I., Westall G.P., Watson A., Holland A.E. Oxygen therapy for interstitial lung disease: A systematic review. Eur. Respir. Rev. 2017;26:160080. doi: 10.1183/16000617.0080-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dowman L., Hill C.J., May A., Holland A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2021;2:CD006322. doi: 10.1002/14651858.CD006322.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauhin W., Levade T., Vanier M.T., Froissart R., Lidove O. Prevalence of cancer in acid sphingomyelinase deficiency. J. Clin. Med. 2021;10:5029. doi: 10.3390/jcm10215029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ordieres-Ortega L., Galeano-Valle F., Mallén-Pérez M., Muñoz-Delgado C., Apaza-Chavez J.E., Menárguez-Palanca F.J., Alvarez-Sala Walther L.A., Demelo-Rodríguez P. Niemann-Pick disease type-B: A unique case report with compound heterozygosity and complicated lipid management. BMC Med. Genet. 2020;21:94. doi: 10.1186/s12881-020-01027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arenz C. Small molecule inhibitors of acid sphingomyelinase. Cell Physiol. Biochem. 2010;26:1–8. doi: 10.1159/000315100. [DOI] [PubMed] [Google Scholar]
- 40.Bonanni P., Grazzini M., Niccolai G., Paolini D., Varone O., Bartoloni A., Bartalesi F., Santini M.G., Baretti S., Bonito C., et al. Recommended vaccinations for asplenic and hyposplenic adult patients. Hum. Vaccin. Imunother. 2017;13:359–368. doi: 10.1080/21645515.2017.1264797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee G.M. Preventing infections in children and adults with asplenia. Hematol. Am. Soc. Hematol. Educ. Program. 2020;2020:328–335. doi: 10.1182/hematology.2020000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y.D., Ding M., Dong X., Zhang J.-J., Azkur A.K., Azkur D., Gan H., Sun Y.-L., Fu W., Li W., et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 43.Vanier M.T. Prenatal diagnosis of Niemann-Pick diseases types A, B and C. Prenat. Diagn. 2002;22:630–632. doi: 10.1002/pd.368. [DOI] [PubMed] [Google Scholar]
- 44.Wasserstein M.P., Schulman E.H. Acid sphingomyelinase deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Mirzaa G., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, WA, USA: 2006. pp. 1993–2021. [PubMed] [Google Scholar]
- 45.Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) Autorisations Temporaires d’Utilisation (ATU) [(accessed on 18 July 2021)]. Available online: https://archiveansm.integra.fr/Activites/Autorisations-temporaires-d-utilisation-ATU/Qu-est-ce-qu-une-autorisation-temporaire-d-utilisation/(offset)/1.
- 46.Jones S.A., McGovern M., Lidove O., Giugliani R., Mistry P.K., Dionisi-Vici C., Munoz-Rojas M.V., Nalysnyk L., Schecter A.D., Wasserstein M. Clinical relevance of endpoints in clinical trials for acid sphingomyelinase deficiency enzyme replacement therapy. Mol Genet Metab. 2020;131:116–123. doi: 10.1016/j.ymgme.2020.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.