Abstract
Background: In cardiopulmonary exercise testing (CPET), oxygen uptake (V’O2) is calculated using the product of minute ventilation (V’E) and the difference between inspiratory and expiratory O2 concentrations (ΔFO2). However, little is known about the response of ΔFO2 to pulmonary rehabilitation (PR). The aim of the present study was (1) to investigate whether PR increases peak V’O2, based on whether ΔFO2 or V’E at peak exercise increase after PR, and (2) to investigate whether an improvement in ΔFO2 correlates with an improvement in ventilatory efficiency. Methods: A total of 38 patients with severe and very severe COPD, whose PR responses were evaluated by CPET, were retrospectively analyzed. Results: After PR, peak V’O2 was increased in 14 patients. The difference in ΔFO2 at peak exercise following PR correlated with the difference in peak V’O2 (r = 0.4884, p = 0.0019), the difference in V’E/V’CO2-nadir (r = −0.7057, p < 0.0001), and the difference in V’E–V’CO2 slope (r = −0.4578, p = 0.0039), but it did not correlate with the difference in peak V’E. Conclusions: The increased O2 extraction following PR correlated with improved exercise tolerance and ventilatory efficiency. In advanced COPD patients, a new strategy for improving O2 extraction ability might be effective in those in whom ventilatory ability can be only minimally increased.
Keywords: dyspnea, exercise training, oxygen uptake, ventilation
1. Introduction
Since 2018, chronic obstructive pulmonary disease (COPD) has become the world’s third leading cause of death [1]. The most frequent and intractable problem in patients with COPD is exercise intolerance due to wasted ventilation [2,3,4,5], which leads to poor disease prognosis [6]. Although several measures have been attempted to improve exercise tolerance, they remain insufficient.
It is widely accepted that pulmonary rehabilitation (PR), serving as an effective non-pharmacological intervention for COPD, improves endurance, quality of life and exertional dyspnea [7,8]. Consequently, since the survival of patients with COPD is also improved, PR is considered a very useful treatment for COPD patients [9,10]. The response to PR, however, varies significantly between patients, and PR including exercise training does not necessarily increase peak oxygen uptake (V’O2), especially in patients with advanced COPD [11,12]. Physical exercise requires gas exchange of both oxygen (O2) and carbon dioxide (CO2) and involves the interaction of pulmonary, cardiovascular and muscle crosstalk in the body [6,13]. Hence, V’O2, as determined by cardiopulmonary exercise testing (CPET), is calculated using the product of minute ventilation (V’E), as a measure of ventilatory ability, and the difference between inspired and expired O2 concentrations (ΔFO2), as a measure of O2 extraction ability. Therefore, peak V’O2 is one of the variables used to characterize total exercise ability and might be informative for assessing the efficacy of PR. In addition, reduced peak V’O2 has been proven to predict a poor prognosis in patients with COPD [14,15], and effective evaluation of peak V’O2 might serve as a guide for decision-making to confirm the pathophysiological condition and choose suitable treatment for COPD.
In several chronic cardiopulmonary diseases, ventilatory inefficiency, indicated by a high V’E versus volume of exhaled carbon dioxide (V’CO2) relationship during exercise, i.e., a high V’E–V’CO2 slope, is used as a prognostic marker and is commonly associated with a low arterial carbon dioxide partial pressure (PaCO2) during exercise [16,17,18]. Recently, we reported that a high V’E–V’CO2 slope was more strongly associated with a low ΔFO2 at peak exercise, as a gas exchange parameter related to O2, as compared to CO2-related variables, such as PaCO2 and partial pressure of end-tidal CO2 (PetCO2), in patients with COPD [19]. Furthermore, we reported that the dependence of reduced peak V’O2 on ΔFO2 becomes relatively high with the progression of COPD, due to a decrease in its dependence on ventilatory ability at peak exercise, and that increasing ΔFO2 might be an attractive approach for improving exercise tolerance and ventilatory efficiency, especially in advanced COPD patients [19].
The aim of the present study was: (1) to investigate whether PR increases exercise tolerance, evaluated by assessment of peak V’O2 during incremental CPET, and to assess the effect of PR based on the variables that are more significantly associated with improvement in peak V’O2, i.e., ΔFO2 or V’E at peak exercise and (2) to investigate whether an improvement in ΔFO2 is predictive of an improvement in ventilatory efficiency, indicated by the V’E–V’CO2 slope and the lowest value of V’E/V’CO2 during exercise in patients with severe and very severe COPD.
2. Materials and Methods
2.1. Study Design
This retrospective study was conducted by analyzing data obtained from severe and very severe COPD patients who underwent PR while in hospital and were evaluated using CPET before and after PR at the National Hospital Organization (NHO) Osaka Toneyama Medical Center from April 2000 to July 2021. This study included data from previous ethically-approved studies performed as screening for studies on COPD at our institution. Thus, a total of 38 patients diagnosed and classified as stage III or IV COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [20] were analyzed. Patients were excluded if they met the following criteria: (1) if they had a diagnosis of bronchial asthma, active infection, or severe heart disease, (2) had a history of lung resection, (3) if their drug regimens were changed within 4 weeks before PR, (4) if new treatment was added during PR, and (5) if their conditions were unstable due to other reasons. This study was performed in accordance with the Declaration of Helsinki and the institutional review board of the NHO Osaka Toneyama Medical Center approved this study (approval number: TNH-A-2021022). Written, informed consent was obtained from each patient before the first CPET evaluation.
2.2. Pulmonary Rehabilitation (PR)
Education and instruction, physical therapy, exercise training and occupational therapy were included in the PR program and performed in hospital. The patients were instructed to use educational material to increase their knowledge of the disease and to improve their management of it. They underwent three sets of exercise training per day with electromechanically braked cycle ergometers, from three to five days a week for one to two months (20 days), with high-intensity targets as previously described [21]. The initial exercise level was set for 6 min a set at the work rate equivalent to 60% of the baseline peak V’O2 before PR. If they could tolerate the exercise, the exercise duration was first increased to 10 min a set, following which the work rate was increased by 5 W and if possible, increased to the work rate equivalent to 80% of the baseline peak V’O2. If the patients could not tolerate the exercise, their exercise levels were reduced to the previous setting.
2.3. Pulmonary Function Tests (PFTs)
Post-bronchodilator spirometry (CHESTAC 8800; CHEST M.I. Inc., Tokyo, Japan) was performed according to the recommendations of the American Thoracic Society [22]. PFTs were performed within 1 week before and after PR.
2.4. Six-Minute Walk Test
The six-minute walk distance (6-MWD) was measured as described previously [23]. The patients were instructed to walk at their own pace but to cover as much ground as possible in 6 min without encouragement.
2.5. Cardiopulmonary Exercise Testing (CPET)
Symptom-limited incremental exercise tests were performed using an electrically braked cycle ergometer (CV-1000SS; Lode, Groningen, The Netherlands) and a CPET system (Aero monitor AE310S; Minato Medical Science Co., Ltd., Osaka, Japan) before and after PR with the same protocol, i.e., the two-minute stage, 10-watt step protocol. Before CPET, patients were instructed to perform to their maximal effort but were advised that the exercise could be stopped at any time. During exercise, CPET was performed without encouragement until the subject was exhausted in order to achieve reliable data. All patients were instructed to maintain a speed of approximately 60 rpm on the cycle ergometer by observing the rpm meter. Resting measurements before exercise were obtained during the steady-state period after at least 3 min of rest after preparation for CPET. Ventilatory values were measured on a breath-by-breath basis using a face mask and are shown as 30-s averages at rest, at two-minute intervals during exercise and at the end of exercise. Severity of dyspnea (10-point modified Borg category-ratio scale) was evaluated at rest, during the last 15 s of each exercise stage and at the end of exercise, and all patients were asked which symptoms (exertional dyspnea, leg discomfort, or others) caused them to stop the exercise. The V’E–V’CO2 slope was calculated by linear regression, excluding the nonlinear part of the data after the onset of the respiratory compensation point. If the respiratory compensation point could not be determined, the V’E–V’CO2 slope was calculated from the data from the start to the end of the exercise. The V’E–V’CO2 nadir was defined as the lowest value of the ratio between V’E and V’CO2 during exercise. The V’E–V’CO2 intercept was defined as the nonzero point on the Y-axis, i.e., V’E [19]. The physiological dead space to tidal volume ratio (VD/VT) was estimated based on Enghoff’s modification of Bohr’s equation [17], using the non-invasive parameter of PetCO2 as an approximation of PaCO2. VD-intercept was estimated as V’E–V’CO2 intercept/fR–V’CO2 intercept during exercise, and the fR–V’CO2 intercept was defined as the nonzero point on the Y-axis, i.e., fR [24]. The time-slope was calculated as the ratio of exercise time until exhaustion to ΔV’O2 (peak minus resting V’O2) obtained during CPET [12]. The predicted maximal voluntary ventilation (MVV) was calculated as FEV1 × 35. The predicted maximum heart rate was calculated as 220—age in years [13]. The percent predicted peak V’O2 was calculated using the equations of Itoh et al. [13]. Isotime was defined as the time the shortest test ended. An inflection point of VT during exercise was determined for each subject using the 30-s averaged data [25]. The anaerobic threshold was identified using the V-slope method [13].
2.6. Statistical Analysis
The data are expressed as means ± standard deviation (SD). First, all patients were divided into two groups based on whether or not peak V’O2 increased after PR. Based on the results of variables with significant differences between the peak V’O2 increase group (peak V’O2 before PR< peak V’O2 after PR) and the non-increase group (peak V’O2 before PR ≥ peak V’O2 after PR), and since V’O2 is calculated using the product of V’E and ΔFO2, all patients were then divided into two groups based on whether V’E or ΔFO2 at peak exercise had increased after PR. That is, the increase group was defined as V’E or ΔFO2 before PR was less than that after PR, and the non-increase group was defined as V’E or ΔFO2 before PR was equal to or greater than that after PR. Fisher’s exact test was used to compare baseline characteristics before PR between the two groups and evaluate the reasons for stopping exercise before and after PR. Mann–Whitney’s U test was used to compare baseline characteristics before PR and compare the mean differences before and after PR between the two groups. The Wilcoxon signed-rank test was used to compare the results after PR with the results before PR within each group. Univariate analysis using Spearman’s rank correlation coefficient as a non-parametric test was used to evaluate the correlations of ΔFO2 at peak exercise with the other clinical variables. A p value < 0.05 was considered to indicate significance (JMP software, version 11, SAS Institute Inc., Cary, NC, USA).
3. Results
A total of 38 patients with severe to very severe airway obstruction according to the GOLD stages were evaluated before and after PR (Table 1). All patients were ex-smokers. Before PR, all patients performed incremental CPET (mean exercise time 7.0 min, maximum value 12.9 min and minimum value 2.8 min), and their mean peak V’O2 was 13.3 mL·min−1·kg−1, suggesting obvious exercise intolerance.
Table 1.
Patient baseline characteristics (n = 38).
| All Patients (n = 38) | |
|---|---|
| Age, years | 68.9 (9.1) |
| Sex, male/female | 37/1 |
| BMI, kg·m−2 | 20.1 (3.7) |
| GOLD stage, III/IV | 21/17 |
| Pulmonary function | |
| FEV1, L | 0.84 (0.29) |
| %FEV1, %predicted | 31.6 (10.3) |
| FEV1/FVC, % | 39.5 (9.2) |
| VC, L | 2.69 (0.62) |
| %VC, %predicted | 83.3 (16.9) |
| IC, L | 1.71 (0.46) |
| 6-MWD *, m | 262.8 (113.3) |
| Incremental CPET | |
| Peak V’O2, mL·min−1·kg−1 | 13.3 (3.7) |
| Percent predicted peak V’O2, % | 57.8 (16.4) |
| SAMA | 17 |
| LAMA | 9 |
| SABA | 6 |
| LABA | 14 |
| ICS | 9 |
| LAMA·LABA/ICS·LABA/Triple therapy | 7/4/1 |
Data are presented as means (standard deviation) unless otherwise specified. BMI: body mass index; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; VC: vital capacity; IC: inspiratory capacity; six-MWD: six-minute walk distance; CPET: cardiopulmonary exercise testing; V’O2: oxygen uptake; SAMA: short-acting muscarinic antagonist; LAMA: long-acting muscarinic antagonist; SABA: short-acting β2-agonist; LABA: long-acting β2-agonist; ICS: inhaled corticosteroid. * The data of 6-MWD were not obtained from two patients.
Although the difference following PR in six-MWD, which was evaluated as endurance, was a positive value in 81% of the patients, the difference following PR in peak V’O2 was positive in only 14 (37%) patients (Table 2). In all patients, the mean difference following PR in peak V’O2 was not increased (mean difference from pre-PR: −0.02 mL·min−1·kg−1). Of these 14 patients, ΔFO2 at peak exercise during CPET after PR was increased in 11 patients (79%), while in the remaining three patients, V’E increased despite a decrease in ΔFO2 at peak exercise after PR. In the peak V’O2 increase group, of the CPET variables obtained, the changes in the ΔFO2 and V’E at peak exercise after PR were significantly higher than in the peak V’O2 non-increase group among CPET variables obtained (Table 2).
Table 2.
Changes in cardiopulmonary variables after pulmonary rehabilitation in the peak V’O2 increase and peak V’O2 non-increase groups (n = 38).
| Peak V’O2 Inc. Group (n = 14) |
Peak V’O2 Non-Inc. Group (n = 24) |
p-Value (Between the Two Groups) | |||
|---|---|---|---|---|---|
| Pre-PR | Difference | Pre-PR | Difference | ||
| Pulmonary function | |||||
| FEV1, L | 0.77 (0.26) | +0.16 (0.19) * | 0.87 (0.30) | +0.02 (0.13) | 0.0514 |
| IC, L | 1.58 (0.42) | +0.16 (0.34) | 1.78 (0.47) | −0.10 (0.33) | 0.0735 |
| 6-MWD †, m | 292.3 (104.6) | +43.2 (53.6) ** | 243.2 (117.0) | +56.8 (65.8) *** | 0.6373 |
| Incremental CPET at peak exercise | |||||
| Dyspnea, Borg scale | 6.6 (2.5) | −1.1 (2.0) | 6.3 (2.6) | −1.3 (2.2) ** | 0.8299 |
| Work rate, watts | 39 (9) | +9 (8) ** | 39 (14) | +2 (10) | 0.0183 |
| V’O2 at anaerobic threshold ††, mL·min−1 | 521.5 (152.6) | +33.6 (52.6) | 547.4 (80.0) | −31.4(51.5) * | 0.0045 |
| R | 1.04 (0.08) | +0.03 (0.08) | 1.03 (0.11) | −0.01 (0.05) | 0.0863 |
| V’E, L·min−1 | 29.3 (7.4) | +4.0 (7.3) ** | 29.8 (6.6) | −1.2 (2.5) * | 0.0008 |
| VT, mL | 986 (182) | +133 (154) *** | 1025 (258) | +15 (153) | 0.0062 |
| fR, breaths·min−1 | 31 (8) | −1 (6) | 30 (5) | −1 (6) | 0.9516 |
| Ti/Ttot | 0.37 (0.04) | +0.01 (0.04) | 0. 36 (0.07) | +0.02 (0.05) | 0.7038 |
| VD/VT | 0.37 (0.08) | −0.02 (0.04) | 0.35 (0.06) | +0.02 (0.05) * | 0.0185 |
| HR, beats·min−1 | 116 (18) | +6 (10) | 119 (20) | + 1 (16) | 0.1112 |
| O2 pulse, mL·beats−1 | 6.7 (1.2) | +0.4 (0.7) * | 6.0 (1.5) | −0.5 (0.5) **** | 0.0001 |
| SpO2, % | 90.4 (4.6) | 0 (2.5) | 89.5 (5.5) | −0.6 (3.2) | 0.4565 |
| PetCO2, mmHg | 37.8 (6.7) | +0.4 (3.2) | 37.3 (4.9) | −1.3 (3.5) | 0.1463 |
| ΔFO2, % | 2.79 (0.50) | +0.15 (0.33) | 2.91 (0.42) | −0.20 (0.30) ** | 0.0037 |
| VD-intercept/VT | 0.46 (0.20) | −0.01 (0.41) | 0.40 (0.25) | +0.01 (0.23) | 0.5701 |
| V’E/V’CO2 | 43.8 (7.8) | -1.1 (4.2) | 42.5 (7.6) | +2.6 (4.2) ** | 0.0168 |
| V’E/V’CO2-nadir | 43.3 (8.0) | −1.4 (3.1) | 42.3 (7.6) | +2.4 (4.2) ** | 0.0062 |
| V’E−V’CO2-slope | 31.7 (7.2) | +1.3 (4.0) | 34.3 (10.2) | +3.3 (4.7) ** | 0.3884 |
Data are presented as means (standard deviation). V’O2: oxygen uptake; inc.: increase; PR: pulmonary rehabilitation; FEV1: forced expiratory volume in one second; IC: inspiratory capacity; 6-MWD: six-minute walk distance; CPET: cardiopulmonary exercise testing; R: gas exchange ratio; V’E: minute ventilation; VT: tidal volume; fR: breathing frequency; Ti/Ttot: inspiratory duty cycle; VD/VT: physiological dead space/tidal volume ratio; HR: heart rate; SpO2: percutaneous oxygen saturation; PetCO2: partial pressure of end-tidal carbon dioxide; ΔFO2: difference between inspiratory and expiratory O2 concentrations; VD-intercept: VD calculated as V’E-axis intercept/fR-axis intercept, obtained from V’E vs. V’CO2 and fR vs. V’CO2 relationships (see the Methods section for details); V’CO2: carbon dioxide output; V’E/V’CO2-nadir: lowest value of the ratio between V’E and V’CO2 during exercise (see the Methods section for details); V’E–V’CO2-slope: the slope was determined by linear regression analysis of V’E to V’CO2 relationship (see the Methods for details). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001: compared with pre-PR values (within-group difference). † The data of six-MWD from 2 patients were not obtained. ††: 27 patients (peak V’O2 inc. group, n = 11; peak V’O2 non-increase group, n = 16), whose anaerobic thresholds were obtained before and after pulmonary rehabilitation were analyzed.
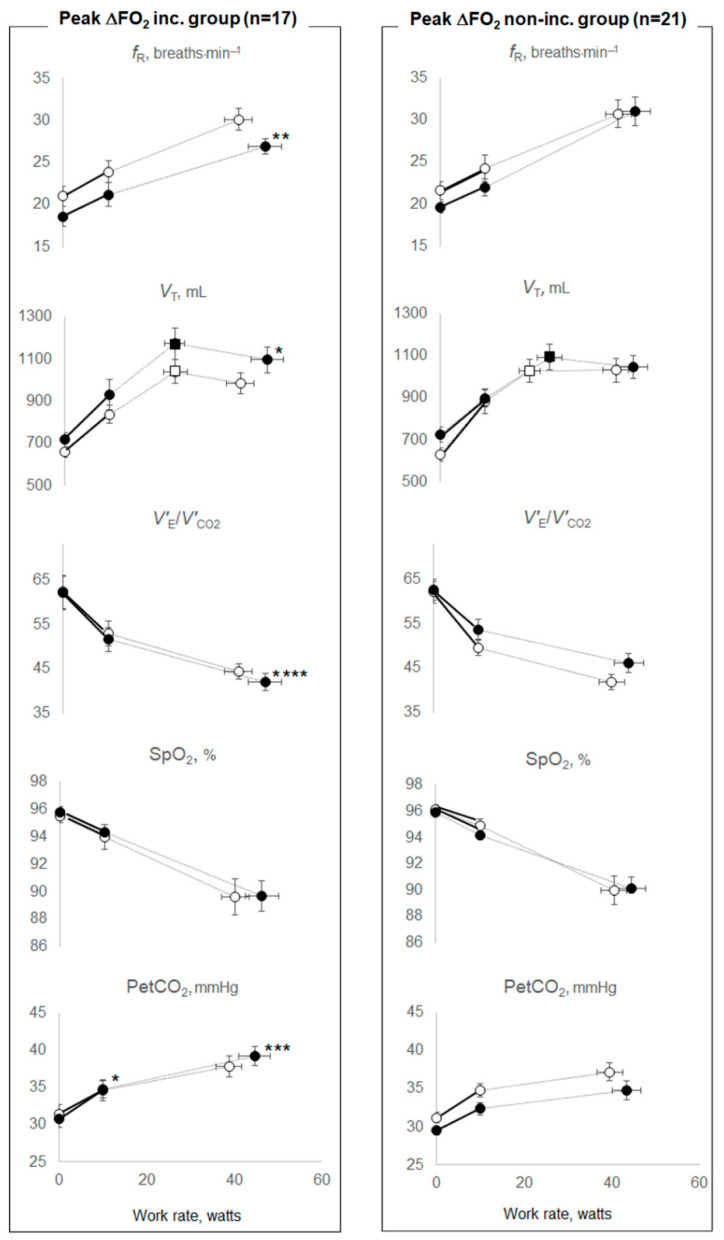
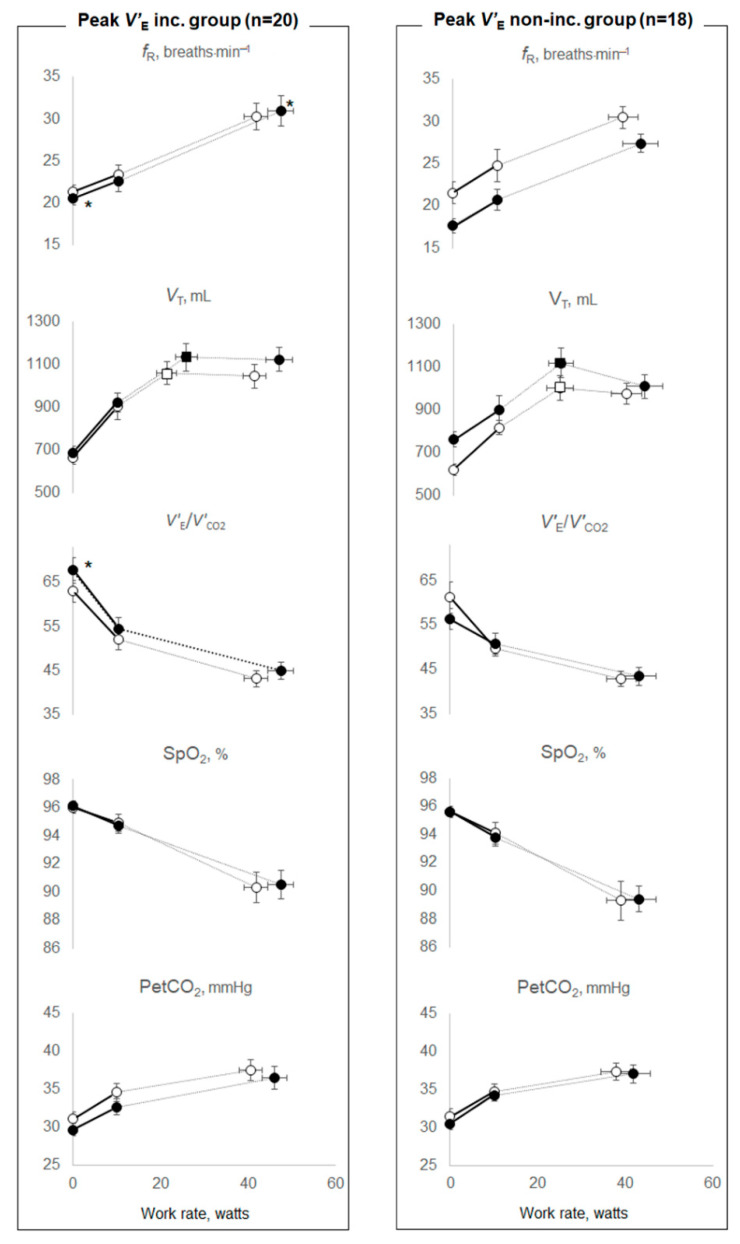
Therefore, we investigated the effects of PR on O2 extraction or ventilatory ability by dividing the patient cohort into two groups based on whether or not they experienced an increase in the ΔFO2 or V’E at peak exercise after PR (Table 3, Table 4 and Table 5). The groups divided according to the increase in the ΔFO2 or V’E at peak exercise were well matched for age, sex and body mass index (BMI), GOLD stages and resting pulmonary function, although the number of subjects with dual or triple inhalation therapy was low in each group (Table 3). In the peak ΔFO2 increase group, compared with the change following PR in the peak ΔFO2 non-increase group, (1) the mean differences following PR in peak V’O2 (p = 0.0136), V’O2 at anaerobic threshold (p < 0.0001), and PetCO2 at peak exercise (p = 0.0007) were significantly increased, and those in V’E/V’CO2-nadir (p < 0.0001) during exercise and V’E–V’CO2-slope (p = 0.0413) were significantly improved, although V’E–V’CO2 intercept was not changed (Table 4), (2) notwithstanding that the mean difference following PR in VT at peak exercise was increased (p = 0.0167), and in the fR at peak exercise was reduced (p = 0.0049), the mean difference following PR in the inspiratory duty cycle (Ti/Ttot) at peak exercise (p = 0.4788) and in VT at the inflection point during exercise (p = 0.1798) were not changed (Table 4 and Figure 1). Then, the mean difference following PR in V’E did not increase significantly (p = 0.7134) (Table 4), and (3) the mean difference following PR in O2-pulse was not significantly changed (p = 0.2218) (Table 4). In the peak ΔFO2 non-increase group, (i) dyspnea at peak exercise was significantly reduced, as seen in the within-group evaluation (p = 0.0006), (ii) the time-slope was significantly lower before PR (p = 0.0277) and increased after PR compared with the peak ΔFO2 increase group (p = 0.0302) (Table 4). In contrast, in the peak V’E increase group, compared with the mean difference following PR in the peak V’E non-increase group, although the mean difference following PR in peak V’O2 was significantly increased (p = 0.0109), those in V’O2 at the anaerobic threshold (p = 0.6429), in V’E/V’CO2-nadir during exercise (p = 0.5685), in V’E–V’CO2-slope (p = 1.0000), in ΔFO2 (p = 0.4558), and in PetCO2 (p = 0.5200) at peak exercise did not change, and significant tachypnea at peak exercise (p = 0.0415) was seen (Table 5 and Figure 2).
Table 3.
Comparison between groups stratified according to the change in peak ΔFO2 or peak V’E (n = 38) after pulmonary rehabilitation.
| Peak ΔFO2 Inc. Group (n = 17) | Peak ΔFO2 Non-Inc. Group (n = 21) | p-Value | |
|---|---|---|---|
| Age, years | 71.4 (8.5) | 66.8 (9.2) | 0.1301 |
| Sex, male/female | 16/1 | 21/0 | 0.2600 |
| BMI, kg·m−2 | 19.7 (3.1) | 20.5 (4.2) | 0.6918 |
| GOLD stage, III/IV | 9/8 | 12/9 | 0.7956 |
| Pulmonary function | |||
| FEV1, L | 0.78 (0.27) | 0.88 (0.30) | 0.3250 |
| %FEV1, %predicted | 30.6 (10.2) | 32.4 (10.5) | 0.6281 |
| FEV1/FVC, % | 41.0 (7.0) | 38.3 (10.6) | 0.3041 |
| VC, L | 2.57 (0.62) | 2.77 (0.62) | 0.2838 |
| %VC, %predicted | 81.8 (17.3) | 84.5 (16.8) | 0.6073 |
| IC, L | 1.57 (0.41) | 1.81 (0.48) | 0.1487 |
| Incremental CPET | |||
| peak V’O2, mL·min−1·kg−1 | 13.0 (4.1) | 13.6 (3.3) | 0.6073 |
| LAMA·LABA/ICS·LABA/Triple | 2/3/0 | 5/1/1 | 0.3836 |
| Peak V’E Inc. group (n = 20) | Peak V’E Non-Inc. group (n = 18) | p-Value | |
| Age, years | 67.5 (10.0) | 70.4 (8.0) | 0.3960 |
| Sex, male/female | 19/1 | 18/0 | 0.3363 |
| BMI, kg·m−2 | 20.6 (4.7) | 19.7 (2.4) | 0.6295 |
| GOLD stage, III/IV | 12/8 | 9/9 | 0.5359 |
| Pulmonary function | |||
| FEV1, L | 0.88 (0.30) | 0.78 (0.27) | 0.2856 |
| %FEV1, %predicted | 33.1 (10.2) | 30.0 (10.4) | 0.4047 |
| FEV1/FVC, % | 40.6 (9.4) | 38.2 (9.0) | 0.5296 |
| VC, L | 2.79 (0.66) | 2.55 (0.56) | 0.2193 |
| %VC, % | 86.5 (17.5) | 79.7 (15.8) | 0.1561 |
| IC, L | 1.74 (0.51) | 1.68 (0.42) | 0.6808 |
| Incremental CPET | |||
| peak V’O2, mL·min−1·kg−1 | 13.1 (3.6) | 13.6 (3.9) | 0.7366 |
| LAMA·LABA/ICS·LABA/Triple | 1/3/1 | 6/1/0 | 0.0855 |
Data are presented as means (standard deviation) unless otherwise specified. ΔFO2: difference between inspiratory and expiratory O2 concentrations; V’E: minute ventilation; inc.: increase; BMI: body mass index; GOLD: Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; VC: vital capacity; IC: inspiratory capacity; CPET: cardiopulmonary exercise testing; V’O2: oxygen uptake; LAMA: long-acting muscarinic antagonist; LABA: long-acting β2-agonist; ICS: inhaled corticosteroid.
Table 4.
Changes in the results of exercise testing after pulmonary rehabilitation in the peak ΔFO2 increase and non-increase groups.
| Peak ΔFO2 Inc. Group (n = 17) | Peak ΔFO2 Non-Inc. Group (n = 21) | p-Value (Between the Two Groups) | |||
|---|---|---|---|---|---|
| Pre-PR | Difference | Pre-PR | Difference | ||
| 6-MWD ‡, m | 265.1 (128.4) | +52.2 (70.7) | 260.7 (100.7) | +50.6 (51.8) | 0.4779 |
| Incremental CPET at peak exercise | |||||
| Dyspnea, Borg scale | 6.3 (2.9) | −0.8 (2.4) | 6.5 (2.3) | −1.6 (1.8) *** | 0.2457 |
| V’O2, ml·min−1·kg−1 | 13.0 (4.1) | +0.7 (1.7) | 13.6 (3.3) | −0.6 (2.2) | 0.0136 |
| Exercise time, sec | 416 (154) | +58 (72) ** | 418 (160) | +51 (113) | 0.8717 |
| Work rate, watts | 39 (12) | +6 (7) ** | 40 (13) | +4 (12) | 0.5296 |
| V’O2 at anaerobic threshold ††, mL·min−1 | 502.3 (111.8) | +50.3 (38.7) *** | 564.5 (110.3) | −49.1 (30.0) **** | <0.0001 |
| R | 1.02 (0.10) | +0.02 (0.06) | 1.03 (0.10) | +0.00 (0.08) | 0.3104 |
| V’E, L·min−1 | 29.2 (8.0) | −0.1 (2.7) | 29.9 (5.9) | +1.4 (6.8) | 0.7134 |
| VT, mL | 986 (206) | +110 (153) ** | 1031 (253) | +16 (159) | 0.0167 |
| VT at inflection point, mL | 1040 (204) | +155 (195) * | 1029 (231) | +76 (194) | 0.1798 |
| fR, breaths·min−1 | 30 (5) | −3 (4) ** | 31 (7) | 0 (7) | 0.0049 |
| Ti/Ttot | 0.37 (0.05) | 0 (0.04) | 0.36 (0.07) | +0.02 (0.05) | 0.4788 |
| VD/VT | 0.38 (0.06) | −0.01(0.04) | 0.34 (0.07) | +0.02 (0.05) * | 0.0534 |
| HR, beats·min−1 | 113 (20) | +6 (9) * | 122 (18) | 0 (17) | 0.2210 |
| O2 pulse, mL·beats−1 | 5.8 (1.5) | −0.1 (0.6) | 5.9 (1.4) | −0.3 (0.8) * | 0.2218 |
| SpO2, % | 89.6 (5.4) | −0.9 (2.5) | 90.0 (5.0) | +0.0 (3.3) | 0.3656 |
| PetCO2, mmHg | 37.8 (5.8) | +1.4 (3.0) | 37.2 (5.5) | −2.4 (2.8) *** | 0.0007 |
| V’E/V’CO2 | 44.4 (7.1) | −2.4 (2.8) ** | 418 (8.0) | +4.2 (3.4) **** | <0.0001 |
| V’E–V’CO2-intercept, L·min−1 | 6.6 (2.6) | −0.5 (1.6) | 6.0 (3.4) | −0.8 (2.7) | 0.9298 |
| VD-intercept/VT | 0.44 (0.26) | +0.10 (0.36) | 0.41 (0.20) | −0.07 (0.24) | 0.4738 |
| V’E/V’CO2-nadir | 44.0 (7.4) | −2.1 (2.6) ** | 41.6 (7.9) | +3.5 (3.5) **** | <0.0001 |
| V’E–V’CO2-slope | 32.5 (6.7) | +0.8 (3.9) | 34.0 (10.9) | +4.1 (4.5) *** | 0.0413 |
| Time slope, sec·mL−1·min | 1.04 (0.29) † | +0.02 (0.33) | 0.91 (0.30) | +0.20 (0.20) *** | 0.0302 |
| The causes to stop during CPET | Pre-PR | Post-PR | Pre-PR | Post-PR | Not evaluated |
| Dyspnea/leg fatigue | 10/7 | 9/8 | 15/6 | 11/10 * | Not evaluated |
Data are presented as means (standard deviation). Peak ΔFO2: difference between inspiratory and expiratory O2 concentrations at peak exercise; inc.: increase; PR: pulmonary rehabilitation; six-MWD: six-minute walk distance; CPET: cardiopulmonary exercise testing; V’O2: oxygen uptake; R: gas exchange ratio; V’E: minute ventilation; VT: tidal volume; fR: breathing frequency; Ti/Ttot: inspiratory duty cycle; VD/VT: physiological dead space/tidal volume ratio; HR: heart rate; O2 pulse: V’O2/HR; SpO2: percutaneous oxygen saturation; PetCO2: partial pressure of end-tidal carbon dioxide; V’CO2: carbon dioxide output; VD-intercept: VD calculated as V’E-axis intercept/fR-axis intercept, obtained from V’E vs. V’CO2 and fR vs. V’CO2 relationships (see the Methods section for details); V’E/V’CO2-nadir: lowest value of the ratio between V’E and V’CO2 during exercise (see the Methods section for details); V’E–V’CO2-slope: the slope was determined by linear regression analysis of V’E to V’CO2 obtained during exercise (see the Methods section for details); Time slope: ratio of exercise time until exhaustion to ΔV’O2 (peak minus resting V’O2) during CPET (see the Methods section for details). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001: compared with pre-PR values (within-group difference). † p < 0.05: compared with pre-PR values between the two groups. ‡ The data of 6-MWD were not obtained from two patients. ††: 27 patients (peak ΔFO2 inc. group, n = 12; peak ΔFO2 non-increase group, n = 15), whose anaerobic thresholds were obtained before and after pulmonary rehabilitation were analyzed.
Table 5.
Changes in the results of exercise testing after pulmonary rehabilitation in the peak V’E increase and non-increase groups.
| Peak V’E Inc. Group (n = 20) | Peak V’E Non-Inc. Group (n = 18) | p-Value (Between the Two Groups) | |||
|---|---|---|---|---|---|
| Pre-PR | Difference | Pre-PR | Difference | ||
| 6-MWD †, m | 268.9 (102.8) | +43.7 (55.1) | 256.4 (126.4) | +59.5 (67.0) | 0.5860 |
| Incremental CPET at peak exercise | |||||
| Dyspnea, Borg scale | 7.3 (2.0) | −1.8 (2.1) *** | 5.5 (2.8) | −0.6 (2.0) | 0.0778 |
| V’O2, ml·min−1·kg−1 | 13.1 (3.6) | +0.8 (2.3) | 13.6 (3.9) | −0.9 (1.4) ** | 0.0109 |
| Exercise time, sec | 422 (147) | +77 (110) ** | 411 (167) | +29 (72) * | 0.1077 |
| Work rate, watts | 41 (11) | +6 (11) | 38 (14) | +4 (8) | 0.8725 |
| V’O2 at anaerobic threshold ††, mL·min−1 | 536.0 (124.6) | −1.7 (64.1) | 538.0 (102.7) | −9.0 (58.1) | 0.6429 |
| R | 1.04 (0.10) | +0.02 (0.07) | 1.02 (0.11) | −0.01 (0.06) | 0.3404 |
| VT, mL | 1043 (251) | +79 (160) * | 976 (209) | +35 (165) | 0.1932 |
| VT at inflection point, mL | 1058 (224) | +102 (167) * | 1003 (212) | +118 (231) | 0.9683 |
| fR, breaths·min−1 | 30 (7) | +1 (5) | 30 (6) | −3 (6) * | 0.0415 |
| Ti/Ttot | 0.36 (0.05) | +0.01 (0.04) | 0. 36 (0.07) | +0.02 (0.05) | 0.6593 |
| VD/VT | 0.36 (0.08) | +0.00 (0.05) | 0.36 (0.05) | +0.00 (0.05) | 0.9415 |
| HR, beats·min−1 | 122 (17) | +6 (12) | 114 (21) | −1 (16) | 0.4035 |
| O2 pulse, mL·beats−1 | 5.6 (1.2) | +0.1 (0.7) | 6.1 (1.6) | −0.5 (0.6) ** | 0.0108 |
| SpO2, % | 90.3 (4.7) | +0.1 (2.8) | 89.3 (5.6) | −0.9 (3.1) | 0.1723 |
| PetCO2, mmHg | 37.5 (6.3) | −1.0 (2.8) | 37.4 (4.7) | −0.4 (4.1) | 0.5200 |
| ΔFO2, % | 2.85 (0.50) | −0.10 (0.34) | 2.89 (0.41) | −0.03 (0.37) | 0.4558 |
| V’E/V’CO2 | 43.2 (8.3) | +1.8 (4.9) | 42.8 (7.1) | +0.6 (4.2) | 0.2792 |
| V’E–V’CO2-intercept, L·min−1 | 6.7 (2.7) | −0.2 (2.0) | 5.9 (3.3) | −1.2 (2.4) * | 0.1883 |
| VD-intercept/VT | 0.44 (0.20) | −0.01 (0.37) | 0.41(0.27) | 0.03 (0.22) | 0.6674 |
| V’E/V’CO2-nadir | 42.9 (8.3) | +1.2 (4.4) | 42.4 (7.1) | +0.8 (4.1) | 0.5685 |
| V’E–V’CO2-slope | 32.8 (8.5) | +2.6 (4.6) * | 33.9 (10.2) | +2.7 (4.5) * | 1.0000 |
| Time slope, sec·mL−1·min | 0.96 (0.28) | +0.07 (0.30) | 0.98 (0.34) | +0.19 (0.25) ** | 0.3310 |
| The causes to stop during CPET | Pre-PR | Post-PR | Pre-PR | Post-PR | Not done |
| Dyspnea/leg fatigue | 14/6 | 11/9 | 11/7 | 9/9 | Not done |
Data are presented as means (standard deviation). Peak V’E: minute ventilation at peak exercise; inc.: increase; PR: pulmonary rehabilitation; six-MWD: six-minute walking distance; CPET: cardiopulmonary exercise testing; V’O2: oxygen uptake; R: gas exchange ratio; VT: tidal volume; fR: breathing frequency; Ti/Ttot: inspiratory duty cycle; VD/VT: physiological dead space/tidal volume ratio; HR: heart rate; O2 pulse: V’O2/HR; SpO2: percutaneous oxygen saturation; PetCO2: partial pressure of end-tidal carbon dioxide; ΔFO2: difference between inspiratory and expiratory O2 concentrations; V’CO2: carbon dioxide output; VD-intercept: VD calculated as V’E-axis intercept/fR-axis intercept, obtained from V’E vs. V’CO2 and fR vs. V’CO2 relationships (see the Methods section for details); V’E/V’CO2 nadir: the lowest value of the ratio between V’E and V’CO2 during exercise (see the Methods section for details); V’E–V’CO2 slope: the slope was determined by linear regression analysis of V’E to V’CO2 obtained during exercise (see the Methods section for details); Time slope: ratio of exercise time until exhaustion to ΔV’O2 (peak minus resting V’O2) during CPET (see the Methods section for details). * p < 0.05, ** p < 0.01, *** p < 0.001: compared with pre-PR values (within-group difference). † The data of 6-MWD were not obtained from two patients. ††: 27 patients (peak V’E inc. group, n = 15; peak V’E non-increase group, n = 12), whose anaerobic thresholds were obtained before and after pulmonary rehabilitation were analyzed.
Figure 1.
Exercise variables before and after pulmonary rehabilitation between the peak ΔFO2 increase and non-increase groups. Data are presented as means (standard error). All patients were divided into two groups based on whether or not ΔFO2 at peak exercise had increased after pulmonary rehabilitation. Peak ΔFO2: difference between inspiratory and expiratory O2 concentrations at peak exercise; inc.: increase; fR: breathing frequency; VT: tidal volume; V’E: minute ventilation; V’CO2: carbon dioxide output; SpO2: percutaneous oxygen saturation; PetCO2: partial pressure of end-tidal carbon dioxide. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001: mean differences between pre-and post-pulmonary rehabilitation values at each exercise phase were compared between peak ΔFO2 increase and non-increase groups. Open symbols: before pulmonary rehabilitation. Closed symbols: after pulmonary rehabilitation. Squares represent VT–ventilation inflection points.
Figure 2.
Exercise variables before and after pulmonary rehabilitation between the peak V’E increase and non-increase groups. Data are presented as means (standard error). All patients were divided into two groups based on whether or not V’E at peak exercise had increased after pulmonary rehabilitation. V’E: minute ventilation; inc.: increase; fR: breathing frequency; VT: tidal volume; V’CO2: carbon dioxide output; SpO2: percutaneous oxygen saturation; PetCO2: partial pressure of end-tidal carbon dioxide. * p < 0.05: mean differences between pre-and post-pulmonary rehabilitation values at each exercise phase were compared between peak V’E increase and non-increase groups. Open symbols: before pulmonary rehabilitation. Closed symbols: after pulmonary rehabilitation. Squares represent VT–ventilation inflection points.
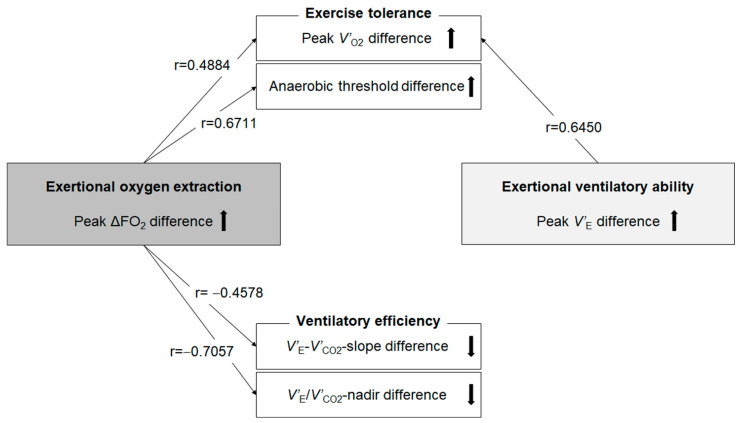
Next, we investigated whether the change in ΔFO2 at peak exercise following PR correlated with changes in the other CPET variables, as shown in Table 6 and Figure 3. The difference in ΔFO2 at peak exercise resulting from PR correlated positively with the difference following PR in peak V’O2 (r = 0.4884, p = 0.0019) and V’O2 at the anaerobic threshold (r = 0.6711, p = 0.0001) and correlated negatively with the difference following PR in fR (r = −0.3894, p = 0.0157), the difference in V’E/V’CO2-nadir during exercise (r = −0.7057, p < 0.0001) and the difference in V’E–V’CO2-slope (r = −0.4578, p = 0.0039). The change in PetCO2 at peak exercise following PR correlated with the difference in V’E/V’CO2-nadir during exercise (r = −0.5227, p < 0.0001) and the difference in V’E–V’CO2-slope (r = −0.3448, p = 0.0340), although the significance of this was lower than the correlation with the change in ΔFO2. In addition, the change in ΔFO2 at peak exercise following PR was positively correlated with the time-slope before PR (r = 0.4120, p = 0.0102). No significant correlation was confirmed between the change in V’E at peak exercise and the change in V’E/V’CO2-nadir during exercise (r = 0.0795, p = 0.6352), and the change in V’E–V’CO2-slope (r = 0.0915, p = 0.5850) or between the change in V’E and the change in ΔFO2 at peak exercise following PR (r = −0.0988, p = 0.5552) (Table 6).
Table 6.
Correlations between the change in the ΔFO2 at peak exercise resulting from pulmonary rehabilitation and other parameters (n = 38).
| r | p-Value | |
|---|---|---|
| Dyspnea at peak exercise diff., Borg scale | 0.1361 | 0.4153 |
| Peak V’O2 diff., mL·min−1 | 0.4884 | 0.0019 |
| V’O2 at anaerobic threshold diff., mL·min−1 | 0.6711 | 0.0001 |
| V’E at peak exercise diff., L·min−1 | −0.0988 | 0.5552 |
| VT at peak exercise diff., mL | 0.2655 | 0.1072 |
| fR at peak exercise diff., breaths·min−1 | −0.3894 | 0.0157 |
| VD/VT at peak exercise diff. | −0.2428 | 0.1419 |
| O2 pulse at peak exercise diff., mL·beats−1 | 0.2547 | 0.1228 |
| V’E/V’CO2-nadir diff. | −0.7057 | <0.0001 |
| V’E–V’CO2-slope diff. | −0.4578 | 0.0039 |
| Time slope diff., s·mL−1·min | −0.4518 | 0.0044 |
ΔFO2: difference between inspiratory and expiratory O2 concentrations; Diff.: value after pulmonary rehabilitation minus the value before pulmonary rehabilitation; V’O2: oxygen uptake; V’E: minute ventilation; VT: tidal volume; fR: breathing frequency; VD/VT: physiological dead space/tidal volume ratio; O2 pulse: V’O2/heart rate; V’CO2: carbon dioxide output; V’E/V’CO2-nadir: lowest value of the ratio between minute ventilation and carbon dioxide output during exercise (see the Methods section for details); V’E–V’CO2-slope: the slope was determined by linear regression analysis of minute ventilation to carbon dioxide output obtained during exercise (see the Methods section for details); Time slope: ratio of exercise time until exhaustion to obtained ΔV’O2 (peak minus resting V’O2) during cardiopulmonary exercise testing (see the Methods section for details).
Figure 3.
Correlations related to exercise tolerance and ventilatory efficiency. Difference: between before and after pulmonary rehabilitation; ΔFO2: difference between inspired and expired O2 concentration; V’E: minute ventilation; V’E/V’CO2-nadir: lowest value of the ratio between minute ventilation and carbon dioxide output during exercise (see the Methods section for details); V’E–V’CO2-slope: the slope was determined by linear regression analysis of minute ventilation to carbon dioxide output observed during exercise (see the Methods section for details); V’O2: oxygen uptake. Black arrows mean the up and down differences obtained from pulmonary rehabilitation.
4. Discussion
The present study aimed to investigate whether PR leads to an increase in incremental effort evaluated by CPET and whether the resultant change in O2 extraction affects the exertional pathophysiological conditions after PR in advanced COPD patients. The main observations were as follows. First, peak V’O2 was increased in only 14 (37%) patients. Second, in the peak ΔFO2 increase group (17 of 38 patients), exercise tolerance and ventilatory efficiency were improved by PR without an increase of V’E. In the peak V’E increase group (20 of 38 patients), although peak V’O2 was significantly increased, ventilatory efficiency did not improve. Third, in all the patients, the difference in O2 extraction at peak exercise before and after PR correlated positively with the difference in exercise tolerance, and negatively with the difference in ventilatory efficiency.
Increasing O2 extraction, which is evaluated as ΔFO2 in CPET, would help improve exercise tolerance, including the anaerobic threshold and ventilatory efficiency, particularly in patients with advanced COPD (Figure 3). Based on the Fick principle, V’O2 is the product of cardiac output and the difference between arterial and mixed venous oxygen content. The difference in arteriovenous oxygen content reflects O2 extraction by the muscles [26]. In CPET, only ventilatory flow and the inspired and expired concentrations of O2 and CO2 are directly measured at the mouth; all other variables are calculated using these measurements. In CPET, V’O2 is calculated using the product of V’E and ΔFO2 [6,13]. The latter reflects total O2 extraction related to cardiopulmonary–muscle crosstalk in the body. Of note, ΔFO2 did not correlate with V’E at peak exercise in our previous study [19]. Furthermore, the change in ΔFO2 at peak exercise following PR did not correlate with the change in V’E after PR in the present study (Table 6). These findings suggest that ΔFO2 might be usable for CPET evaluations independent of V’E. However, little is known about whether this total O2 extraction affects exertional pathophysiological conditions including airflow limitation, cardiac dysfunction and metabolic changes in COPD patients. In the peak ΔFO2 increase and non-increase groups, no significant treatment changes in VT at the inflection point during exercise, in Ti/Ttot at peak exercise and in O2-pulse, i.e., the product of the stroke volume and arterial–venous O2 content difference, were observed (Table 4 and Figure 1); that is, the changed levels following PR of the mechanical constraints on VT and the prolonged expiration pattern during exercise due to wasted ventilation and the changed levels of cardiac dysfunction were similar between the two groups. In addition, the difference in ΔFO2 at peak exercise resulting from PR correlated with the difference following PR in peak V’O2 and the anaerobic threshold (Table 6 and Figure 3), but it did not correlate with the difference following PR in V’E or O2-pulse at peak exercise (Table 6). The response that the higher oxygen extraction obtained from PR improved exercise tolerance shifting the anaerobic threshold point to the late exercise phase might be caused by improved muscle condition related to O2 extraction rather than a direct cardiopulmonary mechanism in the cardiopulmonary-muscle crosstalk. Our previous report [19] and the present study demonstrated that ΔFO2 at peak exercise and the change in ΔFO2 following PR, which is a gas exchange parameter related to O2, rather than CO2-related variables, such as PetCO2, had a stronger inverse relationship with V’E–V’CO2 slope and the change in the V’E–V’CO2 slope following PR, respectively. These findings suggest that the V’E–V’CO2 slope is a comprehensive variable that reflects not only CO2 gas exchangeability, but also O2 extraction ability. Not only that, they might illustrate the response, as shown in Figure 3, that higher oxygen extraction from PR had on improving ventilatory efficiency, as well as shifting the highest V’O2 point without developing an exertional acidosis to the late exercise phase, and they were associating with each other. The response might be the reason why fR at peak exercise was reduced after PR in the peak ΔFO2 increase group (Table 4). In contrast, in the peak V’E increase group, peak V’O2 increased, but the increase in V’E after PR depended on tachypnea, and ventilatory efficiency was not improved after PR (Table 5 and Figure 2). Given the evidence that V’E–V’CO2 slope is a prognostic factor in several chronic cardiopulmonary diseases independent of other exercise-related variables such as peak V’O2 [18], the present results suggest that much attention should be paid to the clinical information about O2 extraction. In terms of pre-PR parameters, only the time-slope in the peak ΔFO2 increase group was higher than that in the peak ΔFO2 non-increase group. In addition, the change in ΔFO2 at peak exercise resulting from PR was positively correlated with the time-slope before PR. These findings indicate that the more gently patients exercised to obtain a certain V’O2 before PR, the larger was the change in O2 extraction obtained after PR. Furthermore, in our previous study, the ΔFO2–V’CO2 slope during exercise correlated positively with the PaCO2–V’CO2 slope, that is, the degree of exertional respiratory acidosis in COPD patients [19]. Although a slower rate of change in V’O2 for a given change in work rate is generally recognized in CPET responses, indicating poor O2 delivery or extraction [13], these findings suggest that increasing O2 extraction would not only improve exercise tolerance and ventilatory efficiency but might also play a compensatory or protective role during exercise in advanced COPD patients.
Improving exercise tolerance, especially incremental effort, following PR might be difficult in some patients, particularly in those with advanced COPD [11,12], although various strategies for improving exercise tolerance in COPD patients have been studied. Surprisingly, in the present study, PR resulted in a decrease in dyspnea at peak exercise even in the peak ΔFO2 non-increase group, despite the lack of improvement in incremental effort. This could be explained by the assumption that less incremental effort required for exercise resulting from PR might have led to a decrease in dyspnea reducing O2 extraction because exercise tolerance and ventilatory efficiency could not improve sufficiently following PR. Meanwhile, given that international position statements recommend that PR programs should offer exercise training for 8–10 weeks, which is longer than the duration of PR in the present study [7], we speculate that a longer PR program might increase ΔFO2, leading to improved exercise tolerance and hence might be needed in certain advanced COPD patients in whom an inability to improve incremental effort is expected. Though the investigation did not specifically address for treatment change in ΔFO2 evaluated by CPET, we fortunately found that oxygen extraction was improved by the administration of ghrelin [27], which was first discovered to have a variety of effects including direct effects of vasodilation [28] and an increase in cardiac output [29]. We previously reported that activated ghrelin (acyl ghrelin) treatment without PR improved peak V’O2 in patients with severe and very severe COPD and that this effect might be attributed to the resultant improvements in cardiac function by O2 pulse and an increase in ΔFO2 rather than V’E [30]. Thus, developing appropriate strategies for improving ΔFO2, using the interaction of the pulmonary, cardiovascular and muscle crosstalk in the body, might lead to a clear understanding of the mechanism by which increasing O2 extraction improves exercise tolerance and gas exchange in COPD patients.
The present study has some limitations. First, this was a single-center study with a small number of patients, and the number of female patients was disproportionately low. Second, detailed evaluation of O2 delivery–utilization in skeletal muscles was not performed in the present study, although it has been reported that the skeletal muscle area measured by computed tomography correlates with ventilatory efficiency during CPET [31], and hence, would have correlated with O2 extraction during exercise in the present study and our previous study [19]. Third, the study included some COPD patients who regularly took short-acting muscarinic antagonists rather than long-acting muscarinic antagonists, and the number of patients receiving dual or triple therapy including inhalation therapy was low, which might have affected the results. Fourth, blood samples for blood gas analyses were not collected during CPET for the estimation of dead space values during exercise and elucidation of the pathophysiological mechanisms during exercise. Admittedly, two non-invasive estimations of dead space volume were used, but in both the peak ΔFO2 or V’E increase and non-increase groups, no significant differences in the mean change following PR in VD/VT estimated by Enghoff’s modification of Bohr’s equation and VD-intercept/VT were observed (Table 4 and Table 5). Fifth, given that the optimal test duration for CPET should be from eight to twelve minutes [13,32], the relatively short test duration in the present study might have affected the results, especially for the calculations using exertional ventilatory variables vs. V’CO2 relationship, such as the slope and Y-axis intercept. Sixth, no significant difference in improvement in exertional dyspnea at peak exercise was observed between the peak ΔFO2 increase and non-increase groups. In addition, isotime comparisons showed that Borg scale scores tended to decrease after PR (mean Borg scale score, pre-PR 6.1 ± 2.9, post-PR 4.4 ± 2.9; within-group comparison, p = 0.0797) in the peak ΔFO2 increase group, indicating that the exertional dyspnea was reduced at isotime. However, the change was not significantly different between the peak ΔFO2 increase and non-increase groups (p = 0.6177). The resultant endurance effort rather than the incremental effort obtained from PR might affect the decrease in exertional dyspnea in the peak ΔFO2 non-increase group, although it may be difficult to distinguish the effect of endurance effort if an incremental effort was not obtained from PR. Further studies including a specific treatment strategy targeting oxygen extraction might be necessary to confirm whether increasing oxygen extraction is useful for improving exertional dyspnea.
5. Conclusions
PR resulted in increased exercise tolerance in only 37% of patients with advanced COPD in the present study. The change in O2 extraction ability resulting from PR, evaluated as the ΔFO2 during exercise, correlated positively with the post-PR change in exercise tolerance and the anaerobic threshold, and negatively with the change in ventilatory efficiency. In patients with advanced COPD, it is often difficult to increase ventilatory ability because of the dynamically hyperinflated lungs. Hence, new strategies involving the improvement of O2 extraction ability are needed for better exercise tolerance and improved ventilatory efficiency.
Acknowledgments
The authors would like to thank S. Sakaguchi, S. Ito, and K. Koyama for their help with the CPET measurements.
Abbreviations
CPET: cardiopulmonary exercise testing; ΔFO2, difference between inspiratory and expiratory oxygen concentrations; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SAMA, short-acting muscarinic antagonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting β2-agonist; LABA, long-acting β2-agonist; ICS, inhaled corticosteroid.
Author Contributions
All authors contributed substantially to this article. A.M. and K.M. were responsible for the study conception and design. A.M., K.M., R.M., K.T., H.H., H.Y., M.M., T.K., T.N., T.M. and H.K. were responsible for data acquisition, analysis and interpretation. A.M., K.M., R.M., K.T., H.H., H.Y., M.M., T.K., T.N., T.M. and H.K. were responsible for drafting and revising the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Clinical Research from the National Hospital Organization (number, not applicable). The funder had no role in the study design, data collection and analysis, or preparation of the manuscript.
Institutional Review Board Statement
The institutional review board of the National Hospital Organization Osaka Toneyama Medical Center approved the study protocol (approval number: TNH-A-2021022) and the protocol was in accordance with the Declaration of Helsinki for experiments involving human subjects.
Informed Consent Statement
The patients/participants provided their written informed consent to participate in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization The Top 10 Causes of Death. [(accessed on 15 October 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Laviolette L., Laveneziana P. Dyspnoea: A multidimensional and multidisciplinary approach. Eur. Respir. J. 2014;43:1750–1762. doi: 10.1183/09031936.00092613. [DOI] [PubMed] [Google Scholar]
- 3.Miki K. Motor Pathophysiology Related to Dyspnea in COPD Evaluated by Cardiopulmonary Exercise Testing. Diagnostics. 2021;11:364. doi: 10.3390/diagnostics11020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell D.E., Milne K.M., James M.D., de Torres J.P., Neder J.A. Dyspnea in COPD: New Mechanistic Insights and Management Implications. Adv. Ther. 2020;37:41–60. doi: 10.1007/s12325-019-01128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley C.M., Sciurba F.C. Diagnosis and Outpatient Management of Chronic Obstructive Pulmonary Disease: A Review. JAMA. 2019;321:786–797. doi: 10.1001/jama.2019.0131. [DOI] [PubMed] [Google Scholar]
- 6.Laviolette L., Laveneziana P. Exercise Testing in the prognostic evaluation of patients with lung and heart diseases. In: Palange P., Laveneziana P., Neder J.A., Ward S.A., editors. Clinical Exercise Testing (ERS Monograph) European Respiratory Society; Sheffield, UK: 2018. pp. 222–234. [Google Scholar]
- 7.Garvey C., Bayles M.P., Hamm L.F., Hill K., Holland A., Limberg T.M., Spruit M.A. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: An Official Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016;36:75–83. doi: 10.1097/HCR.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 8.Spruit M.A., Singh S.J., Garvey C., ZuWallack R., Nici L., Rochester C., Hill K., Holland A.E., Lareau S.C., Man W.D., et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 9.Camillo C.A., Langer D., Osadnik C.R., Pancini L., Demeyer H., Burtin C., Gosselink R., Decramer M., Janssens W., Troosters T. Survival after pulmonary rehabilitation in patients with COPD: Impact of functional exercise capacity and its changes. Int. J. Chronic Obstr. Pulm. Dis. 2016;11:2671–2679. doi: 10.2147/COPD.S113450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maekura R., Hiraga T., Miki K., Kitada S., Miki M., Yoshimura K., Yamamoto H., Kawabe T., Mori M. Personalized pulmonary rehabilitation and occupational therapy based on cardiopulmonary exercise testing for patients with advanced chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:1787–1800. doi: 10.2147/COPD.S86455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burtin C., Saey D., Saglam M., Langer D., Gosselink R., Janssens W., Decramer M., Maltais F., Troosters T. Effectiveness of exercise training in patients with COPD: The role of muscle fatigue. Eur. Respir. J. 2012;40:338–344. doi: 10.1183/09031936.00111811. [DOI] [PubMed] [Google Scholar]
- 12.Miki K., Maekura R., Kitada S., Miki M., Yoshimura K., Yamamoto H., Kawabe T., Kagawa H., Oshitani Y., Satomi A., et al. Pulmonary rehabilitation for COPD improves exercise time rather than exercise tolerance: Effects and mechanisms. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1061–1070. doi: 10.2147/COPD.S131061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserman K., Hansen J., Sue D., Stringer W., Sietsema K., Sun X.-G. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. 5th, ed. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2012. [Google Scholar]
- 14.Oga T., Nishimura K., Tsukino M., Sato S., Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: Role of exercise capacity and health status. Am. J. Respir. Crit. Care Med. 2003;167:544–549. doi: 10.1164/rccm.200206-583OC. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K., Maekura R., Hiraga T., Miki K., Kitada S., Miki M., Tateishi Y., Mori M. Identification of three exercise-induced mortality risk factors in patients with COPD. COPD J. Chronic Obstr. Pulm. Dis. 2014;11:615–626. doi: 10.3109/15412555.2014.898038. [DOI] [PubMed] [Google Scholar]
- 16.Neder J.A., Berton D.C., Arbex F.F., Alencar M.C., Rocha A., Sperandio P.A., Palange P., O’Donnell D.E. Physiological and clinical relevance of exercise ventilatory efficiency in COPD. Eur. Respir. J. 2017;49:1602036. doi: 10.1183/13993003.02036-2016. [DOI] [PubMed] [Google Scholar]
- 17.Phillips D.B., Collins S., Stickland M.K. Measurement and Interpretation of Exercise Ventilatory Efficiency. Front. Physiol. 2020;11:659. doi: 10.3389/fphys.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weatherald J., Sattler C., Garcia G., Laveneziana P. Ventilatory response to exercise in cardiopulmonary disease: The role of chemosensitivity and dead space. Eur. Respir. J. 2018;51:1700860. doi: 10.1183/13993003.00860-2017. [DOI] [PubMed] [Google Scholar]
- 19.Miki K., Tsujino K., Maekuara R., Matsuki T., Miki M., Hashimoto H., Kagawa H., Kawasaki T., Kuge T., Kida H. Oxygen Extraction Based on Inspiratory and Expiratory Gas Analysis Identifies Ventilatory Inefficiency in Chronic Obstructive Pulmonary Disease. Front. Physiol. 2021;12:703977. doi: 10.3389/fphys.2021.703977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GOLD Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report) [(accessed on 15 October 2021)]. Available online: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf.
- 21.Miki K., Maekura R., Nagaya N., Nakazato M., Kimura H., Murakami S., Ohnishi S., Hiraga T., Miki M., Kitada S., et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: A multicenter, randomized, double-blind, placebo-controlled trial. PLoS ONE. 2012;7:e35708. doi: 10.1371/journal.pone.0035708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crapo R.O., Hankinson J.L., Irvin C., MacIntyre N.R., Voter K.Z., Wise R.A., Graham B., O’Donnell C., Paoletti P., Roca J., et al. Standardization of Spirometry: 1994 Update. Am. J. Respir. Crit. Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 23.Woo M.A., Moser D.K., Stevenson L.W., Stevenson W.G. Six-minute walk test and heart rate variability: Lack of association in advanced stages of heart failure. Am. J. Crit. Care. 1997;6:348–354. doi: 10.4037/ajcc1997.6.5.348. [DOI] [PubMed] [Google Scholar]
- 24.Gargiulo P., Apostolo A., Perrone-Filardi P., Sciomer S., Palange P., Agostoni P. A non invasive estimate of dead space ventilation from exercise measurements. PLoS ONE. 2014;9:e87395. doi: 10.1371/journal.pone.0087395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hey E.N., Lloyd B.B., Cunningham D.J., Jukes M.G., Bolton D.P. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir. Physiol. 1966;1:193–205. doi: 10.1016/0034-5687(66)90016-8. [DOI] [PubMed] [Google Scholar]
- 26.Whipp B.J., Ward S.A. Cardiopulmonary coupling during exercise. J. Exp. Biol. 1982;100:175–193. doi: 10.1242/jeb.100.1.175. [DOI] [PubMed] [Google Scholar]
- 27.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 28.Okumura H., Nagaya N., Enomoto M., Nakagawa E., Oya H., Kangawa K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J. Cardiovasc. Pharmacol. 2002;39:779–783. doi: 10.1097/00005344-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Nagaya N., Uematsu M., Kojima M., Ikeda Y., Yoshihara F., Shimizu W., Hosoda H., Hirota Y., Ishida H., Mori H., et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430–1435. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 30.Miki K., Kitada S., Miki M., Hui S.P., Shrestha R., Yoshimura K., Tsujino K., Kagawa H., Oshitani Y., Kida H., et al. A phase II, open-label clinical trial on the combination therapy with medium-chain triglycerides and ghrelin in patients with chronic obstructive pulmonary disease. J. Physiol. Sci. 2019;69:969–979. doi: 10.1007/s12576-019-00716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tashiro H., Takahashi K., Tanaka M., Sadamatsu H., Kurihara Y., Tajiri R., Takamori A., Naotsuka H., Imaizumi H., Kimura S., et al. Skeletal muscle is associated with exercise tolerance evaluated by cardiopulmonary exercise testing in Japanese patients with chronic obstructive pulmonary disease. Sci. Rep. 2021;11:15862. doi: 10.1038/s41598-021-95413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchfuhrer M.J., Hansen J.E., Robinson T.E., Sue D.Y., Wasserman K., Whipp B.J. Optimizing the exercise protocol for cardiopulmonary assessment. J. Appl. Physiol. 1983;55:1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.