Abstract
We investigated changes in symptoms and quality of life (QoL) in men with moderate-to-severe lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) receiving the hexanic extract of Serenoa repens (HESr) and compared results with a matched group on watchful waiting (WW). Data was from a real-world, open-label, prospective, multicenter study. This sub-group analysis included patients with moderate-to-severe symptoms receiving either the HESr 320 mg/daily for six months (HESr) or who remained untreated for LUTS/BPH (WW). Changes in urinary symptoms and QoL were measured by IPSS and BII questionnaires. Two statistical approaches (iterative matching and propensity score pairing) were used to maximize between-group comparability at baseline. Tolerability was assessed in the HESr group. After iterative matching, data for analysis was available for 783 patients (102 WW, 681 HESr). IPSS scores improved by a mean (SD) of 3.8 (4.4) points in the HESr group and by 2.2 (4.5) points in the WW group (p = 0.002). Changes in BII score were 1.8 (2.4) points and 1.0 (2.2) points, respectively (p < 0.001). Three patients (0.9%) treated with the HESr reported mild adverse effects. Moderate-severe LUTS/BPH patients treated for six months with the HESr showed greater improvements in symptoms and QoL than matched patients on WW, with a very low rate of adverse effects.
Keywords: moderate-severe LUTS, BPH, hexanic extract of Serenoa repens, watchful waiting, quality of life, sexual function, tolerability, adverse effects
1. Introduction
Lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) are very common in men aged >40 years [1,2]. The presence of such symptoms negatively impacts patients’ quality of life [3,4], as well as the lives of patients’ partners, who have been shown to suffer sleep problems and increased anxiety deriving from their partners’ LUTS, among other effects [2,5].
Several medical treatments are available to manage patients with LUTS/BPH. Alpha-adrenergic blockers (AB), 5-alpha-reductase inhibitors (5ARI), 5-phosphodiesterase inhibitors, antimuscarinic drugs, beta-3 agonists are used to relieve LUTS, though several of them can also negatively affect sexual function and/or are associated with dizziness [1,6]. Phytotherapy compounds, which form a heterogeneous group that includes extracts from plants such as Serenoa repens, Pygeum africanum, Urtica dioica, Cucurbita pepo and others, are also commonly used to treat LUTS/BPH [1]. The hexanic extract of S. repens (HESr) in particular has been shown to be as effective as AB [7,8,9,10,11] and 6-month treatment with 5ARI [8,9] in improving symptoms and QOL in men with LUTS/BPH, but without their negative side effects, as recently recognized in the EAU Guidelines. It is frequently administered to patients with mild or moderate LUTS/BPH who wish to avoid adverse events which may be associated with other medical treatments, especially those related to sexual function [1].
Watchful waiting (WW) is an acceptable management approach for men with mild-to-moderate non-bothersome LUTS as only a relatively small proportion progress to AUR or show complications [1]. In patients with moderate-severe LUTS/BPH included in the placebo arm of the MTOPS study [12], the cumulative incidence of overall clinical progression was reported as being 17% over 4 years. Symptom worsening was the main progression event, with a cumulative incidence of 14% over that period [13].
Clearly, one advantage of WW is that it avoids the side effects associated with treatments such as AB and 5ARIs, or interactions with drugs used to treat concurrent diseases. This is important, given that it has been shown that men undergoing medical treatment for LUTS/BPH consistently prefer treatment options with a low risk of adverse events; a recent study reported that 93% prefer a treatment with no sexual side effects [14]. In that regard, the HESr has been shown to improve symptoms and QoL in BPH patients with minimal side effects which do not impact sexual function [1,7,8,15].
Given that some patients who visit the urologist with bothersome symptoms decide to remain on WW, possibly due to concerns about the adverse effects of treatments [14], or potential drug interactions, it is of interest to evaluate how the HESr compares to WW in terms of change in symptoms and QoL, and whether additional benefits are associated with any impact on sexual function or severe AEs. To date, there are no published studies reporting a comparison of the HESr vs. WW, with adequate follow-up specifically focusing on QoL.
The objective of the present study was therefore to investigate whether 6 months of treatment with the HESr in men with moderate-or-severe LUTS/BPH can improve their QoL and symptoms without negatively affecting sexual function, and to compare the outcomes with those of a matched group on WW.
2. Materials and Methods
2.1. Patients and Study Design
Data for this analysis was from the QUALIPROST study [16] (ISRCTN11815680), a longitudinal, prospective, non-interventional, multicenter, 6-month follow-up study to evaluate change in symptoms and QoL in patients with moderate to severe LUTS/BPH (baseline IPSS score ≥8 points) managed in a urological setting. The study conformed to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (http://www.strobe-statement.org/; accessed on 2 October 2021) and is described in detail in Alcaraz A et al. [16]. Patients were excluded from QUALIPROST if they had received medical treatment for BPH in the 6 months prior to inclusion, if they had received any drug treatment with a known effect on BPH symptoms (i.e., diuretics, antihistamines, or tricyclic antidepressants) at any time in the 4 weeks prior to inclusion, if they had other urinary disorders, or if they had previously undergone surgery of the lower urinary tract. As QUALIPROST was a real-world study of patient management, investigators could prescribe any of the commercially available treatments according to their usual practice. Participating clinicians could also recommend WW if considered clinically appropriate, or if preferred by the patient. Informed consent was obtained individually from all patients included in the study.
Data for the present sub-analysis was from men ≥40 years with LUTS/BPH and an IPSS score ≥8 who were treated with the HESr (Permixon® or Sereprostat® at a recommended dose of 320 mg/day) throughout the 6-month follow-up or who remained on WW over the same period.
2.2. Study Variables
Key endpoints in the QUALIPROST study were change in the mean score on the International Prostate Symptom Score (IPSS) and in the impact on QoL assessed using the Benign Prostatic Hyperplasia Impact Index (BII).
The IPSS includes 8 questions, seven of which assess symptoms of LUTS/BPH, while the eighth assesses QoL associated with the condition. The symptom items assess problems with both storage (urgency, frequency, nocturia) and voiding (incomplete emptying, intermittency, weak stream, and straining to void). The overall score on the IPSS ranges from 0 to 35 for the symptom items, with a higher score indicating more severe symptoms, and from 0 to 5 for the QoL item (item 8). Separate sub-scores can also be calculated for the storage and voiding symptoms. An improvement of >3 points on IPSS items 1–7 is considered clinically relevant [17].
The BII is a self-administered questionnaire consisting of 4 questions measuring the impact of urinary symptoms on physical discomfort, worries about health, symptom bother, and interference with usual activities during the past month [18]. Items are answered using a Likert scale, with 4 or 5 response options per item and scores range from 0 (best QoL) to 13 (worst QoL). An improvement of >0.4 points on the BII score is considered clinically relevant [17].
Both the BII and the IPSS were self-completed by patients at baseline and at the 6-month follow-up visit.
Sociodemographic data collected at baseline included age, as well as weight and height, which were used to calculate body mass index (BMI). Clinical data collected included date of onset of urinary symptoms, year of LUTS/BPH diagnosis, IPSS score, results from diagnostic tests (digital rectal exam, prostate volume, Qmax, urine analysis, serum analysis, PSA), treatment received, and information on co-morbidities and their treatment. Adverse effects potentially associated with treatment were recorded with study-specific questions at follow-up.
Treatment compliance was assessed in the HESr group using the validated Spanish version of the Haynes–Sackett questionnaire [19] which asks about: (a) patients’ difficulty taking the medication, and (b) the number of tablets they have taken in the previous month. Patients taking between 80% and 100% of the prescribed dose are considered to show good adherence.
2.3. Statistical Methods
To optimize comparability between the HESr and WW patients, the two groups were matched using two different methods. In the first one, an iterative matching procedure was used to ensure they were comparable at an aggregate level in terms of baseline IPSS and BII scores, maximum urinary flow (Qmax), prostate-specific antigen (PSA), and prostate volume. Patients were removed one by one from the HESr or WW group and the two groups were continually compared using t-tests until there were no statistically significant differences (p > 0.10) between them on any of the relevant baseline characteristics. To determine the success of the matching procedure, pre- and post-matching baseline between-group differences on the IPSS and BII total scores, the IPSS voiding and storage sub-scores, and IPSS item 7 (nocturia) were assessed using Student’s t-test for the overall sample, and for sub-groups defined by severity. The second method was a propensity score matching procedure, in which each patient in the HESr group was paired with a patient from the WW group with a similar likelihood of receiving HESr estimated using logistic regression and including the same baseline characteristics used in the iterative procedure as independent variables.
Change over time within the different treatment groups and between-group differences in the size of change on the IPSS and BII were assessed using paired t-tests or independent samples t-test, respectively. Outcomes on the IPSS storage and voiding sub-scores were also analyzed and compared between groups.
With the selected sample size of 102 controls and 680 treated patients, the study had a power of 80% to detect a 6-month minus baseline IPSS difference of at least 1.35 units, assuming a type I error of 5% and a standard deviation of 4.5 units for the between-group difference in change scores.
Quality of life (BII) analyses were performed for the samples as a whole and for two sub-groups defined by baseline severity, i.e., a moderate group with a baseline IPSS score of 8–13 and a more severe group with a baseline IPSS score of ≥14 points.
Adverse events in the HESr group were analyzed in terms of frequencies and proportions.
In all comparisons, results were considered statistically significant at p < 0.05. Statistical analyses were carried out using R 4.1.1 statistical software (https://www.r-project.org; accessed on 2 October 2021).
3. Results
3.1. Iterative Matching Sample
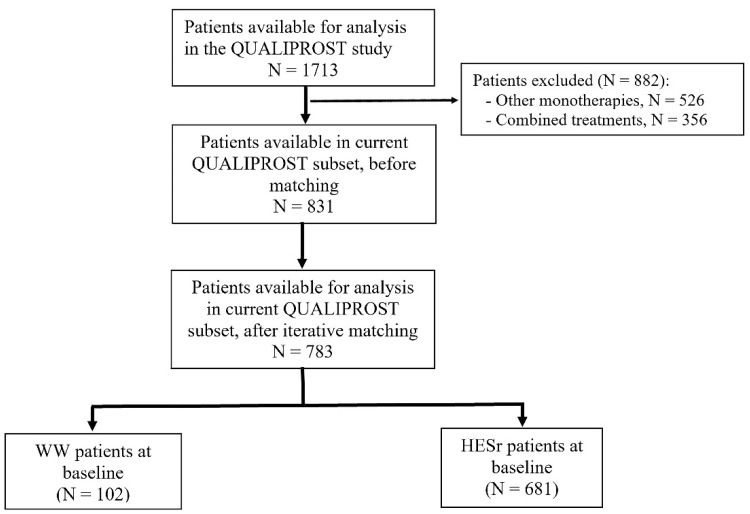
After iterative matching, data was available for analysis from a total of 783 patients (681 HESr, 102 WW) (Figure 1). Table 1 shows the sociodemographic and clinical characteristics for the HESr and WW groups at baseline. After the matching process, there were no statistically significant differences between the two groups on any of the parameters analyzed. In the HESr group, 37.7% of the patients (n = 224) at baseline had a prostate volume > 40 cm3 and a PSA ≥ 1.5 ng/mL compared to 43.5% (n = 40) in the WW group (p = 0.346) (Table S1) showing the percentage of patients with risk of progression in each group. The percentage of patients with severe LUTS/BPH was 14.7% and 17.2% in the WW and HESr groups, respectively. Rates of concomitant diseases at baseline were similar in the groups, with hypertension and dyslipidemia being the most prevalent (Table S2).
Figure 1.
Study flow-chart (iterative matching sample). HESr: hexanic extract of Serenoa repens; WW: watchful waiting.
Table 1.
Patient baseline characteristics by study group (iterative matching sample).
| HESr | WW | p Value | |||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Men (SD) | ||
| Age, years | 610 | 61.7 (9.1) | 74 | 61.9 (9.4) | 0.868 |
| BMI, Kg/m2 | 604 | 26.5 (2.9) | 72 | 26.3 (2.5) | 0.647 |
| IPSS | 681 | 15.2 (5.0) | 102 | 14.4 (4.5) | 0.114 |
| IPSS voiding subscore | 681 | 8.6 (3.2) | 102 | 8.2 (3.1) | 0.208 |
| IPSS storage subscore | 681 | 6.6 (2.4) | 102 | 6.2 (2.0) | 0.117 |
| BII | 681 | 6.0 (2.6) | 102 | 5.7 (2.5) | 0.237 |
| Time since diagnosis, years | 608 | 1.1 (2.6) | 74 | 1.4 (2.7) | 0.402 |
| Qmx, mL/s | 338 | 13.9 (3.9) | 50 | 13.7 (3.6) | 0.701 |
| Prostate volume, cc | 584 | 43.3 (15.8) | 91 | 46.2 (15.9) | 0.107 |
| PSA, ng/mL | 630 | 2.1 (1.3) | 98 | 2.1 (1.3) | 0.840 |
HESr: hexanic extract of Serenoa repens; WW: watchful waiting; BMI: body mass index; IPSS: International Prostate Symptom Score; BII: Benign Prostatic Hyperplasia Impact Index; Qmax: maximum urinary flow rate; PSA: prostate-specific antigen.
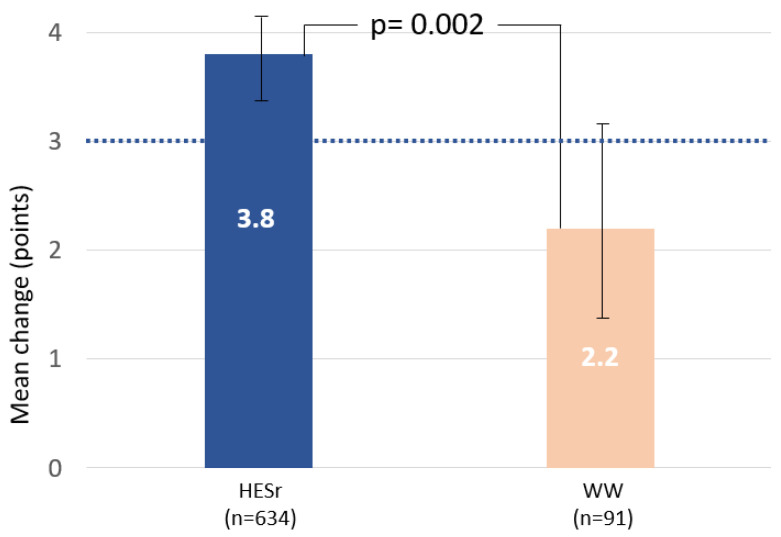
Table 2 shows the change in symptoms and QoL for the HESr and WW groups after six months of follow-up. The IPSS improvement was larger in the HESr group with a mean (SD) improvement of 3.8 (4.4) compared to 2.2 (4.5) in the WW group (p = 0.002). The changes correspond to a mean percentage improvement on the IPSS of 25% in the HESr group versus a change of 15.3% in the WW group. Symptom relief on the IPSS voiding and storage sub-scores were also showed a statistically significant improvement greater in the HESr group compared to patients in WW (p < 0.05). Mean change on the IPSS together with 95% CI is shown in Figure 2 for the two study groups.
Table 2.
Improvements from baseline to 6-month follow-up in symptoms and quality of life (iterative matching sample).
| HESr | WW | p Value | |||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | % * | N | Mean (SD) | % * | ||
| IPSS total | 634 | 3.8 (4.4) | 25.0 | 91 | 2.2 (4.5) | 15.3 | 0.002 |
| IPSS voiding subscore | 634 | 2.1 (2.9) | 24.4 | 91 | 1.0 (2.8) | 12.2 | 0.001 |
| IPSS storage subscore | 634 | 1.7 (2.1) | 25.8 | 91 | 1.2 (2.1) | 19.4 | 0.039 |
| BII total | 632 | 1.8 (2.4) | 30.0 | 91 | 1.0 (2.2) | 17.5 | <0.001 |
| Physical discomfort | 631 | 0.4 (0.8) | 26.7 | 91 | 0.3 (0.8) | 20.0 | 0.119 |
| Worry about the health | 631 | 0.5 (0.9) | 31.3 | 91 | 0.3 (0.7) | 18.8 | 0.002 |
| Bothered with urination | 631 | 0.4 (0.8) | 28.6 | 91 | 0.2 (0.7) | 15.4 | 0.015 |
| Daily activity limitation | 631 | 0.5 (0.8) | 35.7 | 91 | 0.2 (0.8) | 15.4 | 0.002 |
HESr: hexanic extract of Serenoa repens; WW: watchful waiting; IPSS: International Prostate Symptom Score; BII: Benign Prostatic Hyperplasia Impact Index; * Percentage of improvement over initial values.
Figure 2.
Mean (95% CI) improvement in IPSS total score from baseline to 6 months for the HESr and WW groups. Dotted line indicates the level at which a clinically relevant difference is observed. HESr: hexanic extract of Serenoa repens; WW: watchful waiting; IPSS: International Prostate Symptom Score.
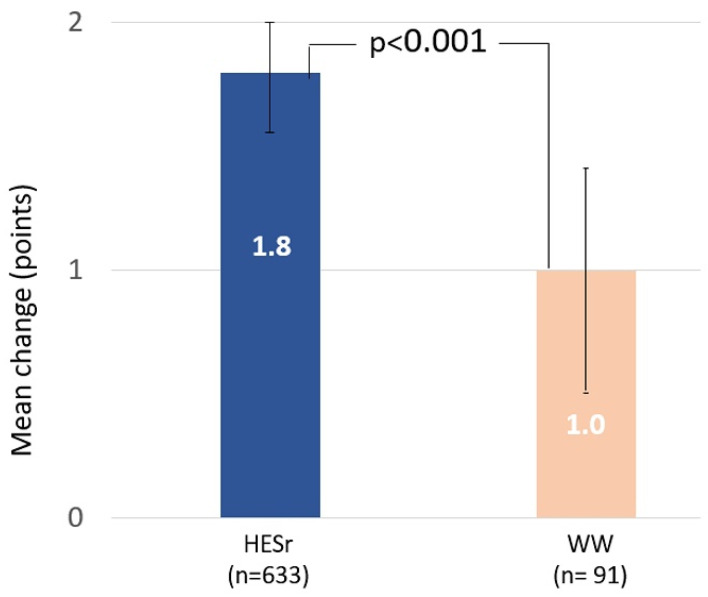
The improvement in QoL was also greater in the HESr group, with a total mean (SD) change score of 1.8 (2.4) points on the BII, representing a 30% improvement, compared to a mean change of 1.0 (2.2) point, or a 17.5% improvement, in the WW group (p < 0.001). BII change for each study group with its 95% CI is shown in Figure 3. The HESr group showed statistically significant greater improvements on 3 of the 4 BII items compared to those on WW, with larger reductions in score (improved QoL) on the items assessing concern about health, bother with urination, and impact on daily activities (Table 2). When analyzing the BII results based on the severity of baseline symptoms (IPSS 8–13 or IPSS ≥ 14), we observed that the improvement in QoL was greater in the HESr patients than in the WW group in both cases (p < 0.04, Table S3).
Figure 3.
Mean (95% CI) improvement in BII total score from baseline to 6 months for the HESr and WW groups. HESr: hexanic extract of Serenoa repens; WW: watchful waiting; BII: Benign Prostatic Hyperplasia Impact Index.
As this was an observational study in which clinicians applied their usual criteria for requesting clinical tests, the number of patients with Qmax and PSA data available at follow-up was substantially smaller than the number with full IPSS and BII results at the same time point. In the WW group, the number of patients with follow-up Qmax and PSA data (n = 20) was too small to allow for appropriate analysis, so follow-up results are not provided here. Good adherence was reported by 98.7% of the patients in the HESr group.
Six (0.9%) patients reported AEs, with five patients (0.7%) reporting constipation and one patient (0.2%) nausea.
3.2. Propensity Score Matching
The propensity score sample was composed of a total of 140 patients (92 HESr, 48 WW) (Figure S1). There were no statistically significant differences in any baseline characteristics between the two study groups (Table S4) nor in the percentage of patients showing risk of progression characteristics in each group (Table S5). Prevalence of concomitant diseases at baseline is described in Table S6.
Results from the propensity score-matched sample were very similar to those reported for the iteratively matched sample and are shown in Table S7. IPSS (SD) scores improved by 4.2 (4.2) points in the HESr group and by 1.6 (3.3) points in the WW patients (p < 0.001) (Figure S2). IPSS voiding and storage sub-scores also improved significantly in the HESr group compared to the WW group (p < 0.004). For QoL, mean (SD) BII scores improved by 2.1 (2.3) and 0.8 (1.7) points in the HESr and WW groups, respectively (p < 0.001) (Figure S3). The biggest differences between the two study groups were on the BII items assessing bother with urination, and limitations on daily activities.
The pattern of AEs reported was similar to that seen in the iteratively matched sample, with 3 patients (3.3%) reporting an AE, either constipation (2 patients, 2.2%) or nausea (1 patient, 1.1%).
4. Discussion
To date, this is the first study to compare changes in symptoms and QoL in patients treated with the HESr vs. WW. Patients included had moderate-severe LUTS/BPH at baseline and data were collected in a real-world setting, which may make the results more generalizable. After six months of follow-up, patients receiving the HESr reported better outcomes in terms of symptoms and QoL than patients managed with watchful waiting. The focus on QoL in the present study and the inclusion of patients with moderate-severe symptoms adds to evidence from earlier studies which studied the effects of the HESr in patients with mild symptoms [20], or reported the effect on clinical symptoms, rather than QoL [21,22], or which focused on the HESr’s anti-inflammatory action [23].
The improvement in symptoms observed in the present sub-analysis was similar to that previously observed in RCTs which have investigated the HESr. In particular, Debruyne et al. [7] reported a decrease in IPSS score of 4.4 points in patients receiving the HESr after 12 months of treatment and Latil et al. [24] found a reduction of 4.3 points on the IPSS in patients receiving the HESr. The reduction in IPSS score is in line with that seen in previous articles evaluating the efficacy of the HESr. In a study comparing the HESr with tamsulosin to treat LUTS/BPH, the IPSS score improved by 4.5 points in the HESr group [11]. In another publication comparing the HESr and tamsulosin versus a combination of the two, the IPSS score decreased by 5.4 points in the HESr group [10].
Other studies have reported no difference between an alcoholic extract of S. repens [25] or carbon dioxide Serenoa repens extract [26] and placebo in terms of the effect on symptoms and placebo. However, it should be noted that, as stated in the EAU Guidelines [1], there are differences between these extracts and the HESr which means that findings observed with one brand of S. repens cannot be extrapolated to other brands. In a recently updated report, the European Medicines Agency states that, as opposed to alcoholic and carbon dioxide extracts, the HESr is the sole S. repens extract with sufficient evidence to support it as well-established for treatment of LUTS/BPH [15].
Another important finding of the present analysis is that patients receiving the HESr showed greater improvement in QoL than those on WW. A decrease of >0.4 points on the BII has been shown to be considered relevant by patients [17], whereas the difference in the size of change on the BII between our two study groups was 0.8 points, which considerably exceeds that threshold, in favor of the HESr group. The improvement of 1.8 points on the BII in the HESr group is similar to that observed for other drugs used to treat LUTS/BPH, such as dutasteride and tamsulosin, which showed improvements of 1.7 points and 1.5 points, respectively, after 2 years of treatment in the COMBAT trial [27]. Improvements on the BII were reported in other publications evaluating the efficacy of the HESr; Alcaraz et al. [11] reported a mean reduction of 2.2 points for patients receiving the HESr in a comparison with tamsulosin and a mean improvement of 2.7 points in an analysis comparing the HESr with tamsulosin and with a combination of both drugs [10].
A question that arises when analyzing these data is why patients with moderate-or-severe LUTS who visit a urologist, and who have a risk of progression (i.e., prostate volume > 40 cm3 and a PSA ≥ 1.5 ng/mL) in over 43% of cases, do not receive medical treatment. In the case of the QUALIPROST Study, such patients represented 8.9% of the total sample [16]. Reasons may include a fear of suffering adverse effects, especially on sexual function, or a desire to avoid the risk of interactions with other drugs the patient is taking; approximately 40% of patients in the QUALIPROST sample had at least one concomitant disease. A recent publication [14] indicated that men with LUTS prefer to receive a drug treatment that presents a low risk of erectile dysfunction or adverse effects related to sexual function. Other studies have reported that approximately 20% of LUTS/BPH patients treated for 12 months abandon treatment due to adverse events [28]. If clinicians and patients are aware of a medication, the HESr which is recommended in the EAU Guidelines as a treatment “for men with LUTS who want to avoid any potential adverse events especially related to sexual function” [1] and which is associated with symptom relief and improved QoL without any known drug interactions, that could potentially facilitate treatment adherence.
Finally, it should be borne in mind that LUTS affects not only patient QoL but also that of their partners. For example, Mitropoulos and colleagues found that sleep impairment was reported by 28% of partners of patients with symptomatic BPH, and alteration of social activities by 30% [5]. Findings were correlated with IPSS scores, and sex life can also be affected. Therefore, a spillover effect of the improvement seen in patients’ LUTS could be an improvement in their partners’ QoL, though that requires further investigation.
As has been shown in several previous studies [7,10,11,16], the improvements in symptoms and QoL associated with the HESr do not come at the expense of any relevant incidence of adverse effects. In the present sub-analysis, only 1% of patients receiving HESr reported an AE, and they were all classified as mild. This is an important point to consider when discussing treatment options with patients.
Strengths of the present study include the matching approaches used, which, as indicated by the lack of any statistically significant differences between groups at baseline, ensured a high level of comparability between the treatment arms. The fact that two different types of matching approaches showed similar outcomes also helps to support the reliability of the results. Another strength is that the analysis used data collected in conditions of daily clinical practice, which means the findings may better reflect patient experience than findings from randomized clinical trials, which are often performed under very controlled conditions.
The present study had also some limitations. Data were obtained without the use of randomization or blinding; patients were therefore allocated to a specific management approach based on clinician judgment and shared decision-making with patients. This could lead to a selection bias although the use of two matching strategies should help to minimize any potential bias. The limited percentage of uroflowmetries, which is similar for both groups, could also be considered a limitation although it reflects current clinical practice, which was one of the objectives of the study. Finally, another limitation could be the unbalanced baseline sample as there were more patients available in the HESr group than in the WW one, using the iterative matching approach. However, selecting a larger sample in this approach also increased statistical power. Furthermore, the propensity score matching procedure led to a more balanced sample in terms of numbers in the two groups and the results are similar to those observed using the iterative matching procedure, with no relevant differences.
Carrying out an RCT in men with moderate/severe LUTS/BPH who refuse medical treatment for their condition to avoid potential adverse events associated with LUTS/BPH drugs or drug interactions would be of interest.
5. Conclusions
As an initial treatment option in men with moderate to severe LUTS/BPH, the HESr shows relevant advantages over WW as it is associated with clinically significant improvements in both urinary symptoms and QoL with a negligible rate of only mild adverse effects.
Acknowledgments
We thank Isaac Subirana for his help with the statistical analysis, Magí Abellán Mercadé and Núria Ayala López for their management support throughout the study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11040967/s1. Figure S1: Study flow-chart (propensity score matching); Figure S2: Mean (95% CI) improvement in IPSS total score from baseline to 6 months for the HESr and WW groups (propensity score sample); Figure S3: Mean (95% CI) improvement in BII total score from baseline to 6 months for the HESr and WW groups (propensity score sample); Table S1: Patients with prostate volume > 40 cm3 and/or PSA ≥ 1.5 ng/mL at baseline by treatment group, in the iterative matched sample; Table S2: Concomitant diseases at baseline by treatment group (iterative matching sample); Table S3: Quality of life improvement by means of BII according to IPSS baseline value by treatment group (iterative matching sample); Table S4: Patient baseline characteristics by study group (propensity score sample); Table S5: Patients with prostate volume >40 cm3 and/or PSA ≥1.5 ng/mL at baseline by treatment group (propensity score sample). Table S6: Concomitant diseases at baseline by treatment group (propensity score sample); Table S7: Improvements from baseline to 6-month follow-up in symptoms and quality of life (propensity score sample).
Appendix A
The following investigators participated in the QUALIPROST Study Group:
A Coruña: Dr. L. Busto Castañón; Dr. J.E. Duarte Novo; Dr. M. Montes Couceiro; Dr. A. Rodríguez Alonso; Alicante: Dr. J.A. Canovas Ivorra; Dr. C. López López; Dr. L. Prieto Chaparro; Almería: Dr. F. Gómez Berjón; Dr. J. Hortelano Parras; Asturias: Dr. A. M. Huescar; Dr. P.L. Muntañola Armora; Badajoz: Dr. E. Godoy Rubio; Dr. J. Mariño del Real; Barcelona: Dr. J. Armona Mani; Dr. F.J. Blasco Casares; Dr. Ll. Cortadellas Angel; Dr. J. Fernández Zuazu; Dr. J.M. Malet Carreras; Dr. S. Mando Dakkar; Dr. J.M. Prats Puig; Dr. M. Puyol Pallás; Dr. J.A. Romero Martín; Dr. J. Sáenz de Cabezón Martí; Dr. J. Sánchez Macías; Dr. C. Vargas Blasco; Dr. J.M. Vayreda Martija; Bilbao: Dr. J.A. Gallego Sánchez; Dr. N. Prieto Ugidos; Cádiz: Dr. J.M. Arroyo Maestre; Dr. S. Garrido Insua; Dr. A. Gutiérrez de Pablo; Dr. M. Marmol Ruíz; Dr. F. Reyes Martínez; Castellón: Dr. J. Beltrán Persiva; Dr. L. Monzonis Rey; Dr. J. Palacios Castañeda; Ciudad Real: Dr. N. Jiménez López-Lucendo; Dr. D. Rodríguez Leal; Córdoba: Dr. J.C. Regueiro López; Granada: Dr. M. Cabezas Zamora; Guipúzcoa: Dr. G. Garmendia Olaizola; Dr. J.A. Rodríguez Andrés; Jaén: Dr. J. Jiménez Verdejo; Dr. E.J. Zarate Rodríguez; León: Dr. S.C. Gómez Cisneros; Dr. M. Lozano Rebollo; Lleida: Dr. J. Cortada Robert; Dr. L.M. Flavian Domenech; Logroño: Dr. A. Fernández Fernández; Lugo: Dr. F.J. Neira Pampin; Madrid: Dr. A. Abdallah Merhi; Dr. F. Arias Funes; Dr. I.T. Castillón Vela; Dr. J.M. Duarte Ojeda; Dr. M.J. García-Matres Cortés; Dr. J.F. Hermina Gutierrez; Dr. J.J. López-Tello García; Dr. C. Martín García; Dr. B. M.El-Awadeh; Dr. J.D. Rendón Sánchez; Dr. R. Rodríguez-Patron Rodríguez; Dr. J.C. Ruíz de la Roja; Dr. J. Vallejo Herrador; Dr. D. Vázquez Alba; Málaga: Dr. A. Bonilla Maldonado; Dr. J.M. Fernández Montero; Dr. A. Galacho Bech; Dr. C. Marchal Escalona; Dr. P. Rodero García; Dr. G. Sanz; Dr. J.D. Vázquez Cervilla; Murcia: Dr. G. Server Pastor; Pontevedra: Dr. D. Jamardo González; Dr. A. Selas Pérez; Salamanca: Dr. F. Díaz Alférez; Dr. M.F. Lorenzo Gómez; Santander: Dr. J.A. Portillo Martín; Sevilla: Dr. F.J. Giráldez Puig; Dr. M.A. Gutiérrez González; Dr. J.I. Huesa Martínez; Dr. Y. Ismail Tomaizeh; Dr. J. Leal Arenas; Dr. J. Martín Calero; Dr. J.L. Moyano Calvo; Dr. A. Ortiz Gámiz; Dr. J.M. Poyato Galán; Dr. J.A. Valero Puerta; Dr. E. Vilches Cocovi; Tarragona: Dr. P.L. Álvarez de la Red; Dr. J. Benagues Pamies; Dr. M. Prados Saavedra; Tenerife: Dr. T. Concepción Masip; Dr. C. Gómez de Segura Melcon; Dr. E. González de Chaves; Dr. J. Postius Robert; Toledo: Dr. F. Álvarez Fernández; Dr. A. Iglesias Justo; Dr. A. Melchor Galán; Valencia: Dr. J.E. Blasco Alfonso; Dr. M.A. Bonillo García; Dr. A. Collado Serra; Dr. J.A. García Cebrián; Dr. L.García Reboll; Dr. Y. Pallás Costa; Dr. M.J. Sánchez Sanchiz; Dr. J. Santamaría Meseguer; Zaragoza: Dr. A. García de Jalón Martínez; Dr. J.M. Gil Fabra; Dr. F. Monzón Alebesque; Dr. A. Ucar Terren.
Author Contributions
Conceptualization, V.F., A.A. and J.M.; methodology, V.F., M.H. and J.M.; validation, M.G., V.F., J.C.A. and J.M.-P. formal analysis, M.G., M.H. and J.M.; investigation, S.B.-T., J.C.-R., A.A. and the QUALIPROST Study Group; data curation, A.A., J.C.-R., J.M.-P. and J.M.; writing—original draft preparation, M.H. and J.M.; writing—review and editing, A.A., M.G., V.F., J.M.-P., A.S., J.M.F.-G., A.C., D.C.-D., A.R.-A., J.C.-R., J.M.C.-O., S.B.-T., N.P.-L., F.J.B.-B., J.M.M.-G., A.F.-P.L., M.H., J.M. and J.C.A.; visualization, M.G., M.H., J.C.A. and J.M.-P., supervision, J.C.A. and M.H.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted educational grant provided by Pierre Fabre Ibérica S.A.
Institutional Review Board Statement
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Ethical approval for the original QUALIPROST Study was obtained from the Ethics Committee of the Puerta de Hierro Majadahonda University Hospital in Madrid, Spain.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Full data will be provided by correspondence author upon reasonable request.
Conflicts of Interest
D.C.-D.: speaker for Astellas, Medtronic, Nobel, Recordati, Boston Scientific and consultant for Bayer; M.G.: trial participation and speaker honorarium for Pierre Fabre; J.M.-P.: speaker for Astellas Pharma, Boston Scientific, Coloplast, Gebro Pharma, GSK, Lacer, Pierre Fabre and Wellspect; F.J.B.-B.: speaker and consultant for GSK, Almirall, Pierre Fabre Ibérica, Astellas, Lacer, Recordati, Lilly, Pfizer, Ferrer and Zambon; M.H.: honoraria or consulting fees from Pierre Fabre, Novartis, and Merck; J.M. is a medical advisor with Pierre Fabre Ibérica S.A., a company that commercializes an extract of Serenoa repens. The other authors do not declare any conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gravas S., Bach T., Bachmann A., Drake M., Gacci M., Gratzke C., Herrmann T.R.W., Mamoulakis C., Rieken M., Speakman M.J., et al. EAU Guidelines on Management of Non-Neurogenic Male LUTS incl. Benign Prostatic Obstruction. [(accessed on 16 December 2021)]. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-on-Management-of-Non-Neurogenic-Male-LUTS-2021.pdf.
- 2.Speakman M., Kirby R., Doyle S., Ioannou C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)-focus on the UK. BJU Int. 2015;115:508–519. doi: 10.1111/bju.12745. [DOI] [PubMed] [Google Scholar]
- 3.Coyne K.S., Wein A.J., Tubaro A., Sexton C.C., Thompson C.L., Kopp Z.S., Aiyer L.P. The burden of lower urinary tract symptoms: Evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 4.Song H.J., Han M.A., Kang H.C., Park K.S., Kim K.S., Kim M.K., Kang J., Park E.O., Hyun M.Y., Kim C.S. Impact of lower urinary tract symptoms and depression on health-related quality of life in older adults. Int. Neurourol. J. 2012;16:132–138. doi: 10.5213/inj.2012.16.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitropoulos D., Anastasiou I., Giannopoulou C., Nikolopoulos P., Alamanis C., Zervas A., Dimopoulos C. Symptomatic benign prostate hyperplasia: Impact on partners’ quality of life. Eur. Urol. 2002;41:240–245. doi: 10.1016/S0302-2838(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 6.Gacci M., Ficarra V., Sebastianelli A., Corona G., Serni S., Shariat S.F., Maggi M., Zattoni F., Carini M., Novara G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex Med. 2014;11:1554–1566. doi: 10.1111/jsm.12525. [DOI] [PubMed] [Google Scholar]
- 7.Debruyne F., Koch G., Boyle P., Da Silva F.C., Gillenwater J.G., Hamdy F.C., Perrin P., Teillac P., Vela-Navarrete R., Raynaud J.P. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur. Urol. 2002;41:497–506. doi: 10.1016/S0302-2838(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 8.Novara G., Giannarini G., Alcaraz A., Cózar-Olmo J.M., Descazeaud A., Montorsi F., Ficarra V. Efficacy and Safety of Hexanic Lipidosterolic Extract of Serenoa repens (Permixon) in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: Systematic Review and Meta-analysis of Randomized Controlled Trials. Eur. Urol. Focus. 2016;2:553–561. doi: 10.1016/j.euf.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Vela-Navarrete R., Alcaraz A., Rodríguez-Antolín A., López B.M., Fernández-Gómez J.M., Angulo J.C., Diaz D.C., Romero-Otero J., Brenes F.J., Carballido J., et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomized controlled trials and observational studies. BJU Int. 2018;122:1049–1065. doi: 10.1111/bju.14362. [DOI] [PubMed] [Google Scholar]
- 10.Alcaraz A., Rodríguez-Antolín A., Carballido-Rodríguez J., Castro-Díaz D., Esteban-Fuertes M., Cózar-Olmo J.M., Ficarra V., Medina-López R., Fernández-Gómez J.M., Angulo J.C., et al. Clinical benefit of tamsulosin and the hexanic extract of Serenoa Repens, in combination or as monotherapy, in patients with moderate/severe LUTS-BPH: A subset analysis of the QUALIPROST study. J. Clin. Med. 2020;9:2909. doi: 10.3390/jcm9092909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alcaraz A., Rodríguez-Antolín A., Carballido-Rodríguez J., Castro-Díaz D., Medina-Polo J., Fernández-Gómez J.M., Ficarra V., Palou J., Ponce de León Roca J., Angulo J.C., et al. Efficacy and tolerability of the hexanic extract of Serenoa repens compared to tamsulosin in moderate-severe LUTS-BPH patients. Sci. Rep. 2021;11:19401. doi: 10.1038/s41598-021-98586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McConnell J.D., Roehrborn C.G., Bautista O.M., Andriole G.L., Jr., Dixon C.M., Kusek J.W., Lepor H., McVary K.T., Nyberg L.M., Jr., Clarke H.S., et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N. Engl. J. Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick J.M. The natural history of benign prostatic hyperplasia. BJU Int. 2006;97:3–6. doi: 10.1111/j.1464-410X.2006.06097.x. [DOI] [PubMed] [Google Scholar]
- 14.Malde S., Umbach R., Wheeler J.R., Lytvyn L., Cornu J.N., Gacci M., Gratzke C., Herrmann T.R.W., Mamoulakis C., Rieken M., et al. A Systematic Review of Patients’ Values, Preferences, and Expectations for the Diagnosis and Treatment of Male Lower Urinary Tract Symptoms. Eur. Urol. 2021;79:796–809. doi: 10.1016/j.eururo.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency . Assessment Report on Serenoa Repens (W. Bartram) Small, Fructus. European Medicines Agency; Amsterdam, The Netherlands: 2015. [(accessed on 25 October 2021)]. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-serenoa-repens-w-bartram-small-fructus_en.pdf. [Google Scholar]
- 16.Alcaraz A., Rodriguez J.C., Unda-Urzaiz M., Medina-Lopez R., Ruiz-Cerdá J.L., Rodríguez-Rubio F., García-Rojo D., Brenes-Bermúdez F.J., Cózar-Olmo J.M., Baena-González V., et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: Change over time in real-life practice according to treatment–the QUALIPROST study. Int. Urol. Nephrol. 2016;48:645–656. doi: 10.1007/s11255-015-1206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry M.J., Williford W.O., Chang Y., Machi M., Jones K.M., Walker-Corkery E., Lepor H. Benign prostatic hyperplasia specific health status measures in clinical research: How much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J. Urol. 1995;154:1770–1774. doi: 10.1016/S0022-5347(01)66780-6. [DOI] [PubMed] [Google Scholar]
- 18.Barry M.J., Fowler F.J., Jr., O’Leary M.P., Bruskewitz R.C., Holtgrewe H.L., Mebust W.K. Measuring disease-specific health status in men with benign prostatic hyperplasia. Measurement Committee of The American Urological Association. Med. Care. 1995;33:AS145–AS155. [PubMed] [Google Scholar]
- 19.Haynes R.B., Sackett D.L., Gibson E.S., Taylor D.W., Hackett B.C., Roberts R.S., Johnson A.L. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1:1265–1268. doi: 10.1016/S0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 20.Djavan B., Fong Y.K., Chaudry A., Reissigl A., Anagnostou T., Bagheri F., Waldert M., Fajkovic H., Marihart S., Harik M., et al. Progression delay in men with mild symptoms of bladder outlet obstruction: A comparative study of phytotherapy and watchful waiting. World J. Urol. 2005;23:253–256. doi: 10.1007/s00345-005-0005-7. [DOI] [PubMed] [Google Scholar]
- 21.Vela Navarrete R., Garcia Cardoso J.V., Barat A., Manzarbeitia F., López Farré A. BPH and inflammation: Pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double-blind pilot clinical assay. Eur. Urol. 2003;44:549–555. doi: 10.1016/S0302-2838(03)00368-3. [DOI] [PubMed] [Google Scholar]
- 22.Samarinas M., Karatzas A., Tzortzis V., Gravas S. The clinical Impact of hexanic extract of Serenoa repens in men with prostatic inflammation: A post hoc analysis of a randomized biopsy study. J. Clin. Med. 2020;9:957. doi: 10.3390/jcm9040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gravas S., Samarinas M., Zacharouli K., Karatzas A., Tzortzis V., Koukoulis G., Melekos M. The effect of hexanic extract of Serenoa repens on prostatic inflammation: Results from a randomized biopsy study. World J. Urol. 2019;37:539–544. doi: 10.1007/s00345-018-2409-1. [DOI] [PubMed] [Google Scholar]
- 24.Latil A., Pétrissans M.T., Rouquet J., Robert G., de la Taille A. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate. 2015;75:1857–1867. doi: 10.1002/pros.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry M.J., Meleth S., Lee J.Y., Kreder K.J., Avins A.L., Nickel J.C., Roehrborn C.G., Crawford E.D., Foster H.E., Jr., Kaplan S.A., et al. Effect of increasing doses of saw palmetto extract on lower urinary tract symptoms: A randomized trial. JAMA. 2011;306:1344–1351. doi: 10.1001/jama.2011.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bent S., Kane C., Shinohara K., Neuhaus J., Hudes E.S., Goldberg H., Avins A.L. Saw palmetto for benign prostatic hyperplasia. N. Engl. J. Med. 2006;354:557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 27.Barkin J., Roehrborn C.G., Siami P., Haillot O., Morrill B., Black L., Montorsi F., CombAT Study Group Effect of dutasteride, tamsulosin and the combination on patient-reported quality of life and treatment satisfaction in men with moderate-to-severe benign prostatic hyperplasia: 2-year data from the CombAT trial. BJU Int. 2009;103:919–926. doi: 10.1111/j.1464-410X.2009.08196.x. [DOI] [PubMed] [Google Scholar]
- 28.Koh J.S., Cho K.J., Kim H.S., Kim J.C. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int. J. Clin. Pract. 2014;68:197–202. doi: 10.1111/ijcp.12241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full data will be provided by correspondence author upon reasonable request.