Abstract
In children with systemic lupus erythematosus on immunosuppressive therapy, infection is a known complication. We present a case of a 12-year-old girl who was previously diagnosed with lupus nephritis but had stopped taking allopathic medications and had been on herbal medicines for a year. She was referred to us with persistent fever and disease activity in spite of restarting immunosuppressive treatment. Results of blood tests and bone marrow aspiration were suggestive of macrophage activation syndrome. Imaging of her chest and abdomen showed features suggestive of miliary tuberculosis (TB) in the lungs and granulomas in the spleen. Mycobacterium tuberculosis was identified in bone marrow cultures, resulting in a diagnosis of disseminated TB. She was successfully treated with intravenous steroids, anti-tuberculous therapy and intravenous immunoglobulin. Mycophenolate mofetil was added after 6 weeks. The patient recovered from TB and her lupus was under control during follow-up.
Keywords: Disseminated tuberculosis, Macrophage activation syndrome, Systemic lupus erythematosus, Nephritis, Child
INTRODUCTION
Systemic lupus erythematosus (SLE) is a multisystem autoimmune disease that can be associated with significant comorbidities. Children with SLE are at an increased risk of infection as there maybe underlying immunologic defects due to the disease and also due to the immunosuppressive therapy that is required to keep the disease under control [1]. Intercurrent infection like tuberculosis (TB) may mimic disease activity in children with lupus and also contribute to the development of macrophage activation syndrome (MAS) in these patients. In tropical countries, latent TB is common and exposure to immunosuppressive therapy may result in dissemination of the infection [2].
CASE REPORT
A 12-year-old girl presented to us with a history of fever, facial rashes with hyperpigmentation, oral ulcers and increased hair loss for several months. She had been diagnosed with lupus nephritis Class IV a few years ago at another hospital. She received two doses of cyclophosphamide and then was lost to follow-up. The parents had opted for treatment with herbal medicines, which she received for 1.5 years. During this period, she continued to have intermittent fevers, oral ulcers, alopecia, weight loss and rashes on her face. In the 6 months prior to being referred to us, the parents decided to switch back to allopathic medicine and the patient was seen at another hospital. She was started on steroids, hydroxychloroquine and mycophenolate mofetil. The mother reported that her fever would subside on being administered intravenous pulse steroids but would return on switching to oral steroids. Her fever became more persistent and she continued to have a drop in haemoglobin (Hb), leukopenia, thrombocytopenia and clinical features of active lupus.
On admission to the hospital, she was febrile and had hyperpigmentation of her face, oral ulcers, alopecia, cervical lymphadenopathy and mild hepatosplenomegaly. There was no evidence of serositis and she had mild tachypnoea and tachycardia. Her O2 saturation in air was 85%.
Investigations revealed Hb of 5.4 gm/dl (reference range [RR] =11.5-15.5 gm/dl), white blood cell count (WBC) 1.9 × 109/l (RR = 4.5-13.5 ×109/l), platelet count of 4 × 109/l (RR = 150-450 × 109/l); differential count: neutrophils 90% (RR = 50%-60%), lymphocyte 8% (RR = 24%-54%), erythrocyte sedimentation rate 57 mm/h (RR = 4-10 mm/h), aspartate aminotransferase (AST or serum glutamic oxaloacetic transaminase) 158 U/L (normal <35 U/l), alanine aminotransferase (ALT or serum glutamic pyruvic transaminase) 98 U/l (RR = 13-45 U/l), C-reactive protein 17 mg/dl (RR = 0-0.8 mg/dl), serum ferritin 7,466 ng/ml (RR = 11-307 ng/ml), fasting triglycerides 283 mg/dl (normal <150 mg/dl) and serum fibrinogen 57 mg/dl (RR = 170-405 mg/dl). She had elevated D-dimer of 2.82 mg/l (normal < 0.55 mg/l Fibrinogen Equivalent Units), very low C3 at 32 mg/dl (RR = 90-180 mg/dl) and C4 of <6 mg/dl (RR = 10-40 mg/dl), strong positive dsDNA and elevated urine protein creatinine ratio of 3. A bone marrow aspiration showed evidence of haemophagocytosis. The blood and bone marrow investigations therefore confirmed MAS.
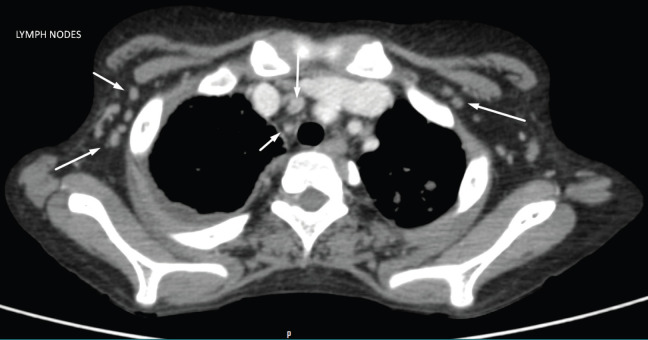
Chest X-ray revealed diffuse reticular changes (Figure 1); ultrasonography (USG) of abdomen showed changes suggestive of multiple granulomas in the spleen (Figure 2); and computed tomography (CT) scan of the chest showed hilar and axillary lymphadenopathy and features of miliary TB (Figures 3 and 4).
Figure 1.
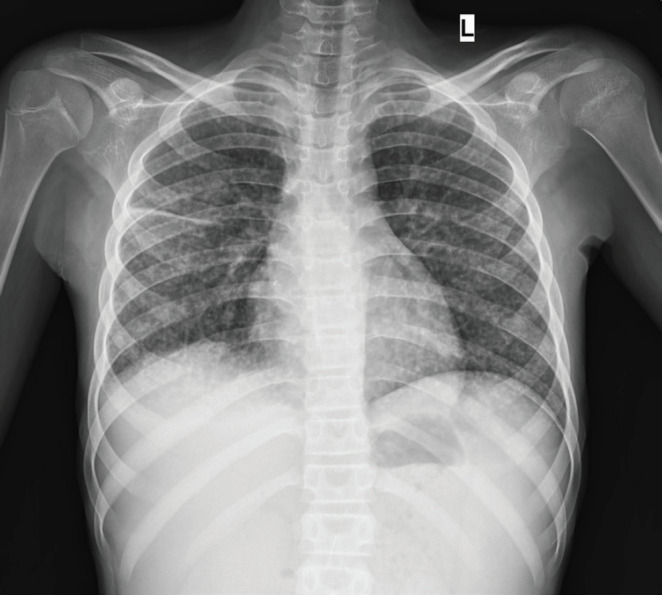
Chest X-ray showing diffuse reticular changes.
Figure 2.
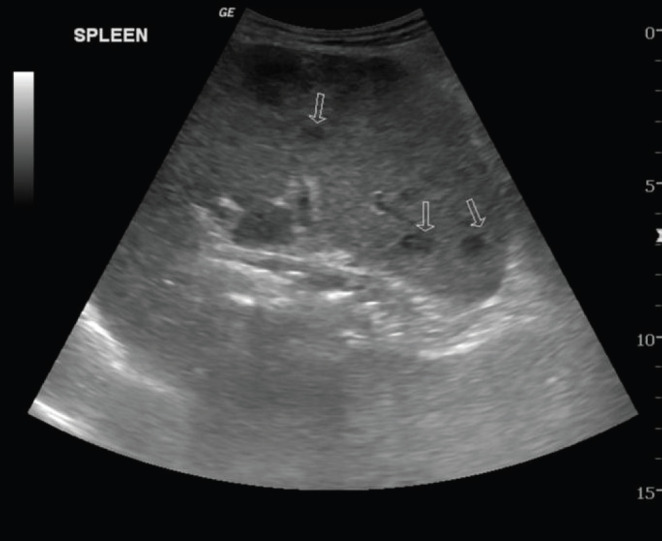
USG of abdomen showed changes suggestive of multiple granulomas in the spleen (arrows pointing to granulomas).
Figure 3.
CT scan of the chest showing hilar and axillary lymphadenopathy (arrows pointing to lymph nodes).
Figure 4.
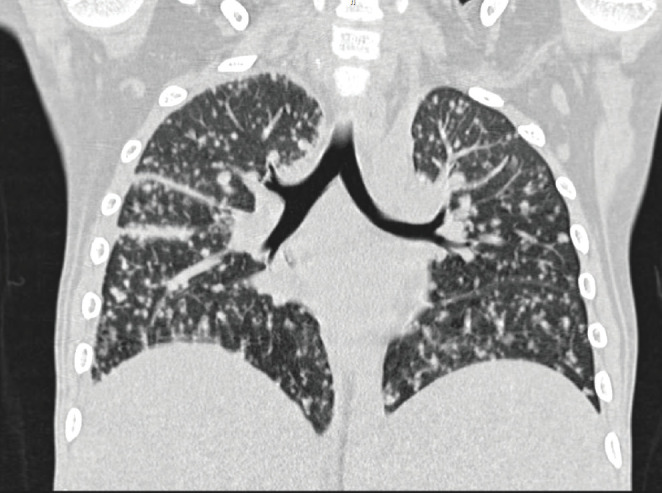
CT scan of the chest showing features of miliary tuberculosis.
GeneXpert for TB was positive in sputum sample. Sputum and bone marrow aspiration smear were negative for acid fast bacilli (AFB). QuantiFERON-TB Gold Plus (QFT-Plus) test was positive. Automated MYCO/F Lytic bottle inoculated with bone marrow aspirate, signalled positive after 17 days of incubation and showed the presence of AFB. Drug susceptibility testing conducted at the National Institute for Research in Tuberculosis, Chennai, confirmed susceptibility to the primary drugs: isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E).
The patient needed a prolonged hospital stay of 6 weeks and was treated with oxygen via mask and later with nasal prongs, pulse methylprednisolone therapy 10 mg/kg once a day for 3 days, followed by oral prednisolone 2 mg/kg in two divided doses, intravenous immunoglobulin (IVIG), presumptive intravenous antibiotics and platelet transfusions. She was commenced on intensive phase of anti-tuberculous therapy with four drugs isoniazid (H), rifampicin(R), pyrazinamide (Z) and ethambutol (E) (HRZE). The pulse steroid therapy was repeated the following 3 weeks due to falling WBC and platelet counts possibly due to MAS.
The patient made a gradual recovery and was discharged on oral steroids, hydroxychloroquine and four anti-tuberculous medications. Mycophenolate mofetil was added to her treatment after she had completed 6 weeks of anti-tuberculous therapy. Enalapril was added to treat proteinuria. The patient is currently well, a year after her initial presentation to us, and continues treatment for lupus with mycophenolate mofetil, hydroxychloroquine, and prednisolone 5 mg on alternate days. She has completed four drug (HRZE) anti-tuberculous therapies for 2 months and three drugs isoniazid (H), rifampicin(R), and ethambutol (E) for 9 months.
DISCUSSION
In young patients with SLE, apart from the disease itself, bacterial infections constitute an important cause of mortality. Extrapulmonary TB or the miliary pattern is the most common form of TB infection in patients with SLE [3]. Prolonged uses of steroids and nephritis have been identified as risk factors for the development of TB [3]. Cell-mediated immunity which is important in the defence against TB is defective in SLE patients [4]. Both SLE and TB can present with a wide range of clinical manifestations and can mimic other diseases. Systemic features like fever, weight loss, fatigue, arthralgia, lymphadenopathy and serositis can be present in both conditions [5]. If coexistent TB infections are not identified, these patients stand a potential risk of being subjected to further increase in immune suppressive treatment as has happened to this case before being seen by us. Disseminated TB may cause a flare of the disease; and this flare can lead to the spread of TB and in turn increase the disease activity in lupus leading to a vicious cycle. MAS, a potentially fatal complication of both SLE and disseminated TB in this scenario can be further challenging [6]. High doses of parenteral steroids maybe needed in the initial stages to bring MAS under control, but this treatment could also lead to further dissemination of TB. A balanced approach is, therefore, required to control the MAS and treat TB simultaneously. Compared to the high dose of parenteral pulse steroids that is normally recommended for management of MAS, we opted for 10 mg/kg/day for 3 days of methylprednisolone, but we repeated the pulse steroid therapy for 3 days again the following 3 weeks to get MAS under control. The standard therapy for TB is normally of 6 months duration. We extended the treatment to 9 months considering the severity at presentation and the immune-suppressed condition of the patient [7].
Although miliary and disseminated TB has been previously reported in SLE patients, to our knowledge, this is the first report of disseminated TB and MAS in a child with SLE. It is likely that lupus nephritis and the immune-suppressed state lead to TB infection and both SLE and TB contributed to the development of MAS in this patient. MAS is a known complication in paediatric rheumatic diseases particularly in systemic juvenile idiopathic arthritis and SLE, and is known to occur when there is persistent uncontrolled disease activity with macrophage stimulation resulting in a cytokine storm [8]. It is sometimes the presenting manifestation of these diseases. In addition, MAS can also occur as a complication of infections including TB. Differentiating a disease flare from an infection can be challenging [9]. Our patient had nephritis, a known risk factor for TB infection. In this scenario, the additional risk factor was the herbal treatment the child was exposed to, the contents of which were unknown.
CONCLUSION
In children with SLE, persistent fever may signify disease activity or an infection. Disseminated TB may mimic clinical features of SLE or may cause a flare of the disease in a patient with SLE, and both the conditions can contribute to the pathogenesis of MAS. Aggressive treatment of the infection, with simultaneous cautious immune suppression to manage disease activity, is the key to successful management of patients with SLE and TB.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
FUNDING
No financial support was received.
ETHICAL APPROVAL
The study was approved, and consent has been obtained from the Institutional Ethics Committee. Consent was obtained from the parents of the patient to publish the case history and images. Confidentiality was maintained at all levels.
REFERENCES
- 1.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38:473–85. doi: 10.1080/08916930500285352. https://doi.org/10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya PK, Jamil M, Roy A, Talukdar KK. SLE and tuberculosis: a case series and review of literature. J Clin Diagn Res. 2017;11:OR01–3. doi: 10.7860/JCDR/2017/22749.9398. https://doi.org/10.7860/JCDR/2017/22749.9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam LS, Li EK, Wong SM, Szeto CC. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. 2002;31:296–300. doi: 10.1080/030097402760375205. https://doi.org/10.1080/030097402760375205. [DOI] [PubMed] [Google Scholar]
- 4.Prabu V, Agrawal S. Systemic lupus erythematosus and tuberculosis: a review of complex interactions of complicated diseases. J Postgrad Med. 2010;56:244–50. doi: 10.4103/0022-3859.68653. https://doi.org/10.4103/0022-3859.68653. [DOI] [PubMed] [Google Scholar]
- 5.Li JC, Fong W, Wijaya L, Leung YY. Disseminated tuberculosis masquerading as a presentation of systemic lupus erythematosus. Int J Rheum Dis. 2018;21:352–5. doi: 10.1111/1756-185X.13195. https://doi.org/10.1111/1756-185X.13195. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Liang G, Qin H, Li Y, Zeng X. Tuberculosis-associated hemophagocytic lymphohistiocytosis with initial presentation of fever of unknown origin in a general hospital: an analysis of 8 clinical cases. Medicine. 2017;96:e6575. doi: 10.1097/MD.0000000000006575. https://doi.org/10.1097/MD.0000000000006575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashmat M, Rana RS, Mahmud TE, Rasheed A, Rehman AU, Pirzada SAR, et al. Case report of a lupus patient with a severe flare and miliary tuberculosis: need for proper guidelines for management. Oxford Med Case Rep. 2017;7:omx030. doi: 10.1093/omcr/omx030. https://doi.org/10.1093/omcr/omx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deane S, Selmi C, Teuber SS, Gershwin ME. Macrophage activation syndrome in autoimmune disease. Int Arch Allergy Immunol. 2010;153:109–20. doi: 10.1159/000312628. https://doi.org/10.1159/000312628. [DOI] [PubMed] [Google Scholar]
- 9.Ospina FE, Echeverri A, Zambrano D, Suso JP, Martínez-Blanco J, Cañas CA, et al. Distinguishing infections vs flares in patients with systemic lupus erythematosus. Rheumatology. 2017;56:i46–54. doi: 10.1093/rheumatology/kew340. [DOI] [PubMed] [Google Scholar]