Abstract
Introduction: Despite the recent progress in the malaria burden, climatic factors are important if the world will achieve the set target of its eradication. Hence, this study determined the impact of climatic conditions on childhood severe malaria in a tertiary health facility in northern Nigeria.
Methodology: This was a retrospective descriptive study that involved children with severe malaria managed between July 2016 and August 2017. The diagnosis of severe malaria was according to the World Health Organization 2015 guidelines. We extracted relevant data from case records and obtained the weather information from the Nigerian Meteorological Agency and www.worldweatheronline.com. Data were entered in Microsoft Excel 2013 and analyzed with Statistical Package for the Social Sciences version 20.
Results: A total of 483 cases of children with severe malaria were managed. The median age was 4.0 (2.5-8.0) years. Males were 261 (54.0%). In the wet season, 375 (77.6%) cases were recorded, while 108 (22.4%) cases occurred during the dry season. The odds of malaria occurring during the wet season were 2.057 (95% CI, 1.613-2.622). Temperature patterns were not related to malaria cases. Malaria cases showed significant moderate positive cross-correlation at 2- and 3-months lag for the rainfall pattern (best cross-correlation occurred at 3 months lag with a coefficient of 0.598, p = 0.045).
Conclusion: This study demonstrated marked seasonality of childhood severe malaria infection with 77% of cases during the wet season. Malaria was associated with only rainfall at a 2 to 3 months lag amongst the climatic variables. We recommend the urgent implementation of seasonal malaria chemoprophylaxis.
Keywords: Child, Severe malaria, Rainfall, Temperature patterns, Nigeria
INTRODUCTION
Malaria is a parasitic infection of public health significance with some progress in the past few decades [1]. In the 2019 malaria reports, an estimated 228 million malaria cases occurred in 2018 in contrast to 251 million cases in 2010 [2,3]. This translated to a reduction of malaria incidence from 71 cases to 57 cases per thousand population between 2010 and 2018. Similarly, malaria deaths declined from 585,000 in 2010 to 405,000 in 2018 [2,3]. At present, the greatest burden of malaria is borne by the Africa region and account for more than 90% of cases including the deaths [4]. To ensure the efforts on global eradication of malaria are on track, 11 countries including Nigeria were identified for ‘high burden to high impact’, a special program jointly launched by World Health Organization (WHO) and roll back malaria partnership in 2018 [5]. The 11 countries were responsible for 155 million out of the 228 million malaria cases in 2018, with Nigeria having the highest-burden (25%) [2]. The 2019 malaria report also indicated Nigeria had 3.4 million more cases of malaria, making the country a top priority in the global effort at curtailing malaria [2].
Whereas there are concerted renew global efforts towards eradication of malaria across the globe as advocated by the WHO, a critical component is a role of climatic and weather in malaria in the tropical regions [6]. Evidence in the literature suggested that temperature, humidity and rainfall have varying roles on the mosquitoes’ survival and malaria transmission across Africa [7]. The changes in the climatic and weather conditions also partly account for seasonal variations and malaria prevalence across Nigeria. Studies from the southern part of Nigeria showed that malaria occurred throughout the year with an increased prevalence in the rain season [8-11]. There are very few studies in Northern Nigeria that have explored the role of climatic conditions in the seasonal prevalence which were limited to uncomplicated malaria [12,13]. Besides, very few studies from Northern Nigeria also showed heterogeneity in the malaria incidence in northern Nigeria. For instance, studies in Maiduguri [13] and Kaduna [12] indicated that malaria occurred throughout the year, while a study in Kano [14] indicated that malaria occurred more in the rain season. Besides, most of the studies from Nigeria did not consider the time series analysis between the different weather conditions and malaria incidence [8-11,13,14]. Hence, we determined the impact of rainfall, and temperature patterns on children with severe malaria in a tertiary health facility in Katsina, Northwestern Nigeria.
MATERIALS AND METHODS
This was a retrospective descriptive study that involved children with severe malaria managed between July 2016 and August 2017 in a tertiary health facility in northern Nigeria. The hospital is the only tertiary health facility in the state (Katsina) with an estimated population of 8 million people including children in 2018 [15]. The facility receives referrals within the state and parts of the adjoining states. Katsina is one of the states in the Northwestern part of Nigeria with a semi-arid climate that spanned part of the Sahel, Sudan, and northern savanna zones [16]. The state lies within 11°08ʹ North and 13°22ʹ North and longitude 6°52ʹ East and 9°20ʹ East with a landmass of about 23,983 km2 [16]. The typical climatic condition is that of tropical wet and dry season [17]. The wet season is characterized by rainfall that spans May to September with a peak in August. The dry season spans from late October to April with a harmattan period in December and January [17]. The annual average rainfall ranges from 550 to 700 mm with a significant variation and occasional drought [18]. The annual daily temperature also varies with a peak of 39°C-40°C in the months of April and May and least temperature of 16°C-18°C observed in the months of December and January [18].
This study included children with severe malaria based on WHO Malaria Treatment Guidelines, 2015 [19]. The WHO definition includes positive parasitological confirmation and one or more of the following features-Impaired consciousness (a Glasgow score of less than 11 or Blantyre coma score less than 3 for younger children); Prostration (generalized weakness so that the person is unable to sit, stand or walk without assistance; Multiple convulsion (more than two episodes within 24 hours); Acidosis (a base deficit of >8 mEq/l or if not available, a plasma bicarbonate level of <15 mmol/l or venous plasma lactate of ≥5 mmol/l, and severe acidosis manifests clinically as respiratory distress-rapid, deep, labored breathing); Hypoglycemia (blood or plasma glucose <2.2 mmol or 40 mg/dl); Severe malarial anemia (hemoglobin concentration less than or equal 5 g/dl or hematocrit of ≤15% in children <12 years of age (<7 g/dl and 20%, respectively, in adults) with a parasite count >10,000/μl; Renal impairment (plasma or serum creatinine >265 umol/l or 3 mg/dl or blood urea >20 mmol/l; Jaundice (plasma or serum bilirubin >50 umol/l or 3 mg/dl with a parasite count >100,000/Ul; Pulmonary edema (radiologically confirmed or oxygen saturation <92% on room air with a respiratory rate >30/minute, often with chest indrawing and crepitations on auscultation; Significant bleeding (including recurrent or prolonged bleeding from the nose, gums or venipuncture sites; hematemesis or melena; Shock (compensated shock is defined as capillary refill greater or equal 3 seconds or temperature gradient on leg [mid to proximal limb] but no hypotension, and decompensated shock is defined as systolic blood pressure <70 mm Hg in children or <80 mm Hg in adults, with evidence of impaired perfusion (cool peripheries or prolonged capillary refill]). Hyperparasitemia (Plasmodium falciparum parasitemia >10%) [19]. The parasitological evidence of malaria was based on a positive rapid diagnostic test for malaria using malaria P. falciparum histidine-rich protein (CareStartTM, Access Bio, USA) and or malaria microscopy.
We retrieved the case records of children aged 14 years and below admitted between July 2016 and August 2017. Children with other diagnoses were excluded from the study. We extracted the demographics and other relevant information from the case records. Weather data were obtained from Nigerian Meteorological Agency and www.worldweatheronline.com [20]. We classified the weather into two seasons; ‘wet season’ from May to September and the ‘dry season’ from October to April [17]. The number of malaria cases admitted daily were noted and collated to generate the monthly malaria cases.
We analyzed using the IBM Statistical Package for the Social Sciences® software and r studio. The age of the children was summarized as median with interquartile range (not normally distributed). The monthly malaria cases, monthly temperature patterns and rainfall were depicted using charts. The relationship between the mean rainfall, minimum temperature, maximum temperature, mean temperature, diurnal temperature variation and monthly malaria cases were evaluated using cross-correlation. A p-value of less than 0.05 was taken as the level of statistically significant.
The ethical review committee of the Federal Medical Centre, Katsina approved this study and being a retrospective one, the committee waived the informed consents with anonymity of the data including absolute confidentiality in the handling of the data.
RESULTS
Out of 1,733 children admitted over the study period, 483 cases were severe malaria, giving a prevalence of 27.9% (95% CI 25.8-30.1%). The median age (interquartile range) of the children admitted with severe malaria was 4.0 (2.5-8.0 years) with a range of 5 months to 14 years. Children less than 5 years were 263 (54.5%) and the males were 261 (54.0%).
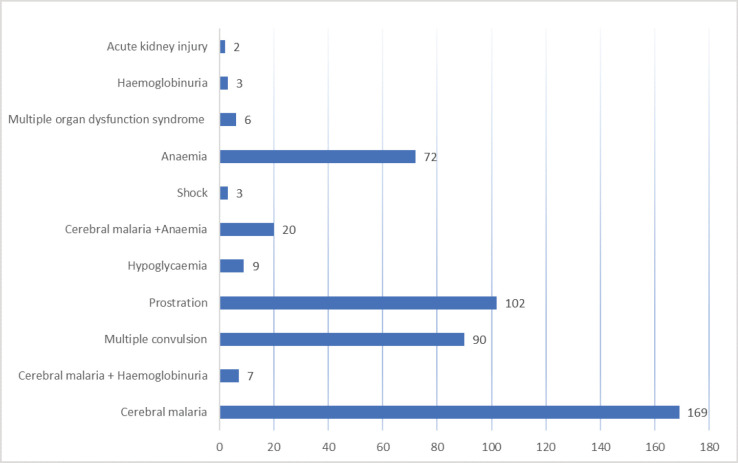
Based on the index of severity, of 483 cases of severe malaria, cerebral malaria (169; 35.0%) was the most common, followed by prostration (102; 21.2%) and multiple convulsion (90; 18.6%) while acute kidney injury was the least common (2; 0.4%). Further details are shown in Figure 1.
Figure 1.
Components of severe malaria in the study children.
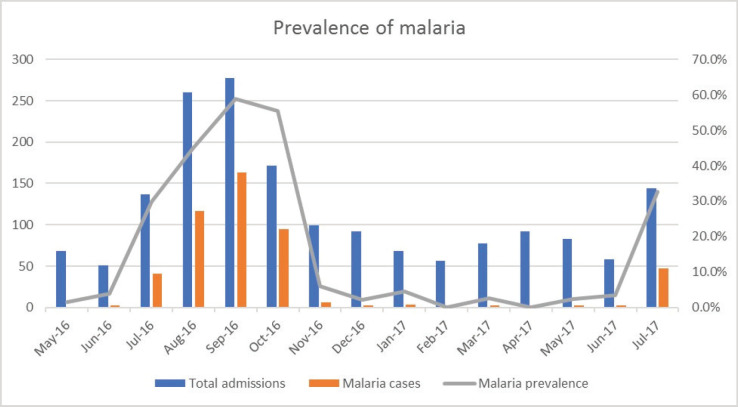
Out of 483 cases of severe malaria, 375 (77.6%) were recorded during the wet season, while 108 (22.4%) cases occurred during the dry season (Figure 2).
Figure 2.
Monthly distribution of severe malaria.
The prevalence of malaria was significantly higher during the wet season 32.3%; (375/1160) compared with the dry season of 18.8%; (108/573), χ2 = 34.665, p = <0.001 (Table 1). The odds of malaria occurring during the wet season were 2.057 (95% CI 1.613-2.622) as shown in Table 1.
Table 1.
Comparison of the malaria based on the season of the year.
| Season | Malaria n (%) | Non-malaria n (%) | χ 2 | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Wet (May to September) (N) = 1,160 | 375 (32.3%) | 785 (67.7%) | 34.665 | 2.057 | 1.613 to 2.622 | <0.001 |
| Dry season (October to April) (N) = 573 | 108 (18.8%) | 465 (81.2%) | ||||
| Total (N) = 1,733 | 483 | 1,250 |
CI, confidence interval; n, number; N, total number; OR, odds ratio.
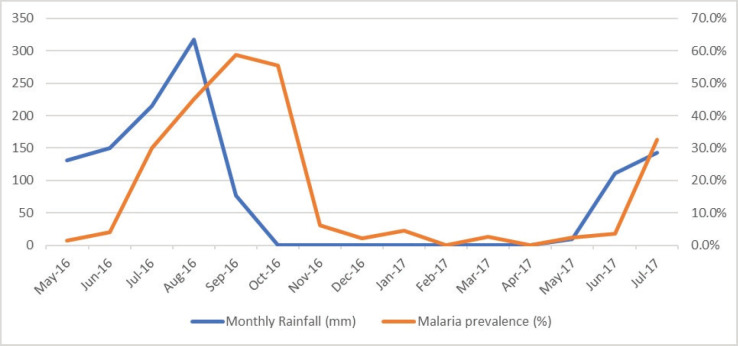
Out of 108 cases during the dry season, 89 cases (82.4%) occurred in the month of October. Further breakdown showed that the highest prevalence of 60% (165/277) was in the month of September, followed by October with 52.0% (89/171) while zero cases were observed in February (0/56) and April (0/92) as shown in Figure 3.
Figure 3.
Monthly rainfall and malaria prevalence.
During the study period, the annual rainfall was 889.1 mm, with the least rainfall of 76.3 mm in September and peak rainfall of 317.2 mm in the month of August (Figure 3). Figure 3 shows that from October 2016 to March 2017, there was no rainfall observed in the state. Also, the number of severe malaria cases declined from 95 in October 2016 to 2 in December 2016, 3 in January 2017, 0 in February 2017 and 3 in March 2017.
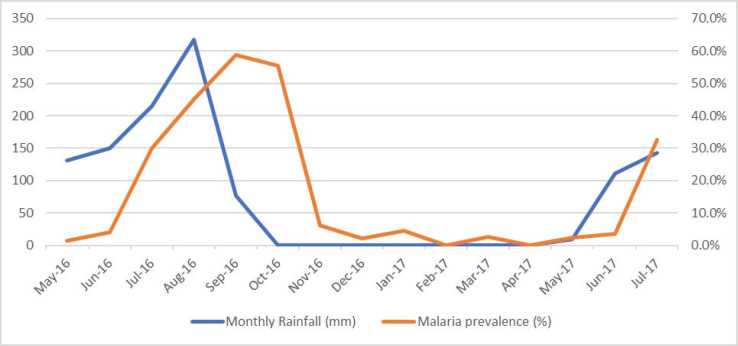
During the study period, the maximum daily temperature was 40°C in the month of April and May, while the least temperature of 23°C was recorded in January and February. The average daily temperature fluctuates between 15°C in January and February to 28°C in May and June (Figure 4). Diurnal temperature (the differences between the maximum and minimum temperature) fluctuate between 4°C in July to 11°C in February and March.
Figure 4.
Monthly temperature and severe malaria prevalence.
Although the pattern of malaria cases correlated with the monthly temperature patterns and monthly rainfall (Table 2), it was only the rainfall pattern that was significantly associated with malaria cases. The number of malaria cases showed a significant moderate cross-correlation at 2- and 3-months lag for the rainfall pattern. The best cross-correlation occurred at 3 months lag with a coefficient of 0.598 (p = 0.045).
Table 2.
Cross correlation of number of malaria cases per month with mean monthly rainfall and temperature parameters.
| Lag (month) | Mean rainfall | Diurnal temperature | Minimum temperature | Maximum temperature | Mean temperature | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cross correlation | p | Cross correlation | p | Cross correlation | p | Cross correlation | p | Cross correlation | p | |
| -5 | -0.520 | 0.898 | 0.206 | 0.307 | 0.071 | 0.431 | 0.179 | 0.331 | -0.029 | 0.528 |
| -4 | -0.220 | 0.720 | 0.357 | 0.172 | 0.193 | 0.305 | 0.382 | 0.156 | 0.091 | 0.405 |
| -3 | 0.598 | 0.045 | -0.082 | 0.592 | 0.138 | 0.348 | 0.102 | 0.387 | 0.128 | 0.359 |
| -2 | 0.553 | 0.046 | -0.483 | 0.926 | -0.169 | 0.694 | -0.421 | 0.897 | -0.088 | 0.604 |
| -1 | 0.142 | 0.327 | -0.419 | 0.908 | -0.265 | 0.799 | -0.489 | 0.939 | -0.069 | 0.586 |
| 0 | -0.156 | 0.697 | -0.290 | 0.832 | 0.151 | 0.308 | 0.010 | 0.487 | 0.244 | 0.209 |
| 1 | -0.190 | 0.726 | 0.030 | 0.462 | 0.438 | 0.083 | 0.471 | 0.068 | 0.452 | 0.076 |
| 2 | -0.133 | 0.655 | 0.218 | 0.257 | 0.165 | 0.310 | 0.283 | 0.198 | 0.133 | 0.345 |
| 3 | -0.059 | 0.567 | 0.058 | 0.434 | -0.274 | 0.780 | -0.255 | 0.765 | -0.372 | 0.853 |
| 4 | -0.002 | 0.502 | 0.116 | 0.379 | -0.740 | 0.975 | -0.712 | 0.970 | -0.771 | 0.979 |
| 5 | -0.001 | 0.500 | 0.538 | 0.094 | -0.700 | 0.957 | -0.456 | 0.868 | -0.676 | 0.951 |
DISCUSSION
Malaria remains a disease of public health significance despite the recent progress which makes some factors including the climatic conditions important if the world will achieve eradication. Our study showed that the malaria occurred twice in the wet seasons with a peak prevalence of 60% in the month of September. This finding contrasts the observation in Maiduguri, northeast Nigeria where more malaria occurred throughout the year, although with a peak during the wet season [13]. A study in Benin, south-south Nigeria did not observe any difference in the prevalence of malaria during the wet and dry seasons [9], while a similar study in Port Harcourt also reported malaria cases throughout the year with a peak of 75% in the months of June and July [10]. The observations of a distinct pattern of malaria in Katsina further reinforced the earlier observation that there is heterogeneity in incidence of malaria in Africa inclusive of Nigeria and further called for a locally generated approach that takes into consideration the uniqueness of a geographical area in the global effort to curtail and eradicate malaria [7].
Furthermore, the malaria prevalence in this study showed ‘marked seasonality’ (75% of cases within 6 months) [21] with 78% cases observed in the wet seasons. This called for the urgent implementation of seasonal malaria chemoprophylaxis adopted by the country [22]. Studies had shown that the periodic administration anti-malaria in areas where at least 60% of malaria cases occurred over 4 months could lead to a substantial decline in the burden of childhood malaria [23,24].
Our study also showed a positive moderate correlation between malaria cases and rainfall and the cases lagged behind the rainfall by 2 to 3 months. This finding is similar to the study in Ethiopia, where malaria cases lagged behind rainfall by 2 to 4 months [25]. Similarly, in Ghana, there was a weak correlation between malaria cases and rainfall and the peak of malaria cases lagged by 2 to 4 months with rainfall [26]. A retrospective study in Sri Lanka between malaria and rainfall also showed a lagged period of 1 to 3 months [27]. We could not compare further with the other Nigerian studies that evaluated the relationship between the malaria cases and rainfall due to the fact they did not consider time series in their analysis [8-11]. The lagging behind of malaria cases observed is not unexpected since the rainfall is necessary for the collection of pockets of water that will serve as breeding sites for mosquitoes, the vector for transmission of malaria parasite [28]. Furthermore, the developments of mosquitoes in the water take a couple of weeks, hence the lagging behind of malaria cases compared with rainfall [28].
This study did not observe any significant relationship between the maximum daily temperature, minimum daily temperature, mean daily temperature, daily diurnal temperature variation and monthly malaria. In contrast, in Swaziland, the warmer temperature was found to be associated with malaria cases at zero lag [29]. In Ghana, only the mean monthly maximum temperature was found to be related to malaria cases at 0 to 4 months lag [26], while in Ethiopia both the mean monthly maximum and minimum were related to malaria cases at a lag of 0 to 4 months [25]. The inability to find the relationship between the patterns of temperature and cases of malaria in this study may be due to extreme of temperature that tends to occur in the early and late part of the year. Extreme temperature with very high and low temperature has been found not to support mosquitoes survival as the best temperature for the mosquito to thrive is usually 25°C to 28°C [30].
In conclusion, this study demonstrated marked seasonality of childhood severe malaria in Katsina, Northwestern Nigeria with 77% of cases occurring between May and September (wet season). Malaria was associated with only rainfall at 2 to 3 months lag amongst the climatic variables. We recommend the urgent implementation of seasonal malaria chemoprophylaxis, using WHO recommended combination drugs (sulfadoxine-pyrimethamine and amodiaquine) for children less than 5 years.
ACKNOWLEDGEMENT
The authors would like to thank the staff of pediatric department and hospital records for their roles in the management and retrieval of the records of the patients.
FUNDING
None.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
The Federal Medical Centre ethical review committee, Katsina, Nigeria gave approval for this study. Also, the committee granted waiver for the informed consents being a retrospective study and data were extracted without the subjects’ names. We ensured absolute confidentiality in the data managements.
REFERENCES
- 1.Maduka O. End malaria for good: a review of current strategies and future novelties for malaria elimination in Nigeria. Malaria World J. 2018;9(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: World Health Organization; 2019. [2021 March]. World malaria report 2019. Available from: https://www.who.int/publications/i/item/9789241565721. [Google Scholar]
- 3.World Health Organization. Geneva, Switzerland: World Health Organization; 2013. [2021 Jan]. Summary of the wWorld mMalaria rReport 2012. Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/en/index.html. [Google Scholar]
- 4.African Union Assembly, Roll Back Malaria Partnership. African union malaria progress report. 2018 Africa. 2018. [2020 Dec]. Available from: http://ndmalaria.org/sites/default/files/African%20Union%20Malaria%20Progress%20Report%202019.pdf.
- 5.World Health Organization. Geneva, Switzerland: World Health Organization; 2018. [2020 Nov]. World mMalaria Rreport 2018. Available from: https://www.who.int/malaria/publications/world-malaria-report-2018/en/ [Google Scholar]
- 6.Roll Back Malaria. Climate change and malaria. 2015. [2021 Feb]. Available from: https://endmalaria.org/sites/default/files/RBM_Climate_Change_Fact-Sheet_170915.pdf.
- 7.Mabaso ML, Craig M, Ross A, Smith T. Environmental predictors of the seasonality of malaria transmission in Africa: the challenge. Am J Trop Med Hyg. 2007;76(1):33–8. https://doi.org/10.4269/ajtmh.2007.76.33. [PubMed] [Google Scholar]
- 8.Weli VE, Efe SI. Climate and eEpidemiology of Mmalaria in Port Harcourt Region, Nigeria. Am J Clim Change. 2015;4(1):40–7. https://doi.org/10.4236/ajcc.2015.41004. [Google Scholar]
- 9.Enosolease ME, Awodu OA. Seasonal variation of malaria parasitaemia in an urban tropical city. Niger J Clin Pract. 2003;6(1):30–3. [Google Scholar]
- 10.Eberechukwu YI, Oluwajenyo AA. A review of the pattern of mMalaria in cChildren above nNeonatal age at the University of Port Harcourt Teaching Hospital (2006–2011) Univers J Clin Med. 2018;6(1):10–4. https://doi.org/10.13189/ujcm.2018.060102. [Google Scholar]
- 11.Anumudu IC, Dozie IN, Iwuala CC, Iwuoha G, Ede AO. Seasonal vVariations and the pPrevalence of malaria in Imo State. J Nurs Health Sci. 2019;8(6):4–10. [Google Scholar]
- 12.Bajoga UA, Balarabe HS, Olufemi AA, Dalhat MM, Sule IB, Ibrahim MS, et al. Trend of malaria cases in Kaduna State using routine surveillance data, 2011-2015. Pan Afr Med J. 2019;32(Suppl 1):8. doi: 10.11604/pamj.supp.2019.32.1.13735. https://doi.org/10.11604/pamj.supp.2019.32.1.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balogun ST, Sandabe UK, Okon KO, Akanmu AO, Fehintola FA. Malaria burden and pre-hospital medication among subjects with malaria in Maiduguri, Northeast Nigeria. Heliyon. 2019;5(8):e02280. doi: 10.1016/j.heliyon.2019.e02280. https://doi.org/10.1016/j.heliyon.2019.e02280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yahaya A, Aminu F, Tukur AI. Seasonal vVariation of mMalaria iInfection among oOut- pPatients attending Wudil General Hospital, Kano State, Nigeria. Int J Appl Res Technol. 2012;7(1):79–84. [Google Scholar]
- 15.Ppopulation.City. Katsina population [Internet] 2018. [2018 Dec 2]. Available from: population.city.%3Eniigeria%3Ekatsina.
- 16.James GK, Jega IM, Halilu AS, Olojo OO, Oyewunmi AS, Shar JT, et al. Assessment of eEnvironmental sSensitivity to desertification in Katsina State, Nigeria. Environ Ecol Res. 2018;6(6):545–55. https://doi.org/10.13189/eer.2018.060604. [Google Scholar]
- 17.Ezeh CU, Obeta MC, Anyadike RN. Variations in the sequences of daily rainfall across Nigeria [Internet] Arab J Geosci. 2016;9(681):1–8. https://doi.org/10.1007/s12517-016-2719-9. [Google Scholar]
- 18.Anaje IB, Onu V, Abashiya M, Oyatayo KT, Ibrahim AA, Ati OF, et al. Climate cChange vVulnerability assessment in the Northern Part of Katsina State, Nigeria: aA quantitative approach. Dutse J Pure Appl Sci. 2017;3(1):1–14. [Google Scholar]
- 19.World Health Organization. 3rd. Geneva, Switzerland: World Health Organization; 2015. [2021 March]. Guidelines for the treatment of malaria. Available from: https://apps.who.int/iris/ [Google Scholar]
- 20.Monthly weather forecast and climate, Katsina, Nigeria. [2020 Oct]. Available from: https://www.weather-atlas.com/en/nigeria/katsina-climate.
- 21.Roca-Feltrer A, Schellenberg JR, Smith L, Carneiro I. A simple method for defining malaria seasonality. Malar J. 2009;8(276):276. doi: 10.1186/1475-2875-8-276. https://doi.org/10.1186/1475-2875-8-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malaria consortium. Seasonal mMalaria cChemoprevention Pprogramme Sstart-Uup gGuide. 2015. [2020 Dec]. p. 234. Accessed on Dec, 2020 Available from: http://www.malariaconsortium.org.
- 23.Nglass IN, Ozor L, Olotu O, Momoh A, Onuekwe CE, Owilli C. Effect of sSeasonal mMalaria chemoprevention and dData mManagement in hHealth fFacilities in tThree study LGAs of Adamawa State, Nigeria. J Trop Dis. 2016;7(6):1–9. [Google Scholar]
- 24.Coldiron ME, Von Seidlein L, Grais RF. Seasonal malaria chemoprevention: successes and missed opportunities [Internet] Malar J. 2017;16(1):481. doi: 10.1186/s12936-017-2132-1. https://doi.org/10.1186/s12936-017-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sena L, Deressa W, Ali A. Correlation of cClimate variability and mMalaria: Aa rRetrospective cComparative study, Southwest Ethiopia. Ethiop J Health Sci. 2015;25(2):129–38. doi: 10.4314/ejhs.v25i2.5. https://doi.org/10.4314/ejhs.v25i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klutse NA, Aboagye-Antwi F, Owusu K, Ntiamoa-Baidu Y. Assessment of pPatterns of cClimate variables and malaria cCases in tTwo ecological zones of Ghana. Open J Ecol. 2014;4(12):764–75. https://doi.org/10.4236/oje.2014.412065. [Google Scholar]
- 27.Briët OJ, Vounatsou P, Gunawardena DM, Galappaththy GN, Amerasinghe PH. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J. 2008;7(1):77. doi: 10.1186/1475-2875-7-77. https://doi.org/10.1186/1475-2875-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okunlola OA, Oyeyemi OT. Spatio-temporal analysis of association between incidence of malaria and environmental predictors of malaria transmission in Nigeria [Internet] Sci Rep. 2019;9(1):17500. doi: 10.1038/s41598-019-53814-x. https://doi.org/10.1038/s41598-019-53814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chuang TW, Soble A, Ntshalintshali N, Mkhonta N, Seyama E, Mthethwa S, et al. Assessment of climate-driven variations in malaria incidence in Swaziland: toward malaria elimination. Malar J. 2017;16(1):232. doi: 10.1186/s12936-017-1874-0. https://doi.org/10.1186/s12936-017-1874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mordecai EA, Paaijmans KP, Johnson LR, Balzer C, Ben-Horin T, de Moor E, et al. Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecol Lett. 2013;16(1):22–30. doi: 10.1111/ele.12015. https://doi.org/10.1111/ele.12015. [DOI] [PubMed] [Google Scholar]