Abstract
Objective
Previous studies have investigated the relationship between alcohol and ventricular structure; however, few studies have evaluated the relation between alcohol consumption and the atrium size. In this study, we aimed to test the association between alcohol consumption and left atrium (LA) size in the general population.
Methods
A population-based sample of 10,211 subjects aged ≥35 years and free from hypertension at baseline were followed from January 2012 to August 2013. Left atrial enlargement (LAE) was defined as the ratio of LA diameter to body surface area exceeding 2.4 cm/m2 in both the sexes. Independent factors for LAE were estimated by multiple logistic regression analyses.
Results
The study included 10,211 participants (4,751 men and 5,460 women). Left atrial diameter/body surface area (LAD/BSA) was higher in the moderate and heavy alcohol consumption groups than in the non-drinker group (non-drinker, 20.5±0.03 cm/m2; moderate, 20.8±0.09 cm/m2; and heavy, 20.6±0.06 cm/m2; p<0.001). Both the groups of moderate and heavy drinkers had a higher incidence of LAE than the non-drinker group (6.9% of non-drinkers, 9.9% of moderate drinkers, and 8.4% of heavy drinkers; p<0.001). After adjusting for related risk factors, multiple logistic regression analyses showed that moderate drinkers had an approximately 1.4-fold higher risk of LAE [odds ratio (OR): 1.387, 95% confidence interval (CI) 1.056–1.822, p=0.019] compared with the non-drinkers, and the heavy drinkers had an approximately 1.2-fold higher risk of LAE (OR: 1.229, 95% CI: 1.002–1.508, p=0.047) compared with that of the non-drinkers.
Conclusion
Both heavy and moderate drinkers had increased odds for LAE compared with participants with no alcohol consumption in the general population.
Keywords: left atrial enlargement, alcohol consumption, general population
INTRODUCTION
The association between alcohol drinking and cardiovascular disease (CVD) is a controversial topic. Previous studies had linked alcohol consumption to a reduced risk of coronary heart disease (1, 2), whereas other surveys report that alcohol use is associated with increased adverse cardiovascular events (3–5).
The evaluation of left atrial (LA) size can predict CVD outcomes (6, 7). Both volume and pressure overload can increase atrial size, and LA dilatation can also cause pressure overload resulting from fibrosis and/or calcification of the LA (8). Previous studies have indicated that ethanol increases blood pressure, which might lead to volume and pressure overload and could disturb myocardial metabolism, including myocardial fibrosis (9–11). On the basis of these theories, we hypothesized that alcohol was associated with heart structure. Although many studies have investigated the relationship between alcohol and ventricular structure, few studies have evaluated the relation between alcohol consumption and atrium size. Therefore, in this study, we aimed to evaluate the association between alcohol consumption and LA size in the general population with the goal of providing a population-based evidence for a link between alcohol and cardiovascular mortality risk.
METHODS
The methods of this study has been published elsewhere (12, 13).
Study population
The study was a cross-sectional survey and was conducted in 3 counties (Dawa, Zhangwu, and Liaoyang) and 26 rural villages in the Liaoning Province, China, from January 2012 to August 2013. The survey used a multilevel stratified random sampling design to select representative samples of the general population aged 35 years or older. Participants who were pregnant and those who had malignant tumors or mental disorders were not included. In the selected villages, every eligible permanent villager was invited to participate in the investigation, a total of 14,016 individuals. In addition, 11,956 individuals took part in the study. Thus, the response rate was 85.3%. The Ethics Committee of the China Medical University (Shenyang, China, AF-SDP-07–1, 0–01) approved the study. All the processes followed ethical standards. All the participants signed informed consents after being informed of the study details. This study used only baseline data for the study that contains data from individuals with complete analytical variables related to this study.
Data collection and measurements
Data were collected through questionnaires and face-to-face interviews by trained cardiologists and nurses. All the participants were questioned about demographic characteristics, lifestyle risk factors, history of heart disease [atrial fibrillation (AF), prevalent valvular heart disease, heart failure, and coronary heart disease], medication use (anti-hypertensive, anti-diabetic, and anti-hyperlipidemia drug treatments), and family income through standardized questionnaires in the last two weeks. Self-reported sleep duration was composed of the duration of nighttime sleep and naps. Physical activity included occupational and leisure activities, and the other study has described the measures in detail (14). After the integration of occupational and leisure activities, they were reclassified as low (participants who had light recreational sports and occupational manual labor), moderate (individuals who had moderate or high level of recreational sports and occupational manual labor), and high (those who had moderate or high level of recreational sports and occupational manual labor). Height and weight measurements were accurate to 0.1 cm and 0.1 kg, respectively. The subjects were required to take off their shoes and wear light clothes when taking the measurements. Body mass index was calculated using the standard formula, kg/m2.
After fasting for at least 12 hours, blood was drawn from the antecubital vein using BD Vacutainer tubes with EDTA (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Samples were rapidly serum-separated, stored at −20°C and transferred to the central certified laboratory with an Olympus AU640 Auto-Analyzer (Olympus Corp., Kobe, Japan) to detect blood biochemical indicators. It was also important to calibrate all the devices and use blinded duplicate samples. Cardiologists used a MAC 5 500 (GE Healthcare, Little Chalfont, Buckingham-shire, UK) with the MUSE Cardiology Information System, version 7.0.0 (GE Healthcare) to perform a 12-lead electrocardiogram on each participant.
Three cardiac sonographers performed echocardiogram on each participant using a Doppler echocardiograph (Vivid, GE Health-care, USA) with a 3.0-MHz transducer and analyzed the data, and if necessary, two superior specialists were requested to assist in the diagnosis. The transthoracic echocardiogram was performed in the supine position, and the report of the echocardiogram was described after integrating the results of two-dimensional (2D) measurements, M-mode measurements, color Doppler checks, and spectral Doppler imaging measurements. The 2D and M-mode measurements of the root of the aorta and the inner diameter and wall thickness of the left ventricle and left atrium were displayed by the parasternal acoustic window. The four- and five-chamber images were displayed by the parasternal acoustic window, and valvular regurgitation were captured by color Doppler. Procedures described in other articles were used to verify the correct orientation of imaging planes and Doppler recordings (15). According to the recommendations of the American Society of Echocardiogram, LV internal dimensions, posterior wall thickness (PWT), and interventricular septal thickness (IVST) were measured at end-systole and end-diastole. The M-mode measurement of the LA posteroanterior dimension under 2D guidance should be made from the parasternal long-axis view. The LV ejection fraction was estimated by the equation area product × ventricular length, which was measured from the four-chamber apical projection. At the end of the left ventricle diastole, the LV end-diastolic diameter (LVDd) was measured from the LV minor axis. The formula for calculating the left ventricle mass (LVM) was LVM = 0.8 × [1.04{(LVIDd + PWTd + SWTd)3 - LVIDd3}] + 0.6 g (16). Left ventricle mass index (LVMI) = LVM/standardized body surface area (BSA) (17). The diameter of the left ventricle was indexed for the BSA (18). The left ventricular ejection fraction was measured from the four-chamber apical projection by area product × ventricular length.
Alcohol consumption assessment
A questionnaire was used to assess alcohol consumption by the average amount of alcohol consumption per day, whether alcohol was consumed regularly, and how many days per month they consumed alcohol. The alcohol content of each drink was not the same, for example, the alcohol content of beer was 5%, alcohol content of red wine was 12.5%, and alcohol content of hard liquor was 45%. Every 15 g of ethanol was 1 drink (19). We used the concept of daily alcohol consumption developed by the National Institute on Alcohol Abuse and Alcoholism to classify and group people as non-drinkers (abstainers, no alcohol consumption history), moderate drinkers (≤1 drink/day for women and ≤2 drinks/day for men), and heavy drinkers (20). The conclusions of this classification method were the same as those of WHO, although different classification methods might lead to different statistical results. According to the concept of daily alcohol consumption developed by the National Institute on Alcohol Abuse and Alcoholism, cut-off values were found and were used to rank the participants’ level of alcohol consumption: non-drinkers (abstainers, no alcohol consumption history), moderate drinkers (≤1 drink/day for women and ≤2 drinks/day for men), but otherwise heavy drinkers.
Definitions
LAE was defined as the ratio of LA diameter to BSA exceeding 2.4 cm/m2 in both sexes (21). AF was defined as shown by electrocardiogram or an AF history with no evidence on the electrocardiogram. The definition of dyslipidemia was based on the National Cholesterol Education Program - Third Adult Treatment Panel (ATP III) guidelines (22). Diabetes mellitus was diagnosed according to the WHO criteria (23): FPG ≥7 mmol/L (126 mg/dL) and/or on treatment for diabetes. Anemia was a condition where the hemoglobin (Hb) concentration was <110 g/L in women or Hb <120 g/L in men on the basis of the China expert consensus.
Statistical analysis
Categorical variables were described by numbers and ratios, and continuous variables were expressed as mean ± standard deviation. The Scheffe method was used to test the differences between the groups. The χ2-test, ANOVA, or t-test were used to assess differences between categories. Independent factors for LAE were estimated by multiple logistic regression analyses, which could also estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs). R version 3.6.3 software (https://www.R-project.org, the R Foundation) was used for mediating effect analysis to explore whether LA enlargement was a mediating effect of increased risk of AF caused by alcohol consumption. The rest of the statistical analysis was done using the Statistical Package for Social Sciences version 25.0 software (IBM Corp., Armonk, New York, USA) and the level of significance was p<0.05. If 0 was not included between the upper and lower limits of the 95% CI, it was statistically significant.
RESULTS
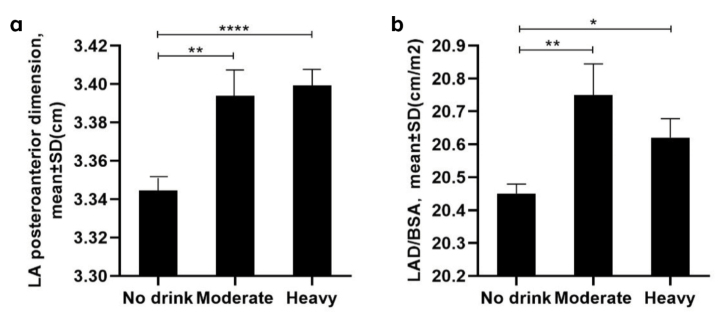
The baseline characteristics of the included participants stratified according to different alcohol consumption levels are shown in Table 1. A total of 10,211 participants aged 35 years or older were included (4,751 men and 5,460 women). There were significant differences in the echocardiography parameters, including LA posteroanterior dimension among the three alcohol consumption groups (Fig. 1a) (p<0.05). The LAD/BSA was higher in the moderate and heavy alcohol consumption groups than in the non-drinker group (non-drinker, 20.5±0.03 cm/m2; moderate, 20.8±0.09 cm/m2; and heavy, 20.6±0.06 cm/m2; p<0.001) (Fig. 1b). The prevalence of LAE was higher in both the moderate and heavy drinker groups than in the non-drinker group (6.9% of non-drinkers, 9.9% of moderate drinkers, and 8.4% of heavy drinkers, p<0.001) (Fig. 2).
Table 1.
Baseline characteristics of the study population according to the different alcohol consumption levels
| Variables | Alcohol consumption | P-value | ||
|---|---|---|---|---|
|
| ||||
| Non-drinker (n=7614) | Moderate (n=795) | Heavy (n=1802) | ||
| Age (years) | 53.25±10.40 | 54.55±10.29 | 54.19±10.35 | <0.001 |
| Race (Han) | 7237 (95.0%) | 752 (94.6%) | 1702 (94.5%) | 0.534 |
| Male sex | 3465 (45.5%) | 385 (48.4%) | 901 (50.0%) | 0.001 |
| Education | 0.039 | |||
| Primary school or below | 3705 (48.7%) | 394 (49.6%) | 932 (51.7%) | |
| Middle school | 3161 (41.5%) | 324 (40.8%) | 731 (40.6%) | |
| High school or above | 748 (9.8%) | 77 (9.7%) | 139 (7.7%) | |
| Family income (CNY/year) | 2.23±0.64 | 2.20±0.65 | 2.19±0.64 | 0.046 |
| Total sleep (hours/day) | 7.25±1.67 | 7.23±1.69 | 7.28±1.70 | 0.731 |
| Physical activity | 0.457 | |||
| Low | 2214 (29.1%) | 235 (29.6%) | 507 (28.1%) | |
| Moderate | 4957 (65.1%) | 509 (64.0%) | 1204 (66.8%) | |
| High | 443 (5.8%) | 51 (6.4%) | 91 (5.0%) | |
| BMI (kg/m2) | 24.66±3.64 | 24.94±3.66 | 24.97±3.60 | 0.002 |
| BSA (m2) | 1.65±0.18 | 1.65±0.18 | 1.66±0.17 | 0.024 |
| SBP (mm Hg) | 139.55±22.05 | 142.45±23.62 | 143.56±23.34 | <0.001 |
| DBP (mm Hg) | 81.33±11.27 | 82.30±11.74 | 82.82±11.69 | <0.001 |
| HR (beats/minute) | 71.84±12.60 | 71.62±12.88 | 71.91±12.23 | 0.866 |
| FPG (mmol/L) | 5.88±1.60 | 6.00±1.75 | 5.95±1.63 | 0.035 |
| TC (mmol/L) | 5.21±1.07 | 5.29±1.09 | 5.27±1.10 | 0.032 |
| HDL-C (mmol/L) | 1.41±0.38 | 1.40±0.37 | 1.41±0.40 | 0.706 |
| LDL-C (mmol/L) | 2.91±0.81 | 2.96±0.85 | 2.95±0.84 | 0.036 |
| TG (mmol/L) | 1.62±1.48 | 1.63±1.24 | 1.64±1.46 | 0.733 |
| Hemoglobin (g/L) | 138.44±18.29 | 139.57±21.27 | 139.98±20.00 | 0.004 |
| Diabetes mellitus | 725 (9.7%) | 99 (12.7%) | 204 (11.5%) | 0.005 |
| Anemia | 969 (13.0%) | 89 (11.4%) | 194 (11.0%) | 0.042 |
| Dyslipidemia | 2676 (35.9%) | 295 (37.9%) | 662 (37.4%) | 0.315 |
| Atrial fibrillation | 75 (1.0%) | 12 (1.5%) | 23 (1.3%) | 0.254 |
| Heart failure | 69 (0.1%) | 9 (1.1%) | 26 (1.4%) | 0.118 |
| Coronary heart disease | 339 (4.5%) | 34 (4.3%) | 111 (6.2%) | 0.007 |
| Anti-hypertensive drug treatment | 980 (12.9%) | 122 (15.3%) | 298 (16.5%) | <0.001 |
| Anti-diabetic drug treatment | 279 (3.7%) | 41 (5.2%) | 66 (3.7%) | 0.106 |
| Anti-hyperlipidemia drug treatment | 231 (3%) | 29 (3.6%) | 57 (3.2%) | 0.629 |
| Alcohol consumption (g/d) | 0.00±0.00 | 15.84±7.39 | 84.55±50.20 | <0.001 |
| LA posteroanterior dimension (cm) | 3.34±0.39 | 3.39±0.42 | 3.40±0.39 | <0.001 |
| LVEF | 0.62±0.10 | 0.62±0.09 | 0.62±0.10 | 0.621 |
| E/A | 1.05±1.19 | 1.01±0.58 | 0.99±0.55 | 0.147 |
| LVW (g) | 132.06±29.17 | 141.77±29.28 | 143.14±28.65 | <0.001 |
| IVST (cm) | 0.86±0.10 | 0.89±0.11 | 0.89±0.10 | <0.001 |
| LVDD (cm) | 4.66±0.38 | 4.75±0.39 | 4.78±0.38 | <0.001 |
| PWT (cm) | 0.84±0.09 | 0.87±0.09 | 0.87±0.09 | <0.001 |
| IVST (cm) | 3.08±0.40 | 3.13±0.41 | 3.15±0.41 | <0.001 |
| Prevalent valvular heart disease | 395 (5.2%) | 51 (6.4%) | 119 (6.6%) | 0.032 |
| Left ventricular hypertrophy | 925 (12.1%) | 85 (10.7%) | 232 (12.9%) | 0.291 |
Data are expressed as mean ± standard deviation (SD) or as n (%).
A - mitral A peak flow; BMI - body mass index; BSA - body surface area; SBP - systolic blood pressure; DBP - diastolic blood pressure; E - mitral E peak flow; E/A - mitral E peak flow/mitral A peak flow; FPG - fasting plasma glucose; HDL-C - high-density lipoprotein cholesterol; IVST - interventricular septal thickness; LDL-C - low-density lipoprotein cholesterol; LVEF - left ventricular ejection fraction; LVDD - left ventricular internal diastolic dimensions; LVSD - left ventricular internal systolic dimensions; PWT - posterior wall thickness; TG - triglyceride; WC - waist circumference
Figure 1.
a: Left atrial posteroanterior dimension among the three alcohol consumption groups (P<0.05). b: Left atrial diameter/body surface area among the different alcohol consumption groups (P<0.001).
Figure 2.
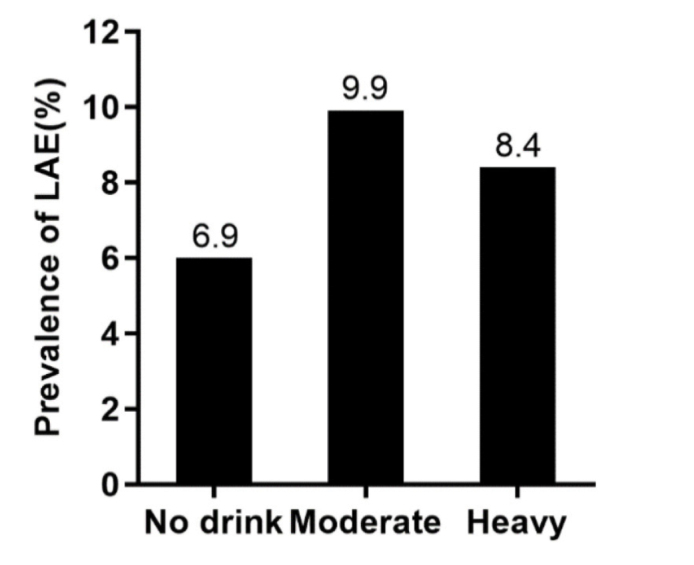
The prevalence of left atrial enlargement among the different alcohol consumption groups (P<0.001).
Table 2 presents the characteristics of the study participants with and without LAE. Alcohol consumption was much higher in the LAE than in the non-LAE participants (19.4±42.2 g/d vs. 15.9±38.0 g/d, p=0.001). In the population with LAE, 75.0% were non-drinkers, 7.6% were moderate drinkers, and 17.5% were heavy drinkers. In the population without LAE, 69.5% were non-drinkers, 10.5% were moderate drinkers, and 20.0% were heavy drinkers.
Table 2.
Characteristics of study participants with and without left atrial enlargement
| Variables | Left atrial enlargement | P-value | |
|---|---|---|---|
|
| |||
| No (n=9660) | Yes (n=551) | ||
| Age (years) | 52.9±10.2 | 61.0±10.5 | 0.981 |
| Male sex | 4567 (48.3%) | 184 (24.4%) | <0.001 |
| Race (Han) | 489 (5.2%) | 31 (4.1%) | 0.203 |
| Income (RMB/year) | 2.2±0.6 | 2.0±0.6 | <0.001 |
| Education | <0.001 | ||
| Primary school or below | 4499 (47.6%) | 532 (70.6%) | |
| Middle school | 4027 (42.6%) | 189 (25.1%) | |
| High school or above | 931 (9.8%) | 33 (4.4%) | |
| Current smoking | 3400 (36.0%) | 215 (28.5%) | <0.001 |
| Total sleep | 7.3±1.7 | 7.0±1.9 | 0.002 |
| Physical activity | <0.001 | ||
| Low | 2652 (28.0%) | 304 (40.3%) | |
| Moderate | 6265 (66.2%) | 405 (53.7%) | |
| High | 540 (5.7%) | 45 (6.0%) | |
| BMI (kg/m2) | 24.8±3.6 | 23.4±3.6 | 0.112 |
| SBP (mm Hg) | 139.9±22.1 | 148.1±25.5 | <0.001 |
| DBP (mm Hg) | 81.8±11.3 | 80.5±12.3 | 0.012 |
| HR (beats/minute) | 71.8±12.5 | 72.1±13.5 | 0.030 |
| LA posteroanterior dimension (cm) | 3.3±0.4 | 3.8±0.5 | <0.001 |
| E/A | 1.0±1.1 | 0.9±0.3 | <0.001 |
| Left ventricular hypertrophy | 1159 (12.3%) | 83 (11.0%) | 0.313 |
| Prevalent valvular heart disease | 497 (5.14%) | 68 (12.34%) | <0.001 |
| Diabetes mellitus | 924 (10.0%) | 104 (14.1%) | <0.001 |
| Dyslipidemia | 3373 (36.4%) | 260 (35.3%) | 0.565 |
| Anemia | 1102 (11.9%) | 150 (20.4%) | <0.001 |
| Atrial fibrillation | 58 (0.6%) | 52 (7.0%) | <0.001 |
| Heart failure | 80 (0.8%) | 24 (4.4%) | <0.001 |
| Coronary heart disease | 437 (4.5%) | 37 (8.5%) | <0.001 |
| Anti-hypertensive drug treatment | 347±3.6 | 39±7.1 | <0.001 |
| Anti-diabetic drug treatment | 1242 (12.9%) | 158 (28.7%) | <0.001 |
| Anti-hyperlipidemia drug treatment | 291 (3%) | 26 (4.7%) | 0.025 |
| Alcohol consumption (g/d) | 15.9±38.0 | 19.4±42.2 | 0.001 |
Data are expressed as mean ± standard deviation (SD) or as n (%).
BMI - body mass index; SBP - systolic blood pressure; DBP - diastolic blood pressure; A - mitral A peak flow; E -mitral E peak flow; E/A - mitral E peak flow/mitral A peak flow
Table 3 presents the multiple logistic regression analyses of the risk of LAE according to the different levels of alcohol consumption. After adjusting for factors, including demographic characteristics; lifestyles; echocardiography parameters; AF; prevalent valvular heart disease; heart failure and coronary heart disease; anti-hypertensive, anti-diabetic, and anti-hyperlipidemia drug treatments; hypertension; diabetes mellitus; anemia; and family income, the moderate drinkers had an approximately 1.4-fold higher risk of LAE (OR: 1.387, 95% CI: 1.056–1.822, p=0.019) than the non-drinkers, and the heavy drinkers had an approximately 1.2-fold higher risk of LAE (OR: 1.229, 95% CI: 1.002–1.508, p=0.047) than the non-drinkers. The occurrence of AF had no significant difference in the occurrence of LAE.
Table 3.
Effect of alcohol consumptions on LAE
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Sex | 4.423 (3.498–5.593) | <0.001 |
| Age (years) | 1.061 (1.050–1.072) | <0.001 |
| Education | ||
| Primary school or below | 1.000 (reference) | 0.068 |
| Middle school | 1.535 (1.030–2.288) | 0.035 |
| High school or above | 1.339 (0.889–2.017) | 0.162 |
| Family income (CNY/year) | 0.836 (0.807–0.966) | 0.043 |
| Total sleep | 0.957 (0.914–1.003) | 0.067 |
| Physical activity | ||
| Low | 1.000 (reference) | 0.252 |
| Moderate | 0.924 (0.754–1.132) | 0.446 |
| High | 1.114 (0.886–1.400) | 0.355 |
| Current smoking | 0.978 (0.806–1.187) | 0.822 |
| Current drinking | 0.715 (0.543–0.943) | 0.017 |
| BMI (kg/m2) | 0.786 (0.753–0.813) | <0.001 |
| E/A | 1.015 (0.929–1.109) | 0.743 |
| Hypertension | 1.268 (1.055–1.524) | <0.001 |
| Prevalent valvular heart disease | 0.572 (0.473–0.691) | <0.001 |
| Atrial fibrillation | 0.804 (0.354–1.823) | 0.601 |
| Heart failure | 0.520 (0.275–0.986) | |
| Coronary heart disease | 1.353 (0.942–1.944) | 0.102 |
| Anti-hypertensive drug treatment | 1.032 (0.865–1.232) | 0.724 |
| Anti-diabetic drug treatment | 1.361 (0.849–2.182) | 0.201 |
| Anti-hyperlipidemia drug treatment | 2.102 (1.114–3.968) | 0.022 |
| Diabetes mellitus | 0.825 (0.622–1.095) | 0.182 |
| Anemia | 0.453 (0.316–0.649) | <0.001 |
| Dyslipidemia | 1.165 (0.982–1.382) | 0.081 |
| Alcohol consumption group | ||
| Non-drinker | 1.000 (reference) | 0.017 |
| Moderate | 1.387 (1.056–1.822) | 0.019 |
| Heavy | 1.229 (1.002–1.508) | 0.047 |
Multiple adjusted model: demographic characteristics, lifestyles, echocardiography parameters, atrial fibrillation, prevalent valvular heart disease, heart failure and coronary heart disease, anti-hypertensive drug treatment, anti-diabetic drug treatment, anti-hyperlipidemia drug treatment, hypertension, diabetes mellitus, anemia, and family income.
BMI - body mass index; A - mitral A peak flow; E - mitral E peak flow; E/A - mitral E peak flow/mitral A peak flow; OR - odds ratio; CI - confidence interval
Table 4 presents the multiple logistic regression analyses of the risk of AF according to the different levels of alcohol consumption. In the multiple adjusted model without LAD/BSA adjusted; factors including demographic characteristics, lifestyles, prevalent valvular heart disease, heart failure and coronary heart disease, anti-hypertensive drug treatment, anti-diabetic drug treatment, anti-hyperlipidemia drug treatment, hypertension, diabetes mellitus, and family income were adjusted. Moderate drinkers had a 4.150 (1.187–14.503) and heavy drinkers had a 4.724 (1.456–15.327) times risk of AF than non-drinkers. After further adjusting LAD/BSA, heavy drinkers had a 3.281 (1.154–9.326) times risk of AF than non-drinkers. However, there was no significant difference in the risk of AF between moderate and non-drinkers. Thus, the size of the left atrium may explain the risk of AF in drinkers.
Table 4.
Effect of alcohol consumption on atrial fibrillation
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Multiple adjusted model without adjusted LAD/BSA | ||
| Alcohol consumption group | ||
| Non-drinker | 1.000 (reference) | 0.024 |
| Moderate | 4.150 (1.187–14.503) | 0.026 |
| Heavy | 4.724 (1.456–15.327) | 0.010 |
| Multiple adjusted model with adjusted LAD/BSA | ||
| Alcohol consumption group | ||
| Non-drinker | 1.000 (reference) | 0.023 |
| Moderate | 3.998 (0.857–18.641) | 0.078 |
| Heavy | 3.281 (1.154–9.326) | 0.026 |
Multiple adjusted models without adjusted LAD/BSA: adjusting for factors including demographic characteristics, lifestyles, prevalent valvular heart disease, heart failure and coronary heart disease, anti-hypertensive drug treatment, anti-diabetic drug treatment, anti-hyperlipidemia drug treatment, hypertension, diabetes mellitus and family income. Multiple adjusted models with adjusted LAD/BSA: adjusting for factors including LAD/BSA, demographic characteristics, life styles, prevalent valvular heart disease, heart failure and coronary heart disease, anti-hypertensive drug treatment, anti-diabetic drug treatment, anti-hyperlipidemia drug treatment, hypertension, diabetes mellitus, and family income.
LAD - left atrial diameter; BSA - body surface area; OR - odds ratio; CI - confidence interval
DISCUSSION
In this study, we evaluated the association between alcohol consumption, LA size, and AF in the general Chinese population. We found that both heavy and moderate drinkers had increased odds for LAE compared with that of participants with no alcohol consumption. Alcohol consumption caused AF by causing the enlargement of the left atrium.
Alcohol use has been shown to have numerous effects on the cardiovascular system, including arrhythmia, hypertension, stroke, and sudden death (24–27). LA size has been shown to have a significant prognostic value for cardiovascular events such as heart failure, AF or stroke, and increased cardiovascular and all-cause mortality rates (28, 29). However, few studies have focused on the association between alcohol consumption and LA size, and their results are inconsistent. In a biracial cohort of young adults, researchers concluded that alcohol consumption was not a significant risk predictor for LAE (30). In patients with coronary heart disease, researchers reported that heavy drinking correlated with a 5-year increase in LAV (31). Another study in an elderly community-based population reported that increased alcohol intake was associated with increased LA size (32). In a cohort study of 5,220 people, McManus et al. (33) included LA diameter instead of LA size in their analysis. They found that LA size was not significantly associated with alcohol consumption; however, it accounted for 24% of the incidence of AF associated with alcohol consumption (33).
In the echocardiographic assessment, there are several methods to evaluate the size of LA, including volume and dimension. The use of echocardiography to evaluate the parameters of the left atrium was subjective to a certain extent, but we adopted the method of step-by-step diagnosis by multiple physicians to ensure the accuracy of the assessment. Moreover, our study subjects involved different populations, and the following adjustments were made to reduce the occurrence of bias. The LA posteroanterior dimension represents the LA size, which increases with increasing body size but is influenced by sex (34). Therefore, the LA size should be indexed to body size measurement. LA size indexing is often measured by BSA (35). In our study, we observed that the LA posteroanterior dimension without correction by BSA increased with the level of alcohol consumed. The prevalence of LAE based on the LA posteroanterior dimension corrected by BSA was slightly higher in moderate drinkers than in heavy drinkers. We believe that this increase may be caused by the drinking duration, which can also affect the structure of the left atrium. However, we did not obtain information concerning drinking duration from each participant. Therefore, this hypothesis needs to be verified in a follow-up study. One explanation for the inconsistency of our results and the previous studies may be the different population characteristics and different definitions for LAE. We graded alcohol consumption to verify its association with LAE. However, our study gives the first evidence of the association between atrial size and alcohol consumption based on a general Chinese population. We also demonstrated that not only heavy but also moderate alcohol consumption can affect the atrial size. According to statistics, the prevalence of AF in people aged 35 years or older in China was 0.71%. According to the analysis of this study, alcohol consumption was not associated with a significant direct effect on AF but was associated with AF through the development of LA enlargement. Case reports suggest that LAE may be caused by fibrosis (36), although some studies have shown that alcohol consumption can promote atrial fibrosis (37). Framingham’s study showed that the risk of AF increased by 39% for each 5 mm increase in the anterior and posterior diameter of the atrium. Therefore, it is consistent with our conclusion that LAE is the intermediate effect of alcohol consumption on AF. The results show no direct effect of alcohol consumption on AF, which may be related to the low prevalence of AF in our study population.
The proposed mechanisms for the result may be the inhibition of protein synthesis, inhibition of oxidative phosphorylation, fatty acid ester accumulation, inhibition of calcium-myofilament interaction, disruption of cell membrane structure, and activation of the renin-angiotensin system by alcohol (38–41). However, the exact mechanism underlying the effect of alcohol on LAE requires an in-depth study.
Study limitations
Our study had some limitations. First, the data were not representative of the adult population throughout China, although this study provides the first information on the effect of alcohol consumption on LA size in a general population. Second, the duration of alcohol consumption was not included in the analyses, which might have affected the results. We did not analyze the type of alcohol consumption separately. We also did not get the parameter of LA volume to double confirm the conclusion of this report. Finally, our results were obtained using a cross-sectional design; therefore, no cause-and-effect relationship could be established.
CONCLUSION
In summary, our study results prove that alcohol consumption is associated with LA size in a general population using the indexed LA diameter from echocardiogram. LA size has been confirmed to have a significant effect on the prognosis of adverse CVD and AF. Follow-up in-depth studies are required to confirm the result and explore the related mechanisms.
HIGHLIGHTS
This study is based on a large-scale epidemiological study of 10,211 Chinese participants.
Both heavy and moderate drinkers had increased odds for left atrial enlargement than participants with no alcohol consumption.
Alcohol consumption may cause atrial fibrillation through enlargement of the left atrium.
Footnotes
Ethics approval and consent to participate: The Ethics Committee of the China Medical University (Shenyang, China AF-SDP-07-1, 0-01) approved the study, and all the processes followed ethical standards. All the participants signed informed consents after being informed of the study details.
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Institutional and Financial Support: This work was supported by the National Science and Technology Program during the twelfth five-year plan period (grant number 2013021090) and the National Science and Technology Support Program of China (grant number 2012BAJ18B02).
Author contributions: Concept – L.M.; Design – X.G., Y.B.; Supervision – X.G., Y.B.; Fundings – Y.S.; Materials – Y.S.; Data collection &/or processing – L.M., X.G.; Analysis &/or interpretation – X.G., G.S., Z.L.; Literature search – Y.B., Y.S.; Writing – L.M., Y.S., Z.L.; Critical review – Y.S., Z.L.
REFERENCES
- 1.Jelinek MV, Santamaria JD, Best JD, Thompson DR, Tonkin AM, Vale MJ. Reversing social disadvantage in secondary prevention of coronary heart disease. Int J Cardiol. 2014;171:346–50. doi: 10.1016/j.ijcard.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Lima MC, Kerr-Côrrea F, Rehm J. Alcohol consumption pattern and coronary heart disease risk in metropolitan São Paulo: analyses of GENACIS Project. Rev Bras Epidemiol. 2013;16:49–57. doi: 10.1590/S1415-790X2013000100005. [Article in Portuguese] [DOI] [PubMed] [Google Scholar]
- 3.Almeida OP, McCaul K, Hankey GJ, Yeap BB, Golledge J, Flicker L. Excessive alcohol consumption increases mortality in later life: a genetic analysis of the health in men cohort study. Addict Biol. 2017;22:570–8. doi: 10.1111/adb.12340. [DOI] [PubMed] [Google Scholar]
- 4.Bobak M, Malyutina S, Horvat P, Pajak A, Tamosiunas A, Kubinova R, et al. Alcohol, drinking pattern and all-cause, cardiovascular and alcohol-related mortality in Eastern Europe. Eur J Epidemiol. 2016;31:21–30. doi: 10.1007/s10654-015-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuckit MA. Alcohol-use disorders. Lancet. 2009;373:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- 6.Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–64. doi: 10.1016/j.ahj.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laonigro I, Correale M, Di Biase M, Altomare E. Alcohol abuse and heart failure. Eur J Heart Fail. 2009;11:453–62. doi: 10.1093/eurjhf/hfp037. [DOI] [PubMed] [Google Scholar]
- 10.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81:349–72. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey DG, Spence JD, Edgar B, Bayliff CD, Arnold JM. Ethanol enhances the hemodynamic effects of felodipine. Clin Invest Med. 1989;12:357–62.. [PubMed] [Google Scholar]
- 12.Sun GZ, Guo L, Wang XZ, Song HJ, Li Z, Wang J, et al. Prevalence of atrial fibrillation and its risk factors in rural China: a cross-sectional study. Int J Cardiol. 2015;182:13–7. doi: 10.1016/j.ijcard.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Guo X, Bai Y, Sun G, Guan Y, Sun Y, et al. The Association Between Alcohol Consumption and Left Ventricular Ejection Fraction: An Observational Study on a General Population. Medicine (Baltimore) 2016;95:e3763. doi: 10.1097/MD.0000000000003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rödjer L. Physical activity: Prescription in healthcare and relationship to different health measures. 2015 [Google Scholar]
- 15.Masiukiewicz M, Anweiler S. Two-phase flow phenomena assessment in minichannels for compact heat exchangers using image analysis methods. J Energy Conversion and Management. 2015;104:44–54. doi: 10.1016/j.enconman.2015.03.055. [DOI] [Google Scholar]
- 16.Cuspidi C, Facchetti R, Bombelli M, Sala C, Grassi G, Mancia G. Differential value of left ventricular mass index and wall thickness in predicting cardiovascular prognosis: data from the PAMELA population. Am J Hypertens. 2014;27:1079–86. doi: 10.1093/ajh/hpu019. [DOI] [PubMed] [Google Scholar]
- 17.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Cataliotti A, et al. Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol. 2001;12:2768–74. doi: 10.1681/ASN.V12122768. [DOI] [PubMed] [Google Scholar]
- 18.Su G, Cao H, Xu S, Lu Y, Shuai X, Sun Y, et al. Left atrial enlargement in the early stage of hypertensive heart disease: a common but ignored condition. J Clin Hypertens (Greenwich) 2014;16:192–7. doi: 10.1111/jch.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildman RP, Gu D, Muntner P, Huang G, Chen J, Duan X, et al. Alcohol intake and hypertension subtypes in Chinese men. J Hypertens. 2005;23:737–43. doi: 10.1097/01.hjh.0000163141.82212.5f. [DOI] [PubMed] [Google Scholar]
- 20.Miller WR, Tonigan JS, Longabaugh R, Mattson ME, Marshall LA Project MATCH Monograph Series. The drinker inventory of consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse: Test manual. 1995. National Institutes of Health Publication No. 95–3911. [DOI]
- 21.Bangalore S, Yao SS, Chaudhry FA. Role of left atrial size in risk stratification and prognosis of patients undergoing stress echocardiography. J Am Coll Cardiol. 2007;50:1254–62. doi: 10.1016/j.jacc.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Petersen JL, Roe MT, Mulgund J, Blazing MA, Foody JM, Smith SC, Jr, et al. Abstract 4165: Impact of the National Cholesterol Education Program Third Adult Treatment Panel Lipid-Lowering Recommendations on the Management of Patients With Non-ST-Segment Elevation Acute Coronary Syndromes. Circulation. 2006;114(suppl_18):II_899.. [Google Scholar]
- 23.Copeland KC, Silverstein J, Moore KR, Prazar GE, Raymer T, Shiffman RN, et al. American Academy of Pediatrics. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364–82. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 24.Estruch R, Lamuela-Raventós RM. Wine, alcohol, polyphenols and cardiovascular disease. Nutrition and Aging. 2014;2:101–9. doi: 10.3233/NUA-140039. [DOI] [Google Scholar]
- 25.Acharjee S, Purushottam B, Figueredo VM. Chapter 13 - Toxic Effects of Alcohol on the Heart. Heart and Toxins. 2015:407–36. doi: 10.1016/B978-0-12-416595-3.00013-X. [DOI] [Google Scholar]
- 26.Veselka J, Krejčí J, Tomašov P, Zemánek D. Long-term survival after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a comparison with general population. Eur Heart J. 2014;35:2040–5. doi: 10.1093/eurheartj/eht495. [DOI] [PubMed] [Google Scholar]
- 27.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, et al. Long-term alcohol and caffeine intake and risk of sudden cardiac death in women. Am J Clin Nutr. 2013;97:1356–63. doi: 10.3945/ajcn.112.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bombelli M, Facchetti R, Cuspidi C, Villa P, Dozio D, Brambilla G, et al. Prognostic significance of left atrial enlargement in a general population: results of the PAMELA study. Hypertension. 2014;64:1205–11. doi: 10.1161/HYPERTENSIONAHA.114.03975. [DOI] [PubMed] [Google Scholar]
- 29.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JA, 3rd, Ilkhanoff L, Soliman EZ, Prineas R, Liu K, Ning H, et al. Natural history of the early repolarization pattern in a biracial cohort: CARDIA (Coronary Artery Risk Development in Young Adults) Study. J Am Coll Cardiol. 2013;61:863–9. doi: 10.1016/j.jacc.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh KJ, Cohen BE, Na B, Regan M, Schiller NB, Whooley MA. Alcohol consumption and 5-year change in left atrial volume among patients with coronary heart disease: results from the Heart and Soul study. J Card Fail. 2013;19:183–9. doi: 10.1016/j.cardfail.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Gonçalves A, Jhund PS, Claggett B, Shah AM, Konety S, Butler K, et al. Relationship between alcohol consumption and cardiac structure and function in the elderly: the Atherosclerosis Risk In Communities Study. Circ Cardiovasc Imaging. 2015;8:e002846. doi: 10.1161/CIRCIMAGING.114.002846. doi: 10.1161/CIRCIMAGING.114.002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc. 2016;5:e004060. doi: 10.1161/JAHA.116.004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudy Y, Lindsay BD. Electrocardiographic imaging of heart rhythm disorders: from bench to bedside. Card Electrophysiol Clin. 2015;7:17–35. doi: 10.1016/j.ccep.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS) Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 36.Mehta S, Charbonneau F, Fitchett DH, Marpole DG, Patton R, Sniderman AD. The clinical consequences of a stiff left atrium. Am Heart J. 1991;122:1184–91. doi: 10.1016/0002-8703(91)90498-7. [DOI] [PubMed] [Google Scholar]
- 37.Piano MR, Rosenblum C, Solaro RJ, Schwertz D. Calcium sensitivity and the effect of the calcium sensitizing drug pimobendan in the alcoholic isolated rat atrium. J Cardiovasc Pharmacol. 1999;33:237–42. doi: 10.1097/00005344-199902000-00009. [DOI] [PubMed] [Google Scholar]
- 38.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP) J Biol Chem. 2002;277:29342–7. doi: 10.1074/jbc.M202411200. [DOI] [PubMed] [Google Scholar]
- 39.Tikellis C, Pickering RJ, Tsorotes D, Huet O, Chin-Dusting J, Cooper ME, et al. Activation of the Renin-Angiotensin system mediates the effects of dietary salt intake on atherogenesis in the apolipoprotein E knockout mouse. Hypertension. 2012;60:98–105. doi: 10.1161/HYPERTENSIONAHA.112.191767. [DOI] [PubMed] [Google Scholar]
- 40.Musso G, Gambino R, Cassader M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog Lipid Res. 2013;52:175–91. doi: 10.1016/j.plipres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667–85. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]