Abstract
Fucoidan is a polysaccharide obtained from marine brown algae, with anti-inflammatory, anti-viral, and immune-enhancing properties, thus, fucoidan may be used as an alternative treatment (complementary to prescribed medical therapy) for COVID-19 recovery. This work aimed to determine the ex-vivo effects of treatment with fucoidan (20 µg/mL) on mitochondrial membrane potential (ΔΨm, using a cationic cyanine dye, 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3)) on human peripheral blood mononuclear cells (HPBMC) isolated from healthy control (HC) subjects, COVID-19 patients (C-19), and subjects that recently recovered from COVID-19 (R1, 40 ± 13 days after infection). In addition, ex-vivo treatment with fucoidan (20 and 50 µg/mL) was evaluated on ΔΨm loss induced by carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 150 µM) in HPBMC isolated from healthy subjects (H) and recovered subjects at 11 months post-COVID-19 (R2, 335 ± 20 days after infection). Data indicate that SARS-CoV-2 infection induces HPBMC loss of ΔΨm, even 11 months after infection, however, fucoidan promotes recovery of ΔΨm in PBMCs from COVID-19 recovered subjects. Therefore, fucoidan may be a potential treatment to diminish long-term sequelae from COVID-19, using mitochondria as a therapeutic target for the recovery of cellular homeostasis.
Keywords: fucoidan, SARS-CoV-2, mitochondrial membrane potential, CCCP
1. Introduction
The coasts of the Caribbean Sea experience large upwelling of sargasso, which has become a problem for the recreational use of beaches since it causes obstruction [1]. However, the incidence of sargasso can be an opportunity for the extraction of biologically active metabolites such as fucoidans, which are a kind of sulfated carbohydrate rich in fucose found in brown seaweeds [2]; the macroalgae genus Sargassum has approximately 450 species and is among the largest in tropical zones [3].
Recent researches on fucoidan have brought to light various biological activities, such as anti-cancer, anti-coagulant, anti-oxidant, anti-bacterial, anti-inflammatory, and immunomodulatory [4,5,6,7,8,9,10,11]. In addition, fucoidan has been reported to exhibit anti-viral therapeutic activities [12,13,14], so these compounds have been proposed as potential candidates for alternative treatment in coronavirus 2019 (COVID-19) disease recovery [15], complementary to medically prescribed treatment. In this regard, Song et al. [15] reported that fucoidan (15.6 μg/mL) inhibits (in vitro) SARS-CoV-2 infection in Vero E6 cells through its sulfated polysaccharides, which bind tightly to SARS-CoV-2 protein S, preventing viral internalization.
On the other hand, other authors have reported that fucoidan inhibits phosphorylation of the PI3K-Akt pathway and the subsequent expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [16,17,18,19,20], parameters that are found to be increased in patients with COVID-19. Recent studies performed by our research group demonstrated that leukocytes from women who recently recovered from COVID-19 present loss of mitochondrial membrane potential; however, when these cells were treated ex-vivo with a commercial formulation of Fucoidan (Alquimar®, Cancún, Mexico), the mitochondrial membrane potential of these cells recovered [21].
The present investigation aimed to evaluate the ex-vivo treatment of fucoidan (20 µg/mL) on human peripheral blood mononuclear cells (HPBMC) from healthy control subjects (HC), patients with active COVID-19 (C-19), and subjects recently recovered from COVID-19. Furthermore, in this study, the ex-vivo effect of fucoidan (20 and 50 µg/mL) on carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 150 µM)-induced ΔΨm loss in HPBMC from healthy subjects (H) and subjects recovered at 11 months post-COVID-19 (long COVID-19 patient) was evaluated.
2. Results
2.1. Phase 1
2.1.1. Characteristics of the Study Population
Seventy-six subjects (30 males and 46 females) with an average age of 40 (range = 18–64) years old participated in the study. The healthy control group (HC, n = 24) was composed of 8 men and 16 women with an average age of 40 (range = 23–70) years. Also, of the SARS-CoV-2-infected patients (C-19, n = 31), 13 were males and 18 females with an average age of 38 (range = 18–65) years, and the subjects that had recently recovered from COVID-19 (R1, n = 21) had an average age of 40 (26–64) years, of which 9 were men and 12 women (Table 1).
Table 1.
Characteristics of the study subjects.
| Subject | n | Female | Male | Age | SAR-CoV-2 Detection (qRT-PCR Result) |
|---|---|---|---|---|---|
| Phase 1 | |||||
| HC | 24 | 16 | 8 | 40 (23–70) | − |
| C-19 | 31 | 18 | 13 | 38 (18–65) | + |
| R1 | 21 | 12 | 9 | 40 (26–64) | − |
| Phase 2 | |||||
| H | 19 | 11 | 8 | 41 (21–69) | − |
| R2 | 19 | 11 | 8 | 41 (24–74) | − |
HC: Group of healthy controls subjects; C-19: patients with COVID-19; R1: Subjects recently recovered from COVID-19 (40 ± 13 days after infection); H: healthy subjects; R2: Recovered subjects at 11 months post-COVID-19 (335 ± 20 days after infection).
2.1.2. Ex-Vivo Fucoidan Treatment in HPBMCs of HC, C-19, and R1 Subjects
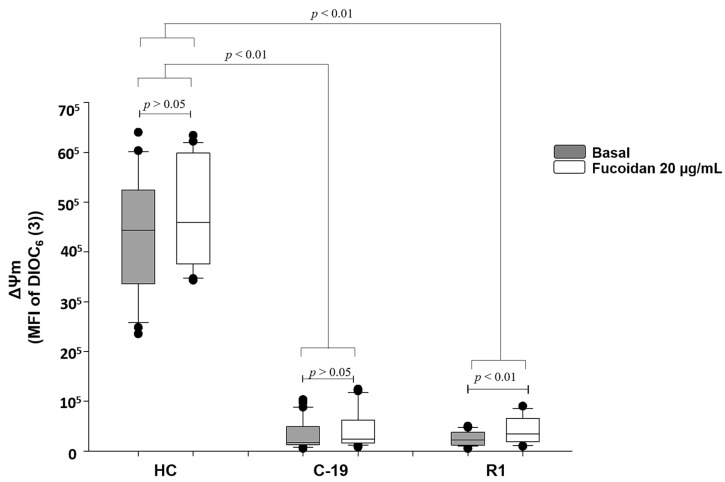
Data indicate that C19 patients and R1 group (patients with active COVID-19 and recently recovered from the infection, respectively), present loss of ΔΨm up to 96% vs. HC group (See gray boxes Figure 1). However, fucoidan treatment (20 µg/mL) induces significant recovery of ΔΨm (70%) in R1 group subjects (basal R1 vs. R1 with fucoidan), but, this parameter still is not restored at HC group level. In C-19 patients, the treatment of fucoidan did not significantly restore ΔΨm (basal C-19 vs. C-19 with fucoidan) (Figure 1).
Figure 1.
Mitochondrial membrane potential (MFI of DIOC6(3)) basal (gray boxes) and with fucoidan treatment ex-vivo (20 µg/mL, white boxes) during 48 h of HPBMC from healthy controls (HC) subjects, patients with COVID-19 (C-19), and subjects recently recovered from COVID-19 (R1) at 40 ± 13 days after infection. Data are reported as medians (horizontal bars) with 25–75% interquartile ranges. p < 0.01 indicates statistically significant difference. Non-parametric Kruskal–Wallis and Dunn’s multiple comparison tests were performed.
2.2. Phase 2
2.2.1. Characteristics of the Study Population
Thirty-eight subjects (16 males and 22 females) with an average age of 41 (range = 21–74) years old participated in the study. The healthy group (H, n = 19) was composed of 8 men and 11 women with an average age of 41 (range = 21–69) years. The recovered subjects at 11 months post-COVID-19 (R2, n = 19) had an average age of 41 (range = 24–74) years, of which 8 were men and 11 women (Table 1).
2.2.2. Ex-Vivo Induction of ΔΨm Loss in HPBMC
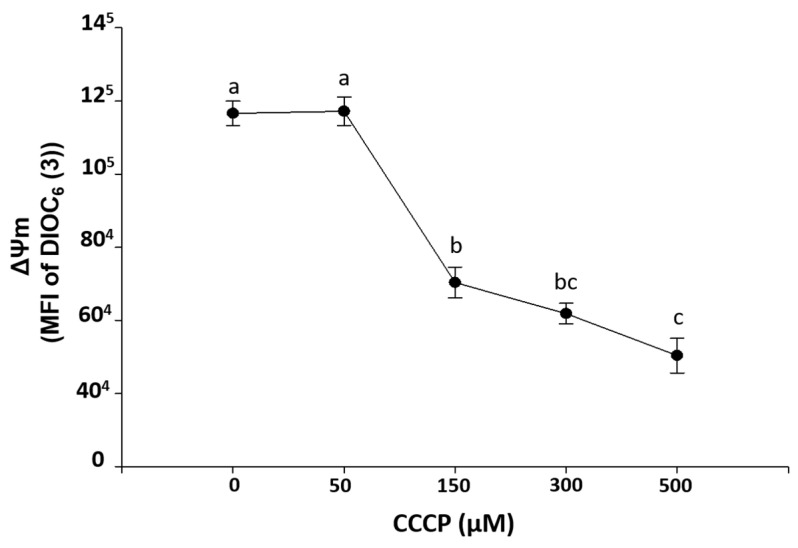
Figure 2 shows an ex-vivo exposure curve to CCCP on HPBMC, indicating that concentrations of 150, 300, and 500 µM significantly (p < 0.001) induced loss of ΔΨm with respect to control HPBMC (without CCCP). This is in contrast to the 50 µM concentration, which did not cause loss of ΔΨm.
Figure 2.
Induction of ΔΨm loss mitochondrial membrane potential (MFI of DIOC6(3)) by carbonyl cyanide 3-chlorophenylhydrazone exposure (CCCP, 0, 50, 150, 300, and 500 µM) during 15 min on HPBMC from healthy subjects (H). Graphs represent the mean ± SEM of the data from three SARS-CoV-2 negative subjects by duplicate. Different letters indicate statistically significant differences of p < 0.01. For normal data distribution, an analysis of variance (ANOVA) was applied followed by a Bonferroni subtest.
2.2.3. Ex-Vivo Treatment with Fucoidan on HPBMCs with CCCP-Induced Loss of ΔΨm from H and R2 Group Subjects
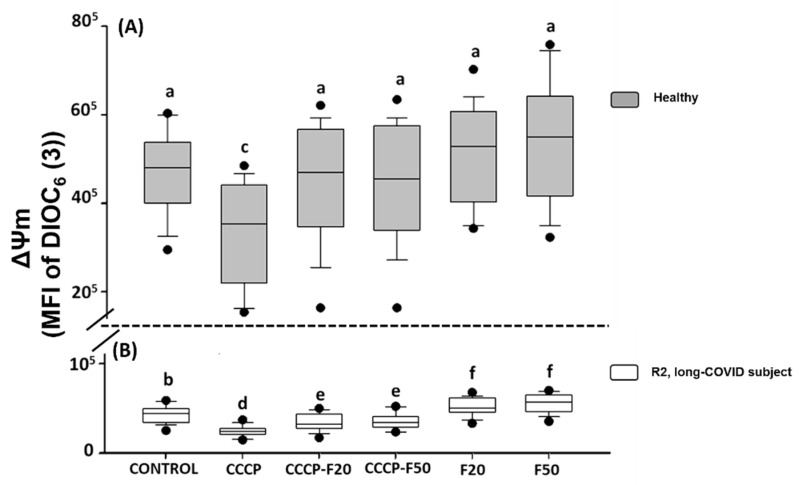
The results indicate that CCCP (150 µM) significantly induces loss of the ΔΨm in HPBMCs of H (30%) and R2 subject (44%) (Figure 3A,B). However, treatment with fucoidan induces restoration of the ΔΨm in HPBMCs in the different experimental groups of H subject (CCCP + F20 (22%), CCCP + F50 (23%), F20 (37%) and F50 (40%)) (Figure 3A) and R2 subjects (CCCP + F20 (24%), CCCP + F50 (25%), F20 (62%) and F50 (70%)) (Figure 3B).
Figure 3.
Mitochondrial membrane potential (MFI of DIOC6(3)) of HPBMC isolated from (A) healthy subjects (gray boxes) and (B) recovered subjects (white boxes) at 11 months post-COVID-19 (R2, long-COVID), under the following conditions: Control: without CCCP and fucoidan exposure; CCCP: exposed to 150 µM of CCCP; CCCP-F20: exposed to 150 µM of CCCP + fucoidan 20 µg/mL; CCCP-F50: exposed to 150 µM of CCCP + fucoidan 50 µg/mL; F20: fucoidan 20 µg/mL and F50: fucoidan 50 µg/mL. Data are reported as medians (horizontal bars) with 25–75% interquartile ranges. Different letters indicate statistically significant differences of p < 0.01. Non-parametric Kruskal–Wallis and Dunn’s multiple comparison tests were performed.
On the other hand, Figure 3 shows that the ΔΨm is significantly decreased in R2 with respect to healthy subjects, however, when these cells were treated with fucoidan 20 and 50 µg/mL, HPBMCs significantly improved this parameter with respect to R2 control, however, the ΔΨm was not restored with respect to the healthy control group.
3. Discussion
COVID-19 induces an elevated inflammatory/oxidative state in organisms, leading to mitochondrial dysfunction and subsequent cell death [22,23,24,25,26]. In the case of patients recovering from acute SARS-CoV-2 infection, they may develop long-term sequelae (pulmonary injury, inflammatory, neurodegenerative, etc.) caused by mitochondrial dysfunction [27,28,29,30,31,32,33,34]. Therefore, the search for alternative treatments is essential to reintegrate cellular homeostasis in SARS-CoV-2 infected subjects.
Obtained data demonstrate that HPBMC of patients with active SARS-CoV-2 infection (C-19) and subjects recently recovered from COVID-19 (R1) show loss of ΔΨm. This loss of mitochondrial membrane potential was also detected in subjects recovered 11 months after infection (R2 group) to SARS-CoV-2, which may be related to long-term sequelae (long-COVID), a phenomenon that may be a predisposing factor for chronic degenerative diseases, such as cardiovascular diseases and other inflammatory conditions [35,36,37,38]. In this respect, it has been reported that mitochondrial dysfunction has been implicated in the pathophysiology of several diseases, such as diabetes, cardiovascular diseases, and gastrointestinal disorders [35,38,39,40]. In addition, mitochondrial dysfunction is directly linked to neuromuscular disorders and increasing evidence has linked mitochondrial dysfunction to neurodegenerative and neurodevelopmental disorders such as Alzheimer’s Disease, Parkinson’s Disease, Rett Syndrome, and Autism Spectrum Disorders [41,42].
Evidence suggests that SARS-CoV-2 infection induces mitochondrial dysfunction [23,43,44]. Gibellini et al. [44] demonstrated that monocytes from SARS-CoV-2-infected patients show signs of bioenergetic alteration and mitochondrial dysfunction (reduced basal and maximal respiration, reduced reserve respiratory capacity, and decreased proton leakage). In addition, a significantly high number of monocytes have depolarized mitochondria and abnormal mitochondrial ultrastructure. Also, Ajaz et al. [43] demonstrated that SARS-CoV-2 infection causes mitochondrial dysfunction through metabolic alterations with increased glycolysis and elevated mitokine levels in HPBMC of infected patients. Other studies report that SARS-CoV-2 induces oxidative stress [23,45,46,47], which leads to mitochondrial dysfunction. Therefore, as mitochondria are the center of cellular oxidative homeostasis, research should be focused on new therapeutic strategies on mitochondrial metabolic pathways and redox balance, which could be useful to restore mitochondrial function [26,48] and thus achieve cellular homeostasis in SARS-CoV-2-infected patients.
Since the onset of the COVID-19 pandemic, there has been a growing interest in applying safe and effective natural approaches that support immune system function and, in addition, have direct anti-viral effects [49]. In this regard, several polysaccharides have been shown to exhibit potential biological activities, such as fucoidan, which is a sulfated polysaccharide that exhibits anti-viral, anti-oxidant, and immunomodulatory activities [50,51]. Furthermore, fucoidans have been reported to present potential applications in the antiviral activity of many enveloped viruses such as herpes simplex virus type 1 (HSV-1) [49], human immunodeficiency virus (HIV) [52], influenza A virus [53], and different kind of paramyxoviruses such as Newcastle disease virus (NDV) and canine distemper virus (CDV) [54,55]. In addition, it has been reported that in vitro and in vivo activity of fucoidan exerts several biological functions against DNA and RNA viruses including dengue virus (DENV), HIV, human cytomegalovirus (HCMV), measles virus, HSV-1, and HSV-2 [56]. Similarly, fucoidan has been reported to inhibit SARS-CoV-2 infection in Vero E6 cells through its sulfated polysaccharides (in vitro), which tightly bind to SARS-CoV-2 S protein, thus preventing viral internalization [15].
Moreover, the immunomodulatory and antioxidant properties of fucoidans have also been reported [57,58,59,60,61,62,63,64,65,66]. Fitton et al. [67] proposed fucoidan as a potential supplementary agent to reduce the harm after viral infections caused by SARS-CoV-2, as this compound restores innate immune function and inhibits inflammation. In this regard, Phase 1 results indicate that treatment with 20 µg/mL fucoidan restores the ΔΨm of recovered subjects at 40 ± 13 days after infection to COVID-19 (basal R1 vs. R1 with fucoidan), likewise, Phase 2 results indicate that treatment with 20 and 50 µg/mL fucoidan restored the ΔΨm of recovered subjects 11 months post-COVID-19 (basal R2 vs. R2 with fucoidan); therefore, the results of the present investigation suggest that Alquimar® fucoidan could be an alternative treatment to improve the mitochondrial function of subjects recovered from COVID-19, which could prevent long-term sequelae.
In phase 2, the ex-vivo treatment of fucoidan on CCCP-induced ΔΨm loss in HPBMC was also evaluated; results indicate that fucoidan (20 and 50 µg/mL) significantly restores the ΔΨm on damaged cells. This suggests that fucoidan treatment may be useful in improving cellular homeostasis in SARS-CoV-2-infected individuals. These results are in agreement with a previous study conducted by this research group, showing that fucoidan (20 µg/mL) significantly improved the ΔΨm of women recently recovered from COVID-19. Also, Han et al. [68] reported that fucoidan inhibits MPP+ (1-methyl-4-phenyl-pyridinium, a neurotoxin used to model Parkinson’s disease)-induced generation of ROS, dysfunction of mitochondrial oxidative phosphorylation, and cellular apoptosis in SH-SY5Y cells through regulation of AMPK-PGC-1α. Previous studies showed that the consequences of AMPK activation include the acute modulation of metabolism as a result of phosphorylation of downstream metabolic enzymes and chronic changes in gene expression and mitochondrial biogenesis [69,70]. Effects of AMPK on mitochondrial genes and PGC-1α are almost entirely dependent on the function of PGC-1α protein, and it is effective in promoting mitochondrial biogenesis in various somatic cells [70,71].
Obtained data showed that fucoidan may have an immunomodulatory effect and antioxidant activity on PBMCs of COVID-19-recovered subjects, due to the treatment with a fucoidan formulation, which restored the ∆Ψm, suggesting that fucoidan allows the recovery of cellular homeostasis [72,73]. However, more studies are required to determine the immunomodulatory and antioxidant role of fucoidan in COVID-19.
4. Materials and Methods
4.1. Samples and Data Collection
All patients signed an informed consent form before biological sample collection. Each individual filled a questionnaire to determine patient characteristics, clinical history, and evolution of COVID-19 recovery.
This study was carried out following the guidelines stated in the Declaration of Helsinki and was approved by the local bioethics commission (Bioethics Commission of Nayarit State, Mexico—Registry number CEBN/01/21).
4.2. Phase 1
4.2.1. Study Design and Participants
For the first phase of the study, patients who came to the LANIIA-Nayarit laboratory (laboratory accredited by the Mexican Ministry of Health) to request a molecular test for SARS-CoV-2 detection by real-time qRT-PCR (swab sampling) were invited to donate blood (10 mL) and freely and voluntarily participate in this study. Likewise, adults who had not presented COVID-19 disease were invited to participate freely and voluntarily.
After conducting a SARS-CoV-2 screening, patients were classified into three groups: (1) Group of healthy control (HC) subjects: negative for SARS-CoV-2 and any symptoms or signs related to COVID-19 were declared (n = 24); (2) group of patients with COVID-19 (C-19): subjects with SARS-CoV-2 active infection (qRT-PCR positive) (n = 31); and (3) group of subjects recently recovered from COVID-19 (R1): individuals who were diagnosed SARS-CoV-2 positive in our laboratory facilities, came back after several weeks of recovery (40 ± 13 days after infection) and resulted as negative to SARS-CoV-2 molecular tests (n = 21). All participants were adults (18 to 70 years).
4.2.2. HPBMCs Isolation
HPBMCs were obtained from 10 mL of blood collected in an EDTA tube. Immediately after sample collection, the blood was diluted 1:1 with PBS (7.2 pH), then the HPBMCs were separated by density gradient using Histopaque-1077 (Sigma, Louis, MO, USA) in a 2:1 ratio (blood-to-histopaque), which was centrifuged for 25 min at 1700 rpm. Subsequently, the HPBMCs were precipitated at 7 min at 2000 rpm. Once the cell button was obtained, it was resuspended in RPMI medium supplemented with 10% of fetal bovine serum (Gibco™, New York, NY, USA) and 1% of penicillin/streptomycin (Sigma, Louis, MO, USA). Cell counting was performed on the BD Accuri C6TM (BD Biosciences, San Jose, CA, USA) flow cytometer, identifying the cell population of interest by forward (FSC) vs. side (SSC) scatter.
Subsequently, cell cultures were performed on 24-well sterile plates, in brief, 1 × 106 cells were resuspended in 1 mL of RPMI medium (Sigma, Louis, MO, USA) supplemented with fetal bovine serum (10%) and penicillin/streptomycin (1%). Cells were incubated at 37 °C and 5% CO2 for 24 h.
4.2.3. Ex-Vivo Fucoidan Treatment in HPBMCs from HC, C-19, and R1 Group Subjects
Fucoidan (Alquimar®), a dietary supplement extracted from Sargassum, was used for this study. Chemical analysis of the formulation Alquimar® (250.61 kDa) used in current research contains sulfates (145.40 ± 6.85 mg/g fucoidan), phenols (51.09 ± 0.71 mg gallic acid equivalents/g fucoidan), and total sugars (663.89 ± 26.19 mg glucose equivalents/g fucoidan). Regarding the quantification of monomers, it consists of glucose (4.85 ± 0.47 mg/100 mg fucoidan), fucose (3.16 ± 0.54 mg/100 mg fucoidan), galactose (10.95 ± 0.30 (mg/100 mg fucoidan), and mannitol (10.45 ± 0.71 mg/100 mg fucoidan). Concentrations were determined by HPLC (Agilent 87H column).
For the preparation of a stock solution (10,000 µg/mL), one capsule of fucoidan (Alquimar®) was resuspended in 5 mL of PBS (pH 7.2) and shaken until a homogeneous mixture was obtained. Afterward, the mixture was diluted at 1:10.
For the first phase of the study, treatments of 20 µg/mL fucoidan were used for each experimental group. Briefly, 2 µL of a stock solution of fucoidan (10,000 µg/mL) were added to each well containing 1 × 106 cells/mL, then the HPBMCs were incubated for a period of 48 h at 37 °C and 5% CO2. HPBMCs from HC, C-19, and R1 subjects were maintained under the same conditions, but without fucoidan treatment.
Before fucoidan treatment, cell viability was assessed by propidium iodide (PI; BD Pharmingen, Franklin Lakes, NJ, USA) staining by flow cytometry. Fucoidan treatment was added on cultured HPBMCs with viability greater than 90%.
4.2.4. Mitochondrial Membrane Potential Determination (ΔΨm)
The ΔΨm determination was performed using a cationic cyanine dye, 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3)) (Invitrogen™, Burlington, ON, Canada) [74]. 1 × 106 HPBMC/mL from each experimental group were centrifuged at 2000 rpm for 7 min, subsequently to the cell button, 500 μL of PBS and DiOC6(3) 20 nM was added. The samples were incubated at room temperature for 15 min. Finally, each sample was analyzed in the flow cytometer (10,000 events) with the 488 nm excitation laser.
4.3. Phase 2
4.3.1. Study Design and Participants
Healthy adults (H) who had not presented COVID-19 disease were freely and voluntarily invited to participate in this investigation. Likewise, according to the LANIIA-Nayarit laboratory database, patients who tested positive for a SARS-CoV-2 molecular test in October–November 2020 (recovered subjects at 11 months post-COVID-19, R2 group) were freely and voluntarily invited to participate in this study. Individuals came to the LANIIA-Nayarit laboratory facilities to donate 10 mL of blood to determine mitochondrial membrane potential. HPBMC were obtained by density gradient as mentioned above, then cell cultures were performed and the HPBMC were incubated at 37 °C and 5% CO2 for 24 h.
After the incubation period, cell viability was assessed by propidium iodide (PI) staining by flow cytometry. Samples with viability >90% were exposed to carbonyl cyanide 3-chlorophenylhydrazone (CCCP; Sigma, Louis, MO, USA), a classic mitochondrial membrane uncoupler.
4.3.2. Induction of ΔΨm Loss Ex-Vivo in HPBMC
To define the concentration of CCCP to be used, a curve was performed with different concentrations (0, 50, 150, 300, and 500 μM) of said compound. Subsequently, 1 × 106 cells/mL were exposed to 150 μM of CCCP for 15 min (37 °C and 5% CO2); the cells were harvested and washed with PBS at 2000 rpm for 7 min. Finally, the cells were seeded in a 24-well culture plate for subsequent treatment with fucoidan.
4.3.3. Ex-Vivo Treatment with Fucoidan in HPBMCs with Loss of ΔΨm Induced by CCCP of H Subjects and R2 Group
For phase 2 of this study, two experimental groups were used, each with six specific treatments: (1) Group of healthy (H) subjects: negative for SARS-CoV-2 and any symptoms or signs related to COVID-19 were declared (n = 19), and (2) group of recovered subjects at 11 months post-COVID-19 (R2): individuals who were diagnosed as SARS-CoV-2 positive in our laboratory facilities, came back after 335 ± 20 days after infection (n = 19).
To evaluate ex-vivo fucoidan treatment on ΔΨm recovery, 20 and 50 µg/mL concentrations of fucoidan were used; the six treatments were as follows: (1) Control: HPBMC with no CCCP exposure or fucoidan treatment; (2) CCCP: HPBMC exposed to 150 µM CCCP for 15 min; (3) CCCP-F20: HPBMC exposed to CCCP (150 µM) for 15 min and treated with 20 µg/mL fucoidan; (4) CCCP-F50: HPBMC exposed to CCCP (150 µM) for 15 min and treated with 50 µg/mL fucoidan; (5) F20: HPBMC treated with 20 µg/mL fucoidan; and (6) F50: HPBMC treated with 50 µg/mL fucoidan. All samples were incubated for 48 h at 37 °C and 5% CO2. After incubating, the ΔΨm was determined in HPBMC as mentioned above using DiOC6(3) dye.
4.3.4. Statistical Analysis
Data were processed with FlowJo v10 Software, while statistical analyses were completed using Prism 6® software (GraphPad Software Inc. San Diego, CA, USA). The normality and homogeneity of the data variances were determined with the Kolmogorov–Smirnov test and the Levene F test. For normal data distribution, analysis of variance (ANOVA) followed by a Bonferroni subtest were used. To compare the differences between three or more non-parametric data groups, a Kruskal–Wallis test and Dunn’s Test for Multiple Comparisons were used. The statistical difference was determined with a level of p < 0.05.
5. Conclusions
Fucoidan significantly restores the ΔΨm of HPBMC, suggesting that fucoidan treatment can be useful to improve mitochondrial homeostasis after SARS-CoV-2 infection. Thus, fucoidan may constitute a potential treatment to prevent long-term sequelae of COVID-19, with mitochondria being a therapeutic target for the recovery of cellular homeostasis.
Author Contributions
K.J.G.D.-R.: Design of the study, development of the clinical survey, coordination of sample processing, data analysis, data interpretation, and writing of the manuscript; C.E.C.-R.: Writing—review and editing; A.B.B.-T.: Investigation and data analysis; M.S.N.-M.: Application of clinical survey and sample processing; F.F.R.-C.: Application of clinical survey and sample processing; C.D.C.-C.: Sample processing; E.J.F.-D.: Sample processing; D.A.P.-D.: Collection of sample, development, and application de clinical survey, data analysis; M.V.D.-B.: Collection of sample, development, and application of clinical survey and data analysis and sample processing; M.Z.-S.: Sample processing and investigation; G.H.V.-R.: Collection of sample and investigation; A.R.-C.: Comments on the final version of the paper; D.A.-E.: Comments on the final version of the paper; M.I.G.-P. Conceptualization and comments on the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the “Consejo Nacional de Ciencia y Tecnología (CONACYT)” with project number 313590 “Characterisation and validation of the immunomodulatory properties of a Mexican formulation of fucoidan as a potential alternative treatment for COVID-19”.
Institutional Review Board Statement
This study was carried out following the guidelines stated in the Declaration of Helsinki and was approved by the local bioethics commission (Bioethics Commission of Nayarit State, Mexico—Registry number CEBN/01/21).
Informed Consent Statement
Informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
All data supporting the findings of this study are available with its corresponding author, M.I.G.-P., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ortegón-Aznar I., Ávila-Mosqueda S.V. Arribazón de sargazo en la península de Yucatán: ¿Problema local, regional o mundial? Bioagrociencias. 2020;13:28–37. [Google Scholar]
- 2.Berteau O., Mulloy B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 3.Nava Jiménez I.A., Sánchez Hernández H. El sargazo del mar Caribe mexicano. Ciencia. 2020;71:58–61. [Google Scholar]
- 4.Rupérez P., Ahrazem O., Leal J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002;50:840–845. doi: 10.1021/jf010908o. [DOI] [PubMed] [Google Scholar]
- 5.Chen H.Y., Huang T.C., Lin L.C., Shieh T.M., Wu C.H., Wang K.L., Hong Y.H., Hsia S.M. Fucoidan inhibits the proliferation of leiomyoma cells and decreases extracellular matrix-associated protein expression. Cell. Physiol. Biochem. 2018;45:1970–1986. doi: 10.1159/000493660. [DOI] [PubMed] [Google Scholar]
- 6.Vishchuk O.S., Sun H., Wang Z., Ermakova S.P., Xiao J.J., Lu T., Xue P.P., Zvyagintseva T.N., Xiong H., Shao C., et al. PDZ-binding kinase/T-LAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget. 2016;7:18763–18773. doi: 10.18632/oncotarget.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan M.D., Yao C.J., Chow J.M., Chang C.L., Hwang P.A., Chuang S.E., Whang-Peng J., Lai G.M. Fucoidan elevates MicroRNA-29b to regulate DNMT3B-MTSS1 axis and inhibit EMT in human hepatocellular carcinoma cells. Mar. Drugs. 2015;13:6099–6116. doi: 10.3390/md13106099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang P.A., Hung Y.L., Chien S.Y. Inhibitory activity of Sargassum hemiphyllum sulfated polysaccharide in arachidonic acid-induced animal models of inflammation. J. Food Drug Anal. 2015;23:49–56. doi: 10.1016/j.jfda.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho T.M., Kim W.J., Moon S.K. AKT signaling is involved in fucoidan-induced inhibition of growth and migration of human bladder cancer cells. Food Chem. Toxicol. 2014;64:344–352. doi: 10.1016/j.fct.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Cho M., Lee D.J., Kim J.K., You S. Molecular characterization and immunomodulatory activity of sulfated fucans from Agarum cribrosum. Carbohydr. Polym. 2014;113:507–514. doi: 10.1016/j.carbpol.2014.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Cumashi A., Ushakova N.A., Preobrazhenskaya M.E., D’Incecco A., Piccoli A., Totani L., Tinari N., Morozevich G.E., Berman A.E., Bilan M.I., et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 12.Pereira L., Critchley A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020;32:1875–1877. doi: 10.1007/s10811-020-02143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sansone C., Brunet C., Noonan D.M., Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants. 2020;9:392. doi: 10.3390/antiox9050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Q., Wang A., Lu Z., Qin C., Hu J., Yin J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohyd. Res. 2017;453:1–9. doi: 10.1016/j.carres.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Song S., Peng H., Wang Q., Liu Z., Dong X., Wen C., Ai C., Zhang Y., Wang Z., Zhu B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020;11:7415–7420. doi: 10.1039/D0FO02017F. [DOI] [PubMed] [Google Scholar]
- 16.Boo H.J., Hong J.Y., Kim S.C., Kang J.I., Kim M.K., Kim E.J., Hyun J.W., Koh S., Yoo E.S., Kwon J.M., et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs. 2013;11:2982–2999. doi: 10.3390/md11082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei C.M., Xiao Q., Kuang X.Y., Zhang T., Yang Z.S., Wang L. Fucoidan inhibits proliferation of the SKM-1 acute myeloid leukaemia cell line via the activation of apoptotic pathways and production of reactive oxygen species. Mol. Med. Rep. 2015;12:6649–6655. doi: 10.3892/mmr.2015.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J.Q., Riby J.E., Conde L., Grizzle W.E., Cui X.Q., Skibola C.F. A Fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines. BMC Complement. Altern. Med. 2016;16:151. doi: 10.1186/s12906-016-1129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Weelden G., Bobiński M., Okła K., Van Weelden W.J., Romano A., Pijnenborg J. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar Drugs. 2019;17:32. doi: 10.3390/md17010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae H., Lee J.Y., Yang C., Song G., Lim W. Fucoidan derived from Fucus vesiculosus inhibits the development of human ovarian cancer via the disturbance of calcium homeostasis, endoplasmic reticulum stress, and angiogenesis. Mar. Drugs. 2020;18:45. doi: 10.3390/md18010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Díaz-Resendiz K.J.G., Toledo-Ibarra G.A., Ruiz-Manzano R., Girón Pérez D.A., Covantes-Rosales C.E., Benitez-Trinidad A.B., Girón-Pérez M.I. Ex vivo treatment with fucoidan of mononuclear cells from SARS-CoV-2 infected patients. Int. J. Environ. Health Res. 2021;31:1–19. doi: 10.1080/09603123.2021.1982875. [DOI] [PubMed] [Google Scholar]
- 22.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 23.Saleh J., Peyssonnaux C., Singh K.K., Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. doi: 10.1016/j.mito.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phua J., Weng L., Ling L., Egi M., Lim C.M., Divatia J.V., Shrestha B.R., Arabi Y.M., Ng J., Gomersall C.D., et al. Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alarcón-Rodríguez J., Fernández-Velilla M., Ureña-Vacas A., Martín-Pinacho J.J., Rigual-Bobillo J.A., Jaureguízar-Oriol A., Gorospe-Sarasúa L. Manejo y seguimiento radiológico del paciente post-COVID-19. Radiología. 2021;63:258–269. doi: 10.1016/j.rx.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernando J.E.C. Seguimiento de los pacientes con secuelas no respiratorias de la COVID-19. FMC Form. Med. Contin. Aten. Primaria. 2021;28:81–89. doi: 10.1016/j.fmc.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26:e928996-1. doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buonsenso D., Di Giuda D., Sigfrid L., Pizzuto D.A., Di Sante G., De Rose C., Lazzareschi I., Sali M., Baldi F., Pia D., et al. Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc. Health. 2021;5:677–680. doi: 10.1016/S2352-4642(21)00196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., Okell T., Sheerin F., Xie C., Mahmod M., et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato K., Sinclair J.E., Sadeghirad H., Fraser J.F., Short K.R., Kulasinghe A. Cardiovascular disease in SARS-CoV-2 infection. Clin. Transl. Immunol. 2021;10:e1343. doi: 10.1002/cti2.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciolac D., Racila R., Duarte C., Vasilieva M., Manea D., Gorincioi N., Condrea A., Crivorucica I., Zota E., Efremova D., et al. Clinical and radiological deterioration in a case of Creutzfeldt–Jakob disease following SARS-CoV-2 infection: Hints to Accelerated Age-Dependent Neurodegeneration. Biomedicines. 2021;9:1730. doi: 10.3390/biomedicines9111730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idrees D., Kumar V. SARS-CoV-2 spike protein interactions with amyloidogenic proteins: Potential clues to neurodegeneration. Biochem. Biophys. Res. Commun. 2021;554:94–98. doi: 10.1016/j.bbrc.2021.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh K.K., Chaubey G., Chen J.Y., Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol. Cell Physiol. 2020;319:C258–C267. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melser S., Lavie J., Bénard G. Mitochondrial degradation and energy metabolism. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015;1853:2812–2821. doi: 10.1016/j.bbamcr.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Blake R., Trounce I.A. Mitochondrial dysfunction and complications associated with diabetes. Biochim. Biophys. Acta Gen. Subj. 2014;1840:1404–1412. doi: 10.1016/j.bbagen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Mohlke K.L., Jackson A.U., Scott L.J., Peck E.C., Suh Y.D., Chines P.S., Watanabe R.M., Buchanan T.A., Conneely K.N., Erdos M.R., et al. Mitochondrial polymorphisms and susceptibility to type 2 diabetes related traits in Finns. Hum. Genet. 2005;118:245–254. doi: 10.1007/s00439-005-0046-4. [DOI] [PubMed] [Google Scholar]
- 39.Shi T.T., Yang F.Y., Liu C., Cao X., Lu J., Zhang X.L., Yuan M.X., Chen C., Yang J.K. Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic β-cells. Biochem. Biophys. Res. Commun. 2018;495:860–866. doi: 10.1016/j.bbrc.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 40.Valero T. Editorial (thematic issue: Mitochondrial biogenesis: Pharmacological approaches) Curr. Pharm. Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 41.Nicoletti V., Palermo G., Del Prete E., Mancuso M., Ceravolo R. Understanding the Multiple role of mitochondria in Parkinson’s disease and related disorders: Lesson from genetics and protein–interaction network. Front. Cell Dev. Biol. 2021;9:493. doi: 10.3389/fcell.2021.636506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castora F.J. Mitochondrial Function and Abnormalities Implicated in the Pathogenesis of ASD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;92:83–108. doi: 10.1016/j.pnpbp.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Ajaz S., McPhail M.J., Singh K.K., Mujib S., Trovato F.M., Napoli S., Agarwal K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am. J. Physiol. Cell Physiol. 2021;320:C57–C65. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibellini L., De Biasi S., Paolini A., Borella R., Boraldi F., Mattioli M., Tartaro D., Fidanza L., Caro-Maldonado A., Meschiari M., et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020;12:e13001. doi: 10.15252/emmm.202013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baqi H.R., Farag H.A.M., El Bilbeisi A.H.H., Askandar R.H., El Afifi A.M. Oxidative stress and its association with COVID-19: A narrative review. KJAR. 2020;5:97–105. doi: 10.24017/covid.11. [DOI] [Google Scholar]
- 46.Chernyak B.V., Popova E.N., Prikhodko A.S., Grebenchikov O.A., Zinovkina L.A., Zinovkin R.A. COVID-19 and oxidative stress. Biochemistry. 2020;85:1543–1553. doi: 10.1134/S0006297920120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De las Heras N., Martín Giménez V.M., Ferder L., Manucha W., Lahera V. Implications of oxidative stress and potential role of mitochondrial dysfunction in COVID-19: Therapeutic effects of vitamin D. Antioxidants. 2020;9:897. doi: 10.3390/antiox9090897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., Van Goor H., et al. The reactive species interactome: Evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxid. Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed S.F., Agili F.A. Antiviral sulphated polysaccharide from brown algae Padina pavonia characterization and structure elucidation. Int. J. Chemtech Res. 2013;5:1469–1476. [Google Scholar]
- 50.Niedzwiecki A., Rath M. Scientific basis of micronutrient applications as an effective, safe and affordable global public health strategy to help to control the coronavirus pandemic. J. Cell. Med. Nat. Health. 2021;15:1–5. [Google Scholar]
- 51.Sen I.K., Chakraborty I., Mandal A.K., Bhanja S.K., Patra S., Maity P. A review on antiviral and immunomodulatory polysaccharides from Indian medicinal plants, which may be beneficial to COVID-19 infected patients. Int. J. Biol. 2021;181:462–470. doi: 10.1016/j.ijbiomac.2021.03.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thuy T.T.T., Ly B.M., Van T.T.T., Quang N.V., Tu H.C., Zheng Y., Seguin-Devaux C., Mi B., Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 53.Kim M., Yim J.H., Kim S.Y., Kim H.S., Lee W.G., Kim S.J., Kang P.S., Lee C.K. In vitro inhibition of influenza, a virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012;93:253–259. doi: 10.1016/j.antiviral.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Elizondo-Gonzalez R., Cruz-Suarez L.E., Ricque-Marie D., Mendoza-Gamboa E., Rodriguez-Padilla C., Trejo-Avila L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle disease virus. Virol. J. 2012;9:307. doi: 10.1186/1743-422X-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trejo-Avila L.M., Morales-Martínez M.E., Ricque-Marie D., Cruz-Suarez L.D., Zapata-Benavides P., Morán-Santibañez K., Rodríguez-Padilla C. In vitro anti-canine distemper virus activity of fucoidan extracted from the brown alga Cladosiphon okamuranus. Virus Dis. 2014;25:474–480. doi: 10.1007/s13337-014-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moran-Santibanez K., Cruz-Suarez L.E., Ricque-Marie D., Robledo D., Freile-Pelegrin Y., Pena-Hernandez M.A., Rodriguez-Padilla C., Trejo-Avila L.M. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. BioMed Res. Int. 2016;2016:8502123. doi: 10.1155/2016/8502123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colona-Vallejos E.H., Alzamora-Gonzales L., Chávez Pérez J., Apumayta Suárez E.V., Chang Avila I. Incremento de la viabilidad, producción de especies reactivas de oxígeno, IL-1 y TNF-α en células mononucleares de sangre periférica humana trata-das con fucoidan de Lessonia trabeculata. Rev. Peru. Biol. 2019;26:291–300. doi: 10.15381/rpb.v26i3.16772. [DOI] [Google Scholar]
- 58.Fitton J.H., Stringer D.N., Karpiniec S.S. Therapies from fucoidan: An update. Mar. Drugs. 2015;13:5920–5946. doi: 10.3390/md13095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitton J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs. 2011;9:1731–1760. doi: 10.3390/md9101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiao G., Yu G., Zhang J., Ewart H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng Y., Song Y., Wang Q., Hu Y., He Y., Ren D., Wu L., Liu S., Cong H., Zhou H. In vitro and in vivo immunomodulatory effects of fucoidan compound agents. Int. J. Biol. Macromol. 2019;127:48–56. doi: 10.1016/j.ijbiomac.2018.12.197. [DOI] [PubMed] [Google Scholar]
- 62.Apostolova E., Lukova P., Baldzhieva A., Katsarov P., Nikolova M., Iliev I., Peychev L., Trica B., Oancea F., Delattre C., et al. Immunomodulatory and anti-inflammatory effects of fucoidan: A review. Polymers. 2020;12:2338. doi: 10.3390/polym12102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borazjani N.J., Tabarsa M., You S., Rezaei M. Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. J. Food Sci. Technol. 2017;54:4016–4025. doi: 10.1007/s13197-017-2867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marudhupandi T., Kumar T.T., Senthil S.L., Devi K.N. In vitro antioxidant properties of fucoidan fractions from Sargassum tenerrimum. Pak. J. Biol. Sci. 2014;17:402–407. doi: 10.3923/pjbs.2014.402.407. [DOI] [PubMed] [Google Scholar]
- 65.Li Q., Jiang S., Shi W., Qi X., Song W., Mou J., Yang J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020;231:115767. doi: 10.1016/j.carbpol.2019.115767. [DOI] [PubMed] [Google Scholar]
- 66.Ashayerizadeh O., Dastar B., Pourashouri P. Study of antioxidant and antibacterial activities of depolymerized fucoidans extracted from Sargassum tenerrimum. Int. J. Biol. Macromol. 2020;151:1259–1266. doi: 10.1016/j.ijbiomac.2019.10.172. [DOI] [PubMed] [Google Scholar]
- 67.Fitton J.H., Park A.Y., Karpiniec S.S., Stringer D.N. Fucoidan and lung function: Value in viral infection. Mar. Drugs. 2021;19:4. doi: 10.3390/md19010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han Y.S., Lee J.H., Lee S.H. Fucoidan suppresses mitochondrial dysfunction and cell death against 1-methyl-4-phenylpyridinum-induced neuronal cytotoxicity via regulation of PGC-1α expression. Mar. Drugs. 2019;17:518. doi: 10.3390/md17090518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong H., Ren J.M., Young L.H., Pypaert M., Mu J., Birnbaum M.J., Shulman G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reznick R.M., Shulman G.I. The role of AMP-activated protein kinase in mitochondrial biogenesis. J. Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bachstetter A.D., Van Eldik L.J. The p38 map kinase family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1:199–211. [PMC free article] [PubMed] [Google Scholar]
- 73.Kwak J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs. 2014;12:851–870. doi: 10.3390/md12020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ortiz-Lazareno P.C., Bravo-Cuellar A., Lerma-Díaz J.M., Jave-Suárez L.F., Aguilar-Lemarroy A., Domínguez-Rodríguez J.R., González-Ramella O., De Célis R., Gómez-Lomelí P., Hernández-Flores G. Sensitization of U937 leukemia cells to doxorubicin by the MG132 proteasome inhibitor induces an increase in apoptosis by suppressing NF-kappa B and mitochondrial membrane potential loss. Cancer Cell Int. 2014;14:13. doi: 10.1186/1475-2867-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available with its corresponding author, M.I.G.-P., upon reasonable request.