Abstract
Objective
High power short duration (HPSD) ablation strategy is proposed to be more effective than low power long duration (LPLD) for radiofrequency ablation of atrial fibrillation. Although small trials abound, data from a large cohort are lacking. This meta-analysis compares all the existing studies comparing these two approaches to evaluate perceived advantages of one over the other.
Methods
A systematic search of PubMed, EMBASE, and Cochrane databases identified studies comparing HPSD to LPLD ablation. All the analyses used the random-effects model.
Results
Ablation settings varied widely across 20 studies comprising 2,136 patients who underwent HPSD and 1,753 patients who underwent LPLD. The pooled incidence of atrial arrhythmia recurrence after HPSD ablation was 20% [95% confidence interval (CI): 0.16–0.25; I2=88%]. Atrial arrhythmia recurrences were significantly less frequent with HPSD ablation (incidence risk ratio=0.66; 95% CI: 0.49–0.88; I2=72%; p=0.004). Procedural, fluoroscopy, and ablation times were significantly shorter with HPSD ablation. First-pass pulmonary vein isolations (PVIs) were significantly more [odds ratio (OR)=2.94; 95% CI: 1.50–5.77; I2=89%; p=0.002), and acute pulmonary vein reconnections (PVRs) were significantly lesser (OR=0.41; 95% CI: 0.28–0.62; I2=62%; p<0.001) in the HPSD group. Although radiofrequency energy was significantly higher, esophageal thermal injuries (ETI) were lower with HPSD ablation. Acute complications, including steam-pops, were rare and statistically similar in both the groups.
Conclusion
HPSD ablation enables faster first-pass PVI with fewer PVRs, similar ETI rates, rare collateral damage, and lower recurrence of atrial arrhythmia in the long term than LPLD. Randomized controlled studies with a larger cohort are indicated both to confirm the benefit of HPSD ablation and standardize the ablation protocol.
Keywords: atrial fibrillation, catheter ablation, esophageal injury, pulmonary vein reconnections, recurrence
INTRODUCTION
Given the recent advances in mapping and catheter technologies, catheter ablation for atrial fibrillation (AF) has become the standard of care. Growing evidence indicates that early ablation for AF may be preferable to antiarrhythmic drug therapy with regard to morbidity and mortality (1, 2). Newer balloon technologies also are showing promising results with similar efficacy but shorter procedural times (3, 4). Intracardiac echocardiography (ICE) has reduced the need for fluoroscopy, contact-force (CF) ablation catheters have lessened collateral damage, and jet ventilation has improved catheter stability.
The cornerstone of AF ablation is durable pulmonary vein (PV) isolation (PVI), which requires creation of a transmural lesion. From a biophysical standpoint, conventional low-power long-duration (LPLD) ablation produces a small area of resistive heating but a large area of conductive heating that can cause collateral damage (although CF catheter use has made these complications infrequent in contemporary electrophysiology practice) (5).
Conversely, high-power short-duration (HPSD) ablation produces a much larger area of resistive heating to create the transmural lesion and a smaller area of conductive heating to alleviate complications associated with posterior wall isolation (PWI) (6). Initial results from HPSD ablation include shorter procedure and ablation times and equivalent safety and efficacy compared with conventional ablation, which has popularized the HPSD approach (7).
Although randomized studies comparing the two ablation strategies are lacking, comprehensive prospective and retrospective nonrandomized data on HPSD ablation have been published (8). In this study, our aim was to determine the pooled incidence of atrial arrhythmia recurrence after HPSD ablation and to compare it with results of LPLD ablation. We also compared procedural parameters, acute efficacy, and safety outcomes between the two ablation strategies.
METHODS
Search strategy
A systematic review of the existing literature (before January 2021) was performed. Two physician reviewers (AH and SD) queried PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases for published literature, using the search terms, “high power short duration,” “atrial fibrillation,” “ablation,” “radiofrequency ablation,” “pulmonary vein isolation,” and combinations thereof. Additional literature was sought by searching the references of eligible articles. Any discrepancies were resolved by a third reviewer (DK). Ethics Committee approval and informed consent were not required as this was a meta-analysis and review.
Study selection
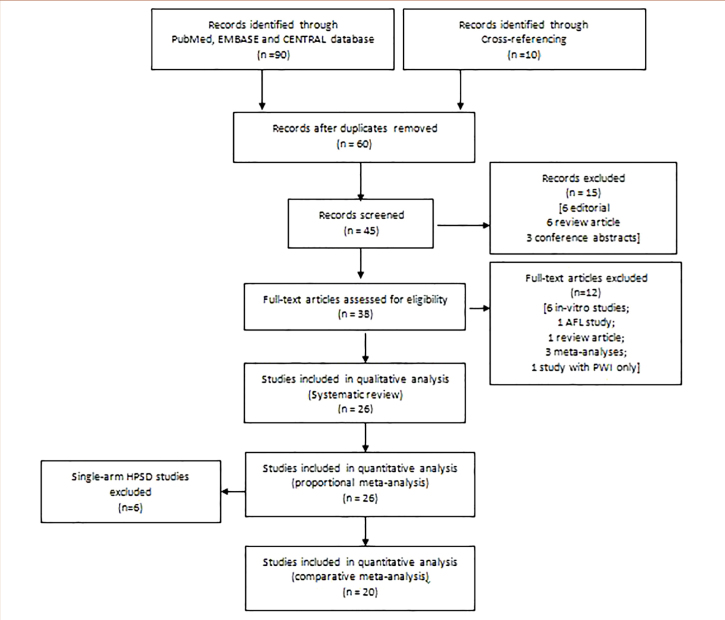
We defined HPSD ablation as that exceeding 30 W–35 W in intensity and lasting <30 seconds (5). For the systematic review and qualitative analysis, we selected prospective, retrospective, and randomized studies that described acute or long-term outcomes of HPSD ablation for AF. For the proportional meta-analysis, comparative and single-arm HPSD studies were pooled. For the comparative meta-analysis, studies that compared the effects of HPSD versus LPLD ablation were selected. Case reports, case series, in vitro studies, review articles, and atrial flutter (AFL) ablation studies were excluded. Non-randomized studies were critically appraised using the ROBINS-I tool (9), retrospective studies using the Newcastle-Ottawa scale (10), and randomized studies using the RoB2 scale (11) (Fig. 1).
Figure 1.
PRISMA flow diagram.
Data extraction
Data on baseline characteristics, procedural details, and safety and efficacy outcomes of HPSD and LPLD ablation were extracted from each study. Baseline characteristics included number of patients, study design, follow-up duration, patient demographics, echocardiography parameters, thrombosis and bleeding risks, and type of AF. Procedural characteristics included mapping and ablation strategies, hardware, and parameters. Safety outcomes included acute complications, particularly esophageal thermal injury (ETI), and efficacy outcomes included atrial arrhythmia recurrence, first-pass PVIs, and acute PV reconnections (PVRs).
Statistical analysis
All the data analyses were performed using R software random-effects modeling (12). To pool the incidence of atrial arrhythmia recurrence across all the selected studies, the “metaprop” function was used. Outcome odds ratios (ORs), risk ratios (RRs), or incidence risk ratio (IRR) as appropriate, were derived by comparing differences in binary events using the Mantel Haenszel method with the “metabin” and “metainc” packages; mean differences with standard deviations were derived by comparing differences in continuous outcomes using the inverse variance method with the “metacont” package. Studies that reported continuous variables in median (range) or mean ± standard deviation were analyzed using the “boxcox” function. To compare HPSD versus LPLD groups’ acute complications, a hypergeometric-normal model was used to approximate the exact likelihood as the number of events in each study was small relative to group size (many zero events) (13). To negate the small study effect, log OR and 95% CIs (expressed as % CI) were calculated by using the “escal” function, which was back-transformed to predicted exponential OR and 95% CIs (expressed as % CI). The DerSimonian and Laird method was used to calculate tau2. Heterogeneity was assessed by using I2 statistics. P values were expressed as two digits after decimal and reported as “significant” if p values were <0.05. Sensitivity analyses were conducted for all the variables. Covariate analyses were performed using the “metareg” function; bubble plots were constructed to visualize moderator effects. Funnel plots were used to assess publication bias.
RESULTS
A total of 26 studies were selected for the systematic review; six single-arm HPSD studies (14–19) and two randomized (20, 21), six retrospective (22–27), and 12 prospective (28–39) studies comparing HPSD and LPLD ablations. Overall, the study quality was good (Supplementary Tables S1–S3). Pooling all the 26 studies yielded 14,014 patients with AF who underwent HPSD ablation. The meta-analysis included 2,136 patients with AF who underwent HPSD ablation and 1,753 patients with AF who underwent LPLD ablation. Follow-up duration ranged from 2 days to 3 years. Among the single-arm studies, Winkle et al. (19) reported the longest follow-up for HPSD ablation (up to 4 years).
Patient profile and ablation settings
Baseline characteristics of the HPSD and LPLD cohorts are shown in Table 1. Matiello et al. (36) and Shin et al. (21) compared 30 W, 40 W, and 50 W ablation strategies with data for 30 W and 40 W combined under LPLD ablation. Leo et al. (35) compared four ablation strategies, including lower and higher lesion size index (LSI) with 20 W (for LPLD) and 40 W (for HPSD). Okamatsu et al. (20) compared high-power, medium-power, and low-power ablation strategies, with medium-power and low-power combined as LPLD ablation. Dhillon et al. (30) compared high-power ablation index guided HPSD ablation with 25 W and 30 W LPLD strategies. Studies were heterogeneous as to power and duration setup and the use of mapping and ablation systems, CF-sensitive catheters, ICE, and esophageal temperature probes.
Table 1.
Baseline patient and ablation characteristics in the selected studies
| Study | Sample size | Age (years) (mean±SD) | Female (%) | CHA2DS2-VASc (mean±SD) | LAD (mm) (mean±SD) | PAF (%) | BMI (kg/m2) (mean±SD) | LVEF (%) (mean±SD) | Ablation setting | Ablation strategy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPSD n | LPLD n | Study design | Follow-up (years) | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | HPSD | LPLD | Comparative studies | |
| Baher et al. 2018 | 574 | 113 | Retrospective | 2.5 | 69±18 | 68.3±11.6 | 32.9 | 40.7 | 2.9±1.7 | 2.5±1.6 | 46.8 | 70.8 | 50W 5s | 35W (AW) 25W (PW) 10–30s |
PVI± PWI± Linear | ||||||
| Berte et al. 2019 | 80 | 94 | Prospective | 0.5 | 62±9 | 63±9 | 38 | 33 | 40%† | 31%† | 65 | 74 | 58±8 | 59±11 | 45W (AW) 35W (PW) |
35W (AW) 25W (PW) |
PVI±CTI CLOSE | ||||
| Bunch et al. 2020 | 402 | 402 | Retrospective | 3 | 67.1±10.5 | 66.4±12.2 | 37.1 | 34.8 | 47.3 | 50.2 | 30.8±7.0 | 30.5±6.8 | 54.6±12.1 | 54.7±12.8 | 50W 2–3s | 30W (AW) 10–20s Eso 25W (PW) 5s |
PVI± PWI± Linear | ||||
| Castrejón-Castrejón et al. 2020 | 48 | 47 | Prospective | 0.25 | 61±10 | 60±10 | 33 | 40 | 15%‡ | 18%‡ | 65 | 64 | 29.4 | 29.5 | 57±9 | 56±11 | 50W in 18 60W in 30 2–3s |
30W 30s |
PVI | ||
| Dhillon et al. 2019 | 50 | 50 | Prospective | 1 | 100 | 100 | CF: 10–20 g AI: 350W (PW) 450W (AW) |
30W (AW) 25W (PW) |
PVI±CTI CLOSE | ||||||||||||
| Ejima et al. 2020 | 60 | 60 | Prospective | 1 | 63.0±11.3 | 66.7±8.9 | 56 | 58 | 1.8±1.4 | 2.2±1.4 | 100 | 100 | 24.9±2.8 | 23.8±3.2 | 57.7±3.9 | 57.4±6.3 | 50W 3–5s |
25–40W 5–10s |
PVI± PWI± Linear± CTI | ||
| Kaneshiro et al. 2020 | 101 | 170 | Prospective | 2 days | 63±10 | 31±10 | 24 | 32 | 40.8±6.3 | 38.8±6.5 | 66 | 79 | 24.9±4.0 | 24.5±3.7 | 45–50W 10–30s |
20–30W 10–15s AI: 500 (AW), 400 (PW) |
PVI | ||||
| Kottmaier et al. 2020 | 97 | 100 | Prospective | 1 | 60.8±13.9 | 60.8±10.5 | 43 | 40 | 1.95 | 1.64 | 100 | 100 | 27.9±4.0 | 28.0±4.5 | 57±5 | 55±9 | 70W 7s (AW) 70W 5s (PW) |
30–40W 20–40s |
PVI | ||
| Kumagai et al. 2020 | 80 | 80 | Prospective | 1 | 63.0±9.1 | 63.1±9.1 | 20 | 14 | 0.7±1.0 | 0.8±0.8## | 41.6±5.1 | 43.3±6.4 | 30 | 24 | 62.5±7.7 | 62.2±7.2 | 50W 5s CF: <10g |
30–40W 30s 20W near Eso |
BOXI | ||
| Leo et al. 2020 (LSI 5) | 20 | 20 | Prospective | 2.4 | 61.3±9.6 | 55.7±10.0 | 30 | 30 | 2 (0–4)§ | 1 (1–2)§ | 43.7±9.3 | 42.4±7.7 | 40 | 30 | 28.8±4.9 | 28.2±4.9 | 57.9±6.4 | 60.0±10.2 | 20W | 40W | PVI± PWI± Linear |
| Leo et al. 2020 (LSI 4) | 20 | 20 | Prospective | 2.4 | 60.1±9.1 | 58.9±9.2 | 40 | 5 | 1 (0–2)§ | 1 (1–3)§ | 41.4±6.5 | 43±6 | 40 | 45 | 27.3±5.0 | 30.8±4.6 | 60±9.2 | 60±11.5 | 20W | 40W | PVI± PWI± Linear |
| Matiello et al. 2008 | 105 | 54 | Prospective | 1 | 52.8±11.3 | 54.5±10.9 | 24.7 | 29.3 | 41.9±5.2 | 40.7±7.1 | 59.5 | 64.3 | Irrigated catheter 45W |
Irrigated catheter 30W |
PVI | ||||||
| Matiello et al. 2008 | 88 | Prospective | 1 | 50.8±11.4 | 16.7 | 41.7±5.7 | 63.3 | 8 mm tip catheter 50W |
PVI | ||||||||||||
| Nilsson et al. 2006 | 45 | 45 | Prospective | 1.25 | 55±10 | 51±11 | 33 | 20 | 57 | 71 | 30W 120s |
45W 20s | PVI | ||||||||
| Okamatsu et al. 2019 | 20 | LP 20 MP 20 | Randomized | 1 | 65±10 | LP: 68±8 MP: 64±8 |
35 | LP: 25 MP: 45 |
2 (1–3)¶ | LP: 2 (1 2)¶ MP: 2 (1 3)¶ |
40±6 | LP:39±6¶ MP: 40±5¶ |
65 | LP: 80 MP: 75 |
24 (22 25)¶ | LP: 24 (21 28)¶ MP: 23 (22 26)¶ |
65 (60 71)¶ | LP:64 (60–67)¶ MP: 64 (59 71)¶ |
50W (AW) 40W (PW) 30W (Eso)# |
LP:30W (AW) 20W (PW) MP:40W (AW) 30W (PW) |
PVI± PWI± Linear |
| Pambrun et al. 2019 | 50 | 50 | Prospective | 1 | 65.0±8.2 | 62.5±10.6 | 30 | 40 | 100 | 100 | 61.7±5.6 | 61.1±4.4 | 40–50W R pattern +2s | 25–30W R pattern+5s | PVI unipolar signal modification | ||||||
| Shin et al. 2020 | 49 | 97 | Randomized | 1 | 58.5±7.9 | 58.7±11.1 | 22 | 34 | 1.6±1.5 | 1.7±1.6 | 39.9±4.6 | 40.7±6.5 | 50 | 48 | 23.8±2.8 | 24.6±2.7 | 55.7±11.4 | 58.8±8.3 | 50W | 40W, 30W (PW) 25–30W max 20s | PVI+ CTI± PWI± Linear |
| Ücer et al 2020 | 25 | 25 | Retrospective | 62.7±10.6 | 36 | 2.5 | 41.7±5.4 | 76 | 57±10 | 50W 6–10s |
Conventional | PVI | |||||||||
| Vassallo et al. 2020 | 71 | 73 | Retrospective | 1 | 59.7 | 60.7 | 29.5 | 31.5 | 2.5 (0 8)¶ | 2.2 (0–7)¶ | 40.4 | 39.1 | 54.9 | 71.2 | 50W (AW) 45W (PW) 6s |
30W (AW) 20W (PW) 30s |
PVI± PWI± Linear± CTI | ||||
| Vassallo et al. 2019 | 41 | 35 | Retrospective | 1 | 54¶ | 46¶ | 17 | 35.3 | 2¶ | 2¶ | 43.3 (28 62)¶ | 41.9 (23 56) | 68.3 | 77 | 27¶ | 28¶ | 50W (AW) 45W (PW) 6–8s Irrigation 17 mL/min |
30W 30s Irrigation 35 mL/min |
PVI± CTI | ||
| Yavin et al. 2020 | 112 | 112 | Prospective | 1.2 vs 1.9 | 62.3±5.2 | 64.8±7.2 | 36.7 | 29.5 | 2.4±1.3 | 2.6±1.4 | 44.2±4.7 | 47±5.1 | 67.8 | 59.8 | 27.6±3.9 | 28.7±4.1 | 66.3±6.1 | 57.8±5.4 | 45–50 W 8–15s |
20–40W 20–30s |
PVI± PWI± Linear± CTI |
| Yazaki et al. 2020 | 32 | 32 | Retrospective | 0.8 | 61±11 | 66±11 | 16 | 37 | 89 | 91 | 55±7 | 56±7 | 50W 5–10s |
25–40W 15–30s |
PVI± PWI± Linear± CTI Imp-min guided Single-arm studies | ||||||
| Chen et al. 2019 | 50 | Prospective | 0.5 | 68.3±9.1 | 60 | 3 (1–4)¶ | 42.2±5.3 | 58 | 58.8±9.3 | 50W AI 550 (AW) 400 (PW) |
PVI | ||||||||||
| Chen et al. 2020 ESO-I | 122 | Prospective | 3 days | 68.1±9.2 | 33.6 | 2.4±1.5 | 40.7±5.8 | 54.9 | 57.4±11.0 | 50W AI 550 (AW) 400 (PW) |
PVI | ||||||||||
| Chen et al. 2020 ESO-II (LET+) | 60 | Prospective | 3 days | 67.5±9.0 | 37 | 2.3±1.3 | 41.7±5.5 | 60 | 58.6±11.0 | 50W AI 550 (AW) 400 (PW) |
PVI | ||||||||||
| Chen et al. 2020 ESO-II (LET−) | 60 | Prospective | 3 days | 68.0±12.0 | 35 | 2.7±1.5 | 40.5±5.8 | 62 | 57.8±10.0 | 50W AI 550 (AW) 400 (PW) |
PVI | ||||||||||
| Reddy et al. 2019 | 52 | Prospective | 0.25 | 62.0±12.1 | 33.3 | 2.0±1.4 | 39.3±5.1 | 100 | 60.8±5.0 | 90W 4s | PVI | ||||||||||
| Winkle et al. 2019 | 10284 | Retrospective | 64±11 | 32 | 2.1±1.4 | 44±7 | 37.2 | 27.9±4.9 | 45–50W 2–15s |
PVI± PWI± Linear± CTI | |||||||||||
| Winkle et al. 2020 | 1250 | Retrospective | 4 | 66.6±10.5 | 30.9 | 3.0±1.4 | 42.6±6.6 | 35.7 | 50W 5–15s |
PVI± PWI± Linear± CTI | |||||||||||
Dilated left atrium (%).
Moderate to severe dilated left atrium.
Median (interquartile range).
Median (minimum-maximum).
AI: 400 (AW), 360 (PW), 260 (Eso)
CHADS2 score
AI - ablation index; AW - anterior wall; BMI - body mass index; BOXI - box isolation; CF - contact force; CHA2DS2-VASc - congestive heart failure, hypertension, age, diabetes, stroke/transient ischemic attack, vascular disease; CTI - cavort tricuspid isthmus ablation; Eso - esophagus; HPSD - high-power short-duration; LAD - left atrial diameter; LET - luminal esophageal temperature monitoring; Linear - mitral/roof/posterior wall lines; LP - low power; LPLD - low-power long-duration; LSI - lesion size index; LVEF - left ventricular ejection fraction; MP - medium power; PAF - paroxysmal atrial fibrillation; PVI - pulmonary vein isolation; PW - posterior wall; PWI - posterior wall isolation
Recurrences of atrial arrhythmia
Pooled incidence
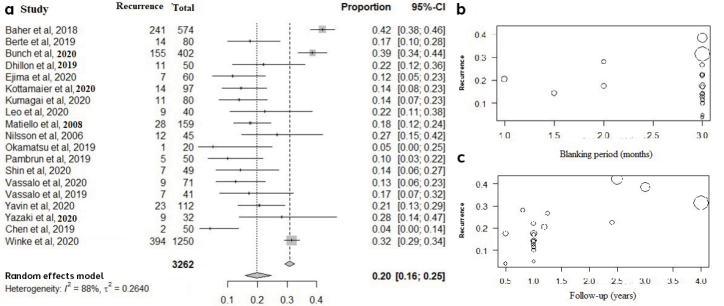
Nineteen studies representing 3,262 patients reported the incidence of AF/atrial tachycardia (AT) recurrence after HPSD ablation. The pooled recurrence rate was 20% (% CI: 016–0.25; I2=88%) (Fig. 2a). Baher et al. (22) had the highest AF/AT recurrence rate (42%), whereas Chen et al. (14) had the lowest (4%). Notably, Baher et al. (22) followed patients for nearly 2.5 years after ablation, whereas Chen et al. (14) followed the patients for only 6 months. In the covariate analysis, recurrences were inversely proportional to the blanking period (the period after myocardial ablation in which arrhythmias are not considered to be recurrences) (Z=−0.25; p=0.31) (Fig. 2b) but were directly proportional to follow-up duration (Z=4.08; p<0.01) (Fig. 2c), without any relation to the study design (p=0.93).
Figure 2.
a) Forest plot showing pooled incidence of atrial arrhythmia recurrence after high-power short-duration atrial fibrillation ablation. b) Bubble plot showing relationship of recurrence with the blanking period. c) Bubble plot showing relationship of recurrence with follow-up duration.
HPSD vs. LPLD
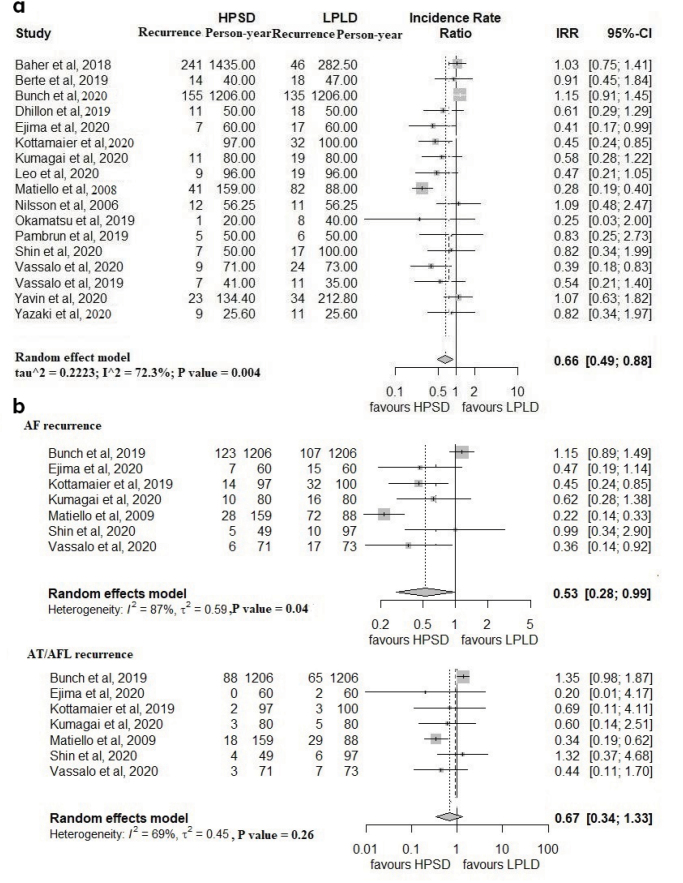
When HPSD and LPLD ablations were compared, incidence of AF/AT recurrence was significantly lower in the HPSD group (IRR=0.66; % CI: 0.49–0.88; I2=72%; p=0.004) (Fig. 3a). The funnel plot showed no asymmetry suggesting publication bias (Supplementary Fig. S1a). The favorable effect of HPSD was maintained across the sensitivity analysis and is reflected in the 95% CI ranges (Supplementary Fig. S1b–S1c).
Figure 3.
Forest plots comparing the incidence rate of atrial arrhythmia recurrence between high-power short-duration and low-power long-duration ablation groups. a) All patients. b) Subgroup analysis of atrial fibrillation and atrial tachycardia/atrial flutter.
Recurrences of AF and AT/AFL were significantly lower with HPSD than with LPLD ablation (Fig. 3b). The recurrence of arrhythmia within the blanking period was similar (p=0.84) (Supplementary Fig. S2a). Arrhythmia recurrence in the HPSD group showed a significantly lower trend in paroxysmal AF (RR=0.70; % CI: 0.43–1.13; I2=73%; p<0.01) and higher trend in persistent AF (RR=1.16; % CI: 0.96–1.41; I2=0%; p=0.64) on the basis of available data (Supplementary Fig. S2b and S2c). However, when the paroxysmal to persistent AF ratio in HPSD to LPLD groups was plotted against the IRR of recurrence across all studies, no significant relationship was found (Supplementary Fig. S2d).
The RR for atrial arrhythmia recurrence was significantly lower with HPSD than LPLD (RR=0.63; 95% CI: 0.47–0.85; I2=83%; p=0.003) (Supplementary Fig. S3a). Regression analysis of relative risk showed no significant correlation with blanking period (p=0.88) or study design (p=0.12), but follow-up duration was a significant moderator (Z=1.95; p=0.05) (Supplementary Fig. S3b).
Procedural outcomes
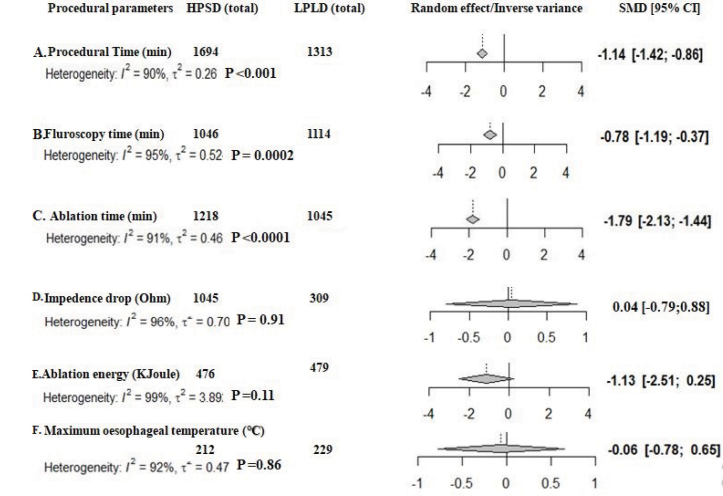
Procedural, fluoroscopy, and ablation times were significantly shorter with HPSD than with LPLD ablation (Fig. 4a–4c). Overall, ablation energy delivery was lower (but not statistically) (Fig. 4d) in the HPSD ablation group (p=0.11). Impedance drop per lesion and maximum esophageal temperatures were similar between HPSD and LPLD groups (p=0.91 and p=0.86, respectively) (Fig. 4e, 4f). High levels of heterogeneity were noted in the procedural parameter comparisons.
Figure 4.
Comparison of procedural parameters between high-power short-duration and low-power long-duration ablation groups. a) Procedural time. b) Fluoroscopy time. c) Ablation time. d) Impedance drop. e) Ablation energy. f) Maximum esophageal temperature.
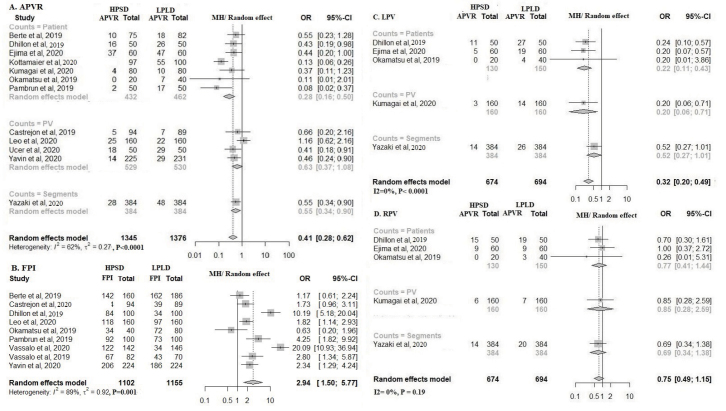
Acute PVRs were significantly less frequent (OR=0.41; % CI: 0.28–0.62; I2=62%; p<0.0001) and first-pass PVIs were significantly more frequent (OR=2.94; % CI: 1.50–5.77; I2=89%; p=0.002) in the HPSD versus the LPLD group (Fig. 5a, 5b). In the presence of provocative tests (adenosine, isoprenaline, or programmed electrical stimulation), incidence of acute PVRs remained significantly lower in the HPSD group (OR=0.41; % CI: 0.28–0.62, I2=62%; p=0.04) (Supplementary Fig. S4). Acute left PVRs were statistically less frequent in HPSD than in LPLD ablation (OR=0.32; % CI: 0.20–0.49; I2=0%; p<0.001); right PVRs were also less frequent (but not statistically) in HPSD (OR=0.75; % CI: 0.49–1.15; I2=0%; p=0.12) (Fig. 5c and 5d).
Figure 5.
Forest plots comparing high-power short-duration (HPSD) and low-power long-duration (LPLD) ablation groups. a) Acute pulmonary vein reconnections (PVR) after pulmonary vein isolation (PVI). b) First-pass PVIs. Subgroup analysis of reconnections between the HPSD and LPLD ablation groups. c) Left PVR. d) Right PVR.
In terms of first-pass PVIs, Dhillon et al. (30) and Vassallo et al. (25) reported very high efficacy for HPSD, which may partially explain the 89% heterogeneity; however, sensitivity analysis did not alter HPSD’s more favorable effect on first-pass PVIs (Supplementary Fig. S5a–5c).
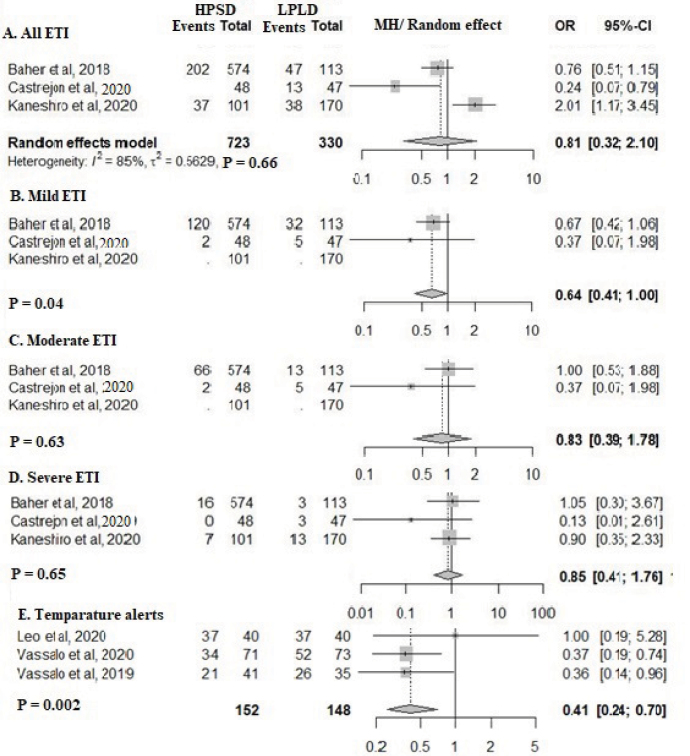
Esophageal thermal injuries
Only few studies explored ETI (22, 29, 32). Analysis of these indicated that ETI incidence and severity were lower in the HPSD group, although not statistically significant (Fig. 6a–6d). Although the maximum esophageal temperatures were similar between the two groups, significantly fewer esophageal temperature alerts occurred in the HPSD group (Fig. 4f and 6e).
Figure 6.
Forest plot comparing esophageal complications between high-power short-duration and low-power long-duration groups. a) Total esophageal thermal injury (ETI). b) Mild ETI. c) Moderate ETIs. d) Severe ETIs. e) Number of esophageal temperature alerts.
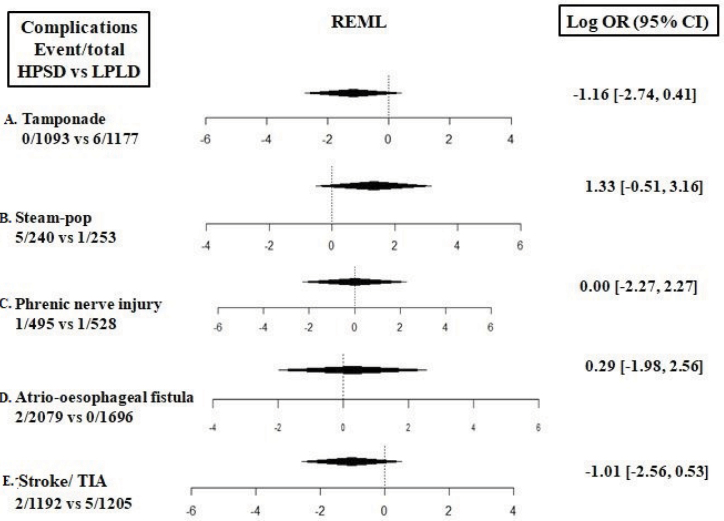
Acute complications
Acute complications related to both ablation strategies, including steam-pops, were numerically rare and statistically similar in both groups (Fig. 7a–7e). When complications data were pooled across the comparative and single-arm studies, incidences of stroke/transient ischemic attack (TIA), phrenic nerve palsy, atrial-esophageal fistula, steam-pop, and tamponade were 1%, 0.17%, 0.09%, 1.4%, 0.3%, respectively (Supplementary Fig. S6a–6e).
Figure 7.
Forest plots comparing acute complications between high-power short-duration and low-power long-duration ablation groups. a) Stroke/transient ischemic attack. b) Phrenic nerve palsy. c) Atrio-esophageal fistula. d) Steam-pop. e) Tamponade.
DISCUSSION
To the best of our knowledge, this is the largest meta-analysis of HPSD ablation for AF to date. We have shown that the HPSD ablation strategy enables faster first-pass PVI and produces lower PVR rates with relatively less radiofrequency energy than does LPLD ablation. ETI rates are similar and collateral damage is rare, resulting in a lower incidence of AF/AT recurrence. Although HPSD ablation outcomes have been studied since 2006 (37), we selected only recent studies for this meta-analysis. As HPSD ablation is rapidly gaining popularity and being adopted globally, the volume of evidence is evolving quickly.
Ablation settings
Ablation settings (power and duration) varied widely across studies and included ultra-high power and ultra-short duration ablation like 70 W for 7 seconds in a study by Kottmaier et al. (33), 90 W for 4 seconds in the study by Reddy et al. (17) (QDOT FAST study). Vassallo et al. (25, 26) used higher irrigation flow in LPLD ablation (30 mL/min) in comparison to the HPLD strategy (17 mL/min). Most of the studies, except the ones by Nilsson et al. (37) and Matiello et al. (36) (2009) used CF and ablation indices or LSI (depending on the electrophysiological system used). Retrospective data from Baher et al. (22), Castrejón-Castrejón et al. (29), and Winkle et al. (18) comprised results of both CF and non-CF ablations. In the first published study with comparative data, Nilsson et al. (37) used 30 W for 120 seconds as LPLD and 45 W for 20 seconds as HPSD.
Ablation strategies also differed across the investigations. Kumagai et al. (34) performed box isolation in all the patients; Shin et al. (21) performed cavotricuspid isthmus ablation in all the patients, along with PVI; Pambrun et al. (38) used unipolar signal modification for PVI, and Yavin et al. (39) and Yazaki et al. (27) guided their ablations by monitoring drop in impedance. In all the studies, non-PV lines were made during ablation depending on the type of ablation, evidence of arrhythmia, and voltage mapping per operator discretion. Both point-by-point ablation and a continuous drag with “perpetual motion” were employed across the studies (19). Given the diversity in ablation settings, we analyzed the data in a random-effects model to avoid assigning undue weight to any particular study, and we conducted sensitivity analyses for all the parameters. Nonetheless, results and estimates did not differ significantly. Even with heterogeneous ablation settings and strategies, HPSD remained substantially favorable than LPLD.
Recurrence of atrial arrhythmia
Atrial arrhythmia recurred less frequently in the HPSD group, probably because HPSD ablation produces more resistive heating, leading to durable lesions (6). In animal studies, lesion sets formed with 50 W–60 W ablations for 5 seconds at 10 g CF were transmural (38). Bourier et al. (40) noted in another ex vivo study that HPSD radiofrequency applications resulted in similar lesion volumes but substantially different lesion geometries (wider but shallower), compared with standard radiofrequency settings.
Recurrence was defined as atrial tachycardia or atrial fibrillation lasting >30 seconds across all the studies. Modalities used for follow-up were mainly ECG and prolonged ambulatory ECG monitoring like Holter in most of the studies; however, Baher et al. (22), Bunch et al. (23), and Kumagai et al. (34) also used event recorders to detect recurrences. The blanking period after AF ablation is conventionally defined as 3 months; however, many studies had shorter durations: Yavin et al. (39) and Ücer et al. (24), 4 weeks; Kottmaier et al. (33), 6 weeks; and Berte et al. (28) and Yazaki et al. (27), 2 months. Vassallo et al. (25) found that AT/AFL was significantly more frequent in the HPSD group during the blanking period, whereas AF was considerably more frequent in the LPLD group. Although AF recurrence during the blanking period may not portend the outcome, our meta-analysis found no significant difference in overall atrial arrhythmia events between the two ablation strategies during the blanking period. It is important to remember that the recurrence of AF after ablation does not only depend on the ablation strategies, operators’ experience, and the use of ablation indices, but also on the patient profile including obesity, obstructive sleep apnea, duration of AF, left atrial size, and the scarring of the left atrial wall.
Practice change for AF ablation will require longer-term efficacy outcomes. Because HPSD ablation is a new approach in the AF ablation armamentarium, most of the studies in our analysis had relatively short follow-up periods. Those with longer follow-up reported more frequent recurrence. Winkle et al. (19) found that 4-year freedom from paroxysmal, persistent, and longstanding AF after multiple ablations was 87.0%, 71.9%, and 64.9%, respectively. Persistent AF tends to be more complicated than paroxysmal AF, but that did not influence HPSD’s favorable outcomes versus LPLD in our meta-analysis. In a multivariate analysis, Winkle et al. (19) found six independent predictors for AF recurrence; older age, female sex, persistent and longstanding AF, larger LA, PWI, and use of CF-sensing catheters.
Procedural outcomes
In these studies, procedure time and time to PVI were shorter in the HPSD group than in the LPLD group. The heterogeneity was probably related to varying procedure-time definitions and whether waiting periods or time for provocation tests were included. Fluoroscopy time also was shorter for HPSD ablation unrelated to ICE as ICE was sparsely used across the studies. Reddy et al. (17) reported substantially shorter total procedure, ablation, fluoroscopy, and radiofrequency application times and less irrigation fluid load with ultra-high power (90 W) and ultra-short duration (4 seconds) ablation. Ablation time and energy used were lower in the HPSD group, which may cause less pain for the patient. This supports the use of conscious sedation and local anesthesia for AF ablation instead of general anesthesia and may improve catheter stability during the procedure.
Pulmonary vein reconnections
Most studies used adenosine to determine PVRs; some also used isoprenaline and/or extra programmed electrical stimulation protocols to assess dormant conduction, primarily in the carina region or ridges (20, 31, 33, 39). Castrejón-Castrejón et al. (29) showed that radiofrequency application characteristics of the lesions responsible for conduction gaps had lower average CF and LSI but slightly better impedance drop in the HPSD group. However, Ücer et al. (24) found no differences in the total number of radiofrequency applications, applied radiofrequency energy, ablation duration, or CF in PVs with or without reconnection. Ablation data between positive and negative adenosine provocation tests were similar (24). In Yavin et al. (39), the incidence of chronic PVRs was significantly lower in the HPSD than the LPLD group (16.6% vs. 52.2%). However, ablation parameters in areas of chronic reconnection were comparable to those in areas without reconnection. Chronic PVR occurred in regions with catheter motion >1 mm for >50% application duration (39). In multivariate analysis, minimum impedance was the only independent predictor of PVR absence after adjusting for maximum inter-lesion distance and minimum ablation index (27).
Esophageal thermal injury
To diagnose ETIs, Baher et al. (22) used same-day MRI; upper gastrointestinal. Endoscopy was used by Castrejón-Castrejón et al. (29) within 48 hours, by Kaneshiro et al. (32) after 48 hours, and by Chen et al. within 72 hours (15, 16). Baher et al. (22) repeated the MRI after 3 months; Kaneshiro et al. (32) repeated the endoscopy after 7 days. One or more of the following have been used to reduce ETI occurrence: intraprocedural esophageal temperature probes for temperature monitoring; computed tomography integration with fluoroscopy mapping; multi-electrode esophageal catheter. The overall incidence of ETI was low, and most patients were asymptomatic (29, 32). Interestingly, in the Chen et al. (16) ISO-II study, ETI in cadence among patients undergoing HPSD ablation was low, with or without the use of esophageal temperature monitoring (often used to decrease ETI occurrences). Castrejón-Castrejón et al. (29) showed that patients with esophageal lesions had higher LSI and CF values during PWI. In Kaneshiro et al. (32), most ETIs in the HPSD group occurred as gastric hypomotility with esophageal ulcers limited to the shallow layer of the periesophageal wall. Larger left inferior PV angle and smaller LA-to-aorta distance independently predicted ETI in the HPSD group (32).
Complications
The acute complication rate was substantially lower in CF-based ablation and with routine use of ICE. In Winkle et al. (18), the largest (10,284 patients) retrospective study on HPSD ablation to date, tamponade, stroke/TIA, phrenic nerve palsy, and atrio-esophageal fistula were observed in 0.24%, 0.04%, 0.01%, and 0.01% of patients, respectively. In our meta-analysis, complications were infrequent; tamponade (0.3%), stroke/TIA (1.0%), phrenic nerve palsy (0.2%), and atrio-esophageal fistula (0.1%), and were similar in both the ablation groups. In an in vitro study by Bhaskaran et al. (7), steam-pops occurred in 8% and 11% of ablations at 40 W/30 s and 80 W/5 s, respectively. Conversely, Barkagan et al. (41) noted no steam-pops in an in vitro study comparing 30 W/30 s ablation with a conventional catheter and 90 W/4 s ablation with a QDOT catheter. In our analysis, the pooled incidence was low (1.4%), suggesting that although steam-pops may increase with higher power, the chances of steam-pop also rise, the incidence of such events is rare in the real-world literature related to AF ablation.
In an in-vitro study by Ali-Ahmed et al. (42), HPSD lesions resulted in inadequate temperature for myocardial lesion formation at 3 mm depth but not at 5 mm, potentially reducing the risk for collateral injury. Leshem et al. (43) demonstrated in an animal study that HPSD ablation using with QDOT catheter resulted in 100% contiguous lines with all transmural lesions. In contrast, standard ablation produced linear gaps in 25% of lines and partial-thickness lesions in 29%. This indicates that HPSD lesions may be durable, which could prevent AF recurrence. The heating is resistive in most parts of the lesion with a meager contribution from conductive heating, which prevents collateral damage.
Study limitations
Our analysis had limitations. Most importantly, we compared the outcomes of AF ablation from several studies in various databases, and our findings may not be reproduced in rigorously designed randomized controlled studies. We identified only two randomized clinical trials comparing HPSD and LPLD ablation. We also found significant heterogeneity among individual studies in terms of ablation settings, overall ablation strategies, blanking period definitions, and follow-up periods, which may have affected outcomes (e.g., AF/AT/AFL recurrences). Few studies reported ETIs, and comparative data for the two ablation strategies were limited. We did not include the outcomes of cavo-tricuspid isthmus ablation with HPSD from a recent study (44). Recurrence rates in the selected studies may have been affected by operator experience, available technology, and ablation workflows.
CONCLUSION
Pulsed-field ablation for PVI is lurking on the horizon as a new and efficient strategy for AF ablation (45). Until that technology is more widely available and empirically supported, HPSD ablation may be the mode of choice for significantly improving productivity by reducing procedure time without compromising recurrence. Compared with LPLD ablation HPSD ablation enables faster first-pass PVI, lower PVR rates, similar ETI rates, and rare collateral damage.
As our understanding of and experience with HPSD ablation evolves, randomized controlled studies comparing long-term outcomes from HPSD versus LPLD ablation for AF will be valuable for confirming the benefits of HPSD ablation over the conventional LPLD strategy and for standardizing ablation settings (46).
HIGHLIGHTS
Recurrence of atrial arrhythmia was significantly less with high-power short-duration (HPSD) ablation.
Procedural, fluoroscopy, and ablation times were significantly shorter with HPSD ablation.
First-pass pulmonary vein isolations (PVIs) were significantly more, and acute pulmonary vein reconnections (PVRs) were significantly lesser in the HPSD group.
Although radiofrequency energy was significantly higher, esophageal thermal injuries (ETI) were lower with HPSD ablation.
Compared with LPLD, HPSD ablation enables faster first-pass PVI with fewer PVRs, similar ETI rates, rare collateral damage, and lower recurrence of atrial arrhythmia in the long term.
Supplementary Data
Comparisons of atrial arrhythmia recurrences between HPSD and LPLD ablation groups. A: Funnel plot. B: Forest plot showing sensitivity analysis. C: Drapery plot.
HPSD - high-power short-duration; LPLD - low-power long-duration.
Forest plots comparing atrial arrhythmia recurrences between HPSD and LPLD ablation groups. A: the blanking period. B: In patients with paroxysmal atrial fibrillation. C. In patients with persistent atrial fibrillation.
HPSD - high-power short-duration; LPLD - low-power long-duration; RR - relative risk.
A: Forest plot comparing recurrence of atrial arrhythmia between HPSD and LPLD ablation groups after the blanking period. B: Bubble plot showing correlation with follow-up duration.
HPSD - high-power short-duration; LPLD - low-power long-duration; RR - relative risk.
Forest plots showing subgroup analysis of acute PVRs between HPSD and LPLD ablation groups in presence and absence of provocation tests.
HPSD - high-power short-duration; LPLD - low-power long-duration; PVR - pulmonary vein reconnection.
Comparison of first-pass PVIs between HPSD and LPLD ablation groups. A: Forest plot showing sensitivity analysis. B: Funnel plot. C: L’Abbe plot.
HPSD - high-power short-duration; LPLD - low-power long-duration; PVI - pulmonary vein isolation.
Forest plots showing pooled incidences of acute complications in the HPSD ablation group. A: Stroke/transient ischemic attack. B: Phrenic nerve palsy. C: Atrio-esophageal fistula. D: Steam-pop. E: Tamponade.
HPSD - high-power short-duration.
Supplementary Table S1.
Nonrandomized studies appraised with the ROBINS-I tool
| Before intervention | At intervention | After intervention | Missing data | Measurement of outcomes | Selection of reported results | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Confounding | Selection of participants | Classification of interventions | Deviation from intended intervention | ||||
| Berte et al., 2019 | 3 to 4 | 1 to 2 | 1 | 2 to 3 | 1 | 1 to 2 | 1 to 2 |
| Bunch et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 | 2 to 3 | 1 to 2 |
| Castrejón Castrejónet al., 2020 | 3 to 4 | 4 | 4 | 3 to 4 | 1 to 2 | 2 to 3 | 3 to 4 |
| Dhillon et al., 2019 | 0 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 1 to 2 | 3 to 4 |
| Ejima et al., 2020 | 1 to 2 | 3 to 4 | 3 to 4 | 1 to 2 | 1 to 2 | 1 to 2 | 1 to 2 |
| Kaneshiro et al., 2020 | 3 to 4 | 1 to 2 | 1 to 2 | 1 to 2 | 1 to 2 | 2 to 3 | 2 to 3 |
| Kottmaier et al., 2020 | 2 to 3 | 1 to 2 | 1 to 2 | 3 to 4 | 4 | 3 to 4 | 3 to 4 |
| Kumagai et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 3 to 4 | 2 to 3 |
| Matiello et al., 2008 | 1 to 2 | 3 to 4 | 3 to 4 | 3 to 4 | 2 to 3 | 2 to 3 | 2 to 3 |
| Nilsson et al., 2006 | 2 to 3 | 2 to 3 | 2 to 3 | 2 to 3 | 1 to 2 | 1 to 2 | 2 to 3 |
| Pambrun et al., 2019 | 0 | 1 to 2 | 1 to 2 | 3 to 4 | 1 to 2 | 4 | 4 |
| Yavin et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 3 to 4 | 3 to 4 |
| Yazaki et al., 2020 | 2 to 3 | 1 to 2 | 1 to 2 | 3 to 4 | 1 to 2 | 2 to 3 | 2 to 3 |
ROBINS-I - Risk of Bias In Nonrandomized Studies of Interventions.
Supplementary Table S2.
Retrospective studies appraised with the Newcastle-Ottawa scale
| Selection | Comparability | Outcome | AHRQ standards | |
|---|---|---|---|---|
| Baher et al., 2018 | ★★★★ | ★★ | ★★★ | Good |
| Ücer et al., 2020 | ★★★ | ★ | ★★ | Good |
| Vassallo et al., 2020 | ★★★★ | ★★ | ★★ | Good |
| Vassallo et al., 2019 | ★★★★ | ★★ | ★★ | Good |
| Chen et al., 2019 | ★★★★ | ★★ | Good | |
| Chen et al., 2020 (ESO-I) | ★★★★ | ★★ | Good | |
| Chen et al., 2020 (ESO-II) | ★★★★ | ★★ | Good | |
| Reddy et al., 2019 | ★★★★ | ★★ | Good | |
| Winkle et al., 2019 | ★★★ | ★★ | Good | |
| Winkle et al., 2020 | ★★★★ | ★★★ | Good |
AHRQ - Agency for Healthcare Research and Quality
Supplementary Table S3.
Randomized controlled studies appraised with the RoB2 scale
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Leo et al., 2020 | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk | None |
| Okamatsu et al., 2019 | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk | None |
| Shin et al., 2020 | Low risk | High risk | High risk | Low risk | Low risk | Low risk | None |
RoB2 - Risk of Bias Tool for Randomized Trials
Acknowledgments
The authors thank Jeanie F. Woodruff, BS, ELS, of the Scientific Publications Department at the Texas Heart Institute for her editorial contributions.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – S.P., M.S., I.B.R.; Design – S.P., M.S., I.B.R.; Supervision – D.K., A.H., S.P., M.S., I.B.R.; Fundings – S.P., M.S., I.B.R.; Materials – D.K., A.H., S.D., A.M.; Data collection &/or processing – D.K., A.H., S.D., A.M.; Analysis &/or interpretation – D.K., A.H.; Literature search – S.D., A.M.; Writing – S.D., A.M.; Critical review – D.K., A.H., S.D., A.M., S.P., M.S., I.B.R.
REFERENCES
- 1.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. CABANA Investigators. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–74. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, et al. CABANA Investigators. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–85. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuck KH, Brugada J, Schlüter M, Braegelmann KM, Kueffer FJ, Chun KRJ, et al. FIRE AND ICE Trial Investigators. The FIRE AND ICE Trial: What We Know, What We Can Still Learn, and What We Need to Address in the Future. J Am Heart Assoc. 2018;7:e010777. doi: 10.1161/JAHA.118.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivasambu B, Hakim JB, Barodka V, Chrispin J, Berger RD, Ashikaga H, et al. Initiation of a High-Frequency Jet Ventilation Strategy for Catheter Ablation for Atrial Fibrillation: Safety and Outcomes Data. JACC Clin Electrophysiol. 2018;4:1519–25. doi: 10.1016/j.jacep.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja DC, Sanders P, Pathak RK. How much is enough? An appraisal of high-power short-duration radiofrequency ablation for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2019;30:2205–8. doi: 10.1111/jce.14124. [DOI] [PubMed] [Google Scholar]
- 6.Winkle RA. High-power short-duration ablation: Turn up the heat to cool down the esophagus. J Cardiovasc Electrophysiol. 2019;30:1884–5. doi: 10.1111/jce.14108. [DOI] [PubMed] [Google Scholar]
- 7.Bhaskaran A, Chik W, Pouliopoulos J, Nalliah C, Qian P, Barry T, et al. Five seconds of 50–60 W radio frequency atrial ablations were transmural and safe: an in vitro mechanistic assessment and force-controlled in vivo validation. Europace. 2017;19:874–80. doi: 10.1093/europace/euw077. [DOI] [PubMed] [Google Scholar]
- 8.Winkle RA. HPSD ablation for AF high-power short-duration RF ablation for atrial fibrillation: A review. J Cardiovasc Electrophysiol. 2021;32:2813–23. doi: 10.1111/jce.14863. [DOI] [PubMed] [Google Scholar]
- 9.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 11.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 12.R Studio Team. RStudio: Integrated Development for R. RStudio, Inc; Boston MA: 2015. [Google Scholar]
- 13.Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–67. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Schmidt B, Bordignon S, Urbanek L, Tohoku S, Bologna F, et al. Ablation index-guided 50W ablation for pulmonary vein isolation in patients with atrial fibrillation: Procedural data, lesion analysis, and initial results from the FAFA AI High Power Study. J Cardiovasc Electrophysiol. 2019;30:2724–31. doi: 10.1111/jce.14219. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Chun KRJ, Tohoku S, Bordignon S, Urbanek L, Willems F, et al. Esophageal endoscopy after catheter ablation of atrial fibrillation using ablation-index guided high-power: Frankfurt AI-HP ESO-I. JACC Clin Electrophysiol. 2020;6:1253–61. doi: 10.1016/j.jacep.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Chen S, Schmidt B, Seeger A, Bordignon S, Tohoku S, Willems F, et al. Catheter ablation of atrial fibrillation using ablation index-guided high power (50 W) for pulmonary vein isolation with or without esophageal temperature probe (the AI-HP ESO II) Heart Rhythm. 2020;17:1833–40. doi: 10.1016/j.hrthm.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Reddy VY, Grimaldi M, De Potter T, Vijgen JM, Bulava A, Duytschaever MF, et al. Pulmonary vein isolation with very high power, short duration, temperature-controlled lesions: the QDOT-FAST trial. JACC Clin Electrophysiol. 2019;5:778–86. doi: 10.1016/j.jacep.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Winkle RA, Mohanty S, Patrawala RA, Mead RH, Kong MH, Engel G, et al. Low complication rates using high power (45–50 W) for short duration for atrial fibrillation ablations. Heart Rhythm. 2019;16:165–9. doi: 10.1016/j.hrthm.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Winkle RA, Mead RH, Engel G, Kong MH, Salcedo J, Brodt CR, et al. High-power, short-duration atrial fibrillation ablations using contact force sensing catheters: outcomes and predictors of success including posterior wall isolation. Heart Rhythm. 2020;17:1223–31. doi: 10.1016/j.hrthm.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Okamatsu H, Koyama J, Sakai Y, Negishi K, Hayashi K, Tsurugi T, et al. High-power application is associated with shorter procedure time and higher rate of first-pass pulmonary vein isolation in ablation index-guided atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2019;30:2751–8. doi: 10.1111/jce.14223. [DOI] [PubMed] [Google Scholar]
- 21.Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace. 2020;22:1495–501. doi: 10.1093/europace/euaa144. [DOI] [PubMed] [Google Scholar]
- 22.Baher A, Kheirkhahan M, Rechenmacher SJ, Marashly Q, Kholmovski EG, Siebermair J, et al. High-power radiofrequency catheter ablation of atrial fibrillation: using late gadolinium enhancement magnetic resonance imaging as a novel index of esophageal injury. JACC Clin Electrophysiol. 2018;4:1583–94. doi: 10.1016/j.jacep.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Bunch TJ, May HT, Bair TL, Crandall BG, Cutler MJ, Mallender C, et al. Long-term outcomes after low power, slower movement versus high power, faster movement irrigated-tip catheter ablation for atrial fibrillation. Heart Rhythm. 2020;17:184–9. doi: 10.1016/j.hrthm.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Ücer E, Jungbauer C, Hauck C, Kaufmann M, Poschenrieder F, Maier L, et al. The low acute effectiveness of a high-power short duration radiofrequency current application technique in pulmonary vein isolation for atrial fibrillation. Cardiol J. 2021;28:663–70. doi: 10.5603/CJ.a2020.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassallo F, Meigre LL, Serpa E, Cunha C, Simoes A, Jr, Carloni H, et al. Changes and impacts in early recurrences after atrial fibrillation ablation in contact force era: comparison of high-power short-duration with conventional technique-FIRST experience data. J Interv Card Electrophysiol. 2021;62:363–71. doi: 10.1007/s10840-020-00911-x. [DOI] [PubMed] [Google Scholar]
- 26.Vassallo F, Cunha C, Serpa E, Meigre LL, Carloni H, Simoes A, Jr, et al. Comparison of high-power short-duration (HPSD) ablation of atrial fibrillation using a contact force-sensing catheter and conventional technique: initial results. J Cardiovasc Electrophysiol. 2019;30:1877–83. doi: 10.1111/jce.14110. [DOI] [PubMed] [Google Scholar]
- 27.Yazaki K, Ejima K, Kanai M, Kataoka S, Higuchi S, Yagishita D, et al. Impedance drop predicts acute electrical reconnection of the pulmonary vein-left atrium after pulmonary vein isolation using short-duration high-power exposure. J Interv Card Electrophysiol. 2020;59:575–84. doi: 10.1007/s10840-019-00691-z. [DOI] [PubMed] [Google Scholar]
- 28.Berte B, Hilfiker G, Russi I, Moccetti F, Cuculi F, Toggweiler S, et al. Pulmonary vein isolation using a higher power shorter duration CLOSE protocol with a surround flow ablation catheter. J Cardiovasc Electrophysiol. 2019;30:2199–204. doi: 10.1111/jce.14122. [DOI] [PubMed] [Google Scholar]
- 29.Castrejón-Castrejón S, Martínez Cossiani M, Ortega Molina M, Escobar C, Froilán Torres C, Gonzalo Bada N, et al. Feasibility and safety of pulmonary vein isolation by high-power short-duration radiofrequency application: short-term results of the POWER-FAST PILOT study. J Interv Card Electrophysiol. 2020;57:57–65. doi: 10.1007/s10840-019-00645-5. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon G, Ahsan S, Honarbakhsh S, Lim W, Baca M, Graham A, et al. A multicentered evaluation of ablation at higher power guided by ablation index: Establishing ablation targets for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2019;30:357–65. doi: 10.1111/jce.13813. [DOI] [PubMed] [Google Scholar]
- 31.Ejima K, Higuchi S, Yazaki K, Kataoka S, Yagishita D, Kanai M, et al. Comparison of high-power and conventional-power radiofrequency energy deliveries in pulmonary vein isolation using unipolar signal modification as a local endpoint. J Cardiovasc Electrophysiol. 2020;31:1702–8. doi: 10.1111/jce.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneshiro T, Kamioka M, Hijioka N, Yamada S, Yokokawa T, Misaka T, et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol. 2020;13:e008602. doi: 10.1161/CIRCEP.120.008602. [DOI] [PubMed] [Google Scholar]
- 33.Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler V, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace. 2020;22:388–93. doi: 10.1093/europace/euz342. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai K, Toyama H. High-power, short-duration ablation during Box isolation for atrial fibrillation. J Arrhythm. 2020;36:899–904. doi: 10.1002/joa3.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leo M, Pedersen M, Rajappan K, Ginks MR, Hunter RJ, Bowers R, et al. Power, lesion size index and oesophageal temperature alerts during atrial fibrillation ablation: a randomized study. Circ Arrhythm Electrophysiol. 2020;13:e008316. doi: 10.1161/CIRCEP.120.008316. [DOI] [PubMed] [Google Scholar]
- 36.Matiello M, Mont L, Tamborero D, Berruezo A, Benito B, Gonzalez E, et al. Cooled-tip vs. 8 mm-tip catheter for circumferential pulmonary vein ablation: comparison of efficacy, safety, and lesion extension. Europace. 2008;10:955–60. doi: 10.1093/europace/eun144. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson B, Chen X, Pehrson S, Svendsen JH. The effectiveness of a high output/short duration radiofrequency current application technique in segmental pulmonary vein isolation for atrial fibrillation. Europace. 2006;8:962–5. doi: 10.1093/europace/eul100. [DOI] [PubMed] [Google Scholar]
- 38.Pambrun T, Durand C, Constantin M, Masse A, Marra C, Meillet V, et al. High-power (40–50 W) radiofrequency ablation guided by unipolar signal modification for pulmonary vein isolation: experimental findings and clinical results. Circ Arrhythm Electrophysiol. 2019;12:e007304. doi: 10.1161/CIRCEP.119.007304. [DOI] [PubMed] [Google Scholar]
- 39.Yavin HD, Leshem E, Shapira-Daniels A, Sroubek J, Barkagan M, Haffajee CI, et al. Impact of high-power short-duration radiofrequency ablation on long-term lesion durability for atrial fibrillation ablation. JACC Clin Electrophysiol. 2020;6:973–85. doi: 10.1016/j.jacep.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Bourier F, Duchateau J, Vlachos K, Lam A, Martin CA, Takigawa M, et al. High-power short-duration versus standard radiofrequency ablation: insights on lesion metrics. J Cardiovasc Electrophysiol. 2018;29:1570–5. doi: 10.1111/jce.13724. [DOI] [PubMed] [Google Scholar]
- 41.Barkagan M, Contreras-Valdes FM, Leshem E, Buxton AE, Nakagawa H, Anter E. High-power and short-duration ablation for pulmonary vein isolation: safety, efficacy, and long-term durability. J Cardiovasc Electrophysiol. 2018;29:1287–96. doi: 10.1111/jce.13651. [DOI] [PubMed] [Google Scholar]
- 42.Ali-Ahmed F, Goyal V, Patel M, Orelaru F, Haines DE, Wong WS. High-power, low-flow, short-ablation duration-the key to avoid collateral injury? J Interv Card Electrophysiol. 2019;55:9–16. doi: 10.1007/s10840-018-0473-5. [DOI] [PubMed] [Google Scholar]
- 43.Leshem E, Zilberman I, Tschabrunn CM, Barkagan M, Contreras-Valdes FM, Govari A, et al. High-power and short-duration ablation for pulmonary vein isolation: biophysical characterization. JACC Clin Electrophysiol. 2018;4:467–79. doi: 10.1016/j.jacep.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Kwon HJ, Lee SS, Park YJ, Park SJ, Park KM, Kim JS, et al. Effectiveness and safety of high-power and short-duration ablation for cavotricuspid isthmus ablation in atrial flutter. Pacing Clin Electrophysiol. 2020;43:941–6. doi: 10.1111/pace.14019. [DOI] [PubMed] [Google Scholar]
- 45.Bradley CJ, Haines DE. Pulsed field ablation for pulmonary vein isolation in the treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2020;31:2136–27. doi: 10.1111/jce.14414. [DOI] [PubMed] [Google Scholar]
- 46.Sauer WH, Tzou WS. With great power comes great responsibility: defining the safety of high-power short-duration atrial ablation. Circ Arrhythm Electrophysiol. 2019;12:e007456. doi: 10.1161/CIRCEP.119.007456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparisons of atrial arrhythmia recurrences between HPSD and LPLD ablation groups. A: Funnel plot. B: Forest plot showing sensitivity analysis. C: Drapery plot.
HPSD - high-power short-duration; LPLD - low-power long-duration.
Forest plots comparing atrial arrhythmia recurrences between HPSD and LPLD ablation groups. A: the blanking period. B: In patients with paroxysmal atrial fibrillation. C. In patients with persistent atrial fibrillation.
HPSD - high-power short-duration; LPLD - low-power long-duration; RR - relative risk.
A: Forest plot comparing recurrence of atrial arrhythmia between HPSD and LPLD ablation groups after the blanking period. B: Bubble plot showing correlation with follow-up duration.
HPSD - high-power short-duration; LPLD - low-power long-duration; RR - relative risk.
Forest plots showing subgroup analysis of acute PVRs between HPSD and LPLD ablation groups in presence and absence of provocation tests.
HPSD - high-power short-duration; LPLD - low-power long-duration; PVR - pulmonary vein reconnection.
Comparison of first-pass PVIs between HPSD and LPLD ablation groups. A: Forest plot showing sensitivity analysis. B: Funnel plot. C: L’Abbe plot.
HPSD - high-power short-duration; LPLD - low-power long-duration; PVI - pulmonary vein isolation.
Forest plots showing pooled incidences of acute complications in the HPSD ablation group. A: Stroke/transient ischemic attack. B: Phrenic nerve palsy. C: Atrio-esophageal fistula. D: Steam-pop. E: Tamponade.
HPSD - high-power short-duration.
Supplementary Table S1.
Nonrandomized studies appraised with the ROBINS-I tool
| Before intervention | At intervention | After intervention | Missing data | Measurement of outcomes | Selection of reported results | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Confounding | Selection of participants | Classification of interventions | Deviation from intended intervention | ||||
| Berte et al., 2019 | 3 to 4 | 1 to 2 | 1 | 2 to 3 | 1 | 1 to 2 | 1 to 2 |
| Bunch et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 | 2 to 3 | 1 to 2 |
| Castrejón Castrejónet al., 2020 | 3 to 4 | 4 | 4 | 3 to 4 | 1 to 2 | 2 to 3 | 3 to 4 |
| Dhillon et al., 2019 | 0 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 1 to 2 | 3 to 4 |
| Ejima et al., 2020 | 1 to 2 | 3 to 4 | 3 to 4 | 1 to 2 | 1 to 2 | 1 to 2 | 1 to 2 |
| Kaneshiro et al., 2020 | 3 to 4 | 1 to 2 | 1 to 2 | 1 to 2 | 1 to 2 | 2 to 3 | 2 to 3 |
| Kottmaier et al., 2020 | 2 to 3 | 1 to 2 | 1 to 2 | 3 to 4 | 4 | 3 to 4 | 3 to 4 |
| Kumagai et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 3 to 4 | 2 to 3 |
| Matiello et al., 2008 | 1 to 2 | 3 to 4 | 3 to 4 | 3 to 4 | 2 to 3 | 2 to 3 | 2 to 3 |
| Nilsson et al., 2006 | 2 to 3 | 2 to 3 | 2 to 3 | 2 to 3 | 1 to 2 | 1 to 2 | 2 to 3 |
| Pambrun et al., 2019 | 0 | 1 to 2 | 1 to 2 | 3 to 4 | 1 to 2 | 4 | 4 |
| Yavin et al., 2020 | 3 to 4 | 3 to 4 | 3 to 4 | 3 to 4 | 1 to 2 | 3 to 4 | 3 to 4 |
| Yazaki et al., 2020 | 2 to 3 | 1 to 2 | 1 to 2 | 3 to 4 | 1 to 2 | 2 to 3 | 2 to 3 |
ROBINS-I - Risk of Bias In Nonrandomized Studies of Interventions.
Supplementary Table S2.
Retrospective studies appraised with the Newcastle-Ottawa scale
| Selection | Comparability | Outcome | AHRQ standards | |
|---|---|---|---|---|
| Baher et al., 2018 | ★★★★ | ★★ | ★★★ | Good |
| Ücer et al., 2020 | ★★★ | ★ | ★★ | Good |
| Vassallo et al., 2020 | ★★★★ | ★★ | ★★ | Good |
| Vassallo et al., 2019 | ★★★★ | ★★ | ★★ | Good |
| Chen et al., 2019 | ★★★★ | ★★ | Good | |
| Chen et al., 2020 (ESO-I) | ★★★★ | ★★ | Good | |
| Chen et al., 2020 (ESO-II) | ★★★★ | ★★ | Good | |
| Reddy et al., 2019 | ★★★★ | ★★ | Good | |
| Winkle et al., 2019 | ★★★ | ★★ | Good | |
| Winkle et al., 2020 | ★★★★ | ★★★ | Good |
AHRQ - Agency for Healthcare Research and Quality
Supplementary Table S3.
Randomized controlled studies appraised with the RoB2 scale
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
|---|---|---|---|---|---|---|---|
| Leo et al., 2020 | Low risk | Unclear risk | High risk | High risk | Low risk | Low risk | None |
| Okamatsu et al., 2019 | Unclear risk | Unclear risk | High risk | High risk | Low risk | Low risk | None |
| Shin et al., 2020 | Low risk | High risk | High risk | Low risk | Low risk | Low risk | None |
RoB2 - Risk of Bias Tool for Randomized Trials