Abstract
Objective
Vascular complications (VCs) contribute to increased morbidity and mortality in patients who have undergone transcatheter aortic valve implantation (TAVI); however, studies on their incidence and predictors show conflicting results. In this study, we sought to assess the incidence, impact, and predictors of VCs in transfemoral (TF) TAVI and also investigated the predictive role of manufacturer’s size charts and a new predictor modified sheath-to-femoral artery ratio.
Methods
A total of 223 patients undergoing TF-TAVI were categorized into 2 groups. The patients were divided as eligible and ineligible according to the manufacturer’s guidelines (MG), and the same patient cohort was dichotomized into eligible and ineligible on the basis of sheath-to-femoral artery ratio (SFAR) value of less than or greater than or equal to modified SFAR (md-SFAR). VCs (defined according to the Valve Academic Research Consortium II criteria) were retrospectively compared.
Results
According to the manufacturer’s size charts, 65 patients were unsuitable; however, 35 patients were ineligible for TF-TAVI per the md-SFAR criteria. Although VCs occurred in 42 (18.8%) patients, 17 (27.7%) of those patients were classified as ineligible according to MG, whereas 14 (41.2%) were classified as ineligible in the md-SFAR group. In a multiple logistic regression analysis that included md-SFAR, MG, SFAR ≥1.05, peripheral artery disease, and minimum iliofemoral artery diameter, only md-SFAR was the independent predictor of VCs (odds ratio=3.71, 95% confidence interval=1.13–12.53, p=0.031).
Conclusion
According to our results, md-SFAR might provide better patient selection to prevent VCs and improve outcomes in TF-TAVI procedures.
Keywords: aortic valve stenosis, aortic valve replacement, complications, heart valve disease, transcatheter aortic valve replacement
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has become an emerging treatment modality for inoperable/high and intermediate-risk patients with severe aortic stenosis (1, 2). TAVI is associated with fewer major bleeding events and similar survival rates compared with those of surgical aortic valve replacement but has an increased incidence of vascular complications (VC) and conduction abnormalities (3). Transfemoral (TF) access is the most commonly used pathway for the vast majority of TAVI procedures, and nearly 89% of patients are deemed suitable for this approach. In daily practice, most of the unsuitable patients are still being treated with the TF approach, given the poor outcomes with non-TF pathways (4–6). The suitability for TF-TAVI is still an issue because artery sizing recommendations determined by manufacturers of TAVI delivery systems for their devices are not evidence based (7).
Even with the decreasing profile of TAVI delivery systems, in conjunction with increasing vascular screening, and operator experience, VCs pose major obstacles to successful outcomes (8) as these predispose a patient to the risk of higher mortality, longer hospital stay, and diminished quality of life.
Center experience, sheath-to-femoral artery ratio (SFAR), defined as the ratio of the outer diameter of the sheath (in millimeters) to minimum iliofemoral lumen diameter (MIFLD) (in millimeters) (≥1.05), circumferential calcifications, peripheral artery disease, and female sex are established as independent predictors particularly for those vulnerable to VCs (9). Of these, SFAR reflects both sheath size and MIFLD and is considered as the strongest predictor of VCs; however, varying SFAR limits were reported on the latter studies with the development of newer TAVI delivery systems (10–12). Therefore, there is still a need to adequately predict VCs to mitigate adverse outcomes.
Considering different SFAR thresholds found by several studies, nonevidence-based recommendations regarding peripheral vessel diameters given by TAVI manufacturers and differences in the manufacturer’s French sizes and outer diameters of the sheath, the question arises as to whether the SFAR cut-off values may differ according to the distribution of TAVI delivery systems. We calculated a modified version of the SFAR [modified SFAR (md-SFAR)] value obtained by dividing the sheath’s outer diameter (in millimeters) to minimum required vessel diameter (in millimeters) derived from each manufacturer sizing chart. The md-SFAR was therefore specific for each valve brand and size (Table 1), and to our knowledge, md-SFAR is a novel tool to predict VC. In this study, we aimed to analyze a single center TAVI experience in terms of VCs and retrospectively compare the current manufacturer’s recommendations with md-SFAR to predict VCs during TF-TAVI procedures.
Table 1.
Outer diameter of sheath and minimum vessel diameters required for TAVI systems
| Valve name | Valve size (mm) | Sheath outer diameter (mm) | Minimum vessel diameter (mm) | Sheath size (F) | Sheath size (mm) | Sheath outer diameter/Minimum vessel diameter ratio (md-SFAR) | Sheath French Size / Minimum vessel diameter ratio |
|---|---|---|---|---|---|---|---|
| SAPIEN XT (Edwards Lifesciences Corporation) | 23 | 6.7 | 6 | 16 | 5.33 | 1.12 | 2.67 |
| 26 | 7.2 | 6.5 | 18 | 6 | 1.11 | 2.77 | |
| 29 | 8 | 7 | 20 | 6.67 | 1.14 | 2.86 | |
| SAPIEN 3 (Edwards Lifesciences Corporation) | 20 | 6 | 5 | 14 | 4.67 | 1.2 | 2.8 |
| 23 | 6 | 5.5 | 14 | 4.67 | 1.09 | 2.55 | |
| 26 | 6 | 5.5 | 14 | 4.67 | 1.09 | 2.55 | |
| 29 | 6.7 | 6 | 16 | 5.33 | 1.12 | 2.67 | |
| Evolut R (Medtronic) | 23 | 6 | 5 | 14 | 4.67 | 1.2 | 2.8 |
| 26 | 6 | 5 | 14 | 4.67 | 1.2 | 2.8 | |
| 29 | 6 | 5 | 14 | 4.67 | 1.2 | 2.8 | |
| 34 | 6.7 | 5.5 | 16 | 5.33 | 1.22 | 2.9 | |
| Portico (Abbot) | 23 | 6 | 6 | 18 | 6 | 1 | 3 |
| 25 | 6 | 6 | 18 | 6 | 1 | 3 | |
| 27 | 6.4 | 6.5 | 19 | 6.33 | 0.98 | 2.92 | |
| 29 | 6.4 | 6.5 | 19 | 6.33 | 0.98 | 2.92 |
md-SFAR - modified sheath-to-femoral artery ratio; TAVI - transcatheter aortic valve implantation
METHODS
Study population and design
In total, 223 consecutive patients undergoing TF-TAVI between 2016 and 2019 were analyzed in this single-center study. A multidisciplinary team with interventional cardiologists, cardiac anesthetists, and cardiovascular surgeons were involved in patient selection and treatment. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Local Research Ethics Committee.
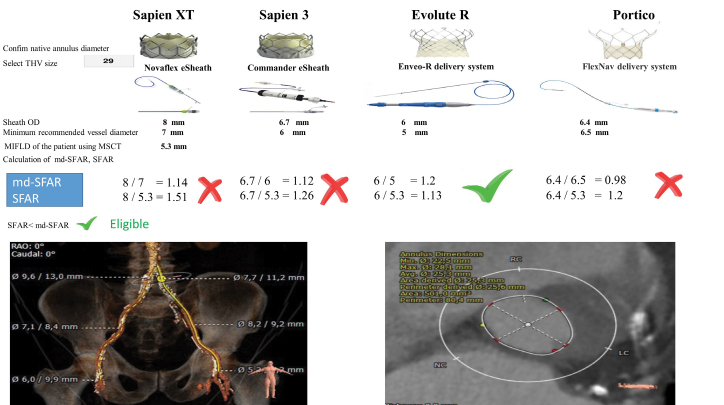
The patients were divided into 2 subgroups, classified as “eligible”’ and “ineligible” on the basis of the MIFLD recommendations given by the valve manufacturers and/or presence of circumferential arterial calcification [manufacturer’s guidelines (MG)]. We also calculated the SFAR value for each patient and compared these values with md-SFAR thresholds to clarify whether the patient was eligible or ineligible for TF-TAVI. Thus, the same patient cohort was dichotomized into those with SFAR <md-SFAR (eligible) versus ≥md-SFAR (ineligible). Eligibility determination according to the SFAR and md-SFAR measures is illustrated with a patient example in Figure 1.
Figure 1.
Annulus diameter and perimeter, sheath OD, MIFLD measured by MSCT and minimum recommended vessel diameter according to 29-mm valve size charts and subsequently derived sheath-to-femoral artery ratio and modified sheath-to-femoral artery-ratio (md-SFAR)- sheath-to-femoral artery ratio as illustrated in a 79-year-old patient
md-SFAR - sheath outer diameter/MIFLD (recommended by the manufacturing company according to the sheath size); MIFLD - minimum iliofemoral lumen diameter; MSCT - multi-slice computed tomography; OD - outer diameter; SFAR - sheath outer diameter/MIFLD (measured by MSCT for each patient)
Devices implanted included balloon-expandable Edwards SAPIEN S3 and Edwards SAPIEN XT™ valves (Edwards Lifesciences Corporation, Irvine, CA, USA), self-expanding Medtronic CoreValve® Evolut™ R System valves (Medtronic, Minneapolis, MN, USA), and Portico valve (St. Jude Medical, Minneapolis, MN, USA). The sheath size was decided according to the manufacturer’s recommendations for each valve size (Table 1).
Vessel access, definitions, and procedural details
Careful screening included baseline characteristics, iliac and femoral artery characteristics (minimal diameter, calcification, and tortuosity), procedural data including sheath type and size, and VCs. Pre-procedural multi-slice CT (MSCT) examinations were performed with ECG-gated scanning and image reconstruction on a 256-slice CT scanner, Brilliance iCT™ (Koninklijke Philips N.V., Amsterdam, The Netherlands) in all patients. Vessel tortuosity and calcifications were also evaluated using MSCT (13, 14). Measurements were performed by an examiner with >20 years of experience in assessing the peripheral vasculature and were cross checked by a physician. Tortuosity was graded as no tortuosity, mild (30°–60°), moderate (60°–90°), and severe (90°). Arterial calcification was graded as no calcification, mild (90° of total circumferential arc), moderate (90°–180° of total circumferential arc), marked (180°–270° of total circumferential arc), and severe calcification (>270° of total circumferential arc). Various md-SFAR thresholds (Table 1) were used to dichotomize the study population different from the definition of conventional SFAR values as previous studies reported (14–16). The Confida™ Brecker (Medtronic, Minneapolis, MN, USA), Amplatz Extra-Stiff APEX (Cook Medical Inc., Bloomington, IN, USA), and Safari2™ (Boston Scientific, Marlborough, MA, USA) wires were used for introducing delivery sheaths for TAVI with no specific selection for ineligible patients. None of the patients had balloon pre-dilatation to enable passage of the delivery system. After valve implantation, hemostasis was achieved by tightening of the Perclose devices. Evaluation of the vessel and puncture site bleeding were assessed using angiography. Access site complications were treated as per the operator’s discretion. Major clinical end points were assessed according to the updated Valve Academic Research Consortium (VARC-II) criteria (17). Careful attention was paid to the collection of vascular complications such as rupture, dissection, perforation, hematoma (>5 cm), and pseudoaneurysm. Vascular interventions were documented as well and included balloon angioplasty, stenting, and the need for unplanned surgical intervention.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics for Windows, version 25 (IBM Corporation, Armonk, NY, USA). The results of continuous variables are expressed as mean (standard deviation). The results of categorical data are reported as frequencies (%). Normality of the distribution of continuous variables was tested using the Kolmogorov–Smirnov goodness-of-fit test. Continuous variables were compared using the student t-test or the Mann–Whitney U test where appropriate. Categorical variables were compared using the chi-square test. Multiple logistic regression was used to identify independent predictors of VCs. Variables found to be significantly related to vascular complications in univariate analysis (p≤0.1) and peripheral artery disease were included to determine the predictors of VARC-II VCs in the final regression model. The level of significance was set at p<0.05.
RESULTS
Patient population
Table 2 compares the baseline characteristics of the patients. The patients were classified as eligible or ineligible groups according to MG (158 patients eligible, 65 patients ineligible) and md-SFAR values (188 patients eligible, 35 patients ineligible). Iliofemoral calcium score, MIFLD, and SFAR values were higher, and peripheral artery disease tended to be more prevalent in ineligible patients for both the groups. Sheath sizes ≥18F, larger sheath outer diameter, and anticoagulation rates were only higher for ineligible patients in the MG group, whereas these parameters did not differ in patients stratified by the md-SFAR criteria. Ineligible patients more likely had tortuous vessels in the md-SFAR group (p=0.072).
Table 2.
Baseline clinical and procedural characteristics of the study population
| Variables | Manufacturer’s guidelines | P-value | md-SFAR value | P-value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| TF-TAVI eligible (n=158) | TF-TAVI ineligible (n=65) | TF-TAVI eligible (n=188) | TF-TAVI ineligible (n=35) | |||
| Age (years) | 78.9±7.08 | 78.95±8.79 | 0.974 | 79.1±7.34 | 78.1±8.98 | 0.475 |
| Men, n (%) | 85 (53.8) | 31 (47.7) | 0.407 | 100 (53.2) | 16 (45.7) | 0.416 |
| logEuroScore | 26.02±4.07 | 25.68±3.57 | 0.556 | 25.79 ±3.85 | 26.62±4.23 | 0.227 |
| NYHA III–IV, n (%) | 85 (53.8) | 32 (49.2) | 0.534 | 100 (52.9) | 17 (50) | 0.756 |
| LVEF % | 49.63±13.8 | 53±10.03 | 0.076 | 50.5±13.2 | 51.2±11.01 | 0.737 |
| BMI, kg/m2 | 26.47±3.63 | 25.68±3.49 | 0.134 | 26.4±3.66 | 25.35±3.13 | 0.147 |
| Hypertension, n (%) | 143 (90.5) | 57 (87.7) | 0.530 | 172 (91) | 28 (80) | 0.063 |
| Diabetes, n (%) | 38 (24.1) | 10 (15.4) | 0.152 | 45 (23.8) | 3 (8.8) | 0.045 |
| CAD, n (%) | 87 (55.1) | 44 (67.7) | 0.082 | 107 (56.9) | 24 (68.6) | 0.198 |
| PAD, n (%) | 26 (16.5) | 18 (27.7) | 0.055 | 33 (17.5) | 11 (31.4) | 0.056 |
| GFR (mL/min/1.73 m2) | 68.7±25.9 | 70.05±25.47 | 0.761 | 69.6±25.6 | 67.1±27.18 | 0.752 |
| Previous MI, n (%) | 41 (25.9) | 18 (27.7) | 0.789 | 48 (25.5) | 11 (31.4) | 0.468 |
| Previous CVO, n (%) | 8 (5.1) | 4 (6.2) | 0.743 | 9 (4.8) | 3 (8.6) | 0.408 |
| Previous PCI, n (%) | 47 (29.7) | 13 (20) | 0.136 | 53 (28) | 7 (20) | 0.316 |
| CABG, n (%) | 29 (18.4) | 13 (20) | 0.775 | 34 (18) | 8 (22.8) | 0.507 |
| AF, n (%) | 43 (27.4) | 13 (20) | 0.249 | 46 (24.5) | 10 (28.6) | 0.619 |
| Anticoagulation, n (%) | 53 (33.8) | 12 (18.5) | 0.024 | 56 (29.8) | 9 (25.7) | 0.626 |
| Edwards SAPIEN XT | 69 (43.7) | 44 (67.7) | 0.001 | 97 (51.6) | 16 (45.7) | 0.016 |
| Edwards SAPIEN 3 | 22 (13.9) | 8 (12.3) | 24 (12.8) | 6 (17.1) | ||
| Evolut R | 54 (34.2) | 6 (9.2) | 55 (29.3) | 5 (14.3) | ||
| Portico | 13 (8.2) | 7 (10.8) | 12 (6.4) | 8 (22.9) | ||
| Iliofemoral calcium score* | 1.24±0.74 | 1.69±1.03 | 0.002 | 1.29±0.78 | 1.82±1.1 | 0.011 |
| Tortuosity score* | 1.34±0.97 | 1.38±0.97 | 0.732 | 1.4±0.98 | 1.1±0.87 | 0.072 |
| MIFLD, mm | 7.66±1.1 | 6.32±0.99 | <0.001 | 7.56±1.05 | 5.66±0.8 | <0.001 |
| SFAR | 0.89±0.11 | 1.13±0.15 | <0.001 | 0.91±0.13 | 1.21±0.17 | <0.001 |
| Sheath ≥18F, n (%) | 67 (42.4) | 41 (63.1) | 0.005 | 90 (47.9) | 18 (51.4) | 0.699 |
| Sheath outer diameter (mm) | 6.71±0.67 | 7.02±0.71 | 0.002 | 6.82±0.71 | 6.68±0.61 | 0.218 |
Measured by MSCT
AF - atrial fibrillation; BMI - body mass index; CABG - coronary artery bypass grafting; CAD - coronary artery disease; CVO - cerebrovascular occlusion; GFR - glomerular filtration rate; LVEF - left ventricular ejection fraction; MI - myocardial infarction; MSCT – multi-splice computed tomography; MIFLD - minimum iliofemoral minimum lumen diameter; NYHA - New York Heart Association; PAD - peripheral artery disease; PCI - percutaneous coronary intervention; SFAR - sheath-to femoral artery ratio; TF-TAVI - transfemoral transcatheter aortic valve implantation
Comparison of vascular complications and in-hospital mortality between the 2 groups
The baseline clinical and anatomical characteristics of patients with and without VC are shown in Table 3. According to the VARC-II criteria, 42 patients suffered VCs. Patients with VCs had higher SFAR levels (1.02±0.21 vs. 0.95±0.15, p=0.009) and were more likely to be deemed ineligible according to the current recommendations (26% vs. 42.9%, p=0.030) and md-SFAR thresholds (11.6% vs. 33.3%, p<0.001).
Table 3.
Baseline characteristics between patients with and without vascular complications
| Variables | Patients without VC (n=181) | Patients with VC (n=42) | P-value |
|---|---|---|---|
| Age (years) | 79.2±7.69 | 77.98±7.2 | 0.353 |
| Female, n (%) | 83 (45.9) | 24 (57.1) | 0.187 |
| logEuroScore | 25.7±3.81 | 26.8±4.33 | 0.103 |
| LVEF % | 50.8±12.78 | 49.6±13.6 | 0.591 |
| BMI, kg/m2 | 26.3±3.5 | 26.1±3.9 | 0.809 |
| Hypertension, n (%) | 164 (90.6) | 36 (85.7) | 0.397 |
| Diabetes, n (%) | 40 (22.1) | 8 (19) | 0.665 |
| CAD, n (%) | 107 (59.1) | 24 (57.1) | 0.815 |
| PAD, n (%) | 32 (17.7) | 13 (28.6) | 0.053 |
| GFR (mL/min/1.73 m2) | 69.43±25.3 | 68.3±27.9 | 0.803 |
| Previous MI, n (%) | 51 (28.2) | 8 (19) | 0.227 |
| Previous CVO, n (%) | 8 (4.4) | 4 (9.5) | 0.246 |
| Previous PCI, n (%) | 51 (28.2) | 9 (21.4) | 0.374 |
| CABG, n (%) | 33 (18.2) | 9 (21.4) | 0.633 |
| AF, n (%) | 46 (25.6) | 10 (23.8) | 0.815 |
| Ineligibility (md-SFAR) | 21 (11.6) | 14 (33.3) | <0.001 |
| Ineligibility (CG) | 47 (26) | 18 (42.9) | 0.030 |
| Calcification ≥moderate | 18 (9.9) | 7 (16.7) | 0.274 |
| Tortuosity ≥ moderate | 73 (81.2) | 17 (59.5) | 0.986 |
| MIFLD, mm | 7.31±1.16 | 6.87±1.41 | 0.035 |
| Sheath ≥18-F, n (%) | 87 (48) | 21 (50) | 0.821 |
| Sheath outer diameter (mm) | 6.8±0.7 | 6.79±0.70 | 0.986 |
| Number of ProGlide per patient | 1.86±0.49 | 1.98±0.71 | 0.199 |
| Single ProGlide, n (%) | 37 (20.4) | 8 (19) | 0.839 |
| Double ProGlide, n (%) | 133 (73.5) | 29 (69) | 0.561 |
| Number of ProGlides ≥3, n (%) | 11 (6.1) | 5 (11.9) | 0.192 |
| Valve types | 0.333 | ||
| SAPIEN XT | 88 (48.6) | 25 (59.5) | |
| SAPIEN 3 | 27 (14.9) | 3 (7.1) | |
| Evolut R | 51 (28.2) | 9 (21.4) | |
| Portico | 15 (8.3) | 5 (11.9) | |
| SFAR | 0.95±0.15 | 1.02±0.21 | 0.009 |
| SFAR ≥1.05 | 43 (23.8) | 16 (38.1) | 0.058 |
| In-hospital death, n (%) | 10 (5.5) | 5 (11.9) | 0.167 |
| 30-day mortality, n (%) | 11 (6.1) | 8 (19) | 0.012 |
AF - atrial fibrillation; BMI - body mass index; CABG - coronary artery bypass grafting; CAD - coronary artery disease; CG - current guidelines; CVO - cerebrovascular occlusion; GFR - glomerular filtrate rate; LVEF - left ventricular ejection fraction; md-SFAR - modified sheath-to-femoral artery ratio; MI - myocadial infarction; MIFLD - minimum iliofemoral lumen diameter; PAD - peripheral artery disease; PCI - percutanious coronary intervention; SFAR - sheath-to-femoral artery ratio; VC - vascular complications
Overall, VCs occurred more frequently in unsuitable patients for both the groups (p=0.030 and p<0.001 for MG and md-SFAR groups, respectively) (Table 4). According to the current guidelines, there was no difference in the incidence of major vascular events (eligible group 5.3% vs. ineligible group 12.3%, p=0.091) or minor vascular events (eligible group 9.5% vs. ineligible group 15.4%, p=0.205). In terms of bleeding rates, a trend for increased minor bleeding was observed in ineligible patients (5.1% vs. 12.8%, p=0.083) in the MG group.
Table 4.
Complication types
| All patients (n=223) | Manufacturer’s guidelines | md-SFAR threshold | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| TF eligible (n=158) | TF ineligible (n=65) | P-value | TF eligible (n=188) | TF ineligible (n=35) | P-value | ||
| Overall VARC-II complication, n (%) | 42 (18.8) | 24 (15.2) | 18 (27.7) | 0.030 | 28 (14.8) | 14 (41.2) | <0.001 |
| Major VARC-II complication, n (%) | 17 (7.6) | 9 (5.3) | 8 (12.3) | 0.091 | 10 (5.3) | 7 (20.6) | 0.007 |
| Minor VARC-II complication, n (%) | 25 (11.2) | 15 (9.5) | 10 (15.4) | 0.205 | 18 (9.5) | 7 (20.6) | 0.083 |
| Major bleeding, n (%) | 21 (9.4) | 12 (7.6) | 9 (13.8) | 0.146 | 14 (7.4) | 7 (20.6) | 0.029 |
| Minor bleeding, n (%) | 16 (7.2) | 8 (5.1) | 8 (12.3) | 0.083 | 10 (5.3) | 6 (17.6) | 0.024 |
| Hematoma, n (%) | 12 (5.4) | 8 (5.1) | 4 (6.2) | 0.749 | 11 (5.9) | 1 (2.9) | 0.697 |
| Aortic dissection, n (%) | 1 | 1 | 0 | - | 1 | 0 | - |
| Rupture, n (%) | 5 (2.2) | 1 (0.6) | 4 (6.2) | 0.026 | 1 (0.5) | 4 (11.4) | 0.002 |
| Stenosis/occlusion | 16 (7.2) | 10 (6.3) | 6 (9.2) | 0.568 | 11 (5.9) | 5 (14.3) | 0.143 |
| Pseudoaneurysm, n (%) | 2 | 1 (0.6) | 1 (1.5) | 0.499 | 1 (0.5) | 1 (2.9) | 0.290 |
| Closure device failure, n (%) | 4 (1.8) | 2 (1.3) | 2 (3.1) | 0.582 | 2 (1.1) | 2 (5.7) | 0.127 |
| Sheath fracture, n (%) | 1 | - | 1 | - | - | 1 | - |
| Annular rupture, n (%) | 1 | 1 | - | 1 | - | - | |
| In-hospital ex, n (%) | 15 (6.7) | 11 (7) | 4 (6.2) | 1 | 12 (6.4) | 3 (8.6) | 0.711 |
| 30-day mortality | 19 (8.1) | 14 (8.9) | 5 (7.7) | 0.776 | 16 (8.5) | 3 (8.6) | 0.991 |
md-SFAR - modified sheath-to-femoral artery ratio; TF - transfemoral; VARC - Valve Academic Research Consortium
Patients in the ineligible group as stratified by md-SFAR had a higher rate of major VCs (5.3% vs. 20.6%, p=0.007) and a tendency toward higher minor VCs (9.50% vs. 20.6%; p=0.083). Major and minor bleeding rates occurred more frequently in ineligible patients (7.4% vs. 20.6%, p=0.029 for major bleeding, 5.3% vs. 17.6%, p=0.024 for minor bleeding). Stenosis/occlusions and hematomas made up the majority of VCs, and rupture rates were more prevalent in ineligible patients for both groups (0.6% vs. 6.2% p=0.026 in the MG group, 0.5% vs. 11.4% p=0.002 in the md-SFAR group).
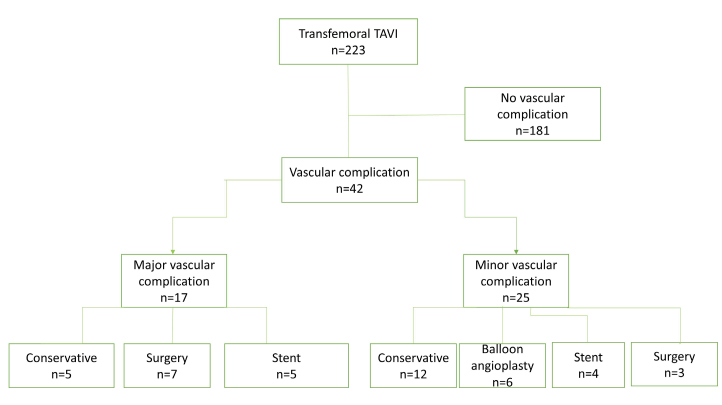
Major VCs (n=17, 7.6%) included 5 vessel ruptures, 3 hematomas, 3 stenosis/occlusions, 2 vascular closure failures, 1 aortic dissection, 1 pseudoaneurysm, 1 annular rupture, and 1 sheath fracture. These serious complications were treated with 5 conservative approaches (manual compression, fluid therapy, and blood transfusions), 5 stenting, and 7 emergent surgical interventions (Fig. 2). Six of the major VCs resulted in death. The access site minor complications were 13 stenosis/occlusions, 9 hematomas, 2 closure device failures, and 1 pseudoaneurysm. Almost half of these patients were treated conservatively (n=12). Of those remaining, 6 had balloon angioplasty, 4 had stenting, and 3 underwent surgical intervention.
Figure 2.
Management of vascular complications
Peripheral artery disease, current guidelines directed ineligibility, unsuitability determined by md-SFAR thresholds, SFAR ≥1.05, calcification ≥moderate, and MIFLD were univariable predictors of overall VCs (Table 5). In the final multiple regression analysis, having SFAR threshold ≥ md-SFAR predicted VCs (OR=3.71, 95% confidence interval=1.13–12.53, p=0.031).
Table 5.
Predictors of overall vascular complications
| Univariate | Multiple | ||
|---|---|---|---|
|
| |||
| P-value | HR (95%CI) | P-value | |
| Ineligibility (md-SFAR) | 0.001 | 3.7 (1.13–12.53) | 0.031 |
| PAD | 0.114 | 1.6 (0.72–3.6) | 0.246 |
| Ineligibility (MG) | 0.032 | 1.1 (0.38–3.28) | 0.849 |
| MIFLD | 0.036 | 1.03 (0.71–1.52) | 0.855 |
| SFAR ≥1.05 | 0.061 | 0.92 (0.29–2.96) | 0.895 |
| Calcification ≥moderate | 0.219 | ||
CI - confidence interval; HR - hazard ratio; md-SFAR - modified sheath-to-femoral artery ratio; MG - manufacturer’s guidelines; MIFLD - minimum iliofemoral artery lumen diameter; PAD - peripheral artery disease; SFAR - sheath-to-femoral artery ratio
DISCUSSION
Our study shows that access site complications are an important source of potential morbidity and mortality in TF-TAVI procedures. Major access site complication rate in this study was 7.6%, which is comparable to those described by other centers, ranging between 5.0% and 7% (1, 18). Our findings are of potential clinical relevance indicating that device recommended usage guidelines and/or expert opinions do not provide individualized risk prediction of VC; however, eligibility based on md-SFAR values was identified as the best predictor of vascular adverse events.
The occurrence of VCs is strongly associated with poor overall clinical outcomes. The rate of major VCs has declined significantly with the transition to newer generation balloon-expandable valves (Edwards SAPIEN vs. SAPIEN XT; 15.18% vs. 8.48%, p<0.00001) (19); however, the incidence of VCs did not differ with either Edwards S3 versus SAPIEN XT devices (0% vs. 7.7%, p=0.15) or Edwards SAPIEN XT versus Medtronic CoreValve (2.8% vs. 3.3%, p=0.66) (20, 21). Newer generation transcatheter heart valves, including Edwards SAPIEN XT, SAPIEN 3, Medtronic Evolut R, and Portico valve systems, were used, and the rate of VCs was comparable among these devices in our study (Table 3). Otherwise, reduced VCs have been driven by a combination of smaller sheath sizes, flexible delivery systems, more frequent use of MSCT, and operator experience (22).
Several variables have been explored as predictors of VCs such as center and surgeon experience, peripheral artery disease, female sex, femoral artery calcification (especially when circumferential), minimal artery diameter, and SFAR ≥1.05 (23, 24). This study adds location of md-SFAR as a new predictor. Another important point is that current expert opinions and/or manufacturer’s size charts should not be the sole criteria for TF-TAVI eligibility.
In contrast to our article, Hayashida et al. (15), who first defined SFAR, evaluated only 58 of 127 (46%) patients undergoing MSCT with the remaining patients being evaluated by 2-dimensional angiography. Previously defined SFAR measures were performed using mainly 22-F and 24-F delivery sheaths, which were bulky compared with contemporary smaller and flexible delivery systems; therefore, different SFAR values were given by the latter studies using different TAVI delivery systems (12, 14, 15). In the regression analysis, we found that md-SFAR could be more accurate in predicting overall VCs than a standard SFAR level.
Some reports showed that iliofemoral calcification was an important predictor of major VCs in patients undergoing TF-TAVI (11, 25), whereas others did not report any such interaction (14, 26). When calcifications are concentric, even in the presence of vessels of good caliber, this was considered as a potential contraindication for TF access. According to these recommendations, together with manufacturer’s minimum vessel diameters, 65 of 223 (29%) of our cohort were deemed ineligible for TF-TAVI. In contrast, the unsuitable patient population almost halved 35 of 223 (15.6%) on the basis of md-SFAR levels. Even with alternative pathways being preferred for those who had small iliofemoral vessel diameters and/or iliofemoral circumferential calcifications, we acknowledge that the current size charts did not completely reflect the true patients who were at risk for VCs and major and minor bleedings. The use of md-SFAR minimized the risk of both VCs and bleedings leading to reduced vascular injury.
Similar to our study, Reinthaler et al. (11) showed that patients deemed unsuitable for TF-TAVI experienced slightly higher major VCs during TF-TAVI (10.7% vs. 2.6%, p=0.07). That study found circumferential iliofemoral calcifications and SFAR to be predictors of major vascular events. Interestingly, we found SFAR was predictive, but circumferential iliofemoral calcification was not found as a predictor of VCs. Sheath diameters used in this study (≥18-F, 51.9%) were smaller than those used in the study conducted by Reinthaler et al. (11) using either ProGlide or Prostar vascular closure devices (≥18-F, 89%). The ProGlide-based vascular closure strategy was associated with lower rates of major VCs, bleeding, and kidney injury when compared with Prostar XL-based vascular closure strategy (16). In our opinion, the impact of iliofemoral calcifications rather than SFAR over access site complications could have been mitigated by the use of lower-profile delivery systems and ProGlide as a default closure device (16).
Study limitations
This was a retrospective, non-randomized study, which meant selection bias could not be excluded. A randomized prospective study or propensity score matching would provide a more reliable analysis. In addition, our study was performed as a single-center analysis and included a limited study population. Finally, the physical properties of the 4 sheaths varying by manufacturers and models could have affected outcomes.
CONCLUSION
Vascular access site complications continue to be a significant issue in patients undergoing TF-TAVI. In comparison with MG and suggested fixed SFAR thresholds, those with SFAR ≥md-SFAR have increased vascular or bleeding complications; therefore, this criterion may mitigate some of the risks of VCs in those at higher risk and safely expand the indications beyond current recommendations.
HIGHLIGHTS
Up to one-third of the transcatheter aortic valve implantation (TAVI) procedures were considered as unsuitable for the transfemoral (TF) approach.
Manufacturer’s size charts for TF TAVI have a poor role in the selection of suitable patients.
Modified sheath-to-femoral artery ratio can play a distinct role in predicting overall vascular events preceding TF TAVI.
Footnotes
Declaration: It was presented as a poster presentation at the EUROPCR Congress held in Paris between 18–21 May 2021.
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – B.Ç.; Design – S.Ç., B.Ç.; Supervision – B.Ç., Ö.U.Ö.; Fundings – None; Materials – Y.G., B.B.; Data collection &/or processing – S.Ç., A.Y.; Analysis &/or interpretation – O.K., F.K.Y.; Literature search – H.M.G.; Writing – B.Ç., O.K., H.M.G., F.K.Y.; Critical review – B.B.
REFERENCES
- 1.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 2.Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, et al. PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 3.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4.Basir MB, Velez C, Fuller B, Wyman J, Paone G, Wang DD, et al. Rates of vascular access use in transcatheter aortic valve replacement: A look into the next generation. Catheter Cardiovasc Interv. 2016;87:E166–71. doi: 10.1002/ccd.26116. [DOI] [PubMed] [Google Scholar]
- 5.Sousa O, Ponte M, Caeiro D, Carvalho M, Leite D, Rocha J, et al. Transcatheter aortic valve implantation: is anatomy still the limiting factor? Rev Port Cardiol. 2013;32:281–6. doi: 10.1016/j.repce.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Madigan M, Atoui R. Non-transfemoral access sites for transcatheter aortic valve replacement. J Thorac Dis. 2018;10:4505–15. doi: 10.21037/jtd.2018.06.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piazza N, Lange R, Martucci G, Serruys PW. Patient selection for transcatheter aortic valve implantation: patient risk profile and anatomical selection criteria. Arch Cardiovasc Dis. 2012;105:165–73. doi: 10.1016/j.acvd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Reidy C, Sophocles A, Ramakrishna H, Ghadimi K, Patel PA, Augoustides JG. Challenges after the first decade of transcatheter aortic valve replacement: focus on vascular complications, stroke, and paravalvular leak. J Cardiothorac Vasc Anesth. 2013;27:184–9. doi: 10.1053/j.jvca.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Seiffert M, Schnabel R, Conradi L, Diemert P, Schirmer J, Koschyk D, et al. Predictors and outcomes after transcatheter aortic valve implantation using different approaches according to the valve academic research consortium definitions. Catheter Cardiovasc Interv. 2013;82:640–52. doi: 10.1002/ccd.24751. [DOI] [PubMed] [Google Scholar]
- 10.Millán X, Azzalini L, Khan R, Cournoyer D, Dorval JF, Ibrahim R, et al. Efficacy of a balloon-expandable vascular access system in transfemoral TAVI patients. Catheter Cardiovasc Interv. 2016;88:1145–52. doi: 10.1002/ccd.26514. [DOI] [PubMed] [Google Scholar]
- 11.Reinthaler M, Aggarwal SK, De Palma R, Landmesser U, Froehlich G, Yap J, et al. Predictors of clinical outcome in transfemoral TAVI: circumferential iliofemoral calcifications and manufacturer-derived recommendations. Anatol J Cardiol. 2015;15:297–305. doi: 10.5152/akd.2014.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca P, Almeida J, Bettencourt N, Ferreira N, Carvalho M, Ferreira W, et al. Incidence and predictors of vascular access site complications following transfemoral transcatheter aortic valve implantation. Rev Port Cardiol. 2017;36:747–53. doi: 10.1016/j.repc.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Eltchaninoff H, Kerkeni M, Zajarias A, Tron C, Godin M, Sanchez Giron C, et al. Aorto-iliac angiography as a screening tool in selecting patients for transfemoral aortic valve implantation with the Edwards SAPIEN bioprosthesis. EuroIntervention. 2009;5:438–42. doi: 10.4244/EIJV5I4A69. [DOI] [PubMed] [Google Scholar]
- 14.Krishnaswamy A, Parashar A, Agarwal S, Modi DK, Poddar KL, Svensson LG, et al. Predicting vascular complications during transfemoral transcatheter aortic valve replacement using computed tomography: a novel area-based index. Catheter Cardiovasc Interv. 2014;84:844–51. doi: 10.1002/ccd.25488. [DOI] [PubMed] [Google Scholar]
- 15.Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4:851–8. doi: 10.1016/j.jcin.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Barbash IM, Barbanti M, Webb J, Molina-Martin De Nicolas J, Abramowitz Y, Latib A, et al. Comparison of vascular closure devices for access site closure after transfemoral aortic valve implantation. Eur Heart J. 2015;36:3370–9. doi: 10.1093/eurheartj/ehv41717. [DOI] [PubMed] [Google Scholar]; Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33:2403–18. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 18.Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, et al. Perioperative Results and Complications in 15,964 Transcatheter Aortic Valve Replacements: Prospective Data From the GARY Registry. J Am Coll Cardiol. 2015;65:2173–80. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Rahhab Z, Ramdat Misier K, El Faquir N, Kroon H, Ziviello F, Kardys I, et al. Vascular Complications after Transfemoral Transcatheter Aortic Valve Implantation: A Systematic Review and Meta-Analysis. Structural Heart. 2020;4:62–71. doi: 10.1080/24748706.2019.1694730. [DOI] [Google Scholar]
- 20.Nijhoff F, Abawi M, Agostoni P, Ramjankhan FZ, Doevendans PA, Stella PR. Transcatheter aortic valve implantation with the new balloon-expandable Sapien 3 versus Sapien XT valve system: a propensity score-matched single-center comparison. Circ Cardiovasc Interv. 2015;8:e002408. doi: 10.1161/CIRCINTERVENTIONS.115.002408. [DOI] [PubMed] [Google Scholar]
- 21.Di Mario C, Eltchaninoff H, Moat N, Goicolea J, Ussia GP, Kala P, et al. Transcatheter Valve Treatment Sentinel Registry (TCVT) Investigators of the EURObservational Research Programme (EORP) of the European Society of Cardiology. The 2011–12 pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation: in-hospital results in 4,571 patients. EuroIntervention. 2013;8:1362–71. doi: 10.4244/EIJV8I12A209. [DOI] [PubMed] [Google Scholar]
- 22.Carroll JD, Vemulapalli S, Dai D, Matsouaka R, Blackstone E, Edwards F, et al. Procedural Experience for Transcatheter Aortic Valve Replacement and Relation to Outcomes: The STS/ACC TVT Registry. J Am Coll Cardiol. 2017;70:29–41. doi: 10.1016/j.jacc.2017.04.056. [DOI] [PubMed] [Google Scholar]
- 23.Masson JB, Kovac J, Schuler G, Ye J, Cheung A, Kapadia S, et al. Transcatheter aortic valve implantation: review of the nature, management, and avoidance of procedural complications. JACC Cardiovasc Interv. 2009;2:811–20. doi: 10.1016/j.jcin.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Webb J, Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J. 2011;32:140–7. doi: 10.1093/eurheartj/ehq453. [DOI] [PubMed] [Google Scholar]
- 25.Czerwińska-Jelonkiewicz K, Michałowska I, Witkowski A, Dąbrowski M, Księżycka-Majczyńska E, Chmielak Z, et al. Vascular complications after transcatheter aortic valve implantation (TAVI): risk and long-term results. J Thromb Thrombolysis. 2014;37:490–8. doi: 10.1007/s11239-013-0996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raju S, Eisenberg N, Montbriand J, Cusimano RJ, Feindel C, Ouzounian M, et al. Vascular Complications and Procedures Following Transcatheter Aortic Valve Implantation. Eur J Vasc Endovasc Surg. 2019;58:437–44. doi: 10.1016/j.ejvs.2019.03.014. [DOI] [PubMed] [Google Scholar]