Abstract
Introduction: Salmonella infection is one of the most common causes of gastroenteritis worldwide. It is associated with high morbidity and mortality if not treated properly. It has developed resistance to multiple antibiotics. These developments are concerning. This study sought to observe common patterns of invasive Salmonella infections, such as the common serotypes involved, presentation, sensitive investigations, and effective treatment. This study also aimed to examine the risk factors that can worsen the infection and increase morbidity and mortality.
Methodology: This retrospective analysis included all patients who were diagnosed with invasive Salmonella infection at King Khalid University Hospital from May 2017 to December 2018. Most patients in this report were immunocompromised; however, a few previously healthy patients. Different types of specimens were collected. Twenty-two patients with invasive Salmonella were included in this study.
Results: Sixteen of them had underlying conditions. The most common presenting symptoms of illness were fever (n = 13), vomiting (n = 6), and diarrhea (n = 4). Most blood samples (94%) were positive for Salmonella. All patients were discharged except three who died; these were all older patients with comorbidities. Although four different antimicrobial resistance patterns were noticed in this study, ciprofloxacin was the highest significant percentage (62.5%). Of five patients initially treated with ciprofloxacin, three of them expressed resistance to this antibiotic.
Conclusion: The findings of this study support that immunocompromised patients and people with extreme ages are more likely to have serious medical illnesses and at higher risk of infection with Salmonella spp. Therefore, this study emphasises the importance of antimicrobial judicious utilisation. Tackling the escalating antibiotic resistance could be approached by implementing advanced public education levels to maintain high standards of food and water safety. Moreover, the urge to investigating newer drugs against Salmonella with an acceptable safety profile is a cornerstone to attenuate the rapidly acquired bacterial resistance precisely for those who are immunocompromised. Furthermore, predicting the mortality, morbidity and the clinical response is feasible according to the patient parameters and comorbidities.
Keywords: Immunocompromised, invasive, ciprofloxacin resistance, Salmonella, Saudi Arabia
INTRODUCTION
Salmonella are a Gram-negative group of bacteria that are from the Enterobacteriaceae family. The group consists of two approved species and one under consideration [1]. These bacteria carry special importance in medicine because of their association with infections in humans, and are a common cause of gastroenteritis worldwide. They also lead to cross-infection between humans and animals; for example, undercooked chicken can cause Salmonella enteritidis infection [1]. Salmonella infections are a serious concern because of their high prevalence and the morbidity and mortality associated with them. Salmonella causes around 93.8 million cases of foodborne disease worldwide every year, and is also associated with 155,000 deaths annually [2]. Of all infections caused by Salmonella, around 99% are caused by Salmonella enterica. Common infections include non-invasive non-typhoidal (NTS) salmonellosis, invasive NTS (iNTS) salmonellosis, and typhoid fever. Patients can also experience sepsis in Salmonella infection [3]. This paper will specifically review invasive salmonellosis. Invasive infections are caused by S. enterica serotypes named Salmonella Typhi and Salmonella Paratyphi A, B, and C. Salmonella Typhi and S. Paratyphi A and B cause enteric fever, while S. Paratyphi C causes sepsis and secondary infections. Some non-typhoidal types of Salmonella iNTS, such as S. Enteritidis and S. Typhimurium, can also cause invasive infections [4].
The symptoms of Salmonella infection are not constant; they vary according to the host and serotype. However, most cases include diarrhea [1]. Patients with typhoidal Salmonella may be asymptomatic or may initially present with influenza-like symptoms and diarrhea, but when the infection reaches the blood, they will develop fever and vomiting in severe cases [5]. NTS patients usually present with gastrointestinal manifestations like nausea, vomiting, and diarrhea, while in the case of iNTS, the common presentation will be febrile illness sometimes accompanied by diarrhea [5]. The prevalence of infection caused by different subtypes also varies. For example, S. Typhi has a lower prevalence of infection (11-20 million cases and 128,000–161,000 deaths per year worldwide) [6].
In Saudi Arabia, Salmonella infection is highly prevalent during hajj and Umrah seasons due to the gathering of people [7]. This fact implies that the Salmonella outbreak can occur in crowded places. Moreover, invasive NTS salmonella infections affect immunocompromised and undernourished people, leading to a high mortality rate [4].
With time, multi-drug resistant (MDR) strains of Salmonella are emerging that are unresponsive to commonly used antibiotic treatments. Moreover, the disease caused by these MDR strains is more severe, prolonged, and associated with higher mortality [2]. Enteric fever is also the most common infectious cause of disability globally [4]. Therefore, our aim is to investigate the pattern of Salmonella infections along with their management and outcome to confront these emerging worldwide problems. This study is a case series of invasive Salmonella infection that will review different patterns of invasive Salmonella infections associated with high morbidity and mortality.
METHODS
This is a retrospective descriptive study, a case-series analysis conducted in King Khalid University Hospital (KKUH), Riyadh, Saudi Arabia between May 2017 and December 2018. The ethics review board of the hospital (KKUH IRB) approved the study and due to its retrospective study design allowed it to proceed without obtaining patient consent. Data collection was carried out from the medical records available at KKUH. Patient identities were not included in the data.
All patients admitted with invasive Salmonella infection during the selected time period were enrolled in the study. A total of 22 cases were included, with ages ranging from 8 months to 74 years, the majority of patients (59%) were children. Diagnosis of Salmonella infection in these patients was confirmed by performing cultures using conventional methods on blood, CSF, stool, urine, biopsied tissue, pus, and peritoneal fluid. All the isolates were identified using the automated Microscan system (Siemens Healthcare Diagnostics, Deerfield, IL) and the API 20 system (bioMérieux, Marcy l’Etoile, France). Serotyping was performed using Salmonella antisera (Welcome, KS) using the Kauffmann-White classification system. Antimicrobial susceptibility for ciprofloxacin, ceftriaxone, ampicillin, and trimethoprim/sulfamethoxazole was assessed with the automated Microscan system (Dade Behring). The E-test method (AB Biodisk, Solna, Sweden) was used to determine the ciprofloxacin minimum inhibitory concentrations (MIC) with interpretation of ≥0.06 to indicate resistance to ciprofloxacin.
The data were analyzed using Microsoft Excel 2016 and Statistical Package for the Social Sciences ‘IBM Statistics for Windows, version 26 (IBM Corp., Armonk, NY,)’.
RESULTS
Age and gender of the patients
Twenty-two patients with invasive Salmonella infection were detected. Fifteen (68%) of them were females and seven (32%) were males. The range of ages was from 8 months to 74 years, and the standard deviation was 25.3. However, there were two infant patients under the age of 1 year without chronic diseases or underlying risk factors.
Clinical presentation
The most common clinical diagnosis was gastroenteritis [(4/22 (18.18%)], followed by wound infection [3/22 (13.64%)]. Meningitis, urinary tract infection and abscess were diagnosed in one patient for each [1/20 (4.55)]. Various clinical manifestations were found in this series, including fever, vomiting, cough, fatigue, and headache, as shown in Table 1. Fever was the most common clinical manifestation, present in more than half of the patients (13/22; 61.9%), especially in children. Patients with Salmonella Typhi presented with prolonged fever. Vomiting and diarrhea were the second most common presentation. Diarrhea was more common in children than in adults. Other clinical manifestations were found to be related to the underlying conditions of each patient (Table 1).
Table 1.
Frequency of different presenting symptoms of Salmonella infection in 22 patients.
| Clinical presentations | Adult (n = 9) | Children typhi (n = 3) | Children non-typhi (n = 10) | Frequency (n = 22) | Percentage |
|---|---|---|---|---|---|
| Fever | 2 | 3 | 8 | 13 | 59.09 |
General symptoms
|
10 | 3 | 8 | 21 | 95.45 |
GIT symptoms
|
6 | 2 | 9 | 17 | 77.27 |
Urinary tract symptoms
|
3 | 0 | 4 | 7 | 31.82 |
Respiratory symptoms
|
0 | 1 | 3 | 4 | 18.18 |
For general symptoms, GIT symptoms, urinary symptoms or respiratory symptoms, indicates that the patient had one or more of the mentioned symptoms.
N, Number; LL edema, lower limb edema.
Underlying diseases
Sixteen patients (72%) had underlying conditions (Table 2). The most common comorbidities were systemic lupus erythematosus (SLE) (18%), and malignancies were found in four patients (leukemia, non-Hodgkin lymphoma, and bladder and prostate cancer) (18%); all of them were found to be on immunosuppressive agents. Other fewer common comorbidities were included.
Table 2.
Risk factors of invasive Salmonella infection and underlying conditions in included cases.
| Clinical characteristics | Number | Percentage |
|---|---|---|
| Malignancy / chemotherapy | 4 | 18.18 |
| Extreme age | 4 | 18.18 |
| Surgery / trauma | 2 | 9.10 |
| Chronic renal failure | 1 | 4.55 |
| Sickle cell anemia | 2 | 9.10 |
| Diabetes mellitus | 3 | 13.64 |
| Autoimmune disease (SLE) | 4 | 18.18 |
| Steroid treatment | 8 | 36.36 |
| Total patients | 22 | 100.00 |
SLE, Systemic lupus erythematosus.
Type of specimens
Samples were taken from patients are shown in Table 3. All the positive samples for Salmonella were identified initially as non-lactose fermenter on MacConkey agar.
Table 3.
Types of positive cultures of invasive Salmonella infection.
| Patient specimens | Frequency | Percentage | Positive culture | Percentage |
|---|---|---|---|---|
| Urine | 21 | 95.45 | 4/21 | 19 |
| Blood | 17 | 77.27 | 16/17 | 94 |
| Stool | 14 | 63.64 | 6/14 | 42 |
| CSF | 4 | 18.18 | 1/4 | 25 |
| Biopsy | 1 | 4.55 | 1/1 | 100 |
| Peritoneal fluid | 1 | 4.55 | 1/1 | 100 |
| Wound | 1 | 4.55 | 1/1 | 100 |
| Sputum | 1 | 4.55 | 0/1 | 0 |
| Total patients | 22 | 100 | 100 |
CSF, Cerebrospinal fluid.
Blood cultures were the most likely to be returned as positive (16/17; 94%), followed by stool cultures (6/14; 42%) and urine cultures (4/21, 19%). This can imply that blood culture is highly sensitive for invasive Salmonella infection.
Type of salmonella species
Different serotypes of Salmonella infection were observed (Table 4). The most common serotype was Salmonella non-typhi and non-paratyphi group B (7/22; 31.8%), which was more common in adults, especially those with severe underlying conditions such as SLE, adenocarcinoma, Sickle cell anemia (SCA), and non-Hodgkin lymphoma. In addition, three younger patients with underlying conditions (SLE nephritis, acute leukemia, and ulcerative colitis) were infected with Salmonella non-typhi and non-paratyphi group B as well. Salmonella non-typhi and non-paratyphi Group D was the second most common cause of infection (4/22; 18.18%) but unlike Salmonella non-typhi and non-paratyphi group B, was more common in children than in adults. It was found in patients with SCA, SLE, and previously healthy individuals. Salmonella Typhi was found in three children with no medical issues. Other groups, such as Salmonella non-typhi and non-paratyphi group A and group C and Salmonella paratyphi group A infected one patient each. Five patients were infected by other species.
Table 4.
Frequency of different Salmonella serotypes isolated from 22 patients with invasive Salmonella infection.
| Type of isolate | Serogroup | Frequency | Percentage |
|---|---|---|---|
| Non-typhi, non-para typhi | Group A | 1 | 4.55 |
| Group B | 7 | 31.8 | |
| Group C | 1 | 4.55 | |
| Group D | 4 | 18.18 | |
| Other species | - | 5 | 22.73 |
| Salmonella typhi | - | 3 | 13.64 |
| Salmonella paratyphoid | Group A | 1 | 4.55 |
| Total | 22 | 100 | |
Treatment and management
Treatment regimens prescribed for patients with invasive Salmonella infection and their susceptibility patterns are shown in Table 5.
Table 5.
Treatment regimens prescribed for patients with invasive Salmonella infection and their susceptibility patterns.
| Treatment | Susceptibility pattern for all positive extra-intestinal specimens (24 specimens) | No of patient treated with this drug | Percentage | |||||
|---|---|---|---|---|---|---|---|---|
| S | I | R | ||||||
| N | % | N | % | N | % | |||
| Ciprofloxacin | 6 | 25 | 3 | 12.5 | 15 | 62.5 | 5 | 27.78 |
| Ceftriaxone | 24 | 100 | 0 | 0 | 0 | 0 | 5 | 27.78 |
| Sulfamethoxazole-trimethoprim | 21 | 87.5 | 0 | 0 | 3 | 12.5 | 4 | 22.22 |
| Meropenem. | 24 | 100 | 0 | 0 | 0 | 0 | 3 | 13.64 |
| Pipracillin/Tazobactam | 24 | 100 | 0 | 0 | 0 | 0 | 3 | 13.64 |
| Ampicillin | 19 | 79.17 | 0 | 0 | 5 | 20.83 | 1 | 5.56 |
| Imipenem | 24 | 100 | 0 | 0 | 0 | 0 | 1 | 5.56 |
| Amoxicillin-clavulanate | 23 | 95.83 | 0 | 0 | 1 | 4.17 | 1 | 5.56 |
| Total | 24 | 18/22 | 81.81 | |||||
| Rehydration only | 4 | 4/22 | 18.18 | |||||
S, Sensitive; I, Intermediate; R, Resistant; N, Number; %, Percentage.
The majority of patients in this study were treated with an anti-microbial agent (18/22; 82%). Third-generation cephalosporins (Ceftriaxone) and a fluoroquinolone (Ciprofloxacin) were the most common antibiotics administered to the patients (5/18; 28%). Sulfamethoxazole-trimethoprim was used in four cases (22%) as monotherapy or combination therapy, with two lethal outcomes. Four patients (18.18%) were not prescribed any antibiotics and were rehydrated only. For the susceptibility pattern, we have included only one of the positive extra-intestinal specimens for the 22 patients, excluding 2 patients where we have included 2specimens for each one of them due to the differences in the susceptibility patterns of the isolated Salmonella species which may represent different serovars.
Resistance to ciprofloxacin (62.5%) represents a major clinical concern. E-tests were performed to measure MIC to determine resistance to ciprofloxacin treatment (if MIC ≥ 0.06). Of five patients initially treated with ciprofloxacin, three patients expressed resistance to this treatment.
Complications and outcomes
All the patients were discharged except three who died (13.6%), all of whom were elderly patients with comorbidities. Two of them were late-stage cancer patients who had septic shock secondary to Salmonella infection; those patients were both also found to be resistant to ciprofloxacin. The third was a 44-year-old female known to have sickle cell disease who presented with severe generalised headache, personality changes, and prolonged vomiting. Brain imaging via computerised tomography (CT) showed a left frontal space-occupying lesion, and magnetic resonance imaging with contrast confirmed the finding of heterogenous mass with a cystic component suspected as high-grade glioma (Figure 1). This patient underwent craniotomy and excisional biopsy, and after histopathology was diagnosed with invasive sinus aspergillosis and intracerebral aspergilloma. Initially, the patient was stable and responding to the antifungal medication (voriconazole and caspofungin), but after 1 week, her condition started to deteriorate. She had seizures and a low level of consciousness due to intracerebral hemorrhage, ventriculitis, and sepsis caused by invasive group B Salmonella. Although the treatment was extensive, the patient died of severe infection and respiratory arrest. Another complicated case was a 58 years old immunocompromised female known to have diabetes mellitus and hypertension. She underwent surgery as management for chronic pancreatitis complicated post-operatively with multiple abdominal and pelvic abscesses (Figure 2). The abscess was observed to have heavy growth of Salmonella species (group C) and moderate growth of Escherichia coli, MDR extended-spectrum beta lactamase producer . However, she received ciprofloxacin as an IV antibiotic after the drainage of the abscess.
Figure 1.
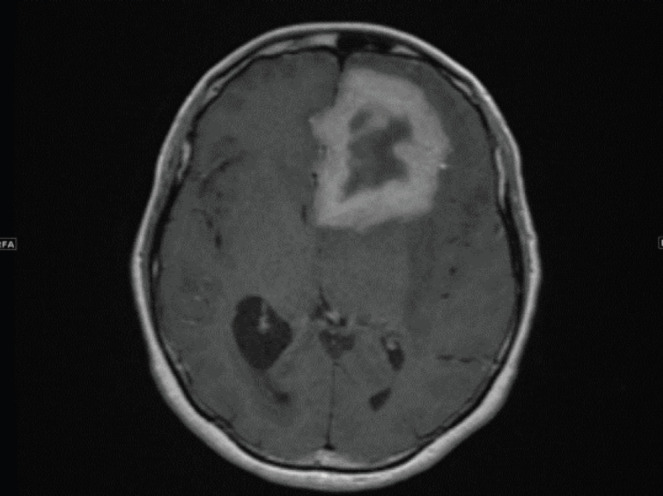
Axial T1WI post contrast administration revealed left frontal intra-axial mass thick-walled ring enhancing lesion with extensive surrounding edema is possibly aggressive neoplastic lesion, or less likely contiguous inflammatory / infectious process from skull base.
Figure 2.
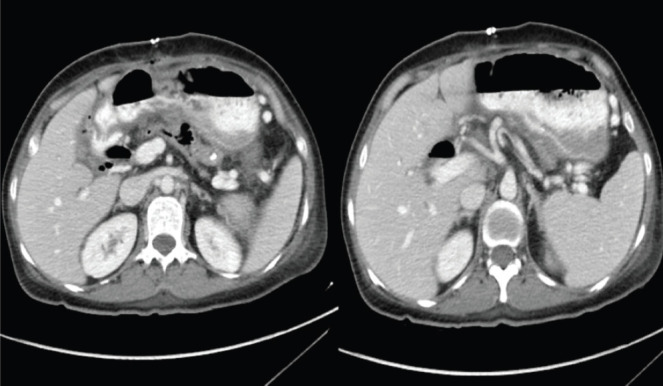
CT abdomen and pelvis revealed multiple abdomen and pelvic collections.
DISCUSSION
Salmonella is one of the most common causes of gastroenteritis in humans globally [8]. The high disease burden is not only associated with morbidity and mortality, but also causes a significant economic burden, somewhere between $ 400 million and $ 3.5 billion annually in the US alone [8]. In the Global Burden of Disease study 2010 (GBD 2010), typhoid and paratyphoid infections were found to have caused the loss of 12.2 million disability-adjusted life years and 190,200 deaths [5]. These statistics stress the importance of understanding this disease and developing strategies accordingly to fight high incidence and associated outcomes.
This study included 22 cases of Salmonella infection and analyzed various characteristics of these cases, such as age, gender, symptoms, outcomes, etc., to determine the pattern of this disease and understand the factors associated with the risk of infection and outcomes. We found more females to be affected by this infection than males; the available literature also confirms this fact. In a population study conducted in a city in Pakistan, the incidence of infection in the female population was significantly higher than in males, 54.5% versus 45.5% [9]. In this study, S. typhi affected almost equal numbers of both genders, but of all paratyphi-infected individuals, 62.5% were female [9]. Another study found that females have a higher incidence of Salmonella infection and are infected by more serotypes, while men tend to be infected by common serotypes [10]. Another study conducted in Tanzania also reported a higher prevalence in females (58.2%) than males (41.8%) [11]. This higher incidence in the female population is largely unexplained, but may result from biological susceptibility differences. However, more research is required here to reach a definite answer [12].
Another important point observed in this study was the presence of underlying conditions in most cases. Most patients were suffering from conditions affecting immunity or taking immunosuppressive agents. Similar results have been obtained in other studies where Salmonella infection prevalence was found to be higher in people with low immunity due to diseases like human immunodeficiency virus or malignancies, or immunosuppressive treatment [5]. Also, Salmonella has been observed to have a highly variable presentation in immunocompromised individuals, and the rate of extra-intestinal manifestations is also higher in these patients [13]. Being immunocompromised is also an important risk factor for disseminated infections caused by NTS Salmonella [14].
Salmonella infection presents with a range of symptoms. In this study, fever was found to be the most common symptom, followed by vomiting and diarrhea. All three are common symptoms of gastroenteritis, the most common manifestation of Salmonella [15]. Moreover, Salmonella group B was the most common serotype found in the study. However, this frequency can differ according to different regions and other characteristics [16,17]. One study reported that the most infections were caused by group D (60.0%), followed by group B (25.3%), group C (9.3%), and group E (5.3%) [18]. That study also reported that there was the highest resistance to ciprofloxacin followed by ceftriaxone, two commonly used antibiotics. The emergence of ciprofloxacin resistance has also been reported in other countries [19], so this is an important point of concern that can affect treatment outcomes. Another study also reported an outbreak of ceftriaxone-resistant S. typhi in pregnant women traveling from Pakistan to Germany; ceftriaxone is a commonly used antibiotic in pregnancy [20]. There is susceptibility reported for cefixime, azithromycin, and ceftriaxone in most cases. Carbapenems (e.g., imipenem, meropenem, and ertapenem) and tigecycline could be alternatives in MDR salmonellosis [21]. However, the susceptibility to antibiotics is continuously changing, and therefore there is a need for continuous evaluation [22].
Based on the results we obtained, it concluded that the resistance of Salmonella to ciprofloxacin was 62.5%. Similarly, according to a previous study which concluded that the resistance of Salmonella to ciprofloxacin was 47.2% [23].
CONCLUSION
In this case series analysis, we report a higher incidence of Salmonella in females that should be investigated further. We also document unusual presentations of salmonellosis involving infected wounds with no detectable bacteremia.
We found resistance to ciprofloxacin in three of five cases treated with this antibiotic. Thus, we encourage the wise use of antibiotics and restriction of their prescription to only those individuals who are at high risk of invasive infections. There is a need for continuous evaluation of antibiotic susceptibility to improve treatment outcomes. Moreover, we found the frequency and severity of salmonella infection to be high in immunocompromised patients. Therefore, there is a special need to take steps, such as clean food and water, to prevent infection in this group.
The burden of Salmonella infection is high globally and must be addressed. For this purpose, there is a need to conduct more research studies and examine the hidden aspects of this microorganism and the infections it causes. Our study has several strong points, one is the detected incidence of Salmonella infection among females. Also, the observation of a unique clinical manifestation of salmonellosis. moreover, the antimicrobial agents’ susceptibility and resistance have been fully described. Nonetheless, the sample size may diminish the generalisability of this study.
ACKNOWLEDGEMENT
The authors acknowledge Dr. Fawzia Alotaibi for reviewing the manuscript.
CONFLILCT OF INTERESTS
The authors declare that there is no conflict of interest.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICAL APPROVAL
An IRB request was submitted to the IRB committee for approval, and it has been approved without official number according to the IRB policy in our hospital, retrospective studies that not involving human or animal experiments require no IRB.
AUTHOR’S CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
REFERENCES
- 1.Ajmera A, Shabbir N. Salmonella [Internet] 2020. [2020 June 6]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555892/
- 2.Eng SK, Pusparajah P, Ab Mutalib NS, Ser HL, Chan KG, Lee LH. Salmonella: a review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci. 2015;8(3):284–93. [Google Scholar]
- 3.Kurtz JR, Goggins JA, McLachlan JB. Salmonella infection: interplay between the bacteria and host immune system. Immunol Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews JR, Ryan ET. Diagnostics for invasive Salmonella infections: current challenges and future directions. Vaccine. 2015;33:C8–15. doi: 10.1016/j.vaccine.2015.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;34(28):901–37. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geneva, Switzerland: WHO Press; 2018. World Health Organization (WHO): fact sheet/typhoid. Available from: https://www.who.int/mediacentre/factsheets/typhoid/en/ [Google Scholar]
- 7.Abd El Ghany M, Alsomali M, Almasri M, Padron Regalado E, Naeem R, Tukestani A, et al. Enteric infections circulating during hajj seasons, 2011–2013. Emerg Infect Dis. 2017;23(10):1640–9. doi: 10.3201/eid2310.161642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabo DA, Granier SA, Diguimbaye CD, Marault M, Brisabois A, Mama B, et al. Are Salmonella-induced gastroenteritis neglected in developing countries? feedback from microbiological investigations in N’Djamena Hospitals, Chad. PLoS One. 2015;10(8):e0136153. doi: 10.1371/journal.pone.0136153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanum S, Saba NV, Qayyum MI, Qazilbash AA. Distribution patterns of Salmonella infection in Rawalpindi/Islamabad area and the risks factors associated with the disease prevalence. J Biol Sci. 2006;6:253–60. [Google Scholar]
- 10.Judd MC, Hoekstra RM, Mahon BE, Fields PI, Wong KK. Epidemiologic patterns of human Salmonella serotype diversity in the USA, 1996–2016. Epidemiol Infect. 2019;147:e187. doi: 10.1017/S0950268819000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ngogo FA, Abade AM, Rumisha SF, Mizinduko MM, Majigo MV. Factors associated with Salmonella infection in patients with gastrointestinal complaints seeking health care at Regional Hospital in Southern Highland of Tanzania. BMC Infect Dis. 2020;20:135. doi: 10.1186/s12879-020-4849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reller ME, Tauxe RV, Kalish LA, Mølbak K. Excess salmonellosis in women in the United States: 1968–2000. Epidemiol Infect. 2008;136(8):1109–17. doi: 10.1017/S0950268807009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyyur Aravamudan V, Kee Fong P, Singh P, Sze Chin J, Sam YS, Tambyah PA. Extraintestinal Salmonellosis in the immunocompromised: an unusual case of pyomyositis. Case Rep Med. 2017;2017:5030961. doi: 10.1155/2017/5030961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lokken KL, Walker GT, Tsolis RM. Disseminated infections with antibiotic-resistant non-typhoidal Salmonella strains: contributions of host and pathogen factors. Pathog Dis. 2016;74(8):ftw103. doi: 10.1093/femspd/ftw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashurst JV, Truong J, Woodbury B. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Salmonella Typhi. [Updated 2020 Apr 21] Available from: https://www.ncbi.nlm.nih.gov/books/NBK519002/ [Google Scholar]
- 16.Gutema FD, Agga GE, Abdi RD, Duchateau L, DeZutter L, Gabriël S. Prevalence and serotype diversity of Salmonella in apparently healthy cattle: systematic review and meta-analysis of published studies, 2000–2017. Front Vet Sci. 2019;6:102. doi: 10.3389/fvets.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy SP, Wang H, Adams JK, Feng PC. Prevalence and characteristics of Salmonella serotypes isolated from fresh produce marketed in the United States. J Food Prot. 2016;79(1):6–16. doi: 10.4315/0362-028X.JFP-15-274. [DOI] [PubMed] [Google Scholar]
- 18.Park HK, Rhie K, Yeom JS, Park JS, Park ES, Seo JH, et al. Differences in clinical and laboratory findings between group D and non-group D non-typhoidal Salmonella gastroenteritis in children. Pediatr Gastroenterol Hepatol Nutr. 2015;18(2):85–93. doi: 10.5223/pghn.2015.18.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Fernández A, Gallina S, Owczarek S, Dionisi AM, Benedetti I, Decastelli L, et al. Emergence of ciprofloxacin-resistant Salmonella enterica serovar typhi in Italy. PLoS One. 2015;10(6):e0132065. doi: 10.1371/journal.pone.0132065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engsbro AL, Jespersen HS, Goldschmidt MI, Mollerup S, Worning P, Pedersen MS, et al. Ceftriaxone-resistant Salmonella enterica serotype typhi in a pregnant traveller returning from Karachi, Pakistan to Denmark, 2019 Euro Surveill. 2019;24(21):1900289. doi: 10.2807/1560-7917.ES.2019.24.21.1900289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil N, Mule P. Sensitivity pattern of Salmonella typhi and paratyphi A isolates to chloramphenicol and other anti-typhoid drugs: an in vitro study. Infect Drug Resist. 2019;12:3217–25. doi: 10.2147/IDR.S204618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassetti M, Peghin M, Vena A, Giacobbe DR. Treatment of infections due to MDR gram-negative bacteria. Front Med (Lausanne) 2019;6:74. doi: 10.3389/fmed.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peruzy MF, Capuano F, Proroga YTR, Cristiano D, Carullo MR, Murru N. Antimicrobial susceptibility testing for Salmonella serovars isolated from food samples: five-year monitoring (2015-2019) Antibiotics (Basel) 2020;9(7):365. doi: 10.3390/antibiotics9070365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated or analyzed during this study are included in the manuscript.


