Abstract
The close contact between people and their pets has generated the exchange of skin microbiota, accompanied by bacteria that present resistance to antibiotics. Staphylococcus spp., opportunistic pathogens present in the skin and mucosa of mammals, have had their importance recognized in human and veterinary medicine. The objectives of this study were to identify Staphylococcus spp. present in isolates from the nostrils of healthy humans, dogs and cats as well as to determine their phenotype of resistance to methicillin. Strain identification was performed by MALDI-TOF mass spectrometry and antimicrobial susceptibility was determined using a disk diffusion assay for 12 antibiotics. Sixty humans (veterinary and technicians), sixty dogs and sixty cats were sampled; of them, 61.6%, 56.6% and 46.6%, respectively, carried Staphylococcus spp. in their nostrils, and only two people carried two different species of Staphylococcus in the only anatomical site sampled. A methicillin-resistant phenotype was present in 48.7% of the humans, 26.5% of the dogs and 57.1% of the cats, and sampled. These results demonstrate the presence of Staphylococcus spp. strains resistant to methicillin in personnel who work in contact with animals, as well as in dogs and cats that entered the same hospital or veterinary clinic, which alerts us to the potential transfer of these strains to or between people, dogs and/or cats.
Keywords: antimicrobial susceptibility testing, human, dogs, cats, Staphylococcus spp., resistance
1. Introduction
The genus Staphylococcus is composed of Gram-positive and facultative anaerobic bacteria present in cutaneous and mucous membrane microbiota of mammals and birds [1,2]. The genus includes clinically relevant opportunistic pathogens in both human and veterinary medicine [3,4,5,6]. The species belonging to this genus have traditionally been grouped and differentiated according to the production of the enzyme coagulase, capable of converting fibrinogen into fibrin, a characteristic that is easily detectable in the laboratory and allows for a practical classification [7]. In general, coagulase-positive staphylococci (CoPS), such as S. aureus, S. intermedius and S. pseudointermedius, among others, are usually pathogenic, even though in some cases they can cause asymptomatic colonization in healthy individuals, whereas coagulase-negative staphylococci (CoNS) [8], represented by a larger group of species, have been associated with opportunistic infections [3,4,9,10,11,12,13].
For decades, S. aureus has been considered the most important pathogen of the genus [14,15,16,17]. In people, it can be found in community settings [18] or hospital premises, constituting an important source of infections associated with healthcare [19]. The bacterium can produce infections in humans associated with skin and soft tissue, pneumonia, septicemia and osteomyelitis [19], which have also been reported in animals [20]. However, S. pseudointermedius nevertheless is a common cause of skin and soft tissue infections in dogs, cats and humans [5,6,21]. In recent years, CoNS species, such as S. epidermidis [22,23,24], S. haemolyticus [25] and S. lugdunensis [26], have also been associated with opportunistic infections in humans [12,23,27,28,29]. The recognition of some CoNS as pathogens in veterinary medicine has emerged with S. epidermidis and some subspecies of S. schleiferi causing skin and ear infections in dogs [30,31,32,33], as well as S. felis related to lower urinary tract disease, eye infections and otitis in cats [34,35].
Pathogen transmission between species is recognized as being of clinical relevance and zoonotic. The transmission of commensal Staphylococcus spp., including those resistant to methicillin and other antibiotics, has been recognized from animals to humans and vice versa [35], particularly among domestic animals and their owners [32,36,37,38,39,40,41,42,43,44]. Importantly, the transmission also occurs among staff working in veterinary hospitals and their patients, as well as among patients who are in the same hospital [5,45,46,47,48,49,50]. The objective of this study was to isolate and identify Staphylococcus spp. obtained from healthy humans, dogs and cats.
2. Materials and Methods
2.1. Ethical Approval
This study was approved by the Bioethics Committee of the Faculty of Life Sciences, Universidad Andrés Bello (Approval Certificate #019/2020), and was carried out in a veterinary hospital and a veterinary clinic, located in Colina and Independencia, respectively, Santiago, Metropolitan Region, Chile (S 33°27′24.98″ O 70°38′53.77″). Samples were collected between October 2020 and March 2021.
2.2. Subjects and Inclusion Criteria
Sixty healthy adult dogs and sixty healthy adult cats of any breed and sex were included, who attended, with their owners, the hospital or veterinary clinic to comply with their vaccination schedule. Similarly, 60 people were sampled during the same period. Sampled people were veterinary doctors and technicians who worked regularly at or visited the same hospital or clinic. Included subjects or enrolled pets were under no antibiotic treatment for at least 3 months before obtaining the sample.
2.3. Isolation and Identification
Each sample was obtained with prior authorization by means of informed consent. For each sampled subject, the use of a face shield, mask and sterile gloves was taken into consideration, materials which were discarded between each participant. In humans, a single swab was inserted by a maximum of 1 cm, rotated in each nostril and rubbed with support on the septum. In animals the same procedure was carried out, considering that in small breeds and cats the swab was introduced by a maximum of 0.5 cm and employed Stuart Transport media (Linsan, Santiago, Chile). Each swab was seeded on mannitol salt agar (Becton Dickinson, Heidelberg, Germany) and incubated at 37 °C for 24 h; a semi-quantitative evaluation was made of the different morphotypes grown on mannitol salt agar, such that those that showed abundant growth in the second quadrant of the clock sowing were selected. Gram- and catalase-positive morphotypes were isolated on blood agar (Linsan, Santiago, Chile); additionally, they were identified using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis (MALDI Biotyper, Bruker, Billerica, MA, USA) following the manufacturer’s instructions and as described previously [51,52].
2.4. Antimicrobial Susceptibility Testing
All isolates confirmed as Staphylococcus were tested against a panel of 12 antibiotics using the disk diffusion Kirby–Bauer method following CLSI guidelines in the M100 and VET01S documents [53,54]. The tested antibiotics included cefoxitin (FOX, 30 µg), oxacillin (OX, 1 µg), imipenem (IPM, 10 μg), ciprofloxacin (CIP, 5 μg), vancomycin (VA, 30 μg), doxycycline (DO, 30 μg), erythromycin (E, 15 μg), amikacin (AMK, 30 μg), gentamicin (GEN, 10 μg), trimethoprim/sulfamethoxazole (SXT, 1.25/23.75 μg), amoxicillin/clavulanic acid (AMC, 30 μg) and clindamycin (DA, 2 μg), all of which were supplied by OXOID (Hampshire, UK). Methicillin-resistant phenotype in strains of human origin were evaluated using a FOX disc for all species. However, in cats and dogs, OX for S. pseudointermedius and CoNS as well as FOX for S. aureus were used.
S. aureus ATCC 25923 was included as a reference strain. Bacterial isolates resistant to three or more antimicrobial classes were cataloged as multidrug-resistant (MDR) following previously standardized criteria [55].
2.5. Data Analysis
Data were analyzed and plots were made utilizing python3 software and the pandas package, release 1.3.4; upset plots [56] were made by employing the UpSetPlot package (https://github.com/jnothman/UpSetPlot (accessed on 27 October 2021), release 0.6.0.
3. Results
3.1. Study Population
The group of 60 people that were sampled consisted of 35 women and 25 men with an average age of 30 years (range of 21 to 44 years), who carried out different activities within the clinic or hospital. Activities recorded were surgeon, surgeon assistant, animal care and treatment, management of hospitalized, medical consultation, wound treatment, student veterinary, nurse, animal rehabilitation, feline care and analysis of animal samples, among others.
A total of 120 pets (60 dogs and 60 cats) were sampled from a veterinary hospital and clinic in Santiago, Chile. Half of the dogs were crossbreeds (30), while the others were German Shepherds (6), Poodles (4), Schnauzers (3), Cocker Spaniels (3), Yorkshires (3), Beagles (2), Labradors (2), Chihuahuas (2), a Dachshund (1), a Maltese (1), a Saint Bernard (1), a Pug (1) and an Akita (1). Their average age was 4.4 years (range of 2 to 9 years). The cats had an average of 4.3 years (range of 2 to 8 years), and most of them were domestic short hairs (41), domestic long hairs (18) and a Siamese (1).
3.2. Detection of Staphylococcus spp.
Of the total number of humans, 61.6% of the subjects sampled (37 of 60) carried a Staphylococcus spp. in their nostrils; only two people carried two different species of Staphylococcus spp. in the only anatomical site sampled, meaning that a total of 39 isolated strains were obtained. Of the dogs and cats, 56.6% (34 of 60) and 46.6% (28 of 60) were carriers of Staphylococcus spp., respectively.
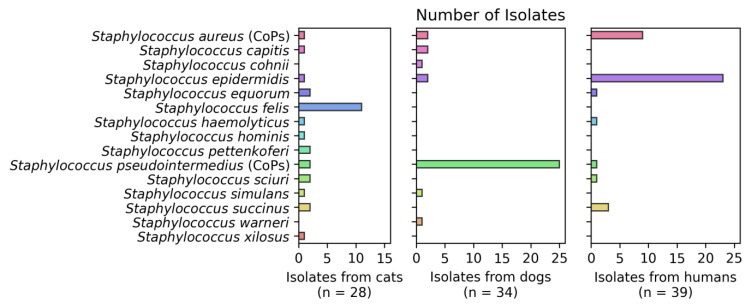
A total of 13 species of CoNS were identified, the most prevalent being S. epidermidis (26.1%), S. felis (11.1%), S. succinus (4.8%), S. sciuri (2.9%) and S. equorum (2.9%), distributed among the humans and animals sampled (Figure 1).
Figure 1.
Number of Staphylococcus isolates per group. In total, 15 different species from the Staphylococcus genus were isolated from the studied subjects. Considering the frequency, 28 Staphylococcus spp. were isolated from the 60 healthy adult cats, 34 Staphylococcus spp. were isolated from the 60 healthy adult dogs and 39 Staphylococcus spp. were isolated from the 60 healthy adult human participants of the study.
In humans, most of the isolates corresponded to CoNS represented by S. epidermidis, with 58.9% being of this type (23 of 39 isolates). This also occurred in cats, where 39.3% of the isolates were S. felis (11 of 28). On the contrary, in dogs two species of CoPS were identified, represented by S. pseudointermedius (25 of 34) and S. aureus (two of thirty-four) (Figure 1).
3.3. Resistance Phenotype
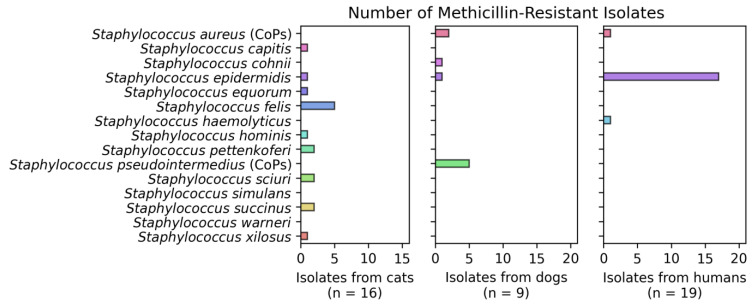
Of all the isolates obtained from humans, 48.7% (19 of 39) showed a methicillin-resistant phenotype, mainly in isolates of S. epidermidis (17), S. aureus (1) and S. haemolyticus (1). The strains isolated from dogs showed 26.5% resistance, where S. pseudointermedius (5) was the predominant species, followed by S. aureus (2), S. cohnii (1) and S. epidermidis (1). Likewise, 57.1% of the isolates from felines showed this phenotype in the species S. felis (5), followed by S. sciuri (2), S. pettenkoferi (2), S. succinus (2), S. capitis (1), S. xilosus (1), S. epidermidis (1), S. hominis and S. equorum (1) (Figure 2).
Figure 2.
Number of methicillin-resistant isolates per group (cats, dogs and humans).
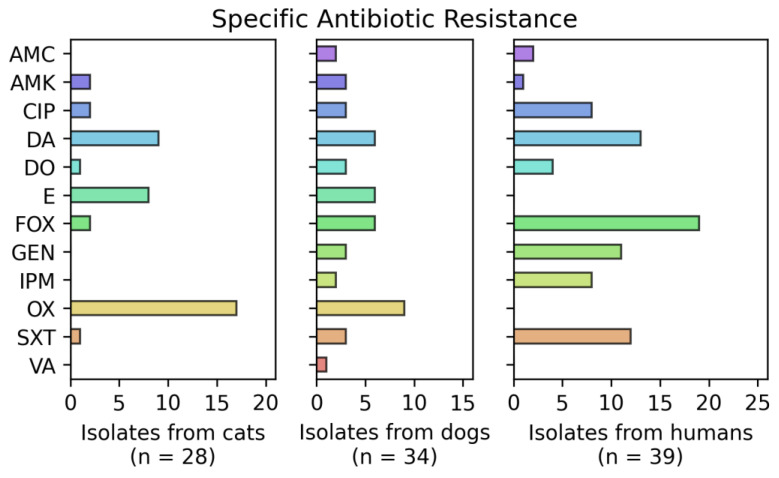
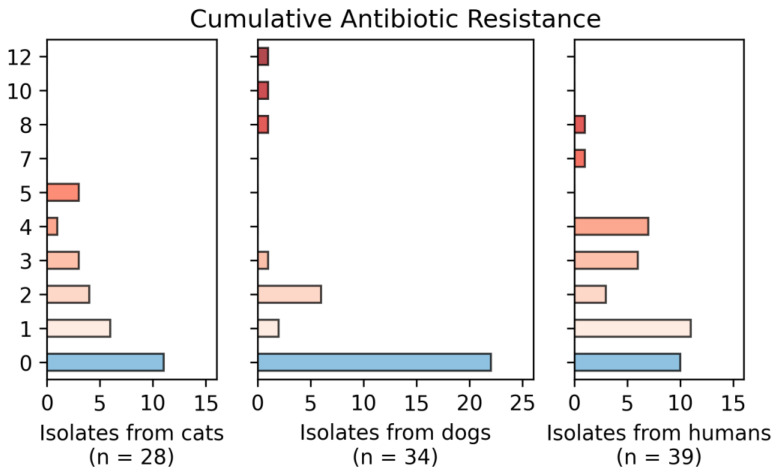
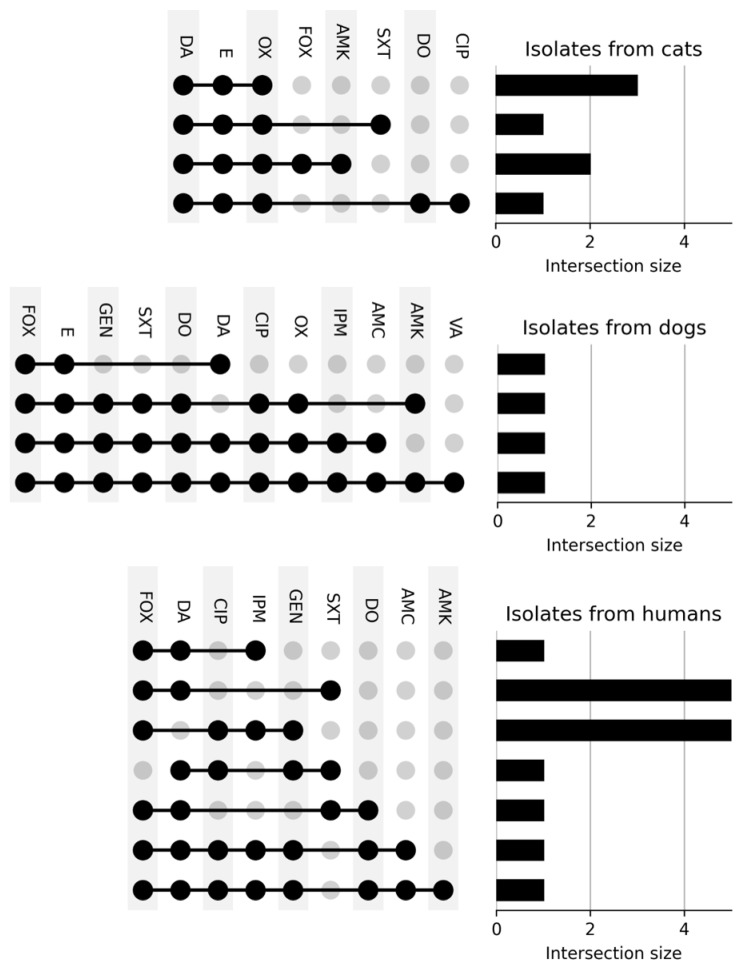
Figure 3, Figure 4 and Figure 5 show the number of isolates obtained from dogs, cats and humans that were resistant against the different antibiotics tested. It is noted that 11 isolates from cats, 22 from dogs and 2 from human participants showed resistance to zero antimicrobials; seven isolates from cats, four isolates from dogs and fifteen isolates from humans showed resistance to three or more antibiotics. All cat isolates showed resistance to OX, E and DA. Isolates from dogs showed high multidrug resistance, with all of them resistant to E and DA and three of them also resistant to GEN, SXT, DO, CIP and OX. Of all the isolates there was a pan-resistant S. pseudointermedius strain, isolated from a dog.
Figure 3.
Number of resistant isolates obtained from dogs, cats and humans. All isolates were tested for antimicrobial susceptibility employing the disk diffusion method following CLSI guidelines. Colors represent the total number of Staphylococcus isolates that showed resistance to cefoxitin (FOX, 30 µg), oxacillin (OX, 1 µg), imipenem (IPM, 10 μg), ciprofloxacin (CIP, 5 μg), vancomycin (VA, 30 μg), doxycycline (DO, 30 μg), erythromycin (E, 15 μg), amikacin (AMK, 30 μg), gentamicin (GEN, 10 μg), trimethoprim/sulfamethoxazole (SXT, 1.25/23.75 μg), amoxicillin/clavulanic acid (AMC, 30 μg) and clindamycin (DA, 2 μg).
Figure 4.
Cumulative resistance of the number of isolates obtained from dogs, cats and humans against the antibiotics used. In total, 11 isolates from cats, 22 from dogs and 2 from human participants showed resistance to zero antimicrobials. Two isolates showed resistance to nine, and one isolate showed resistance to 10, 11 and 12 antimicrobials.
Figure 5.
Specific multidrug resistance of isolates. In total, seven isolates from cats, four isolates from dogs, and fifteen isolates from human participants showed resistance to three or more antibiotics. All isolates from cats showed resistance to OX, E and DA. Isolates from dogs showed elevated multidrug resistance, with all isolates being resistant to E as well as DA and three of them also showing resistance to GEN, SXT, DO, CIP and OX. Finally, most of the isolates from human participants showed resistance to FOX and DA.
4. Discussion
This is the first study carried out in Santiago de Chile that informs on the diversity of CoPS and CoNS species present in the nostrils of healthy humans, dogs and cats, and that also reports the resistance of those obtained against 12 antibiotics.
In general, the frequency of isolates was similar in the three groups sampled; however, the presence of CoPS or CoNS differs between them and is consistent with previous studies; for example, one carried out in Trinidad and Tobago shows a general carriage of Staphylococcus in 53.4% of pets and 46.6% of their owners, indicating that these strains can act as a reservoir of resistance genes between dogs and humans [46]. Other reports have evaluated the carriage of CoPS, mainly S. aureus and S. pseudointermedius in animals, with quantities that fluctuate between 8.7 and 43.8% [6,21,28,40]. Likewise, some authors have focused on the detection of CoNS obtained from healthy animals, finding a carriage that varies between 12.8 and 28% [12,21,22,30].
In Latin America there is little information about the carriage and resistance levels of Staphylococcus spp. in companion animals; a retrospective study conducted in Argentina analyzed a total of 23,922 isolates recovered from clinical samples of dogs and cats between 2011 and 2017, of which 30.8% corresponded to three species of Staphylococcus spp., with S. pseudointermedius being the most frequent [57] and therefore in agreement with what was reported in this work.
Different species implicated in human and animal infections were isolated, such as S. aureus, S. epidermidis and S. pseudointermedius [22,23,24]. Additionally, other pathogens considered to be emerging, such as S. sciuri, S. simulans, S. haemolyticus, S. schleiferi and S. lugdunensis, were isolated [12,23,27,30,31,32]. In humans, most of the isolates corresponded to CoNS represented by S. epidermidis, considered to be the most abundant species that lives on skin [55]. In recent years this species has been associated with infections in humans, dogs and cats; it has also been related to resistance to methicillin, which makes some infections difficult to treat [12,22,25,31]. In cats, S. felis (CoNS) is a species that has been reported as the most frequent [21,28,33,34,35], and has been associated with lower urinary tract disease, eye infections and otitis in these pets [34,35].
Of CoPS species, S. pseudointermedius was the most isolated in this study mainly in dogs, a worldwide concordant finding, reflecting the adaptation of this Staphylococcus to dogs as the major host species [28,40,43,46,48]. Furthermore, it has acquired great importance in these patients as it is one of the main species causing deep pyoderma [33,58]. The isolation of this species in felines differs enormously and depends on the anatomical site sampled: cases in the nostrils of healthy animals are lower compared to skin scraping [33].
A significant number of isolates were found to have a methicillin-resistant phenotype present, specifically in 26.5% of the dogs, 57.1% of the cats and 48.7% of the humans sampled. These figures are higher compared with previous studies carried out in Africa [21], Spain [58] and Australia; additionally, the latter indicates the absence of the phenotype in felines [33].
S. aureus and S. pseudointermedius were isolated from humans and dogs. One isolate of S. aureus from humans and two isolates from dogs were methicillin-resistant. However, only S. pseudointermedius isolates from dogs showed resistance to methicillin. Regarding this, previous studies have detected variable numbers of resistance in pets from 2.6% [33] to 27.4% [6], while others mention 13.3% and 15.1% in CoPS and CoNS, respectively [45]. This scenario shows us limited therapeutic options to treat infections generated by methicillin-resistant Staphylococcus either in humans or animals; in such cases the options are limited to drugs such as vancomycin, oxazolidinones, daptomycin, tigecycline and novel cephalosporins [59,60].
Interestingly, Rossi et al. show a dynamic of horizontal transfer of antimicrobial resistance genes from CoNS to other Staphylococcus species [22], while other authors point to S. sciuri (a colonizer found in dogs) as the source of the mecA gene present in S. aureus [61]. Regarding the risk factors that affect the carrying of a methicillin-resistant genotype, hospitalization, previous bacterial infections and the density of the human population have been reported, indicating a positive association between these variables [28].
Since, in 2017, the WHO classified those strains of S. aureus resistant to methicillin and vancomycin as “high priority” [53], in this study we obtained one isolate with this type of resistance corresponding to S. pseudointermedius obtained from a dog, an antecedent that we must consider since these microorganisms can transmit resistance genes to other species. On the other hand, of all the strains obtained 20 of them were MDR. Previously, it has been reported that 55% of the isolates of S. aureus and S. pseudointermedius obtained from pets were MDR [6], while other authors have obtained these results in 100% of the strains [5].
Interestingly, in people who carry Staphylococcus spp., resistance figures increase when they have been in direct contact with pets [39,42,58] that have been previously treated with antibiotics, as well as in those who work in hospitals or veterinary clinics [5], [32] expressing clonal lineages similar to those identified in humans and other animals [36,43,46,48]. Other authors report a prevalence of methicillin-resistant S. aureus between 0.2 and 15.3% among medical students [49] a figure that increases to 79% when the wardrobe, mainly long-sleeved gowns, has been sampled [47].
Considering that pets are a probable source of transmission of these agents, it is important to have trained professionals to supervise cleaning and disinfection protocols within a hospital or clinic, and on the other hand have detection, classification and isolation systems for high-risk patients in addition to contact tracing to prevent possible outbreaks by medical personnel.
Acknowledgments
We appreciate the support of the PURINA scientific funds and ANID, PAI # 77190079.
Author Contributions
Conceptualization, P.T.; methodology, P.T., P.G., J.M., D.I., C.Y., C.d.R., F.C., A.N. and C.F.-Y.; software, R.S.; validation, P.G. and R.S.; formal analysis, R.S.; investigation, C.d.R., J.M., D.I., C.Y., F.C., A.N. and C.F.-Y.; resources, P.G. and P.T.; writing—original draft preparation, C.d.R. and P.T.; writing—review and editing, P.G., R.S. and P.T.; visualization, A.N.; supervision P.G. and P.T.; project administration, P.T.; funding acquisition, P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the scientific funds PURINA 2020-2021 and ANID, PAI # 77190079.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Faculty of Life Sciences, Universidad Andrés Bello (Approval Certificate #019/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vire F.P., Akpaka P.E., Unakal C. Molecular characterization of methicillin-resistant Staphylococcus aureus isolates from rural community settings in Trinidad and Tobago. Niger J. Clin. Pract. 2018;21:1596–1601. doi: 10.4103/njcp.njcp_269_18. [DOI] [PubMed] [Google Scholar]
- 2.Tortora G., Berdell F., Case C., Weber D., Bair W. Microbiology: An Introduction. 13th ed. Pearson; Boston, MA, USA: 2019. [Google Scholar]
- 3.Pasachova J., Ramírez S., Muñoz L. Staphylococcus aureus: Generalidades, mecanismos de patogenicidad y colonización celular. Nova. 2019;17:25–38. doi: 10.22490/24629448.3631. [DOI] [Google Scholar]
- 4.Tam K., Torres V.J. Staphylococcus aureus Secreted Toxins and Extracellular Enzymes. Microbiol. Spectr. 2019;7:2. doi: 10.1128/microbiolspec.GPP3-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papić B., Golob M., Zdovc I., Kušar D., Avberšek J. Genomic insights into the emergence and spread of methicillin-resistant Staphylococcus pseudintermedius in veterinary clinics. Vet. Microbiol. 2021;258:109119. doi: 10.1016/j.vetmic.2021.109119. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Ripa L., Simón C., Ceballos S., Ortega C., Zarazaga M., Torres C., Gómez-Sanz E. S. pseudintermedius and S. aureus lineages with transmission ability circulate as causative agents of infections in pets for years. BMC Vet. Res. 2021;17:1. doi: 10.1186/s12917-020-02726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Martín M., Corbera J.A., Suárez-Bonnet A., Tejedor-Junco M.T. Virulence factors in coagulase-positive staphylococci of veterinary interest other than Staphylococcus aureus. Vet. Q. 2020;40:118–131. doi: 10.1080/01652176.2020.1748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steining E.J., Duchene S., Robinson D.A. Evolution and Global Transmission of a Multidrug-Resistant, Community-Associated Methicillin-Resistant Staphylococcus aureus Lineage from the Indian Subcontinent. mBio. 2019;10:e01105-19. doi: 10.1128/mBio.01105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu S., Song P.I., Seo C.H., Cheong H., Park Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014;15:8753–8772. doi: 10.3390/ijms15058753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argudín M.A., Vanderhaeghen W., Butaye P. Antimicrobial resistance and population structure of Staphylococcus epidermidis recovered from pig farms in Belgium. Vet. J. 2015;203:302–308. doi: 10.1016/j.tvjl.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 11.LoPinto A., Mohammed H., Ledbetter E. Prevalence and risk factors for isolation of methicillin-resistant Staphylococcus in dogs with keratitis. Vet. Ophthalmol. 2015;18:297–303. doi: 10.1111/vop.12200. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Moein K.A., Zaher H.M. The Nasal Carriage of Coagulase-Negative Staphylococci among Animals and Its Public Health Implication. Vector Borne Zoonotic Dis. 2020;20:897–902. doi: 10.1089/vbz.2020.2656. [DOI] [PubMed] [Google Scholar]
- 13.Rich M. Staphylococci in animals: Prevalence, identification and antimicrobial susceptibility, with an emphasis on methicillin-resistant Staphylococcus aureus. Br. J. Biomed. Sci. 2005;62:98–105. doi: 10.1080/09674845.2005.11732694. [DOI] [PubMed] [Google Scholar]
- 14.Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 15.Chambers H.F. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seas C., Garcia C., Salles M.J., Labarca J., Luna C., Alvarez-Moreno C., Mejía-Villatoro C., Zurita J., Guzmán-Blanco M., Rodríguez-Noriega E., et al. Staphylococcus aureus bloodstream infections in Latin America: Results of a multinational prospective cohort study. J. Antimicrob. Chemother. 2018;73:212–222. doi: 10.1093/jac/dkx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung G., Bae J.S., Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12:547–569. doi: 10.1080/21505594.2021.1878688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero L.C., Souza da Cunha M. Insights into the epidemiology of community-associated methicillin-resistant Staphylococcus aureus in special populations and at the community-healthcare interface. Braz. J. Infect. Dis. 2021;25:101636. doi: 10.1016/j.bjid.2021.101636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor T., Unakal C. Staphylococcus aureus. StatPearls; Treasure Island, FL, USA: 2021. [Google Scholar]
- 20.Algammal A.M., Hetta H.F., Elkelish A., Alkhalifah D., Hozzein W.N., Batiha G.E., El Nahhas N., Mabrok M.A. Methicillin-resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect. Drug Resist. 2020;13:3255–3265. doi: 10.2147/IDR.S272733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elnageh H., Hiblu M., Abbassi M., Abouzeed Y., Ahmed M. Prevalence and antimicrobial resistance of Staphylococcus species isolated from cats and dogs. Open Vet. J. 2021;10:452–456. doi: 10.4314/ovj.v10i4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi C., Souza-Silva T., Araújo-Alves A., Giambiagi-deMarval M. CRISPR-Cas Systems Features and the Gene-Reservoir Role of Coagulase-Negative Staphylococci. Front. Microbiol. 2017;8:1545. doi: 10.3389/fmicb.2017.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanoukon C., Argemi X., Sogbo F., Orekan J., Keller D., Affolabi D., Schramm F., Riegel P., Baba-Moussa L., Prévost G. Pathogenic features of clinically significant coagulase-negative staphylococci in hospital and community infections in Benin. Int. J. Med. Microbiol. 2017;307:75–82. doi: 10.1016/j.ijmm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Fišarová L., Pantůček R., Botka T., Doškař J. Variability of resistance plasmids in coagulase-negative staphylococci and their importance as a reservoir of antimicrobial resistance. Res. Microbiol. 2019;170:105–111. doi: 10.1016/j.resmic.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Malinis A.J., Milagro A., Torres Sopena L., Gilaberte Y. Staphylococcus lugdunensis Skin Infection: Report of 16 Cases. Actas Dermosifiliogr. 2021;112:261–265. doi: 10.1016/j.ad.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado E., Acuña M., Álvarez A.M., Avilés C., Maza V., Salgado C., Tordecilla J., Varas M., Venegas M., Villarroel M., et al. Microorganismos aislados de hemocultivos en niños con cáncer y neutropenia febril de alto riesgo en cinco hospitales de Santiago, Chile, período 2012–2015. Rev. Chil. Infectol. 2018;35:140–146. doi: 10.4067/s0716-10182018000200140. [DOI] [PubMed] [Google Scholar]
- 28.Rynhoud H., Meler E., Gibson J.S., Price R., Maguire T., Farry T., Bennett E., Hartono J., Magalhaes R.J. Epidemiology of methicillin resistant Staphylococcus species carriage in companion animals in the Greater Brisbane Area, Australia. Res. Vet. Sci. 2021;136:138–142. doi: 10.1016/j.rvsc.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Alshaikh B., Yee W., Lodha A., Henderson E., Yusuf K., Sauve R. Coagulase-negative Staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J. Perinatol. 2014;34:125–129. doi: 10.1038/jp.2013.155. [DOI] [PubMed] [Google Scholar]
- 30.Chah K.F., Gómez-Sanz E., Nwanta J.A., Asadu B., Agbo I.C., Lozano C., Zarazaga M., Torres C. Methicillin-resistant coagulase-negative staphylococci from healthy dogs in Nsukka, Nigeria. Braz. J. Microbiol. 2014;45:215–220. doi: 10.1590/S1517-83822014005000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherifi S., Byl B., Deplano A., Nagant C., Nonhoff C., Denis O., Hallin M. Genetic characteristics and antimicrobial resistance of Staphylococcus epidermidis isolates from patients with catheter-related bloodstream infections and from colonized healthcare workers in a Belgian hospital. Ann. Clin. Microbiol. Antimicrob. 2014;13:1. doi: 10.1186/1476-0711-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison E.M., Weinert L.A., Holden M.T., Welch J.J., Wilson K., Morgan F.J., Harris S.R., Loeffler A., Boag A.K., Peacock S.J., et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. mBio. 2014;5:3. doi: 10.1128/mBio.00985-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma G.C., Worthing K.A., Ward M.P., Norris J.M. Commensal Staphylococci Including Methicillin-Resistant Staphylococcus aureus from Dogs and Cats in Remote New South Wales, Australia. Microb. Ecol. 2020;79:164–174. doi: 10.1007/s00248-019-01382-y. [DOI] [PubMed] [Google Scholar]
- 34.Litster A., Moss S.M., Honnery M., Rees B., Trott D.J. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: Recognition of Staphylococcus felis as a possible feline urinary tract pathogen. Vet. Microbiol. 2007;121:182–188. doi: 10.1016/j.vetmic.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Worthing K., Pang S., Trott D.J., Abraham S., Coombs G.W., Jordan D., McIntyre L., Davies M.R., Norris J. Characterization of Staphylococcus felis isolated from cats using whole genome sequencing. Vet. Microbiol. 2018;222:98–104. doi: 10.1016/j.vetmic.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira J.P., Anderson K.L., Correa M.T., Lyman R., Ruffin F., Reller L.B., Fowler V.G., Jr. Transmission of mrsa between companion animals and infected human patients presenting to outpatient medical care facilities. PLoS ONE. 2011;6:e26978. doi: 10.1371/journal.pone.0026978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvey R.G., Marples R., Noble W. Nasal Carriage of Staphylococcus intermedius in Humans in Contact with Dogs. Microb Ecol. Health Dis. 1994;7:225–227. doi: 10.3109/08910609409141358. [DOI] [Google Scholar]
- 38.Van Duijkeren E., Wolfhagen M.J., Box A.T., Heck M.E., Wannet W.J., Fluit A.C. Human-to-Dog Transmission of Methicillin-Resistant Staphylococcus aureus. Emerg. Infect. Dis. 2004;10:2235–2237. doi: 10.3201/eid1012.040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boost M., O’Donoghue M.M., James A. Prevalence of Staphylococcus aureus carriage among dogs and their owners. Epidemiol. Infect. 2008;136:953–964. doi: 10.1017/S0950268807009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gómez-Sanz E., Torres C., Ceballos S., Lozano C., Zarazaga M. Clonal Dynamics of Nasal Staphylococcus aureus and Staphylococcus pseudintermedius in Dog-Owning Household Members. Detection of MSSA ST398. PLoS ONE. 2013;8:e69337. doi: 10.1371/journal.pone.0069337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vengust M., Anderson M.E., Rousseau J., Weese J.S. Methicillin-resistant staphylococcal colonization in clinically normal dogs and horses in the community. Lett. Appl. Microbiol. 2006;43:602–606. doi: 10.1111/j.1472-765X.2006.02018.x. [DOI] [PubMed] [Google Scholar]
- 42.Weese S.J., Nichols J., Jalali M., Litster A. The oral and conjunctival microbiotas in cats with and without feline immunodeficiency virus infection. Vet. Res. 2015;46:21. doi: 10.1186/s13567-014-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanselman B.A., Kruth S.A., Rousseau J., Weese J.S. Article Coagulase positive staphylococcal colonization of humans and their household pets. Can. Vet. J. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- 44.Van Duijkeren E., Kamphuis M., Van der Mije I.C., Laarhoven L.M., Duim B., Wagenaar J.A., Houwers D.J. Transmission of methicillin-resistant Staphylococcus pseudintermedius between infected dogs and cats and contact pets, humans and the environment in households and veterinary clinics. Vet. Microbiol. 2011;150:338–343. doi: 10.1016/j.vetmic.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Suepaul S., Georges K., Unakal C., Boyen F., Sookhoo J., Ashraph K., Yusuf A., Butaye P. Determination of the frequency, species distribution and antimicrobial resistance of staphylococci isolated from dogs and their owners in Trinidad. PLoS ONE. 2021;16:e0254048. doi: 10.1371/journal.pone.0254048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gortel K., Campbell K., Kakoma I., Whittem T., Schaeffer D., Weisiger R. Methicillin resistance among staphylococci isolated from dogs. Am. J. Vet. Res. 1999;60:1526–1530. [PubMed] [Google Scholar]
- 47.Grönthal T., Moodley A., Nykäsenoja S., Junnila J., Guardabassi L., Thomson K., Rantala M. Large outbreak caused by methicillin resistant Staphylococcus pseudintermedius ST71 in a Finnish Veterinary Teaching Hospital—from out-break control to outbreak prevention. PLoS ONE. 2014;9:e110084. doi: 10.1371/journal.pone.0110084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lena P., Ishak A., Karageorgos S.A., Tsioutis C. Presence of methicillin-resistant Staphylococcus aureus (Mrsa) on healthcare workers’ attire: A systematic review. Trop. Med. Infect. Dis. 2021;6:42. doi: 10.3390/tropicalmed6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loncaric I., Tichy A., Handler S., Szostak M.P., Tickert M., Diab-Elschahawi M., Spergser J., Künzel F. Prevalence of methicillin-resistant Staphylococcus sp. (MRS) in different companion animals and determination of risk factors for colonization with MRS. Antibiotics. 2019;8:36. doi: 10.3390/antibiotics8020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira E., Carvalho A., Ferreira A.M., Moura L., Valle A., Freitas D., Moura M. Colonization of methicillin-resistant Staphylococcus aureus among healthcare students: An integrative review. Sao Paulo Med. J. 2021;139:607–614. doi: 10.1590/1516-3180.2020.0564.r2.22042021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tchamba C., Rao A.S., Boyen F., Haesebrouck F., Jean Noel D., Theron L. Comparison of quantitative PCR and MALDI-TOF mass spectrometry assays for identification of bacteria in milk samples from cows with subclinical mastitis. J. Appl. Microbiol. 2019;127:683–692. doi: 10.1111/jam.14358. [DOI] [PubMed] [Google Scholar]
- 52.Uchida-Fujii E., Niwa H., Kinoshita Y., Nukada T. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS) for Identification of Bacterial Isolates from Horses. J. Equine Vet. Sci. 2020;95:103276. doi: 10.1016/j.jevs.2020.103276. [DOI] [PubMed] [Google Scholar]
- 53.CLSI, VET01S . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolate from Animals. 5th ed. Clinical and Laboratory Standards Institute; Philadelphia, PA, USA: 2020. [Google Scholar]
- 54.CLSI, M100 . Performance Standards for Antimicrobial Susceptibility Testing. 31th ed. Clinical and Laboratory Standards Institute; Philadelphia, PA, USA: 2021. [Google Scholar]
- 55.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 56.Lex A., Gehlenborg N., Strobelt H., Vuillemot R., Pfister H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput Graph. 2014;20:1983–1992. doi: 10.1109/TVCG.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rumi M.V., Nuske E., Mas J., Argüello A., Gutkind G., Di Conza J. Antimicrobial resistance in bacterial isolates from companion animals in Buenos Aires, Argentina: 2011–2017 retrospective study. Zoonoses Public Health. 2021;68:516–526. doi: 10.1111/zph.12842. [DOI] [PubMed] [Google Scholar]
- 58.Pahlavanzadeh S., Khoshbakht R., Kaboosi H., Moazamian E. Antibiotic resistance and phylogenetic comparison of human, pet animals and raw milk Staphylococcus aureus isolates. Comp. Immunol. Microbiol. Infect. Dis. 2021;79:101717. doi: 10.1016/j.cimid.2021.101717. [DOI] [PubMed] [Google Scholar]
- 59.Avedissian S.N., Rhodes N.J., Shaffer C.L., Tran L., Bradley J.S., Le J. Antimicrobial prescribing for treatment of serious infections caused by Staphylococcus aureus and methicillin-resistant Staphylococcus aureus in pediatrics: An expert review. Expert Rev. Anti Infect. Ther. 2021;19:1107–1116. doi: 10.1080/14787210.2021.1886923. [DOI] [PubMed] [Google Scholar]
- 60.Castañeda X., García-De-la-Mària C., Gasch O., Pericàs J.M., Soy D., Cañas-Pacheco M.A., Falces C., García-González J., Hernández-Meneses M., Vidal B., et al. Effectiveness of vancomycin plus cloxacillin compared with vancomycin, cloxacillin and dap-tomycin single therapies in the treatment of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in a rabbit model of experimental endocarditis. J. Antimicrob. Chemother. 2021;76:1539–1546. doi: 10.1093/jac/dkab069. [DOI] [PubMed] [Google Scholar]
- 61.Hiramatsu K., Cui L., Kuroda M. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2021;9:486–493. doi: 10.1016/S0966-842X(01)02175-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.