Abstract
In previous studies, we identified a common site of retroviral integration designated Fli-2 in Friend murine leukemia virus (F-MuLV)-induced erythroleukemia cell lines. Insertion of F-MuLV at the Fli-2 locus, which was associated with the loss of the second allele, resulted in the inactivation of the erythroid cell- and megakaryocyte-specific gene p45NFE2. Frequent disruption of p45NFE2 due to proviral insertion suggests a role for this transcription factor in the progression of Friend virus-induced erythroleukemias. To assess this possibility, erythroleukemia was induced by F-MuLV in p45NFE2 mutant mice. Since p45NFE2 homozygous mice mostly die at birth, erythroleukemia was induced in +/− and +/+ mice. We demonstrate that +/− mice succumb to the disease moderately but significantly faster than +/+ mice. In addition, the spleens of +/− mice were significantly larger than those of +/+ mice. Of the 37 tumors generated from the +/− and +/+ mice, 10 gave rise to cell lines, all of which were derived from +/− mice. Establishment in culture was associated with the loss of the remaining wild-type p45NFE2 allele in 9 of 10 of these cell lines. The loss of a functional p45NFE2 in these cell lines was associated with a marked reduction in globin gene expression. Expression of wild-type p45NFE2 in the nonproducer erythroleukemic cells resulted in reduced cell growth and restored the expression of globin genes. Similarly, the expression of p45NFE2 in these cells also slows tumor growth in vivo. These results indicate that p45NFE2 functions as an inhibitor of erythroid cell growth and that perturbation of its expression contributes to the progression of Friend erythroleukemia.
The transcription factor NFE2 (nuclear factor erythroid 2) plays a critical role in the regulation of erythroid cell-specific gene expression (25). This nuclear factor binds to AP1-like consensus binding sites located in the enhancers and promoters of several erythroid cell- and megakaryocyte-specific genes, including the β-globin locus control region (15, 17, 23, 31), human porphobilinogen deaminase (19), ferrochelatase (29, 34), and thromboxane synthase (9). NFE2 is a heterodimer complex of two basic leucine zipper proteins, consisting of 45-kDa (p45NFE2) and 18-kDa (p18NFE2) subunits (1). The expression of the large subunit, p45NFE2, has been found to be tissue specific, with expression restricted to erythroid cells, megakaryocytes, and mast cells (1). However, p18NFE2, a member of the Maf oncoprotein family (13), is widely expressed in many tissues (2).
p45NFE2-deficient mice display mild abnormalities in erythropoiesis, including hypochromia, anisocytosis, and reticulocytosis (27). However, these mice are severely thrombocytopenic due to arrest in late megakaryocyte maturation which results in hemorrhage after birth. Although most p45NFE2-deficient mice die at birth, a small fraction survives and develops primary or secondary phenotypes such as severe megakaryocytosis, splenomegaly, and bone marrow hypercellularity (16).
Previously, we have reported that the p45NFE2 gene resides in the Fli-2 locus, a common site for retroviral integration identified in erythroleukemias induced by both FV-P and F-MuLV strains of Friend virus (17). In one erythroleukemia cell line, CB3, proviral insertion within one allele of the p45NFE2 gene was associated with loss of the second allele, resulting in complete inactivation of p45NFE2 expression (17). Loss of p45NFE2resulted in significant reduction in the expression of both α- and β-globin genes. When p45NFE2 was reintroduced into CB3 cells, expression of both α- and β-globin was restored, providing evidence that p45NFE2 is a positive regulator of globin gene expression (15, 17). Since proviral integration within the p45NFE2 gene was also identified in other cell lines (17), these results raised the intriguing possibility that this transcription factor functions as a suppressor of tumor growth in Friend virus-induced erythroleukemias.
Friend virus-induced erythroleukemia has been an excellent animal model to identify genes involved in multistep malignancies. The induction and progression of erythroleukemias by Friend virus are mainly due to the ability of proviruses to activate cellular oncogenes or inactivate tumor suppressor genes (3). In Friend virus-induced erythroleukemias, the expression of p53 was first shown to be lost by mechanisms such as proviral integration, mutation, and rearrangement (7, 10, 12, 21, 22). Subsequently, the Ets-related transcription factors Spi-1 and Fli-1 were identified, and their expression was shown to be induced as a result of integration of spleen focus-forming virus or F-MuLV adjacent to these genes, respectively (5, 20). While insertional activation of either Fli-1 or Spi-1 is an early and critical event during the induction of these two types of erythroleukemia, p53 mutation is associated with late stages in the progression of the disease (35)
In this study, we utilized p45NFE2 mutant mice to study the role of this gene in the progression of Friend virus induced-erythroleukemias. Our results support a role for p45NFE2 as a negative regulator of cell growth in Friend virus-induced erythroleukemia.
MATERIALS AND METHODS
Breeding and F-MuLV inoculation of newborn mice.
p45NFE2 heterozygous (+/−) mice of the 129/Sv strain (28) were mated with BALB/c mice (Jackson Laboratories) for six generations in order to confer upon them sensitivity to Friend virus-induced erythroleukemias (35). The offspring were genotyped by Southern blot analysis of tail DNA as described previously (28). +/− newborn mice from the BALB/c cross were then tested for sensitivity to F-MuLV by a single intraperitoneal injection at birth (26). It was found that these mice were susceptible to F-MuLV-induced erythroleukemia. Accordingly, two breeding pairs of the +/− mice were used to generate offspring for viral inoculation.
Tumor induction and establishment of cell lines.
Newborn pups were injected intraperitoneally with F-MuLV clone 57 as described elsewhere (26). They were monitored for (i) enlarged abdomens causing hunched postures, a symptom of splenomegaly, and (ii) a lack of movement, reflecting low energy due to severe anemia, and were sacrificed when moribund. Mice displaying these marked symptoms rarely survive for over a day. For genotyping, tail tissues and tumor cells were processed for Southern analysis. The spleen cells from these erythroleukemias were cultured in α-minimum essential medium supplemented with 15% heat-inactivated fetal bovine serum (Gibco BRL). Cells were cultured in this medium alone or supplemented with either 1 U of erythropoietin (Epo)/ml, 10% stem cell factor (SCF) conditioned medium, or both growth factors as described elsewhere (35). SCF was obtained from SCF-producing BHK-MKL cells (provided by S. Tsai) (33). These conditions were maintained until cells were established in culture, at which time fetal bovine serum was reduced to 10% and growth factors were removed in order to determine the growth factor dependency of the cell lines. To examine cellular growth rates, erythroleukemic cells were cultured in triplicate in the presence of Epo, SCF, or Epo plus SCF and viable cells were determined by trypan blue dye exclusion at various times.
DNA isolation and Southern analysis.
High-molecular-weight DNA was isolated from homogenized spleen tissue, tail samples, or cell lines as previously described (12). Fifteen micrograms of genomic DNA from tumors or cell lines was digested overnight with appropriate restriction enzymes and separated on 1% agarose gels. DNA was acid depurinated in 0.1 M HCl for 15 min before denaturation and capillary transferred onto nylon membranes using 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membranes were hybridized with 100 ng of random-primed DNA probe in a mixture of 50% formamide, 5× SSPE (20× SSPE is 3 M NaCl, 200 mM NaH2PO4·H2O, and 20 mM EDTA), 1× Denhardt's solution (0.02% bovine serum albumin, 0.02% Ficoll, 0.02% polyvinylpyrolidone), and 5% dextran sulfate at 42°C. The filters were washed for 15 min twice at room temperature in 2× SSC–0.5% sodium dodecyl sulfate (SDS) and then twice at 65°C in 0.2× SSC–0.1% SDS.
RNA isolation and Northern analysis.
Two micrograms of poly(A)+ mRNA isolated from cell lines was dissolved in 2.2 M formamide, incubated at 55°C for 15 min, and separated in a 1% agarose gel containing 0.66 M formaldehyde. Gels were washed twice in transfer buffer (10× SSC) for 20 min and transferred overnight onto nylon filters. The filters were hybridized with 2 × 106 cpm of 32P-labeled random-primed probe per ml of hybridization mixture that contained 50% formamide, 10% dextran sulfate, 1.5× SSC, and 5× Denhardt's solution at 42°C. The filters were washed twice with 2× SSC–0.2% SDS at room temperature for 20 min and then twice with 0.2× SSC–0.1% SDS at 65°C for 15 min. Hybridized probe was removed from the filters by two 30-min washes with a mixture of 0.1% SDS, 10 mM Tris (pH 7.5), and 1 mM EDTA at 70°C.
DNA probes.
The F-MuLV env probe is a 0.8-kb BamHI segment of plasmid pHC6 (8). The Fli-1 cDNA probe is a 1.7-kb EcoRI fragment of the BB4 plasmid (5). The α-globin probe is a 0.5-kb EcoRI fragment from plasmid PB1. The p45NFE2 probe used in Northern blot analysis is a 1.5-kb EcoRI cDNA fragment derived from the CB7 erythroleukemia cell line (17). For Southern analysis a genomic fragment corresponding to the HindIII-EcoRV fragment of the p45NFE2 gene was used (28). The p53 probe is a 0.9-kb BglII-PstI cDNA fragment from mouse clone 27.1a (14). The 750-bp PstI-XbaI fragment of mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was used to normalize the RNA loaded.
p45NFE2 viral vector and cell infection.
The 1.5-kbp EcoRI fragment corresponding to full-length p45NFE2 cDNA was cloned into the EcoRI site of pMX-puro retroviral expression vector (24) and designated pMX-NFE2. To generate the retroviruses, pMX-puro and pMX-NFE2 constructs were transfected into amphotropic GP+envAM12 helper-free packaging cells (18) by the Lipofectin transfection method (Life Technologies). Cells resistant to puromycin (2 μg/ml) were pooled and cocultured with erythroleukemia cell lines for two days. The nonadherent leukemic cells were then removed and selected for 1 week with 0.3 to 0.5 μg of puromycin per ml, and resistant cells were used for expression and cell growth analyses.
In vivo assays.
NKH18-C4A cells (106) infected with pMX-puro or pMX-NFE2 were injected into nude mice via tail vein injection. Mice were monitored for the development of leukemia and were sacrificed when they exhibited terminal stages of the disease, as described above.
Statistical analysis.
Comparisons between two groups were made with Student's t test. Differences were considered significant at P < 0.05.
RESULTS
In vivo progression of erythroleukemias in p45NFE2mutant and control mice.
To render sensitivity to Friend virus, the p45NFE2 knockout mice originally generated in the C57BL/6 and 129Sv strains of mice (28) were crossed into the susceptible BALB/c background. After six consecutive crosses, heterozygous breeding pairs of p45NFE2 mice were mated and the pups were infected with F-MuLV. Of 39 infected pups, 2 died around 2 weeks after viral infection. The remaining mice were monitored for the development of erythroleukemias and were sacrificed when they became moribund. Genotype analysis indicated that 26 of these erythroleukemias originated from +/− mice, and the remaining 11 were from +/+ mice. −/− mice were not used in this analysis due to their high perinatal mortality (28). The average times between viral injection and sacrifice in +/+ and +/− mice were 44 (standard deviation [SD] = 3.8) and 41 (SD = 4.2) days, respectively. Statistical analysis comparing the spectrum of +/+ and +/− revealed a moderate but significant difference (P < 0.049) in the time until these mice succumbed to the disease. In addition, unpaired t test comparison of spleen weights between p45NFE2 heterozygous (mean, 1.78 g; SD = 0.25) and p45NFE2 wild-type mice (mean, 1.552; g; SD = 0.34) indicated a significant difference (P < 0.03) between these populations. The shorter time span for tumor development and the significant increase in the volume of tumorigenic spleen suggest that in +/− infected mice the leukemogenic process is accelerated.
Growth of erythroleukemic cells in culture is associated with the loss of the p45NFE2 gene.
Retroviral insertional activation of the Fli-1 gene has been detected in the majority of F-MuLV-induced erythroleukemias (4, 12). Thus, we examined the genomic organization of the Fli-1 locus in tumors induced in p45NFE2 mutant mice. Southern analysis revealed rearrangements of the Fli-1 locus in all tumors derived from +/+ and +/− mice (data not shown). Our previous studies demonstrated that F-MuLV-induced erythroleukemic cells that have acquired activated Fli-1 undergo apoptosis when they are introduced into cell culture (11). However, when F-MuLV was injected into p53-deficient mice, tumorigenic cell lines were established from the majority of the induced erythroleukemias, although their growth was dependent on the presence of Epo and/or SCF (35). To examine whether the absence of p45NFE2 could also be correlated with the immortalization of primary erythroleukemic cells, tumor cells removed from +/− and +/+ mice were grown in culture medium supplemented with 15% fetal bovine serum alone or further supplemented with both Epo and SCF. After 4 weeks of culturing in the presence of Epo plus SCF, 10 independent cell lines that originated from +/− tumors were established (Table 1). None of the +/+ tumors gave rise to cell lines in the same culture period. Optimal growth of eight of these established cell lines was dependent on the presence of Epo, although two cell lines, NKH18-C and NKH2-C, were capable of growing in the presence of either Epo or SCF (Table 1). Since tumors used to establish these cell lines contained a rearranged Fli-1 gene, high levels of Fli-1 mRNA were detected in all of them (see Fig. 3).
TABLE 1.
Summary of cell lines established from +/− primary F-MuLV-induced erythroleukemiasa
| Tumor | p45NFE2 genotype in tumors | Cell line | p45NFE2 genotype in cell lines | Growth factor responsiveness |
|---|---|---|---|---|
| NKH2-T | +/− | NKH2-C | +/− | Epo or SCF |
| NKH17-T | +/− | NKH17-C | −/− | Epo |
| NKH18-T | +/− | NKH18-C2 | −/− | Epo or SCF |
| NKH19-T | +/− | NKH19-C | −/− | Epo |
| NKH23-T | +/− | NKH23-C | −/− | Epo |
| NKH24-T | +/− | NKH24-C | −/− | Epo |
| NKH25-T | +/− | NKH25-C | −/− | Epo |
| NKH26-T | +/− | NKH26-C | −/− | Epo |
| NKH31-T | +/− | NKH31-C | −/− | Epo |
| NKH34-T | +/− | NKH34-C | −/− | Epo |
The properties of tumors which gave rise to permanent cell lines are indicated. Growth factors upon which cells are dependent for growth in culture are indicated. Tumors and cell lines were genotyped by Southern analysis.
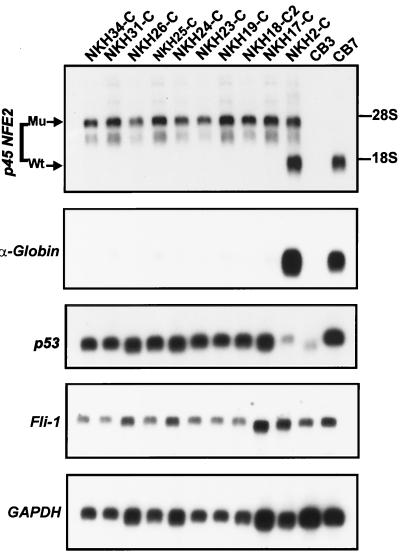
FIG. 3.
Analysis of gene expression in erythroleukemia cell lines derived from p45NFE2 mutant mice. Two micrograms of poly(A)+ mRNA isolated from the indicated erythroleukemia cell lines was denatured in formamide, electrophoresed on 1% agarose gels, blotted onto nylon filters, and hybridized with the p45NFE2 probe. The same blot was subsequently stripped and hybridized with α-globin, p53, or Fli-1 probe and with GAPDH probe to normalize for RNA loading per lane.
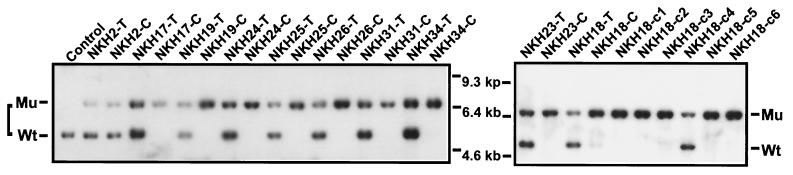
These observations raised the possibility that p45NFE2 deficiency contributes to the establishment of erythroleukemic cells in culture. Therefore, we assessed whether the genomic structure of p45NFE2 was altered in these cell lines. Southern blot analysis demonstrated that eight cell lines derived from +/− tumors had lost the remaining wild-type allele after less than 1 month in culture (Fig. 1; summarized in Table 1). In one cell line, NKH18-C, the intensity of the hybridized band corresponding to the p45NFE2 wild-type allele was significantly reduced compared to its corresponding tumor (Fig. 1), suggesting an oligoclonal process. Indeed, genomic analysis of the six individual clones isolated from NKH18-C by a limiting dilution experiment resulted in the identification of five cell lines that were homozygous and one (NKH18-C4) that was heterozygous for the p45NFE2 gene (Fig. 1, right panel). NKH2-C was the only cell line that maintained heterozygosity after 2 months in culture (Fig. 1, left panel).
FIG. 1.
p45NFE2 genotype in F-MuLV-induced erythroleukemias and their derivative cell lines. Fifteen micrograms of genomic DNA isolated from the indicated tumors (T) and their derivative cell lines (C) was digested with EcoRI, electrophoresed in 1% agarose, transferred onto a nylon filter, and hybridized with p45NFE2 probe. Bands corresponding to the mutated (Mu) p45NFE2 from the targeted allele and wild-type (Wt) p45NFE2 allele are marked. We also included the derivative cell clones isolated from the NKH18-C cell line (designated NKH18-C1 to -C6). Kidney DNA from BALB/c mice was used as a control.
To assess the clonal relationship between primary tumors and their respective cell lines, we examined the pattern of proviral integration by hybridizing genomic DNA with a F-MuLV-specific env probe (12, 35). As shown in Fig. 2 (upper panel), the pattern of proviral integration was similar in cell lines NKH18-C2, NKH19-C, NKH24-C, NKH26-C, NHK31-C and their corresponding tumors. However, five cell lines, NKH2-C, NKH17-C, NKH23-C, NKH25-C and NKH34-C, were clonally unrelated to the dominant cell population present in tumors. Therefore, these cell clones were likely derived from a minor subpopulation of tumor cells that survived in culture following loss of the wild-type p45NFE2 allele. A clonal relationship was also seen between two representative cell clones isolated from the NKH18-C cell line and its corresponding tumor (Fig. 2, lower panel). A similar clonal relationship between tumors and their corresponding cell lines was previously noted in tumors and cell lines derived from p53 mutant mice (35).
FIG. 2.
Clonal analysis of tumors and their derivative cell lines. Fifteen micrograms of genomic DNA isolated from tumors and their derivative cell lines was digested with EcoRI, transferred to a nylon filter, and hybridized with the F-MuLV env-specific probe. Normal kidney DNA from BALB/c mice was used as a control.
Loss of the p45NFE2 gene in erythroleukemias suppresses globin gene expression.
Northern blot analysis demonstrated that the nine erythroleukemia cell lines that had lost the wild-type p45NFE2 allele expressed only the mutated p45NFE2 mRNA from the targeted allele (Fig. 3). Both normal-size p45NFE2 mRNA from the wild-type allele and mutant mRNA from the targeted allele were detected in the NKH2-C cell line, which still retained one of the wild-type alleles (Fig. 3). Since p45NFE2 is an erythroid cell- and megakaryocyte-specific gene, the expression of either mutant or wild-type p45NFE2 in these cells attests to their erythroid origin.
Our studies have indicated that the p53 gene is altered in essentially all Friend virus-induced erythroleukemias cell lines (12, 35). Thus, we examined the status of this tumor suppressor gene in the NKH cell lines. Southern analysis using the restriction enzymes BamHI and HindIII failed to reveal rearrangement of the p53 gene in any of the cell lines (data not shown). Moreover, normal levels of p53 mRNA were detected in nine of the NKH cell lines, and NKH2-C was the only cell line that expressed lower levels of p53 (Fig. 3). The level of p53 expression was compared to those in the F-MuLV-induced erythroleukemia cell lines CB3 and CB7, which lost a functional p53 through deletion and point mutation, respectively (8, 22).
Although globin gene expression was not altered in p45NFE2 null mice (28), the expression of globin genes was significantly compromised in the CB3 erythroleukemia cell line, which has a homozygous loss of p45NFE2 (17). To determine the generality of this observation, we determined expression of globin in the established NKH cell lines. A negligible level of globin mRNA was detected in all NKH cell lines except NKH2-C and the control p45NFE2-expressing erythroleukemia cell line CB7 (Fig. 3). These results further reinforce the notion that p45NFE2 is a positive regulator of globin gene expression.
Loss of p45NFE2in erythroleukemic cells accelerates growth in culture.
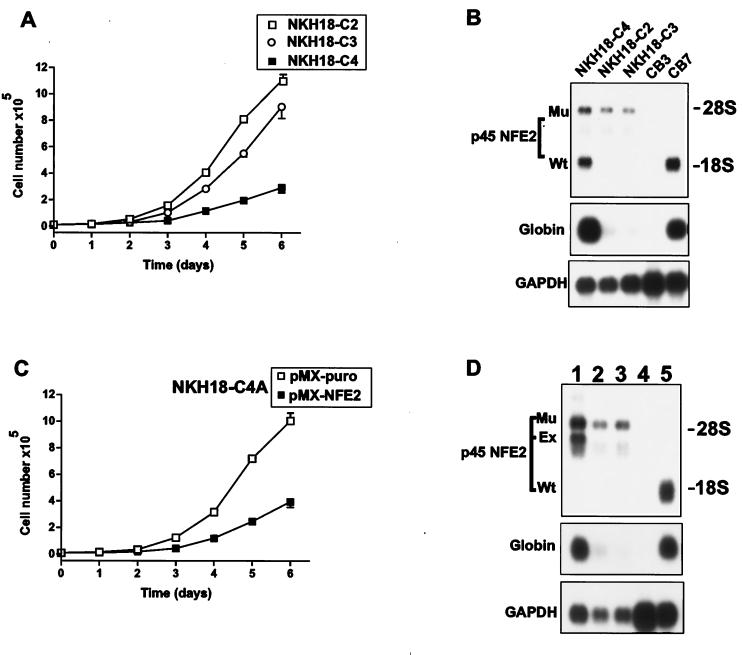
To examine the effect of loss of p45NFE2 on erythroleukemia progression, we first compared the growth rates of the NKH18-C derivative cell lines NKH18-C2 and NKH18-C3, which are p45NFE2 deficient, and NKH18-C4 cells, which are heterozygous (Fig. 1). In the presence of SCF and Epo, p45NFE2-expressing NKH18-C4 cells grew much more slowly than NKH18-C2 and NKH18-C3 cells (Fig. 4A). As expected, NKH18-C4 cells expressed both p45NFE2 and α-globin (Fig. 4B). Interestingly, NKH18-C4 cells, which are heterozygous for p45NFE2, proliferated rapidly after a month in culture. Southern analysis indicated that this new variant of NKH18-C4 (designated NKH18-C4A) had lost the wild-type p45NFE2 allele (data not shown). Loss of p45NFE2 in these cells was associated with the downregulation of α-globin expression (Fig. 4D). To further explore the growth suppressor ability of p45NFE2 on erythroleukemic cells, we reintroduced this gene into the NKH18-C4A cell line using a retroviral vector. As shown in Fig. 4C, p45NFE2-negative cells infected with p45NFE2 retrovirus grew at a much lower rate than did cells infected with vector alone. Expression of p45NFE2in these cells also resulted in up-regulation of α-globin (Fig. 4D). Similar results were obtained when an independent p45NFE2-negative erythroleukemia cell line NKH23-C was infected with the p45NFE2 retrovirus (data not shown). Although globin genes are induced in these cells and are conventionally used as differentiation markers, morphological changes resembling erythroid cell differentiation (32) were not detected in these cells.
FIG. 4.
Expression of p45NFE2 in nonproducer erythroleukemia cell lines suppresses cell proliferation. (A) Triplicate cultures of the indicated cells (104) were cultured in the presence of Epo plus SCF, and the number of viable cells was determined for days 1 to 6 by trypan blue dye exclusion. (B) RNAs extracted from the indicated erythroleukemic cells were Northern blotted and sequentially hybridized with p45NFE2, α-globin, and GAPDH probes. The p45NFE2 producer cell line CB7 and the nonproducer cell line CB3 were used as controls. (C) Triplicate cultures (104) of NKH18-C4A cells that were infected with either PMX-puro or PMX-NFE2 retroviruses were grown in the presence of SCF plus Epo. The growth rate of these cells was determined for days 1 to 6 by trypan blue dye exclusion. (D) Northern blot analysis of NKH18-C4A cells (lane 3) infected with the PMX-puro (lane 2) and PMX-NFE2 (lane 1) retroviruses was performed as described for panel B. Cell lines CB3 (lane 4) and CB7 (lane 5) were used as controls for p45NFE2-negative and -positive cell lines, respectively.
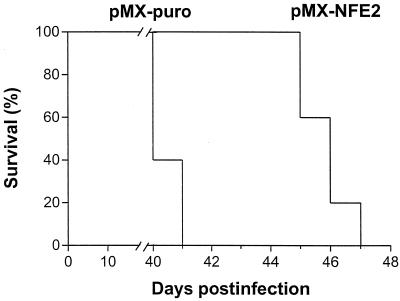
Although the difference in the time for development of Friend disease induced in +/− and +/+ mice was moderate but significant, we examined whether reexpression of p45NFE2 in nonproducer cells can delay tumor growth in vivo. When NKH18-C4A cells infected with the vector alone or the p45NFE2 retrovirus were injected into nude mice, expression of p45NFE2 significantly suppressed growth of erythroleukemic cells in vivo (Fig. 5). Overall, these results confirm the role of p45NFE2 in the regulation of globin gene expression and suggest that this transcription factor functions as a negative regulator of cell proliferation both in vitro and in vivo.
FIG. 5.
Expression of p45NFE2 in nonproducer erythroleukemic cells suppresses growth in vivo. NKH18-C4A cells (106) that were infected with either pMX-puro or pMX-NFE2 retroviruses were injected into nude mice (n = 5 for each group). Days indicate the time postinfection at which the mice succumbed to the disease.
DISCUSSION
Analysis of the sites of proviral integration in Friend virus-induced erythroleukemias led to the isolation of the Fli-2 locus, a common site for retroviral integration localized within the p45NFE2 gene (17). Frequent proviral insertion within the Fli-2 locus, which was associated with the loss of the second allele in a single cell line, suggested a role for p45NFE2 in the progression of erythroleukemias induced by Friend virus. We demonstrate that loss of p45NFE2 in tumor cells enhanced proliferation and was associated with the growth of erythroleukemic cells in culture. Moreover, these results confirm a direct role for p45NFE2 in the regulation of globin gene expression in erythroid cells.
Since both the targeted and wild-type alleles of p45NFE2 are expressed in erythroleukemias, lower expression levels of the functional protein may be responsible for the apparent, albeit modest growth advantage observed in vivo. Alternatively, the presence of a faster-growing subpopulation of erythroleukemic cells that lost the wild-type allele may have increased total cell numbers, resulting in accelerated tumor progression. This hypothesis is consistent with our demonstration that the proliferating erythroleukemic cells, maintained for less than 30 days in culture, are mostly negative for expression of the wild-type p45NFE2 allele and that cells expressing the wild-type p45NFE2 gene grow slower in vivo and in vitro. Moreover, cell lines established from the NKH18 tumors were shown to be a mixed population of both +/− and −/− erythroleukemic cells in which only the p45NFE2 negative cells with higher proliferating rate eventually survived in culture.
We have previously reported a direct association between p53 mutation, in vitro immortalization, and survival in culture of F-MuLV-induced erythroleukemias (11, 12). These observations were further supported when erythroleukemias were induced in the p53 mutant mouse background (35). In these studies, p53-deficient mice infected with F-MuLV died at a higher rate than control mice and growth of erythroleukemias in culture was mainly seen in erythroleukemias induced in p53−/− and p53+/− mice. Data for the leukemogenic potential of p45NFE2 −/− mice are not available because they died at birth or shortly after viral inoculation (28). We identified a strong similarity between in vivo and in vitro progression of erythroleukemias induced in p53+/− and p45NFE2+/− mice as follows. (i) Both p53+/− and p45NFE2+/− mice succumb to the disease more rapidly than p53+/+ and p45NFE2+/+ mice, respectively. (ii) Similar to cell lines derived from p53+/− tumors (35), in vitro establishment of erythroleukemias induced in p45NFE2+/− mice was associated with a loss of heterozygosity in 9 out of 10 established NKH cell lines. (iii) Both the p53 and p45NFE2 genes were frequently targeted for retroviral insertional inactivation, and loss of heterozygosity was commonly seen in erythroleukemias carrying viral integration in the other allele (7). These similarities raise the intriguing possibility that p53 and p45NFE2 have similar or overlapping functions during leukemogenesis and that like p53, p45NFE2 functions as a tumor suppressor gene in Friend virus-induced erythroleukemias.
Of 10 cell lines established from p45NFE2+/− tumors, NKH2-C was the only cell line that remained heterozygous and showed dramatically reduced expression of p53. Similarly, p53 inactivation was also seen in two previously established erythroleukemia cell lines, CB7 and DP28–9, which were also heterozygous for p45NFE2 due to proviral insertion within the Fli-2 locus (6, 22). Interestingly, the other nine cell lines with the p45NFE2−/− genotype did not appear to have abnormalities in p53 expression, and sequence analysis of one of these cell lines confirmed wild-type p53 status (unpublished results). To our knowledge, this is the first demonstration of an erythroleukemia cell line that expresses wild-type p53 (35). These results suggest that mutations within either p53 or p45NFE2 may be required for immortalization of erythroleukemias.
The strong selective advantage for p45NFE2 nullizygosity raises the possibility that expression of this gene in erythroleukemic cells negatively regulates their proliferative capability. Indeed, reintroduction of p45NFE2 into the nonproducer erythroleukemia cell lines significantly attenuated cell growth rates in vitro and in vivo. In addition, in the case of the NKH18-T tumor that contained a clonally related population of +/− and −/− cells, erythroleukemic cells lacking this transcription factor proliferated faster in culture. This suggests that p45NFE2 expression confers a negative growth advantage to erythroleukemic cells. The higher rate of tumor development and the increase in the size of tumorigenic spleens observed in +/− infected mice suggest that erythroleukemias induced in these mice may contain heterogeneous populations of both +/− and −/− cells. Since retroviral insertional activation of Fli-1 is required for the initial transformation of erythroblasts by F-MuLV, the loss of p45NFE2 in a subpopulation of these leukemic cells could result in the appearance of a cell population with a higher proliferative capability. Although clonal dominance of the −/− cells was not obvious by Southern analysis of the primary tumors, this could be explained by the early (∼40 days) death of mice after viral inoculation, due to the development of severe anemia (35). This is supported by the observation that only −/− cells survive after 3 weeks of growth in culture. Furthermore, while the number of erythroid burst-forming unit and erythroid CFU progenitor cells were identical in +/+ and −/− mice (27), splenomegaly with active erythropoiesis throughout life has been observed in the surviving adult −/− mice (16). Together, these results support a negative role for p45NFE2 in the proliferation of erythroblasts.
Previous studies using transgenic mice and cell lines indicated that p45NFE2 plays a major role in globin gene expression (15, 17, 30). However, in mice lacking p45NFE2, erythroid lineages were mildly affected and a small decrease in the hemoglobin content per cell was detected. Interestingly, −/− erythroleukemia cell lines independently derived from +/− mice were severely defective in globin gene expression, and reintroduction of p45NFE2 into these cells resulted in restored globin expression. These observations support the notion that p45NFE2 expression is critical for globin gene expression and further suggest that in −/− mice, the function of this protein may be compensated for by another factor capable of restoring globin gene expression. In contrast to adults, severe erythrocyte abnormalities, including extensive reticulocytosis, hypochromia, target cells, and dysmorphic cell forms, were detected in −/− neonates (27). Since F-MuLV induces erythroleukemias when injected into newborn mice, it is possible that this virus targets a subpopulation of erythroid progenitors in which globin expression is independent of p45NFE2. It is also possible that these neonate-derived erythroid cells do not express the compensatory factor that overlaps the p45NFE2 function. Therefore, identification of such a factor could significantly enhance our understanding of globin gene regulation.
In summary, the results of this study demonstrate that loss of p45NFE2 expression is required for the establishment of permanent erythroleukemic cells in culture. The absence of p45NFE2 in erythroleukemic cells promotes tumor growth by accelerating the rate of cellular proliferation. Moreover, we provided comprehensive and direct evidence to support the requirement of p45NFE2 in globin gene expression.
ACKNOWLEDGMENTS
We thank Jorge Filmus for his comments on the manuscript and Lynda Woodcock for help in preparation of the manuscript.
This work was supported by a grant from the Medical Research Council of Canada to Y.B.-D. and the National Cancer Institute of Canada to M.A. R.A.S. was supported in part by a grant from the National Institutes of Health. P.A.N. was supported in part by National Institutes of Health grant ROI DK53469, NIH Cancer Center Support grant p30 (CA21762), and the American Lebanese-Syrian Associated Charities. B.J.P and Y.-J.L. were supported by fellowships from the Sunnybrook Trust Fund for Medical Research. R.R.H. was supported by fellowships from the Natural Science and Engineering Research Council (Canada) and the Leukemia Research Fund of Canada.
REFERENCES
- 1.Andrews N C, Erdjument-Bromage H, Davidson M B, Tempst P, Orkin S H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993;362:722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N C, Kotkow K J, Ney P A, Erdjument-Bromage H, Tempst P, Orkin S H. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci USA. 1993;90:11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-David Y, Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991;66:831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- 4.Ben-David Y, Giddens E B, Bernstein A. Identification and mapping of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci USA. 1990;87:1332–1336. doi: 10.1073/pnas.87.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-David Y, Giddens E G, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5:908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- 6.Ben-David Y, Lavigueur A, Cheong G Y, Bernstein A. Insertional inactivation of the p53 gene during Friend leukemia: a new strategy for identifying tumor suppressor genes. New Biol. 1990;2:1015–1023. [PubMed] [Google Scholar]
- 7.Ben-David Y, Prideaux V R, Chow V, Benchimol S, Bernstein A. Inactivation of the p53 oncogene by internal deletion or retroviral integration in erythroleukemic cell lines induced by Friend leukemia virus. Oncogene. 1988;3:179–185. [PubMed] [Google Scholar]
- 8.Chow V, Ben-David Y, Bernstein A, Benchimol S, Mowat M. Multi-stage Friend erythroleukemia: independent origin of tumor clones with normal or rearranged p53 cellular oncogenes. J Virol. 1987;61:2777–2781. doi: 10.1128/jvi.61.9.2777-2781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deveaux S, Cohen-Kaminsky S, Shivdasani R A, Andrews N C, Filipe A, Kuzniak I, Orkin S H, Romeo P H, Mignotte V. p45 NF-E2 regulates expression of thromboxane synthase in megakaryocytes. EMBO J. 1997;16:5654–5661. doi: 10.1093/emboj/16.18.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicks G G, Mowat M. Integration of Friend murine leukemia virus into both alleles of the p53 oncogene in an erythroleukemia cell line. J Virol. 1988;62:4752–4755. doi: 10.1128/jvi.62.12.4752-4755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard J C, Ung Y, Adachi D, Ben-David Y. p53-independent tumor growth and in vitro cell survival for F-MuLV-induced erythroleukemias. Cell Growth Differ. 1996;7:1651–1660. [PubMed] [Google Scholar]
- 12.Howard J C, Yousefi S, Cheong G, Bernstein A, Ben-David Y. Temporal order and functional analysis of mutations within the Fli-1 and p53 genes during the erythroleukemias induced by F-MuLV. Oncogene. 1993;8:2721–2729. [PubMed] [Google Scholar]
- 13.Igarashi K, Kataoka K, Itoh K, Hayashi N, Nishizawa M, Yamamoto M. Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature. 1994;367:568–572. doi: 10.1038/367568a0. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins J R, Rudge K, Redomond S, Wade-Evans A. Cloning and expression analysis of full length mouse cDNA sequences encoding the transformation associated protein p53. Nucleic Acids Res. 1984;12:5609–5626. doi: 10.1093/nar/12.14.5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotkow K J, Orkin S H. Dependence of globin gene expression in mouse erythroleukemia cells on the NF-E2 heterodimer. Mol Cell Biol. 1995;15:4640–4647. doi: 10.1128/mcb.15.8.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin J, Peng J P, Baker G R, Villeval J L, Lecine P, Burstein S A, Shivdasani R A. Pathophysiology of thrombocytopenia and anemia in mice lacking transcription factor NF-E2. Blood. 1999;94:3037–3047. [PubMed] [Google Scholar]
- 17.Lu S J, Rowan S, Bani M R, Ben-David Y. Retroviral integration within the FLi-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc Natl Acad Sci USA. 1994;91:8398–8402. doi: 10.1073/pnas.91.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mignotte V, Wall L, deBoer E, Grosveld F, Romeo P H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989;17:37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreau-Gachelin F, Tavitian A, Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukemias. Nature. 1988;331:277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- 21.Mowat M, Cheng A, Kimura N, Bernstein A, Benchimol S. Rearrangements of the cellular p53 gene in erythroleukemic cells transformed by Friend virus. Nature. 1985;314:633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- 22.Munroe D G, Peacock J W, Benchimol S. Inactivation of the cellular p53 gene is a common feature of Friend erythroleukemia: relation to dominant transforming alleles. Mol Cell Biol. 1990;10:3307–3313. doi: 10.1128/mcb.10.7.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ney P A, Sorrentino B P, Lowrey C H, Nienhuis A W. Inducibility of the HS II enhancer depends on binding of an erythroid specific nuclear protein. Nucleic Acids Res. 1990;18:6011–6017. doi: 10.1093/nar/18.20.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nosaka T, Kawashima T, Misawa K, Ikuta K, Mui A L, Kitamura T. STAT5 as a molecular regulator of proliferation, differentiation and apoptosis in hematopoietic cells. EMBO J. 1999;18:4754–4765. doi: 10.1093/emboj/18.17.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orkin S H. Globin gene regulation and switching: circa 1990. Cell. 1990;63:665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya T, Mak T. Isolation and induction of erythroleukemic cell lines with properties of erythroid progenitor burst-forming cell (BFU-E) and erythroid precursor cell (CFU-E) Proc Natl Acad Sci USA. 1983;80:3721–3725. doi: 10.1073/pnas.80.12.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shivdasani R A, Orkin S H. Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc Natl Acad Sci USA. 1995;92:8690–8694. doi: 10.1073/pnas.92.19.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivdasani R A, Rosenblatt M F, Zucker-Franklin D, Jackson C W, Hunt P, Saris C J M, Orkin S H. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 29.Taketani S, Inazawa J, Nakahashi Y, Abe T, Tokunaga R. Structure of the human ferrochetalase gene: exon/intron gene organization and location of the gene to chromosome 18. Eur J Biochem. 1992;205:217–222. doi: 10.1111/j.1432-1033.1992.tb16771.x. [DOI] [PubMed] [Google Scholar]
- 30.Talbot D, Grosveld F. The 5 prime HS2 of the globin locus control region enhances transcription through the interaction of a multimeric complex binding at two functionally distinct NF-E2 binding sites. EMBO J. 1991;10:1391–1398. doi: 10.1002/j.1460-2075.1991.tb07659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talbot D, Philipsen S, Fraser P, Grosveld F. Detailed analysis of the site 3 region of the human beta-globin dominant control region. EMBO J. 1990;9:2169–2178. doi: 10.1002/j.1460-2075.1990.tb07386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamir A, Howard J, Higgins R R, Li Y J, Berger L, Zacksenhaus E, Reis M, Ben-David Y. Fli-1, an ets-related transcription factor, regulates erythropoietin-induced erythroid proliferation and differentiation: evidence for direct transcriptional repression of the Rb gene during differentiation. Mol Cell Biol. 1999;19:4452–4464. doi: 10.1128/mcb.19.6.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 1994;8:2831–2841. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 34.Tugores A, Magness S T, Brenner D A. A single promoter directs both housekeeping and erythroid preferential expression of the human ferrochelatase gene. J Biol Chem. 1994;269:30789–30797. [PubMed] [Google Scholar]
- 35.Wong K S, Li Y J, Howard J, Ben-David Y. Loss of p53 in F-MuLV induced-erythroleukemias accelerates the acquisition of mutational events that confers immortality and growth factor independence. Oncogene. 1999;18:5525–5534. doi: 10.1038/sj.onc.1202938. [DOI] [PubMed] [Google Scholar]