Abstract
Neurodegenerative diseases (NDs) are one of the most challenging public health issues. Despite tremendous advances in our understanding of NDs, little progress has been made in establishing effective treatments. Natural products may have enormous potential in preventing and treating NDs by targeting microglia; yet, there have been several clinical concerns about their usage, primarily due to a lack of scientific evidence for their efficacy, molecular targets, physicochemical properties, and safety. To solve this problem, the secondary bioactive metabolites derived from neuroprotective medicinal plants were identified and selected for computational predictions for anti-inflammatory activity, possible molecular targets, physicochemical properties, and safety evaluation using PASS online, Molinspiration, SwissADME, and ProTox-II, respectively. Most of the phytochemicals were active as anti-inflammatory agents as predicted using the PASS online webserver. Moreover, the molecular target predictions for some phytochemicals were similar to the reported experimental targets. Moreover, the phytochemicals that did not violate important physicochemical properties, including blood-brain barrier penetration, GI absorption, molecular weight, and lipophilicity, were selected for further safety evaluation. After screening 54 neuroprotective phytochemicals, our findings suggest that Aromatic-turmerone, Apocynin, and Matrine are the most promising compounds that could be considered when designing novel neuroprotective agents to treat neurodegenerative diseases via modulating microglial polarization.
Keywords: medicinal plants, neurological diseases, microglia polarization, neuroinflammation, ADME, target production, immune response
1. Introduction
Once the body is exposed to damage caused by external or internal harmful stimuli, the immune system will defend against these threats and initiate the repairing process [1,2]. After recognition of foreign agents, inflammatory processes will begin where many inflammatory mediators are released, such as tumor necrosis factor-α (TNF-α), interleukins (ILs), leukotrienes, nitric oxide (NO), and prostaglandin E2 (PGE2), besides the activation of inflammatory pathways such as nuclear factor-kappa-B (NF-κB), mitogen-activated protein kinase (MAPK), and Janus kinase signal transducer and activator of transcription (JAK/STAT) to minimize the impending of the damage [1]. After that, inflammation resolution is mediated by reducing mediators’ production, which leads to diluting the chemokine gradients and reducing the white blood cells (WBC) sensation at the site of damage. Although this biological response, inflammation, is a vital defensive mechanism of the body, especially in acute conditions, it also plays a significant role in several pathophysiological disorders [3,4]. If the resolution process fails and the inflammatory response continues, it may progress into persistent and chronic inflammation, as the excess production of cytokines and inflammatory mediators is associated with many neurodegeneration diseases [5,6,7].
Neurodegeneration Diseases (NDs) is a phrase that refers to the loss of neurons in diseases of the central nervous system such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Multiple sclerosis (MS). More recent attention has focused on the role of microglia-mediated inflammatory singling in the onset and progression of neurodegenerative disease [8]. The polarization of activated microglia into the M1 phenotype has been linked to the release of pro-inflammatory mediators that promote neuroinflammation and neuronal damage [9]. The interest that activated microglia contributes to the progression of chronic neurodegeneration was first postulated in brain samples of AD patients [10]. Studies showed an extracellular deposition of the protein amyloid-beta [Aβ]-containing plaques and the development of intracellular neurofibrillary tangles (NFT) composed of hyper-phosphorylated tau proteins [11,12]. Upon the accumulation of Aβ, microglia are activated as phagocytic cells and are believed to clear Aβ deposits initially; however, as the disease progresses, microglia produce pro-inflammatory mediators and reactive oxygen species (ROS), as well as lose their ability to clear Aβ, promoting neuronal degeneration and disease progression [13]. Moreover, pro-inflammatory microglia have exacerbated tau pathology by increasing its phosphorylation [14]. In the case of PD, studies reported the accumulation of Lewy bodies, which are intracellular inclusions containing α-synuclein, as well as the loss of dopaminergic neurons in the substantia nigra, which are the hallmarks of PD [15,16]. Microglial cells have been observed to be gradually activated in the substantia nigra of PD patients [17]. Moreover, in early PD, the degree of microglial activity was linked to dopaminergic terminal loss [18]. Additionally, MS is characterized by neuroaxonal degeneration, which results in irreversible neurological impairment [19]. Microglia have been shown to play a direct role in the progression of MS, in which pro-inflammatory mediators produced by activated microglia contribute to myelin destruction [20,21].
Microglia are specialized innate immune cells that function in the brain in place of macrophages. It maintains the central nervous system’s homeostasis by regulating two cycles classified into M1 and M2 based on their metabolism and secretory mediators [22,23,24]. M1 is the pro-inflammatory phase induced by interferon-gamma combined with lipopolysaccharide, INF-γ/LPS, resulting in the production of mediators such as IL-1β, IL-6, IL-12, IL-18, and IL-23, as well as TNF-α, which cause neuronal damage [24,25]. M2, on the contrary, is an anti-inflammatory phase that is triggered by, but not limited to, Toll-like receptors agonists (TLRs agonists), Transforming growth factor-beta (TGF-β), and glucocorticoids, resulting in the release of mediators such as interleukins IL-4, IL10, and IL-13, as well as Arginase-1 (ARG1), which relieve inflammatory responses and enhance neuronal repair [24,25]. Hence, suppressing inflammatory responses via targeting the microglia is a promising approach in managing neuroinflammatory-based diseases. In this context, several natural products have such properties and may influence the prevention, incidence, and severity of neurodegenerative illness.
Only palliative treatments are available for these neurodegenerative disorders, none of which can appreciably slow or cure the underlying cause [26]. Therefore, new treatments and novel therapeutic approaches are urgently needed; regulation of microglial polarization from M1 to M2 phenotypes seems to be a viable strategy for NDs treatment and prevention. As per the World Health Organization (WHO), neurodegenerative illnesses that affect motor function are estimated to become the second-leading cause of mortality in the next 20 years [27]. Thus, in this study, we aspire to shed some insight into phytochemical compounds used to treat neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Multiple sclerosis (MS) by investigating their pharmacokinetic properties, predicting their biological targets, assessing their safety/toxicity profiles, and cytochrome enzyme inhibition using computational techniques.
2. Study Design
Below is the study design that involves several steps, as shown in Figure 1.
Figure 1.
The steps involved in the study design of neuroprotective phytochemicals.
3. Results
3.1. Proposed Mechanisms Involved in the Neuroprotective Effects of Phytochemicals in Neurodegenerative Diseases Based on the Reported Literature
3.1.1. AD
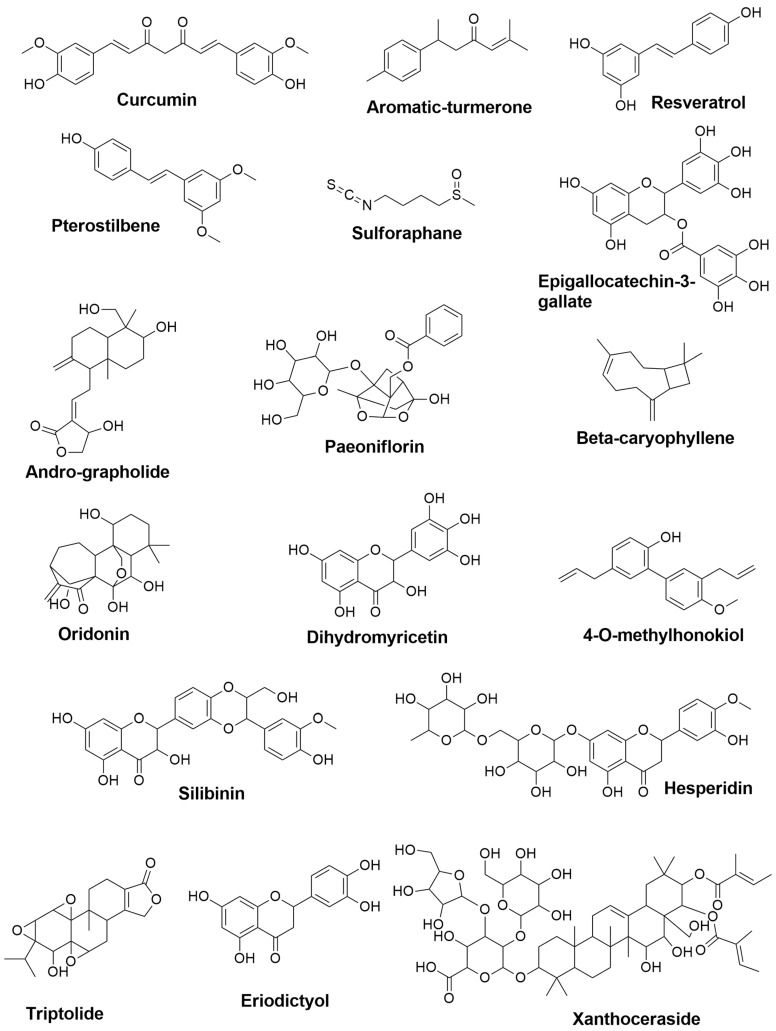
The prevalence of AD greatly rises with age [28], and in 1997, approximately 2.32 million people in the United States had Alzheimer’s disease, and by 2047, it is expected that 8.64 million individuals will be diagnosed with AD, resulting in a massive societal and economic burden [29]. Although no treatments are available to stabilize or reverse the neurodegenerative process, several palliative disease-modifying medicines are now in development with early clinical investigations [30]. Natural products are a viable treatment option. A wide range of phytochemical compounds and secondary bioactive metabolites has been studied pre-clinically and clinically to prevent and attenuate the multifactorial pathologies of AD (chemical structures are summarized in Figure 2) via microglial modulation.
Figure 2.
The 2D chemical structures of the neuroprotective phytochemical used for AD treatments.
In the case of physiological conditions, microglia’s number and functions are tightly regulated. Nonetheless, if stimuli bind to the pattern-recognition receptors [PRRs] on the surface of microglia [31], microglia will be over-activated to respond to the insult through shifting into different functional states, modifying its proliferation, morphology, phagocytic activity, antigen presentation, and the production of inflammatory markers such as cytokines and chemokines [32]. The process involves a diverse set of signaling pathways, including but not limited to tumor necrosis factors (TNFs), interferons (IFNs), chemokines, colony-stimulating factors (CSFs), and interleukins (ILs) [33]. This sustained over-activation of microglia has been observed in various neurodegenerative diseases, and targeting these pathways is one of the proposed mechanisms of multiple phytochemical compounds, as discussed in detail below.
Pattern Recognition Receptors (PRRs)
Pattern recognition receptors (PRRs) are present on the plasma membrane of microglia that are capable of detecting foreign bodies that stimulate microglia. PRR subfamilies that are predominantly expressed by microglia include toll-like receptors (TLR), inflammasome-forming nucleotide-binding oligomerization domain (nod)-like receptors (NLRs), triggering receptor expressed on myeloid cells (TREMs), and other receptors [34]. Inflammatory factors such as IL-1β, IL-6, TNF-α, ROS, and Cyclooxygenase-2 (COX-2) are produced due to the interaction between the ligand and PRR receptor, as well as boosting microglial phagocytic activity in the short term microglial activation. However, chronic activation will impair this protective mechanism and might exacerbate neurodegeneration [34]. TLR4 signaling pathways, for example, are activated in microglia during neuroinflammation, resulting in caspase-8 and caspase-3 activation, nuclear translocation of NF-κB, and expression of genes implicated in the inflammatory response; inhibiting TLR4 activation and signaling is thus a beneficial mechanism.
For instance, Eriodictyol, a natural flavonoid found in citrus fruits and peanuts, has been shown to alleviate neuroinflammation, amyloidogenesis, and memory impairment induced by Lipopolysaccharide (LPS) through many mechanisms, one of which is via inhibiting TLR4 activation [35]. Furthermore, NLRP3, which belongs to the NOD-like receptors (NLRs) family, is another target of Esculentoside A and Pterostilbene according to in-vitro models, where they inhibit the Aβ1−42 induced NLRP3/caspase-1 inflammasome in BV-2 cells, as shown in Table 1 [36,37].
Table 1.
Modulatory Mechanisms of the Neuroprotective Phytochemicals used to Treat AD Based on in-silico Computational Predictions and Reported in-vitro and in-vivo Studies.
| Compound Names | Compound Natural Source | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
| Curcumin | Curcuma longa | 0.677 | 0.019 | Suppression of ERK1/2 and p38 MAPK pathways, and inhibition of IL-1β, IL-6, and TNF-α [38] Induction of HO-1 leading to Inhibition of NO, PGE2, and TNF-α [39] Activation of PPARγ pathway and inhibition of the NF-κB signaling pathway [40] |
Activation of PPARγ pathway and inhibition of the NF-κB signaling pathway [40] |
| Aromatic-turmerone | Curcuma longa | 0.584 | 0.035 | Inhibition of the NF-κB, JNK, and p38 MAPK signaling pathways [41] Suppression of iNOS, COX-2, NO, PGE2, and NF-κB, besides attenuation the levels of TNF-α, IL-1β, IL-,6, and monocyte chemoattractant protein-1(MCP-1) [42] |
Reduction of TNF-α and IL-1β [43] |
| Resveratrol | the skin of grapes and blueberries | 0.554 | 0.042 | Reduction of the expression of mPGES-1, a key enzyme in the synthesis of PGE2 [44] | Inhibition of the NF-κB, STAT1, and STAT3 pathways and inhibition of TNF-α and IL-6 secretions [45] |
| Pterostilbene | Pterocarpus marsupium, blueberries | 0.508 | 0.054 | Inhibition of the NLR family pyrin domain containing-3 (NLRP3)/caspase-1 inflammasome pathway, and reduction of TNF,-α, IL-6, and IL-1β [36] | Inhibition of NO, TNF-α, and IL-6 [46] |
| Sulforaphane | Cruciferous vegetables (e.g., cabbage mustard radish, and broccoli) | NA | NA | Inhibition of JNK/AP-1/NF-κB pathway and activation of Nrf2/HO-1 pathway [47] | Reduction of IL-1β and TNF-α [48] |
| Epigallocatechin-3-gallate | Camellia sinensis | 0.623 | 0.027 | Suppression of iNOS and NO [49] Suppression of TNFα, IL-1β, IL-6 and iNOS [50] |
Inhibition of iNOS and COX-2 [51] |
| Andrographolide | Andrographis paniculate | 0.845 | 0.005 | Activation of Nrf2/Keap1-mediated HO-1 signaling pathway, and downregulation of NF-κB signaling pathway [52] Inhibition of PGE2 and TNF-α, and downregulation of iNOS and COX-2 [53] Inhibition of NF-κB signaling pathway and JNK-MAPK pathway [54] |
- |
| Paeoniflorin | Paeonia lactiflora | 0.578 | 0.036 | Suppression of TNF-α, IL-1β, and IL-6. Inhibition of NF-κB signal activation [55] | Inhibition of IL-1β, IL-6, TNF-α, and NO. Upregulation of IL-10 and TGF-β1. Inhibition of mTOR/NF-κB signaling pathway, and activation of phosphatidylinositol-3-Kinase and Protein/Kinase B (PI3K/Akt) signaling pathway [56] |
| β-caryophyllene | Myristica fragrans, Piper Nigrum, Ribes nigrum, and Syzygium aromaticum | 0.745 | 0.011 | Upregulation of IL-10 and Arg-1, and reduction of L-1β, TNF-α, PGE2, iNOS and NO; Activation of the PPAR-γ pathway [57] | Activation of cannabinoid receptor 2 (CB2R) and PPARγ receptor [58] |
| Oridonin | Rabdosia rubescens | 0.681 | 0.018 | Reduction of NO and attenuation of expression of iNOS, IL-1β, and IL-6 [59] | Inhibition of NF-κB pathway [60] |
| Dihydromyricetin | Ampelopsis, Pinus, and Cedrus species | 0.737 | 0.012 | Inhibition of TLR4/NF-κB signaling pathway [61] | Activation of Adenosine monophosphate-activated protein kinase (AMPK)/NAD-dependent deacetylase sirtuin-1 [SIRT1] pathway [62] Inhibition of NLRP3 inflammasome [63] |
| 4-O-methylhonokiol | Officinalis icinalis | 0.446 | 0.074 | Inhibition of NF-κB pathways [64] | Inhibition of NF-κB pathways [64] |
| Silibinin | Silybum marianum | 0.667 | 0.020 | - | Inhibition of MAPKs pathway [65] |
| Hesperidin | The peel of citrus fruits | 0.691 | 0.017 | Reduction of iNOS and NO [66] Reduction of NO, iNOS, TNF-α and IL-1β [67] |
Inhibition of protein kinase B/glycogen synthase kinase-3β (AKT/GSK-3β) and attenuation of iNOS, NF-κB, TNF-α, IL-1β, IL-4, IL-6, and COX-2 [68] |
| Triptolide | Tripterygium wilfordii | 0.698 | 0.016 | Inhibition of TNF-α and IL-1β [69] | Suppression of MAPKs including p3,8, ERK1/2, and JNK [70] |
| Eriodictyol | A variety of fruits and herbs | 0.691 | 0.017 | Suppression of NF-κB [35] | Inhibition of TLR4, MAPKs, and PI3K/Akt, and activation of SIRT1; thus, blocking NF-κB pathway [35] |
| Xanthoceraside | Xanthoceras sorbifolia | 0.753 | 0.010 | Suppression of IL-1β and TNF-α through inhibition of NF-κB and MAPK pathways [71] | Suppression of MAPK and NF-κB pathways [72] |
| Piperlongumine | Piper longum | 0.435 | 0.079 | Inhibition of NF-κB pathway [73,74] | Inhibition of NF-κB pathway [72] |
| Esculentoside A | Phytolacca esculenta | 0.857 | 0.005 | Inhibition of NF-κB, MAPKs, and NLRP3 pathways [37] | Reduction of iNOS, COX-2, and TNF-α through inhibition of MAPKs pathway [75] |
| Quercetin | Fruits and vegetables (e.g., onions and apples) | 0.689 | 0.017 | Reduction of NO through inhibiting NF-κB pathway [76] | - |
| Apigenin | A variety of fruits and vegetables (e.g., chamomile, tea, and oranges) | 0.644 | 0.024 | Suppression of IFN-γ [77] | - |
Transcription Factors (TFs)
Transcription factors are proteins that are involved in the regulation of the expression of genes. NF-κB represents a family of transcription factors that control the expression of a variety of genes involved in cell death, inflammation, proliferation, and differentiation [78]. Multiple studies have revealed that NF-κB is activated in several NDs and engaged in microglia-mediated Aβ toxicity, making it one of the most important transcription factors for the expressions of pro-inflammatory cytokines [79]. The activation of NF-κB results in the phosphorylation of NF-κB inhibitor, IκB, via the IκB kinase (IKK) signalosome complex leading to transcription of pro-inflammatory mediators, such as iNOS, COX-2, TNF-α, and IL-1β [80,81] Therefore, inhibiting the NF-κB will suppress the release of these inflammatory markers, which is a mechanism of a variety of natural plants, such as Piperlongumine, Aromatic-turmerone, Oridonin, and Andrographolide, as demonstrated in pre-clinical studies that shown in Table 1. Epigallocatechin-3-gallate, a polyphenolic compound found in green tea, has been shown to suppress the expression of TNFα, Il- β, Il-6, and iNOS in Aβ-stimulated EOC 13.31 mouse immortalized microglial cells [49]. It is worth noting that a phase III clinical trial for Epigallocatechin-3-gallate is being conducted to treat the early stages of Alzheimer’s disease; however, the results have not yet been published [82].
Moreover, signal transducer and activator of transcription (STATs), another family of the transcription factors that expressed and mediated various functions, including proliferation, apoptosis, and differentiation in response to cytokines [83]. STAT1 is assumed to be a key signaling regulator via IFNs involved in innate immune responses, including type I and type II IFNs [84]. STAT3, on the other hand, mediates the cells’ survival and proliferation of the IL-6 through regulating the expression of genes involved in the cell cycle and suppression of apoptosis [84]. STAT proteins are phosphorylated by the Janus kinase family, which includes JAK1, JAK2, and TYK2, causing them to translocate to the nucleus and stimulate transcription of their target genes. The abnormal activation of JAK/STAT signaling in innate immune cells has been linked to AD and MS [84].
Resveratrol, a naturally occurring dietary polyphenolic compound found in abundance in the skin of grapes and blueberries, reduced pro-inflammatory IL-6 and TNF-α production via inhibiting STAT1 and STAT3, as well as NF-κB pathways. Additionally, oral administration of Resveratrol suppressed microglial activity associated with the production of cortical amyloid plaques in a mouse model of cerebral amyloid deposition [45]. It is worth mentioning that Resveratrol has undergone a phase II clinical trial to investigate its beneficial role in delaying or altering the deterioration of memory and daily functioning in AD [85].
Activator protein-1 (AP-1) is also another transcription factor that regulates pro-inflammatory genes, including COX-2 and iNOS, and this signaling is inhibited by Sulforaphane, leading to reducing the expression of many inflammatory mediators and pro-inflammatory cytokines [47]. Indeed, multiple transcription factors are potential targets of herbal medicines as the mutations of transcription factors are one of the causes of neurodegenerative diseases, including AD.
Nuclear Receptors (NRs)
Nuclear Receptors are responsible for regulating microglia phenotypes by activating transcription factors such as Peroxisome proliferator-activated receptors (PPARs) and nuclear factor erythroid 2-related factor 2 (Nrf2) [86]. PPARs are a nuclear receptor family composed of three subtypes, one of which is PPARγ, which suppresses the expression of pro-inflammatory mediators such as TNF-α, IL-6, IL-1β, and IL-12 while also promoting the production of anti-inflammatory cytokines such as TGF-β and IL-10 [87]. PPARγ agonists, such as β-caryophyllene and Curcumin, have been shown in pre-clinical trials to alter microglia polarization to the M2 phenotype, as shown in Table 1. Moreoever, it is worth mentioning that Curcumin has been clinically studied. Phase II clinical trials were carried out, one for treating patients with mild to moderate Alzheimer’s disease [88] and the other for studying the combination of Curcumin and Ginkgo for treating mild to severe dementia [89]. The beneficial effects of PPARγ agonists are proposed to be due to the suppression of microglial pro-inflammatory activity as well as the promotion of their phagocytic activity [90,91].
In addition, Nrf2 is a nuclear receptor that governs antioxidant responses initiated in oxidative damage, which is a feature of many neurodegenerative disorders [92]. Nrf2 expression in macrophages directly suppresses inflammation by blocking RNA polymerase II to IL-6 and TNF, as well as modulating antioxidative defense proteins such as heme oxygenase-1 (HO-1) [93]. As a result, Nrf2 activation is hypothesized to be involved in neuroprotection for Alzheimer’s disease patients. An in-vitro study conducted by Yeon Seo, Ji et al. [52] showed that Andrographolide activates the Nrf2/Keap1- mediated HO-1 signaling pathway, leading to a decrease in the expression of iNOS and COX-2 in BV-2 cells [52].
Protein Kinases (PKs)
MAPKs are one of the most important kinase groups in inflammatory cells. They include Extracellular signal-regulated kinase (ERK1/2), also known as p44/42 MAPK, and c-Jun N-terminal kinase (JNK), as well as p38 MAPK pathways [94]. Activation of these MAPK pathways causes phosphorylation of nuclear transcription factors and other cytoplasmic protein kinases, which results in increased expression of target inflammatory genes. For example, p38 MAPK activation via multiple pathways is necessary for the productions of IL-1, IL-6, TNF-α, COX-2, and iNOS, implying that p38 MAPK activity is associated with the hallmark lesions of Alzheimer’s disease [94]. Hence, targeting these activations through suppressing phosphorylation of the proteins is a proposed mechanism of many herbal medicines, such as Curcumin and Aromatic-turmerone [38,41]. Furthermore, Silibinin, Triptolide, Xanthoceraside, and Eriodictyol are natural plants that have been studied in-vitro and in-vivo to treat AD by inhibiting different MAPK pathways, as summarized in Table 1.
Similarly, the mammalian target of rapamycin (mTOR) kinase, a member of the phosphatidylinositol 3-kinase-related kinase (PIKKs) protein kinase family, is implicated in the neuroinflammation process. mTOR activation will eventually result in the activation of the NF-κB signaling pathway. As a result, blocking mTOR can reduce microglial cell activation and enhance M2 phenotypic conversion. Paeoniflorin, a traditional Chinese herb, has been proven in a rat model to suppress the mTOR/NF-κB pro-inflammatory pathway [56].
Cytokines
Cytokines are small proteins that have a role in controlling innate and adaptive immune responses. They are also involved in cell growth, survival, differentiation, and activities regulation [95]. Various types of CNS cells, including tissue infiltrating immune cells, neurons, and astrocytes, have been identified as CNS cytokine sources. However, microglia appears to be a major source of both pro-inflammatory and immune-regulatory cytokines. Several cytokines and their receptors have been discovered to exist and function in the CNS. TNF-α, IFNs, ILs including IL-1, -2, -3, -4, -6, -10, -12, -15, and -18, TGFβ, and CSFs are some of them [96]. During CNS inflammation, microglia produce two main pro-inflammatory cytokines, IL-1 and TNF-α, which are involved in BBB disruption [97]. Thereby, inhibiting activation of microglia and attenuating production of pro-inflammatory and anti-inflammatory cytokines are proposed mechanisms of many phytochemical compounds to treat AD, as shown in Table 1. For example, Oridonin extracted from Rabdosia rubescens has been shown to reduce NO production as well as the attenuation of iNOS, IL-1β, and IL-6 expressions that are involved in the development of neuroinflammation and neurodegeneration [59]. Moreover, Luo et al. (2018) found that the administration of Paeoniflorin, derived from Paeonia lactiflora, inhibits the productions of IL-1β, IL-6, TNF-α, and NO, while upregulating IL-10 and TGF-β1, which promote the transition of M1 to M2 phenotypes in microglia [56].
3.1.2. PD
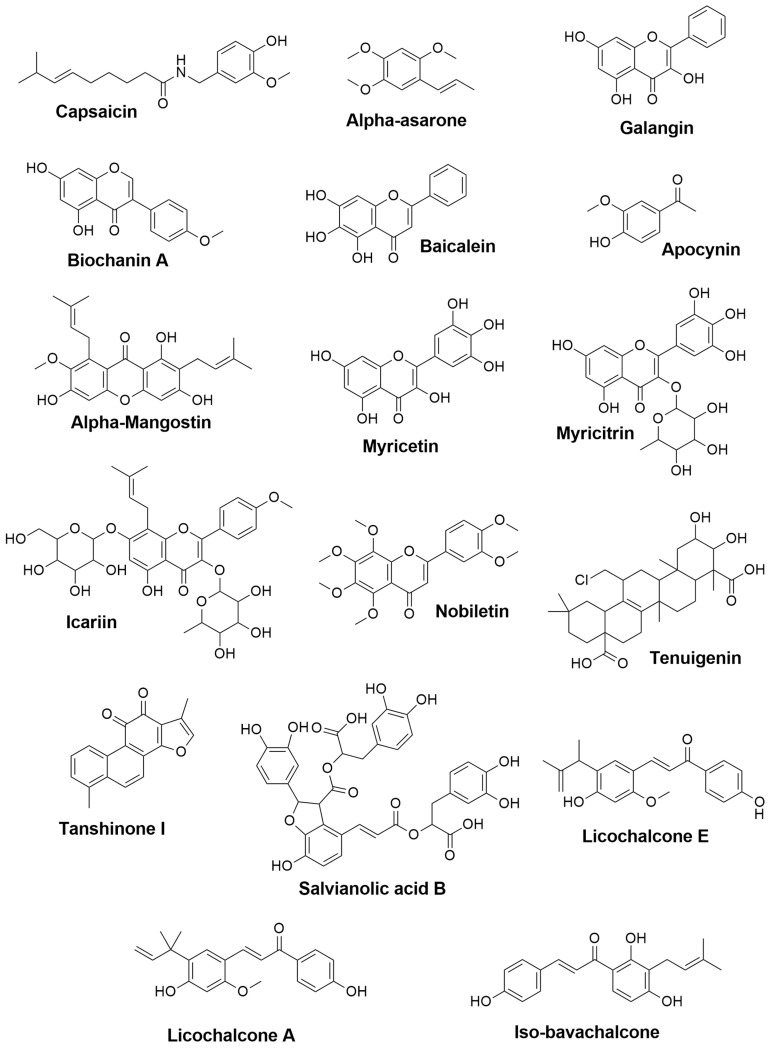
Parkinson’s disease (PD) is a progressive age-related neurodegenerative condition characterized by resting tremors, muscle rigidity, bradykinesia, and postural reflex deficits [98]. There is scientific proof that oxidative stress, peptide misfolding, and the death of dopaminergic neurons in the substantia nigra pars compacta are the fundamental features of Parkinson’s disease pathophysiology [99]. Although Levodopa is the gold standard for symptomatic management of Parkinson’s disease, long-term usage has been linked to the development of dyskinesia. Besides that, there are no pharmacological options that provide neuroprotection or slow the onset of PD. As a result, more efforts are required to discover therapy methods that alter the course of PD progression as well as relieve symptoms [100]. Therefore, numerous studies on phytochemical compounds have been conducted to investigate secondary metabolites’ efficacy and mechanisms in treating PD, some of which will be summarized in Figure 3 and addressed below.
Figure 3.
The 2D chemical structures of the neuroprotective phytochemical for PD treatments.
Pattern Recognition Receptors (PRRs)
Rui W et al. (2020) [101] demonstrated that Baicalein, a flavonoid extracted from Scutellaria baicalensis Georgi, could reverse MPTP-induced motor dysfunction and dopaminergic neurons loss in mice model via blocking the NLRP3/caspase-1/gasdermin D pathway, which suppresses the disease-associated pro-inflammatory cytokine [101]. Moreover, Tenuigenin showed increased striatal dopaminergic levels and reduced motor impairment in the MPTP-induced mice model by suppressing NLRP3 inflammasome activation and decreasing caspase-1 and IL-1β productions as summarized in Table 2 [102].
Table 2.
Modulatory Mechanisms of Phytochemicals used to Treat PD Based on in-silico Computational Predictions and Reported in-vitro and in-vivo Studies.
| Compound Names | Compound Natural Sources | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
| Capsaicin | Capsicum | 0.266 | 0.196 | - | Elevation of the expression of ciliary neurotrophic factor receptor alpha [CNTFRα] [103] Reduction of NO, iNOS, and IL-6 expressions, and elevation of Arg-1 and macrophage mannose receptor (CD206) [104] Reduction of TNF-α and IL-1β expressions [105] |
| α-asarone | Acorus tatarinowii | 0.592 | 0.033 | Inhibition of NF-κB [106] | Inhibition of NF-κB [106] |
| Galangin | Alpinia officinarum | 0.689 | 0.017 | Inhibition of MAPK and NF-κB signaling pathways [107] Inhibition of TNF-α, IL-6, IL-1β, and COX-2 through JNK and NF-κB pathways [108] |
Inhibition of TNF-α, IL-6, IL-1β, and COX-2 through JNK and NF-κB pathways [108] |
| Biochanin A | Legume plants | 0.588 | 0.034 | Inhibition of TNF-α and IL-1β through MAPK pathway [109] | Inhibition of TNF-α and IL-1β through MAPK pathway [109] |
| Baicalein | Scutellaria baicalensis Georgi | 0.674 | 0.019 | Inhibition of TNF-α and IL-6 through MAPK and NF-κB signaling pathways [110] | Suppression of NLRP3/caspase-1/GSDMD pathway [101] |
| Apocynin | Picrorhiza kurroa | 0.496 | 0.058 | - | Inhibition of STAT1 and NF-κB pathways [111] |
| α-Mangostin | Mangosteen pericarp | 0.694 | 0.017 | Inhibition of NF-κB pathway [112] | Reduction of IL-6 and COX-2 [113] |
| Myricetin | Turbinaria ornata | 0.720 | 0.013 | Inhibition of MAPK and NF-κB signaling pathways [114] | Inhibition of MAPK and NF-κB signaling pathways [114] |
| Myricitrin | Myrica cerifera | 0.762 | 0.009 | - | Suppression of TNF-α [115] |
| Icariin | Herba epimedii | 0.732 | 0.012 | Reduction of TNF- α, IL-1β and NO through inhibition of NF-κB pathway [116] | Reduction of TNF- α, IL-1β and NO through inhibition of NF-κB pathway [116] |
| Nobiletin | Citrus fruits | 0.694 | 0.017 | Suppression of TNF-α, IL-1β and NO through inhibition of NF-κB pathway [117] | Attenuation of IL-1β production [118] |
| Tenuigenin | Polygala tenuifolia | 0.841 | 0.005 | Inhibition of NLRP3 inflammasome and downregulation of caspase-1, pro-IL-1β, and IL-1β [102] | Suppression of NLRP3 inflammasome [102] |
| Tanshinone I | Radix salviae miltiorrhizae | 0.515 | 0.053 | Suppression of TNF-α, IL-6, and IL-1β [119] | Attenuation of the increase of TNF-α, and reserving the increase of IL-10 [119] |
| Salvianolic acid B | Salviae miltiorrhizae | 0.313 | 0.149 | Reduction of TNF-α, IL-1β and NO productions [120] | Attenuation of the expressions of TNF-α, IL-1β, and NO [120] |
| Licochalcone E | Glycyrrhiza inflata | 0.523 | 0.050 | Activation of Nrf2/ARE-dependent pathway [107] | Activation of Nrf2/ARE-dependent pathway [107] |
| Licochalcone A | Glycyrrhiza inflata | 0.740 | 0.011 | Inhibition of ERK1/2 and NF-κB p65 through reduction of iNOS, COX-2, TNF-α, IL-1β, and IL-6 expressions [121] | Inhibition of ERK1/2 and NF-κB p65 through reduction of iNOS, COX-2, TNF-α, IL-1β, and IL-6 expressions [121] |
| Isobavachalcone | Psoralea corylifolia | 0.778 | 0.008 | Inhibition of NF-κB pathway through inhibition of TNF-α, IL-6, IL-1β, and IL-10 [122] | Reduction of IL-6 and IL-1β expressions [122] |
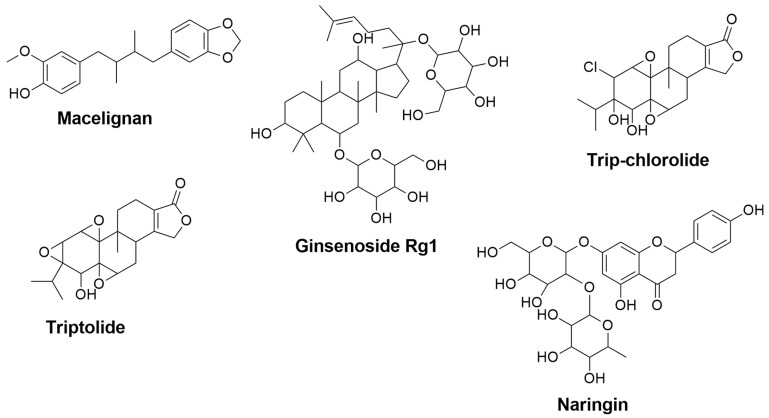
| Macelignan | Myristica fragrans | 0.352 | 0.121 | Suppression of MAPKs and NF-kB via the regulation of IkB [123] | Activation of PPAR-γ [124] |
| Ginsenoside Rg1 | Panax ginseng | 0.801 | 0.007 | Inhibition of NF-κB and MAPK signaling pathways through attenuation of TNF-α, IL-1β, iNOS, and COX-2 mRNA and protein levels [125] | Inhibition of NF-κB and MAPK signaling pathways through reduction of TNF-α, IL-1β, and IL-6 [126] |
| Tripchlorolide | Tripterygium wilfordii Hook F | 0.791 | 0.007 | Attenuation of TNF-α, IL-1β, NO, iNOS, PGE2, and COX-2 [127] | - |
| Triptolide | Tripterygium wilfordii Hook F | 0.698 | 0.016 | Downregulation of NO, iNOS, TNF-α, and IL-1β [128] | - |
| Naringin | Grapefruit, Citrus fruits | 0.700 | 0.016 | - | Inhibition of IL-1β [129] Attenuation of TNF-α [130] |
NA: not applicable.
Transcription Factors (TFs)
Kim et al. (2015) [111] revealed that prophylactic therapy with α-asarone inhibits microglial activation by blocking the NF-κB pathway, which improves PD-like behavioral impairment [106]. Likewise, several phytochemical compounds are have been reported to treat PD in pre-clinical experiments via targeting the transcription factor, NF-κB, such as Apocynin, α-Mangostin, Myricetin, Icariin, Nobiletin, Isobavachalcone, and Ginsenoside Rg1, among other herbs, as shown in Table 2. Further, STAT1 is a potential target for Parkinson’s disease therapy; Apocynin, a herb derived from Picrorhiza kurroa, has been shown to alleviate learning and memory impairments in the mice model through suppression of STAT1 and NF-κB signaling pathways [111].
Nuclear Receptors (NRs)
In PD patients, clinical trials with pioglitazone, a PPARγ agonist, have shown encouraging results [131]. Moreover, Macelignan is a plant-derived from Myristica fragrans that exhibits a PPARγ agonist activity and has been demonstrated to protect dopaminergic neurons [124]. Nrf2, a nuclear receptor that defends against oxidative stress and inflammatory process, is a target for Licochalcone E herb extracted from Glycyrrhiza inflata. Lico-E activates the Nrf2-antioxidant response element (ARE) system and up-regulates HO-1 [132].
Protein Kinases (PKs)
Kim et al. (2019) [121] found that Galangin suppressed the phosphorylation of p38 MAPK and JNK pathways, which significantly reduced the production of NO, iNOS, and IL-1β [107]. Similarly, phytochemical compounds such as Biochanin A, Baicalein, Myricetin, Macelignan, and Ginsenoside Rg1, which are listed in Table 2, have also been shown in pre-clinical studies to treat PD via targeting MAPKs pathways. Further, suppressing the phosphorylation of ERK1/2 is one of the mechanisms of Licochalcone A, according to in-vitro and in-vivo experiments in which the LPS-stimulated production of pro-inflammatory mediators and microglial activation was inhibited [121].
Cytokines
Growing evidence revealed that activation of microglia in the PD brain resulted in higher expression of pro-inflammatory cytokines, in which the productions of IL-1β, IL-6, and TNF-α were enhanced in activated microglia [133]. Several phytochemical compounds have been studied pre-clinically to treat PD, as shown in Table 2, and it has been noted that they exert their activity by inhibiting pro-inflammatory cytokines releases, such as Capsaicin and Icariin.
3.1.3. MS
Multiple Sclerosis (MS) is a chronic degenerative neuroinflammatory disease that affects the central nervous system (CNS) and manifests in a range of clinical presentations. It is characterized by immunological abnormalities that result in myelin degradation in grey and white matter plaques [134,135]. The neurological symptoms are associated with the visible inflammatory lesions made up of lesser amounts of microglia and other types of cells that are all involved in the demyelinating process.
Currently, there is no cure for MS; however, there are two available approaches for management. The first is known as disease-modifying drugs, which include recombinant interferon β-1a and β-1b (e.g., Avonex and Betaferon), in addition to glatiramer acetate [136]. These agents are used to prevent relapses and improve neuropsychological deficits by inhibiting gamma interferon and enhancing the production of anti-inflammatory cells [137,138]. The second approach involves utilizing γ-aminobutyric acid type B (GABA-B) receptor agonists (e.g., baclofen) and α2 adrenergic receptor agonists (e.g., tizanidine) to manage MS symptoms such as pain and spasticity, with moderate benefits [139,140]. Multiple research, on the other hand, has studied the role of bioactive metabolites (Figure 4) as a therapeutic alternative for MS, which will be mentioned below.
Figure 4.
The 2D chemical structures of the neuroprotective phytochemicals used for MS treatments.
Pattern Recognition Receptors (PRRs)
According to Peng H et al. (2016) [141], Dimethyl fumarate, the methyl ester of fumaric acid, is strongly suppressed NF-κB activation, besides other pathways, leading to a reduction of pro-inflammatory cytokines and chemokines production, which eventually improves the survival of oligodendrocytes and neurons [141]. It is worth mentioning that Dimethyl fumarate has been approved by the FDA to manage relapsing-remitting MS.
Nuclear Receptors (NRs)
Some natural plants have been studied to treat MS through activating Nrf2, which modulates the anti-oxidant stress response. As an example, Dimethyl fumarate, it has been reported that activation of Nrf2 receptor will lead to inhibit the phosphorylation of NF-κB signaling [142]. Moreover, Foresti et al. (2013) [143] identified Carnosol, a traditional medicine derived from Rosmarinus officinalis [Rosemary] and Salvia officinalis, to be a potent activator of the Nrf/Ho-1 pathway [143].
Protein Kinases (PKs)
18β-Glycyrrhe acid derived from Glycyrrhiza glabra is demonstrated by Zhou J. et al. (2015) [144] in a mice model to block the release of neurotoxic pro-inflammatory mediators induced by IFN-γ through inhibiting the phosphorylation of the MAPK pathways, ERK1/2 and p38 in microglia [144].
Cytokines
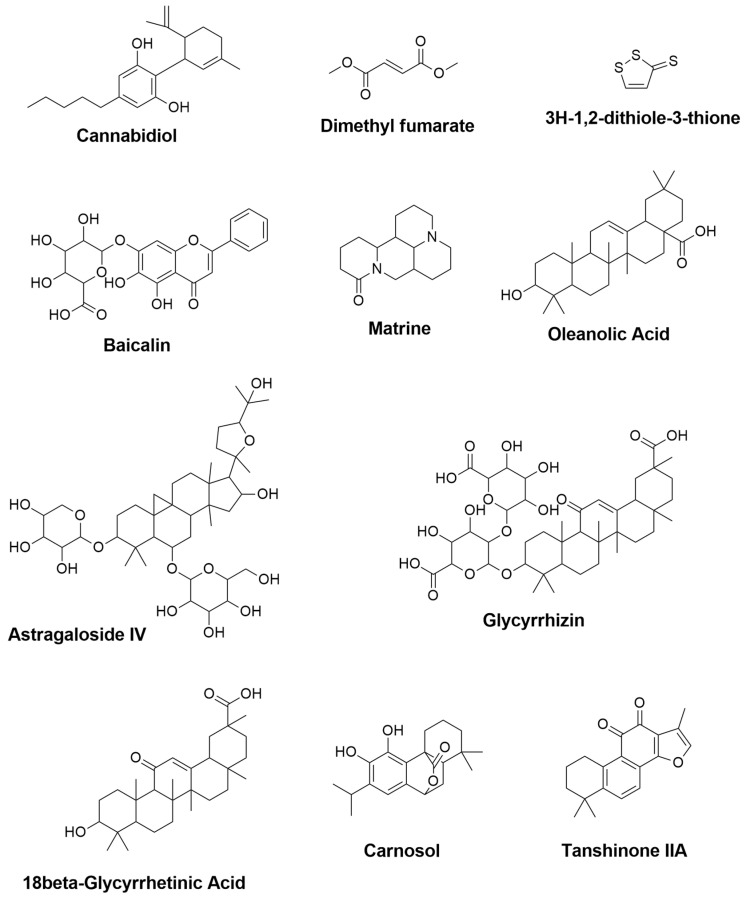
Most of the natural plants proposed to treat MS share the inhibition of IFN-γ cytokines, which function as effector cells damaging CNS cells by phagocytosis and the release of cytotoxic substances such as glutamate, nitric oxide, superoxide, and pro-inflammatory cytokines [145]. As shown in Table 3, Cannabidiol, 3H-1,2-dithiole-3-thione, Oleanolic Acid, Astragaloside IV, and Glycyrrhizin are all compounds that have been studied and found to suppress IFN-γ.
Table 3.
Modulatory Mechanisms of the Neuroprotective Phytochemicals used to Treat MS Based on in-silico Predictions and in-vitro and in-vivo Reported Studies.
| Compound Names | Compound Natural Sources | In-Silico Anti-inflammatory Prediction | Modulatory Mechanism of Microglia Polarization | ||
|---|---|---|---|---|---|
| Pa | Pi | In-Vitro | In-Vivo | ||
| Cannabidiol | Cannabis sativa | 0.427 | 0.082 | - | Reduction of TNF- α, IFN-γ and IL-17 [148] |
| Dimethyl fumarate | Fumaria officinalis | 0.469 | 0.066 | Upregulation of gene expression for IGF-1 and MRC1 [149] Activation of Nrf2 and modulation of NF-κB pathways, leading to reduction of TNF- α and IL-12 productions [141] |
- |
| 3H-1,2-dithiole-3-thione | Cruciferous plants | 0.945 | 0.004 | Suppression of IFN-γ and IL-17 [150] | - |
| Baicalin | Scutellaria baicalensis | 0.674 | 0.019 | - | Reduction of IFN-γ, and elevation of IL-4 [151] Inhibition of STAT/NF-κB pathways [152] |
| Matrine | Radix sophorae flavescentis | NA | NA | - | Reduction of caspase-3, HSPB5 (alpha B-crystallin), and IL-1β [153] |
| Oleanolic Acid | Olea europea, Aralia chinensis, and Rosa woodsia | 0.819 | 0.005 | Suppression of TNF-α, COX-2, and iNOS [154] | Attenuation of TNF-α [154] Reduction of IFN-γ and TNF-α, and elevation of IL-10 [155] |
| Astragaloside IV | Astragalus membranceus | 0.774 | 0.009 | - | Downregulation of iNOS, IFN-γ, TNF-α and IL-6 [156] |
| Glycyrrhizin | 0.849 | 0.005 | - | Reduction of TNF-α, IFN-γ, IL-17A, IL-6 and TGF-β1 and elevation of IL-4 [146] | |
| 18β-Glycyrrhetinic Acid | Glycyrrhiza glabra | 0.863 | 0.005 | - | Suppression of MAPK signal pathway [144] Reduction of TNF- α and IL-1β [157] |
| Carnosol | Rosmarinus officinalis and Salvia pachyphylla | 0.594 | 0.033 | Reduction of NO and TNF-α levels [143] | Reduction of iNOS and elevation of ARG-1 [158] |
| Tanshinone IIA | Salvia miltiorrhiza | 0.432 | 0.080 | - | Downregulation of IL-17 and IL-23 [159] |
NA: not applicable.
Glycyrrhizin, a compound extracted from licorice root, was studied by Sun Y. et al. (2018) [146] who showed that glycyrrhizin had an anti-inflammatory effect against MS through suppressing microglial M1 activation via reducing TGF-β1, IFN-γ, TNF-α, IL-17A, and IL-6 cytokines while increasing IL-4 [146]. On the other hand, Sativex® [Nabiximols®], a derived mixture of delta-9-tetrahydrocannabinol and Cannabidiol, is an investigational product in Phase III for the spasticity and pain associated with MS in the US [147].
3.2. Target Prediction
We have investigated the possible targets of the bioactive metabolites of 54 plants using a Molinspiration webserver that predict the probability of the compound’s activity as G protein-coupled receptors ligand, ion channel modulator, a kinase inhibitor, nuclear receptor ligand, protease inhibitor, and enzyme inhibitor.
3.2.1. GPCR Ligand
G protein-coupled receptors (GPCRs) expressed by microglia had already been exhibited to regulate various aspects of their activation process, such as cell proliferation, migration, and differentiation into M1 or M2 phenotypes [160]. GPCRs, among these numerous different receptor types, play an important role in the modulation of different components of microglial activation. As a direct consequence, the involvement of GPCRs and their subtypes in neurological diseases has been implicated in many studies. Furthermore, many other unstudied GPCR subtypes are highlighted in microglial activation and need to be investigated for their potential therapeutic and molecular activity in Alzheimer’s disease [161,162]. Several types of research have concluded that GPCRs are novel targets for treating neuropsychiatric illnesses such as anxiety, depression, and cognition in Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and schizophrenia.
As shown in Table 4, only compounds Epigallocatechin-3-gallate, Andrographolide, Paeoniflorin, Oridonin, Dihydromyricetin, 4-O-methylhonokiol, Silibinin, Triptolide, Eriodictyol, Piper-longumine, Capsaicin, Tenuigenin, Iso-bavachalcone, Trip-chlorolide, Triptolide, Naringin, Cannabidiol, Matrine, Oleanolic Acid, 18β-Glycyrrhetinic Acid, and Carnosol were active at G protein-coupled receptors (GPCRs). Furthermore, compounds Andrographolide, Cannabidiol, and Carnosol were the most active compounds with scores of 0.32, 0.35, and 0.52, respectively.
Table 4.
Target Predictions of the Neuroprotective Phytochemicals Used for AD, PD, and MS Treatments using Molinspiration Webserver.
| Compound Names | Molinspiration | Reported Target |
|||||
|---|---|---|---|---|---|---|---|
| GPCR ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | ||
| Curcumin | −0.06 | −0.20 | −0.26 | 0.12 | −0.14 | 0.08 | ERK1/2 and p38 MAPK IL-1β, IL-6, and TNF-α NO, PGE2 PPARγ, NF-κB |
| Aromatic-turmerone | −0.68 | −0.46 | −1.36 | −0.14 | −0.80 | −0.25 | NF−κB, JNK, and p38 MAPK iNOS, COX-2, NO, PGE2, NF-κB, TNF-α, IL-1β, IL-,6MCP-1 |
| Resveratrol | −0.20 | 0.02 | −0.20 | 0.01 | −0.41 | 0.02 | mPGES-1 NF-κB, STAT1, STAT3, TNF-α, IL-6 |
| Pterostilbene | −0.13 | −0.06 | −0.12 | 0.08 | −0.33 | 0.01 | NLRP3, NO TNF,-α, IL-6, IL-1β |
| Sulforaphane | −0.35 | −0.59 | −1.98 | −0.84 | −0.72 | 0.44 | JNK/AP-1/NF-κB Nrf2/HO-1, IL-1β, TNF-α |
| Epigallocatechin-3-gallate | 0.16 | 0.02 | 0.06 | 0.33 | 0.13 | 0.25 | iNOS and NO TNFα, IL-1β, IL-6, COX-2 |
| Andrographolide | 0.32 | 0.17 | −0.01 | 0.94 | 0.26 | 0.81 | Nrf2/Keap1-, NF-κB, TNF-α, iNOS, COX-2 JNK-MAPK |
| Paeoniflorin | 0.24 | 0.16 | −0.03 | 0.15 | 0.14 | 0.44 | TNF-α, IL-1β, and IL-6, NF-κB TGF-β1, mTOR, PI3K/Akt |
| β-caryophyllene | −0.34 | 0.28 | −0.78 | 0.13 | −0.60 | 0.19 | IL-10 and Arg-1, L-1β, TNF-α, PGE2. iNOS, NO CB2R, PPARγ |
| Oridonin | 0.1 | 0.27 | −0.19 | 0.73 | 0.08 | 0.53 | NO, iNOS, IL-1β, IL-6 |
| Dihydromyricetin | 0.09 | 0.03 | 0.01 | 0.27 | 0.08 | 0.32 | TLR4/NF-κB, AMPK, SIRT1, NLRP3 |
| 4-O-methylhonokiol | 0.04 | −0.00 | −0.09 | 0.29 | −0.23 | 0.06 | NF-κB |
| Silibinin | 0.07 | −0.05 | 0.01 | 0.16 | 0.02 | 0.23 | MAPKs |
| Hesperidin | −0.01 | −0.59 | −0.36 | −0.20 | −0.00 | 0.06 | iNOS, NO, TNF-α, IL-1β AKT/GSK-3β iNOS, NF-κB, TNF-α, IL-1β, IL-4, IL-6, COX-2 |
| Triptolide | 0.11 | 0.09 | −0.43 | 0.4 | 0.24 | 0.86 | TNF-α, IL-1β, MAPKs p3,8, ERK1/2, and JNK |
| Eriodictyol | 0.07 | −0.20 | −0.22 | 0.46 | −0.09 | 0.21 | TLR4, MAPKs, PI3K/Akt, SIRT1, NF-κB |
| Xanthoceraside | −3.77 | −3.85 | −3.90 | −3.82 | −3.74 | −3.71 | IL-1β and TNF-α, MAPK, NF-κB |
| Piperlongumine | 0.21 | −0.03 | −0.07 | −0.08 | −0.05 | 0.08 | NF-κB |
| Esculen-toside A | −3.50 | −3.71 | −3.73 | −3.63 | −3.16 | −3.36 | TNF-κB, MAPKs, NLRP3 iNOS, COX-2, TNF-α MAPKs |
| Quercetin | −0.06 | −0.19 | 0.28 | 0.36 | −0.25 | 0.28 | NO, NF-κB |
| Apigenin | −0.07 | −0.09 | 0.18 | 0.34 | −0.25 | 0.26 | IFN-γ |
| Capsaicin | 0.03 | −0.01 | −0.28 | 0.01 | −0.02 | 0.07 | CNTFRα CD206 TNF-α and IL-1β |
| α-asarone | −0.71 | −0.43 | −0.72 | −0.47 | −0.97 | −0.39 | NF-κB IL (NADPH) oxidase-2 (NOX2)/NF-κB tyrosine kinase (SRC)/ERK PGE2, COX-2, NO, iNOS IL-6, IL-1β, and TNF-α |
| Galangin | −0.13 | −0.21 | 0.19 | 0.28 | −0.32 | 0.28 | TNF-α and IL-1β |
| Biochanin A | −0.23 | −0.59 | −0.07 | 0.23 | −0.66 | 0.07 | TNF-α and IL-1β |
| Baicalein | −0.12 | −0.18 | 0.19 | 0.17 | −0.35 | 0.26 | TNF-α and IL-6 NLRP3/caspase-1/GSDMD |
| Apocynin | −1.01 | −0.54 | −1.22 | −1.04 | −1.31 | −0.59 | STAT1 and NF-κB |
| α-Mangostin | −0.01 | −0.12 | −0.10 | 0.45 | −0.19 | 0.39 | NF-κB IL-6 and COX-2 |
| Myricetin | −0.06 | −0.18 | 0.28 | 0.32 | −0.20 | 0.3 | MAPK and NF-κB |
| Myricitrin | −0.02 | −0.08 | 0.08 | 0.14 | −0.06 | 0.38 | TNF-α |
| Icariin | −0.41 | −1.25 | −0.75 | −0.59 | −0.34 | −0.36 | TNF- α, IL-1β and NO, NF-κB |
| Nobiletin | −0.13 | −0.04 | 0.09 | 0 | −0.22 | 0.11 | TNF- α, IL-1β and NO, NF-κB |
| Tenuigenin | 0.13 | −0.22 | −0.22 | 0.67 | 0.13 | 0.45 | NLRP3 pro-IL-1β, and IL-1β |
| Tanshinone I | −0.34 | −0.27 | −0.09 | −0.01 | −0.62 | −0.08 | TNF-α, IL-10 IL-6, IL-1β |
| Salvianolic acid B | −0.66 | −1.88 | −1.52 | −1.13 | −0.54 | −1.05 | TNF-α, IL-1β, NO |
| Licochalcone E | −0.13 | −0.20 | −0.37 | 0.27 | −0.23 | −0.03 | Nrf2/ARE- |
| Licochalcone A | −0.05 | −0.03 | −0.21 | 0.18 | −0.25 | 0.1 | ERK1/2 and NF-κB p65 |
| Isobavachalcone | 0.15 | 0.06 | −0.17 | 0.44 | 0.02 | 0.38 | NF-κB, TNF-α, IL-6, IL-1β, and IL-10 |
| Macelignan | 0 | −0.04 | −0.10 | −0.04 | −0.07 | 0.05 | MAPKs and NF-kB, PPAR-γ |
| Ginsenoside Rg1 | −1.34 | −2.52 | −2.34 | −1.94 | −0.92 | −1.36 | NF-κB and MAPK |
| Tripchlorolide | 0.17 | 0.24 | −0.41 | 0.51 | 0.36 | 0.7 | TNF-α, IL-1β, NO, iNOS, PGE2, and COX-2 |
| Triptolide | 0.11 | 0.09 | −0.43 | 0.4 | 0.24 | 0.86 | NO, iNOS, TNF-α and IL-1β |
| Naringin | 0.11 | −0.40 | −0.24 | 0.04 | 0.09 | 0.24 | IL-1β, TNF-α |
| Cannabidiol | 0.35 | −0.14 | −0.48 | 0.38 | −0.19 | 0.33 | TNF- α, IFN-γ, IL-17 |
| Dimethyl fumarate | −1.22 | −0.64 | −1.57 | −1.14 | −1.11 | −0.66 | IGF-1, MRC1 TNF- α, IL-12 |
| 3H-1,2-dithiole-3-thione | −4.02 | −4.01 | −4.03 | −4.03 | −4.01 | −3.67 | IFN-γ and IL-17 |
| Baicalin | −0.12 | −0.18 | 0.19 | 0.17 | −0.35 | 0.26 | IFN-γ, IL-4 STAT/NF-κB |
| Matrine | 0.21 | −0.10 | −0.60 | −0.88 | 0.07 | 0.06 | HSPB5, IL-1β |
| Oleanolic Acid | 0.28 | −0.06 | −0.40 | 0.77 | 0.15 | 0.65 | IFN-γ, TNF-α IL-10 |
| Astragaloside IV | −1.17 | −2.43 | −2.13 | −1.76 | −0.86 | −1.23 | iNOS, IFN-γ, TNF-α and IL-6 |
| Glycyrrhizin | −1.78 | −3.09 | −3.09 | −2.36 | −1.26 | −1.93 | TNF-α, IFN-γ IL-17A, IL-6 TGF-β1, IL-4 |
| 18β-Glycyrrhetinic Acid | 0.24 | −0.09 | −0.59 | 0.79 | 0.21 | 0.7 | MAPK, TNF- α and IL-1β |
| Carnosol | 0.52 | 0.13 | −0.26 | 0.51 | −0.08 | 0.37 | iNOS ARG-1 NO and TNF-α |
| Tanshinone IIA | −0.08 | 0.06 | −0.23 | 0.22 | −0.62 | 0.08 | IL-17 and IL-23 |
Cannabinoid receptor 2 (CB2R) is a subfamily of GPCRs found on cell membranes. Although CB2R is abundant on peripheral immune cells, it is only found in very small amounts in the normal brain, primarily in microglia [163]. Interestingly, Cheng Z et al. (2014) [58] Founded that β-Caryophyllene intragastric administration (48 mg/kg, for 10 weeks) to APP/PS1 rats might prevent cognitive impairments and reverse neurodegeneration [58]. This was linked to a reduction in microglial M1 activation and inflammatory cytokines via the CB2R and PPAR- pathway [58]. However, in the Molinspiration biological predictions, our results showed that β-caryophyllene is not active as GPCR with a result of –0.34, as shown in Table 4.
In-silico predictions suggested compounds Andrographolide, Cannabidiol, and Carnosol are active as GPCR-targeting. However, the reported studies have not investigated these possible targets suggesting further mechanistic studies are warranted.
3.2.2. Ion Channel Modulators
Microglial functions, including the proliferation, morphological alterations, migration, cytokine release, and reactive oxygen species generation, are all regulated by ion channels and transporters, which regulate ionic flux [164]. In microglial cells, ion channel expression is carefully controlled, with most ion channel types expressing differently depending on the cells’ functional state. Even though microglia are non-excitable cells, the abundance of voltage-gated ion channels shows that they play an important role in both normal and pathological conditions. Inflammation in the brain is a hallmark of Alzheimer’s disease, and multiple studies have shown that microglia can directly interact with neurons to cause inflammation [165].
As illustrated in Table 4, the findings of Resveratrol, Epigallocatechin-3-gallate, Andrographolide, Paeoniflorin, β-caryophyllene, Oridonin, Dihydromyricetin, Triptolide, Isobavachalcone, Tripchlorolide, Triptolide, Carnosol, and Tanshinone IIA suggest that these bioactive metabolites could modulate ion channels; however, inadequate published data is investigating phytochemical compounds as ion channel modulators.
As microglia ion channels are key regulators of microglial function and morphology. New evidence on the presence of specific ion channel localization on microglia and the possibility of enhanced ion channel expression in neurodegeneration may open up a new method for selectively targeting microglia and reducing the ongoing inflammatory process [166]. Among the six potential transient receptors (TRP) subfamilies, only the TRPC (canonical), TRPV (vanilloid), TRPM (melastatin) are expressed in microglia [167]. Capsaicin, a TRPV1 agonist, has been demonstrated by Young C et al. (2017) [105] to be useful in treating Parkinson’s disease. Using the in-vivo model, Capsaicin (0.5 mg/kg, i.p.) was found to restore nigrostriatal dopaminergic neurons in MPTP-injected mice, resulting in improved motor function. This, however, did not match our in-silico predictions as shown in Table 4 that Capsaicin had activity as Ion Channel Modulator with a score of −0.15 [105].
Despite the lack of studies that evaluate these natural products, the in-silico prediction illustrated that β-caryophyllene, Oridonin, and Tripchlorolide are considered ion channel modulators with the activity of 0.28, 0.27, and 0.24, respectively.
3.2.3. Kinase Inhibitors
Kinases have become attractive drug targets because they are involved in nearly all cellular activities, such as cell growth, survival, proliferation, differentiation, and metabolism, and dysregulation of their activity has been linked to a variety of diseases, including CNS disorders such as AD, PD, and MS [168].
Unfortunately, most of the compounds showed no activity as a kinase inhibitor. However, Yang et al. (2017) [54] suggested that the Andrographolide suppressed NF-κB nuclear translocation by suppressing NF-κB phosphorylation in BV-2 cells, which were supported by our in-silico study [54]. Moreover, Leung et al. (2005) [169] studied the novel mechanism of inhibition of NF-κB DNA-binding activity by diterpenoids found in the compound Oridonin to treat inflammatory diseases [169]. However, the study did not find Oridonin to be active as a kinase inhibitor. Nevertheless, Oridonin works as a Nuclear Receptor Ligand and Enzyme Inhibitor based on Molinspiration biological predictions. Additionally, using the prediction analysis, only Epigallocatechin-3-gallate, Dihydromyricetin, Silibinin, Quercetin, Apigenin, Galangin, Baicalein, Myricetin, Myricitrin, and Nobiletin showed a good activity as kinase inhibitors. Moreover, Quercetin and Myricetin were the most active, with a score of 0.28 for both. Goldmann et al. demonstrate that 18β-Glycyrrhetinic Acid targeted the MAPK, but this did not represent our in-silico prediction [170].
3.2.4. Nuclear Receptor Ligand
Nuclear receptors have attracted a lot of attention in the last 10 years as prospective therapeutic targets for neurodegenerative diseases. Effective treatments for progressive neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and ALS have eluded researchers for years, making non-traditional therapeutic targets like nuclear receptors an appealing alternative. The involvement of nuclear receptors in several neurodegenerative disorders, most notably Alzheimer’s disease, has been studied extensively in mice models of disease and several therapeutic studies [86].
Our in-silico predictions suggest that Curcumin, Resveratrol, Pterostilbene, Epigallocatechin-3-gallate, Andrographolide, Paeoniflorin, β-caryophyllene, Oridonin, Dihydromyricetin, 4-O-methylhonokiol, Silibinin, Triptolide, Eriodictyol, Quercetin, Apigenin, Capsaicin, Galangin, Biochanin A, Baicalein, α-Mangostin, Myricetin, Myricitrin, Licochalcone E, Licochalcone A, Isobavachalcone, Triptolide, Naringin, Cannabidiol, Baicalin, Oleanolic Acid, 18β-Glycyrrhetinic Acid, Carnosol, and Tanshinone IIA were active as nuclear receptor ligand as summarized in Table 4.
Zun-jing et al. (2016) [86] reported that Curcumin inhibited the NF-κB signaling pathway and reduced the production of pro-inflammatory mediators from M1 microglia by specifically targeting PPAR-γ which is a Nuclear Receptor, and this was obvious in the Molinspiration biological predictions with an activity of 0.12 [86]. Moreover, Cheng et al. (2014) [40] showed that β-caryophyllene intragastric treatment (48 mg/kg, for 10 weeks) to APP/PS1 mice could prevent cognitive decline and reverse neurodegeneration through the activation of the CB2R and PPAR-pathways. This correlates with the reduction in microglial M1 activation and inflammatory cytokines [40]. Interestingly, all these results were supported by the Molinspiration webserver. Moreover, as shown in Table 4, some of the data were favorable as a Nuclear Receptor ligand, especially for compound PD-4. The results of the Galangin matched those of Min-ji and his colleagues in their 2017 study in which authors suggest in LPS-stimulated BV-2 cells, Galangin is a well-known PPAR activator that inhibits M1 inflammatory responses and increases the Nrf2/CREB signaling pathway from 10 to 50 μM [58]. Additionally, Sativex® (Sativex-like combination of Phytocannabinoids) therapy alone exhibited potential results in TMEV-IDD (Theiler’s murine encephalomyelitis virus-induced demyelinating disease) models as a modulatory drug for increasing microglia polarization to M2 phenotype to establish cytoprotective milieu. The therapeutic effects of Sativex may be due to (tetrahydrocannabinol-botanical drug substance) THC-induced upregulation of both CB1R and CB2R expression, as well as CBD-induced PPAR activation, and this matched the in-silico of Cannabidiol which showed a good activity (0.38) as nuclear receptor ligand [171]. Furthermore, compounds Andrographolide, Oridonin, Oleanolic Acid, 18β-Glycyrrhetinic Acid, and Carnosol demonstrated high scores of 0.94, 0.73, 0.77, 0.79, and 0.51 as nuclear receptor ligand, respectively.
3.2.5. Protease Inhibitors
Gene transcription, the initiation process of precursor forms, and interactions with endogenous protease inhibitors are all mechanisms that closely regulate protease activity. Once activated, proteases can cause irreversible breakage of peptide bonds in various proteins. Some substrates are inactivated after cleavage, while others are activated to gain new functionalities. As a result, microglial proteases are thought to have both positive and negative effects. According to Table 4, only compounds Epigallocatechin-3-gallate, Andrographolide, Paeoniflorin, Oridonin, Dihydromyricetin, Silibinin, Triptolide, Tenuigenin, Isobavachalcone, Tripchlorolide, Triptolide, Naringin, Matrine, Oleanolic Acid, and Glycyrrhizin appear to have good activity as protease inhibitors. Defects in proteostasis are thought to be associated with various neurodegenerative disorders, including Parkinson’s disease. While the proteasome fails to destroy large protein aggregates, such as alpha-synuclein (α-SYN) in PD, drug-induced autophagy can effectively remove clusters and prevent dopaminergic neuron degeneration. As a result, maintaining these pathways is critical for preserving all cellular functions that rely on a properly folded proteome [172]. The Molinspiration analysis indicated that Tenuigenin, Isobavachalcone, Tripchlorolide, Triptolide, and Naringin act as Protease Inhibitors.
3.2.6. Enzyme Inhibitors
The aggregation of misfolded amyloid-β and hyperphosphorylated tau and α-synuclein are linked to the pathogenesis of AD and PD, respectively. To cure the diseases, multiple small molecules have been developed to regulate the aggregation pathways of these amyloid proteins. In addition to controlling the aggregation of amyloidogenic proteins, maintaining the levels of the proteins in the brain by amyloid degrading enzymes (ADE); neprilysin (NEP), insulin-degrading enzyme (IDE), asparagine endopeptidase (AEP), and ADAM10 is also essential to cure AD and PD. Therefore, numerous biological molecules and chemical agents have been investigated as either inducers or inhibitors against the levels and activities of amyloid degrading enzymes [173]. All the AD and PD compounds showed enzyme inhibitor activity except Aromatic-turmerone, Xanthoceraside, Esculentoside A. α-asarone, Apocynin, Icariin, Tanshinone I, Salvianolic acid B, Licochalcone E, and Ginsenoside Rg1.
Moreover, reactive oxygen species (ROS) possess a physiological role in various cellular regulation processes. Antioxidant enzyme therapy may be advantageous for treating MS as ROS scavengers may interfere at numerous levels during the formation of MS lesions [174]. Cannabidiol, Baicalin, Matrine, Oleanolic Acid, 18β-Glycyrrhetinic Acid, Carnosol, and Tanshinone IIA demonstrated activity as enzyme inhibitors with an activity of 0.33, 0.26, 0.06, 0.65, 0.70, 0.37, and 0.08, respectively, as shown in Table 4.
3.3. Absorption, Distribution, Metabolism, and Excretion (ADME)
ADME properties were predicted using SwissADME, an online web server. Furthermore, the BBB can prevent chemicals from entering the brain and acts as a natural barrier against numerous poisons and infected cells in the bloodstream, but it also restricts the uptake of diagnostic and therapeutic substances in the brain, diminishing therapeutic efficiency and targeted delivery, therefore, small (often less than 500 Da) and lipophilic compounds can effectively penetrate the BBB and enter the brain. Thus, as disease-targeting strategies molecular weight (MW), blood-brain barrier penetration (BBB), high solubility (logS), and P-glycoprotein substrate, all are essential characteristics of the drug to be promising as a neuroprotective molecule [175].
3.3.1. Molecular Weight (MW)
Considering Lipinski’s rule limit of MW of 500 g/mol, all compounds were within the recommended range, which improves their chances to be absorbed orally in the gastrointestinal tract except for Hesperidin, Xanthoceraside, Esculentoside A, Icariin, Tenuigenin, Salvianolic acid B, Ginsenoside Rg1, Naringin, Astragaloside IV, and Glycyrrhizin, which have molecular weights of 610.56, 1141.29, 973.11, 676.66, 537.13, 718.61, 801.01, 580.53, 784.97, and 822.93 g/mol, respectively [176].
3.3.2. Blood-Brain Barrier (BBB) Permeability
All the studied compounds could not cross the blood-brain barrier (BBB) except for Aromatic-turmerone, Resveratrol, Pterostilbene, 4-O-methylhonokiol, Piperlongumine, Capsaicin, α-asarone, Apocynin, Tanshinone I, Licochalcone E, Licochalcone A, Macelignan, Cannabidiol, Matrine, Carnosol, and Tanshinone IIA. Moreover, these sixteen compounds possess an advantage of blood-brain barrier penetration that allows them to be used in treating neurodegenerative diseases and targeting microglia [177]. Furthermore, α-asarone is one of the most studied compounds to cross the blood-brain barrier in more than one scientific study as an effective treatment for Parkinson’s disease. For example, according to Chinese medicine, Xiao et al. (2015) [178] showed that α-asarone had been used to treat dementia, amnesia, and stroke as an orifice-opening medicinal because of the adequate and appropriate BBB permeability [178]. Similarly, Carnosol can cross through the BBB and subsequently produce an anti-inflammatory effect on M1 microglia in the CNS, according to Xing Li et al. (2018). [158]
3.3.3. Solubility (Log S)
The aqueous solubility of substances that have a direct impact on oral absorption is referred to as Log S. Within the specified range (−6.5 to 0.5), all compounds demonstrated soluble to moderate solubility except for Nobiletin, Tanshinone I, Astragaloside IV, and Tanshinone IIA with log S values of −6.82, −6.91, and −6.71 which were poorly soluble.
3.3.4. P-glycoprotein Substrate
P-glycoprotein (P-gp) has emerged as the transporter that poses the largest barrier to innovative neuroprotective drug delivery among the BBB’s reported transporters. All the compounds are not a P-glycoprotein substrate except for Andrographolide, Paeoniflorin, Oridonin, Hesperidin, Triptolide, Eriodictyol, Xanthoceraside Esculentoside A, Icariin, Tenuigenin, Ginsenoside Rg1, Tripchlorolide, Triptolide, Naringin, Astragaloside IV, Glycyrrhizin, 18β-Glycyrrhetinic Acid, Carnosol, and Tanshinone IIA. All ADME results are summarized in Table 5.
Table 5.
The Pharmacokinetics ADME Properties of the Neuroprotective Phytochemicals Used for AD, PD, and MS Treatments using SwissADME webserver.
| Compounds Names | Molecular Weight | HB Donor | HB Acceptor | Log Po/w [WLOGP] | Log S [SILICO S-IT] | BBB Permeant | GI Absorption | P-gp Substrate | Rule of Five [ROF] |
|---|---|---|---|---|---|---|---|---|---|
| Curcumin | 368.38 g/mol | 2 | 6 | 3.15 | −4.45 | No | High | No | Yes: 0 violation |
| Aromatic-turmerone | 216.32 g/mol | 0 | 1 | 4.02 | −4.45 | Yes | High | No | Yes: 0 violation |
| Resveratrol | 228.24 g/mol | 3 | 3 | 2.76 | −3.29 | Yes | High | No | Yes: 0 violation |
| Pterostilbene | 256.30 g/mol | 1 | 3 | 3.36 | −4.69 | Yes | High | No | Yes: 0 violation |
| Sulforaphane | 177.29 g/mol | 0 | 2 | 2.11 | −2.10 | No | High | No | Yes: 0 violation |
| Epigallocatechin-3-gallate | 458.37 g/mol | 8 | 11 | 1.91 | −2.50 | No | Low | No | No; 2 violations: NorO > 10, NHorOH > 5 |
| Andrographolide | 350.45 g/mol | 3 | 5 | 1.96 | −2.69 | No | High | Yes | Yes: 0 violation |
| Paeoniflorin | 480.46 g/mol | 5 | 11 | −1.36 | −1.15 | No | Low | Yes | Yes; 1 violation: NorO > 10 |
| β-caryophyllene | 204.35 g/mol | 0 | 0 | 4.73 | −3.77 | No | Low | No | Yes; 1 violation: MLOGP > 4.15 |
| Oridonin | 364.43 g/mol | 4 | 6 | 0.38 | −1.60 | No | High | Yes | Yes: 0 violation |
| Dihydromyricetin | 320.25 g/mol | 6 | 8 | 0.57 | −1.44 | No | Low | No | Yes; 1 violation: NHorOH > 5 |
| 4-O-methylhonokiol | 280.36 g/mol | 1 | 2 | 4.52 | −6.17 | Yes | High | No | Yes: 0 violation |
| Silibinin | 482.44 g/mol | 5 | 10 | 1.71 | −4.50 | No | Low | No | Yes: 0 violation |
| Hesperidin | 610.56 g/mol | 8 | 15 | −1.48 | −0.58 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Triptolide | 360.40 g/mol | 1 | 6 | 1.1 | −2.51 | No | High | Yes | Yes: 0 violation |
| Eriodictyol | 288.25 g/mol | 4 | 6 | 1.89 | −2.84 | No | High | Yes | Yes: 0 violation |
| Xanthoceraside | 1141.29 g/mol | 12 | 23 | 0.26 | 0.2 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Piperlongumine | 317.34 g/mol | 0 | 5 | 1.55 | −2.94 | Yes | High | No | Yes: 0 violation |
| Esculentoside A | 973.11 g/mol | 11 | 20 | −1.09 | −0.08 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Quercetin | 302.24 g/mol | 5 | 7 | 1.99 | −3.24 | No | High | No | Yes: 0 violation |
| Apigenin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Capsaicin | 305.41 g/mol | 2 | 3 | 3.64 | −4.87 | Yes | High | No | Yes: 0 violation |
| α-asarone | 208.25 g/mol | 0 | 3 | 2.64 | −3.26 | Yes | High | No | Yes: 0 violation |
| Galangin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Biochanin A | 284.26 g/mol | 2 | 5 | 2.88 | −5.10 | No | High | No | Yes: 0 violation |
| Baicalein | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes: 0 violation |
| Apocynin | 166.17 g/mol | 1 | 3 | 1.6 | −2.28 | Yes | High | No | Yes: 0 violation |
| α-Mangostin | 410.46 g/mol | 3 | 6 | 5.09 | −6.14 | No | High | No | Yes: 0 violation |
| Myricetin | 318.24 g/mol | 6 | 8 | 1.69 | −2.66 | No | Low | No | Yes; 1 violation: NHorOH > 5 |
| Myricitrin | 464.38 g/mol | 8 | 12 | 0.19 | −1.49 | No | Low | No | No; 2 violations: NorO > 10, NHorOH > 5 |
| Icariin | 676.66 g/mol | 8 | 15 | 0.07 | −2.74 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Nobiletin | 402.39 g/mol | 0 | 8 | 3.51 | −6.82 | No | High | No | Yes: 0 violation |
| Tenuigenin | 537.13 g/mol | 4 | 6 | 5.49 | −4.85 | No | Low | Yes | No; 2 violations: MW > 500, MLOGP > 4.15 |
| Tanshinone I | 276.29 g/mol | 0 | 3 | 4.1 | −6.91 | Yes | High | No | Yes; 0 violation |
| Salvianolic acid B | 718.61 g/mol | 9 | 16 | 2.9 | −4.41 | No | Low | No | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Licochalcone E | 338.40 g/mol | 2 | 4 | 4.57 | −5.17 | Yes | High | No | Yes; 0 violation |
| Licochalcone A | 338.40 g/mol | 2 | 4 | 4.57 | −5.17 | Yes | High | No | Yes; 0 violation |
| Isobavachalcone | 324.37 g/mol | 3 | 4 | 4.1 | −4.47 | No | High | No | Yes; 0 violation |
| Macelignan | 328.40 g/mol | 1 | 4 | 4.19 | −5.88 | Yes | High | No | Yes; 0 violation |
| Ginsenoside Rg1 | 801.01 g/mol | 10 | 40 | 1.12 | −0.87 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Tripchlorolide | 396.86 g/mol | 2 | 6 | 1.3 | −2.79 | No | High | Yes | Yes; 0 violation |
| Triptolide | 360.40 g/mol | 1 | 6 | 1.1 | −2.51 | No | High | Yes | Yes; 0 violation |
| Naringin | 580.53 g/mol | 8 | 14 | −1.49 | −0.49 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Cannabidiol | 314.46 g/mol | 2 | 2 | 5.85 | −5.41 | Yes | High | No | Yes: 1 violation: MLOGP > 4.15 |
| Dimethyl fumarate | 144.13 g/mol | 0 | 4 | −0.11 | −0.10 | No | High | No | Yes; 0 violation |
| 3H-1,2-dithiole-3-thione | 134.24 g/mol | 0 | 0 | 2.54 | −1.43 | No | High | No | Yes; 0 violation |
| Baicalin | 270.24 g/mol | 3 | 5 | 2.58 | −4.40 | No | High | No | Yes; 0 violation |
| Matrine | 248.36 g/mol | 0 | 2 | 1.11 | −1.68 | Yes | High | No | Yes; 0 violation |
| Oleanolic Acid | 456.70 g/mol | 2 | 3 | 7.23 | −6.12 | No | Low | No | Yes; 1 violation: MLOGP > 4.15 |
| Astragaloside IV | 784.97 g/mol | 9 | 14 | 0.72 | −1.11 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| Glycyrrhizin | 822.93 g/mol | 8 | 16 | 2.25 | −1.39 | No | Low | Yes | No; 3 violations: MW > 500, NorO > 10, NHorOH > 5 |
| 18β-Glycyrrhetinic Acid | 470.68 g/mol | 2 | 4 | 6.41 | −6.00 | No | High | Yes | Yes; 1 violation: MLOGP > 4.15 |
| Carnosol | 330.42 g/mol | 2 | 4 | 3.96 | −4.45 | Yes | High | Yes | Yes; 0 violation |
| Tanshinone IIA | 294.34 g/mol | 0 | 3 | 4.25 | −6.71 | Yes | High | Yes | Yes; 0 violation |
3.4. Toxicity and Safety Prediction for Neuroprotective Phytochemicals
3.4.1. Inhibition of the Cytochromes P450
Herbs can accelerate or decrease the expected activity of prescribed medication, resulting in undesired side effects or therapeutic failure. Herbal active components can dramatically affect a drug’s pharmacokinetic and pharmacodynamic properties, raising concerns regarding herb-drug interactions. The inhibition or induction of cytochrome P450 (CYP450) has been proposed as one of the key mechanisms for herb-drug interactions. Thus, to evaluate the potential interactions between the bioactive metabolites of natural herbs and cytochrome P450 enzymes SwissADME webserver was utilized [179].
As shown below in Table 6, 4-O-methylhonokiol and Tanshinone IIA strongly inhibited all the CYP groups. Moreover, the safest compound that did not show any inhibition of cytochrome P450 was Aromatic turmerone, Sulforaphane, Epigallocatechin-3-gallate, Andrographolide, Paeoniflorin, Oridonin, Dihydromyricetin, Hesperidin, Triptolide, Xanthoceraside, Piperlongumine, and Esculentoside A, for the PD, they were Apocynin, Myricitrin, Icariin, Tenuigenin, Salvianolic acid B, Ginsenoside Rg1, Tripchlorolide, Triptolide, and Naringin moving to MS they were Dimethyl fumarate, 3H-1,2-dithiole-3-thione, Matrine, Oleanolic Acid, Astragaloside IV, Glycyrrhizin, and 18β-Glycyrrhetinic Acid.
Table 6.
Cytochromes Inhibition Profile of the Neuroprotective Phytochemicals Used for AD, PD, and MS Treatments using SwissADME webserver.
| Compound Names | CYP1A2 | CYP2C19 | CYP2C9 | CYP2D6 | CYP3A4 |
|---|---|---|---|---|---|
| Curcumin | No | No | Yes | No | Yes |
| Aromatic turmerone | No | No | No | No | No |
| Resveratrol | Yes | No | Yes | No | Yes |
| Pterostilbene | Yes | Yes | Yes | Yes | No |
| Sulforaphane | No | No | No | No | No |
| Epigallocatechin-3-gallate | No | No | No | No | No |
| Andrographolide | No | No | No | No | No |
| Paeoniflorin | No | No | No | No | No |
| β-caryophyllene | No | Yes | Yes | No | No |
| Oridonin | No | No | No | No | No |
| Dihydromyricetin | No | No | No | No | No |
| 4-O-methylhonokiol | Yes | Yes | Yes | Yes | Yes |
| Silibinin | No | No | No | No | Yes |
| Hesperidin | No | No | No | No | No |
| Triptolide | No | No | No | No | No |
| Eriodictyol | No | No | No | No | Yes |
| Xanthoceraside | No | No | No | No | No |
| Piperlongumine | No | No | No | No | No |
| Esculentoside A | No | No | No | No | No |
| Quercetin | Yes | No | No | Yes | Yes |
| Apigenin | Yes | No | No | Yes | Yes |
| Capsaicin | Yes | No | No | Yes | Yes |
| α-asarone | Yes | Yes | No | No | No |
| Galangin | Yes | No | No | Yes | Yes |
| Biochanin A | Yes | No | No | Yes | Yes |
| Baicalein | Yes | No | No | Yes | Yes |
| Apocynin | No | No | No | No | No |
| α-Mangostin | No | No | Yes | No | No |
| Myricetin | Yes | No | No | No | Yes |
| Myricitrin | No | No | No | No | No |
| Icariin | No | No | No | No | No |
| Nobiletin | No | No | Yes | No | Yes |
| Tenuigenin | No | No | No | No | No |
| Tanshinone I | Yes | Yes | No | No | Yes |
| Salvianolic acid B | No | No | No | No | No |
| Licochalcone E | Yes | No | Yes | No | Yes |
| Licochalcone A | Yes | No | Yes | No | Yes |
| Isobavachalcone | Yes | No | Yes | No | Yes |
| Macelignan | No | Yes | Yes | Yes | No |
| Ginsenoside Rg1 | No | No | No | No | No |
| Tripchlorolide | No | No | No | No | No |
| Triptolide | No | No | No | No | No |
| Naringin | No | No | No | No | No |
| Cannabidiol | No | Yes | Yes | Yes | Yes |
| Dimethyl fumarate | No | No | No | No | No |
| 3H-1,2-dithiole-3-thione | No | No | No | No | No |
| Baicalin | Yes | No | No | Yes | Yes |
| Matrine | No | No | No | No | No |
| Oleanolic Acid | No | No | No | No | No |
| Astragaloside IV | No | No | No | No | No |
| Glycyrrhizin | No | No | No | No | No |
| 18β-Glycyrrhetinic Acid | No | No | No | No | No |
| Carnosol | No | No | Yes | No | No |
| Tanshinone IIA | Yes | Yes | Yes | Yes | Yes |
3.4.2. Organ Toxicity
During the development of new medicine, the most important consideration is always safety, which includes a variety of toxicities and adverse drug effects that should be assessed during the preclinical and clinical trial phases. Herein, we investigated the direct organ toxicity of bioactive metabolites using computational approaches [180].
We investigated the safety profile of all compounds by conducting toxicity prediction tests with the ProTox-II online tool. This server classified compounds into six toxicity classes [1,2,3,4,5,6], with class 1 being the most toxic and fatal, with an estimated lethal dosage (LD50) of 5, and class 6 demonstrating an LD50 > 5000, indicating the compound is non-toxic. All compounds’ LD50, organ toxicity (hepatotoxicity], toxicity endpoints [carcinogenicity, mutagenicity, immunotoxicity), were predicted, except compound Glycyrrhizin and 18β-Glycyrrhetinic Acid, which were inactive. Furthermore, the toxicity class and the estimated probability of each compound were provided. The oral toxicity prediction findings revealed that the safest compounds were Hesperidin, Apocynin, Tenuigenin, and Astragaloside IV, which were in class 6, and the majority of the compounds were in class 4 and 5, except for compounds Oridonin, and Quercetin, Myricetin, Dimethyl fumarate, and Matrine, which were in class 3. For the most toxic and fatal compounds, they were only compounds Triptolide and Capsaicin, Salvianolic acid B, Tripchlorolide, and Triptolide which were classified as 1 and 2. On the ProTox-II server, the majority of the compounds in Table 7. were predicted to be potentially immunogenic except for Aromatic-turmerone, Resveratrol, Sulforaphane, Epigallocatechin-3-gallate, Paeoniflorin, Dihydromyricetin, Eriodictyol, and Apigenin, Galangin, Biochanin A, Baicalein, Apocynin, Myricetin, and Tanshinone I, Dimethyl fumarate, 3H-1,2-dithiole-3-thione, Baicalin, Matrine, and Oleanolic Acid. Among the compounds investigated, 14 out of the 54 compounds were predicted to be carcinogenic, including Dihydromyricetin, Triptolide, Eriodictyol, Apigenin, Capsaicin, α-asarone, Baicalein, Myricetin, Myricitrin, Enuigenin, Tripchlorolide, Triptolide, Baicalin, Oleanolic Acid, and 18β-Glycyrrhetinic Acid. Furthermore, all compounds showed mutagenicity with probability values ranging from 0.51 to 0.99 except Dihydromyricetin, Apigenin, Capsaicin, Baicalein, Myricetin, Salvianolic acid B, and Baicalin. Finally, there was no remarkable hepatotoxicity except for Licochalcone E and Oleanolic Acid. To conclude, compounds Aromatic-turmerone, Resveratrol, Sulforaphane, Epigallocatechin-3-gallate, Paeoniflorin, Galangin, Biochanin A, Apocynin, Tanshinone I, Dimethyl fumarate, 3H-1,2-dithiole-3-thione, and Matrine could be considered safe according to ProTox-II online tool.
Table 7.
The Toxicity Profiles of the Neuroprotective Phytochemicals Used for AD, PD, and MS Treatments using ProTox-II online Tool.
| Compound Names | Predicted Toxicity Class | Predicted LD50 [mg/kg] | Organ toxicity/ Toxicity endpoints | Probability |
|---|---|---|---|---|
| Curcumin | 4 | 2000 | Hepatotoxicity | 0.61 |
| Carcinogenicity | 0.84 | |||
| Mutagenicity | 0.88 | |||
| Immunotoxicity | 0.92 | |||
| Aromatic-turmerone | 4 | 2000 | Hepatotoxicity | 0.59 |
| Carcinogenicity | 0.64 | |||
| Mutagenicity | 0.93 | |||
| Immunotoxicity | 0.99 | |||
| Resveratrol | 4 | 1560 | Hepatotoxicity | 0.74 |
| Carcinogenicity | 0.71 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.86 | |||
| Pterostilbene | 4 | 1560 | Hepatotoxicity | 0.67 |
| Carcinogenicity | 0.61 | |||
| Mutagenicity | 0.81 | |||
| Immunotoxicity | 0.65 | |||
| Sulforaphane | 4 | 1000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.63 | |||
| Immunotoxicity | 0.99 | |||
| Epigallocatechin-3-gallate | 4 | 1000 | Hepatotoxicity | 0.70 |
| Carcinogenicity | 0.54 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.89 | |||
| Andrographolide | 4 | 1890 | Hepatotoxicity | 0.93 |
| Carcinogenicity | 0.83 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.82 | |||
| Paeoniflorin | 5 | 4000 | Hepatotoxicity | 0.90 |
| Carcinogenicity | 0.85 | |||
| Mutagenicity | 0.61 | |||
| Immunotoxicity | 0.86 | |||
| β-caryophyllene | 5 | 5300 | Hepatotoxicity | 0.80 |
| Carcinogenicity | 0.70 | |||
| Mutagenicity | 0.95 | |||
| Immunotoxicity | 0.54 | |||
| Oridonin | 3 | 120 | Hepatotoxicity | 0.86 |
| Carcinogenicity | 0.69 | |||
| Mutagenicity | 0.56 | |||
| Immunotoxicity | 0.98 | |||
| Dihydromyricetin | 4 | 2000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.59 | |||
| 4-O-methylhonokiol | 4 | 1649 | Hepatotoxicity | 0.71 |
| Carcinogenicity | 0.64 | |||
| Mutagenicity | 0.89 | |||
| Immunotoxicity | 0.50 | |||
| Silibinin | 4 | 2000 | Hepatotoxicity | 0.78 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.97 | |||
| Hesperidin | 6 | 12,000 | Hepatotoxicity | 0.81 |
| Carcinogenicity | 0.93 | |||
| Mutagenicity | 0.90 | |||
| Immunotoxicity | 0.99 | |||
| Triptolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.58 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.97 | |||
| Eriodictyol | 4 | 2000 | Hepatotoxicity | 0.67 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.59 | |||
| Immunotoxicity | 0.71 | |||
| Xanthoceraside | 4 | 590 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.99 | |||
| Piperlongumine | 4 | 1180 | Hepatotoxicity | 0.79 |
| Carcinogenicity | 0.52 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.99 | |||
| Esculentoside A | 5 | 4000 | Hepatotoxicity | 0.95 |
| Carcinogenicity | 0.73 | |||
| Mutagenicity | 0.96 | |||
| Immunotoxicity | 0.99 | |||
| Quercetin | 3 | 159 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.87 | |||
| Apigenin | 5 | 2500 | Hepatotoxicity | 0.86 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.57 | |||
| Immunotoxicity | 0.99 | |||
| Capsaicin | 2 | 47 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.71 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.86 | |||
| α-asarone | 4 | 418 | Hepatotoxicity | 0.63 |
| Carcinogenicity | 0.56 | |||
| Mutagenicity | 0.92 | |||
| Immunotoxicity | 0.67 | |||
| Immunotoxicity | 0.99 | |||
| Galangin | 5 | 3919 | Hepatotoxicity | 0.68 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.52 | |||
| Immunotoxicity | 0.97 | |||
| Biochanin A | 5 | 2500 | Hepatotoxicity | 0.73 |
| Carcinogenicity | 0.65 | |||
| Mutagenicity | 0.94 | |||
| Immunotoxicity | 0.75 | |||
| Baicalein | 5 | 3919 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.99 | |||
| Apocynin | 6 | 9000 | Hepatotoxicity | 0.52 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.99 | |||
| Immunotoxicity | 0.78 | |||
| α-Mangostin | 4 | 1500 | Hepatotoxicity | 0.70 |
| Carcinogenicity | 0.69 | |||
| Mutagenicity | 0.53 | |||
| Immunotoxicity | 0.84 | |||
| Myricetin | 3 | 159 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.86 | |||
| Myricitrin | 5 | 5000 | Hepatotoxicity | 0.73 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.98 | |||
| Icariin | 5 | 5000 | Hepatotoxicity | 0.74 |
| Carcinogenicity | 0.83 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.98 | |||
| Nobiletin | 5 | 5000 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.53 | |||
| Mutagenicity | 0.69 | |||
| Immunotoxicity | 0.51 | |||
| Tenuigenin | 6 | 6176 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.51 | |||
| Mutagenicity | 0.86 | |||
| Immunotoxicity | 0.86 | |||
| Tanshinone I | 4 | 1655 | Hepatotoxicity | 0.63 |
| Carcinogenicity | 0.51 | |||
| Mutagenicity | 0.55 | |||
| Immunotoxicity | 0.66 | |||
| Salvianolic acid B | 2 | 25 | Hepatotoxicity | 0.64 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.55 | |||
| Immunotoxicity | 0.97 | |||
| Licochalcone E | 4 | 1000 | Hepatotoxicity | 0.51 |
| Carcinogenicity | 0.67 | |||
| Mutagenicity | 0.68 | |||
| Immunotoxicity | 0.92 | |||
| Licochalcone A | 4 | 1000 | Hepatotoxicity | 0.62 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.79 | |||
| Immunotoxicity | 0.76 | |||
| Isobavachalcone | 4 | 1000 | Hepatotoxicity | 0.64 |
| Carcinogenicity | 0.72 | |||
| Mutagenicity | 0.76 | |||
| Immunotoxicity | 0.97 | |||
| Macelignan | 5 | 2260 | Hepatotoxicity | 0.75 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.97 | |||
| Ginsenoside Rg1 | 5 | 4000 | Hepatotoxicity | 0.94 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.91 | |||
| Immunotoxicity | 0.88 | |||
| Tripchlorolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.60 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.99 | |||
| Triptolide | 1 | 4 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.58 | |||
| Mutagenicity | 0.75 | |||
| Immunotoxicity | 0.97 | |||
| Naringin | 5 | 2300 | Hepatotoxicity | 0.81 |
| Carcinogenicity | 0.90 | |||
| Mutagenicity | 0.73 | |||
| Immunotoxicity | 0.99 | |||
| Cannabidiol | 4 | 500 | Hepatotoxicity | 0.79 |
| Carcinogenicity | 0.66 | |||
| Mutagenicity | 0.85 | |||
| Immunotoxicity | 0.93 | |||
| Dimethyl fumarate | 3 | 62 | Hepatotoxicity | 0.80 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.71 | |||
| Immunotoxicity | 0.99 | |||
| 3H-1,2-dithiole-3-thione | 4 | 1480 | Hepatotoxicity | 0.68 |
| Carcinogenicity | 0.50 | |||
| Mutagenicity | 0.81 | |||
| Immunotoxicity | 0.99 | |||
| Baicalin | 5 | 3919 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.51 | |||
| Immunotoxicity | 0.99 | |||
| Matrine | 3 | 243 | Hepatotoxicity | 0.92 |
| Carcinogenicity | 0.68 | |||
| Mutagenicity | 0.77 | |||
| Immunotoxicity | 0.96 | |||
| Oleanolic Acid | 4 | 2000 | Hepatotoxicity | 0.52 |
| Carcinogenicity | 0.57 | |||
| Mutagenicity | 0.85 | |||
| Immunotoxicity | 0.79 | |||
| Astragaloside IV | 6 | 23,000 | Hepatotoxicity | 0.92 |
| Carcinogenicity | 0.74 | |||
| Mutagenicity | 0.67 | |||
| Immunotoxicity | 0.99 | |||
| Glycyrrhizin | 4 | 1750 | Hepatotoxicity | 0.88 |
| Carcinogenicity | 0.61 | |||
| Mutagenicity | 0.96 | |||
| Immunotoxicity | 0.99 | |||
| 18β-Glycyrrhetinic Acid | 4 | 560 | Hepatotoxicity | 0.69 |
| Carcinogenicity | 0.55 | |||
| Mutagenicity | 0.90 | |||
| Immunotoxicity | 0.94 | |||
| Carnosol | 4 | 1500 | Hepatotoxicity | 0.76 |
| Carcinogenicity | 0.62 | |||
| Mutagenicity | 0.88 | |||
| Immunotoxicity | 0.99 | |||
| Tanshinone IIA | 4 | 1230 | Hepatotoxicity | 0.71 |
| Carcinogenicity | 0.56 | |||
| Mutagenicity | 0.70 | |||
| Immunotoxicity | 0.80 |
Class 1 Fatal if swallowed [LD50 ≤ 5], Class 2 Fatal if swallowed [5 < LD50 ≤ 50], Class 3 Toxic if swallowed [50 < LD50 ≤ 300], Class 4 Harmful if swallowed [300 < LD50 ≤ 2000], Class 5 It may be harmful if swallowed [2000 < LD50 ≤ 5000],Class 6 Non-toxic [LD50 > 5000].
4. Materials and Methods
4.1. Literature Search
A systematic search was conducted in databases such as PubMed, Google Scholar, and Science Direct to identify relevant studies using key-words such as Microglia, Neurodegenerative diseases, Alzheimer disease, Parkinson disease, Multiple sclerosis, M1, and M2, Neuroprotective, ADME, in-vitro, in-vivo, in-silico, clinical trial. The reported phytochemicals in the studies that demonstrated neuroprotective effects via microglia modulation in neurodegenerative diseases (AD, PD, and MS) were selected.
4.2. Computational Analysis
The 2D chemical structure of each bioactive constituent was drawn using Chemdraw, and the simplified molecular-input line-entry system (SMILES), was utilized to conduct the computational analysis. The following computational tools were used: PASS online, Molinspiration, SwissADME, and ProTox-II webservers.
4.2.1. PASS Online
The activity is predicted by finding similarities between the new compound chemical structure and a well-known biological active substrate in the database. The activity spectrum estimation algorithm uses a Bayesian method. The PASS prediction tool will predict the probability of active [Pa] to probability of inactive [Pi] ratio. According to leave-one-out cross-validation [LOO CV] estimation, the average prediction accuracy is around 95%. PASS prediction accuracy depends on detailed information on the biological activity spectrum for each molecule in the PASS training set, so the biological activity estimate is more accurate. The website ( www.way2drug.com, accessed on 25 May 2021) [181] can be accessed directly with the search term "PASS prediction" in multiple web browsers.
4.2.2. Molinspiration
Molinspiration (www.molinspiration.com, accessed on 26 December 2021) [182] is a free online tool that aids the internet chemistry community by calculating essential chemical characteristics and predicting bioactivity scores for the most important drug targets [GPCR ligands, kinase inhibitors, ion channel modulators, nuclear receptors]. A molecule with a bioactivity score greater than 0.00 is most likely to have significant biological activities, whereas values and scores less than −0.50 are considered inactive.
4.2.3. SwissADME
To enhance drug discovery, this webserver (www.swissadme.ch, accessed on 8 November 2021) [183] allows for computing physicochemical descriptors and estimating absorption, distribution, metabolism, and excretion [ADME] parameters, pharmacokinetic properties, druglike nature, and medicinal chemistry properties of one or more small molecules.
4.2.4. ProTox-II
ProTox-II (http://tox.charite.de/protox_II, accessed on 8 November 2021) [184] uses a total of 33 models based on molecular similarity, fragment propensities, most frequent features, and [fragment similarity-based CLUSTER cross-validation] machine learning to predict various toxicity endpoints like acute toxicity, hepatotoxicity, cytotoxicity, carcinogenicity, mutagenicity, immunotoxicity. Toxicity classifications are determined using the globally harmonized system of classification of labeling of chemicals (GHS); toxic doses are frequently expressed as LD50 values in milligrams per kilogram of body weight. The median lethal dose (LD50) is the dose at which 50% of test subjects die after being exposed to a substance. The following are the classification and the (mg/kg) LD50 values.
| Class 1: | Fatal if swallowed [LD50 ≤ 5] |
| Class 2: | Fatal if swallowed [5 < LD50 ≤ 50] |
| Class 3: | Toxic if swallowed [50 < LD50 ≤ 300] |
| Class 4: | Harmful if swallowed [300 < LD50 ≤ 2000] |
| Class 5: | It may be harmful if swallowed [2000 < LD50 ≤ 5000] |
| Class 6: | Non-toxic [LD50 > 5000] |
5. Conclusions and Future Directions
The reported biological activity of neuroprotective medicinal plants could result from the overall effects of several bioactive molecules on multiple targets that make it difficult to identify the specific biological activity of a phytochemical. Thus, in this study, we screened 54 phytochemicals that have been reported in-vitro and in-vivo to be neuroprotective against NDs, and several parameters important for drug design and development were evaluated.
One of the most crucial factors that limit the therapeutic applications of these phytochemicals for the treatment of NDs is the physicochemical properties. Thus, we have selected phytochemicals that exhibited a good pharmaceutical profile with 0 violation of the rule of five [ROF], and only 34 phytochemicals were selected. The second important criteria that were considered is the safety and toxicity profile; thus, phytochemicals classified as class 4 and above were chosen, and the selection included 27 phytochemicals that passed this criterion. Furthermore, since herb-drug interactions are as important as toxicity, we selected phytochemicals that exhibited no CYP enzymes inhibition, and phytochemicals are Aromatic-turmerone, Sulforaphane, Andrographolide, Piperlongumine, Apocynin, and 3H-1,2-dithiole-3-thione.
To conclude, natural products hold considerable promise for treating various NDs, even though numerous questions concerning their efficacy and safety remain unevaluated. After the screening of 54 phytochemicals with neuroprotective effects in microglia, we can draw a solid conclusion that Aromatic-turmerone, Sulforaphane, Andrographolide, Piperlongumine, Apocynin, and 3H-1,2-dithiole-3-thione are the most promising compounds that could be considered when designing novel biologically active anti-inflammatory agents to treat neurodegenerative diseases via targeting microglial polarization. These six compounds demonstrated excellent ADME properties, safety profile, and promising anti-inflammatory activity that could be utilized as lead compounds for further drug optimization and development.
Acknowledgments
The authors want to express their sincerest gratitude to the College of Pharmacy (COP) at King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) for their continued support.
Abbreviation
| AD | Alzheimer’s disease |
| ADE | amyloid degrading enzymes |
| AEP | asparagine endopeptidase |
| AKT/GSK | 3β: protein kinase B/glycogen synthase kinase-3beta |
| AMPK/SIRT1 | Adenosine monophosphate-activated protein kinase [AMPK]/NAD-dependent deacetylase sirtuin-1 [SIRT1] |
| AP-1 | activator protein-1 |
| ARE | antioxidant response element |
| ARG1 | Arginase-1 |
| Aβ | amyloid-beta |
| BBB | blood-brain barrier penetration |
| CB2R | cannabinoid receptor 2 |
| CD206 | macrophage mannose receptor |
| CNTFRα | ciliary neurotrophic factor receptor alpha |
| COX2 | Cyclooxygenase-2 |
| CSFs | colony-stimulating factors |
| CYP450 | cytochrome P450 |
| ERK1/2 | Extracellular signal-regulated kinase |
| GABA-B | γ-aminobutyric acid type B |
| GPCRs | G protein-coupled receptors |
| HO-1 | heme oxygenase-1 |
| IDE | insulin-degrading enzyme |
| IFNs | interferons |
| IKK | IκB kinase |
| IL | interleukins |
| INF-γ/LPS | interferon-gamma combined with lipopolysaccharide |
| iNOS | Inducible nitric oxide synthase |
| IκB | NF-κB inhibitor |
| JAK-STAT | Janus kinase signal transducer and activator of transcription |
| JNK | c-Jun N-terminal kinase |
| logS | high solubility |
| LOO-CV | leave-one-out cross-validation |
| LPS | Lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| MS | Multiple sclerosis |
| mTOR | mammalian target of rapamycin |
| MW | molecular weight |
| NDs | neurodegeneration diseases |
| NEP | neprilysin |
| NF-κB | nuclear factor-kappa-B |
| NFT | neurofibrillary tangles |
| NLRP3 | NLR family pyrin domain containing 3 |
| NLRs | nucleotide-binding oligomerization domain [nod]-like receptors |
| NO | nitric oxide |
| NOX2 | nicotinamide adenine dinucleotide phosphate [NADPH] oxidase-2 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| P-gp | P-glycoprotein |
| Pa:Pi | active, inactive ratio |
| PASS | predict the activity spectra of substances |
| PD | Parkinson’s disease |
| PGE2 | prostaglandin E2 |
| PI3K/Akt | phosphatidylinositol-3-Kinase and Protein/Kinase B |
| PIKKs | phosphatidylinositol 3-kinase-related kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| PRRs | pattern-recognition receptors |
| ROS | reactive oxygen species |
| SMILES | simplified molecular-input line-entry system |
| SRC | non-receptor protein tyrosine kinase |
| STATs | signal transducer and activator of transcription |
| TGF-β | transforming growth factor-beta |
| TLRs | toll-like receptors |
| TNF-α | tumor necrosis factor-α |
| TREMs | triggering receptor expressed on myeloid cells |
| TRP | potential transient receptors |
| WBC | white blood cells |
| α-SYN | alpha-synuclein |
Author Contributions
Conceptualization, S.S.A. and R.S.S.; Methodology, S.S.A., R.S.S. and G.M.A.; Software, N.A.A., K.M.K. and D.A.A.; Validation, N.A.A., K.K, and D.A.A.; Formal Analysis, S.S.A., R.S.S. and G.M.A.; Investigation, N.A.A., K.M.K. and D.A.A.; Resources, S.S.A. and R.S.S.; Data Curation, S.S.A., N.A.A., K.M.K. and D.A.A.; Writing–Original Draft Preparation, N.A.A., K.M.K. and D.A.A.; Writing–Review & Editing, S.S.A.; Visualization, S.S.A., R.S.S. and G.M.A.; Supervision, S.S.A. and R.S.S.; Project Administration, S.S.A., R.S.S. and Funding Acquisition, G.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by KAIMRC with funding number SP21R/463/12.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. [(accessed on 14 December 2017)];Oncotarget. 2018 9:7204. doi: 10.18632/oncotarget.23208. Available online: https://www.oncotarget.com/article/23208/text/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. [(accessed on 26 December 2021)];Clin. Exp. Immunol. 2017 147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan C., Ding A. Nonresolving Inflammation. Volume 140. Elsevier; Amsterdam, The Netherlands: 2010. [(accessed on 31 May 2021)]. pp. 871–882. Available online: https://pubmed.ncbi.nlm.nih.gov/20303877/ [DOI] [PubMed] [Google Scholar]
- 4.Allison M.C., Howatson A.G., Torrance C.J., Lee F.D., Russell R.I. Gastrointestinal Damage Associated with the Use of Nonsteroidal Antiinflammatory Drugs. [(accessed on 13 February 2021)];N. Engl. J. Med. 1992 327:749–754. doi: 10.1056/NEJM199209103271101. Available online: https://pubmed.ncbi.nlm.nih.gov/1501650/ [DOI] [PubMed] [Google Scholar]
- 5.McGeer P.L., Itagaki S., Boyes B.E., McGeer E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. [(accessed on 26 December 2021)];Neurology. 1988 38:1285–1291. doi: 10.1212/WNL.38.8.1285. Available online: https://pubmed.ncbi.nlm.nih.gov/3399080/ [DOI] [PubMed] [Google Scholar]
- 6.Banati R.B., Daniel S.E., Blunt S.B. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. [(accessed on 26 December 2021)];Mov. Disord. 1998 13:221–227. doi: 10.1002/mds.870130205. Available online: https://pubmed.ncbi.nlm.nih.gov/9539333/ [DOI] [PubMed] [Google Scholar]
- 7.Raine C.S. Multiple Sclerosis: Immune System Molecule Expression in the Central Nervous System. [(accessed on 26 December 2021)];J. Neuropathol. Exp. Neurol. 1994 53:328–337. doi: 10.1097/00005072-199407000-00002. Available online: https://pubmed.ncbi.nlm.nih.gov/8021705/ [DOI] [PubMed] [Google Scholar]
- 8.Zhonghua L., Dong W., Sheng Z., Ye B., Za Z., Zhonghua L., Weisheng Z.Z. Chinese Journal of Industrial Hygiene and Occupational Diseases Publons. [(accessed on 26 December 2021)]. Available online: https://publons.com/journal/18134/zhonghua-lao-dong-wei-sheng-zhi-ye-bing-za-zhi-zho/
- 9.Magni P., Ruscica M., Dozio E., Rizzi E., Beretta G., Facino R.M. Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-α and NF-κB nuclear translocation in BV-2 microglia. Phyther. Res. 2012;26:1405–1409. doi: 10.1002/ptr.3732. [DOI] [PubMed] [Google Scholar]
- 10.Griffin W.S.T., Stanley L.C., Ling C., White L., MacLeod V., Perrot L.J., White C.L., III, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. [(accessed on 26 December 2021)];Proc. Natl. Acad. Sci. USA. 1989 86:7611–7615. doi: 10.1073/pnas.86.19.7611. Available online: https://pubmed.ncbi.nlm.nih.gov/2529544/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Näslund J., Haroutunian V., Mohs R., Davis K.L., Davies P., Greengard P., Buxbaum J.D. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. [(accessed on 26 December 2021)];JAMA. 2000 283:1571–1577. doi: 10.1001/jama.283.12.1571. Available online: https://pubmed.ncbi.nlm.nih.gov/10735393/ [DOI] [PubMed] [Google Scholar]
- 12.Jia Y., Zhao G., Jia J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. [(accessed on 3 April 2021)];J. Ethnopharmacol. 2008 120:181–189. doi: 10.1016/j.jep.2008.08.008. Available online: https://pubmed.ncbi.nlm.nih.gov/18773950/ [DOI] [PubMed] [Google Scholar]
- 13.Hale C., Véniant M., Wang Z., Chen M., McCormick J., Cupples R., Hickman D., Min X., Sudom A., Xu H., et al. Structural characterization and pharmacodynamic effects of an orally active 11beta-hydroxysteroid dehydrogenase type 1 inhibitor. [(accessed on 26 December 2021)];Chem. Biol. Drug. Des. 2008 71:36–44. doi: 10.1111/j.1747-0285.2007.00603.x. Available online: https://pubmed.ncbi.nlm.nih.gov/18069989/ [DOI] [PubMed] [Google Scholar]
- 14.Lee D.C., Rizer J., Selenica M.-L.B., Reid P., Kraft C., Johnson A., Blair L., Gordon M.N., Dickey C., Morgan D. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. [(accessed on 26 December 2021)];J. Neuroinflamm. 2010 7:56. doi: 10.1186/1742-2094-7-56. Available online: https://jneuroinflammation.biomedcentral.com/articles/10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass C.K., Saijo K., Winner B., Marchetto M.C., Gage F.H. Mechanisms underlying inflammation in neurodegeneration. [(accessed on 26 December 2021)];Cell. 2010 140:918–934. doi: 10.1016/j.cell.2010.02.016. Available online: https://pubmed.ncbi.nlm.nih.gov/20303880/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braak H., del Tredici K., Rüb U., de Vos R.J., Steur E.N.H., Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. [(accessed on 26 December 2021)];Neurobiol Aging. 2003 24:197–211. doi: 10.1016/S0197-4580(02)00065-9. Available online: https://pubmed.ncbi.nlm.nih.gov/12498954/ [DOI] [PubMed] [Google Scholar]
- 17.Rocha N.P., de Miranda A.S., Teixeira A.L. Insights into neuroinflammation in Parkinson’s disease: From biomarkers to anti-inflammatory based therapies. Biomed. Res. Int. 2015;2015:628192. doi: 10.1155/2015/628192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouchi Y., Yoshikawa E., Sekine Y., Futatsubashi M., Kanno T., Ogusu T., Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. [(accessed on 26 December 2021)];Ann. Neurol. 2005 57:168–175. doi: 10.1002/ana.20338. Available online: https://pubmed.ncbi.nlm.nih.gov/15668962/ [DOI] [PubMed] [Google Scholar]
- 19.Frohman E.M., Racke M.K., Raine C.S. Multiple Sclerosis—The Plaque and Its Pathogenesis. [(accessed on 26 December 2021)];New Engl. J. Med. 2006 354:942–955. doi: 10.1056/NEJMra052130. Available online: https://www.nejm.org/doi/10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 20.Bsibsi M., Peferoen L.A.N., Holtman I.R., Nacken P.J., Gerritsen W.H., Witte M.E., van Horssen J., Eggen B.J.L., van der Valk P., Amor S., et al. Demyelination during multiple sclerosis is associated with combined activation of microglia/macrophages by IFN-γ and alpha B-crystallin. [(accessed on 26 December 2021)];Acta Neuropathol. 2014 128:215–229. doi: 10.1007/s00401-014-1317-8. Available online: https://pubmed.ncbi.nlm.nih.gov/24997049/ [DOI] [PubMed] [Google Scholar]
- 21.Genain C.P., Cannella B., Hauser S.L., Raine C.S. Identification of autoantibodies associated with myelin damage in multiple sclerosis. [(accessed on 26 December 2021)];Nat. Med. 1999 5:170–175. doi: 10.1038/5532. Available online: https://pubmed.ncbi.nlm.nih.gov/9930864/ [DOI] [PubMed] [Google Scholar]
- 22.Askari V.R., Fereydouni N., Baradaran R.V., Askari N., Sahebkar A.H., Rahmanian-Devin P., Samzadeh-Kermani A. β-Amyrin, the cannabinoid receptors agonist, abrogates mice brain microglial cells inflammation induced by lipopolysaccharide/interferon-γ and regulates Mφ 1/Mφ 2 balances. [(accessed on 26 December 2021)];Biomed. Pharmacother. 2018 101:438–446. doi: 10.1016/j.biopha.2018.02.098. Available online: https://pubmed.ncbi.nlm.nih.gov/29501766/ [DOI] [PubMed] [Google Scholar]
- 23.Correa F., Hernangómez M., Mestre L., Loría F., Spagnolo A., Docagne F., Guaza C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-kappaB. [(accessed on 26 December 2021)];Glia. 2010 58:135–147. doi: 10.1002/glia.20907. Available online: https://pubmed.ncbi.nlm.nih.gov/19565660/ [DOI] [PubMed] [Google Scholar]
- 24.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. [(accessed on 26 December 2021)];F1000Prime Rep. 2014 6:13. doi: 10.12703/P6-13. Available online: http://pmc/articles/PMC3944738/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay T.L., Carrier M., Tremblay M.È. Physiology of microglia. Adv. Exp. Med. Biol. 2019;1175:129–148. doi: 10.1007/978-981-13-9913-8_6. [DOI] [PubMed] [Google Scholar]
- 26.Solanki I., Parihar P., Parihar M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. [(accessed on 26 December 2021)];Neurochem. Int. 2016 95:100–108. doi: 10.1016/j.neuint.2015.11.001. Available online: https://pubmed.ncbi.nlm.nih.gov/26550708/ [DOI] [PubMed] [Google Scholar]
- 27.Durães F., Pinto M., Sousa E. Old Drugs as New Treatments for Neurodegenerative Diseases. [(accessed on 26 December 2021)];Pharmaceuticals. 2018 11:44. doi: 10.3390/ph11020044. Available online: https://www.mdpi.com/1424-8247/11/2/44/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beard C.M., Kokmen E., O’Brien P.C., Kurland L.T. The prevalence of dementia is changing over time in Rochester, Minnesota. [(accessed on 26 December 2021)];Neurology. 1995 45:75–79. doi: 10.1212/WNL.45.1.75. Available online: https://pubmed.ncbi.nlm.nih.gov/7824140/ [DOI] [PubMed] [Google Scholar]
- 29.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. [(accessed on 26 December 2021)];Am. J. Public. Health. 1998 88:1337–1342. doi: 10.2105/AJPH.88.9.1337. Available online: https://pubmed.ncbi.nlm.nih.gov/9736873/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The Tacrine Study Group—PubMed A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. [(accessed on 26 December 2021)];JAMA. 1994 27:985–991. Available online: https://pubmed.ncbi.nlm.nih.gov/8139083/ [PubMed] [Google Scholar]
- 31.Larochelle A., Bellavance M.A., Rivest S. Role of adaptor protein MyD88 in TLR-mediated preconditioning and neuroprotection after acute excitotoxicity. Brain Behav. Immun. 2015;46:221–231. doi: 10.1016/j.bbi.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Bachiller S., Jiménez-Ferrer I., Paulus A., Yang Y., Swanberg M., Deierborg T., Boza-Serrano A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta-Mol. Cell Res. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez-Gómez J.A., Kavanagh E., Engskog-Vlachos P., Engskog M.K.R., Herrera A.J., Espinosa-Oliva A.M. Microglia: Agents of the CNS Pro-Inflammatory Response. [(accessed on 26 December 2021)];Cells. 2020 9:1717. doi: 10.3390/cells9071717. Available online: https://www.mdpi.com/2073-4409/9/7/1717/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He P., Yan S., Zheng J., Gao Y., Zhang S., Liu Z., Liu X., Xiao C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. [(accessed on 26 December 2021)];J. Agric. Food Chem. 2018 66:10205–10214. doi: 10.1021/acs.jafc.8b03731. Available online: https://pubmed.ncbi.nlm.nih.gov/30208700/ [DOI] [PubMed] [Google Scholar]
- 36.Li Q., Chen L., Liu X., Li X., Cao Y., Bai Y., Qi F. Pterostilbene inhibits amyloid-β-induced neuroinflammation in a microglia cell line by inactivating the NLRP3/caspase-1 inflammasome pathway. [(accessed on 26 December 2021)];J. Cell Biochem. 2018 119:7053–7062. doi: 10.1002/jcb.27023. Available online: https://pubmed.ncbi.nlm.nih.gov/29737568/ [DOI] [PubMed] [Google Scholar]
- 37.Yang H., Chen Y., Yu L., Xu Y. Esculentoside A exerts anti-inflammatory activity in microglial cells. Int. Immunopharmacol. 2017;51:148–157. doi: 10.1016/j.intimp.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Shi X., Zheng Z., Li J., Xiao Z., Qi W., Zhang A., Wu Q., Fang Y. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. [(accessed on 26 December 2021)];Neurosci. Lett. 2015 594:105–110. doi: 10.1016/j.neulet.2015.03.045. Available online: https://www.meta.org/papers/curcumin-inhibits-a-induced-microglial/25818332. [DOI] [PubMed] [Google Scholar]
- 39.Jin M., Park S.Y., Shen Q., Lai Y., Ou X., Mao Z., Lin D., Yu Y., Zhang W. Anti-neuroinflammatory effect of curcumin on Pam3CSK4-stimulated microglial cells. [(accessed on 26 December 2021)];Int. J. Mol. Med. 2018 41:521–530. doi: 10.3892/ijmm.2017.3217. Available online: https://pubmed.ncbi.nlm.nih.gov/29115589/ [DOI] [PubMed] [Google Scholar]
- 40.Liu Z.-J., Li Z.-H., Liu L., Tang W.-X., Wang Y., Dong M.-R., Xiao C. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. [(accessed on 26 December 2021)];Front. Pharmacol. 2016 7:1–12. doi: 10.3389/fphar.2016.00261. Available online: https://pubmed.ncbi.nlm.nih.gov/27594837/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park S.Y., Jin M.L., Kim Y.H., Kim Y., Lee S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. [(accessed on 26 December 2021)];Int. Immunopharmacol. 2012 14:13–20. doi: 10.1016/j.intimp.2012.06.003. Available online: https://pubmed.ncbi.nlm.nih.gov/22728094/ [DOI] [PubMed] [Google Scholar]
- 42.Park S.Y., Kim Y.H., Kim Y., Lee S.J. Aromatic-turmerone’s anti-inflammatory effects in microglial cells are mediated by protein kinase A and heme oxygenase-1 signaling. [(accessed on 26 December 2021)];Neurochem. Int. 2012 61:767–777. doi: 10.1016/j.neuint.2012.06.020. Available online: https://pubmed.ncbi.nlm.nih.gov/22766494/ [DOI] [PubMed] [Google Scholar]
- 43.Chen M., Chang Y.Y., Huang S., Xiao L.H., Zhou W., Zhang L.Y., Li C., Zhou R.P., Tang J., Lin L., et al. Aromatic-Turmerone Attenuates LPS-Induced Neuroinflammation and Consequent Memory Impairment by Targeting TLR4-Dependent Signaling Pathway. [(accessed on 26 December 2021)];Mol. Nutr. Food Res. 2018 62:1700281. doi: 10.1002/mnfr.201700281. Available online: https://pubmed.ncbi.nlm.nih.gov/28849618/ [DOI] [PubMed] [Google Scholar]
- 44.Candelario-Jalil E., de Oliveira A.C.P., Gräf S., Bhatia H.S., Hüll M., Muñoz E., Fiebich B.L. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. [(accessed on 26 December 2021)];J. Neuroinflamm. 2007 4:25. doi: 10.1186/1742-2094-4-25. Available online: https://pmc/articles/PMC2100038/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Capiralla H., Vingtdeux V., Zhao H., Sankowski R., Al-Abed Y., Davies P., Marambaud P. Resveratrol mitigates lipopolysaccharide- and Aβ-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. [(accessed on 26 December 2021)];J. Neurochem. 2012 120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. Available online: https://pubmed.ncbi.nlm.nih.gov/22118570/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou Y., Xie G., Miao F., Ding L., Mou Y., Wang L., Wu C. Pterostilbene attenuates lipopolysaccharide-induced learning and memory impairment possibly via inhibiting microglia activation and protecting neuronal injury in mice. [(accessed on 26 December 2021)];Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014 54:92–102. doi: 10.1016/j.pnpbp.2014.03.015. Available online: https://pubmed.ncbi.nlm.nih.gov/24709550/ [DOI] [PubMed] [Google Scholar]
- 47.Subedi L., Lee J.H., Yumnam S., Ji E., Kim S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells. 2019;8:194. doi: 10.3390/cells8020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou T.T., Yang H.Y., Wang W., Wu Q.Q., Tian Y.R., Jia J.P. Sulforaphane Inhibits the Generation of Amyloid-β Oligomer and Promotes Spatial Learning and Memory in Alzheimer’s Disease [PS1V97L] Transgenic Mice. [(accessed on 26 December 2021)];J. Alzheimers Dis. 2018 62:1803–1813. doi: 10.3233/JAD-171110. Available online: https://pubmed.ncbi.nlm.nih.gov/29614663/ [DOI] [PubMed] [Google Scholar]
- 49.Kim C.Y., Lee C., Park G.H., Jang J.H. Neuroprotective effect of epigallocatechin-3-gallate against beta-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. [(accessed on 26 December 2021)];Arch. Pharm. Res. 2009 32:869–881. doi: 10.1007/s12272-009-1609-z. Available online: https://pubmed.ncbi.nlm.nih.gov/19557365/ [DOI] [PubMed] [Google Scholar]
- 50.Cheng-Chung W.J., Huang H.C., Chen W.J., Huang C.N., Peng C.H., Lin C.L. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13.31 microglia. [(accessed on 26 December 2021)];Eur. J. Pharmacol. 2016 770:16–24. doi: 10.1016/j.ejphar.2015.11.048. Available online: https://pubmed.ncbi.nlm.nih.gov/26643169/ [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. [(accessed on 26 December 2021)];J. Nutr. Biochem. 2013 24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. Available online: https://pubmed.ncbi.nlm.nih.gov/22959056/ [DOI] [PubMed] [Google Scholar]
- 52.Seo J.Y., Pyo E., An J.P., Kim J., Sung S.H., Oh W.K. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by A β42 through Nrf2-Related Inflammatory Response. [(accessed on 26 December 2021)];Mediat. Inflamm. 2017 2017:5906189. doi: 10.1155/2017/5906189. Available online: https://pubmed.ncbi.nlm.nih.gov/28373747/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang T., Liu B., Zhang W., Wilson B., Hong J.S. Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. [(accessed on 26 December 2021)];J. Pharmacol. Exp. Ther. 2004 308:975–983. doi: 10.1124/jpet.103.059683. Available online: https://pubmed.ncbi.nlm.nih.gov/14718612/ [DOI] [PubMed] [Google Scholar]
- 54.Yang R., Liu S., Zhou J., Bu S., Zhang J. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK MAPK signaling pathway. [(accessed on 26 December 2021)];Immunopharmacol. Immunotoxicol. 2017 39:276–284. doi: 10.1080/08923973.2017.1344989. Available online: https://pubmed.ncbi.nlm.nih.gov/28669260/ [DOI] [PubMed] [Google Scholar]
- 55.Liu H., Wang J., Wang J., Wang P., Xue Y. Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling pathways. Brain Res. 2015;1618:149–158. doi: 10.1016/j.brainres.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 56.Luo X.Q., Li A., Yang X., Xiao X., Hu R., Wang T.W., Dong Z. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2. [(accessed on 26 December 2021)];Chin. Med. 2018 13:1–17. doi: 10.1186/s13020-018-0173-1. Available online: https://pubmed.ncbi.nlm.nih.gov/29560022/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Askari V.R., Shafiee-Nick R. The protective effects of β-caryophyllene on LPS-induced primary microglia M 1/M 2 imbalance: A mechanistic evaluation. [(accessed on 26 December 2021)];Life Sci. 2019 219:40–73. doi: 10.1016/j.lfs.2018.12.059. Available online: https://pubmed.ncbi.nlm.nih.gov/30620895/ [DOI] [PubMed] [Google Scholar]
- 58.Cheng Y., Dong Z., Liu S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. [(accessed on 26 December 2021)];Pharmacology. 2014 94:1–12. doi: 10.1159/000362689. Available online: https://pubmed.ncbi.nlm.nih.gov/25171128/ [DOI] [PubMed] [Google Scholar]
- 59.Zhang Z.Y., Daniels R., Schluesener H.J. Oridonin ameliorates neuropathological changes and behavioural deficits in a mouse model of cerebral amyloidosis. [(accessed on 26 December 2021)];J. Cell Mol. Med. 2013 17:1566–1576. doi: 10.1111/jcmm.12124. Available online: https://pubmed.ncbi.nlm.nih.gov/24034629/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S., Yang H., Yu L., Jin J., Qian L., Zhao H., Zhu X. Oridonin attenuates Aβ1-42-induced neuroinflammation and inhibits NF-κB pathway. [(accessed on 26 December 2021)];PLoS ONE. 2014 9:e104745. doi: 10.1371/journal.pone.0104745. Available online: https://pubmed.ncbi.nlm.nih.gov/25121593/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jing N., Li X. Dihydromyricetin Attenuates Inflammation through TLR4/NF-kappaB Pathway. [(accessed on 26 December 2021)];Open Med. 2019 14:719–725. doi: 10.1515/med-2019-0083. Available online: https://pubmed.ncbi.nlm.nih.gov/31572805/ [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Sun P., Yin J.-B., Liu L.-H., Guo J., Wang S.-H., Qu C.-H., Wang C.-X. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. [(accessed on 26 December 2021)];Biosci. Rep. 2019 39:BSR20180902. doi: 10.1042/BSR20180902. Available online: https://pmc/articles/PMC6328867/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feng J., Wang J., Du Y., Liu Y., Zhang W., Chen J., Liu Y., Zheng M., Wang K., He G. Dihydromyricetin inhibits microglial activation and neuroinflammation by suppressing NLRP3 inflammasome activation in APP/PS1 transgenic mice. [(accessed on 26 December 2021)];CNS Neurosci. Ther. 2018 24:1207–1218. doi: 10.1111/cns.12983. Available online: https://pubmed.ncbi.nlm.nih.gov/29869390/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee Y.J., Choi D.Y., Choi I.S., Kim K.H., Kim Y.H., Kim H.M., Hong J.T. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. [(accessed on 26 December 2021)];J. Neuroinflamm. 2012 9:35. doi: 10.1186/1742-2094-9-35. Available online: https://pubmed.ncbi.nlm.nih.gov/22339795/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin G., Bai D., Yin S., Yang Z., Zou D., Zhang Z., Li X., Sun Y., Zhu Q. Silibinin rescues learning and memory deficits by attenuating microglia activation and preventing neuroinflammatory reactions in SAMP8 mice. [(accessed on 26 December 2021)];Neurosci. Lett. 2016 629:256–261. doi: 10.1016/j.neulet.2016.06.008. Available online: https://pubmed.ncbi.nlm.nih.gov/27276653/ [DOI] [PubMed] [Google Scholar]
- 66.Ho S.C., Kuo C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel [Citri reticulatae pericarpium] [(accessed on 26 December 2021)];Food Chem. Toxicol. 2014 71:176–182. doi: 10.1016/j.fct.2014.06.014. Available online: https://pubmed.ncbi.nlm.nih.gov/24955543/ [DOI] [PubMed] [Google Scholar]
- 67.Li C., Zug C., Qu H., Schluesener H., Zhang Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. [(accessed on 26 December 2021)];Behav. Brain Res. 2015 281:32–42. doi: 10.1016/j.bbr.2014.12.012. Available online: https://pubmed.ncbi.nlm.nih.gov/25510196/ [DOI] [PubMed] [Google Scholar]
- 68.Justin-Thenmozhi A., Dhivya B.M., Kiruthika R., Manivasagam T., Borah A. Essa MM. Attenuation of Aluminum Chloride-Induced Neuroinflammation and Caspase Activation Through the AKT/GSK-3β Pathway by Hesperidin in Wistar Rats. [(accessed on 26 December 2021)];Neurotox. Res. 2018 34:463–476. doi: 10.1007/s12640-018-9904-4. Available online: https://pubmed.ncbi.nlm.nih.gov/29687202/ [DOI] [PubMed] [Google Scholar]
- 69.Jiao J., Xue B., Zhang L., Gong Y., Li K., Wang H. Triptolide inhibits amyloid-beta1-42-induced TNF-alpha and IL-1beta production in cultured rat microglia. [(accessed on 26 December 2021)];J. Neuroimmunol. 2008 205:32–36. doi: 10.1016/j.jneuroim.2008.08.006. Available online: https://pubmed.ncbi.nlm.nih.gov/19004508/ [DOI] [PubMed] [Google Scholar]
- 70.Cui Y.-Q., Wang Q., Zhang D.-M., Wang J.-Y., Xiao B., Zheng Y., Wang X.M. Triptolide Rescues Spatial Memory Deficits and Amyloid-β Aggregation Accompanied by Inhibition of Inflammatory Responses and MAPKs Activity in APP/PS1 Transgenic Mice. [(accessed on 26 December 2021)];Curr. Alzheimer Res. 2016 13:288–296. doi: 10.2174/156720501303160217122803. Available online: https://pubmed.ncbi.nlm.nih.gov/26906357/ [DOI] [PubMed] [Google Scholar]
- 71.Qi Y., Zou L.B., Wang L.H., Jin G., Pan J.J., Chi T.Y., Ji X.F. Xanthoceraside inhibits pro-inflammatory cytokine expression in Aβ25-35/IFN-γ-stimulated microglia through the TLR2 receptor, MyD88, nuclear factor-κB, and mitogen-activated protein kinase signaling pathways. [(accessed on 26 December 2021)];J. Pharmacol. Sci. 2013 122:305–317. doi: 10.1254/jphs.13031FP. Available online: https://www.researchgate.net/publication/256076333_Xanthoceraside_Inhibits_Pro-inflammatory_Cytokine_Expression_in_Ab25-35IFN-g-Stimulated_Microglia_Through_the_TLR2_Receptor_MyD88_Nuclear_Factor-kB_and_Mitogen-Activated_Protein_Kinase_Signaling_Pathw. [DOI] [PubMed] [Google Scholar]
- 72.Zhou H., Tai J., Xu H., Lu X., Meng D. Xanthoceraside could ameliorate Alzheimer’s disease symptoms of rats by affecting the gut microbiota composition and modulating the endogenous metabolite levels. Front. Pharmacol. 2019;10:1035. doi: 10.3389/fphar.2019.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim N., Do J., Bae J.S., Jin H.K., Kim J.H., Inn K.S., Lee J.K. Piperlongumine inhibits neuroinflammation via regulating NF-κB signaling pathways in lipopolysaccharide-stimulated BV2 microglia cells. [(accessed on 26 December 2021)];J. Pharmacol. Sci. 2018 137:195–201. doi: 10.1016/j.jphs.2018.06.004. Available online: https://pubmed.ncbi.nlm.nih.gov/29970291/ [DOI] [PubMed] [Google Scholar]
- 74.Gu S.M., Lee H.P., Ham Y.W., Son D.J., Kim H.Y., Oh K.W., Hong J.T. Piperlongumine Improves Lipopolysaccharide-Induced Amyloidogenesis by Suppressing NF-KappaB Pathway. NeuroMolecular Med. 2018;20:312–327. doi: 10.1007/s12017-018-8495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang H., Wang S., Yu L., Zhu X., Xu Y. Esculentoside A suppresses Aβ [1–42]-induced neuroinflammation by down-regulating MAPKs pathways in vivo. [(accessed on 26 December 2021)];Neurol. Res. 2015 37:859–866. doi: 10.1179/1743132815Y.0000000066. Available online: https://pubmed.ncbi.nlm.nih.gov/26104317/ [DOI] [PubMed] [Google Scholar]
- 76.Mrvová N., Škandík M., Kuniaková M., Račková L. Modulation of BV-2 microglia functions by novel quercetin pivaloyl ester. [(accessed on 26 December 2021)];Neurochem. Int. 2015 90:246–254. doi: 10.1016/j.neuint.2015.09.005. Available online: https://pubmed.ncbi.nlm.nih.gov/26386394/ [DOI] [PubMed] [Google Scholar]
- 77.Rezai-Zadeh K., Ehrhart J., Bai Y., Sanberg P.R., Bickford P., Tan J., Shytle R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. [(accessed on 26 December 2021)];J. Neuroinflamm. 2008 5:41. doi: 10.1186/1742-2094-5-41. Available online: https://pubmed.ncbi.nlm.nih.gov/18817573/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gilmore T.D. Introduction to NF-kappaB: Players, pathways, perspectives. [(accessed on 26 December 2021)];Oncogene. 2006 25:6680–6684. doi: 10.1038/sj.onc.1209954. Available online: https://pubmed.ncbi.nlm.nih.gov/17072321/ [DOI] [PubMed] [Google Scholar]
- 79.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. [(accessed on 26 December 2021)];Cold Spring Harb. Perspect. Biol. 2009 1 doi: 10.1101/cshperspect.a001651. Available online: https://pubmed.ncbi.nlm.nih.gov/20457564/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghosh S., Karin M. Missing pieces in the NF-kappaB puzzle. [(accessed on 26 December 2021)];Cell. 2002 109((Suppl. 1)):S81–S96. doi: 10.1016/S0092-8674(02)00703-1. Available online: https://pubmed.ncbi.nlm.nih.gov/11983155/ [DOI] [PubMed] [Google Scholar]
- 81.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-κB activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 82.Sunphenon EGCg [Epigallocatechin-Gallate] in the Early Stage of Alzheimer´s Disease—Full Text View—ClinicalTrials.Gov. [(accessed on 26 December 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00951834.
- 83.Kaminska B., Mota M., Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. [(accessed on 26 December 2021)];Biochim. Biophys. Acta. 2016 1862:339–351. doi: 10.1016/j.bbadis.2015.10.026. Available online: https://pubmed.ncbi.nlm.nih.gov/26524636/ [DOI] [PubMed] [Google Scholar]
- 84.Smith J.A., Das A., Ray S.K., Banik N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. [(accessed on 26 December 2021)];Brain Res. Bull. 2012 87:10–20. doi: 10.1016/j.brainresbull.2011.10.004. Available online: https://pubmed.ncbi.nlm.nih.gov/22024597/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Resveratrol for Alzheimer’s Disease—Full Text View—ClinicalTrials.Gov. [(accessed on 26 December 2021)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01504854.
- 86.Skerrett R., Malm T., Landreth G. Nuclear receptors in neurodegenerative diseases. [(accessed on 26 December 2021)];Neurobiol. Dis. 2014 72:104–116. doi: 10.1016/j.nbd.2014.05.019. Available online: https://pubmed.ncbi.nlm.nih.gov/24874548/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Q., Wu X., Yan S., Xie X., Fan Y., Zhang J., Peng C., You Z. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARγ-mediated alteration of microglial activation phenotypes. [(accessed on 26 December 2021)];J. Neuroinflammation. 2016 13:259. doi: 10.1186/s12974-016-0728-y. Available online: https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-016-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., Cole G.M. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. [(accessed on 26 December 2021)];Alzheimer Res. Ther. 2012 4:43. doi: 10.1186/alzrt146. Available online: https://clinicaltrials.gov/ct2/show/NCT00099710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baum L., Lam C.W.K., Cheung S.K.K., Kwok T., Lui V., Tsoh J., Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. [(accessed on 26 December 2021)];J. Clin. Psychopharmacol. 2008 28:110–113. doi: 10.1097/jcp.0b013e318160862c. Available online: https://clinicaltrials.gov/ct2/show/NCT00164749. [DOI] [PubMed] [Google Scholar]
- 90.Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M.P., Heneka M.T. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. [(accessed on 26 December 2021)];J. Neurosci. 2012 32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. Available online: https://pubmed.ncbi.nlm.nih.gov/23197723/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savage J.C., Jay T., Goduni E., Quigley C., Mariani M.M., Malm T., Ransohoff R.M., Lamb B.T., Landreth G.E. Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer’s disease. [(accessed on 26 December 2021)];J. Neurosci. 2015 35:6532–6543. doi: 10.1523/JNEUROSCI.4586-14.2015. Available online: https://pubmed.ncbi.nlm.nih.gov/25904803/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Y., Jiang S., Yan J., Li Y., Xin Z., Lin Y., Qu Y. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. [(accessed on 26 December 2021)];Cytokine Growth Factor Rev. 2015 26:47–57. doi: 10.1016/j.cytogfr.2014.09.002. Available online: https://pubmed.ncbi.nlm.nih.gov/25280871/ [DOI] [PubMed] [Google Scholar]
- 93.Holtman I.R., Skola D., Glass C.K. Transcriptional control of microglia phenotypes in health and disease. [(accessed on 26 December 2021)];J. Clin. Invest. 2017 127:3220–3229. doi: 10.1172/JCI90604. doi: 10.1172/JCI90604. Available online: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koistinaho M., Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. [(accessed on 26 December 2021)];Glia. 2002 40:175–183. doi: 10.1002/glia.10151. Available online: https://pubmed.ncbi.nlm.nih.gov/12379905/ [DOI] [PubMed] [Google Scholar]
- 95.Rothwell N.J., Hopkins S.J. Cytokines and the nervous system II: Actions and mechanisms of action. [(accessed on 26 December 2021)];Trends Neurosci. 1995 18:130–136. doi: 10.1016/0166-2236(95)93890-A. Available online: https://pubmed.ncbi.nlm.nih.gov/7754524/ [DOI] [PubMed] [Google Scholar]
- 96.Hanisch U.K. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 97.Sedgwick J.D., Riminton D.S., Cyster J.G., Körner H. Tumor necrosis factor: A master-regulator of leukocyte movement. [(accessed on 26 December 2021)];Immunol. Today. 2000 21:110–113. doi: 10.1016/S0167-5699(99)01573-X. Available online: https://pubmed.ncbi.nlm.nih.gov/10689296/ [DOI] [PubMed] [Google Scholar]
- 98.Hussain G., Rasul A., Anwar H., Sohail M.U., Kamran S.K.S., Baig S.M., Shabbir A. Epidemiological Data of Neurological Disorders in Pakistan and Neighboring Countries: A Review. [(accessed on 26 December 2021)];Pak. J. Neurol. Sci. 2017 12:52–70. Available online: https://ecommons.aku.edu/pjns/vol12/iss4/12. [Google Scholar]
- 99.Kaur R., Mehan S., Singh S. Understanding multifactorial architecture of Parkinson’s disease: Pathophysiology to management. [(accessed on 26 December 2021)];Neurol. Sci. 2019 40:13–23. doi: 10.1007/s10072-018-3585-x. Available online: https://pubmed.ncbi.nlm.nih.gov/30267336/ [DOI] [PubMed] [Google Scholar]
- 100.Thanvi B., Lo N., Robinson T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. [(accessed on 26 December 2021)];Postgrad. Med. J. 2007 83:384–388. doi: 10.1136/pgmj.2006.054759. Available online: https://pubmed.ncbi.nlm.nih.gov/17551069/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rui W., Li S., Xiao H., Xiao M., Shi J. Baicalein Attenuates Neuroinflammation by Inhibiting NLRP3/Caspase-1/GSDMD Pathway in MPTP-Induced Mice Model of Parkinson’s Disease. [(accessed on 26 December 2021)];Int. J. Neuropsychopharmacol. 2020 23:762–773. doi: 10.1093/ijnp/pyaa060. Available online: https://academic.oup.com/ijnp/article/23/11/762/5881996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan Z., Liang Z., Yang H., Pan Y., Zheng Y., Wang X. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J. Neuroinflamm. 2017;14:256. doi: 10.1186/s12974-017-1036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baek J.Y., Jeong J.Y., Kim K.I., Won S.-Y., Chung Y.C., Nam J., Cho E.J., Ahn T.-B., Bok E., Shin W.-H., et al. Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP+ Neurotoxicity. [(accessed on 26 December 2021)];Int. J. Mol. Sci. 2018 19:3543. doi: 10.3390/ijms19113543. Available online: https://pubmed.ncbi.nlm.nih.gov/30423807/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bok E., Chung Y.C., Kim K.S., Baik H.H., Shin W.H., Jin B.K. Modulation of M1/M2 polarization by capsaicin contributes to the survival of dopaminergic neurons in the lipopolysaccharide-lesioned substantia nigra in vivo. [(accessed on 26 December 2021)];Exp. Mol. Med. 2018 50:1–14. doi: 10.1038/s12276-018-0111-4. Available online: https://www.nature.com/articles/s12276-018-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung Y.C., Baek J.Y., Kim S.R., Ko H.W., Bok E., Shin W.-H., Won S.-Y., Jin B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. [(accessed on 26 December 2021)];Exp. Mol. Med. 2017 49:e298. doi: 10.1038/emm.2016.159. Available online: https://pubmed.ncbi.nlm.nih.gov/28255166/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim B., Koppula S., Kumar H., Park J.-Y., Kim I.-W., More S.V., Kim I.-S., Han S.-D., Kim S.-K., Yoon S.-H., et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. [(accessed on 26 December 2021)];Neuropharmacology. 2015 97:46–57. doi: 10.1016/j.neuropharm.2015.04.037. Available online: https://pubmed.ncbi.nlm.nih.gov/25983275/ [DOI] [PubMed] [Google Scholar]
- 107.Kim M.E., Park P.R., Na J.Y., Jung I., Cho J.H., Lee J.S. Anti-neuroinflammatory effects of galangin in LPS-stimulated BV-2 microglia through regulation of IL-1β production and the NF-κB signaling pathways. [(accessed on 26 December 2021)];Mol. Cell Biochem. 2019 451:145–153. doi: 10.1007/s11010-018-3401-1. Available online: https://pubmed.ncbi.nlm.nih.gov/29995265/ [DOI] [PubMed] [Google Scholar]
- 108.Chen G., Liu J., Jiang L., Ran X., He D., Li Y., Huang B., Wang W., Fu S. Galangin Reduces the Loss of Dopaminergic Neurons in an LPS-Evoked Model of Parkinson’s Disease in Rats. [(accessed on 26 December 2021)];Int. J. Mol. Sci. 2017 19:12. doi: 10.3390/ijms19010012. Available online: https://pubmed.ncbi.nlm.nih.gov/29267220/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang J., He C., Wu W.-Y., Chen F., Li W.-Z., Chen H.-Q., Yin Y.-Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. [(accessed on 26 December 2021)];Pharmacol. Biochem. Behav. 2015 138:96–103. doi: 10.1016/j.pbb.2015.09.013. Available online: https://pubmed.ncbi.nlm.nih.gov/26394281/ [DOI] [PubMed] [Google Scholar]
- 110.Zhang X., Yang Y., Du L., Zhang W., Du G. Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. [(accessed on 26 December 2021)];Int. Immunopharmacol. 2017 50:38–47. doi: 10.1016/j.intimp.2017.06.007. Available online: https://pubmed.ncbi.nlm.nih.gov/28623717/ [DOI] [PubMed] [Google Scholar]
- 111.Hou L., Sun F., Huang R., Sun W., Zhang D., Wang Q. Inhibition of NADPH oxidase by apocynin prevents learning and memory deficits in a mouse Parkinson’s disease model. [(accessed on 26 December 2021)];Redox. Biol. 2019 22:101134. doi: 10.1016/j.redox.2019.101134. Available online: https://pubmed.ncbi.nlm.nih.gov/30798073/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hu Z., Wang W., Ling J., Jiang C. α-Mangostin Inhibits α-Synuclein-Induced Microglial Neuroinflammation and Neurotoxicity. [(accessed on 26 December 2021)];Cell Mol. Neurobiol. 2016 36:811–820. doi: 10.1007/s10571-015-0264-9. Available online: https://pubmed.ncbi.nlm.nih.gov/27002719/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nava C.M., Acero G., Pedraza-Chaverri J., Fragoso G., Govezensky T., Gevorkian G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. [(accessed on 26 December 2021)];J. Neuroimmunol. 2016 297:20–27. doi: 10.1016/j.jneuroim.2016.05.008. Available online: https://pubmed.ncbi.nlm.nih.gov/27397072/ [DOI] [PubMed] [Google Scholar]
- 114.Huang B., Liu J., Ma D., Chen G., Wang W., Fu S. Myricetin prevents dopaminergic neurons from undergoing neuroinflammation-mediated degeneration in a lipopolysaccharide-induced Parkinson’s disease model. J. Funct. Foods. 2018;45:452–461. doi: 10.1016/j.jff.2018.04.018. [DOI] [Google Scholar]
- 115.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Myricitrin Ameliorates 6-Hydroxydopamine-Induced Dopaminergic Neuronal Loss in the Substantia Nigra of Mouse Brain. [(accessed on 26 December 2021)];J. Med. Food. 2016 19:374–382. doi: 10.1089/jmf.2015.3581. Available online: https://pubmed.ncbi.nlm.nih.gov/26991235/ [DOI] [PubMed] [Google Scholar]
- 116.Wang G.-Q., Li D.-D., Huang C., Lu D.-S., Zhang C., Zhou S.-Y., Liu J., Zhang F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 2018;10:441. doi: 10.3389/fnmol.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Cui Y., Wu J., Jung S.C., Park D.B., Maeng Y.H., Hong J.Y., Kim S.J., Lee S.R., Kim S.J., Kim S.J., et al. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. [(accessed on 26 December 2021)];Biol. Pharm. Bull. 2010 33:1814–1821. doi: 10.1248/bpb.33.1814. Available online: https://pubmed.ncbi.nlm.nih.gov/21048305/ [DOI] [PubMed] [Google Scholar]
- 118.Jeong K.H., Jeon M.-T., Kim H.D., Jung U.J., Jang M.C., Chu J.W., Yang S.J., Choi I.Y., Choi M.-S., Kim S.R. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. [(accessed on 26 December 2021)];J. Med. Food. 2015 18:409–414. doi: 10.1089/jmf.2014.3241. Available online: https://pubmed.ncbi.nlm.nih.gov/25325362/ [DOI] [PubMed] [Google Scholar]
- 119.Wang S., Jing H., Yang H., Liu Z., Guo H., Chai L., Hu L. Tanshinone I selectively suppresses pro-inflammatory genes expression in activated microglia and prevents nigrostriatal dopaminergic neurodegeneration in a mouse model of Parkinson’s disease. [(accessed on 26 December 2021)];J. Ethnopharmacol. 2015 164:247–255. doi: 10.1016/j.jep.2015.01.042. Available online: https://pubmed.ncbi.nlm.nih.gov/25666429/ [DOI] [PubMed] [Google Scholar]
- 120.Zhou J., Qu X.-D., Li Z.-Y., Ji W., Liu Q., Ma Y.-H., He J.-J. Salvianolic acid B attenuates toxin-induced neuronal damage via Nrf2-dependent glial cells-mediated protective activity in Parkinson’s disease models. [(accessed on 26 December 2021)];PLoS ONE. 2014 9:e101668. doi: 10.1371/journal.pone.0101668. Available online: https://pubmed.ncbi.nlm.nih.gov/24991814/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang B., Liu J., Ju C., Yang D., Chen G., Xu S., Fu S. Licochalcone A Prevents the Loss of Dopaminergic Neurons by Inhibiting Microglial Activation in Lipopolysaccharide [LPS]-Induced Parkinson’s Disease Models. [(accessed on 26 December 2021)];Int. J. Mol. Sci. 2017 18:2043. doi: 10.3390/ijms18102043. Available online: https://pubmed.ncbi.nlm.nih.gov/28937602/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jing H., Wang S., Wang M., Fu W., Zhang C., Xu D. Isobavachalcone Attenuates MPTP-Induced Parkinson’s Disease in Mice by Inhibition of Microglial Activation through NF-κB Pathway. [(accessed on 26 December 2021)];PLoS ONE. 2017 12:e0169560. doi: 10.1371/journal.pone.0169560. Available online: https://pubmed.ncbi.nlm.nih.gov/28060896/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma J., Hwang Y.K., Cho W.H., Han S.H., Hwang J.K., Han J.S. Macelignan attenuates activations of mitogen-activated protein kinases and nuclear factor kappa B induced by lipopolysaccharide in microglial cells. [(accessed on 26 December 2021)];Biol. Pharm. Bull. 2009 32:1085–1090. doi: 10.1248/bpb.32.1085. Available online: https://www.researchgate.net/publication/26254223_Macelignan_Attenuates_Activations_of_Mitogen-Activated_Protein_Kinases_and_Nuclear_Factor_kappa_B_Induced_by_Lipopolysaccharide_in_Microglial_Cells. [DOI] [PubMed] [Google Scholar]
- 124.Kiyofuji K., Kurauchi Y., Hisatsune A., Seki T., Mishima S., Katsuki H. A natural compound macelignan protects midbrain dopaminergic neurons from inflammatory degeneration via microglial arginase-1 expression. [(accessed on 26 December 2021)];Eur. J. Pharmacol. 2015 760:129–135. doi: 10.1016/j.ejphar.2015.04.021. Available online: https://pubmed.ncbi.nlm.nih.gov/25917324/ [DOI] [PubMed] [Google Scholar]
- 125.Gao X.-Q., Du Z.-R., Yuan L.-J., Zhang W.-D., Chen L., Teng J.-J., Wong M.S., Xie J.-X., Chen W.-F. Ginsenoside Rg1 Exerts Anti-inflammatory Effects via G Protein-Coupled Estrogen Receptor in Lipopolysaccharide-Induced Microglia Activation. Front. Neurosci. 2019;13:1168. doi: 10.3389/fnins.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J.Q., Zhao M., Zhang Z., Cui L.Y., Zhou X., Zhang W., Chen N.H. Rg1 improves LPS-induced Parkinsonian symptoms in mice via inhibition of NF-κB signaling and modulation of M1/M2 polarization. [(accessed on 26 December 2021)];Acta Pharmacol. Sin. 2020 41:523–534. doi: 10.1038/s41401-020-0358-x. Available online: https://www.researchgate.net/publication/340129734_Rg1_improves_LPS-induced_Parkinsonian_symptoms_in_mice_via_inhibition_of_NF-kB_signaling_and_modulation_of_M1M2_polarization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan X.-D., Chen X.-C., Zhu Y.-G., Zhang J., Huang T.-W., Chen L.-M., Ye Q.-Y., Huang H.-P. Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. [(accessed on 26 December 2021)];Biochem. Pharmacol. 2008 76:362–372. doi: 10.1016/j.bcp.2008.05.018. Available online: https://pubmed.ncbi.nlm.nih.gov/18602088/ [DOI] [PubMed] [Google Scholar]
- 128.Huang Y.-Y., Zhang Q., Zhang J.-N., Zhang Y.-N., Gu L., Yang H.-M., Xia N., Wang X.-M., Zhang H. Triptolide up-regulates metabotropic glutamate receptor 5 to inhibit microglia activation in the lipopolysaccharide-induced model of Parkinson’s disease. [(accessed on 26 December 2021)];Brain Behav. Immun. 2018 71:93–107. doi: 10.1016/j.bbi.2018.04.006. Available online: https://pubmed.ncbi.nlm.nih.gov/29649522/ [DOI] [PubMed] [Google Scholar]
- 129.Kim H.D., Jeong K.H., Jung U.J., Kim S.R. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J. Nutr. Biochem. 2016;28:140–146. doi: 10.1016/j.jnutbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 130.Leem E., Nam J., Jeon M.-T., Shin W.-H., Won S.-Y., Park S.-J., Choi M.-S., Jin B.K., Jung U.J., Kim S.R. Naringin protects the nigrostriatal dopaminergic projection through induction of GDNF in a neurotoxin model of Parkinson’s disease. [(accessed on 26 December 2021)];J. Nutr. Biochem. 2014 25:801–806. doi: 10.1016/j.jnutbio.2014.03.006. Available online: https://pubmed.ncbi.nlm.nih.gov/24797334/ [DOI] [PubMed] [Google Scholar]
- 131.Omeragic A., Kara-Yacoubian N., Kelschenbach J., Sahin C., Cummins C.L., Volsky D.J., Bendayan R. Peroxisome Proliferator-Activated Receptor-gamma agonists exhibit anti-inflammatory and antiviral effects in an EcoHIV mouse model. [(accessed on 26 December 2021)];Sci. Rep. 2019 9:9428. doi: 10.1038/s41598-019-45878-6. Available online: https://pubmed.ncbi.nlm.nih.gov/31263138/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim S.S., Lim J., Bang Y., Gal J., Lee S.U., Cho Y.C., Choi H.J. Licochalcone E activates Nrf2/antioxidant response element signaling pathway in both neuronal and microglial cells: Therapeutic relevance to neurodegenerative disease. J. Nutr. Biochem. 2012;23:1314–1323. doi: 10.1016/j.jnutbio.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 133.Sawada M., Imamura K., Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. [(accessed on 26 December 2021)];J. Neural. Transm. Suppl. 2006 :373–381. doi: 10.1007/978-3-211-45295-0_57. Available online: https://pubmed.ncbi.nlm.nih.gov/17017556/ [DOI] [PubMed] [Google Scholar]
- 134.Vlachou S., Nomikos G.G., Stephens D.N., Panagis G. Lack of evidence for appetitive effects of Delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. [(accessed on 26 December 2021)];Behav. Pharmacol. 2007 18:311–319. doi: 10.1097/FBP.0b013e3282186cf2. Available online: https://pubmed.ncbi.nlm.nih.gov/17551324/ [DOI] [PubMed] [Google Scholar]
- 135.Losseff N.A., Webb S.L., O’Riordan J.I., Page R., Wang L., Barker G.J., Thompson A.J. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. [(accessed on 26 December 2021)];Brain. 1996 119:701–708. doi: 10.1093/brain/119.3.701. Available online: https://pubmed.ncbi.nlm.nih.gov/8673483/ [DOI] [PubMed] [Google Scholar]
- 136.Rudick R.A. Disease-modifying drugs for relapsing-remitting multiple sclerosis and future directions for multiple sclerosis therapeutics. [(accessed on 26 December 2021)];Arch. Neurol. 1999 56:1079–1084. doi: 10.1001/archneur.56.9.1079. Available online: https://pubmed.ncbi.nlm.nih.gov/10488808/ [DOI] [PubMed] [Google Scholar]
- 137.Neuropsychological Effects of Interferon Beta-1a in Relapsing Multiple Sclerosis. Multiple Sclerosis Collaborative Research Group—PubMed. [(accessed on 26 December 2021)]; Available online: https://pubmed.ncbi.nlm.nih.gov/11117545/ [PubMed]
- 138.Kasper L.H., Reder A.T. Immunomodulatory activity of interferon-beta. [(accessed on 26 December 2021)];Ann. Clin. Transl. Neurol. 2014 1:622–631. doi: 10.1002/acn3.84. Available online: https://pubmed.ncbi.nlm.nih.gov/25356432/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Solaro C., Trabucco E., Messmer U.M. Pain and multiple sclerosis: Pathophysiology and treatment. [(accessed on 26 December 2021)];Curr. Neurol. Neurosci. Rep. 2013 13:320. doi: 10.1007/s11910-012-0320-5. Available online: https://pubmed.ncbi.nlm.nih.gov/23250765/ [DOI] [PubMed] [Google Scholar]
- 140.Fu X., Wang Y., Wang C., Wu H., Li J., Li M., Ma Q., Yang W. A mixed treatment comparison on efficacy and safety of treatments for spasticity caused by multiple sclerosis: A systematic review and network meta-analysis. undefined. Clin. Rehabil. 2018;32:713–721. doi: 10.1177/0269215517745348. [DOI] [PubMed] [Google Scholar]
- 141.Peng H., Li H., Sheehy A., Cullen P., Allaire N., Scannevin R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. [(accessed on 26 December 2021)];J. Neuroimmunol. 2016 299:35–44. doi: 10.1016/j.jneuroim.2016.08.006. Available online: https://pubmed.ncbi.nlm.nih.gov/27725119/ [DOI] [PubMed] [Google Scholar]
- 142.Qin S.Y., Du R.H., Yin S.S., Liu X.F., Xu G.L., Cao W. Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. [(accessed on 26 December 2021)];Inflamm. Res. 2015 64:537–548. doi: 10.1007/s00011-015-0834-9. Available online: https://pubmed.ncbi.nlm.nih.gov/26049867/ [DOI] [PubMed] [Google Scholar]
- 143.Foresti R., Bains S.K., Pitchumony T.S., De Castro Brás L.E., Drago F., Dubois-Randé J.-L., Bucolo C., Motterlini R. Small molecule activators of the Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. [(accessed on 26 December 2021)];Pharm. Res. 2013 76:132–148. doi: 10.1016/j.phrs.2013.07.010. Available online: https://pubmed.ncbi.nlm.nih.gov/23942037/ [DOI] [PubMed] [Google Scholar]
- 144.Zhou J., Cai W., Jin M., Xu J., Wang Y., Xiao Y., Hao L., Wang B., Zhang Y., Han J., et al. 18β-glycyrrhetinic acid suppresses experimental autoimmune encephalomyelitis through inhibition of microglia activation and promotion of remyelination. [(accessed on 26 December 2021)];Sci. Rep. 2015 5:13713. doi: 10.1038/srep13713. Available online: https://pubmed.ncbi.nlm.nih.gov/26329786/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takeuchi H., Wang J., Kawanokuchi J., Mitsuma N., Mizuno T., Suzumura A. Interferon-gamma induces microglial-activation-induced cell death: A hypothetical mechanism of relapse and remission in multiple sclerosis. [(accessed on 26 December 2021)];Neurobiol. Dis. 2006 22:33–39. doi: 10.1016/j.nbd.2005.09.014. Available online: https://pubmed.ncbi.nlm.nih.gov/16386911/ [DOI] [PubMed] [Google Scholar]
- 146.Sun Y., Chen H., Dai J., Wan Z., Xiong P., Xu Y., Han Z., Chai W., Gong F., Zheng F. Glycyrrhizin Protects Mice Against Experimental Autoimmune Encephalomyelitis by Inhibiting High-Mobility Group Box 1 [HMGB1] Expression and Neuronal HMGB1 Release. Front Immunol. 2018;9:1518. doi: 10.3389/fimmu.2018.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.A Study to Evaluate the Efficacy of Sativex in Relieving Symptoms of Spasticity Due to Multiple Sclerosis—Study Results—ClinicalTrials.Gov. [(accessed on 26 December 2021)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT01599234?view=results.
- 148.Rahimi A., Faizi M., Talebi F., Noorbakhsh F., Kahrizi F., Naderi N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. [(accessed on 26 December 2021)];Neuroscience. 2015 290:279–287. doi: 10.1016/j.neuroscience.2015.01.030. Available online: https://pubmed.ncbi.nlm.nih.gov/25637488/ [DOI] [PubMed] [Google Scholar]
- 149.Kronenberg J., Pars K., Brieskorn M., Prajeeth C.K., Heckers S., Schwenkenbecher P., Skripuletz T., Pul R., Pavlou A., Stangel M. Fumaric Acids Directly Influence Gene Expression of Neuroprotective Factors in Rodent Microglia. [(accessed on 26 December 2021)];Int. J. Mol. Sci. 2019 20:325. doi: 10.3390/ijms20020325. Available online: https://pubmed.ncbi.nlm.nih.gov/30650518/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuo P.-C., Brown D.A., Scofield B.A., Yu I.-C., Chang F.-L., Wang P.-Y., Yen J.-H. 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. [(accessed on 26 December 2021)];Brain Behav. Immun. 2016 57:173–186. doi: 10.1016/j.bbi.2016.03.015. Available online: https://pubmed.ncbi.nlm.nih.gov/27013356/ [DOI] [PubMed] [Google Scholar]
- 151.Zeng Y., Song C., Ding X., Ji X., Yi L., Zhu K. Baicalin reduces the severity of experimental autoimmune encephalomyelitis. [(accessed on 26 December 2021)];Braz. J. Med. Biol. Res. 2007 40:1003–1010. doi: 10.1590/S0100-879X2006005000115. Available online: https://www.researchgate.net/publication/6186224_Baicalin_reduces_the_severity_of_experimental_autoimmune_encephalomyelitis. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y., Li X., Ciric B., Abdolmohamad R., Gran B., Rostami A., Zhang G.-X. Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. [(accessed on 26 December 2021)];Sci. Rep. 2015 5:17407. doi: 10.1038/srep17407. Available online: https://pubmed.ncbi.nlm.nih.gov/26616302/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang M.-R., Zhang X.-J., Liu H.-C., Ma W.-D., Zhang M.-L., Zhang Y., Li X., Dou M.-M., Jing Y.-L., Chu Y.-J., et al. Matrine protects oligodendrocytes by inhibiting their apoptosis and enhancing mitochondrial autophagy. [(accessed on 26 December 2021)];Brain Res. Bull. 2019 153:30–38. doi: 10.1016/j.brainresbull.2019.08.006. Available online: https://pubmed.ncbi.nlm.nih.gov/31404585/ [DOI] [PubMed] [Google Scholar]
- 154.Martín R., Hernández M., Córdova C., Nieto M.L. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. [(accessed on 26 December 2021)];Br. J. Pharmacol. 2012 166:1708. doi: 10.1111/j.1476-5381.2012.01869.x. Available online: https://pmc/articles/PMC3419913/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martín R., Carvalho-Tavares J., Hernández M., Arnés M., Ruiz-Gutiérrez V., Nieto M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: A potential therapeutic role. [(accessed on 26 December 2021)];Biochem. Pharmacol. 2010 79:198–208. doi: 10.1016/j.bcp.2009.08.002. Available online: https://pubmed.ncbi.nlm.nih.gov/19679109/ [DOI] [PubMed] [Google Scholar]
- 156.He Y., Du M., Gao Y., Liu H., Wang H., Wu X., Wang Z. Astragaloside IV Attenuates Experimental Autoimmune Encephalomyelitis of Mice by Counteracting Oxidative Stress at Multiple Levels. [(accessed on 26 December 2021)];PLoS ONE. 2013 8:e76495. doi: 10.1371/journal.pone.0076495. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0076495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kamisli S., Ciftci O., Taslidere A.B., Turkmen N., Ozcan C. The beneficial effects of 18β-glycyrrhetinic acid on the experimental autoimmune encephalomyelitis [EAE] in C57BL/6 mouse model. [(accessed on 26 December 2021)];Immunopharmacol. Immunotoxicol. 2018 40:344–352. doi: 10.1080/08923973.2018.1490318. Available online: https://pubmed.ncbi.nlm.nih.gov/30052483/ [DOI] [PubMed] [Google Scholar]
- 158.Li X., Zhao L., Han J.-J., Zhang F., Liu S., Zhu L., Wang Z.-Z., Zhang G.-X., Zhang Y. Carnosol modulates Th17 cell differentiation and microglial switch in experimental autoimmune encephalomyelitis. Front. Immunol. 2018;9:1807. doi: 10.3389/fimmu.2018.01807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yan J., Yang X., Han D., Feng J. Tanshinone IIA attenuates experimental autoimmune encephalomyelitis in rats. [(accessed on 26 December 2021)];Mol. Med. Rep. 2016 14:1601–1609. doi: 10.3892/mmr.2016.5431. Available online: https://pubmed.ncbi.nlm.nih.gov/27357729/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fung S., Cherry A.E., Xu C., Stella N. Alkylindole-sensitive receptors modulate microglial cell migration and proliferation. [(accessed on 26 December 2021)];Glia. 2015 63:1797–1808. doi: 10.1002/glia.22845. Available online: https://pubmed.ncbi.nlm.nih.gov/25914169/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heng B.C., Aubel D., Fussenegger M. An overview of the diverse roles of G-protein coupled receptors [GPCRs] in the pathophysiology of various human diseases. [(accessed on 26 December 2021)];Biotechnol. Adv. 2013 31:1676–1694. doi: 10.1016/j.biotechadv.2013.08.017. Available online: https://pubmed.ncbi.nlm.nih.gov/23999358/ [DOI] [PubMed] [Google Scholar]
- 162.Guerram M., Zhang L.Y., Jiang Z.Z. G-protein coupled receptors as therapeutic targets for neurodegenerative and cerebrovascular diseases. [(accessed on 26 December 2021)];Neurochem. Int. 2016 101:1–14. doi: 10.1016/j.neuint.2016.09.005. Available online: https://pubmed.ncbi.nlm.nih.gov/27620813/ [DOI] [PubMed] [Google Scholar]
- 163.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. [(accessed on 26 December 2021)];Glia. 2010 58:1017–1030. doi: 10.1002/glia.20983. Available online: https://pubmed.ncbi.nlm.nih.gov/20468046/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schilling T., Eder C. Microglial K [+] channel expression in young adult and aged mice. [(accessed on 26 December 2021)];Glia. 2015 63:664–672. doi: 10.1002/glia.22776. Available online: https://pubmed.ncbi.nlm.nih.gov/25472417/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hashioka S., Klegeris A., McGeer P.L. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. [(accessed on 26 December 2021)];Neuropharmacology. 2012 63:685–691. doi: 10.1016/j.neuropharm.2012.05.033. Available online: https://pubmed.ncbi.nlm.nih.gov/22659089/ [DOI] [PubMed] [Google Scholar]
- 166.Richardson J.R., Hossain M.M. Microglial ion channels as potential targets for neuroprotection in Parkinson’s disease. Neural. Plast. 2013;2013:587418. doi: 10.1155/2013/587418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Eder C. Regulation of microglial behavior by ion channel activity. [(accessed on 26 December 2021)];J. Neurosci. Res. 2005 81:314–321. doi: 10.1002/jnr.20476. Available online: https://pubmed.ncbi.nlm.nih.gov/15929071/ [DOI] [PubMed] [Google Scholar]
- 168.Lee S.H., Suk K. Emerging roles of protein kinases in microglia-mediated neuroinflammation. [(accessed on 26 December 2021)];Biochem. Pharmacol. 2017 146:1–9. doi: 10.1016/j.bcp.2017.06.137. Available online: https://pubmed.ncbi.nlm.nih.gov/28684305/ [DOI] [PubMed] [Google Scholar]
- 169.Leung C.H., Grill S.P., Lam W., Han Q.B., Sun H.D., Cheng Y.C. Novel mechanism of inhibition of nuclear factor-kappa B DNA-binding activity by diterpenoids isolated from Isodon rubescens. [(accessed on 26 December 2021)];Mol. Pharmacol. 2005 68:286–297. doi: 10.1124/mol.105.012765. Available online: https://pubmed.ncbi.nlm.nih.gov/15872117/ [DOI] [PubMed] [Google Scholar]
- 170.Goldmann T., Wieghofer P., Müller P.-F., Wolf Y., Varol D., Yona S., Brendecke S.M., Kierdorf K., Staszewski O., Datta M., et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. [(accessed on 26 December 2021)];Nat. Neurosci. 2013 16:1618–1626. doi: 10.1038/nn.3531. Available online: https://pubmed.ncbi.nlm.nih.gov/24077561/ [DOI] [PubMed] [Google Scholar]
- 171.Choi M.J., Lee E.J., Park J.S., Kim S.N., Park E.M., Kim H.S. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. [(accessed on 26 December 2021)];Biochem. Pharmacol. 2017 144:120–131. doi: 10.1016/j.bcp.2017.07.021. Available online: https://pure.ewha.ac.kr/en/publications/anti-inflammatory-mechanism-of-galangin-in-lipopolysaccharide-sti. [DOI] [PubMed] [Google Scholar]
- 172.Lehtonen Š., Sonninen T.M., Wojciechowski S., Goldsteins G., Koistinaho J. Dysfunction of cellular proteostasis in Parkinson’s disease. Front. Neurosci. 2019;13:457. doi: 10.3389/fnins.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim N., Lee H.J. Target Enzymes Considered for the Treatment of Alzheimer’s Disease and Parkinson’s Disease. Biomed. Res. Int. 2020;2020:2010728. doi: 10.1155/2020/2010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schreibelt G., van Horssen J., van Rossum S., Dijkstra C.D., Drukarch B., de Vries H.E. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. [(accessed on 26 December 2021)];Brain Res. Rev. 2007 56:322–330. doi: 10.1016/j.brainresrev.2007.07.005. Available online: https://pubmed.ncbi.nlm.nih.gov/17761296/ [DOI] [PubMed] [Google Scholar]
- 175.Thangudu S., Cheng F.Y., Su C.H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. [(accessed on 26 December 2021)];Polymers. 2020 12:3055. doi: 10.3390/polym12123055. Available online: https://www.mdpi.com/2073-4360/12/12/3055/htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alghamdi S.S., Suliman R.S., Almutairi K., Kahtani K., Aljatli D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. [(accessed on 26 December 2021)];Drug Des. Devel. Ther. 2021 15:3289–3312. doi: 10.2147/DDDT.S307113. Available online: https://pubmed.ncbi.nlm.nih.gov/34354342/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Feng X.L., Yu Y., Qin D.P., Gao H., Yao X.S. Acorus Linnaeus: A review of traditional uses, phytochemistry and neuropharmacology. [(accessed on 26 December 2021)];RSC Adv. 2014 5:5173–5182. doi: 10.1039/C4RA12049C. Available online: https://pubs.rsc.org/en/content/articlehtml/2015/ra/c4ra12049c. [DOI] [Google Scholar]
- 178.Bors L.A., Erdö F. Overcoming the Blood–Brain Barrier. Challenges and Tricks for CNS Drug Delivery. [(accessed on 26 December 2021)];Sci. Pharm. 2019 87:6. doi: 10.3390/scipharm87010006. Available online: https://www.mdpi.com/2218-0532/87/1/6/htm. [DOI] [Google Scholar]
- 179.Kahraman C., Arituluk Z.C., Irem I., Cankaya T. The Clinical Importance of Herb-Drug Interactions and Toxicological Risks of Plants and Herbal Products. [(accessed on 26 December 2021)];Med. Toxicol. 2020 :1–31. Available online: https://www.intechopen.com/chapters/71771. [Google Scholar]
- 180.Yang H., Sun L., Li W., Liu G., Tang Y. In Silico Prediction of Chemical Toxicity for Drug Design Using Machine Learning Methods and Structural Alerts. Front. Chem. 2018;20:30. doi: 10.3389/fchem.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Way2Drug—Main. [(accessed on 25 May 2021)]. Available online: http://way2drug.com/PassOnline/
- 182.Molinspiration Cheminformatics. [(accessed on 26 December 2021)]. Available online: https://www.molinspiration.com/
- 183.Swiss ADME. [(accessed on 8 November 2021)]. Available online: http://www.swissadme.ch/
- 184.ProTox-II—Prediction of TOXicity of Chemicals. [(accessed on 8 November 2021)]. Available online: https://tox-new.charite.de/protox_II/