Abstract
ΔFOSB is a uniquely stable member of the FOS family of immediate early gene AP1 transcription factors. Its accumulation in specific cell types and tissues in response to a range of chronic stimuli is associated with biological phenomena as diverse as memory formation, drug addiction, stress resilience, and immune cell activity. Causal connections between ΔFOSB expression and the physiological and behavioral sequelae of chronic stimuli have been established in rodent and, in some cases, primate models for numerous healthy and pathological states with such preclinical observations often supported by human data demonstrating tissue-specific ΔFOSB expression associated with several specific syndromes. However, the viability of ΔFOSB as a target for therapeutic intervention might be questioned over presumptive concerns of side effects given its expression in such a wide range of cell types and circumstances. Here, we summarize numerous insights from the past three decades of research into ΔFOSB structure, function, mechanisms of induction, and regulation of target genes that support its potential as a druggable target. We pay particular attention to the potential for targeting distinct ΔFOSB isoforms or distinct ΔFOSB-containing multiprotein complexes to achieve cell type or tissue specificity to overcome off-target concerns. We also cover critical gaps in knowledge that currently limit the exploitation of ΔFOSB’s therapeutic possibilities and how they may be addressed. Finally, we summarize both current and potential future strategies for generating small molecules or genetic tools for the manipulation of ΔFOSB in the clinic.
Keywords: AP1, transcription, addiction, Alzheimer’s disease, depression, stress, learning and memory, protein structure, drug design
Graphical Abstract
INTRODUCTION
Complex organisms have distinct organs and tissues that are composed of many different cell types varying enormously in form and function, yet all cells in a given organism contain essentially identical DNA. During development, the differentiation of these cells depends upon exquisitely regulated changes in gene expression controlled by cell-to-cell signaling, environmental cues, and presumably random factors.1 Likewise, regulation of gene expression occurs within cells of adult organisms to mediate adaptation to the environment throughout life, such as learned behaviors as well as critical oscillatory functions like daily sleep or seasonal mating or migration.2–4 Many complex and interlocking mechanisms contribute to the expression of a particular gene within a eukaryotic cell, including modification of chromatin structure, noncoding RNAs, and a large class of proteins called transcription factors (TFs) that bind to DNA in a sequence-specific manner to control RNA transcription. There are many families of TFs, which bind to the proximal promoter regions of genes to regulate RNA polymerase binding and initiation of transcription as well as to more distant regulatory (e.g., enhancer) sequences to stimulate or repress the expression of genes thousands of base pairs away, potentially even on different chromosomes.5
Activator Protein-1 (AP1) is a prototypical family of TFs critical in virtually every eukaryotic cell.6 Dimeric AP1 protein complexes are master regulators of transcription, responding to a wide variety of extracellular signals to modulate myriad physiological functions, and have been implicated in dozens of diseases from cancer to drug addiction.3,7 Genes encoding AP1 proteins are classified as immediate early genes, a superfamily characterized by their rapid (minutes) expression in response to many forms of cell activation, for instance, viral regulatory proteins that are synthesized following viral infection of a host cell or homeostatic regulators of excitability expressed after rapid firing of neurons.8,9 AP1 complexes expressed transiently in neurons following activation are typically formed as heterodimers of one FOS family and one JUN family protein (see details below). Of the many AP1 proteins, this current Review focuses on ΔFOSB, which is unique among AP1 proteins due to its remarkable stability in the brain.3,10 In neurons, all AP1 proteins, like FOS, are rapidly and robustly induced following high-frequency firing with a half-life ranging from minutes up to a couple of hours.11–13 FOS-containing AP1 complexes are hypothesized to target a variety of genes associated with cell differentiation, cell and synapse development, synaptic plasticity, and learning.14,15 ΔFOSB, on the other hand, has an unusually long half-life, up to 8 days in vivo,16–18 and though it is primarily expressed in neurons of the central nervous system, it is also found in other cells, including microglia in brain19 as well as in osteoblasts in bones, where it functions as an oncoprotein and directly associates with tumorigenesis.7 In the brain, long-lasting ΔFOSB induction in distinct types of neurons is directly linked to addiction,20–22 stress susceptibility, resilience, and antidepressant action,23,24 l-DOPA-induced dyskinesias in Parkinson’s disease,25,26 Alzheimer’s disease (AD) and memory formation,27–29 and sexual behavior,30 among others, with each function linked to the specific brain regions and cell types in which ΔFOSB accumulates.
Although ΔFOSB has been studied extensively for decades and bidirectional genetic manipulation of ΔFOSB expression in the brains of mice, rats, and monkeys has provided causal links between ΔFOSB expression and many physiological responses and behaviors linked to disease, ΔFOSB has yet to be effectively targeted with pharmacological tools. Viral vectors or inducible genetic mutant mice that increase or decrease ΔFOSB function in specific brain cells and circuits have proven effective in reversing animal behaviors relevant to human disease,24,31–33 but these viral and genetic tools are not readily translatable to humans.34 This gap highlights the critical need to develop small molecules that target ΔFOSB, a prospect that appears more feasible than in past years with the recent development of drugs targeting other AP1 TFs.35,36 Here, we review the circumstances and effects of ΔFOSB induction and the current understanding of ΔFOSB structure and function, and we highlight recent discoveries that indicate the suitability of ΔFOSB as a pharmacological target. We also cover the strategies currently being used to uncover compounds that modulate its function and tools that will provide greater understanding of activity-dependent regulation of long-term gene expression and may become vital new treatments for diseases ranging from addiction to AD to cancer.
ΔFOSB INDUCTION IN THE BRAIN AND PERIPHERY
ΔFOSB Induction and Function in Nucleus Accumbens.
ΔFOSB is induced in response to a wide range of stimuli in many different tissues and cell types. For many years, we and others have studied the mechanism and circumstances of ΔFOSB induction in discrete regions of the brain in response to stimuli associated with neurological and psychiatric disease. We originally characterized 35–37 kDa proteins, which we termed “chronic FRAs” (FOS-related antigens) induced in rodent brain after chronic exposure to psychostimulants.22,37,38 We further found that these proteins persisted for weeks after exposure to chronic cocaine, an effect that was especially prominent in the nucleus accumbens (NAc), a key site of dopaminergic signaling modulated by cocaine and critical for the rewarding effects of drugs and other pleasurable stimuli. These FRAs were resolved as unique isoforms of ΔFOSB,38,39 and we went on to demonstrate that ΔFOSB accumulates in medium spiny neurons (MSNs) of the mouse NAc in response to chronic exposure to virtually all drugs of abuse.40–43 This accumulation occurs preferentially in MSNs expressing D1-type dopamine receptors (D1-MSNs) for all drugs of abuse except for opioids, which also induce ΔFOSB in D2-type MSNs (D2-MSNs).44–48 Critically, ΔFOSB is induced in the NAc of human drug addicts,21 indicating that this phenomenon may indeed underlie certain addiction behaviors.
In support of this idea, overexpression of ΔFOSB specifically in D1-MSNs of the NAc and dorsal striatum of mice causes increased locomotor sensitivity to cocaine,20,49 increased cocaine and morphine conditioned place preference,20,49,50 and increased cocaine self-administration and relapse.51,52 Although the downstream mechanisms underlying the effects of NAc D1-MSN ΔFOSB on drug responses are still not fully understood, ΔFOSB is critical for cocaine-dependent changes in D1-MSN dendritic spine shape and number,21,32,49 which are reflected in the altered function of glutamatergic synapses onto D1-MSNs.49 Plasticity at these synapses involving altered AMPA-type glutamate receptor (AMPAR) insertion and activity underlies cocaine reward, seeking, withdrawal, and incubation of craving in rodents,53,54 and indeed, ΔFOSB target genes in the NAc include those that encode certain AMPAR subunits,20,24 Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα),21,23 and cyclin-dependent kinase 5 (CDK5),55 all critical regulators of glutamatergic synaptic strength. It is important to note that constitutive knockout of the Fosb gene in mice also results in increased sensitivity to the behavioral effects of cocaine.38 Because this knockout is global and occurs at the earliest stages of development, a variety of adaptive changes may occur driving this drug-sensitivity phenotype that may be independent of the acute or chronic adult effects of ΔFOSB specifically in NAc MSNs. Moreover, in other cells like hippocampal CA1 pyramidal neurons, both overexpression and inhibition of ΔFOSB in adulthood can cause a similar impairment in spatial learning,27 suggesting that there is a range of ΔFOSB expression that drives normal behavioral outcomes with a deviation above or below this range causing cellular and behavioral abnormalities.
ΔFOSB is likewise induced in rodent NAc by other stimuli, including many forms of chronic stress.24,48,56–61 Transgenic reporter mice allowing visualization of MSN subtypes revealed that chronic social defeat stress (CSDS) induces ΔFOSB in NAc D2-MSNs of animals that are susceptible to depression-like behaviors following stress, while mice that were stressed in the same manner but were behaviorally resilient showed ΔFOSB accumulation only in D1-MSNs.48 Using a novel neuroepigenome-editing approach, it was shown that induction of endogenous ΔFOSB in D1-MSNs of NAc drives the resilient phenotype, whereas such induction in D2-MSNs promotes susceptibility with neuroepigenomic suppression of endogenous ΔFOSB expression having the opposite effects.57 These opposite actions of ΔFOSB in D1- vs D2-MSNs are consistent with the opposite effects seen upon optogenetic activation of these MSN subtypes in controlling resilience vs susceptibility to CSDS.62 Antidepressants like fluoxetine and tranylcypromine also induce ΔFOSB in mouse NAc,22,24,58 and NAc ΔFOSB function is necessary for antidepressant reversal of behavioral deficits after CSDS.24 Chronic electroconvulsive seizures, which are antidepressant in humans as well, induce ΔFosB in this and other brain regions (see below). Meanwhile, ΔFOSB expression levels are reduced in the NAc of depression patients.24,63 It is likely this finding in humans is mediated via D1-MSNs although this remains to be demonstrated directly. The identification of the target genes of ΔFOSB in D1- and D2-MSNs that mediate these various effects is a high priority of current research; known target genes such as those encoding the GLUA2 AMPAR subunit24 and CaMKIIα,23 plus many others that control functional and morphological plasticity of NAc glutamatergic synapses, are plausible candidates.24,64,65
In addition to drugs, stress, and antidepressants, other stimuli that affect the brain’s reward circuitry induce ΔFOSB accumulation in NAc. For instance’ natural rewards like high fat diet increase ΔFOSB protein in NAc,66 potentially in a subregion-specific manner.67,68 However, chronic mild food restriction can also induce ΔFOSB in NAc,60 suggesting that metabolic changes in either direction can affect expression, which likely occurs in different cell types as discussed above. Nevertheless, ΔFOSB expression in NAc appears critical for motivation to consume food, as overexpression of ΔFOSB in NAc enhanced food-reinforced instrumental performance and the progressive ratio response in rats69 and drove the preference for sucrose in mice.70 Nonfood rewards also induce ΔFOSB in NAc, including sexual experience,71,72 and blocking the ΔFOSB function in NAc prevents the acquisition of sexual proficiency in adult male rats,71 while overexpression of ΔFOSB in NAc of female hamsters enhances sexual reward.73 Moreover, ΔFOSB is induced in NAc by exercise, and overexpression of ΔFOSB in NAc D1-MSNs increases voluntary wheel running, while overexpression in D2-MSNs decreases it.74 Taken together, these studies establish cell type-specific ΔFOSB expression in NAc as a key indicator of reward circuit activation and an essential requirement for proper processing of virtually all forms of reward.
ΔFOSB Induction in Other Brain Regions.
ΔFOSB is induced by chronic neuronal activation and, as such, it is induced in many other brain regions upon repeated exposure to stimuli driving regional activity. For instance, chronic exposure to many drugs of abuse, including psychostimulants, ethanol, delta(9)-tetrahydrocannabinol, and morphine, induce ΔFOSB in brain regions as diverse as dorsal striatum, prefrontal cortex, and hippocampus in rodents, and many of these effects have been found in human drug users as well.40,63,75,76 ΔFOSB is also induced throughout the rodent brain by both chronic stress58,61 and chronic antidepressant exposure,58,77 and ΔFOSB is elevated in the prefrontal cortex of depression patients.78 Additionally, antipsychotics induce ΔFOSB throughout the rodent brain, as covered in detail by a recent review,79 and its induction in the prefrontal cortex has been associated with negative behavioral outcomes.80 Along those lines, ΔFOSB induction in response to repeated l-DOPA administration in a dopamine-lesioned rodent or nonhuman primate has been linked directly to abnormal dyskinetic movements that clinically limit l-DOPA therapy.25,26
ΔFOSB induction in the hippocampus has become an important topic in recent years. ΔFOSB accumulates in the hippocampus in response to externally induced electroconvulsive seizures in rats55,81 and multiple mouse genetic and physiological models driving repeated seizures.28,82–84 More recently, our groups have demonstrated that, in glutamatergic pyramidal neurons of the dorsal and ventral hippocampus, ΔFOSB accumulation decreases pyramidal cell excitability.31,85 Importantly, we demonstrated that viral overexpression of ΔFOSB reduces neuronal excitability and that viral expression of a dominant negative construct or knockout of the Fosb gene increases neuronal excitability, indicating that the native ΔFOSB protein is actively engaged in controlling hippocampal neuronal excitability.31,85 Together, these data suggest that ΔFOSB expression in response to high levels of neuronal activity may represent a critical negative feedback mechanism to reduce excitability, as in traditional homeostatic scaling,86 and that ΔFOSB dysfunction could potentially contribute to pathological states of hippocampal hyperexcitability, such as epilepsy. Indeed, Fosb knockout mice are prone to seizures.87
ΔFOSB expression in the dorsal hippocampus is critical for normal learning and memory. We have demonstrated that ΔFOSB is induced in the dorsal CA1 region after spatial learning in both mice and rats and that inhibition of the ΔFOSB function in the hippocampus prevents spatial and contextual learning.27 Critically, we also showed that viral overexpression of ΔFOSB in dorsal hippocampal neurons prevents learning,27 and other groups have gone on to demonstrate that ΔFOSB is highly induced in dorsal hippocampus in human AD patients and mouse models of AD and that inhibition of this ΔFOSB can reverse cognitive deficits in AD mice.28,29,88 The ventral hippocampus, though also important for learning and cognition, is more often correlated with emotional learning and associations between specific experiences and feelings of reward, fear, pain, or pleasure. ΔFOSB is induced in the ventral hippocampus by stress, antidepressants, and drugs of abuse,31,40,58,61,77 and we and others have shown that ventral hippocampal ΔFOSB expression is critical for resilience to stress31 and the antidepressant effects of ketamine.77
ΔFOSB is induced in many subregions of the cortex under conditions associated with their individual functions. For instance, ΔFOSB is induced in the prelimbic area of the mouse medial prefrontal cortex by stress, where it appears to promote stress susceptibility through modulation of specific output circuits.89 In the orbitofrontal cortex, however, ΔFOSB is critical for resilience to cognitive impairments caused by chronic cocaine exposure and impulsivity during subsequent withdrawal.90–92 ΔFOSB is induced in the infralimbic cortex by sexual experience, and viral overexpression of ΔFOSB in the hamster infralimbic cortex is sufficient to induce sexual proficiency and the attendant changes in dendritic spine morphology.30 These and other studies of ΔFOSB in cortex indicate that it accumulates in neurons in response to virtually any chronic behavior or stimulus that engages a particular cortical circuit and is often critical for key neuroadaptations in those cells and synapses underlying the long-term behavioral sequelae of these experiences.
ΔFOSB in Non-neuronal Cells.
Many AP1 proteins are critical for embryonic development and organogenesis, including JUN and FRA-1, but ΔFOSB is among the group including JUND and FOS that appear largely dispensable for embryonic development.7 Although rapidly induced in response to growth factors, ΔFOSB also appears to be dispensable for cell cycle progression, since fibroblasts and embryonic stem cells lacking Fosb gene expression have no proliferation defect.87 However, ectopic expression of ΔFOSB promotes differentiation in osteoblasts and increases bone formation.93,94 Moreover, Fosb is associated with tumorigenesis in bone,7 and rearrangements of the human Fosb gene are found in human bone tumors,95 though to a much lesser extent than rearrangements of FOS.95 FOSB and ΔFOSB can transform cells in culture,96 though the mechanism and transforming potential of ΔFOSB remains enigmatic.7
It is likely that the Fosb gene plays an important role in progenitor cell differentiation in the brain. In the mouse hippocampus, Fosb appears important for progenitor cell division and proliferation as Fosb null mice have impaired hippocampal neurogenesis and specific deletion of Fosb expression in the subgranular zone impairs cell proliferation and migration.97,98 Moreover, ΔFOSB is expressed in microglia in the hippocampus and is required for microglial activation.19 Other immune cells also express the Fosb gene, including mast cells99,100 and T cells, and Fosb induction in response to T cell activation has been implicated in T cell death.101,102 However, the gene targets of ΔFOSB and its function in most immune cells remain largely to be determined.
ΔFOSB STRUCTURE AND FUNCTION
AP1 complexes are formed by pairs of proteins derived from multigene families most commonly including one FOS (FOS, FOSB, FRA-1, and FRA-2) and one JUN (JUN, JUNB, JUND) protein as stated earlier. The heterodimer is bound together by complementary basic leucine zipper (bZIP) domains with each protein containing the requisite DNA-binding basic region and more variable transactivation domains (Figure 1A). When the two DNA binding regions are brought together, they bind the AP1 consensus sequence TGA(C/G)TCA most typically found in the promoter regions of target genes but now being identified increasingly in enhancer regions as they are delineated. FOSB and ΔFOSB are FOS family proteins both produced from the Fosb gene. ΔFOSB is generated by alternative splicing that does not introduce any novel amino acid residues but rather encodes a premature stop codon causing truncation of the C-terminal 101 amino acids in the full-length protein. As a result, ΔFOSB lacks two degron domains that are found in full-length FOSB and all other FOS family proteins and that target these proteins for rapid degradation via ubiquitinylation-dependent and -independent mechanisms.103 The lack of these degron domains is a key factor in ΔFOSB’s enhanced stability: unlike other FOS family proteins whose half-lives range from minutes to hours, ΔFOSB has a half-life in the mouse brain of ~8 days as stated earlier.17,39,104 Thus, while other FOS family members are expressed but quickly degrade after each stimulus, ΔFOSB accumulates in the brain in response to chronic stimuli including drugs, stress, antidepressants, consumption of natural rewards, learning, and seizures, among others24,27,39,58 (Figure 1B22).
Figure 1.
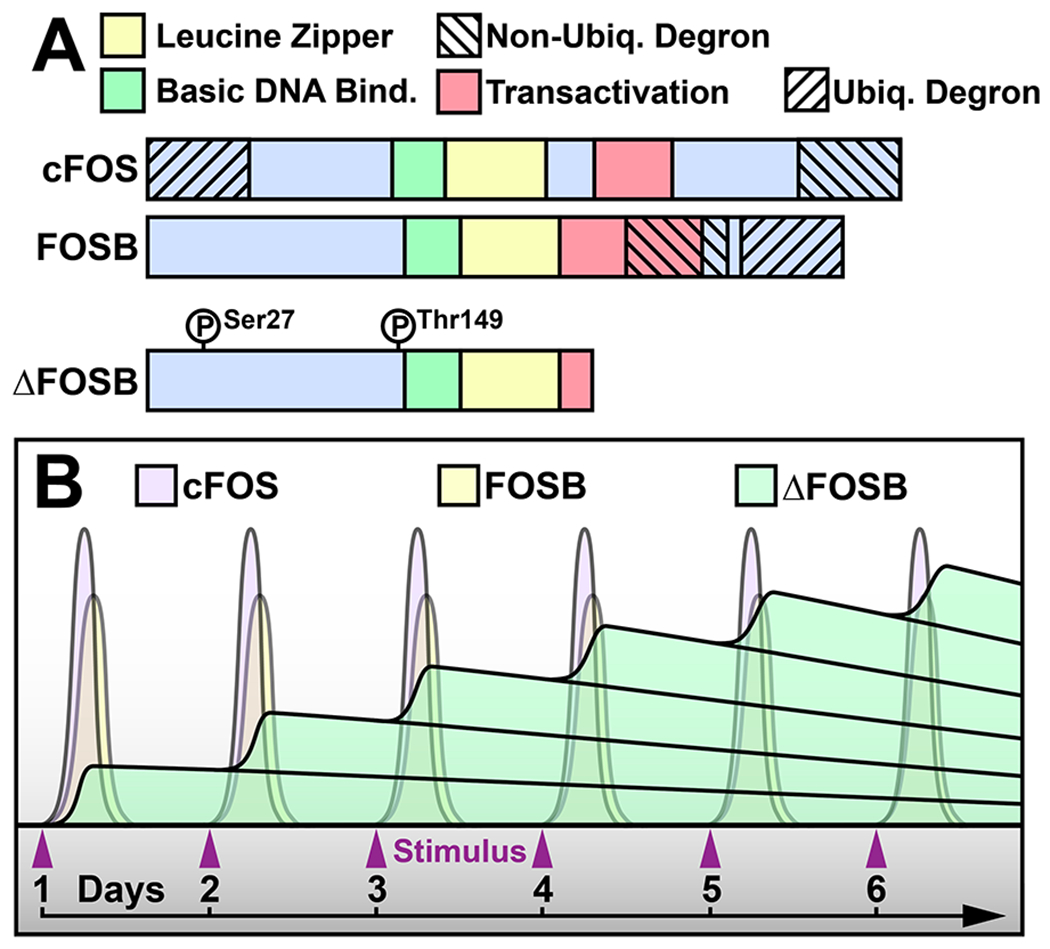
ΔFOSB’s stability allows it to accumulate with chronic stimuli. (A) FOS family protein domain structures. ΔFOSB lacks both ubiquitin-dependent and ubiquitin-independent degron domains present in other FOS family members’ C-terminal regions, reducing ΔFOSB’s proteolytic degradation. Phosphorylation at Ser27 also contributes to ΔFOSB stability. (B) While other FOS family members are strongly but transiently induced by daily stimuli, ΔFOSB is initially induced at lower levels but accumulates over many days of chronic activation.
ΔFOSB is further stabilized by phosphorylation. Multiple sites of ΔFOSB phosphorylation have been uncovered in vitro and in vivo, some of which are increased by stimuli like cocaine and may regulate its ability to transactivate gene expression.105 However, phosphorylation at serine 27 (S27) in the N-terminus of ΔFOSB increases its stability in cells and in the brain.17,103,106,107 S27 of ΔFOSB can be phosphorylated by casein kinase 2 in cells,107 and Ca2+/calmodulin-dependent protein kinase IIα can phosphorylate S27 in cells and appears to mediate ΔFOSB stability in the brain.21 Interestingly, ΔFOSB increases Camk2a gene expression in mouse NAc in response to cocaine, suggesting a positive feed-forward loop mediating long-lasting effects of the drug.21
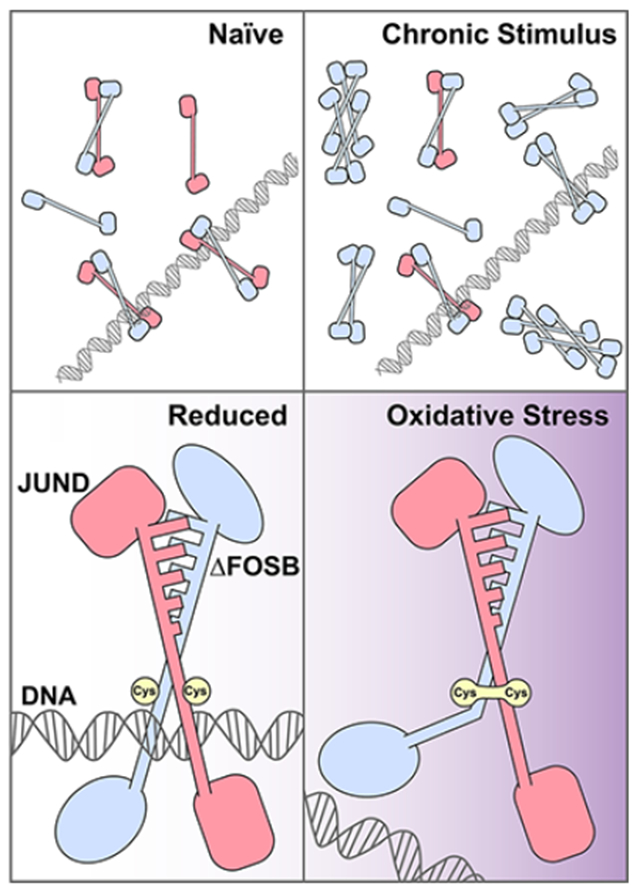
In recent years, X-ray crystallography and in vitro biochemistry have shed new light on the ΔFOSB structure and potential complex formation. Although most data point to other FOS family proteins requiring a JUN family partner to form a function AP1 complex,7 ΔFOSB uniquely can form homodimers that bind AP1 consensus sequences in vitro.108 Further, the bZIP domain of ΔFOSB can even form tetrameric structures that defy canonical AP1 arrangements (Figure 2A), and we have provided some evidence that ΔFOSB is found in complexes consistent with tetramers in cultured cells.109 These observations suggest that, once a chronic stimulus causes induction of the ΔFOSB protein exceeding a threshold within a cell, perhaps swamping ambient concentrations of JUND, its major binding partner in vivo,110 self-assembly occurs generating several noncanonical molecular arrangements whose function and potential gene targets have yet to be determined. Moreover, ΔFOSB complexes may be stabilized by oxidation, as recent in vitro (cell-free and cell-based) experiments suggest that ΔFOSB cysteine 172 (C172) can form a disulfide bond with C279 of JUND in the canonical heterodimeric AP1 complex and that ΔFOSB C172 may form a disulfide bond with C172 of a partner ΔFOSB in a noncanonical homodimeric complex.109,111
Figure 2.

ΔFOSB structure and function are dynamic. (A) Under naive conditions, ΔFOSB predominantly forms high-affinity heterodimers with JUND. Upon accumulation in response to chronic stimuli, ΔFOSB forms homodimers or larger oligomers that may have different DNA binding affinity or target sites. (B) ΔFOSB and JUND heterodimerize via a leucine zipper motif, allowing DNA binding and bringing cysteines 172 and 285 into close proximity. Upon oxidative stress, a disulfide bond forms between the two cysteines, causing a kink in the ΔFOSB helical structure and preventing the complex from binding DNA.
These properties, some unique to ΔFOSB and others shared by FOS/JUN family members, have made ΔFOSB an intriguing target of study in fields ranging from neuropsychiatric disease to oncology for decades. However, to fully elucidate the role of ΔFOSB in physiology and disease and to potentially exploit it as a target for therapeutic intervention, many outstanding questions remain to be addressed.
OUTSTANDING QUESTIONS OF ΔFOSB FUNCTION
Although a great deal has been learned about the functional relevance of ΔFOSB by overexpressing or inhibiting it with transgenic mice or viral vectors and by deleting the Fosb gene, our understanding of the mechanism by which ΔFOSB controls cellular function remains largely inadequate due to our incomplete knowledge of ΔFOSB target genes. Early studies of ΔFOSB targets in brain used gene expression microarrays to probe the effects of ΔFOSB overexpression on the profiles of hundreds or thousands of candidate genes in homogenates from a specific brain region. This approach identified targets like Cdk5 in the hippocampus and Fos in the NAc.33,55 Other early studies used a candidate gene approach, uncovering targets like Gria2 using Western blotting of its gene product, GLUA2, in NAc of mice overexpressing ΔFOSB with the preexisting rationale that NAc AMPA receptors were already known to be critical for many of the same behavioral responses to cocaine regulated by ΔFOSB.20 Later studies often confirmed these targets in a brain region-specific manner using chromatin immunoprecipitation (ChIP) and qPCR.24 An earlier study employed promoter microarrays to identify genomic binding targets of endogenous ΔFOSB in NAc under control and chronic cocaine conditions.112 However, these approaches were limited as they relied upon preconceived rationales for choosing candidate target genes, were restricted to a very small subset of genomic regions (e.g., proximal promoters) as putative ΔFOSB-binding sites, and lacked the quantitative power of more recently developed sequencing-based technologies.
More recently, ChIP has been combined with next generation sequencing (ChIPseq) to enable an unbiased and genome-wide survey of ΔFOSB targets. This approach yielded some exciting results in the hippocampus in models of seizure and AD,28,29,88 in one case uncovering Calb1 (which encodes calbindin-1) as a key gene target in the dentate gyrus mediating the severity of cognitive impairment in a mouse model of AD.28 However, the use of ChIPseq to determine ΔFOSB targets in other brain regions, most notably NAc, has remained challenging, likely due to the very small amount of tissue available and the lack of selective high-affinity antibodies for ΔFOSB. For this reason, our groups and others have begun to adopt the Cleavage Under Targets and Release Using Nuclease (CUT&RUN) technique,113 a strategy in which antibody-targeted controlled cleavage by micrococcal nuclease releases the ΔFOSB–DNA complexes such that the specifically bound DNA can then be sequenced and identified. This technique avoids the cross-linking and solubilization issues inherent to ChIP, and we have found that it indeed produces a stronger signal-to-noise ratio requiring much less input chromatin and less sequencing depth than traditional ChIP in both brain samples and cultured cells.114 For example, it is now possible to obtain high quality CUT&RUN maps of genome-wide binding patterns of endogenous ΔFOSB in isolated D1-MSNs and D2-MSNs of mouse NAc.114 Critical advances that may arise from these unbiased sequencing approaches include not only determining novel gene targets underlying the biological effects of ΔFOSB but also identifying the sites where ΔFOSB binds to enhancers, other intergenic regions, intragenic regions, or other elements that may be as crucial as gene promoters.
One of the key factors complicating the study of ΔFOSB is that it, like any TF, has different gene targets in different cell types and brain regions. For instance, ΔFOSB regulates Calb1 in hippocampus,28,55,88 but has not been shown to bind this gene in NAc. Conversely, multiple studies have confirmed that ΔFOSB exerts many of its effects on NAc D1-MSNs and subsequent reward behaviors through binding and regulation of Gria2,20,24,115,116 but Gria2 does not appear to be a ΔFOSB target in hippocampus, a brain region wherein AMPA receptor expression is absolutely central to synaptic function and learning. Such cell type-specific effects of ΔFOSB were highlighted in a recent paper that showed the very different range of target genes induced or suppressed upon induction of endogenous ΔFOSB in D1-MSNs vs D2-MSNs of mouse NAc by use of locus-specific neuroepigenome-editing tools.117 It is not clear why ΔFOSB or other TFs have different targets in different cell types or brain regions, but the likely explanation is that the chromatin state at those genes is differentially regulated in the different cells, allowing or preventing ΔFOSB (or another TF) access to target sequences in a cell-specific manner. This epigenetic explanation is supported by findings that altered histone acetylation at the promoter region of Camk2a in the NAc is required for fluoxetine-dependent regulation of ΔFOSB binding at this site and subsequent behavioral effects of the drug.23
Similar questions have arisen from the observation that different stimuli can cause ΔFOSB regulation of distinct target genes in the same cells or brain region. For example, chronic exposure to either cocaine or morphine strongly induces ΔFOSB expression in NAc D1-MSNs, but even within this single cell type, the two drugs cause ΔFOSB transactivation of partly distinct target genes. For instance, Sirt1 (which encodes the protein deacetylase sirtuin-1), identified originally as a target for ΔFOSB in the context of chronic cocaine exposure in an early ChIP-promoter array study,112 was found to be similarly induced via ΔFOSB in NAc by chronic cocaine and by chronic morphine, while its isoform Sirt2 was found to be bound by ΔFOSB and induced in NAc in response to chronic cocaine only.118 Current research is investigating whether cocaine induces another factor that is required for ΔFOSB binding to Sirt2 or whether morphine induces a factor that prevents that binding.
It has long been observed that ΔFOSB can function as either a transcriptional activator, as in the case with Gria2,20,24 or a transcriptional repressor, as with Fos or Pdyn,28,50,119 but the mechanisms controlling this dichotomy are only partially understood, not only for ΔFOSB but also for most TFs. One potential mechanism could again be epigenetic control of the 3D conformation of chromatin. At the Cdk5 gene, ΔFOSB recruits the SWItch/Sucrose Non-Fermentable (SWI/SNF) chromatin remodeling complex as well as histone acetyltransferases with the combined effect of destabilizing DNA–histone interactions and increasing nucleosome spacing along the Cdk5 gene, which along with other changes render the gene in a conformation permissive for transcription.120 In contrast, chronic amphetamine drives increased ΔFOSB binding to the Fos gene where it recruits specific histone deacetylases, decreasing histone acetylation and presumably shifting the chromatin into a tighter, more repressed structure that reduces Fos expression.118 It will be critical in future studies to overlay RNaseq and DNA regions with altered histone modifications with genome-wide ΔFOSB binding sites, perhaps using CUT&RUN, to determine the specific sites at which ΔFOSB drives permissive vs repressive chromatin modification to control differential gene expression (Figure 3).
Figure 3.
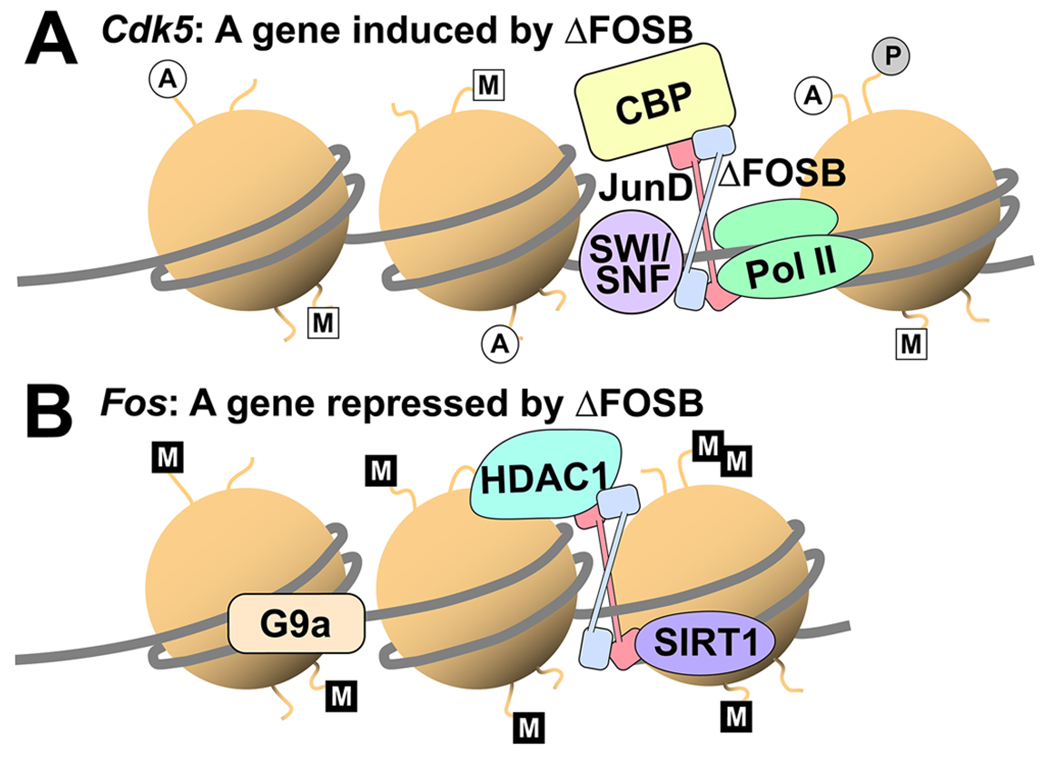
ΔFOSB as a transcriptional activator and repressor. (A) At some genes, like Cdk5, ΔFOSB/JUND (blue and pink) acts to recruit histone acetyltransferases like CREB binding protein (CBP), promoting histone acetylation (white circles) and binding of the SWI/SNF chromatin remodeling complex, which allows greater separation of nucleosomes. The resulting permissive chromatin conformation, including the activating trimethylation of H3 at lysine 4 (white squares) and histone phosphorylation (gray circle), allows binding of RNA polymerase (Pol II) and the basal transcription complex with subsequent gene expression. (B) At other genes, such as Fos, ΔFOSB/JUND recruits histone deacetylases like HDAC1 and sirtuin-1 (SIRT1), resulting in reduced histone acetylation and allowing histone methyltransferases like G9a to dimethylate H3 at lysine 9 (black squares). This results in a repressive chromatin state in which the gene is not readily expressed.
For many decades, immunohistochemical staining of immediate early gene products, like FOS, has been used to identify neuronal populations active just prior to the death of an animal, helping to identify cell types or brain regions whose activity is acutely associated with specific stimuli or behaviors. More recently, the Fos promoter has been used to drive expression of tools allowing labeling of neuronal ensembles encoding specific memories or behaviors. Critically, these FOS-expressing ensembles can be subsequently manipulated to affect related behavioral outcomes.121 For instance, disruption of the activity of NAc neurons expressing FOS in response to cocaine prevents locomotor sensitization to the drug.122 ΔFOSB’s unique stability can likewise be exploited to identify cell populations that have been chronically stimulated.40,58,61 This raises the intriguing possibility that ΔFOSB could be exploited as a molecular handle to identify and then specifically manipulate neuronal ensembles chronically activated by specific stimuli, allowing a better understanding of functional regulation over time and causal connection to the behavioral sequelae of chronic conditions. Indeed, there is some evidence that ΔFOSB accumulates in repeatedly activated NAc ensembles that are specifically associated with the environment in which the drug is administered,123 indicating that functionally manipulating ΔFOSB could selectively affect behaviors associated with these ensembles.
The mechanisms by which ΔFOSB is induced are only partially understood. For instance, we know that ΔFOSB induction in NAc by chronic cocaine is dependent upon three TFs: cAMP response element binding protein (CREB), serum response factor (SRF), and E2F3a.124,125 In contrast, its induction in the same brain region by chronic stress appears to require only SRF.126 Moreover, epigenetic control of the chromatin state at the Fosb gene plays a clear role in cocaine-mediated ΔFOSB expression in both NAc and hippocampus.32,75,127 However, the TFs and epigenetic modifiers controlling Fosb gene expression at baseline or after stimulation in specific cell types are not known. It may prove critical to uncover cell type-specific upstream pathways driving ΔFOSB expression, as they could be exploited systemically to control the function of specific neurons underlying a syndrome associated with ΔFOSB without affecting the critical functions of ΔFOSB in other, unaffected cells.
ΔFOSB AS A MEDICINAL TARGET
As we have described, ΔFOSB is expressed in many different cell types and tissues in response to stimuli both natural and artificial, and its functions range from mediating responses to psychotropic drugs, to controlling stress susceptibility vs resilience, to facilitating learning and memory, to transforming bone cells to drive tumor growth, and beyond. As such, ΔFOSB has often been questioned as a viable target for therapeutic intervention in the many disease states with which it is associated due to presumptive concerns of side effects in any of these diverse areas of function. However, it has become apparent that TFs with a similar diversity of functions and gene targets are viable pharmacological targets for the treatment of disease.128 One of the clearest examples of this advance has been the selective inhibition of FOS-containing AP1 complexes by the drug T-5224. This compound has been proven to be effective in reversing arthritis and spinal cartilage degeneration in preclinical mouse models,35,36 and Toyoma Chemical has entered this compound in Phase II trials for the treatment of rheumatoid arthritis.
One of the key mechanisms allowing the targeting of many proteins previously considered “undruggable” has been the advent of compounds that covalently bind to cysteine residues. For example, the chemotherapeutic afatinib targets cysteines in epidermal growth factor receptor and has been proven to be effective for treating specific forms of lung cancer.129 The binding of ΔFOSB to its AP1 response element in DNA is modulated by oxidation of a cysteine residue, and targeting this site could become a key strategy for finding compounds that modulate ΔFOSB function but avoid some of the potential side effects. Specifically, the ΔFOSB bZIP domain must undergo a large conformational rearrangement that is controlled by a “redox switch” at C172 when converting from an oxidized to a reduced state in order to adopt a DNA-binding compatible form111 (Figure 2B), and studies with purified proteins suggest that a ΔFOSB/JUND dimer undergoes this structural transition and can no longer bind to an AP1 DNA sequence when it is oxidized and the disulfide bond is present, suggesting that the ΔFOSB function is sensitive to cellular redox homeostasis.111 This is consistent with reports that AP1 complexes of FOS and JUN are also oxidized at conserved cysteine residues, which prevents DNA binding,130 suggesting that such disulfide bonds may be a conserved mechanism for controlling ΔFOSB transactivation in response to the cellular redox state. It is possible to conceive of compounds that allow modulation of the specific transcriptional effects of ΔFOSB downstream of oxidation for the treatment of diseases associated with increased oxidative stress. For instance, it is well-known that many neurodegenerative diseases cause oxidative stress in affected brain regions, and it is likely that this contributes to neurodegeneration,131 although antioxidant therapies have been largely unsuccessful.132,133 It is possible that some of the deleterious effects of oxidative stress driving memory impairments, aberrant behavior, or even neurodegeneration occur via long-term changes in gene expression through altered ΔFOSB expression28,29 and also perhaps redox-regulated function. Thus, compounds targeting the ΔFOSB redox switch could prevent or reverse changes in gene expression in specifically affected brain regions but might have fewer “off-target” effects on ΔFOSB in brain regions where oxidative stress is lower or in bone or immune cells, where neurodegenerative diseases may have no effect on redox state at all. Of course, many other syndromes in which ΔFOSB plays a central or potential role, like cocaine addiction or epilepsy, also involve alterations in redox state,134,135 and thus compounds targeting the ΔFOSB redox switch may prove to have multiple therapeutic but safe uses by only affecting tissues where ΔFOSB accumulates concomitant with oxidative stress.
Excitingly, collaborations involving our groups, structural biologists, and medicinal chemists have uncovered compounds targeting ΔFOSB,115 and multiple classes of compounds targeting ΔFOSB/DNA interactions are rapidly coming to light.136 We are employing a strategy informed by protein structural data, taking advantage of the findings from our groups and others demonstrating novel ΔFOSB conformations, oligomerization partners (e.g., heterodimers vs homodimers and potential multimers), and post-translational modifications like oxidation or phosphorylation. We are using cell-free, cell-based, and mouse model assays to screen existing compound libraries to find compound scaffolds that can then be further modified by medicinal chemistry to refine and improve specificity, efficacy, and bioavailability in an iterative process. We are using this approach with the goal of uncovering both ΔFOSB antagonists and potentiators, as inhibiting ΔFOSB as it accumulates in one cell type in NAc could prove useful for reducing the rewarding effects of abused drugs and decreasing relapse to addiction, for instance, while potentiating the function of ΔFOSB as it accumulates in the hippocampus after seizures could reduce neuronal excitability and prevent recurrence of seizure activity.
The challenge, of course, will be to modulate the function of ΔFOSB in cells or tissues requiring treatment while minimizing effects on ΔFOSB underlying normal cell functions and physiological or behavioral outputs, like memory formation or bone growth. The very nature of ΔFOSB accumulation may provide some traction in this regard. Because ΔFOSB accumulates due to chronic cell activity, its function is by nature exaggerated in the cells and ensembles associated with the stimuli causing ΔFOSB production. For instance, epileptiform activity induces ΔFOSB in the regions that are initial foci of the seizure, like the hippocampus, for example.28,29,55,137 In contrast, ethanol exposure causes a greater accumulation of ΔFOSB in the nucleus accumbens than in the hippocampus.40 Thus, while ΔFOSB has many functions throughout the brain in behaviors unrelated to each particular disease state, systemically administered compounds that modulate ΔFOSB function may have the greatest effects on the cells that have ΔFOSB accumulation, and those cells may drive the physiological and behavioral outcomes specifically associated with the disease, such as seizures or alcohol abuse. The key to targeting the cells and systems underlying a disease state with minimal effects on those expressing ΔFOSB as a function of normal physiology may therefore be accurate dosing or potency of ΔFOSB-targeting compounds. This idea is not without precedent, as low doses of acetylsalicylic acid (aspirin, 75 to 81 mg/day) are sufficient to irreversibly acetylate serine 530 of cyclooxygenase (COX)-1 and produce antithrombotic effects, whereas higher doses (650 mg to 4 g/day) inhibit COX-1 and COX-2, blocking prostaglandin (PG) production and having analgesic and antipyretic effects.138
As mentioned above, it is possible that specificity could also be partially achieved by small molecules targeting the redox switch or a specific arrangement of ΔFOSB oligomerization. However, it is also conceivable that systemically administered viral vectors that cross the blood–brain barrier could drive expression or inhibition of ΔFOSB with cell type-specific promoters to selectively target the affected cell populations. Indeed, in mouse brain, we have used viral vectors in combination with CRISPR technology to achieve knockdown of ΔFOSB expression in specific circuits,31 and we have used viral vector epigenetic remodeling of the Fosb promoter to knock down ΔFOSB in specific cell types.57,127 The transition of such technology from injections into mouse brain to safe and systemic treatments in the clinic will be an enormous challenge, but there is reason to expect that viral gene therapy for the treatment of neurological and psychiatric disorders will become a reality in the coming decades.139–142
ACKNOWLEDGMENTS
A.J.R. and E.J.N. acknowledge support from the National Institutes of Mental Health (R01MH111604, R01MH051399) and the National Institutes of Drug Abuse (R01DA040621, R01DA007359, P01DA0047233). A.J.R. also acknowledges support from the National Institutes of Neurological Disease and Stroke (NS085171) and the National Institute of Child Health and Human Development (HD072968). E.J.N. also received support from the Hope for Depression Research Foundation.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acschemneuro.1c00723
Contributor Information
Alfred J. Robison, Department of Physiology, Michigan State University, East Lansing, Michigan 48824, United States.
Eric J. Nestler, Nash Family Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, New York 10029, United States
REFERENCES
- (1).Phillips T Regulation of Transcription and Gene Expression in Eukaryotes. Nature Education 2008, 1 (1), 199. [Google Scholar]
- (2).Tiffon C The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci 2018, 19 (11), 3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Robison AJ; Nestler EJ Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci 2011, 12 (11), 623–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Abel T; Havekes R; Saletin JM; Walker MP Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol 2013, 23 (17), R774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lambert SA; Jolma A; Campitelli LF; Das PK; Yin Y; Albu M; Chen X; Taipale J; Hughes TR; Weirauch MT The Human Transcription Factors. Cell 2018, 175 (2), 598–599. [DOI] [PubMed] [Google Scholar]
- (6).Bejjani F; Evanno E; Zibara K; Piechaczyk M; Jariel-Encontre I The AP-1 transcriptional complex: Local switch or remote command?. Biochim Biophys Acta Rev Cancer 2019, 1872 (1), 11–23. [DOI] [PubMed] [Google Scholar]
- (7).Jochum W; Passegue E; Wagner EF AP-1 in mouse development and tumorigenesis. Oncogene 2001, 20 (19), 2401–12. [DOI] [PubMed] [Google Scholar]
- (8).Manning CE; Williams ES; Robison AJ Reward Network Immediate Early Gene Expression in Mood Disorders. Front Behav Neurosci 2017, 11, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sheng M; Greenberg ME The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4 (4), 477–85. [DOI] [PubMed] [Google Scholar]
- (10).Nestler EJ FosB: A transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol 2015, 753, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sheng M; Greenberg ME The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4 (4), 477–485. [DOI] [PubMed] [Google Scholar]
- (12).Kovács KJ Invited review c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int 1998, 33 (4), 287–297. [DOI] [PubMed] [Google Scholar]
- (13).Ferrara P; Andermarcher E; Bossis G; Acquaviva C; Brockly F; Jariel-Encontre I; Piechaczyk M The structural determinants responsible for c-Fos protein proteasomal degradation differ according to the conditions of expression. Oncogene 2003, 22 (10), 1461–1474. [DOI] [PubMed] [Google Scholar]
- (14).Alberini CM Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev 2009, 89 (1), 121–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).West AE; Greenberg ME Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harbor Perspectives in Biology 2011, 3 (6), a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Andersson M; Westin JE; Cenci MA Time course of striatal ΔFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur. J. Neurosci 2003, 17 (3), 661–666. [DOI] [PubMed] [Google Scholar]
- (17).Ulery-Reynolds PG; Castillo MA; Vialou V; Russo SJ; Nestler EJ Phosphorylation of DeltaFosB mediates its stability in vivo. Neuroscience 2009, 158 (2), 369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hope BT; Nye HE; Kelz MB; Self DW; Iadarola MJ ; Nakabeppu Y; Duman RS; Nestler EJ Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 1994, 13 (5), 1235–1244. [DOI] [PubMed] [Google Scholar]
- (19).Nomaru H; Sakumi K; Katogi A; Ohnishi YN; Kajitani K ; Tsuchimoto D; Nestler EJ; Nakabeppu Y Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia. Glia 2014, 62 (8), 1284–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kelz MB; Chen J; Carlezon WA Jr; Whisler K; Gilden L; Beckmann AM; Steffen C; Zhang YJ; Marotti L; Self DW; Tkatch T; Baranauskas G; Surmeier DJ; Neve RL; Duman RS; Picciotto MR; Nestler EJ Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature 1999, 401 (6750), 272–6. [DOI] [PubMed] [Google Scholar]
- (21).Robison AJ; Vialou V; Mazei-Robison M; Feng J; Kourrich S; Collins M; Wee S; Koob G; Turecki G; Neve R; Thomas M; Nestler EJ Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J. Neurosci 2013, 33 (10), 4295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hope BT; Nye HE; Kelz MB; Self DW; Iadarola MJ; Nakabeppu Y; Duman RS; Nestler EJ Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 1994, 13 (5), 1235–44. [DOI] [PubMed] [Google Scholar]
- (23).Robison AJ; Vialou V; Sun HS; Labonte B; Golden SA; Dias C; Turecki G; Tamminga C; Russo S; Mazei-Robison M; Nestler EJ Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 2014, 39 (5), 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Vialou V; Robison AJ; Laplant QC; Covington HE 3rd; Dietz DM; Ohnishi YN; Mouzon E; Rush AJ 3rd; Watts EL; Wallace DL; Iniguez SD; Ohnishi YH; Steiner MA; Warren BL; Krishnan V; Bolanos CA; Neve RL; Ghose S; Berton O; Tamminga CA; Nestler EJ DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci 2010, 13 (6), 745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Feyder M; Sodersten E; Santini E; Vialou V; LaPlant Q; Watts EL; Spigolon G; Hansen K; Caboche J; Nestler EJ; Fisone G A Role for Mitogen- and Stress-Activated Kinase 1 in L-DOPA-Induced Dyskinesia and FosB Expression. Biol. Psychiatry 2016, 79 (5), 362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Berton O; Guigoni C; Li Q; Bioulac BH; Aubert I; Gross CE; Dileone RJ; Nestler EJ; Bezard E Striatal overexpression of DeltaJunD resets L-DOPA-induced dyskinesia in a primate model of Parkinson disease. Biol. Psychiatry 2009, 66 (6), 554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Eagle AL; Gajewski PA; Yang M; Kechner ME; Al Masraf BS; Kennedy PJ; Wang H; Mazei-Robison MS; Robison AJ Experience-Dependent Induction of Hippocampal DeltaFosB Controls Learning. J. Neurosci 2015, 35 (40), 13773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).You JC; Muralidharan K; Park JW; Petrof I; Pyfer MS; Corbett BF; LaFrancois JJ; Zheng Y; Zhang X; Mohila CA; Yoshor D; Rissman RA; Nestler EJ; Scharfman HE; Chin J Epigenetic suppression of hippocampal calbindin-D28k by DeltaFosB drives seizure-related cognitive deficits. Nat. Med 2017, 23 (11), 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Corbett BF; You JC; Zhang X; Pyfer MS; Tosi U; Iascone DM; Petrof I; Hazra A; Fu CH; Stephens GS; Ashok AA; Aschmies S; Zhao L; Nestler EJ; Chin J DeltaFosB Regulates Gene Expression and Cognitive Dysfunction in a Mouse Model of Alzheimer’s Disease. Cell reports 2017, 20 (2), 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).De Lorme KC; Staffend-Michael NA; Simmons SC; Robison AJ; Sisk CL Pubertal Testosterone Programs Adult Behavioral Adaptations to Sexual Experience through Infralimbic Cortex DeltaFosB. eNeuro 2019, 6 (3), ENEURO.0176-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Eagle AL; Manning CE; Williams ES; Bastle RM; Gajewski PA; Garrison A; Wirtz AJ; Akguen S; Brandel-Ankrapp K; Endege W; Boyce FM; Ohnishi YN; Mazei-Robison M; Maze I; Neve RL; Robison AJ Circuit-specific hippocampal DeltaFosB underlies resilience to stress-induced social avoidance. Nat. Commun 2020, 11 (1), 4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Maze I; Covington HE 3rd; Dietz DM; LaPlant Q; Renthal W; Russo SJ; Mechanic M; Mouzon E; Neve RL; Haggarty SJ; Ren Y; Sampath SC; Hurd YL; Greengard P; Tarakhovsky A; Schaefer A; Nestler EJ Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 2010, 327 (5962), 213–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).McClung CA; Nestler EJ Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci 2003, 6 (11), 1208–15. [DOI] [PubMed] [Google Scholar]
- (34).Arjmand B; Larijani B; Sheikh Hosseini M; Payab M; Gilany K; Goodarzi P; Parhizkar Roudsari P; Amanollahi Baharvand M; Hoseini Mohammadi NS The Horizon of Gene Therapy in Modern Medicine: Advances and Challenges. Adv. Exp. Med. Biol 2019, 1247, 33–64. [DOI] [PubMed] [Google Scholar]
- (35).Makino H; Seki S; Yahara Y; Shiozawa S; Aikawa Y; Motomura H; Nogami M; Watanabe K; Sainoh T; Ito H; Tsumaki N; Kawaguchi Y; Yamazaki M; Kimura T A selective inhibition of c-Fos/activator protein-1 as a potential therapeutic target for intervertebral disc degeneration and associated pain. Sci. Rep 2017, 7 (1), 16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Aikawa Y; Morimoto K; Yamamoto T; Chaki H; Hashiramoto A; Narita H; Hirono S; Shiozawa S Treatment of arthritis with a selective inhibitor of c-Fos/activator protein-1. Nat. Biotechnol 2008, 26 (7), 817–23. [DOI] [PubMed] [Google Scholar]
- (37).Hope B; Kosofsky B; Hyman SE; Nestler EJ Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc. Natl. Acad. Sci. U. S. A 1992, 89 (13), 5764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hiroi N; Brown JR; Haile CN; Ye H; Greenberg ME; Nestler EJ FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. Proc. Natl. Acad. Sci. U. S. A 1997, 94 (19), 10397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Chen J; Kelz MB; Hope BT; Nakabeppu Y; Nestler EJ Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J. Neurosci 1997, 17 (13), 4933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Perrotti LI; Weaver RR; Robison B; Renthal W; Maze I; Yazdani S; Elmore RG; Knapp DJ; Selley DE; Martin BR; Sim-Selley L; Bachtell RK; Self DW; Nestler EJ Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse 2008, 62 (5), 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Nye HE; Nestler EJ Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol. Pharmacol 1996, 49 (4), 636–645. [PubMed] [Google Scholar]
- (42).Pich EM; Pagliusi SR; Tessari M; Talabot-Ayer D; Hooft van Huijsduijnen R; Chiamulera C Common neural substrates for the addictive properties of nicotine and cocaine. Science 1997, 275 (5296), 83–86. [DOI] [PubMed] [Google Scholar]
- (43).McDaid J; Graham MP; Napier TC Methamphetamine-induced sensitization differentially alters pCREB and DeltaFosB throughout the limbic circuit of the mammalian brain. Mol. Pharmacol 2006, 70 (6), 2064–74. [DOI] [PubMed] [Google Scholar]
- (44).Nye HE; Hope BT; Kelz MB; Iadarola M; Nestler EJ Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J. Pharmacol Exp Ther 1995, 275 (3), 1671–1680. [PubMed] [Google Scholar]
- (45).Lee KW; Kim Y; Kim AM; Helmin K; Nairn AC; Greengard P Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A 2006, 103 (9), 3399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Muller DL; Unterwald EM D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J. Pharmacol Exp Ther 2005, 314 (1), 148–54. [DOI] [PubMed] [Google Scholar]
- (47).Moratalla R; Elibol B; Vallejo M; Graybiel AM Network-level changes in expression of inducible Fos-Jun proteins in the striatum during chronic cocaine treatment and withdrawal. Neuron 1996, 17 (1), 147–56. [DOI] [PubMed] [Google Scholar]
- (48).Lobo MK; Zaman S; Damez-Werno DM; Koo JW; Bagot RC; DiNieri JA; Nugent A; Finkel E; Chaudhury D; Chandra R; Riberio E; Rabkin J; Mouzon E; Cachope R; Cheer JF; Han MH; Dietz DM; Self DW; Hurd YL; Vialou V; Nestler EJ DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J. Neurosci 2013, 33 (47), 18381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Grueter BA; Robison AJ; Neve RL; Nestler EJ; Malenka RC FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (5), 1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zachariou V; Bolanos CA; Selley DE; Theobald D; Cassidy MP; Kelz MB; Shaw-Lutchman T; Berton O; Sim-Selley LJ; Dileone RJ; Kumar A; Nestler EJ An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat. Neurosci 2006, 9 (2), 205–11. [DOI] [PubMed] [Google Scholar]
- (51).Colby CR; Whisler K; Steffen C; Nestler EJ; Self DW Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine. J. Neurosci 2003, 23 (6), 2488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Larson EB; Akkentli F; Edwards S; Graham DL; Simmons DL; Alibhai IN; Nestler EJ; Self DW Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. J. Neurochem 2010, 115 (1), 112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Thomas MJ; Beurrier C; Bonci A; Malenka RC Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci 2001, 4 (12), 1217–23. [DOI] [PubMed] [Google Scholar]
- (54).Pierce RC; Wolf ME Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. Cold Spring Harbor perspectives in medicine 2013, 3 (2), a012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Chen J; Zhang Y; Kelz MB; Steffen C; Ang ES; Zeng L; Nestler EJ Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of [Delta]-FosB. J. Neurosci 2000, 20 (24), 8965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Cooper SE; Kechner M; Caraballo-Perez D; Kaska S; Robison AJ; Mazei-Robison MS Comparison of chronic physical and emotional social defeat stress effects on mesocorticolimbic circuit activation and voluntary consumption of morphine. Sci. Rep 2017, 7 (1), 8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Hamilton PJ; Burek DJ; Lombroso SI; Neve RL; Robison AJ; Nestler EJ; Heller EA Cell-Type-Specific Epigenetic Editing at the Fosb Gene Controls Susceptibility to Social Defeat Stress. Neuropsychopharmacology 2018, 43 (2), 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Vialou V; Thibault M; Kaska S; Cooper S; Gajewski P; Eagle A; Mazei-Robison M; Nestler EJ; Robison AJ Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 2015, 99, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Staples LG; McGregor IS; Hunt GE Long-lasting FosB/DeltaFosB immunoreactivity in the rat brain after repeated cat odor exposure. Neurosci. Lett 2009, 462 (2), 157–61. [DOI] [PubMed] [Google Scholar]
- (60).Stamp JA; Mashoodh R; van Kampen JM; Robertson HA Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008, 1204, 94–101. [DOI] [PubMed] [Google Scholar]
- (61).Perrotti LI; Hadeishi Y; Ulery PG; Barrot M; Monteggia L; Duman RS; Nestler EJ Induction of deltaFosB in reward-related brain structures after chronic stress. J. Neurosci 2004, 24 (47), 10594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Francis TC; Chandra R; Friend DM; Finkel E; Dayrit G; Miranda J; Brooks JM; Iniguez SD; O’Donnell P; Kravitz A; Lobo MK Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry 2015, 77 (3), 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Gajewski PA; Turecki G; Robison AJ Differential Expression of FosB Proteins and Potential Target Genes in Select Brain Regions of Addiction and Depression Patients. PLoS One 2016, 11 (8), No. e0160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Christoffel DJ; Golden SA; Dumitriu D; Robison AJ; Janssen WG; Ahn HF; Krishnan V; Reyes CM; Han MH; Ables JL; Eisch AJ; Dietz DM; Ferguson D; Neve RL; Greengard P; Kim Y; Morrison JH; Russo SJ IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci 2011, 31 (1), 314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Dong C; Zhang JC; Yao W; Ren Q; Ma M; Yang C; Chaki S; Hashimoto K Rapid and Sustained Antidepressant Action of the mGlu2/3 Receptor Antagonist MGS0039 in the Social Defeat Stress Model: Comparison with Ketamine. Int. J. Neuropsychopharmacol 2017, 20 (3), 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Sharma S; Fernandes MF; Fulton S Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int. J. Obes (Lond) 2013, 37 (9), 1183–91. [DOI] [PubMed] [Google Scholar]
- (67).Tobiansky DJ; Kachkovski GV; Enos RT; Schmidt KL; Murphy EA; Soma KK Sucrose consumption alters steroid and dopamine signalling in the female rat brain. J. Endocrinol 2020, 245 (2), 231–246. [DOI] [PubMed] [Google Scholar]
- (68).Teegarden SL; Nestler EJ; Bale TL Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol. Psychiatry 2008, 64 (11), 941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Olausson P; Jentsch JD; Tronson N; Neve RL; Nestler EJ; Taylor JR DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J. Neurosci 2006, 26 (36), 9196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Wallace DL; Vialou V; Rios L; Carle-Florence TL; Chakravarty S; Kumar A; Graham DL; Green TA; Kirk A; Iniguez SD; Perrotti LI; Barrot M; DiLeone RJ; Nestler EJ; Bolanos-Guzman CA The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J. Neurosci 2008, 28 (41), 10272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Pitchers KK; Frohmader KS; Vialou V; Mouzon E; Nestler EJ; Lehman MN; Coolen LM DeltaFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav 2010, 9 (7), 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Pitchers KK; Vialou V; Nestler EJ; Laviolette SR; Lehman MN; Coolen LM Natural and drug rewards act on common neural plasticity mechanisms with DeltaFosB as a key mediator. J. Neurosci 2013, 33 (8), 3434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hedges VL; Chakravarty S; Nestler EJ; Meisel RL Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav 2009, 8 (4), 442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Werme M; Messer C; Olson L; Gilden L; Thoren P; Nestler EJ; Brene S Delta FosB regulates wheel running. J. Neurosci 2002, 22 (18), 8133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Gajewski PA; Eagle AL; Williams ES; Manning CE; Lynch H; McCornack C; Maze I; Heller EA; Robison AJ Epigenetic Regulation of Hippocampal Fosb Expression Controls Behavioral Responses to Cocaine. J. Neurosci 2019, 39 (42), 8305–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Huggett SB; Stallings MC Genetic Architecture and Molecular Neuropathology of Human Cocaine Addiction. J. Neurosci 2020, 40 (27), 5300–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Mastrodonato A; Martinez R; Pavlova IP; LaGamma CT; Brachman RA; Robison AJ; Denny CA Ventral CA3 Activation Mediates Prophylactic Ketamine Efficacy Against Stress-Induced Depressive-like Behavior. Biol. Psychiatry 2018, 84, 846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Teyssier JR; Ragot S; Chauvet-Gelinier JC; Trojak B; Bonin B Activation of a DeltaFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. J. Affect Disord 2011, 133 (1–2), 174–8. [DOI] [PubMed] [Google Scholar]
- (79).Kiss A; Osacka J Extra-forebrain impact of antipsychotics indicated by c-Fos or FosB/DeltaFosB expression: A minireview. Endocr Regul 2021, 55 (2), 120–130. [DOI] [PubMed] [Google Scholar]
- (80).Dietz DM; Kennedy PJ; Sun H; Maze I; Gancarz AM; Vialou V; Koo JW; Mouzon E; Ghose S; Tamminga CA; Nestler EJ DeltaFosB induction in prefrontal cortex by antipsychotic drugs is associated with negative behavioral outcomes. Neuropsychopharmacology 2014, 39 (3), 538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Hope BT; Kelz MB; Duman RS; Nestler EJ Chronic electroconvulsive seizure (ECS) treatment results in expression of a long-lasting AP-1 complex in brain with altered composition and characteristics. J. Neurosci 1994, 14 (7), 4318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Lee TS; Li AY; Rapuano A; Mantis J; Eid T; Seyfried TN; de Lanerolle NC Gene expression in the epileptic (EL) mouse hippocampus. Neurobiol Dis 2021, 147, 105152. [DOI] [PubMed] [Google Scholar]
- (83).Giordano C; Vinet J; Curia G; Biagini G Repeated 6-Hz Corneal Stimulation Progressively Increases FosB/DeltaFosB Levels in the Lateral Amygdala and Induces Seizure Generalization to the Hippocampus. PLoS One 2015, 10 (11), No. e0141221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Biagini G; D’Arcangelo G; Baldelli E; D’Antuono M; Tancredi V; Avoli M Impaired activation of CA3 pyramidal neurons in the epileptic hippocampus. Neuromolecular Med. 2005, 7 (4), 325–42. [DOI] [PubMed] [Google Scholar]
- (85).Eagle AL; Williams ES; Beatty JA; Cox CL; Robison AJ DeltaFosB Decreases Excitability of Dorsal Hippocampal CA1 Neurons. eNeuro 2018, 5 (4), ENEURO.0104-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Turrigiano GG; Leslie KR; Desai NS; Rutherford LC; Nelson SB Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 1998, 391 (6670), 892–6. [DOI] [PubMed] [Google Scholar]
- (87).Gruda MC; van Amsterdam J; Rizzo CA; Durham SK; Lira S; Bravo R Expression of FosB during mouse development: normal development of FosB knockout mice. Oncogene 1996, 12 (10), 2177–2185. [PubMed] [Google Scholar]
- (88).You JC; Stephens GS; Fu CH; Zhang X; Liu Y; Chin J Genome-wide profiling reveals functional diversification of FosB gene targets in the hippocampus of an Alzheimer’s disease mouse model. PLoS One 2018, 13 (2), No. e0192508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Vialou V; Bagot RC; Cahill ME; Ferguson D; Robison AJ; Dietz DM; Fallon B; Mazei-Robison M; Ku SM; Harrigan E; Winstanley CA; Joshi T; Feng J; Berton O; Nestler EJ Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of DeltaFosB. J. Neurosci 2014, 34 (11), 3878–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Winstanley CA; Green TA; Theobald DE; Renthal W; LaPlant Q; DiLeone RJ; Chakravarty S; Nestler EJ DeltaFosB induction in orbitofrontal cortex potentiates locomotor sensitization despite attenuating the cognitive dysfunction caused by cocaine. Pharmacol., Biochem. Behav 2009, 93 (3), 278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Winstanley CA; Bachtell RK; Theobald DE; Laali S; Green TA; Kumar A; Chakravarty S; Self DW; Nestler EJ Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontal cortex. Cereb Cortex 2009, 19 (2), 435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Winstanley CA; LaPlant Q; Theobald DE; Green TA; Bachtell RK; Perrotti LI; DiLeone RJ; Russo SJ; Garth WJ; Self DW; Nestler EJ DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J. Neurosci 2007, 27 (39), 10497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Sims NA; Sabatakos G; Chen JS; Kelz MB; Nestler EJ; Baron R Regulating DeltaFosB expression in adult Tet-Off-DeltaFosB transgenic mice alters bone formation and bone mass. Bone 2002, 30 (1), 32–9. [DOI] [PubMed] [Google Scholar]
- (94).Sabatakos G; Sims NA; Chen J; Aoki K; Kelz MB; Amling M; Bouali Y; Mukhopadhyay K; Ford K; Nestler EJ; Baron R Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat. Med 2000, 6 (9), 985–90. [DOI] [PubMed] [Google Scholar]
- (95).Fittall MW; Mifsud W; Pillay N; Ye H; Strobl AC; Verfaillie A; Demeulemeester J; Zhang L; Berisha F; Tarabichi M; Young MD; Miranda E; Tarpey PS; Tirabosco R; Amary F; Grigoriadis AE; Stratton MR; Van Loo P; Antonescu CR; Campbell PJ; Flanagan AM; Behjati S Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat. Commun 2018, 9 (1), 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Kovary K; Rizzo CA; Ryseck RP; Noguchi T; Raynoschek C; Pelosin JM; Bravo R Constitutive expression of FosB and its short form, FosB/SF, induces malignant cell transformation in rat-1A cells. New Biol. 1991, 3 (9), 870–879. [PubMed] [Google Scholar]
- (97).Manning CE; Eagle AL; Kwiatkowski CC; Achargui R; Woodworth H; Potter E; Ohnishi Y; Leinninger GM; Robison AJ Hippocampal Subgranular Zone FosB Expression Is Critical for Neurogenesis and Learning. Neuroscience 2019, 406, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Yutsudo N; Kamada T; Kajitani K; Nomaru H; Katogi A; Ohnishi YH; Ohnishi YN; Takase K; Sakumi K; Shigeto H; Nakabeppu Y fosB-null mice display impaired adult hippocampal neurogenesis and spontaneous epilepsy with depressive behavior. Neuropsychopharmacology 2013, 38 (5), 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Novotny V; Prieschl EE; Csonga R; Fabjani G; Baumruker T Nrf1 in a complex with fosB, c-jun, junD and ATF2 forms the AP1 component at the TNF alpha promoter in stimulated mast cells. Nucleic Acids Res. 1998, 26 (23), 5480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Alim MA; Grujic M; Ackerman PW; Kristiansson P; Eliasson P; Peterson M; Pejler G Glutamate triggers the expression of functional ionotropic and metabotropic glutamate receptors in mast cells. Cell Mol. Immunol 2020, 17, 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Yoza BK; Brooks JW; Mizel SB Induction of AP-1 transcription factor components during T-cell activation by interleukin 1 and phorbol esters. Cell Growth Differ. 1992, 3 (10), 677–684. [PubMed] [Google Scholar]
- (102).Baumann S; Hess J; Eichhorst ST; Krueger A; Angel P; Krammer PH; Kirchhoff S An unexpected role for FosB in activation-induced cell death of T cells. Oncogene 2003, 22 (9), 1333–9. [DOI] [PubMed] [Google Scholar]
- (103).Carle TL; Ohnishi YN; Ohnishi YH; Alibhai IN; Wilkinson MB; Kumar A; Nestler EJ Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur. J. Neurosci 2007, 25 (10), 3009–19. [DOI] [PubMed] [Google Scholar]
- (104).Alibhai IN; Green TA; Potashkin JA; Nestler EJ Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007, 1143, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Cates HM; Thibault M; Pfau M; Heller E; Eagle A; Gajewski P; Bagot R; Colangelo C; Abbott T; Rudenko G; Neve R; Nestler EJ; Robison AJ Threonine 149 phosphorylation enhances DeltaFosB transcriptional activity to control psychomotor responses to cocaine. J. Neurosci 2014, 34 (34), 11461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Ulery PG; Nestler EJ Regulation of DeltaFosB transcriptional activity by Ser27 phosphorylation. Eur. J. Neurosci 2007, 25 (1), 224–30. [DOI] [PubMed] [Google Scholar]
- (107).Ulery PG; Rudenko G; Nestler EJ Regulation of DeltaFosB stability by phosphorylation. J. Neurosci 2006, 26 (19), 5131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Jorissen HJ; Ulery PG; Henry L; Gourneni S; Nestler EJ; Rudenko G Dimerization and DNA-binding properties of the transcription factor DeltaFosB. Biochemistry 2007, 46 (28), 8360–72. [DOI] [PubMed] [Google Scholar]
- (109).Yin Z; Venkannagari H; Lynch H; Aglyamova G; Bhandari M; Machius M; Nestler EJ; Robison AJ; Rudenko G Self-assembly of the bZIP transcription factor DeltaFosB. Curr. Res. Struct Biol 2020, 2, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Hiroi N; Marek GJ; Brown JR; Ye H; Saudou F; Vaidya VA; Duman RS; Greenberg ME; Nestler EJ Essential role of the fosB gene in molecular, cellular, and behavioral actions of chronic electroconvulsive seizures. J. Neurosci 1998, 18 (17), 6952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Yin Z; Machius M; Nestler EJ; Rudenko G Activator Protein-1: redox switch controlling structure and DNA-binding. Nucleic Acids Res. 2017, 45, 11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Renthal W; Kumar A; Xiao G; Wilkinson M; Covington HE 3rd; Maze I; Sikder D; Robison AJ; LaPlant Q; Dietz DM; Russo SJ; Vialou V; Chakravarty S; Kodadek TJ; Stack A; Kabbaj M; Nestler EJ Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 2009, 62 (3), 335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Skene PJ; Henikoff S An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife 2017, 6, e21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Yeh S; Estill MS; Shen L; Nestler EJ Molecular basis of ΔFosb action in drug addiction: mapping ΔFosb binding sites in nucleus accumbens medium spiny neurons. In Society for Neuroscience Annual Meeting, 2021; P732. [Google Scholar]
- (115).Wang Y; Cesena TI; Ohnishi Y; Burger-Caplan R; Lam V; Kirchhoff PD; Larsen SD; Larsen MJ; Nestler EJ; Rudenko G Small molecule screening identifies regulators of the transcription factor DeltaFosB. ACS chemical neuroscience 2012, 3 (7), 546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Peakman MC; Colby C; Perrotti LI; Tekumalla P; Carle T; Ulery P; Chao J; Duman C; Steffen C; Monteggia L; Allen MR; Stock JL; Duman RS; McNeish JD; Barrot M; Self DW; Nestler EJ; Schaeffer E Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003, 970 (1–2), 73–86. [DOI] [PubMed] [Google Scholar]
- (117).Lardner CK; van der Zee Y; Estill MS; Kronman HG; Salery M; Cunningham AM; Godino A; Parise EM; Kim JH; Neve RL; Shen L; Hamilton PJ; Nestler EJ Gene-Targeted, CREB-Mediated Induction of DeltaFosB Controls Distinct Downstream Transcriptional Patterns Within D1 and D2Medium Spiny Neurons. Biol. Psychiatry 2021, 90 (8), 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Ferguson D; Koo JW; Feng J; Heller E; Rabkin J; Heshmati M; Renthal W; Neve R; Liu X; Shao N; Sartorelli V; Shen L; Nestler EJ Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J. Neurosci 2013, 33 (41), 16088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Renthal W; Carle TL; Maze I; Covington HE 3rd; Truong HT; Alibhai I; Kumar A; Montgomery RL; Olson EN; Nestler EJ, Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J. Neurosci 2008, 28 (29), 7344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Kumar A; Choi KH; Renthal W; Tsankova NM; Theobald DE; Truong HT; Russo SJ; Laplant Q; Sasaki TS; Whistler KN; Neve RL; Self DW; Nestler EJ Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 2005, 48 (2), 303–14. [DOI] [PubMed] [Google Scholar]
- (121).Cruz FC; Javier Rubio F; Hope BT Using c-fos to study neuronal ensembles in corticostriatal circuitry of addiction. Brain Res. 2015, 1628 (Pt A), 157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Koya E; Golden SA; Harvey BK; Guez-Barber DH; Berkow A; Simmons DE; Bossert JM; Nair SG; Uejima JL; Marin MT; Mitchell TB; Farquhar D; Ghosh SC; Mattson BJ; Hope BT Targeted disruption of cocaine-activated nucleus accumbens neurons prevents context-specific sensitization. Nat. Neurosci 2009, 12 (8), 1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Mattson BJ; Koya E; Simmons DE; Mitchell TB; Berkow A; Crombag HS; Hope BT Context-specific sensitization of cocaine-induced locomotor activity and associated neuronal ensembles in rat nucleus accumbens. Eur. J. Neurosci 2008, 27 (1), 202–12. [DOI] [PubMed] [Google Scholar]
- (124).Vialou V; Feng J; Robison AJ; Ku SM; Ferguson D; Scobie KN; Mazei-Robison MS; Mouzon E; Nestler EJ Serum Response Factor and cAMP Response Element Binding Protein Are Both Required for Cocaine Induction of {Delta}FosB. J. Neurosci 2012, 32 (22), 7577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Cates HM; Lardner CK; Bagot RC; Neve RL; Nestler EJ Fosb Induction in Nucleus Accumbens by Cocaine Is Regulated by E2F3a. eNeuro 2019, 6 (2), ENEURO.0325-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Vialou V; Maze I; Renthal W; LaPlant QC; Watts EL; Mouzon E; Ghose S; Tamminga CA; Nestler EJ Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J. Neurosci 2010, 30 (43), 14585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Heller EA; Cates HM; Pena CJ; Sun H; Shao N; Feng J; Golden SA; Herman JP; Walsh JJ; Mazei-Robison M; Ferguson D; Knight S; Gerber MA; Nievera C; Han MH; Russo SJ; Tamminga CS; Neve RL; Shen L; Zhang HS; Zhang F; Nestler EJ Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nat. Neurosci 2014, 17 (12), 1720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Bushweller JH Targeting transcription factors in cancer - from undruggable to reality. Nat. Rev. Cancer 2019, 19 (11), 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Keating GM Afatinib: a review of its use in the treatment of advanced non-small cell lung cancer. Drugs 2014, 74 (2), 207–21. [DOI] [PubMed] [Google Scholar]
- (130).Abate C; Patel L; Rauscher FJ 3rd; Curran T Redox regulation of fos and jun DNA-binding activity in vitro. Science 1990, 249 (4973), 1157–61. [DOI] [PubMed] [Google Scholar]
- (131).Markesbery WR The role of oxidative stress in Alzheimer disease. Arch Neurol 1999, 56 (12), 1449–52. [DOI] [PubMed] [Google Scholar]
- (132).Persson T; Popescu BO; Cedazo-Minguez A Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxid. Med. Cell. Longevity 2014, 2014, 427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Forman HJ; Zhang H Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov 2021, 20 (9), 689–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Pearson-Smith JN; Patel M Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci 2017, 18 (11), 2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Thornton C; Grad E; Yaka R The role of mitochondria in cocaine addiction. Biochem. J 2021, 478 (4), 749–764. [DOI] [PubMed] [Google Scholar]
- (136).Li Y; Liu Z; Aglyamova G; Chen J; Chen H; Bhandari M; White MA; Rudenko G; Zhou J Discovery of phenanthridine analogues as novel chemical probes disrupting the binding of DNA to DeltaFosB homodimers and DeltaFosB/JunD heterodimers. Bioorg. Med. Chem. Lett 2020, 30 (16), 127300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Chen J; Nye HE; Kelz MB; Hiroi N; Nakabeppu Y; Hope BT; Nestler EJ Regulation of delta FosB and FosB-like proteins by electroconvulsive seizure and cocaine treatments. Mol. Pharmacol 1995, 48 (5), 880–889. [PubMed] [Google Scholar]
- (138).Pillinger MH; Capodici C; Rosenthal P; Kheterpal N; Hanft S; Philips MR; Weissmann G Modes of action of aspirin-like drugs: salicylates inhibit erk activation and integrin-dependent neutrophil adhesion. Proc. Natl. Acad. Sci. U. S. A 1998, 95 (24), 14540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Joshi CR; Labhasetwar V; Ghorpade A Destination Brain: the Past, Present, and Future of Therapeutic Gene Delivery. J. Neuroimmune Pharmacol 2017, 12 (1), 51–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Lesch KP Gene transfer to the brain: emerging therapeutic strategy in psychiatry? Biol. Psychiatry 1999, 45 (3), 247–53. [DOI] [PubMed] [Google Scholar]
- (141).Yim YY; Teague CD; Nestler EJ In vivo locus-specific editing of the neuroepigenome. Nat. Rev. Neurosci 2020, 21 (9), 471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Bedbrook CN; Deverman BE; Gradinaru V Viral Strategies for Targeting the Central and Peripheral Nervous Systems. Annu. Rev. Neurosci 2018, 41, 323–348. [DOI] [PubMed] [Google Scholar]


