Abstract
Signal Transducer and Activator of Transcription (STAT) proteins have been identified as drivers of prostate cancer (PCa) progression and development of aggressive castration-resistant phenotypes. In particular, STAT3, 5, and 6 have been linked to resistance to androgen receptor inhibition and metastasis in in vitro and in vivo models. This descriptive study aimed to validate these preclinical data in tissue obtained from patients with PCa before and while under androgen-deprivation therapy. Therefore, STAT3, 5, and 6 expressions and activity were assessed by immunohistochemistry. The data revealed that STAT3 and 5 changed in PCa. However, there was no relationship between expression and survival. Moreover, due to the heterogeneous nature of PCa, the preclinical results could not be transferred congruently to the patient’s material. A pilot study with a longitudinal patient cohort could also show this heterogeneous influence of systemic therapy on STAT3, 5, and 6 expressions and activity. Even if the main mechanisms were validated, these data demonstrate the urge for better patient-near preclinical models. Therefore, these data reflect the need for investigations of STAT proteins in a longitudinal patient cohort to identify factors responsible for the diverse influence of system therapy on STAT expression.
Keywords: STAT3, STAT5, STAT6, androgen deprivation therapy, novel hormonal therapy, chemotherapy, HSPC, CRPC, therapy resistance
1. Introduction
Prostate cancer (PCa) is one of the most common cancers in men, with an estimated number of over 1.4 million new cases worldwide in 2020. In addition, with an estimated 375,000 new deaths worldwide, PCa is also one of the leading causes of death in men [1]. Organ-confined PCa (TMN stage 1 and 2) is treated with curative intent by radical prostatectomy or radiation therapy [2]. For these patients, 5-year survival is 100% [3]. However, in patients with metastasized PCa (TMN stage 3 and 4) 5-year survival is ~30% [3]. Since PCa is androgen dependent in its growth, first-line therapy includes androgen-deprivation therapy (ADT), which is indicated for metastasized hormone-sensitive PCa (mHSPC) and can be combined with taxan-based chemotherapy (CTx) or novel hormonal therapy (NHT) as apalutamide, abiraterone, or enzalutamide [2,4]. Unfortunately, resistance to ADT inevitably occurs, and after a medium response duration of 18 months, tumors progress to castration-resistant PCa (CRPC). Treatment options for non-metastasized CPRC are the antiandrogens apalutamide, darolutamide, and enzalutamide [4]. Treatment for metastasized CRPC (mCRPC) includes taxane-based chemotherapy with docetaxel or cabazitaxel, abiraterone, and the antiandrogen enzalutamide [4]. The PARP-inhibitor Olaparib was recently approved for patients with mCRPC and alteration of one or more homologous recombination repair genes [4]. Unfortunately, despite recent improvements in treatment options, patients suffering from mCRPC only have a median life expectancy of 19 months due to tumor progress and emerging therapy resistance [5].
The Signal Transducers and Activators of Transcription (STATs) are a group of transcription factors, which reportedly are involved in those life-threatening processes in PCa [5]. The group contains seven members, of which STAT3, STAT5, and STAT6 are involved in tumor progress, metastasis, and therapy resistance.
STAT3 is highly expressed and active in advanced PCa and PCa bone metastases [6,7]. In addition, the transcription factor is involved in the transdifferentiation processes of the PCa cell line LNCaP to ADT and antiandrogen-resistant neuroendocrine PCa cells [8,9]. Moreover, in vitro studies revealed that crosstalk between the STAT3 and the androgen receptor (AR) axis potentiates the androgen-dependent transactivation of the AR and therefore mediates resistance to the antiandrogen enzalutamide, which can be reversed by STAT3 inhibition [10,11].
STAT5 expression correlates with high Gleason scores (GS) and predicts early recurrence of PCa after radical prostatectomy [12,13]. Furthermore, Thomas et al. has reported that ADT leads to increased STAT5 expression in PCa tissue [12]. Moreover, STAT5 influences the AR activity directly by regulating the AR stability, an important factor in antiandrogen response [12,14,15]. In addition, increased STAT5 expression and transactivity have been revealed in enzalutamide-resistant cell lines and siRNA-mediated STAT5-knockdown resensitized the enzalutamide resistant MR49F cells to enzalutamide [16,17].
Both STAT3 and STAT5 have been reported to be increased in docetaxel-resistant cell lines compared to their sensitive controls. However, their function in docetaxel-resistant cells has not yet been investigated [18].
STAT6 expression is elevated in PCa compared to benign prostate, while STAT6 expression correlates with higher GS and larger tumor size [19,20]. In vitro experiments revealed a possible role of STAT6 in metastasis. However, involvement in therapy resistance has not yet been described [19].
As most findings of STAT proteins in therapy resistance have been revealed in cell line experiments, data about expression and activity in therapy-resistant PCa tissue are limited. Therefore, this descriptive study aimed to examine the expression and activity of STAT3, STAT5, and STAT6 in PCa patients undergoing systemic therapies, including ADT, enzalutamide, abiraterone, and docetaxel.
2. Materials and Methods
2.1. Patient Material and Immunohistochemistry (IHC)
Patients’ samples were selected from the Tumor and Normal Tissue Bank of the University Cancer Center Dresden. The Ethics Committee of the Technische Universität Dresden approved the use of archived material (Study no. EK59032007). According to statutory provisions, written consent was obtained from all patients and documented in the database. The cohort contained 154 formalin-fixed and paraffin-embedded (FFPE) tissue specimens of 97 PCa patients and 32 patients with benign prostate hyperplasia (BPH) undergoing TURP. Tissue blocks were cut in serial sections of 1–2 µm thickness; sections were deparaffinized with BenchMark XT (Ventana Medical Systems, Oro Valley, AZ, USA) and then exposed to a heat-induced epitope retrieval. The antibodies listed in Table 1 were used for staining, followed by counterstaining with hematoxylin, dehydration, and mounting of the slides. IHC was evaluated by the department of pathology Dresden using the Remmele Score [21]. Therefore, the immunoreactivity score (IRS) was calculated according to the following parameters: staining intensity was scored 0–3 (0 = absent, 1 = low intensity, 2 = average intensity, 3 = intense). The percentage of positively stained cells was scored 0–4 (0 = absent; 1 ≤ 10%; 2 ≤ 50%; 3 ≤ 80%; 4 > 80%). Both scores were multiplied to obtain the IRS, ranging from 0–12. The percentage of cells with nuclear STAT was assessed to investigate STAT activity. Therefore, the activity of unphosphorylated and phosphorylated STAT was included in the study. IHC slides were digitalized using a Pannoramic Scan II (3DHistech LTD, Budapest, Hungary) scanner. IRS data from the different cohorts were displayed as box and whisker diagrams (min to max). The IRS data from the longitudinal patient cohort were displayed as individual data points. The corresponding IRS values of one patient have been connected with a line.
Table 1.
Antibodies used in the study.
| Name | Company | Lot | Dilution |
|---|---|---|---|
| STAT3 (124H6) Mouse mAb #9139 | Cell Signaling Technology, Danvers, MA, USA | 16 | 1:300 |
| Anti-STAT5a + STAT5b antibody [EPR16671-40] | Abcam, Cambridge, United Kingdom | GR3247129-1 | 1:4000 |
| STAT6 (EP325) | Bio SB, Inc., Santa Barbara, CA, USA | 3425QLD05 | 1:25 |
2.2. Statistical Analysis
Prism 9.3.1 (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. Differences between treatment groups were analyzed using ordinary one-way ANOVA or Student’s t-test. p-values of ≤0.05 were considered statistically significant. A Kaplan–Meier estimate was used for overall survival analysis. The Pearson correlation coefficient (r) was calculated and interpreted by the guidelines suggested by Schober et al. for correlation analysis [21]. All differences highlighted by asterisks were statistically significant as encoded in figure legends (* p ≤ 0.05; ** p ≤ 0.01; *** p ≤ 0.001).
3. Results
3.1. Description of the Patient’s Cohort
For this study, 97 PCa patients (26 HSPC patients, 71 ADT patients, of which 25 received ADT only, 33 received ADT + NHT, and 13 received ADT + CTx) and 32 BPH patients (a non-malignant control cohort) were retrospectively selected. In the used cohort, ADT was induced by treatment with buserelin, triptorelin, degarelix, or leuprorelin. For ADT + NHT, ADT combined with enzalutamide or abiraterone was administered. For ADT + CTx, ADT combined with docetaxel or cabazitaxel was administered. The treatment regimen was used for at least one month. Patients were recruited between 2011 and 2020. In total, 154 FFPE PCa tissue specimens were taken after palliative TURP. Baseline patient characteristics are summarized in Table 2.
Table 2.
Baseline characteristics of PCa-patients cohort.
| All | HSPC | mCRPC | |||
|---|---|---|---|---|---|
| ADT Only | ADT + NHT | ADT + CTx | |||
| Patient Number | 97 | 26 | 25 | 33 | 13 |
| Median age at primary diagnosis, years | 73 | 72 | 74 | 71 | 66 |
| Median PSA at primary diagnosis, ng/mL (Interquartile range IQR) | 18 (6.9; 79.0) |
6.3 (2.7; 10.2) |
15.6 (7.2; 87.6) |
49.0 (11.2;128.0) |
60.4 (28.7; 92.7) |
| Neuroendocrine differentiation at primary diagnosis, % | 1.0 | 0.0 | 0.0 | 3.0 | 0.0 |
| Presence of bone metastases at primary diagnosis, % | 25.0 | 3.8 | 20.0 | 30 | 62 |
| Presence of lymph node metastases at primary diagnosis, % | 14 | 12 | 8.0 | 18 | 15 |
| Presence of organ metastases at primary diagnosis, % | 1.00 | 0.00 | 0.0 | 3.0 | 0.0 |
| Median overall survival since the start of primary therapy, months | 59 | 47 | 81 | 67 | 86 |
Kaplan–Meier analysis revealed a median overall survival (OS) of 201 months (Hazard Ratio, 95% Confidence Interval: 1.5; 0.8–2.9) for patients with HSPC and 136 months (0.7; 0.3–1.3) for patients undergoing ADT (Figure 1A). Patients receiving additional treatment (Figure 1B) with NHT (OS: 187 months) or CTx (OS: 187 months) had a non-significant increase in OS compared to the ADT patients (OS: 128 months).
Figure 1.
Kaplan–Meier analysis of the PCa cohort. (A) Kaplan–Meier analysis of the HSPC vs. the ADT therapy cohort; (B) Kaplan–Meier analysis of the different ADT cohorts.
Therefore, treatment did not influence OS in this cohort.
3.2. Change in STAT 3, 5, and 6 Expressions in PCa Compared to BPH
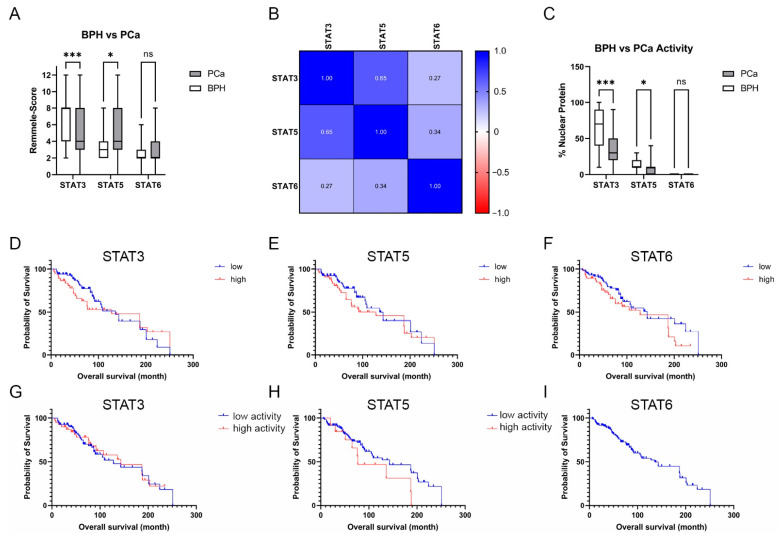
STAT protein expression was reported to change during malignant transformation of the prostate [5]. Therefore, to evaluate STAT3 (Figure 2), 5 (Figure 3) and 6 protein expression (Figure 4) patterns in BPH and PCa, tissue of the presented patient cohort was investigated using the Remmele Score. Therefore, an immune reactivity score (IRS) was calculated using the grade of staining intensity and the fraction of positive cells was evaluated (Figure 5) [21].
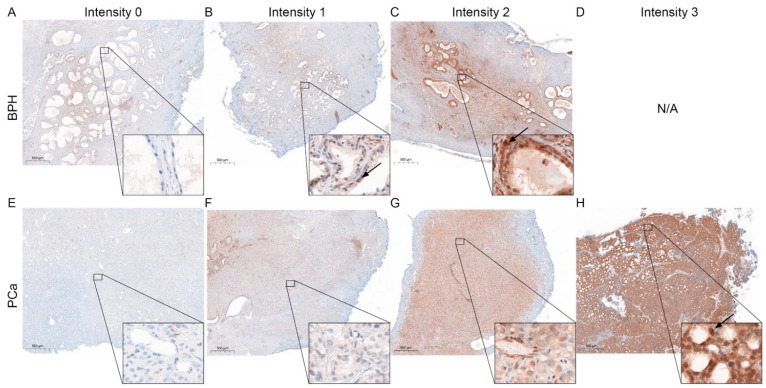
Figure 2.
Representative STAT3 staining in BPH and PCa. (A–D) Immunohistochemical staining for STAT3 of representative benign tissue with different staining intensities cores. Scale bar = 500 μM. (E–H) Immunohistochemical staining for STAT3 of representative malignant tissue with different staining intensities cores. Arrows mark representative nuclear localization. Scale bar = 500 μM.
Figure 3.
Representative STAT5 staining in BPH and PCa. (A–D) Immunohistochemical staining for STAT5 of representative benign tissue with different staining intensities cores. Scale bar = 500 μM. (E–H) Immunohistochemical staining for STAT5 of representative malignant tissue with different staining intensities cores. Arrows mark representative nuclear localization. Scale bar = 500 μM.
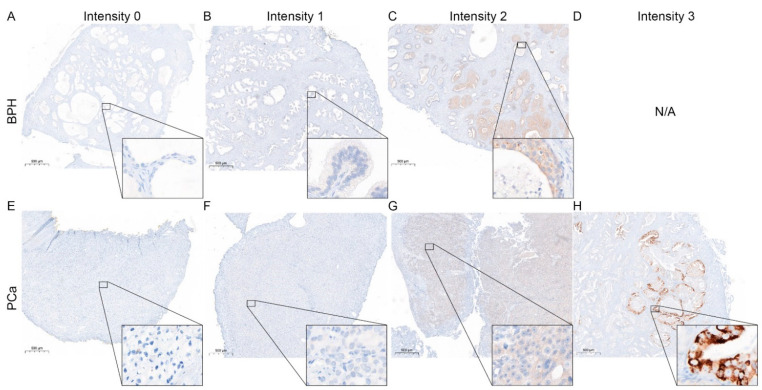
Figure 4.
Representative STAT6 staining in BPH and PCa. (A–D) Immunohistochemical staining for STAT6 of representative benign tissue with different staining intensities cores. Scale bar = 500 μM. (E–H) Immunohistochemical staining for STAT6 of representative malignant tissue with different staining intensities cores. Scale bar = 500 μM.
Figure 5.
Evaluation of the STAT3, 5, and 6 protein staining in BPH and PCa. (A) Evaluation of the expression of STAT3,5, and 6 of BPH and PCa tissue using the Remmele score. Data are shown as box and whisker diagrams (ns: not significant,*: p ≤ 0.05, ***: p ≤ 0.001). (B) Pearson correlation of STAT3, 5, and 6 in PCa tissue. The r-values are displayed in a heat map. (C) Evaluation of the % STAT3, 5, and 6 nuclear localization in BPH and PCa tissue as a surrogate for the activity of the transcription factors. Data are shown as box and whisker diagrams (ns—not significant; *: p ≤ 0.05, ***: p ≤ 0.001). (D–F) OS analysis of patients with low and high STAT3, 5, or 6 expressions (D) Kaplan–Meier curves indicating OS according to the STAT3 expression level of the PCa cohort. The median STAT3-IRS was chosen as the threshold. (E) Kaplan–Meier curves indicate OS according to STAT3 expression level. The median STAT5-IRS was selected as the threshold. (F) Kaplan–Meier curves indicate OS according to STAT6 expression level. The median STAT6-IRS was chosen as the threshold. (G–I) OS analysis of patients with low and high STAT3, 5, or 6 activity. (G) Kaplan–Meier curves indicating OS according to the STAT3 expression level of the PCa cohort. The median % of nuclear STAT3 was chosen as the threshold. (H) Kaplan–Meier curves indicate OS according to STAT3 expression level. The median % of nuclear STAT5 was selected as the threshold. (I) Kaplan–Meier curves indicate OS according to STAT6 activity.
The IRS evaluation of STAT3 (Figure 5A) revealed a significant downregulation of STAT3 in PCa (mean IRS ± SD: 4.9 ± 3.0) compared to BPH (mean IRS ± SD: 7.1 ± 2.5). In contrast, IRS evaluation of STAT5 showed a significant increase in STAT5 in PCa (mean IRS ± SD: 5.2 ± 2.1) compared to BPH (mean IRS ± SD: 3.9 ± 2.1). Assessment of STAT6 showed no significant difference in the STAT6-IRS between the PCa (mean IRS ± SD: 2.0 ± 1.4) and BPH (mean IRS ± SD: 2.9 ± 2.2) groups. Correlation analysis (Figure 5B) showed no significant correlation between the STAT protein IRS scores in PCa. To assess the activity of the investigated STATs, the percentage of nuclear localization was examined in the malignant areas. The analysis of nuclear STAT revealed a significant decrease in nuclear localization in STAT3 and STAT5, whereas STAT6 showed almost no nuclear localization at all (Figure 5C). Correlation analysis revealed no correlation between the investigated STAT proteins IRS scores and activity in PCa (Figure S1 in Supplementary Material). Kaplan–Meier survival analysis showed that STAT3, 5, or 6 expressions did not affect OS for all examined STAT proteins. STAT3 activity did not affect OS (Figure 5G). Even if not significant, patients with low STAT5 activity (Figure 5H) had a longer OS than patients with high STAT5 activity (143 months vs. 77 months). No STAT6 activity could be detected, so no Kaplan–Meier estimation between high and low activity could be performed (Figure 5I).
Taken together, STAT 3 and 5 expression and activity changes in PCa, but does not significantly influence OS.
3.3. Influence of Systemic Therapy on STAT3, 5, and 6 Protein Levels in PCa
Systemic therapy such as ADT, NHT, or CTx was reported to change STAT expression and activity and promote tumor progression and therapy resistance [5,10,12,16,17,22]. Therefore, the ADT PCa cohort was divided into HSPC and ADT subgroups to verify these findings. As a result of this subgrouping, the ADT subgroup includes patients receiving ADT, ADT+ NHT, and ADT + CTx. Even if not significant, IRS evaluation revealed a change from HSPC to the ADT subgroup from 4.6 ± 2.5 to 5.0 ± 3.1 for STAT3, 4.5 ± 3.0 to 5.4 ± 2.7 for STAT5, and 3.0 ± 2.4 to 2.9 ± 2.2 for STAT6 (Figure 6A). In addition, no significant but minor increase in the nuclear localization of STAT3 and 5 could be detected, whereas no nuclear localization could be seen for STAT6 (Figure 6B). However, expression and activity are highly heterogeneous in the evaluated cohorts (Figure 6).
Figure 6.
Evaluation of the STAT3, 5, and 6 levels and activity in HSPC and ADT subgroups. (A) Evaluation of the expression of STAT3,5, and 6 in PCa tissue of the HSPC and ADT subgroups using the Remmele score. Data are shown as box and whisker diagrams (ns.: not significant). (B) Evaluation of the % STAT3, 5, and 6 nuclear localization in PCa tissue of the HSPC and ADT subgroups as a surrogate for the activity of the transcription factors. Data are shown as box and whisker diagrams (ns.: not significant).
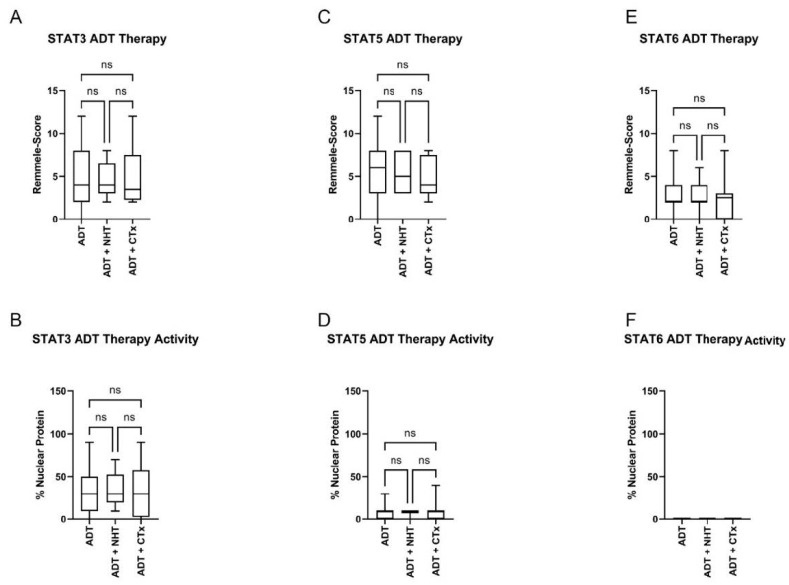
To investigate if further treatment with NHT or CTx affects STAT3, 5, or 6 expressions, the ADT subgroup was further divided into ADT, ADT + NHT, and ADT + CTx (Figure 7). IRS evaluation of STAT3 did not show any change after treatment with ADT + NHT and ADT + CTx (Figure 7C). Additional therapy with NHT or CTx did not change STAT3 nuclear localization compared to the ADT-only subgroup (Figure 7B). Additionally, the assessment of STAT5 (Figure 7C,D) and STAT6 (Figure 7C,D) did not reveal any change in expression or activity between the three subgroups. Analysis of the public PRAD SU2C 2019 cohort also indicated no change in STAT3, 5, and 6 expressions in the ADT, ADT + NHT, and ADT + CTx subgroups (Figure S1).
Figure 7.
Evaluation of the STAT3, 5, and 6 levels (A+C+E) and activity in HSPC and ADT subgroups (B+D+F). (A+C+E) Evaluation of the expression of STAT3 (A), 5 (C), and 6 (E) in PCa tissue of the HSPC and ADT subgroups using the Remmele score. Data are shown as box and whisker diagrams (ns.: not significant). (B+D+F) Evaluation of the % STAT3 (B), 5 (D), and 6 (F) nuclear localization in PCa tissue of the HSPC and ADT subgroups as a surrogate for the activity of the transcription factors. Data are shown as box and whisker diagrams (ns.: not significant).
In summary, no association between system therapy and STAT expression and activity can be established.
3.4. Influence of Systemic Therapy on STAT3, 5, and 6 Protein Levels in PCa
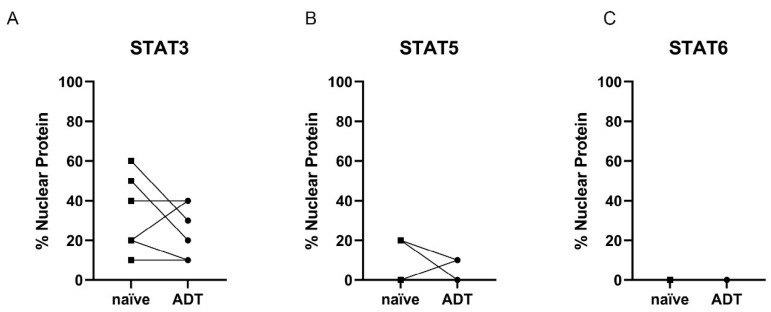
As a general comparison of the cohorts did not show any difference in STAT3, 5, and 6 expressions between the ADT subgroups, a pilot study was performed with TURP tissue of six patients obtained before and under ADT. These tissue specimens were investigated for STAT levels and activity. STAT3 expression was elevated after ADT treatment in three out of six patients (Figure 8A), whereas two patients decreased STAT3 levels. Likewise, STAT5 expression was increased in two out of six cases, whereas in one patient, STAT5 was reduced (Figure 8B). In contrast, STAT-6 expression was elevated and decreased in two patients (Figure 8C).
Figure 8.
Evaluation of the STAT3, 5, and 6 levels in HSPC (naïve) and ADT subgroups. Evaluation of the expression of STAT3 (A), 5 (B), and 6 (C) in PCa tissue of the HSPC and ADT subgroups using the Remmele score. Individual data points are shown. Corresponding patient data before and under ADT have been connected.
For STAT3, investigation of the STAT activity revealed a decrease in nuclear localization in three out of six patients and only one increase in nuclear localization after ADT (Figure 9B). STAT5 nuclear localization decreased in two patients and increased in only one patient (Figure 9B). STAT6 showed no nuclear localization, independent of treatment status (Figure 9C).
Figure 9.
Evaluation of the STAT3, 5, and 6 activity in HSPC (naïve) and ADT subgroups. (A+B+C) Evaluation of the % STAT3 (A), 5 (B), and 6 (C) nuclear localization in PCa tissue of the HSPC and ADT subgroups as a surrogate for the activity of the transcription factors. Individual data points are shown. Corresponding patient data before and under ADT have been connected.
4. Discussion
STAT proteins influence multiple biological processes such as immune response, mitogenesis, wound healing, cell survival, and cell growth [5,23]. After activation, the transcription factors transmit their signals from the cell membrane through growth factors, cytokines, or hormones into the nucleus. They bind to specific response elements on the DNA in the nucleus, thereby inducing gene transcription [23]. Therefore, the STAT proteins interfere with several health conditions such as autoimmune diseases and cancer, including PCa. In PCa, the STAT protein family promotes tumor progression and mediates therapy resistance by regulating oncogene and pro-survival gene transcription (e.g., BCL2L1, MCL1, MYC, CCND1) [5]. However, most findings of STAT proteins in PCa are obtained from preclinical models, and data about expression and activity in advanced and therapy-resistant PCa tissue are limited. This study investigated three specific STAT proteins, STAT3, STAT5, and STAT6, in PCa tissue from patients undergoing systemic therapies. The effects of systemic treatment on the selected STAT proteins shown in the preclinical models are evident and well described. These changes should already be reflected in a small cohort as presented in this study [5].
STAT3 is the most investigated STAT protein in PCa. It is aberrantly activated in ~50% of PCa patients and modulates androgen receptor expression and activity [5,6,7,8]. STAT3 is activated by several proinflammatory cytokines, including interleukin-6 (IL-6) which is elevated in PCa patients and is the driver of PCa progression [24,25,26]. In vitro data support the oncogenic and growth-promoting role of IL-6 and STAT3 [11,27]. Furthermore, increased activated STAT-3 is associated with clinicopathological parameters such as high T-stage and increased Gleason score [28,29]. Moreover, IHC analysis of CRPC revealed increased STAT3 expression compared to BPH tissue [30]. In contrast to these findings, the IHC analysis revealed a significant decrease in STAT3 expression and activity in primary PCa tissue compared to BPH. This finding is in line with the studies published by Pencik and colleagues [31]. The group demonstrated that STAT3 exerts a tumor-suppressive function by activating senescence via the p19ARF–Mdm2–p53 axis at an early stage of PCa development. Based on these data, STAT3 may also play a tumor suppressor role and promote different functions depending on the tumor stage described for STAT1 [32]. These data are supported by Handle et al., revealing an increase in STAT3 activity but not STAT3 expression in castration-resistant and androgen-responsive patient-derived xenografts [10].
STAT5, which refers to the proteins STAT5a and STAT5b, was reported to play an essential role in the progression of PCa to CRPC and the development of enzalutamide resistance [5]. Similar to STAT3, there is evidence for an increase in STAT5 in CRPC [12,30]. Here, an elevated STAT5 level could be confirmed in primary PCa tissue compared to BPH. Furthermore, the growth hormone–STAT5 axis was linked to malignant transformation in several cancers, including PCa [33,34,35,36,37]. Therefore, the increased STAT5 indicates the involvement of the transcription factor in the oncogenic transformation of prostate cells. However, more investigations are necessary to validate this hypothesis.
STAT6 in vitro data suggested a role of the IL-4/STAT6 in disease relapse by providing a favorable niche for the clonogenic growth of tumor-inducing PCa cells [19]. In PCa, STAT6 has increased expression and activity in malignant regions compared to adjective normal areas [19]. However, compared to BPH tissue, the PCa assessed here did not show a difference in STAT6 levels. Moreover, no activation could be detected in both cohorts. This result agrees with the role of the IL-4/STAT6 axis in metastasis as described earlier [19,38,39]. Moreover, activation of STAT6 was linked to high serum levels of IL-4 or increased M2 macrophage infiltration, which has not been assessed in the present study [40,41].
Kaplan–Meier estimations revealed a role of STAT proteins in biochemical recurrence and OS of PCa patients [5]. High levels of activated STAT3 and high STAT3 expression are linked to biochemical recurrence and shorter OS [42,43]. Increased STAT5 is associated with lower disease-free survival after RP in PCa [44]. Only high STAT5 activity was linked to a shorter OS in the present study. However, it must be noted that possible statistical effects are hidden due to the small cohort size. Additionally, treatment failure and biochemical recurrence were chosen as endpoints in most cases. Several studies used the phosphorylated STAT protein and not the localization. However, when evaluating phosphorylated STAT3 and STAT5, the activity of unphosphorylated STATs is not taken into account [45].
Multiple reports have associated STAT proteins with the development of therapy resistance. Preclinical studies have linked STAT3 to ADT, enzalutamide, and docetaxel resistance in PCa [5]. It is suggested that under ADT, active STAT3 enhances AR activity in the presence of low levels of androgens and therefore enhances PCa progression [10,46,47]. In this study, increased STAT3 levels and activity could also be observed in patients receiving ADT. This result confirms the role of STAT3 in PCa progress [11,27,28,29]. In vitro studies also revealed a function of the STAT3 signal pathway in enzalutamide and docetaxel resistance [11,48,49]. Here we could not show a further increase in STAT3 expression and activity in PCa tissue obtained from patients after receiving NHT or CTx. However, the absence of further change in STAT3 expression and activity does not rule out involvement of STAT3 in NHT or CTx resistance.
STAT5 promotes resistance to ADT and the development of the aggressive CRPC [12,50]. Therefore, it is suggested that STAT5 stabilizes the AR and thus promotes AR activity and PCa progression. AR stability was identified as a main regulator of AR after androgen deprivation and antiandrogen treatment [14]. In line with the data from Thomas and colleagues, an increase in STAT5 levels and activity could be observed in patients after receiving ADT [12]. The amplification of the STAT5 gene may partially explain this increase during ADT [51]. Enzalutamide has also been described to increase STAT5 activity by activating an AR-induced JAK2/STAT5 feed-forward loop [16,52]. Additionally, increased STAT5 levels have been demonstrated in docetaxel-resistant Du145 cells compared to their docetaxel-sensitive controls [18]. However, additional treatment with NHT or CTx increased neither STAT5 levels nor activity.
STAT6 has not been linked to the development of therapy resistance in PCa so far. Additionally, treatment with ADT or ADT combined with NHT and CTx did not influence STAT6 activity and expression in this study.
Tumor multifocality and heterogeneity are among the biggest challenges in primary PCa research and management. Therefore, many findings from preclinical models cannot be directly applied to primary tumor material, and statistical significance can hardly be achieved. Additionally, the data presented in this study reveal high heterogeneous expression and activity of the investigated STAT proteins. This heterogeneity may mask treatment-induced changes previously seen in in vitro and in vivo experiments [5]. For this reason, PCa tissue from six patients obtained from palliative TURP before and during ADT was analyzed for STAT3, 5, and 6 expression and activity changes. None of the investigated STATs showed a homogenous response to ADT in this small cohort. This finding is in line with the data from Handle et al. or Bishop et al. revealing multiple independent mechanisms in developing enzalutamide-resistant LNCaP cells and demonstrating numerous response possibilities to one treatment despite the same cellular background [53,54].
Preclinical cell models have been used to investigate signal pathways and mimic tumor behavior to identify new therapeutic strategies. In particular, cell line models such as LNCaP cells have been used as they are easy to handle and the results are highly reproducible [55,56]. However, most cell line models have been intensively cultured over decades, and they have changed due to accumulations of mutations and chromosomal aberrations [56,57]. In addition, culturing them in 2D without the supporting tumor microenvironment led to adaption to the cell culture conditions not representing their natural environment [55]. Due to these adaptions, most findings identified in 2D cell culture models cannot be translated directly into natural tumor biology. Therefore, more complex and patient near cell models are mandatory to increase the physiological relevance of data identified in basic research. Among other model systems, ex vivo tissue slice models, organoid models, and patient-derived xenografts are highly recommended to maintain physiological relevance. Although these models have shown to be expensive and difficult to handle, they represent the tumor heterogeneity and physiological relevance best. For example, it could be demonstrated that the tumor environment alters tumor metabolism as well as glutamine and glucose dependence. Therefore, findings identified in cell line models need to be validated in a patient near complex model to be described as a novel mechanism.
One limitation of this study is the low sample number, especially from the longitudinal patient cohort. Moreover, cancer-specific survival and mortality would have been desirable. Finally, expression data about the known STAT targets gene would also have been an excellent addition to estimating the tissue’s STAT activity.
5. Conclusions
Most STAT proteins’ functional role in PCa progression was obtained in preclinical in vitro and in vivo models. Therefore, this study attempted to transfer the preclinical observations to patient material. Although there was no relationship between expression and survival, the data revealed that STAT 3 and 5 changed in PCa. The biggest hurdle to transfer the in vitro and in vivo data to the patient situation is the tumor heterogeneity and the different tumor response to the treatments, which can only be represented to a limited extent in preclinical models. Therefore, these data show the need for investigations of STAT3 and 5 in a longitudinal patient cohort to identify factors responsible for the diverse influence of system therapy on STAT3, 5, and 6 expressions.
Acknowledgments
The authors would also like to thank the patients who kindly provided samples. The Department of Urology at the Technische Universität Dresden is acknowledged for its technical support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12020240/s1: Figure S1: Correlation Matrix displaying the r-Values of STAT expression and STAT activity; Figure S2: mRNA expression data of the SU2C PRAD cohort
Author Contributions
Conceptualization, A.B., U.S. and H.H.H.E.; methodology, P.H., K.J., A.B., U.S. and H.H.H.E.; software, A.-M.K.B., C.S. and H.H.H.E.; validation, C.E., A.-M.K.B., C.S., G.B.B., K.J., A.B., U.S. and H.H.H.E.; formal analysis, C.E., A.-M.K.B., G.B.B., K.J., A.B., U.S., C.T. and H.H.H.E.; investigation, C.E., A.B., U.S., and H.H.H.E.; resources, G.B.B. and C.T.; data curation, C.E., G.B.B., K.J., A.B., U.S. and H.H.H.E.; writing—original draft preparation, H.H.H.E.; writing—review and editing, C.E., A.-M.K.B., C.S., P.H., G.B.B., K.J., C.T., A.B. and U.S.; visualization, A.-M.K.B., P.H. and H.H.H.E.; supervision, A.B., U.S. and H.H.H.E.; project administration, A.B., U.S. and H.H.H.E.; funding acquisition, A.B., U.S. and H.H.H.E. All authors have read and agreed to the published version of the manuscript.
Funding
AKB and CE were supported by the Deutsche Krebshilfe through an MD-student fellowship of the Mildred Scheel Early Career Center Dresden.
Institutional Review Board Statement
Patients samples were selected from the Tumor and Normal Tissue Bank of the University Cancer Center Dresden. The Ethics Committee approved the use of archived material of the Medical University of Dresden (Study no. EK195092004).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N., van den Bergh R.C.N., Briers E., van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 3.Siegel D.A., O’Neil M.E., Richards T.B., Dowling N.F., Weir H.K. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity—United States, 2001–2017. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1473–1480. doi: 10.15585/mmwr.mm6941a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornford P., van den Bergh R.C.N., Briers E., van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II—2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Ebersbach C., Beier A.-M.K., Thomas C., Erb H.H.H. Impact of STAT Proteins in Tumor Progress and Therapy Resistance in Advanced and Metastasized Prostate Cancer. Cancers. 2021;13:4854. doi: 10.3390/cancers13194854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora L.B., Buettner R., Seigne J., Diaz J., Ahmad N., Garcia R., Bowman T., Falcone R., Fairclough R., Cantor A., et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: Direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 7.Don-Doncow N., Marginean F., Coleman I., Nelson P.S., Ehrnström R., Krzyzanowska A., Morrissey C., Hellsten R., Bjartell A. Expression of STAT3 in Prostate Cancer Metastases. Eur. Urol. 2016;71:313–316. doi: 10.1016/j.eururo.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiotto M.T., Chung T.D. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(SICI)1097-0045(20000215)42:3<186::AID-PROS4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 9.Oelrich F., Junker H., Stope M.B., Erb H.H., Walther R., Venz S., Zimmermann U. Gelsolin Governs the Neuroendocrine Transdifferentiation of Prostate Cancer Cells and Suppresses the Apoptotic Machinery. Anticancer Res. 2021;41:3717–3729. doi: 10.21873/anticanres.15163. [DOI] [PubMed] [Google Scholar]
- 10.Handle F., Puhr M., Schaefer G., Lorito N., Hoefer J., Gruber M., Guggenberger F., Santer F.R., Marques R.B., Van Weerden W.M., et al. The STAT3 Inhibitor Galiellalactone Reduces IL6-Mediated AR Activity in Benign and Malignant Prostate Models. Mol. Cancer Ther. 2018;17:2722–2731. doi: 10.1158/1535-7163.MCT-18-0508. [DOI] [PubMed] [Google Scholar]
- 11.Liu C., Zhu Y., Lou W., Cui Y., Evans C.P., Gao A.C. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate. 2013;74:201–209. doi: 10.1002/pros.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C., Zoubeidi A., Kuruma H., Fazli L., Lamoureux F., Beraldi E., Monia B.P., MacLeod A.R., Thüroff J.W., Gleave M.E. Transcription Factor Stat5 Knockdown Enhances Androgen Receptor Degradation and Delays Castration-Resistant Prostate Cancer Progression In vivo. Mol. Cancer Ther. 2011;10:347–359. doi: 10.1158/1535-7163.MCT-10-0850. [DOI] [PubMed] [Google Scholar]
- 13.Mirtti T., Leiby B.E., Abdulghani J., Aaltonen E., Pavela M., Mamtani A., Alanen K., Egevad L., Granfors T., Josefsson A., et al. Nuclear Stat5a/b predicts early recurrence and prostate cancer–specific death in patients treated by radical prostatectomy. Hum. Pathol. 2012;44:310–319. doi: 10.1016/j.humpath.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siciliano T., Simons I.H., Beier A.-M.K., Ebersbach C., Aksoy C., Seed R.I., Stope M.B., Thomas C., Erb H.H.H. A Systematic Comparison of Antiandrogens Identifies Androgen Receptor Protein Stability as an Indicator for Treatment Response. Life. 2021;11:874. doi: 10.3390/life11090874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb H.H.H., Oster M.A., Gelbrich N., Cammann C., Thomas C., Mustea A., Stope M.B. Enzalutamide-induced Proteolytic Degradation of the Androgen Receptor in Prostate Cancer Cells Is Mediated Only to a Limited Extent by the Proteasome System. Anticancer Res. 2021;41:3271–3279. doi: 10.21873/anticanres.15113. [DOI] [PubMed] [Google Scholar]
- 16.Erb H.H.H., Bodenbender J., Handle F., Diehl T., Donix L., Tsaur I., Gleave M., Haferkamp A., Huber J., Fuessel S., et al. Assessment of STAT5 as a potential therapy target in enzalutamide-resistant prostate cancer. PLoS ONE. 2020;15:e0237248. doi: 10.1371/journal.pone.0237248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udhane V., Maranto C., Hoang D.T., Gu L., Erickson A., Devi S., Talati P.G., Banerjee A., Iczkowski K.A., Jacobsohn K., et al. Enzalutamide-Induced Feed-Forward Signaling Loop Promotes Therapy-Resistant Prostate Cancer Growth Providing an Exploitable Molecular Target for Jak2 Inhibitors. Mol. Cancer Ther. 2019;19:231–246. doi: 10.1158/1535-7163.MCT-19-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puhr M., Hoefer J., Neuwirt H., Eder I.E., Kern J., Schäfer G., Geley S., Heidegger I., Klocker H., Culig Z. PIAS1 is a crucial factor for prostate cancer cell survival and a valid target in docetaxel resistant cells. Oncotarget. 2014;5:12043–12056. doi: 10.18632/oncotarget.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nappo G., Handle F., Santer F.R., McNeill R.V., Seed R.I., Collins A.T., Morrone G., Culig Z., Maitland N.J., Erb H.H.H. The immunosuppressive cytokine interleukin-4 increases the clonogenic potential of prostate stem-like cells by activation of STAT6 signalling. Oncogenesis. 2017;6:e342. doi: 10.1038/oncsis.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das S., Roth C.P., Wasson L.M., Vishwanatha J.K. Signal transducer and activator of transcription-6 (STAT6) is a constitutively expressed survival factor in human prostate cancer. Prostate. 2007;67:1550–1564. doi: 10.1002/pros.20640. [DOI] [PubMed] [Google Scholar]
- 21.Remmele W., Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 22.Patterson S.G., Wei S., Chen X., Sallman D.A., Gilvary D.L., Zhong B., Pow-Sang J., Yeatman T., Djeu J.Y. Novel role of Stat1 in the development of docetaxel resistance in prostate tumor cells. Oncogene. 2006;25:6113–6122. doi: 10.1038/sj.onc.1209632. [DOI] [PubMed] [Google Scholar]
- 23.Lim C.P., Cao X. Structure, function, and regulation of STAT proteins. Mol. BioSyst. 2006;2:536–550. doi: 10.1039/b606246f. [DOI] [PubMed] [Google Scholar]
- 24.Culig Z. Cytokine disbalance in common human cancers. Biochim. Biophys. Acta. 2011;1813:308–314. doi: 10.1016/j.bbamcr.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Culig Z., Puhr M. Interleukin-6: A multifunctional targetable cytokine in human prostate cancer. Mol. Cell. Endocrinol. 2012;360:52–58. doi: 10.1016/j.mce.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Culig Z. Proinflammatory cytokine interleukin-6 in prostate carcinogenesis. Am. J. Clin. Exp. Urol. 2014;2:231–238. [PMC free article] [PubMed] [Google Scholar]
- 27.Culig Z., Bartsch G., Hobisch A. Interleukin-6 regulates androgen receptor activity and prostate cancer cell growth. Mol. Cell. Endocrinol. 2002;197:231–238. doi: 10.1016/S0303-7207(02)00263-0. [DOI] [PubMed] [Google Scholar]
- 28.Liu X., He Z., Li C.-H., Huang G., Ding C., Liu H. Correlation Analysis of JAK-STAT Pathway Components on Prognosis of Patients with Prostate Cancer. Pathol. Oncol. Res. 2011;18:17–23. doi: 10.1007/s12253-011-9410-y. [DOI] [PubMed] [Google Scholar]
- 29.Horinaga M., Okita H., Nakashima J., Kanao K., Sakamoto M., Murai M. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology. 2005;66:671–675. doi: 10.1016/j.urology.2005.03.066. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty S.K., Yagiz K., Pradhan D., Luthringer D.J., Amin M.B., Alkan S., Cinar B. STAT3 and STAT5A are potential therapeutic targets in castration-resistant prostate cancer. Oncotarget. 2017;8:85997–86010. doi: 10.18632/oncotarget.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pencik J., Schlederer M., Gruber W., Unger C., Walker S.M., Chalaris A., Marié I.J., Hassler M.R., Javaheri T., Aksoy O., et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat. Commun. 2015;6:7736. doi: 10.1038/ncomms8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meissl K., Macho-Maschler S., Müller M., Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2015;89:12–20. doi: 10.1016/j.cyto.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Iglesias-Gato D., Chuan Y.-C., Wikström P., Augsten S., Jiang N., Niu Y., Seipel A., Danneman D., Vermeij M., Fernandez-Perez L., et al. SOCS2 mediates the cross talk between androgen and growth hormone signaling in prostate cancer. Carcinogenesis. 2013;35:24–33. doi: 10.1093/carcin/bgt304. [DOI] [PubMed] [Google Scholar]
- 34.Perry J.K., Emerald B.S., Mertani H.C., Lobie P.E. The oncogenic potential of growth hormone. Growth Horm. IGF Res. 2006;16:277–289. doi: 10.1016/j.ghir.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Roberts C.T., Jr. IGF-1 and prostate cancer. Novartis Found Symp. 2004;262:193–199; discussion 199–204, 265–198. [PubMed] [Google Scholar]
- 36.Barash I. Stat5 in breast cancer: Potential oncogenic activity coincides with positive prognosis for the disease. Carcinogenesis. 2012;33:2320–2325. doi: 10.1093/carcin/bgs362. [DOI] [PubMed] [Google Scholar]
- 37.Boutillon F., Pigat N., Sala L.S., Reyes-Gomez E., Moriggl R., Guidotti J.-E., Goffin V. STAT5a/b Deficiency Delays, but does not Prevent, Prolactin-Driven Prostate Tumorigenesis in Mice. Cancers. 2019;11:929. doi: 10.3390/cancers11070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrand-Rosenberg S., Clements V.K., Terabe M., Park J.M., Berzofsky J.A., Dissanayake S.K. Resistance to Metastatic Disease in STAT6-Deficient Mice Requires Hemopoietic and Nonhemopoietic Cells and Is IFN-γ Dependent. J. Immunol. 2002;169:5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 39.Wang N., Tao L., Zhong H., Zhao S., Yu Y., Yu B., Chen X., Gao J., Wang R. miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol. Lett. 2015;11:543–550. doi: 10.3892/ol.2015.3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise G.J., Marella V.K., Talluri G., Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000;164:722–725. doi: 10.1016/S0022-5347(05)67289-8. [DOI] [PubMed] [Google Scholar]
- 41.Sheng J., Yang Y., Cui Y., He S., Wang L., Liu L., He Q., Lv T., Han W., Yu W., et al. M2 macrophage-mediated interleukin-4 signalling induces myofibroblast phenotype during the progression of benign prostatic hyperplasia. Cell Death Dis. 2018;9:755. doi: 10.1038/s41419-018-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tam L., McGlynn L.M., Traynor P., Mukherjee R., Bartlett J.M.S., Edwards J. Expression levels of the JAK/STAT pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br. J. Cancer. 2007;97:378–383. doi: 10.1038/sj.bjc.6603871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberhuber M., Pecoraro M., Rusz M., Oberhuber G., Wieselberg M., Haslinger P., Gurnhofer E., Schlederer M., Limberger T., Lagger S., et al. STAT 3 -dependent analysis reveals PDK 4 as independent predictor of recurrence in prostate cancer. Mol. Syst. Biol. 2020;16:e9247. doi: 10.15252/msb.20199247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haddad B.R., Erickson A., Udhane V., LaViolette P.S., Rone J.D., Kallajoki M.A., See W.A., Rannikko A., Mirtti T., Nevalainen M.T. Positive STAT5 Protein and Locus Amplification Status Predicts Recurrence after Radical Prostatectomy to Assist Clinical Precision Management of Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2019;28:1642–1651. doi: 10.1158/1055-9965.EPI-18-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wingelhofer B., Neubauer H.A., Valent P., Han X., Constantinescu S.N., Gunning P.T., Müller M., Moriggl R. Implications of STAT3 and STAT5 signaling on gene regulation and chromatin remodeling in hematopoietic cancer. Leukemia. 2018;32:1713–1726. doi: 10.1038/s41375-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Miguel F., Lee S.O., Onate S.A., Gao A.C. Stat3 enhances transactivation of steroid hormone receptors. Nucl. Recept. 2003;1:3. doi: 10.1186/1478-1336-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malinowska K., Neuwirt H., Cavarretta I., Bektic J., Steiner H., Dietrich H., Moser P.L., Fuchs D., Hobisch A., Culig Z. Interleukin-6 stimulation of growth of prostate cancer in vitro and in vivo through activation of the androgen receptor. Endocr. Relat. Cancer. 2009;16:155–169. doi: 10.1677/ERC-08-0174. [DOI] [PubMed] [Google Scholar]
- 48.Liu Q., Tong D., Liu G., Xu J., Do K., Geary K., Zhang D., Zhang J., Zhang Y., Li Y., et al. Metformin reverses prostate cancer resistance to enzalutamide by targeting TGF-β1/STAT3 axis-regulated EMT. Cell Death Dis. 2017;8:e3007. doi: 10.1038/cddis.2017.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zemskova M., Sahakian E., Bashkirova S., Lilly M. The PIM1 Kinase Is a Critical Component of a Survival Pathway Activated by Docetaxel and Promotes Survival of Docetaxel-treated Prostate Cancer Cells. J. Biol. Chem. 2008;283:20635–20644. doi: 10.1074/jbc.M709479200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tan S.-H., Dagvadorj A., Shen F., Gu L., Liao Z., Abdulghani J., Zhang Y., Gelmann E.P., Zellweger T., Culig Z., et al. Transcription Factor Stat5 Synergizes with Androgen Receptor in Prostate Cancer Cells. Cancer Res. 2008;68:236–248. doi: 10.1158/0008-5472.CAN-07-2972. [DOI] [PubMed] [Google Scholar]
- 51.Haddad B.R., Gu L., Mirtti T., Dagvadorj A., Vogiatzi P., Hoang D.T., Bajaj R., Leiby B., Ellsworth E., Blackmon S., et al. STAT5A/B Gene Locus Undergoes Amplification during Human Prostate Cancer Progression. Am. J. Pathol. 2013;182:2264–2275. doi: 10.1016/j.ajpath.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Udhane V., Maranto C., Hoang D., Erickson A., Devi S., Jacobsohn K., Iczkowski K., See W., Kilari D., Mirtti T., et al. The role of enzalutamide-induced hyperactive Jak2-Stat5 feed-forward signaling loop on enzalutamide-resistant prostate cancer growth and as a therapeutic target for second-line treatment. J. Clin. Oncol. 2019;37:221. doi: 10.1200/JCO.2019.37.7_suppl.221. [DOI] [Google Scholar]
- 53.Handle F., Prekovic S., Helsen C., Van den Broeck T., Smeets E., Moris L., Eerlings R., El Kharraz S., Urbanucci A., Mills I.G., et al. Drivers of AR indifferent anti-androgen resistance in prostate cancer cells. Sci. Rep. 2019;9:13786. doi: 10.1038/s41598-019-50220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop J.L., Sio A., Angeles A., Roberts M., Azad A.A., Chi K.N., Zoubeidi A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget. 2014;6:234–242. doi: 10.18632/oncotarget.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muir A., Danai L.V., Heiden M.G.V. Microenvironmental regulation of cancer cell metabolism: Implications for experimental design and translational studies. Dis. Model. Mech. 2018;11 doi: 10.1242/dmm.035758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maitland N.J. Getting closer to prostate cancer in patients—What scientists should want from clinicians. J. Cancer Metastasis Treat. 2017;3:262. doi: 10.20517/2394-4722.2017.23. [DOI] [Google Scholar]
- 57.Masters J.R. HeLa cells 50 years on: The good, the bad and the ugly. Nat. Cancer. 2002;2:315–319. doi: 10.1038/nrc775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.