Abstract
The vaginal microbiome of healthy women contains nondiphtheria corynebacteria. The role and functions of nondiphtheria corynebacteria in the vaginal biotope are still under study. We sequenced and analysed the genomes of three vaginal C. amycolatum strains isolated from healthy women. Previous studies have shown that these strains produced metabolites that significantly increased the antagonistic activity of peroxide-producing lactic acid bacteria against pathogenic and opportunistic microorganisms and had strong antimicrobial activity against opportunistic pathogens. Analysis of the C. amycolatum genomes revealed the genes responsible for adaptation and survival in the vaginal environment, including acid and oxidative stress resistance genes. The genes responsible for the production of H2O2 and the synthesis of secondary metabolites, essential amino acids and vitamins were identified. A cluster of genes encoding the synthesis of bacteriocin was revealed in one of the annotated genomes. The obtained results allow us to consider the studied strains as potential probiotics that are capable of preventing the growth of pathogenic microorganisms and supporting colonisation resistance in the vaginal biotope.
Keywords: Corynebacterium amycolatum, vaginal microbiome, genome, secondary metabolism, bacteriocin
1. Introduction
The vaginal microbiome is an open complex multicomponent system in dynamic equilibrium [1]. The vaginal microbiome is represented by various microbial communities containing bacteria that can synthesize organic acids, including lactic acid, maintaining a vaginal pH of 3.8–4.4, thereby supporting women’s health [2,3]. It is generally accepted that the main microorganism responsible for maintaining the stability of the vaginal microbiome is the dominant microbe lactobacilli. Lactobacilli produce lactate, hydrogen peroxide and various bacteriocins and bacteriocin-like substances, thereby inhibiting the growth of obligate anaerobes and opportunistic microorganisms [2,3,4].
However, using culture-independent methods based on sequencing of the 16S (rRNA) gene, researchers demonstrated that a significant proportion (7–33%) of healthy women lack lactobacilli in their vagina [5,6]. It is known that the absence of lactobacilli is accompanied by the presence of other microorganisms, such as Gardnerella vaginalis, or various species of Peptostreptococcus spp., Prevotella spp., Pseudomonas spp., Streptococcus spp. and/or Corynebacterium spp. Such changes in the structure of the vaginal microbiome are not considered a pathological disorder [7,8].
The Corynebacterium genus contains approximately 130 different species of diverse and ecologically significant microorganisms. The well-known typical representatives of this genus are the pathogenic species C. diphtheriae, C. ulcerans, and C. pseudotuberculosis [9], and the roles of these species in the development of human infection have been proven. In addition, a large group of nondiphtheria corynebacteria is part of the resident microflora of human skin and mucous membranes and most often stands out from clinical samples [10]. For the last seven years, the number of publications on the important role of individual strains of nondiphtheria corynebacteria in protecting human mucous membranes from infection have increased. It was shown that certain types of nondiphtheria corynebacteria produce various bacteriocins, bacteriocin-like substances and biosurfactants, which inhibit the growth of opportunistic microorganisms and their biofilm formation [11,12]. Individual strains have pronounced bactericidal activity against opportunistic microorganisms, including MRSA [13]. Certain strains of nondiphtheria corynebacteria were recommended for use as probiotic microorganisms [14]. There are few reports on the use of nondiphtheria corynebacteria as immunomodulators in tumour immunotherapy [15].
Nondiphtheriae corynebacteria in vaginal biotopes were found in women regardless of age and microecological status [16,17,18]. Nondiphtheriae corynebacteria along with Staphylococcus epidermidis constitute the main part, approximately 80% of the vaginal microbiota, in prepubescent girls [19]. The number of nondiphtheriae corynebacteria in pregnant and postpartum women is also increased [20]. Despite the high frequency of nondiphtheriae corynebacteria occurrence in the female genital tract, studies on this topic are limited mainly to the description of pathogens [21,22].
Corynebacterium amycolatum was isolated for the first time by Collins and Burton from clinical specimens in 1988 [23]. Based on our observations, C. amycolatum is rather frequently isolated from vaginal biotopes of healthy women, and features a high probiotic potential. Particularly, we isolated three strains of corynebacteria from the vaginal contents of healthy women. All of them were identified as C. amycolatum. Metabolites of these strains greatly increased the antagonistic activity of peroxide-producing lactobacilli against pathogenic and opportunistic microorganisms and had strong antimicrobial activity against opportunistic pathogens such as Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa [24,25]. These strains showed the greatest adhesive ability to vaginal epithelial cells and human fibronectin under low pH conditions [25]. Due to their useful properties, we selected these strains for high-throughput sequencing (HTS) genome annotation. To better understand the ability of corynebacteria to survive in the vaginal biotope under eubiosis and to show their beneficial properties, we sequenced and analysed the genomes of three isolated strains. In addition, we should note that the genomic characteristics of Corynebacterium amycolatum have not yet been previously described.
2. Materials and Methods
2.1. DNA Preparation, Genome Sequencing and Assembly
Strains of C. amycolatum ICIS 5, ICIS 9 and ICIS 53 were previously isolated from vaginal smears of healthy women of reproductive age. The strains are deposited in the Collection of Microorganisms of the Institute for Cellular and Intracellular Symbiosis UrB RAS (Orenburg, Russia) under the same accession names. The phenotypic characteristics of these isolates have been previously described in detail [24,25]. The strains were kept at −80 °C in 20% (v/v) glycerol before experiment. The isolates were grown in tryptic soy broth (TSB) at 37 °C for 24 h.
Overnight bacterial cultures were used for extraction genomic DNA with the phenol-chloroform method. DNA libraries were prepared and sequenced at the Center of Shared Scientific Equipment “Persistence of microorganisms” at the Institute for Cellular and Intracellular Symbiosis UrB RAS (Orenburg, Russia). The Nextera XT DNA library preparation kit (Illumina, San Diego, CA, USA) was used according to the manufacturer’s instructions. High-throughput sequencing of the DNA libraries was carried out in the MiSeq sequencer (Illumina, USA) using the MiSeq reagent kit v3 2 × 300 cycles (Illumina, USA). The reads were quality-trimmed with the Trimmomatic tool [26]. De novo genome assembly was carried out with SPAdes (version 3.9.0) [27].
2.2. Genome Annotation
Functional annotation of the genomes was carried out by the RAST server (Rapid Annotation using Subsystem Technology) [28] and NCBI Prokaryotic Genome Annotation Pipeline (PGAAP) [29]. Clusters of orthologous groups (COGs) of proteins were used for functional classifications performed with the eggNOG (version 4.5) database (http://eggnog.embl.de/version_3.0/, accessed on 19 December 2021) [30]. The bioinformatic tools BAGEL4 [31] and AntiSMASH 5 [32] were used to determine potential clusters of secondary metabolites with antimicrobial activity. Antibiotic resistance genes in the genomes were predicted using the RGI (Resistance Gene Identifier) tool [33]. The presence of putative virulence genes in the genomes was investigated using the Virulence Factor of Bacterial Pathogens Database (VFDB) [34]. The CRISPR regions were identified with a CRISPR online detection tool, CRISPR finder [35].
2.3. Phylogenetic Analysis
Phylogenetic analysis was conducted based on the 16S rDNA sequences retrieved from draft genomes of the C. amycolatum strains ICIS 5 (WGS Project: SSOR01), ICIS 9 (MTPT01) and ICIS 53 (MIFV01), draft genomes of nine C. amycolatum strains currently available in the NCBI database (Supplementary Table S1), including SK46 (WGS Project: ABZU01), NCTC7243 (UFXE01), UMB0042 (PKHS01), UMB0338 (PKHT01), UMB7760 (VYVQ01), UMB9184 (VYVF01), UMB1182 (VYWH01), UMB1310 (VYWB01) and UMB9256 (VYVD01), and 16S rDNA sequences of the 28 type strains of Corynebacterium spp. most often isolated from human clinical samples and 2 strains of Euzebya spp. as an out group. The type strains of Corynebacterium spp. were selected from the List of Prokaryotic Names with Standing in Nomenclature website (http://www.bacterio.net/index.html, accessed on 19 December 2021) [36]. The 16S gene sequences of type strains were downloaded from the National Center for Biotechnology Information at https://www.ncbi.nlm.nih.gov/, accessed on 19 December 2021). Sequences were aligned by MUSCLE [37] with Unipro UGENE software (version 34.0) [38] using default parameters. The phylogenetic tree was constructed by MrBayes, V. 3.27 using the GTR replacement model on the Unipro UGENE software platform (version 35.0).
Genome similarity of the strains ICIS 5, ICIS 9 and ICIS 53 and other C. amycolatum strains currently available at NCBI was determined by calculating the average nucleotide identity (ANI) and orthologous average nucleotide identity (OrthoANI) using OAT (version v. 0.93.1) software [39].
2.4. Nucleotide Sequence Accession Numbers
The annotated genome sequences were deposited in the GenBank database as sequencing project PRJNA339674 with accession numbers SSOR00000000, MTPT00000000 and MIFV00000000 for C. amycolatum ICIS 5, ICIS 9 and ICIS 53, respectively. The strains C. amycolatum ICIS 9 and ICIS 53 were deposited in the culture collection of the All-Russian Collection of Microorganisms at the G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms (Russian Academy of Sciences, Pushchino, Russia) under registration no. VKM Ac-2843D and VKM Ac-2844D, respectively.
3. Results
3.1. General Genome Features
As shown in Table 1, the draft genome of strain ICIS 5 was composed of 2,474,151 bp, with an N50 length of 164,886 bp, an L50 of 6, and a G + C content of 58.8%. The final assembled genome consisted of 115 contigs.
Table 1.
The characteristics of the assembly and genomes of Corynebacterium amycolatum strains.
| Statistics | ICIS 5 | ICIS 9 | ICIS 53 |
|---|---|---|---|
| Assembly | |||
| Number of contigs | 115 | 181 | 41 |
| N50 | 164,886 | 45,496 | 170,644 |
| L50 | 6 | 18 | 4 |
| Depth of coverage | 278 | 22 | 100 |
| Draft genome sequences | |||
| Genome size (b.p.) | 2,474,151 | 2,587,830 | 2,460,257 |
| GC contents (%) | 58.80 | 58.60 | 59.00 |
| Genes (total) | 2195 | 2392 | 2173 |
| CDSs (total) | 2109 | 2330 | 2110 |
| Genes (coding) | 2062 | 2277 | 2076 |
| CDSs (with protein) | 2062 | 2277 | 2076 |
| rRNAs (5S, 16S, 23S) | 30 (4, 20, 6) | 6 (4, 1, 1) | 7 (5, 1, 1) |
| complete rRNAs | 3, 1 (5S, 16S) | 1, 1, 1 (5S, 16S, 23S) | 5, 1, 1 (5S, 16S, 23S) |
| tRNAs | 53 | 53 | 53 |
| Pseudo Genes (total) | 47 | 53 | 34 |
Genome annotation was performed using the National Center for Biotechnology Information (NCBI) Prokaryotic Genome Annotation Pipeline (PGAP) (http://www.ncbi.nlm.nih.gov/genome/annotation_prok (19 December 2021)), and 2195 coding sequences, including 2062 proteins (CDSs), 47 pseudogenes, complete rRNAs (3, 1 (5S, 16S) and 53 tRNAs, were identified. The identified coding proteins were classified into 26 functional categories based on COG classification (Supplementary Table S2). Of the 2062 protein-coding genes in ICIS 5, 1908 were assigned to COGs, and 154 genes were not assigned. The percentage of proteins with unknown function, including “Function unknown (S)” and “Not assigned (−)”, was 31.7%. Most genes belonged to the categories: Amino acid transport and metabolism (7.27% of CDS), Inorganic ion transport and metabolism (6.98% of CDS), Translation, ribosomal structure and biogenesis (6.84% of CDS), Replication, recombination and repair (6.3% of CDS) and Transcription (4.8% of CDS).
The genome of strain ICIS 9 was slightly larger than that of ICIS 5. It was composed of 2,587,830 bp, with an N50 length of 45,496 bp, an L50 of 18 and a G + C content of 58.6%. The final assembled genome consisted of 181 contigs. Genome annotation identified 2392 coding sequences, including 2277 proteins, 53 pseudogenes, complete rRNAs 1, 1, 1 (5S, 16S, 23S) and 53 tRNAs (Table 1). The identified coding proteins were classified into 26 functional categories based on COG classification (Supplementary Table S2). Of the 2277 protein-coding genes in ICIS 9, 2044 were assigned to COGs, and 233 genes were not assigned. The percentage of proteins with unknown function, including “Function unknown (S)” and “Not assigned (–)”, was 33.2%. Unlike ICIS 5, the distribution of identified coding proteins into categories based on COG classification was as follows: genes were mostly involved in the categories, Replication, recombination and repair (10.01% of CDS), Inorganic ion transport and metabolism (6.19% of CDS), Translation, ribosomal structure and biogenesis (6.19% of CDS), Amino acid transport and metabolism (6.05% of CDS) and Coenzyme transport and metabolism (4.61% of CDS).
The draft genome of strain ICIS 53 was composed of 2,460,257 bp, with an N50 length of 170,410 bp, an L50 of 4, and a G + C content of 59.0%. The final assembled genome consisted of 41 contigs. Genome annotation identified 2173 coding sequences, including 2076 proteins, 34 pseudogenes, 5, 1, and 1 complete rRNAs (5S, 16S, 23S) and 53 tRNAs (Table 1). The identified coding proteins were classified into 26 functional categories based on COG classification (Supplementary Table S2). Of the 2076 protein-coding genes in ICIS 53, 1884 were assigned to COGs, and 189 genes were not assigned. The percentage of proteins with unknown function, including “Function unknown (S)” and “Not assigned (–)”, was 33.9%. The identified coding proteins according to the COG classification were distributed as follows: Amino acid transport and metabolism (7.32% of CDS), Inorganic ion transport and metabolism (6.65% of CDS), Translation, ribosomal structure and biogenesis (6.55% of CDS), Replication, recombination and repair (4.82% of CDS) and Energy production and conversion (4.72% of CDS).
3.2. Phylogenetic Analysis
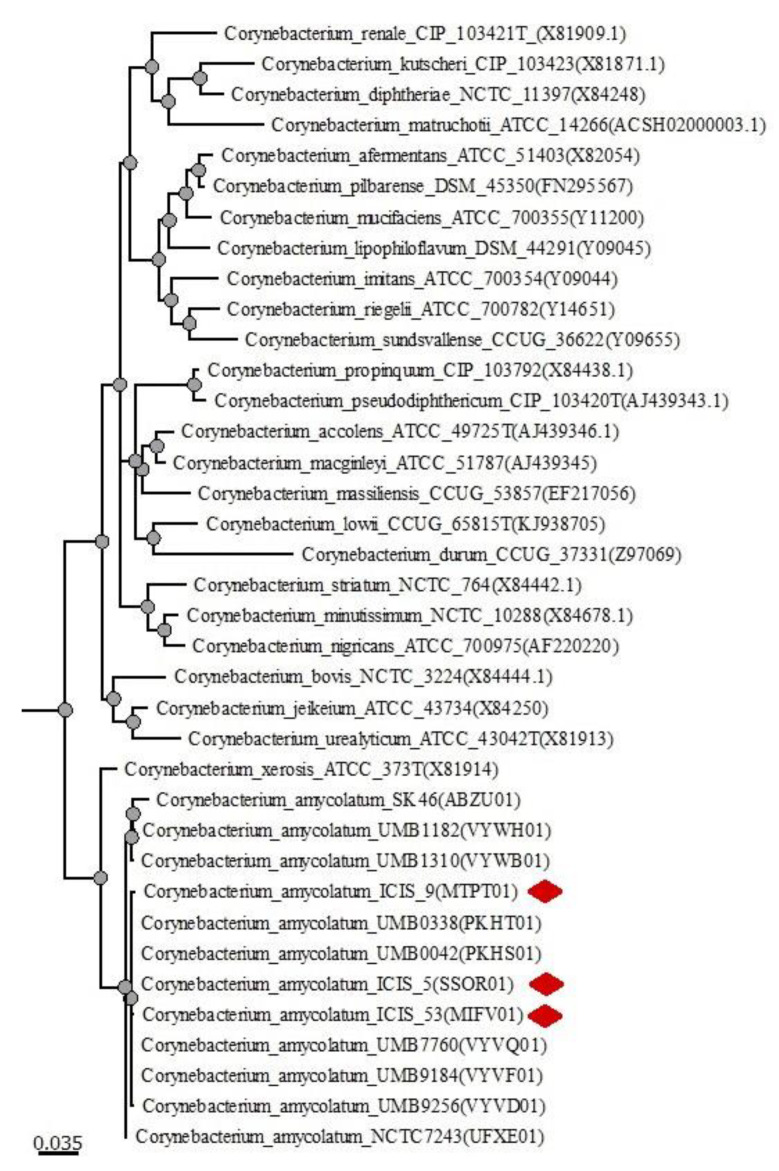
As shown in the phylogenetic tree constructed based on the 16S rRNA gene sequences, strains ICIS 5, ICIS 9, and ICIS 53 formed a common clade with nine strains of C. amycolatum from the NCBI database. The, C. amycolatum clade with a sister branch represented by Corynebacterium xerosis ATCC 373T was clearly separated from another clade containing all other species of Corynebacterium spp. (Figure 1). The data obtained are in good agreement with those described previously [40,41].
Figure 1.
Phylogenetic tree highlighting the position of C. amycolatum ICIS 5, ICIS 9, and ICIS 53 (denoted with red diamonds) relative to the 28 type strains of Corynebacterium spp. most often isolated from human clinical samples and 2 strains of Euzebya spp. as an out group. The phylogenetic tree was constructed by MrBayes, V. 3.27 using the GTR replacement model on the Unipro UGENE software platform (version 35.0). Corresponding NCBI accession numbers are shown in parentheses.
The similarity scores between ICIS 5, ICIS 9 and ICIS 53 and other C. amycolatum strains exceed 99% based on 16S rRNA gene phylogeny. The average nucleotide identity (ANI) between ICIS 5, ICIS 9 and ICIS 53 ranged from 96.79% to 97.87%, and the average nucleotide orthology (OrthoANI) ranged from 96.89% to 97.93% (Table 2).
Table 2.
Heatmap showing relative average nucelotide identity (ANI) and average nucleotide orthology (OrthoANI) between C. amycolatum species.
| (OrthoANI) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICIS5 | ICIS9 | ICIS53 | NCTC7243 | SK46 | UMB0042 | UMB0338 | UMB1182 | UMB7760 | UMB9184 | UMB1310 | UMB9256 | |
| ICIS5 | 96.87 | 97.93 | 94.97 | 95.38 | 97.95 | 97.51 | 94.96 | 97.69 | 97.57 | 95.13 | 97.40 | |
| ICIS9 | 96.79 | 96.90 | 95.71 | 95.05 | 96.83 | 96.79 | 95.42 | 96.83 | 96.76 | 95.55 | 96.66 | |
| ICIS53 | 97.87 | 96.84 | 94.77 | 95.37 | 98.07 | 97.74 | 94.95 | 97.82 | 97.83 | 94.96 | 97.47 | |
| NCTC7243 | 94.90 | 95.66 | 94.74 | 94.30 | 94.77 | 95.13 | 96.87 | 95.03 | 95.08 | 96.77 | 95.21 | |
| SK46 | 95.20 | 94.93 | 95.29 | 94.22 | 95.35 | 95.09 | 94.42 | 95.16 | 95.17 | 94.43 | 95.12 | |
| UMB0042 | 97.85 | 96.78 | 98.05 | 94.67 | 95.29 | 97.54 | 94.73 | 97.66 | 97.55 | 94.88 | 97.26 | |
| UMB0338 | 97.48 | 96.71 | 97.67 | 95.02 | 95.05 | 97.55 | 95.04 | 97.89 | 97.85 | 95.10 | 97.66 | |
| UMB1182 | 94.83 | 95.37 | 94.75 | 96.83 | 94.26 | 94.62 | 94.90 | 95.06 | 94.96 | 98.70 | 95.20 | |
| UMB7760 | 97.69 | 96.74 | 97.82 | 95.00 | 95.12 | 97.66 | 97.81 | 94.93 | 98.10 | 95.12 | 97.98 | |
| UMB9184 | 97.58 | 96.75 | 97.79 | 94.97 | 95.11 | 97.60 | 97.80 | 94.90 | 98.03 | 95.07 | 97.68 | |
| UMB1310 | 95.02 | 95.50 | 94.85 | 96.68 | 94.28 | 94.82 | 95.07 | 98.64 | 95.06 | 95.03 | 95.23 | |
| UMB9256 | 97.33 | 96.58 | 97.47 | 95.18 | 95.06 | 97.30 | 97.58 | 95.08 | 97.95 | 97.67 | 95.18 | |
| (Original ANI) | ||||||||||||
ANI values between our three strains and a type SK 46 strain of C. amycolatum varied from 94.93% to 95.29%, and orthoANI values varied from 95.05% to 95.37% and were over the species boundary value (ANI > 95–96%, orthoANI > 95–96%) [39,42]. Relative to other C. amycolatum strains, ANI values ranged from 94.26% to 98.02%, OrthoANI values ranged from 94.3% to 98.1%.
3.3. Genome Annotation of ICIS 5, ICIS 9 and ICIS 53
3.3.1. Genes and Properties Allowing, C. amycolatum to Survive in the Vaginal Ecosystem
The vaginal ecosystem is an aggressive environment for most microorganisms. The acidic environment in the vagina creates a natural filter; as a result, most pathogens and opportunistic microbes die. In order to survive and successfully colonise this ecosystem, microorganisms of the genus Corynebacterium spp. must have evolved mechanisms of adaptation. In the studied genomes, we identified a large number of genes encoding proteins involved in stress response. These stresses included pH, temperature, osmotic pressure, nitrosative and oxidative stress. The detailed analysis of genes coding for proteins involved in stress response in the genomes of ICIS 5, ICIS 9 and ICIS 53 is shown in Table 3.
Table 3.
Genes coding for proteins involved in stress response detected in vaginal isolates Corynebacterium amycolatum strains.
| Stresses | Gene | Product | ICIS 5 Locus_tag | ICIS 9 Locus_tag | ICIS 53 Locus_tag |
|---|---|---|---|---|---|
| pH | atpB | F0F1 ATP synthase subunit A | E7L51_RS04555 | BXT90_RS01980 | BGC22_RS01625 |
| – | F0F1 ATP synthase subunit B | E7L51_RS04545 | BXT90_RS01970 | BGC22_RS01615 | |
| F0F1 ATP synthase subunit C | |||||
| – | F0F1 ATP synthase subunit alpha | E7L51_RS04535 | BXT90_RS01960 | BGC22_RS01605 | |
| atpD | F0F1 ATP synthase subunit beta | E7L51_RS04525 | BXT90_RS01950 | BGC22_RS01595 | |
| – | F0F1 ATP synthase subunit gamma | E7L51_RS04530 | BXT90_RS01955 | BGC22_RS01600 | |
| – | F0F1 ATP synthase subunit delta | E7L51_RS04540 | BXT90_RS01965 | BGC22_RS01610 | |
| – | F0F1 ATP synthase subunit epsilon | E7L51_RS04520 | BXT90_RS01945 | BGC22_RS01590 | |
| F0F1 ATP synthase protein I | |||||
| – | L-lactate dehydrogenase | E7L51_RS05660, E7L51_RS02260 | BXT90_RS06700, BXT90_RS07585 | BGC22_RS01270, BGC22_RS10270 | |
| – | Glucose-6-phosphate isomerase | E7L51_RS06505 | BXT90_RS00390 | BGC22_RS07690 | |
| – | GTP pyrophosphokinase | E7L51_RS00625 | BXT90_RS01675 | BGC22_RS04770 | |
| pyk | Pyruvate kinase | E7L51_RS00810, E7L51_RS02265 | BXT90_RS02290, BXT90_RS06705 | BGC22_RS04585, BGC22_RS10275 | |
| clpX | ATP-dependent Clp protease ATP-binding subunit | E7L51_RS09205 | BXT90_RS08190, BXT90_RS06490 | BGC22_RS03025, BGC22_RS06215 | |
| – | Na+/H+ antiporter subunit A | E7L51_RS03140 | BXT90_RS04255 | BGC22_RS02670 | |
| – | Na+/H+ antiporter subunit D | E7L51_RS03130 | BXT90_RS04265 | BGC22_RS02680 | |
| – | Na+/H+ antiporter subunit E | E7L51_RS03125 | BXT90_RS04270 | BGC22_RS02685 | |
| Temperature | hrcA | Heat-inducible transcriptional repressor | E7L51_RS03385 | BXT90_RS03730 | BGC22_RS05570 |
| grpE | Heat shock protein GrpE | E7L51_RS02840 | BXT90_RS09385 | BGC22_RS02385 | |
| dnaK | Heat shock protein DnaK | E7L51_RS02835 | BXT90_RS09390 | BGC22_RS02390 | |
| dnaJ | Heat shock protein DnaJ | E7L51_RS02845, E7L51_RS03380, E7L51_RS06605 | BXT90_RS03725, BXT90_RS09380 | BGC22_RS02380, BGC22_RS07585, BGC22_RS05565 | |
| – | Molecular chaperone GroES | E7L51_RS06730 | BXT90_RS00160 | BGC22_RS07460 | |
| groL | Molecular chaperone GroEL | E7L51_RS03170, E7L51_RS06725 | BXT90_RS00165, BXT90_RS06205 | BGC22_RS02645, BGC22_RS07465 | |
| Osmotic stress | betA | Choline dehydrogenase (EC 1.1.99.1) | E7L51_RS02520 | BXT90_RS09090 | BGC22_RS06865 |
| Nitrosative stress | – | Nitrate reductase | E7L51_RS05075 | BXT90_RS02975 | BGC22_RS00655 |
| Oxidative stress | – | Catalase (EC 1.11.1.6) | E7L51_RS10255 | BXT90_RS07525 | BGC22_RS03550 |
| – | Thiol peroxidase | E7L51_RS01250 | BXT90_RS05315 | BGC22_RS08510 | |
| trxA | Thioredoxin | E7L51_RS08530, E7L51_RS08670 | BXT90_RS04815, BXT90_RS10200 | BGC22_RS02030, BGC22_RS02165 | |
| trxB | Thioredoxin-disulfide reductase | E7L51_RS08665 | BXT90_RS04820 | BGC22_RS02160 | |
| – | Thioredoxin-dependent thiol peroxidase | E7L51_RS10400 | BXT90_RS08035 | BGC22_RS04010 | |
| – | Thioredoxin domain-containing protein | E7L51_RS09080, E7L51_RS10075 | BXT90_RS09275, BXT90_RS07735 | BGC22_RS03780, BGC22_RS08125 | |
| – | Glutathione peroxidase | E7L51_RS08875 | BXT90_RS09805 | BGC22_RS06430 | |
| – | Hydrogen peroxide-inducible genes activator | E7L51_RS05005 | BXT90_RS03045 | BGC22_RS00585 | |
| – | Superoxide dismutase | E7L51_RS02250 | BXT90_RS06690 | BGC22_RS10260 | |
| mshA | D-inositol-3-phosphate glycosyltransferase | E7L51_RS05705 | BXT90_RS09175 | BGC22_RS06775 | |
| mshB | N-acetyl-1-D-myo-inositol-2-amino-2-deoxy-alpha-D-glucopyranoside deacetylase | E7L51_RS04900 | BXT90_RS09615 | BGC22_RS01970 | |
| mshC | Cysteine--1-D-myo-inosityl 2-amino-2-deoxy-alpha-D-glucopyranoside ligase | E7L51_RS01445 | BXT90_RS02610 | BGC22_RS08710 | |
| mshD | Mycothiol synthase | E7L51_RS08935 | BXT90_RS09425 | BGC22_RS06490 | |
| mca | Mycothiol conjugate amidase | E7L51_RS03790 | BXT90_RS06270 | BGC22_RS05930 | |
| mtr | Mycothione reductase | E7L51_RS05350 | BXT90_RS05885 | BGC22_RS00925 | |
| nrdH | Gutaredoxin-like protein | E7L51_RS09855 | BXT90_RS10670 | BGC22_RS03865 |
ICIS 5, ICIS 9 and ICIS 53 contain 7 genes that encode F0F1-ATPase. Membrane-bound ATP synthases (F0F1-ATPases) of bacteria serve two important physiological functions. The enzyme catalyses the synthesis of ATP from ADP and inorganic phosphate utilizing the energy of an electrochemical ion gradient. On the other hand, under conditions of low driving force, ATP synthases function as ATPases, thereby generating a transmembrane ion gradient at the expense of ATP hydrolysis [43]. Such activity protects cells from damage induced by an acidic environment; 3 genes encoding Na+/H+ antiporters are membrane proteins that play a major role in pH and Na+ homeostasis of cells [44]. The analysis of the genomes revealed the presence of genes that encode L-lactate dehydrogenase. This enzyme catalyses the conversion of lactate to pyruvate with the formation of NADH. As a result, it restored the NAD/NADH balance and subsequently increased ATP production. The concomitant surplus of ATP is used to drive the F0F1-ATPases, resulting in enhanced acid tolerance in bacteria [45]. Furthermore, the ICIS 5, ICIS 9 and ICIS 53 genomes encode glucose-6-phosphate isomerase, GTP pyrophosphokinase, pyruvate kinase, ATP-dependent Clp protease ATP-binding subunit, which are proteins involved in the acid resistance of various bacteria [46,47]. We also identified a number of genes related to temperature stress. A cluster of heat shock proteins was identified, hrcA-grpE-dnaK-dnaJ and chaperonin system GroEL-GroES, which are present in all kingdoms of life and rescue proteins from improper folding and aggregation upon internal and external stress conditions, including high temperatures and pressures [48,49].
A large number of genes associated with oxidative stress were identified in the studied genomes. They can play a crucial significance for survival and adaptation of the bacteria in the vaginal niche. The genomes contain genes that encode catalase, thiol peroxidase and glutathione peroxidase, which are antioxidant protective enzymes capable to detoxify reactive oxygen species [50,51,52]. The genomes also harbour genes encoding superoxide dismutase (SOD) and the complete thioredoxin system. It is known that SOD and thioredoxin (Trx) systems are key antioxidant systems in cellular protection against oxidative stress conditions [53,54,55,56]. In addition, genes nrdH and MSH encoding glutaredoxin and mycothiol, respectively, were identified. Similar to glutathione, mycothiol is one of key metabolites providing protection of bacteria from oxidative stress, as well as detoxication of xenobiotics [57,58]. Recently, significance of MSH has been shown for resistance of Corynebacterium glutamicum to antibiotics, alkylating agents, ethanol and heavy metals [59,60]. The genomes of strains ICIS 5, ICIS 9 and ICIS 53 contained genes encoding four enzymatic steps of mycothiol biosynthesis: production of GlcNAc-Ins-P using D-inositol-3-phosphate glycosyltransferase (MshA), deacetylation using N-acetyl-1-D-myo-inositol-2-amino-2-deoxy-alpha-D-glucopyranoside deacetylase (MshB) to form GlcN-Ins, binding to cysteine via cysteine-1-D-myo-inosityl-2-amino-2-deoxy-alpha-D-glucopyranoside ligase (MshC), and acetylation of mycothiol synthase (MshD) to give MSH.
3.3.2. Biologically Active Secondary Metabolite-Related Genes
In order to survive and successfully colonise the vaginal biotope, nonpathogenic corynebacteria must have the ability to produce secondary metabolites with antimicrobial activity and determine their competitive advantage [61]. In the studied genomes, we found the presence of gene clusters potentially involved in the biosynthesis of secondary metabolites. Gene clusters were predicted for T3pks (type III polyketide synthases), Nrps (nonribosomal peptide), Nrps-like and terpene. Each of the three genomes contained one T3pks gene cluster, which was associated with the biosynthesis of polyketides. Polyketides are natural metabolites that comprise the basic chemical structure of various anticancer, antifungal and anticholesteremic agents, antibiotics, parasiticides and immunomodulators [62,63]. These T3pks gene clusters encoded the biosynthesis of merochlorin A–D-like compounds (Supplementary Figures S1–S3). Merochlorins A–D, cyclic meroterpenoid antibiotics, were first described in the marine bacterium Streptomyces sp. strain CNH-189 [64]. The genomes of strains ICIS 5 and ICIS 53 contained one Nrps gene cluster, which was associated with the biosynthesis of phthoxazolin-like compounds (Supplementary Figures S4 and S5). Phthoxazolin, an oxazole-containing polyketide, has a broad spectrum of anti-oomycete activity and herbicidal activity [65]. Additionally, each genome contained one terpene gene cluster and an Nrps-like gene cluster, but substances were not identified. Terpenes or isoprenoids are the largest and structurally most diverse class of secondary metabolites. Terpenes are involved in a wide range of vital biological functions, including electron transport, cellular respiration, photosynthesis, membrane biosynthesis, signalling and growth regulation [66]. In accordance with their structural diversity, the functions of terpenoids range from mediating symbiotic or antagonistic interactions between organisms to electron transfer, protein prenylation, or contribution to membrane fluidity [67]. In addition, an increasing number of terpenes have been utilised for pharmaceuticals [68,69,70]. We checked the “Terpenoid backbone biosynthesis (map00900)” pathway in the genomes of strains ICIS 5, ICIS 9 and ICIS 53 and identified six key enzymes distributed in the mevalonate (MVA) pathway. The obtained data confirm the previously described MVA pathway for terpenoid backbone biosynthesis in C. amycolatum [71]. The core enzymes involved in the MVA pathway are listed in Table 4.
Table 4.
Genes coding for proteins involved in terpenoid backbone biosynthesis in Corynebacterium amycolatum strains.
| Product/EC No. | ICIS 5 Locus_tag | ICIS 9 Locus_tag | ICIS 53 Locus_tag |
|---|---|---|---|
| acetyl-CoA C-acetyltransferase (EC:2.3.1.9) | E7L51_RS02615 E7L51_RS08850 |
BXT90_RS00480 | BGC22_RS02560 BGC22_RS06405 BGC22_RS07780 |
| hydroxymethylglutaryl-CoA synthase (EC:2.3.3.10) | E7L51_RS06810 | BXT90_RS00080 | BGC22_RS07380 |
| hydroxymethylglutaryl-CoA reductase (NADPH) (EC:1.1.1.34) | E7L51_RS06815 | BXT90_RS00075 | BGC22_RS07375 |
| mevalonate kinase (EC:2.7.1.36) | E7L51_RS06830 | BXT90_RS00060 | BGC22_RS07360 |
| phosphomevalonate kinase (EC:2.7.4.2) | E7L51_RS06820 | BXT90_RS00070 | BGC22_RS07370 |
| diphosphomevalonate decarboxylase (EC:4.1.1.33) | E7L51_RS06825 | BXT90_RS00065 | BGC22_RS07365 |
| isoprenyl transferase (undecaprenyl diphosphate synthase) uppS (EC:2.5.1-) | E7L51_RS03345 E7L51_RS03775 |
BXT90_RS03690 BXT90_RS06255 |
BGC22_RS05530 BGC22_RS05915 |
| isopentenyl-diphosphate Delta-isomerase (EC:5.3.3.2) | E7L51_RS06835 | BXT90_RS00055 | BGC22_RS07355 |
| polyprenyl synthetase family protein | E7L51_RS05960 | BXT90_RS05620 | BGC22_RS04055 |
| geranylgeranyl pyrophosphate synthase (EC:2.5.1.1 2.5.1.10 2.5.1.29) | E7L51_RS00410 | BXT90_RS01380 | BGC22_RS04980 |
| farnesyl-diphosphate farnesyltransferase (EC:2.5.1.21) | E7L51_RS00395 | BXT90_RS01365 | BGC22_RS04995 |
All enzymes are encoded by a single gene, except isoprenyl transferase (undecaprenyl diphosphate synthase), which is encoded by two gene copies. Isoprenyl transferase catalyses the condensation of isopentenyl diphosphate (IPP) with allylic pyrophosphates, generating different types of terpenoids [72]. Farnesyl-diphosphate farnesyltransferase (squalene synthase) is a precursor of steroids, cholesterol, sesquiterpenes, farnesylated proteins, heme and vitamin K12 [73].
3.3.3. Bacteriocin-Related Genes
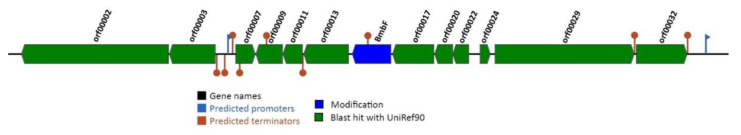
Bacteriocins are antimicrobial peptides ribosomally produced in bacteria, either processed or not by additional post-translational modification (PTM) enzymes, and exported to the extracellular medium [74,75]. Of the three genomes analysed, only the genome of strain ICIS 9 had one area of interest (AOI) that included genes encoding a bacteriocin of the class Sactipeptide (Figure 2 and Supplementary Table S3).
Figure 2.
Sactipeptide BmbF encoded by gene bmbF (orf00016) predicted in ICIS 9 genome with BAGEL4.
Sactipeptides are a new class of synthesized in ribosomes and post-translationally modified peptides (RiPPs). Sactipeptides are known as antibiotics with narrow spectrum capable to inhibit Clostridia and some human multidrug-resistant bacterial pathogens [76]. The revealed features allow to consider sactipeptides promising scaffolds for the creation of new antibiotics [77]. The presence of an AOI encoding sactipeptide in the genome of strain ICIS 9 suggests that this strain may produce the sactipeptide. However, this assumed feature should be checked further through isolation and characteristics of this peptide.
3.3.4. H2O2-Related Genes
H2O2 is one of the key factors in maintaining vaginal biotope homeostasis [78]. In the studied genomes, we identified a number of genes encoding the production of hydrogen peroxide: cytochrome d ubiquinol oxidase subunit I (locus_tag: E7L51_RS08995, BXT90_RS09475, BGC22_RS06540 and subunit II (E7L51_RS08990, BXT90_RS09470, BGC22_RS06535), glutathione peroxidase family protein (E7L51_RS08875, BXT90_RS09805, BGC22_RS06430), pyridoxamine 5′-phosphate oxidase (E7L51_RS09830, BXT90_RS11320, BGC22_RS08135), fumarate reductase (E7L51_RS09150, BXT90_RS09225, BGC22_RS06825), and NADH-flavin reductase family protein (E7L51_RS07920, BXT90_RS06560, BGC22_RS02940). As shown in the Lactobacillus acidophilus group [79], these enzymes are directly involved in the production of hydrogen peroxide, along with well-known enzymes such as NADH oxidase or lactate oxidase.
3.3.5. Nutrient Synthesis (Vitamins and Essential Amino Acids)-Related Genes
Microorganisms that colonize various biotopes of the human body form multi-species communities and represent a kind of “organ”, which, in turn, affects the functioning of all organs and systems that play an important role in maintaining the health of the host. Using intestinal microbiota as an example, commensal bacteria have been shown to be important sources of vitamins and amino acids. In addition to their nutritional/physiological properties, many of these vitamins are also involved in the development and functioning of host immune cells, as there is a direct link between biosynthetic biosynthesis intermediates derived from commensal bacteria and immune cells that directly recognise them [80,81]. The biosynthesis of vitamins and essential amino acids by probiotic strains has recently been an important aspect in the development of probiotic products and pharmaceuticals [82,83]. The genomes of strains ICIS 5, ICIS 9 and ICIS 53 contain functionally active biosynthetic gene clusters that encode all the enzymes required for the synthesis of B vitamins such as B2 (riboflavin), B6 (pyridoxin), B7 (biotin), B9 (folate) and B12 (cobalamin) (Table 5), and essential amino acids such as histidine, arginine, methionine, threonine, lysine, leucine and tryptophan (Table 6).
Table 5.
Vitamin biosynthetic proteins detected in Corynebacterium amycolatum ICIS 5, ICIS 9 and ICIS 53.
| Vitamin | Biosynthesis Protein/Gene/EC No. |
|---|---|
| Biotin | Transmembrane component BioN of energizing module of biotin ECF transporter Predicted biotin repressor from TetR family Substrate-specific component BioY of biotin ECF transporter Adenosylmethionine-8-amino-7-oxononanoate aminotransferase (EC 2.6.1.62) 8-amino-7-oxononanoate synthase (EC 2.3.1.47) Dethiobiotin synthetase (EC 6.3.3.3) Biotin synthase (EC 2.8.1.6) Long-chain-fatty-acid--CoA ligase (EC 6.2.1.3) Biotin synthesis protein BioC 3-ketoacyl-CoA thiolase (EC 2.3.1.16) ATPase component BioM of energizing module of biotin ECF transporter |
| Cobalamin | Cobalt-precorrin-6x reductase (EC 1.3.1.54) Cobalamin biosynthesis protein BluB L-threonine 3-O-phosphate decarboxylase (EC 4.1.1.81) Adenosylcobinamide-phosphate guanylyltransferase (EC 2.7.7.62) Cobalt-precorrin-8x methylmutase (EC 5.4.1.2) Cobalt-precorrin-2 C20-methyltransferase (EC 2.1.1.130) Cobyric acid synthase (EC 6.3.5.10) Cobalt-precorrin-4 C11-methyltransferase (EC 2.1.1.133) Cobalt-precorrin-3b C17-methyltransferase Nicotinate-nucleotide--dimethylbenzimidazole phosphoribosyltransferase (EC 2.4.2.21) Adenosylcobinamide-phosphate synthase (EC 6.3.1.10) Cob(I)alamin adenosyltransferase (EC 2.5.1.17) Cobyrinic acid A,C-diamide synthase |
| Riboflavin | FMN adenylyltransferase (EC 2.7.7.2) hypothetical protein YebC C-terminal domain of CinA type S 6,7-dimethyl-8-ribityllumazine synthase (EC 2.5.1.78) Riboflavin transporter PnuX 5-amino-6-(5-phosphoribosylamino)uracil reductase (EC 1.1.1.193) tRNA pseudouridine synthase B (EC 4.2.1.70) Riboflavin kinase (EC 2.7.1.26) GTP cyclohydrolase II (EC 3.5.4.25) Diaminohydroxyphosphoribosylaminopyrimidine deaminase (EC 3.5.4.26) 3,4-dihydroxy-2-butanone 4-phosphate synthase (EC 4.1.99.12) FMN adenylyltransferase (EC 2.7.7.2) Riboflavin synthase eubacterial/eukaryotic (EC 2.5.1.9) |
| Pyridoxine | Pyridoxine biosynthesis glutamine amidotransferase, synthase subunit (EC 2.4.2.-) D-3-phosphoglycerate dehydrogenase (EC 1.1.1.95) Pyridoxal kinase (EC 2.7.1.35) Phosphoserine aminotransferase (EC 2.6.1.52) NAD-dependent glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12) |
| Folate | Dihydropteroate synthase (EC 2.5.1.15) tRNA(Ile)-lysidine synthetase (EC 6.3.4.19) Aspartate 1-decarboxylase (EC 4.1.1.11) Cell division protein FtsH (EC 3.4.24.-) GTP cyclohydrolase I (EC 3.5.4.16) type 1 Pantoate--beta-alanine ligase (EC 6.3.2.1) 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase (EC 2.7.6.3) Dihydroneopterin aldolase (EC 4.1.2.25) 5-formyltetrahydrofolate cyclo-ligase (EC 6.3.3.2) Dihydrofolate reductase (EC 1.5.1.3) Thymidylate synthase thyX (EC 2.1.1.-) Dihydrofolate synthase (EC 6.3.2.12) Para-aminobenzoate synthase, aminase component (EC 2.6.1.85) |
Table 6.
Amino acid biosynthetic proteins detected in Corynebacterium amycolatum ICIS 5, ICIS 9 and ICIS 53.
| Amino Acid | Biosynthesis Protein/Gene/EC No. |
|---|---|
| Histidine | Phosphoribosylformimino-5-aminoimidazole carboxamide ribotide isomerase (EC 5.3.1.16) Phosphoribosyl-ATP pyrophosphatase (EC 3.6.1.31) Imidazole glycerol phosphate synthase amidotransferase subunit (EC 2.4.2.-) Histidinol-phosphatase [alternative form] (EC 3.1.3.15) Histidinol-phosphate aminotransferase (EC 2.6.1.9) Imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) Imidazole glycerol phosphate synthase cyclase subunit (EC 4.1.3.-) Phosphoribosyl-AMP cyclohydrolase (EC 3.5.4.19) ATP phosphoribosyltransferase (EC 2.4.2.17) Histidinol dehydrogenase (EC 1.1.1.23) |
| Arginine | N-succinyl-L,L-diaminopimelate desuccinylase (EC 3.5.1.18) Glutamate N-acetyltransferase (EC 2.3.1.35) Acetylglutamate kinase (EC 2.7.2.8) Arginine pathway regulatory protein ArgR Argininosuccinate lyase (EC 4.3.2.1) N-acetylglutamate synthase (EC 2.3.1.1) Argininosuccinate synthase (EC 6.3.4.5) N-acetyl-gamma-glutamyl-phosphate reductase (EC 1.2.1.38) Ornithine carbamoyltransferase (EC 2.1.3.3) Acetylornithine aminotransferase (EC 2.6.1.11) |
| Methionine | Methionine ABC transporter ATP-binding protein S-adenosylmethionine synthetase (EC 2.5.1.6) O-succinylhomoserine sulfhydrylase (EC 2.5.1.48) Serine acetyltransferase (EC 2.3.1.30) Homoserine kinase (EC 2.7.1.39) 5-methyltetrahydropteroyltriglutamate--homocysteine methyltransferase (EC 2.1.1.14) 5-methyltetrahydrofolate--homocysteine methyltransferase (EC 2.1.1.13) Cystathionine beta-lyase, type II (EC 4.4.1.8) Cysteine synthase (EC 2.5.1.47) O-acetylhomoserine sulfhydrylase (EC 2.5.1.49) 5,10-methylenetetrahydrofolate reductase (EC 1.5.1.20) Homoserine dehydrogenase (EC 1.1.1.3) Methionine ABC transporter substrate-binding protein Homoserine O-acetyltransferase (EC 2.3.1.31) Methionine ABC transporter permease protein Adenosylhomocysteinase (EC 3.3.1.1) |
| Threonine | Homoserine dehydrogenase (EC 1.1.1.3) Aspartate-semialdehyde dehydrogenase (EC 1.2.1.11) Aspartate aminotransferase (EC 2.6.1.1) Threonine synthase (EC 4.2.3.1) Homoserine kinase (EC 2.7.1.39) Aspartokinase (EC 2.7.2.4) |
| Lysine | N-succinyl-L,L-diaminopimelate desuccinylase (EC 3.5.1.18) N-acetyl-L,L-diaminopimelate deacetylase (EC 3.5.1.47) 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase (EC 2.3.1.117) Diaminopimelate epimerase (EC 5.1.1.7) Aspartate-semialdehyde dehydrogenase (EC 1.2.1.11) 4-hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7) Meso-diaminopimelate D-dehydrogenase (EC 1.4.1.16) Diaminopimelate decarboxylase (EC 4.1.1.20) N-succinyl-L,L-diaminopimelate aminotransferase alternative (EC 2.6.1.17) 2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-acetyltransferase (EC 2.3.1.89) 4-hydroxy-tetrahydrodipicolinate reductase (EC 1.17.1.8) Aspartokinase (EC 2.7.2.4) |
| Leucine | 3-isopropylmalate dehydratase small subunit (EC 4.2.1.33) 3-isopropylmalate dehydratase large subunit (EC 4.2.1.33) 2-isopropylmalate synthase (EC 2.3.3.13) Branched-chain amino acid aminotransferase (EC 2.6.1.42) 3-isopropylmalate dehydrogenase (EC 1.1.1.85) |
| Tryptophan | Anthranilate synthase, amidotransferase component (EC 4.1.3.27) Aminodeoxychorismate lyase (EC 4.1.3.38) Tryptophan-associated membrane protein Tryptophan synthase alpha chain (EC 4.2.1.20) Anthranilate phosphoribosyltransferase (EC 2.4.2.18) Tryptophan synthase beta chain (EC 4.2.1.20) Acting phosphoribosylanthranilate isomerase (EC 5.3.1.24) Indole-3-glycerol phosphate synthase (EC 4.1.1.48) Anthranilate synthase, aminase component (EC 4.1.3.27) Para-aminobenzoate synthase, aminase component (EC 2.6.1.85) Para-aminobenzoate synthase, amidotransferase component (EC 2.6.1.85) |
3.3.6. Antibiotic Resistance- and Virulence-Related Genes
We searched the genomes of vaginal isolates of C. amycolatum strains for antibiotic resistance genes and found genes encoding resistance to antibiotics in only two strains, ICIS 5 and ICIS 9. The genome of strain ICIS 5 contained genes encoding resistance to chloramphenicol and aminoglycosides (Supplementary Table S4). The genome of strain ICIS 9 contained genes encoding resistance to macrolides, lincosamide, streptogramin, tetracycline, chloramphenicol and aminoglycosides (Supplementary Table S4). The results confirmed the antibiotic resistance profile of these strains, which was determined earlier with a disc susceptibility assay [84]. VFDB software was used to predict virulence factors in ICIS 5, ICIS 9 and ICIS 53. VFDB predicted 27 virulence factors in ICIS 5 and ICIS 9 and 23 virulence factors in ICIS 53 (Supplementary Table S5). The virulence genes of ICIS 5, ICIS 9 and ICIS 53 can be classified into ten categories: adherence, iron uptake, regulation, amino acid and purine metabolism, antiphagocytosis, cell surface components, immune evasion, lipid and fatty acid metabolism, protease and secretion system. However, all identified genes were not true virulence factors; only the structural and functional characteristics of microorganisms of the genus Corynebacterium were determined, as well as adaptation to this ecological niche [85,86,87,88,89]. The true virulence genes, such as toxin-related genes (diphtheria toxin and phospholipase D) and haemolysin-related genes characteristic of well-known pathogenic corynebacteria, were not identified.
3.3.7. Phage Defense Systems
CRISPR-Cas modules are adaptive immune systems that are present in most archaea and many bacteria and provide sequence-specific protection against foreign DNA or, in some cases, RNA [90]. Of the three analysed genomes, CRISPR-associated sequence (Cas) systems were identified exclusively in the genome of strain ICIS 5. Type I-E CRISPR consists of 7 cas genes: cas1e (locus_tag: E7L51_RS06945), cas2e (E7L51_RS06950), cas3 E7L51_RS06915, Cse4 (E7L51_RS06930), cas5e (E7L51_RS06935), cas6e (E7L51_RS06940) and cas7e (E7L51_RS06930). The abortive infection (Abi) system is another property of phage resistance that can target different phases of phage development [91]. Abortive infection family proteins were identified in the genomes of strains ICIS 5 (locus_tag: E7L51_RS07100), ICIS 9 (BXT90_RS06835) and ICIS 53 (BGC22_RS10885). The presence of such phage defence systems in the studied strains probably reflects the exposure of these strains to phages in the vagina [92].
4. Conclusions
We presented a comparative study of draft genomes for three C. amycolatum vaginal strains based on the annotation and analysis of genes associated with the physiological functions and adaptation of these microorganisms under the specific conditions of vaginal microbiocenosis. The presence of resistance genes against acid and oxidative stress, which are specific features of the vaginal biotope, has been established. The genes responsible for the synthesis of essential amino acids and vitamins have been identified, demonstrating the involvement of the corynebacteria in host metabolism. The presence of genes associated with H2O2 production and the absence of true virulence genes allows us to consider the studied strains as potential probiotics capable of preventing the growth of pathogens and supporting colonisation resistance in the vaginal biotope. Antibiotic resistance genes revealed in the strains can provide corynebacteria the ability to colonise the vaginal biotope under antibiotic treatment. In addition, the studied strains are biotechnologically promising based on the identified genes for the synthesis of secondary metabolites of polyketides (merochlorins A–D), terpenes and sactipeptides. The analysis of C. amycolatum genomes revealed common genes for all three strains; for example, genes encoding adaptation and survival in the vaginal environment, as well as unique genes determining strain specificity. The presence of an AOI encoding sactipeptide in the genome of strain ICIS 9 suggests that this strain may produce the sactipeptide. However, this assumed feature should be checked further through isolation and characteristics of this peptide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms10020249/s1, Supplementary Table S1: Comparison of genome characteristics of ICIS5, ICIS 9 and ICIS 53 with other Corynebacterium amycolatum strains; Supplementary Table S2: eggNOG categories of coding proteins in C. amycolatum ICIS 5, ICIS 9 and ICIS 53 genomes; Supplementary Table S3: Condensed Gene of ICIS 9; Supplementary Table S4: Antibiotic-resistant genes detected in C. amycolatum ICIS 5 and ICIS 9; Supplementary Table S5: Virulence-related genes detected in C. amycolatum ICIS 5, ICIS 9 and ICIS 53; Supplementary Figure S1: Proposed biosynthetic gene cluster of merochlorins A–D-like compound in the C. amycolatum ICIS 5. The most similar gene cluster from Streptomyces sp. CNH189 is shown, with related genes drawn in the same colour to highlight inter-cluster rearrangements.; Supplementary Figure S2: Proposed biosynthetic gene cluster of merochlorins A–D-like compound in the C. amycolatum ICIS 9. The most similar gene cluster from Streptomyces sp. CNH189 is shown, with related genes drawn in the same colour to highlight inter-cluster rearrangements; Supplementary Figure S3: Proposed biosynthetic gene cluster of merochlorins A–D-like compound in the C. amycolatum ICIS 3. The most similar gene cluster from Streptomyces sp. CNH189 is shown, with related genes drawn in the same colour to highlight inter-cluster rearrangements.; Supplementary Figure S4: Proposed biosynthetic gene cluster of phthoxazolin-like compound in the C. amycolatum ICIS 5. The most similar gene cluster from Streptomyces avermitilis is shown, with related genes drawn in the same colour to highlight inter-cluster rearrangements; Supplementary Figure S5: Proposed biosynthetic gene cluster of phthoxazolin-like compound in the C. amycolatum ICIS 53. The most similar gene cluster from Streptomyces avermitilis is shown, with related genes drawn in the same colour to highlight inter-cluster rearrangements.
Author Contributions
I.V.G. and S.V.C. conceived and designed a study. I.V.G. and S.V.C. carried out the experimental design. Y.A.K. carried out bioinformatic analysis. I.V.G., S.V.C. and A.O.P. analysed data and prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buchta V. Vaginal microbiome. Ceska Gynekol. 2018;83:371–379. [PubMed] [Google Scholar]
- 2.Mendling W. Advances in Experimental Medicine and Biology. Volume 902. Springer; New York, NY, USA: 2016. Vaginal microbiota; pp. 83–93. [DOI] [PubMed] [Google Scholar]
- 3.Smith S.B., Ravel J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017;595:451–463. doi: 10.1113/JP271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Hyman R.W., Fukushima M., Diamond L., Kumm J., Giudice L.C., Davis R.W. Microbes on the human vaginal epithelium. Proc. Natl. Acad. Sci. USA. 2005;102:7952–7957. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhelst R., Verstraelen H., Claeys G., Verschraegen G., Delanghe J., Van Simaey L., De Ganck C., Temmerman M., Vaneechoutte M. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2005;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X., Bent S.J., Schneider M.G., Davis C.C., Islam M.R., Forney L.J. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150:2565–2573. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X., Brown C.J., Abdo Z., Davis C.C., Hansmann M.A., Joyce P., Foster J., Forney L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1:121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 9.Zasada A.A., Mosiej E. Contemporary microbiology and identification ofCorynebacteriaspp. causing infections in human. Lett. Appl. Microbiol. 2018;66:472–483. doi: 10.1111/lam.12883. [DOI] [PubMed] [Google Scholar]
- 10.Araújo C.L., Alves J., Lima A., Dias L., Silva P., Marques J., Azevedo V., Silva A., Folador A.S.A.A. Basic Biology and Applications of Actinobacteria. IntechOpen; London, UK: 2018. The Genus Corynebacterium in the Genomic Era. [DOI] [Google Scholar]
- 11.Bomar L., Brugger S.D., Yost B.H., Davies S.S., Lemon K.P. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio. 2016;7:e01725-15. doi: 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey M.M., Freire M., Gabrilska R.A., Rumbaugh K.P., Lemon K.P. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front. Microbiol. 2016;7:1230. doi: 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy B.L., Dickey S.W., Plaut R.D., Riggins D.P., Stibitz S., Otto M., Merrell D.S. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. mBio. 2019;10:e02491-18. doi: 10.1128/mBio.02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlyshev A.V., Melnikov V. Draft Genome sequence of Corynebacterium pseudodiphtheriticum strain 090104 “Sokolov”. Genome Announc. 2013;1:e00921-13. doi: 10.1128/genomeA.00921-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao S., Liu C., Qu S., Song J., Li J., Zhang P., Wang Q., Guo C., Gao F., Zhang L. Non-cell Corynebacterium parvum generated by nanotechnology: A promising immunomodulator with less side effects. Int. Immunopharmacol. 2007;7:1334–1342. doi: 10.1016/j.intimp.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Ma B., Forney L.J., Ravel J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012;66:371–389. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaban B., Links M.G., Jayaprakash T.P., Wagner E.C., Bourque D.K., Lohn Z., Albert A.Y., van Schalkwyk J., Reid G., Hemmingsen S.M., et al. Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome. 2014;2:23. doi: 10.1186/2049-2618-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce M.M., Hilt E.E., Rosenfeld A.B., Zilliox M.J., Thomas-White K., Fok C., Kliethermes S., Schreckenberger P.C., Brubaker L., Gai X., et al. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. mBio. 2014;5:e01283-14. doi: 10.1128/mBio.01283-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerschlag M.R., Alpert S., Rosner I., Thurston P., Semine D., McComb D., McCormack W.M. Microbiology of the vagina in children: Normal and potentially pathogenic organisms. [(accessed on 19 December 2021)];Pediatrics. 1978 62:57–62. doi: 10.1542/peds.62.1.57. Available online: http://pediatrics.aappublications.org/content/62/1/57.short. [DOI] [PubMed] [Google Scholar]
- 20.Aagaard K., Riehle K., Ma J., Segata N., Mistretta T.-A., Coarfa C., Raza S., Rosenbaum S., Veyver I.V.D., Milosavljevic A., et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS ONE. 2012;7:e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diop K., Nguyen T.T., Delerce J., Armstrong N., Raoult D., Bretelle F., Fenollar F. Corynebacterium fournierii sp. nov., isolated from the female genital tract of a patient with bacterial vaginosis. Antonie Van Leeuwenhoek. 2018;111:1165–1174. doi: 10.1007/s10482-018-1022-z. [DOI] [PubMed] [Google Scholar]
- 22.Chen X., Zhao X., Chen L., Zeng W., Xu H. Vaginitis Caused by Corynebacterium amycolatum in a Prepubescent Girl. J. Pediatr. Adolesc. Gynecol. 2015;28:e165–e167. doi: 10.1016/j.jpag.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Collins M.D., Burton R.A., Jones D. Corynebacterium amycolatum sp. nov. a new mycolic acid-lessCorynebacteriumspecies from human skin. FEMS Microbiol. Lett. 1988;49:349–352. doi: 10.1111/j.1574-6968.1988.tb02755.x. [DOI] [Google Scholar]
- 24.Gladysheva I.V., Cherkasov S.V., Khlopko Y. Antibacterial activities of metabolites from Corynebacterium spp. Strains isolated from the reproductive tract of a healthy woman against human pathogenic bacteria. Int. J. Pharma Bio Sci. 2017;8:549–556. doi: 10.22376/ijpbs.2017.8.3.b549-556. [DOI] [Google Scholar]
- 25.Gladysheva I.V., Cherkasov S.V. Corynebacterium species in the female genital tract–pathogens or potential probiotics. Int. J. Pharma Bio Sci. 2018;9:265–272. doi: 10.22376/ijpbs.2018.9.4.b265-272. [DOI] [Google Scholar]
- 26.Bolger A.M., Lohse M., Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A., Formsma K., Gerdes S.Y., Glass E.M., Kubal M., et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatusova T., DiCuccio M., Badretdin A., Chetvernin V., Nawrocki E.P., Zaslavsky L., Lomsadze A., Pruitt K.D., Borodovsky M., Ostell J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huerta-Cepas J., Szklarczyk D., Forslund K., Cook H., Heller D., Walter M.C., Rattei T., Mende D.R., Sunagawa S., Kuhn M., et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016;44:D286–D293. doi: 10.1093/nar/gkv1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Heel A.J., De Jong A., Song C., Viel J., Kok J., Kuipers O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018;46:W278–W281. doi: 10.1093/nar/gky383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blin K., Medema M.H., Kottmann R., Lee S.Y., Weber T. The antiSMASH database, a comprehensive database of microbial secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 2016;45:D555–D559. doi: 10.1093/nar/gkw960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia B., Raphenya A.R., Alcock B., Waglechner N., Guo P., Tsang K.K., Lago B.A., Dave B.M., Pereira S., Sharma A.N., et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017;45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu B., Zheng D., Jin Q., Chen L., Yang J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2018;47:D687–D692. doi: 10.1093/nar/gky1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grissa I., Vergnaud G., Pourcel C. CRISPRFinder: A web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007;35:W52–W57. doi: 10.1093/nar/gkm360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parte A.C. LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Res. 2013;42:D613–D616. doi: 10.1093/nar/gkt1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okonechnikov K., Golosova O., Fursov M., The UGENE Team Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics. 2012;28:1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 39.Lee I., Kim Y.O., Park S.-C., Chun J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2015;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 40.Pascual C., Lawson P.A., Farrow J.A.E., Gimenez M.N., Collins M.D. Phylogenetic analysis of the genus corynebacterium based on 16S rRNA gene sequences. Int. J. Syst. Bacteriol. 1995;45:724–728. doi: 10.1099/00207713-45-4-724. [DOI] [PubMed] [Google Scholar]
- 41.Ruimy R., Riegel P., Boiron P., Monteil H., Christen R. Phylogeny of the genus corynebacterium deduced from analyses of small-subunit ribosomal DNA sequences. Int. J. Syst. Bacteriol. 1995;45:740–746. doi: 10.1099/00207713-45-4-740. [DOI] [PubMed] [Google Scholar]
- 42.Chun J., Oren A., Ventosa A., Christensen H., Arahal D.R., Da Costa M.S., Rooney A.P., Yi H., Xu X.-W., De Meyer S., et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 43.Deckers-Hebestreit G., Altendorf K. The F0F1-type atp synthases of bacteria: Structure and function of the F0 complex. Annu. Rev. Microbiol. 1996;50:791–824. doi: 10.1146/annurev.micro.50.1.791. [DOI] [PubMed] [Google Scholar]
- 44.Xu N., Wang L., Cheng H., Liu Q., Liu J., Ma Y. In vitro functional characterization of the Na+/H+ antiporters in Corynebacterium glutamicum. FEMS Microbiol. Lett. 2015;363:fnv237. doi: 10.1093/femsle/fnv237. [DOI] [PubMed] [Google Scholar]
- 45.Toyoda K., Teramoto H., Inui M., Yukawa H. The ldha gene, encoding fermentative L-lactate dehydrogenase of corynebacterium glutamicum, is under the control of positive feedback regulation mediated by LlDr. J. Bacteriol. 2009;191:4251–4258. doi: 10.1128/JB.00303-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapse N.G., Engineer A.S., Gowdaman V., Wagh S., Dhakephalkar P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics. 2019;111:921–929. doi: 10.1016/j.ygeno.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 47.Zhang W., Ji H., Zhang D., Liu H., Wang S., Wang J., Wang Y. Complete genome sequencing of lactobacillus plantarum zlp001, a potential probiotic that enhances intestinal epithelial barrier function and defense against pathogens in pigs. Front. Physiol. 2018;9:1689. doi: 10.3389/fphys.2018.01689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goyal K., Qamra R., Mande S.C. Multiple gene duplication and rapid evolution in the groel gene: Functional implications. J. Mol. Evol. 2006;63:781–787. doi: 10.1007/s00239-006-0037-7. [DOI] [PubMed] [Google Scholar]
- 49.Jaworek M.W., Moebitz S., Gao M., Winter R. Stability of the chaperonin system GroEL–GroES under extreme environmental conditions. Phys. Chem. Chem. Phys. 2020;22:3734–3743. doi: 10.1039/C9CP06468K. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.Y., Park C., Jang H.-J., Kim B.-O., Bae H.-W., Chung I.-Y., Kim E.S., Cho Y.-H. Antibacterial strategies inspired by the oxidative stress and response networks. J. Microbiol. 2019;57:203–212. doi: 10.1007/s12275-019-8711-9. [DOI] [PubMed] [Google Scholar]
- 51.Flohé L., Toppo S., Cozza G., Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid. Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 52.Delaunay A., Pflieger D., Barrault M.-B., Vinh J., Toledano M.B. A Thiol Peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/S0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 53.Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 54.El Shafey H., Ghanem S., Merkamm M., Guyonvarch A. Corynebacterium glutamicum superoxide dismutase is a manganese-strict non-cambialistic enzyme in vitro. Microbiol. Res. 2008;163:80–86. doi: 10.1016/j.micres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 56.Su T., Si M., Zhao Y., Liu Y., Yao S., Che C., Chen C. A thioredoxin-dependent peroxiredoxin Q from Corynebacterium glutamicum plays an important role in defense against oxidative stress. PLoS ONE. 2018;13:e0192674. doi: 10.1371/journal.pone.0192674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton G.L., Buchmeier N., Fahey R.C. Biosynthesis and functions of mycothiol, the unique protective thiol of actinobacteria. Microbiol. Mol. Biol. Rev. 2008;72:471–494. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koledin T., Newton G.L., Fahey R.C. Identification of the mycothiol synthase gene (mshD) encoding the acetyltransferase producing mycothiol in actinomycetes. Arch. Microbiol. 2002;178:331–337. doi: 10.1007/s00203-002-0462-y. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y.-B., Long M.-X., Yin Y.-J., Si M.-R., Zhang L., Lu Z.-Q., Wang Y., Shen X.-H. Physiological roles of mycothiol in detoxification and tolerance to multiple poisonous chemicals in Corynebacterium glutamicum. Arch. Microbiol. 2013;195:419–429. doi: 10.1007/s00203-013-0889-3. [DOI] [PubMed] [Google Scholar]
- 60.Si M., Zhao C., Zhang B., Wei D., Chen K., Yang X., Xiao H., Shen X. Overexpression of mycothiol disulfide reductase enhances Corynebacterium glutamicum robustness by modulating cellular redox homeostasis and antioxidant proteins under oxidative stress. Sci. Rep. 2016;6:29491. doi: 10.1038/srep29491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin J.F., Demain A.L. Control of antibiotic biosynthesis. Microbiol. Rev. 1980;44:230–251. doi: 10.1128/mr.44.2.230-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes E.S., Schuch V., Lemos E.G.D.M. Biotechnology of polyketides: New breath of life for the novel antibiotic genetic pathways discovery through metagenomics. Braz. J. Microbiol. 2013;44:1007–1034. doi: 10.1590/S1517-83822013000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Staunton J., Weissman K.J. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]
- 64.Kaysser L., Bernhardt P., Nam S.-J., Loesgen S., Ruby J.G., Skewes-Cox P., Jensen P., Fenical W., Moore B.S. Merochlorins A–D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium-dependent chloroperoxidases. J. Am. Chem. Soc. 2012;134:11988–11991. doi: 10.1021/ja305665f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suroto D.A., Kitani S., Arai M., Ikeda H., Nihira T. Characterization of the biosynthetic gene cluster for cryptic phthoxazolin A in Streptomyces avermitilis. PLoS ONE. 2018;13:e0190973. doi: 10.1371/journal.pone.0190973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helfrich E.J.N., Lin G.-M., Voigt C.A., Clardy J. Bacterial terpene biosynthesis: Challenges and opportunities for pathway engineering. Beilstein J. Org. Chem. 2019;15:2889–2906. doi: 10.3762/bjoc.15.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada Y., Kuzuyama T., Komatsu M., Shin-Ya K., Omura S., Cane D.-E., Ikeda H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA. 2015;112:857–862. doi: 10.1073/pnas.1422108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahizan N.A., Yang S.-K., Moo C.L., Song A.A.-L., Chong C.-M., Chong C.-W., Abushelaibi A., Lim S.-H.E., Lai K.-S. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) Pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravikumar S., Woo H.M., Choi J.-I. Analysis of novel antioxidant sesquarterpenes (C35 terpenes) produced in recombinant Corynebacterium glutamicum. Appl. Biochem. Biotechnol. 2018;186:525–534. doi: 10.1007/s12010-018-2756-9. [DOI] [PubMed] [Google Scholar]
- 70.Vasaturo M., Cotugno R., Fiengo L., Vinegoni C., Piaz F.D., De Tommasi N. The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells. Sci. Rep. 2018;8:16735. doi: 10.1038/s41598-018-35088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lombard J., Moreira D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol. Biol. Evol. 2011;28:87–99. doi: 10.1093/molbev/msq177. [DOI] [PubMed] [Google Scholar]
- 72.Liang P.-H., Ko T.-P., Wang A.H.-J. Structure, mechanism and function of prenyltransferases. JBIC J. Biol. Inorg. Chem. 2002;269:3339–3354. doi: 10.1046/j.1432-1033.2002.03014.x. [DOI] [PubMed] [Google Scholar]
- 73.Tarshis L.C., Proteau P.J., Kellogg B.A., Sacchettini J.C., Poulter C.D. Regulation of product chain length by isoprenyl diphosphate synthases. Proc. Natl. Acad. Sci. USA. 1996;93:15018–15023. doi: 10.1073/pnas.93.26.15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Riley M.A., Wertz J.E. Bacteriocins: Evolution, ecology, and application. Annu. Rev. Microbiol. 2002;56:117–137. doi: 10.1146/annurev.micro.56.012302.161024. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez-Sieiro P., Montalbán-López M., Mu D., Kuipers O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016;100:2939–2951. doi: 10.1007/s00253-016-7343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balty C., Guillot A., Fradale L., Brewee C., Boulay M., Kubiak X., Benjdia A., Berteau O. Ruminococcin C, an anti-clostridial sactipeptide produced by a prominent member of the human microbiota Ruminococcus gnavus. J. Biol. Chem. 2019;294:14512–14525. doi: 10.1074/jbc.RA119.009416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chiumento S., Roblin C., Kieffer-Jaquinod S., Tachon S., Leprètre C., Basset C., Aditiyarini D., Olleik H., Nicoletti C., Bornet O., et al. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci. Adv. 2019;5:eaaw9969. doi: 10.1126/sciadv.aaw9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ocaña V.S., de Ruiz Holgado A.A.P., Nader-Macías M.E. Selection of vaginal H2O2-generating lactobacillus species for probiotic use. Curr. Microbiol. 1999;38:279–284. doi: 10.1007/PL00006802. [DOI] [PubMed] [Google Scholar]
- 79.Hertzberger R., Arents J.C., Dekker H.L., Pridmore R.D., Gysler C., Kleerebezem M., De Mattos M.J.T. H2O2 production in species of the Lactobacillus acidophilus group: A central role for a novel NADH-dependent flavin reductase. Appl. Environ. Microbiol. 2014;80:2229–2239. doi: 10.1128/AEM.04272-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leblanc J.G., Chain F., Martín R., Humaran L.G.B., Courau S., Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gu Q., Li P. Probiotics and Prebiotics in Human Nutrition and Health. InTechOpen; London, UK: 2016. Biosynthesis of vitamins by probiotic bacteria. [DOI] [Google Scholar]
- 82.Ahire J.J., Mokashe N.U., Patil H.J., Chaudhari B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013;50:26–34. doi: 10.1007/s13197-011-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai Z., Wu Z., Hang S., Zhu W., Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 2015;21:389–409. doi: 10.1093/molehr/gav003. [DOI] [PubMed] [Google Scholar]
- 84.Cherkasov S.V., Gladysheva I.V. Antibiotic resistance of coryneform bacteria isolated from the reproductive tract of women. [(accessed on 4 April 2020)];Antibiot. I Khimioterapiia. 2010 55:45–49. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21400755. [PubMed] [Google Scholar]
- 85.Tauch A., Burkovski A. Molecular armory or niche factors: Virulence determinants of Corynebacterium species. FEMS Microbiol. Lett. 2015;362:fnv185. doi: 10.1093/femsle/fnv185. [DOI] [PubMed] [Google Scholar]
- 86.Swierczynski A., Ton-That H. Type III pilus of corynebacteria: Pilus length is determined by the level of its major pilin subunit. J. Bacteriol. 2006;188:6318–6325. doi: 10.1128/JB.00606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baumgart M., Schubert K., Bramkamp M., Frunzke J. Impact of LytR-CpsA-Psr proteins on cell wall biosynthesis in Corynebacterium glutamicum. J. Bacteriol. 2016;198:3045–3059. doi: 10.1128/JB.00406-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wennerhold J., Bott M. The DtxR regulon of Corynebacterium glutamicum. J. Bacteriol. 2006;188:2907–2918. doi: 10.1128/JB.188.8.2907-2918.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bush M.J. The actinobacterial WhiB-like (Wbl) family of transcription factors. Mol. Microbiol. 2018;110:663–676. doi: 10.1111/mmi.14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makarova K.S., Wolf Y.I., Alkhnbashi O.S., Costa F., Shah S.A., Saunders S.J., Barrangou R., Brouns S.J., Charpentier E., Haft D.H., et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 2015;13:722–736. doi: 10.1038/nrmicro3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molineux I.J. Host-parasite interactions: Recent developments in the genetics of abortive phage infections. New Boil. 1991;3:230–236. [PubMed] [Google Scholar]
- 92.Kiliç A.O., Pavlova S.I., Alpay S., Kiliç S.S., Tao L. Comparative study of vaginal lactobacillus phages isolated from women in the united states and turkey: Prevalence, morphology, host range, and DNA homology. Clin. Diagn. Lab. Immunol. 2001;8:31–39. doi: 10.1128/CDLI.8.1.31-39.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this study are available in the article.