Abstract
Pancreatic cancer is the third most common cause of cancer death in the United States and eleventh worldwide. The majority of patients present with advanced disease with five-year overall survival of less than 10%. Traditional chemotherapy has been the mainstay treatment for years, with limited improvement in survival. Relative success has been achieved with agents targeting the DNA damage repair (DDR) mechanisms with poly adenosine diphosphate-ribose polymerase (PARP) inhibitors. The initial benefit was observed in patients with germline breast cancer-associated (BRCA) mutations. Multiple trials are now underway exploring PARP inhibitors in other DDR mutations such as the ataxia-telangiectasia mutated (ATM) gene and the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene (familial atypical multiple mole and melanoma syndrome), mismatch repair genes (Lynch syndrome), and others. PARP inhibitors are being evaluated as a single agent or combination chemotherapy, immunotherapy, and maintenance after chemotherapy. Here, we review current clinical trials targeting various DDR mutations and treatment strategies.
Keywords: overall survival, clinical trials, brca mutation, parp inhibitors, pancreatic cancer
Introduction and background
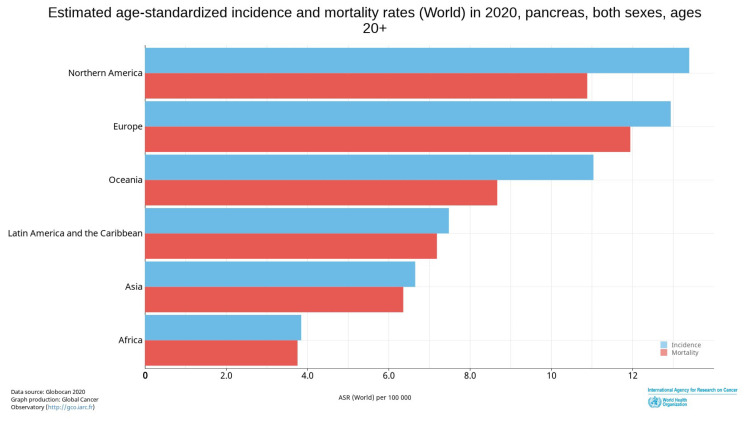
Pancreatic cancer comprises 3.2% of all new cancer cases in North America and 3% of cases worldwide. Mortality is approximately 8% of all cancer deaths in North America and 5% worldwide, making it the third most common cause of cancer death in the United States and seventh worldwide [1]. Estimated age-standardized incidence and mortality are higher among North American and European populations in comparison to the rest of the world (Figure 1) [1]. Overall, 82% of patients are diagnosed with an advanced-stage disease on presentation, with abysmal five-year overall survival of approximately 9% [2]. Traditional chemotherapy has been the mainstay treatment with limited improvement in survival, necessitating the need to identify targetable agents to significantly impact survival. Relatively successful strategies with agents targeting the DNA damage repair (DDR) mechanism, especially among patients with breast cancer-associated (BRCA) mutations, are being examined in the setting of pancreatic cancer.
Figure 1. Estimated age-standardized incidence and mortality rates for the global population in 2020, aged 20+ years, including both sexes for pancreatic cancer.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F: Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 2021, 71:209-249. 10.3322/caac.21660 [1].
This article is available under the Creative Commons CC-BY-NC license and permits non-commercial use, distribution, and reproduction in any medium provided the original work is properly cited.
Incidence of DNA damage repair genes
Pancreatic cancer is largely sporadic, with 5-20% of patients having a germline predisposition [3-7]. Genetic mutations of damaged DNA repair genes such as BRCA1/2, partner and localizer of BRCA2 (PALB2), and the ataxia-telangiectasia mutated (ATM) gene along with the cyclin-dependent kinase inhibitor 2A (CDKN2A) gene (familial atypical multiple mole and melanoma syndrome) and mismatch repair genes (Lynch syndrome) are associated with significantly increased risk of pancreatic cancer. BRCA1 is seen in up to 2.4% of pancreatic cancer patients, with up to a three-fold higher risk of developing pancreatic cancer [3,8]. BRCA2 mutations are seen in about 6% in sporadic and up to 17% in familial pancreatic cancer, with an even higher incidence in the Ashkenazi Jewish population [3,7-10]. BRCA2-mutated patients have a 3.5-6-fold higher risk for developing pancreatic cancer [11,12]. PALB2 is reported to occur in 2-4.9% of familial pancreatic cancer patients and 0.5% sporadically [9,13-15]. ATM mutations have been reported to occur in 2.4% of familial pancreatic cancer patients [16] and 3-4% in a study with a population unselected for family history of cancer [9,17]. Checkpoint kinase 2 (CHEK2) has been observed in up to 4% of patients and CDKN2A in 1.7% of patients [4,9,18-20]. A summary of the above mutations has been presented in Table 1.
Table 1. Incidence of various DNA damage repair genes.
BRCA: breast cancer-associated; ATM: ataxia-telangiectasia mutated; CHEK2: checkpoint kinase 2; CDKN2A: cyclin-dependent kinase inhibitor 2A G: germline; S: sporadic
| BRCA1 | BRCA2 | ATM | CHEK2 | PALB2 | CDKN2A |
| 0.5–3.4% (G&S) | 3–17% (G&S) | 3–4% (G&S) | 3.9% (G&S) | 0.5% (S) and 2–5% (G) | 1.7% (G&S) |
Synthetic lethality and DNA damage repair
The synthetic lethality concept was first introduced by Bryant et al. [21] and Farmer et al. in 2005 [22], which explains synergistic genetic mutations in two or more genes leading to cellular death. There are many DNA repair mechanisms that help restore genetic integrity in cells as well as common repair pathways that sense single-stranded DNA breaks are base excision repair, nucleotide excision repair, and mismatch repair. If the single-stranded break repair is not effective, it leads to the formation of double-stranded DNA breaks. Double-stranded DNA breaks are repaired through homologous recombination (HR) during the last phase of the S and G2 phases of cell cycles and non-homologous end joining (NHEJ) during the G1 phase [23]. Based on this concept, several targeted therapies against tumor-specific gene defects have been used to kill cancer cells. Poly adenosine diphosphate-ribose polymerase (PARP) inhibitors use the concept of synthetic lethality to target BRCA mutations.
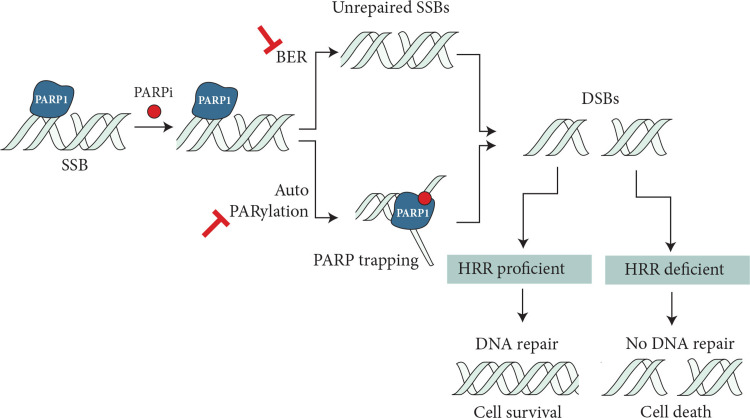
PARP1 senses DNA damage and binds to the region of DNA breaks, inducing signal transduction by producing PAR chains (autoPARylation) on target proteins. PAR chains attract DNA repair effectors, thereby competing for DNA repair [24]. PARP inhibitors trap PARP1 through inhibition of autoPARylation with or without PARP release, which in the presence of HR deficiency leads to the accumulation of double-stranded DNA breaks and ultimately to cell death (Figure 2). Cells lacking BRCA1/2 and PALB2 are predominantly affected by PARP inhibition. Platinum agents create DNA cross-links, disrupting DNA functions, which is further potentiated by the lack of a DNA repair mechanism. Platinum agents are preferred in BRCA-mutated patients as they have been shown to significantly improve overall survival in the advanced or metastatic disease setting [25]. Single-strand breaks, which are repaired through the base excision pathway, are also inhibited by PARP inhibitors, resulting in cell death in HR-deficient cells. Multiple PARP inhibitors are currently available with different PARP trapping potencies.
Figure 2. Proposed mechanism of PARP inhibitors in HRR-deficient cells.
PARP inhibitors trap PARP1 through inhibition of autoPARylation with or without PARP release, which in the presence of HR deficiency leads to the accumulation of double-stranded DNA breaks and ultimately to cell death.
SSBs: single strain breaks; BER: base excision repair; DSBs: DNA double-strand breaks; HRR homologous recombination repair; PARP: poly adenosine diphosphate-ribose polymerase
Mateo J, Lord CJ, Serra V, et al.: A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019, 30:1437-47. 10.1093/annonc/mdz192 [24].
This article is available under the Creative Commons CC-BY-NC license and permits non-commercial use, distribution, and reproduction in any medium provided the original work is properly cited.
Review
PARP inhibitors
Following the detailed introduction, PARP inhibitors sensitize cancer cells to DNA damaging therapies and inhibit DNA repair mechanisms leading to synthetic lethality. Several clinical trials such as the OlampiAD study for breast cancer [26], SOLO study for ovarian cancer [27], and TOPARP-B study for prostate cancers [28] with underlying germline BRCA1/2 mutations have shown promising results with an increase in response rate and progression-free survival (PFS). According to the National Comprehensive Cancer Network (NCCN) practice guidelines, germline testing is recommended for all patients with confirmed pancreatic cancer [29]. The effectiveness of PARP inhibitors can be examined in cancers that possess BRCAness gene defects. BRCAness can be described as tumors that do not have germline line BRCA mutation but have gene defects that share phenotypic similarities with BRCA mutations and have defective HRR. This review focuses mainly on the clinical trials of several PARP inhibitors to date and those currently underway to assess the efficacy of PARP inhibitors in the treatment of pancreatic cancer (Table 2) [30-32].
Table 2. Potency of various PARP inhibitors.
PARP: poly adenosine diphosphate-ribose polymerase
| PARP inhibitor | Potency | PARP target |
| Olaparib | 1 | PARP 1, 2, 3 |
| Rucaparib | 1 | PARP 1, 2, 3 |
| Talazoparib | 100 | PARP 1, 2 |
| Veliparib | 0.1 | PARP 1, 2 |
| Niraparib | 2 | PARP 1, 2 |
| Pamiparib (BGB 290) | 10 | PARP 1, 2 |
Olaparib
Olaparib is currently the only Food and Drug Administration (FDA)-approved PARP inhibitor for use in pancreatic cancer. Multiple clinical trials are underway evaluating olaparib use for pancreatic cancer both as a monotherapy and combination therapy (Tables 3, 4). FDA approval was based on the POLO trial, a phase III randomized, placebo-controlled, double-blinded study that evaluated the efficacy of olaparib as maintenance therapy [33]. In this study, a total of 154 patients had germline BRCA1 or BRCA2-mutated metastatic pancreatic cancer without disease progression during at least 16 weeks of first-line platinum-based chemotherapy. In the study, 92 patients received olaparib and had significantly improved median PFS of 7.4 months versus 3.8 months in the placebo group (p = 0.004). At two years, 22.1% in the olaparib group had no disease progression in comparison to 9.6% of patients in the placebo group. However, planned interim analysis at 46% data maturation showed no difference in OS between the groups. There were certain limitations to the study. Included trial patients had a good response to the platinum agents prior to maintenance PARP treatment and non-responders were not included. Maintenance chemotherapy after six months of intense triple chemotherapy in responders is a common strategy employed with single-agent 5-fluorouracil or capecitabine versus combination with irinotecan or platinum agents, unlike placebo in the control arm. Overall, this study paved the way for multiple therapeutic and maintenance strategies in pancreatic cancer patients.
Table 3. Olaparib as maintenance therapy and single-agent therapy.
PFS: progression-free survival; OS: overall survival; ORR: objective response rate; BRCA: breast cancer-associated; PDAC: pancreatic ductal adenocarcinoma; ATM: ataxia-telangiectasia mutated; PALB2: partner and localizer of BRCA2; FANCB: FA complementation group B; PTEN: phosphatase and tensin homolog; CCNE1: cyclin E1; SD: stable disease; PR: partial response; CR: complete response
| Clinical trial | Study objective | N | PFS | OS | ORR | Germline mutations | Somatic mutations |
| Olaparib as maintenance therapy | |||||||
| NCT02184195 POLO trial [33] | Phase III randomized, placebo-controlled, double-blinded study evaluating the efficacy of olaparib as maintenance therapy | 154 | 7.4 months | No survival benefit versus placebo at 46% data maturity | 20% response rate in the study group, and 10% response in the placebo group | BRCA1 or BRCA2 | Excluded |
| Olaparib as single-agent therapy | |||||||
| NCT02677038 (United States) and NCT02511223 (Israel) [34] | Parallel phase II trials in the United States and Israel evaluating the efficacy of olaparib in advanced PDAC with BRCAness with ≥ prior systemic therapy | 11 patients in the United States and 21 in Israel | Median PFS of ~25 weeks in the United States and 14 weeks in Israel | Not reached | ~ 80% (17/21) from the Israel group and 73% (8/11) from the US group had stable or partial response. No responses were seen in platinum-refractory cases | Excluded | DDR-genetic aberrations (ATM, PALB2, BRCA somatic, FANCB, PTEN, and CCNE1), family history of BRCA without DDR genetic aberrations |
| NCT01078662 [35] | Phase 2 study assessing the efficacy and safety of olaparib in confirmed genetic BRCA1 or BRCA2 mutation patients in advanced tumors with 23 pancreatic patients in the study | 23 | Median PFS of 4.5 months | Median OS of 9.8 months | 57% (SD+PR+CR) at >8 weeks | BRCA1 or BRCA2 | Excluded |
Table 4. Olaparib as combination therapy.
MTD: maximum tolerated dose; BRCA: breast cancer-associated; ATM: ataxia-telangiectasia mutated; HRD: homologous recombination deficiency; DDR: DNA damage repair
| Clinical trial | Phase | Study objective | Germline mutations | Somatic mutations | Outcomes |
| Olaparib with chemotherapy and targeted therapy | |||||
| NCT00515866 [36] | Phase I | Evaluate the safety and MTD of olaparib with gemcitabine versus gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer | Unselected for mutations | Out of 66 patients treated, olaparib 100 mg twice daily with gemcitabine 600 mg/m2 was the most tolerable combination. | |
| NCT01296763 [37] | phase I | Dose escalation trial evaluating olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer | Known BRCA-mutated or Jewish ancestry patients and those with familial and sporadic pancreatic cancer were included | Early closure due to toxicity | |
| NCT03682289 [38] | phase II | Studying the efficacy of ATR kinase inhibitor AZD6738 alone versus combination with olaparib in patients with advanced renal cell, urothelial and pancreatic cancers | N/A | BAF250a-positive received AZD6738 with olaparib and BAF250a-negative or ATM-mutant received AZD6738 only | Currently recruiting |
| NCT02498613 [39] | Phase II | Study evaluating cediranib maleate combined with olaparib in advanced breast, non-small-cell and small-cell lung cancer, and pancreatic cancer patients | Identification of DNA repair genes in tumors using the BROCA panel with a plan to correlate tumor regression with mutations status | Currently recruiting | |
| NCT03205176 [40] | Phase I | Study evaluating MTD and preliminary antitumor activity of AZD5153 in relapsed/refractory malignant solid tumor, including lymphoma patients as a single agent or in combination with olaparib. | Patients are included regardless of BRCA status. Planned to collect BRCA and HRD gene mutational status | Currently recruiting | |
| NCT04005690 [41] | Phase II window of opportunity trial | Evaluating cobimetinib or olaparib response in patients with resectable pancreatic cancer by comparing pretreatment biopsy samples with posttreatment resection specimens | N/A | Currently recruiting | |
| Olaparib with immunotherapy | |||||
| NCT03851614 (DAPPER) [42] | Phase II | Evaluating changes in genomic and immune biomarkers in tumor, peripheral blood, and stool; changes in radiomic profiles, with the combination of durvalumab with olaparib or cediranib | Patients with germline or somatic DDR genes will be evaluated retrospectively but is not an eligibility criteria | Currently recruiting | |
Olaparib as monotherapy: Two ongoing parallel phase 2 trials [34] in the United States (NCT02677038) and Israel (NCT02511223) are evaluating the efficacy of olaparib in advanced pancreatic ductal adenocarcinoma (PDAC) with BRCAness (excluding germline BRCA1/2 mutations) after at least one prior systemic therapy regimen. Of the 11 U.S. patients, two achieved partial response (PR), six reported stable disease (SD), and three have experienced progressive disease (PD), with a median PFS up to 24.7 weeks. Of the 21 Israeli patients, five are with SD, and 12 are with PD, with a median PFS of 14 weeks. Another phase 2, open-label study assessed the efficacy and safety of olaparib in 23 advanced pancreatic cancer patients with confirmed genetic BRCA1 or BRCA2 mutations (NCT01078662) [35]. Over 74% of patients with BRCA2 mutations with a mean of two prior therapies with 65% receiving prior platinum. Overall, 22% of patients had a complete response (CR) or PR, and 35% had SD at eight weeks. The response was independent of BRCA1/2 status as well as response to prior platinum treatment. Median PFS was 4.5 months with a median OS of 9.8 months at the end of the study. OS rate at 12 months was 41%.
Olaparib with chemotherapy, other targeted therapies: NCT00515866 is a phase I study of olaparib with gemcitabine in patients with advanced solid tumors to evaluate the safety and maximum tolerated dose (MTD) of the combination for pancreatic cancer treatment [36]. A total of 68 patients were included in the dose-escalation and expansion phase, with more than 81% of patients with combination treatment experiencing grade ≥3 adverse events, predominantly cytopenias (55%). Based on this study, the ideal combination was olaparib 100 mg twice daily (intermittent dosing) with gemcitabine 600 mg/m2 with manageable toxicities. NCT01296763 [37] is another phase 1 dose-escalation trial evaluating olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Unfortunately, patients in this trial developed significant toxicity with this combination therapy, predominantly cytopenias, resulting in early study closure.
NCT03682289 [38] is a non-randomized phase II trial studying the efficacy of ataxia telangiectasia and Rad3-related protein (ATR) kinase inhibitor AZD6738 alone or in combination with olaparib in patients with advanced renal cell, urothelial, and pancreatic cancers. Patients tested for BAF250a positive expression on immunohistochemistry received AZD6738 in combination with olaparib. BAF250a-negative or ATM-mutant patients received AZD6738 only. NCT02498613 [39] is an open-label phase II trial studying cediranib maleate (vascular endothelial growth factor (VEGF)-tyrosine kinase inhibitor (TKI)) combined with olaparib in advanced breast, non-small-cell and small-cell lung cancer, and pancreatic cancer patients. Apart from the primary objective to assess the response to the treatment, other exploratory objectives include the correlation of response to DNA repair gene mutations, estimating levels of angiogenesis markers such as VEGF at baseline and after treatment, evaluating tumor hypoxia in lung cancer patients, and circulation tumor DNA measures through treatment course. This study unfortunately did not show any meaningful activity.
Other ongoing trials are NCT03205176 [40], a phase I, multicenter, dose-escalation study evaluating AZD5153 (bivalent BRD4/BET bromodomain inhibitor) pharmacokinetics, anti-tumor activity with tolerability both as a monotherapy and in combination with olaparib in patients with relapsed/refractory malignant solid tumors and lymphomas. NCT04005690 [41] is a phase II trial evaluating how cobimetinib or olaparib works in patients with resectable pancreatic cancer by comparing pretreatment biopsy samples with posttreatment resection specimens. The primary goal is to use the results in designing future biomarker-driven trials.
Olaparib with immunotherapy: NCT03851614 [42] (DAPPER) is a phase II basket study in patients with advanced pancreatic cancer, mismatch repair colorectal cancer, and leiomyosarcoma to evaluate changes in genomic and immune biomarkers in the tumor, peripheral blood, and stool samples. The study involves a combination of durvalumab (programmed death-ligand 1 (PD-L1)) with olaparib or cediranib (AZD2171, a small-molecule VEGF-TKI). This study is still ongoing, and results have not been published yet.
Veliparib
Veliparib as monotherapy: There are nine clinical studies involving veliparib (Tables 5, 6). Monotherapy was evaluated as a phase 1 study (NCT00892736) in patients with refractory BRCA1/2-mutated solid cancer; platinum-refractory ovarian, fallopian tube, or primary peritoneal cancer; or basal-like breast cancer. At the MTD, BRCA-positive patients had a response rate of 58%. This study had a limited number of pancreatic cancer patients, and specific data regarding pancreatic cancer outcomes were not available [43].
Table 5. Veliparib as monotherapy.
BRCA: breast cancer-associated
| Clinical trial | Phase | Study objective | Germline mutations | Somatic mutations | Outcomes |
| Veliparib monotherapy | |||||
| NCT00892736 [43] | I | Refractory BRCA1/2-mutated solid cancer; platinum-refractory ovarian, fallopian tube, or primary peritoneal cancer; or basal-like breast cancer | Refractory BRCA1/2-mutated solid cancer | BRCA-positive patients had about a 58% response rate. Pancreatic data are limited | |
Table 6. Veliparib in combination therapy.
MPC: metastatic pancreatic cancer; PFS: progression-free survival; OS: overall survival; BRCA: breast cancer-associated; ATM: ataxia-telangiectasia mutated; PALB2: partner and localizer of BRCA2; FANCB: FA complementation group B; CHEK2: checkpoint kinase 2; IMRT: intensity-modulated radiotherapy; SD: stable disease
| Clinical trial | Phase | N | Study objective | Germline mutations | Somatic mutations | PFS | OS | ORR |
| Veliparib with chemotherapy | ||||||||
| NCT01908478 [44] | I | 30 | Evaluates veliparib (ABT-888) in combination with gemcitabine and IMRT in locally advanced and unresectable pancreatic cancer patients | None | DDR mutations, PARP level, and tumor mutation burden were once enrolled in the trial | 9.8 months | mOS for DDR-deficient was 19 months and DDR-intact was and 14 months | SD was 93% (28/30), PR and 3% (1/30) |
| NCT01489865 [45] | I/II | 64 (57 patients in the final analysis) | Evaluates veliparib in combination with FOLFOX in MPC | Trial included patients with DDR-positive and two mBRCA2 patients | 3.7 months | 8.5 months | 26% | |
| DDR positive 7.2 mo | 11.1 | 50% | ||||||
| NCT01585805 [46] | II | 55 | Evaluates response to gem + cis in combination with beliparib (Arm – A) and without veliparib (Arm – B) | Germline BRCA1/2 or germline PALB2 mutation | NA | No statistically significant difference in both arms with median PFS of ~10 months and median OS of ~16 months | Insufficient power to extrapolate | |
| NCT02890355 [47] | II | 143 | Evaluates response to mFOLFIRI + veliparib versus FOLFIRI alone for second-line MPC patients | 15 patients: BRCA1, BRCA2, ATM, FANC, BLM, SLX4, CHEK2 | 20 patients: BRCA2, PALB2, ATM, CDK12, FANC, BLM, POLD1, RIF1, MSH2, MSH6, and other unclassified DDR. | In biomarker unselected patients, there is no difference in median PFS (~2–3 months), median OS (~5–6 months) along with increased toxicity in veliparib arm | ||
| NCT01233505 [48] | I | 17 | Evaluate safety and preliminary efficacy of the combination of CAPOX with veliparib | Four breast and three ovarian are BRCA1-positive. Information unavailable on pancreatic cancer patient | One pancreatic cancer patient has SD | |||
| NCT00576654 [49] | I | 35 | Evaluate safety and preliminary efficacy of veliparib in combination with irinotecan | Observed BRCA mutations carriers among ovarian cancer patient. | No pancreatic cancer patients on trial so far. PR in ~19% of patients | |||
Veliparib with chemotherapy: NCT01908478 is a phase I study evaluating veliparib (ABT-888) in combination with gemcitabine and intensity-modulated radiation therapy (IMRT) in patients with locally advanced and unresectable pancreatic cancer. This study among 30 patients showed a median OS of 15 months. There were no BRCA patients in the trial. Interestingly, median OS for DDR pathway gene-altered was 19 months and 14 months among DDR-intact patients. Increased expression of the DDR proteins PARP3 showed significantly improved OS; however, DDR pathway mutations did not correlate with OS [44]. The majority of patients had SD (93%), with PR in one patient.
NCT01489865 is a phase I/II study of veliparib in combination with 5-fluorouracil and oxaliplatin (FOLFOX) in patients with metastatic pancreatic cancer. According to the final study results, of the 64 patients, 78% were platinum-naïve, 69% had a family history, and 27% had DDR mutations. Among the total 57 evaluable patients, the objective response rate (ORR) was 26% with median PFS of 3.7 months and median OS of 8.7 months. However, in patients with DDR mutations, ORR (58%) and m PFS (7.2 months) doubled with improvement in OS (11.1 months). The responses further improved in patients with family history and platinum-naïve disease along with DDR deficiency, which appears to be a promising combination with respect to the safety and response in metastatic PDAC [45]. An earlier report from the trial mentioned, two BRCA2 patients were included in the study, one had a PR at 17 months, and the other had CR with normalization of carbohydrate antigen 19-9 at 10 months.
NCT01585805 is a randomized, phase II study of gemcitabine, cisplatin without (Arm A) or with (Arm B) veliparib, and a phase II single-arm study of single-agent veliparib in patients with BRCA- or PALB2-mutated pancreatic adenocarcinoma. Among the enrolled 55 patients, median PFS (~10 months) and median OS (~16 months) were not significantly improved with the addition of veliparib [46].
NCT02890355 is a randomized, phase II study evaluating second-line modified FOLFIRI with veliparib versus FOLFIRI. Out of 143 patients included in the analysis, 30% had DNA repair gene abnormalities and 9% has HR deficiency. Interim futility analysis at 35% of expected PFS events showed that the veliparib arm was unlikely to be superior to control in the biomarker unselected population. Median PFS was approximately two to three and median OS was five to six months. Biomarker-driven efficacy is yet to be published [47]. NCT01233505 is a phase I study evaluating veliparib in combination with oxaliplatin and capecitabine in advanced solid tumors. The trial included one patient with pancreatic cancer who had SD with this combination [48].
NCT00576654 is a phase I study designed to evaluate the safety and preliminary efficacy of veliparib (ABT-888) in combination with Irinotecan among advanced solid tumors. Safety of combination was established, and PR is observed in approximately 19% of patients. However, no pancreatic cancer patients were reported in the study [49].
Rucaparib
According to the literature, four clinical trials have been reported, two as monotherapy, and two as combination therapy. NCT03140670 is a phase II trial of rucaparib evaluating patients with advanced pancreatic cancer with known germline or somatic BRCA or PALB2 mutations who had not progressed on at least 16 weeks of platinum treatment. Out of 24 patients enrolled, 13 had germline BRCA2, three had germline BRCA1, two had germline PALB2, and one had somatic BRCA2 mutation. Median PFS was 9.1 months from starting rucaparib therapy, with an ORR of 36.8% and disease control rate (CR + PR + SD) of 89.5% for at least eight weeks [50]. Overall, the study showed encouraging results that maintenance strategy not only for germline BRCA,as in POLO trial, but in other germline and somatic mutations might be an effective strategy.
NCT02042378 (RUCAPANC) evaluated the efficacy of rucaparib monotherapy in patients with pancreatic cancer with deleterious BRCA mutations. Sixteen of nineteen BRCA1/2 mutations were germline, and three were somatic BRCA2. There were two PR, one CR, with an ORR of 15.8%, and further enrollment was halted in view of the poor response rate [51].
Rucaparib with chemotherapy: NCT03337087 [52] is a phase Ib/II trial evaluating safety along with the efficacy of liposomal irinotecan and fluorouracil with rucaparib in patients with advanced pancreatic, colorectal, biliary, and gastroesophageal cancers. Patients with pancreatic cancer could have received up to two lines of prior therapy. This is planned to evaluate responses based on the HRD mutation status. NCT04171700 [53] is a phase II study of rucaparib as a treatment for solid tumors with deleterious HRD mutations including pancreatic cancer patients. Currently, no published data are available for both studies.
Rucaparib with other targeted therapies: NCT02711137 [54] is an open-label phase I/II dose-escalation/expansion along with safety and tolerability study of INCB057643 bromodomain and extraterminal (BET) inhibitor as a single agent and in combination with multiple interventions, including rucaparib in patients with advanced malignancies. The study is currently terminated in view of safety issues. Table 7 lists the trials involving rucaparib as maintenance therapy, monotherapy, and combination therapy.
Table 7. Rucaparib as maintenance therapy, monotherapy, and combination therapy.
PFS: progression-free survival; OS: overall survival; ORR: objective response rate; BRCA: breast cancer-associated; PALB2: partner and localizer of BRCA2; HRD: homologous recombination deficiency
| Clinical trial | Phase | N | Study objective | Germline mutations | Somatic mutations | PFS | OS | ORR |
| Rucaparib as maintenance therapy | ||||||||
| NCT03140670 [50] | II | 24 | Evaluate maintenance in pancreatic adenocarcinoma not progressing for at least 16 weeks of platinum treatment | 13 germline BRCA2, 3 germline BRCA1, 2 germline PALB2 | 1 somatic BRCA2 | 9.1 | N/A | 37% |
| Rucaparib as monotherapy | ||||||||
| NCT02042378 [51] RUCAPANC | II | 19 | Evaluation monotherapy in advanced pancreatic cancer patients with germline or somatic BRCA patients who received upto two lines of prior chemotherapy. | 12 germline BRCA2, 4 germline BRCA1 | 3 somatic BRCA2 | Halted enrollment (ORR of 16% ) in view of insufficient response rate among the first 15 patients | ||
| Rucaparib with chemotherapy | ||||||||
| NCT03337087 [52] | Ib/II | N/A | Evaluate safety and preliminary efficacy of liposomal irinotecan and fluorouracil with rucaparib in patients with metastatic gastrointestinal cancers including pancreatic cancer | Plan to evaluate response based on HRD mutations | Currently enrolling | |||
| NCT04171700 [53] LODESTAR | II | N/A | Evaluating rucaparib therapy in HRD deficient solid tumors including pancreatic cancer | HRD-deficient tumors | Currently enrolling | |||
| Rucaparib in combination with targeted therapy | ||||||||
| NCT02711137 [54] | I/II | N/A | Safety and BET inhibitor as single agent and in combination with multiple interventions including rucaparib in advanced malignancy pts | Study is currently terminated in view of safety issues. | ||||
Talazoparib
Talazoparib is a selective oral PARPi that is more potent than earlier PARP inhibitors (Table 8). NCT01286987 is a phase I, first-in-human study of talazoparib in patients with advanced or recurrent solid tumors to evaluate antitumor activity and MTD of talazoparib. The study included 13 patients with pancreatic cancer; four out of 13 patients have shown clinical benefit (CR + PR + SD = 31% ≥16 weeks), with 20% (two patients) having PR. Among patients with PR, one had BRCA2 and another had PALB2 mutations. The maximum tolerated dose was found to be 1 mg/day [55]. NCT03637491 [56] is an ongoing phase 1b/II study evaluating the safety and efficacy of avelumab, binimetinib, and talazoparib combination in patients with locally advanced or metastatic Ras-mutant solid tumors including pancreatic cancer patients. DDR mutations will be assessed at baseline. Objective response and dose-limiting toxicities are considered as primary outcomes. Unfortunately, the study was terminated as there was limited antitumor activity, and reaching target study drug dose levels was not feasible.
Table 8. Talazoparib as monotherapy and combination therapy.
BRCA: breast cancer-associated; PALB2: partner and localizer of BRCA2; DDR: DNA damage repair; PFS: progression-free survival; OS: overall survival; ORR: objective response rate
| Clinical trial | Phase | N | Study objective | Germline mutations | Somatic mutations | PFS | OS | ORR | |
| Talazoparib monotherapy | |||||||||
| NCT01286987 [55] | I | 13 | Evaluate safety and preliminary efficacy in advanced or recurrent solid tumors and study has 13 pancreatic cancer patients | BRCA2 PALB2 | - | N/A | 20%. Patients with response were bearing DDR mutations | ||
| Talalzoparib with combination with immunotherapy and targeted therapy | |||||||||
| NCT03637491 [56] | Ib/II | Evaluate and efficacy of avelumab, binimetinib and talazoparib combinations in patients with locally advanced or metastatic Ras-mutant solid tumors including pancreatic cancer pts | DDR mutations will be assessed at baseline | Currently terminated | |||||
Niraparib
There are three ongoing trials of niraparib (two monotherapy and one combination therapy trial). NCT03601923 [57] is a phase 2, proof-of-concept trial evaluating the safety and efficacy of niraparib in patients with advanced pancreatic cancer with germline and somatic HRD mutations. Included patients received at least one line of treatment for their cancer and did not have cancer progression on an oxaliplatin regimen (similar to FOLFIRINOX or FOLFOX). Palliative radiation will be started at least a week before the initiation of niraparib. PFS is the primary outcome of the study, and overall response and survival rate are the secondary outcomes. NIRA-PANC (NCT03553004) [58] is a phase II trial evaluating the efficacy of niraparib in metastatic pancreatic cancer patients with germline or somatic HRD mutations who received at least one prior line of therapy. Currently, patients are actively being enrolled. ORR (overall response to therapy at eight weeks) is the primary outcome, and PFS and OS are the secondary outcomes. Currently, no published data are available for these studies.
Niraparib as combination therapy (with immunotherapy): Parpvax (NCT03404960) is a phase Ib/II study of niraparib plus ipilimumab (phase 2), niraparib plus nivolumab (phase 1) evaluating the safety, effectiveness, and antitumor activity (preventing tumor growth) in patients with advanced pancreatic cancer whose disease has not progressed on platinum-based therapy for at least 16 weeks [59]. PFS is the primary outcome of this trial. This study is actively recruiting patients (Table 9).
Table 9. Niraparib as monotherapy and in combination therapy.
PFS: progression-free survival; OS: overall survival; ORR: objective response rate; BRCA: breast cancer-associated; ATM: ataxia-telangiectasia mutated; PALB2: partner and localizer of BRCA2; HRD: homologous recombination deficiency
| Clinical trial | Phase | N | Study objective | Germline mutations | Somatic mutations | PFS | OS | ORR |
| Niraparib monotherapy | ||||||||
| NCT03601923 [57] | II | Proof-of-concept trial evaluating niraparib in patients with HRD pancreatic cancer progressed on one line of therapy except platinum agents | Germline and somatic mutations in BRCA1, BRCA2, PALB2, CHEK2, or ATM | Currently enrolling | ||||
| NCT03553004 NIRA-PANC [58] | II | Efficacy in metastatic pancreatic cancer patients with HRD mutations who received at least one prior line of therapy. | Germline and somatic HRD mutations | Currently enrolling | ||||
| Niraparib with immunotherapy | ||||||||
| NCT03404960 Parpvax [59] | Ib/II | Study of niraparib plus either ipilimumab or nivolumab in patients with advanced pancreatic cancer whose disease has not progressed on platinum-based therapy | HRD will be identified after enrollment | Currently enrolling. | ||||
Discussion
Pancreatic cancer continues to be one of the most difficult cancer to treat with chemotherapy as the predominant first-line treatment. Immunotherapy was granted accelerated FDA approval in 2017 for MSI-high/MMR-deficient advanced solid tumor patients, and larotrectinib/entrectinib is currently approved for neurotrophic tyrosine receptor kinase gene fusion-positive patients. These therapies are currently recommended by the NCCN guidelines in patients with poor performance as firstline [29]. Targeting the DNA repair mechanism is one of the novel approaches in the treatment of pancreatic cancer, and PARP inhibitors are at the forefront of that approach. Apart from germline BRCA1/2 patients, multiple genetic defects affecting the DNR repair mechanism mentioned as BRCAness are currently under investigation in several cancers with PARP inhibitor therapy.
The most significant success, along with the first FDA approval, was through olaparib (POLO trial) as maintenance therapy in platinum-sensitive germline BRCA-mutated patients. Maintenance rucaparib (NCT03140670) [50] and niraparib (NCT03553004) [58] are currently being evaluated through phase II clinical trials in patients with not only germline mutations but expanding it to somatic HRD mutations. Early results are optimistic, and these trials have the potential to further expand the patient population who can receive significant benefits through maintenance therapy.
PARP inhibitors as monotherapy were assessed among germline BRCA1/2-mutated ovarian cancer patients with olaparib (NCT01078662) [35], which showed durable response rates regardless of platinum sensitivity. Interestingly, two parallel phase II studies (NCT02511223 and NCT02677038) [34] studied olaparib as monotherapy in germline BRCA1/2-negative patients with DDR deficiency and showed a response in platinum-sensitive patients. Talazoparib in a phase Ib trial showed a good response in 20% of patients among germline BRCA1/2-mutated patients [55]. Rucaparib [51] in germline and somatic BRCA patients and veliparib [43] in germline BRCA or PALB2 mutations did not show significant response when used a monotherapy. Niraparib is currently under evaluation in two trials with germline and somatic HRD mutation patients with results yet to be published [57,58].
PARP inhibitors and multiple chemotherapy combinations have been evaluated with mixed results so far, and the majority of trials are still under evaluation. Veliparib, in combination with FOLFOX among metastatic pancreatic cancer patients, shows good tolerability with combination therapy, especially among patients with platinum-naïve, DDR deficiency, and patients with a family history. However, the efficacy of veliparib needs to be evaluated in a randomized fashion [44]. Veliparib with gemcitabine and cisplatin in BRCA/PALB2-mutated patients as well as in combination with FOLFIRI as second-line therapy among DDR deficiency patients did not show any significant benefit from the addition of veliparib [45,46]. There is also increased toxicity with combination therapy, as seen in NCT01296763 [37], of olaparib with irinotecan, cisplatin, and mitomycin.
Combination with immunotherapy, along with additional targeted therapy, is one of the new strategies under development. PARPVAX [59] trial is currently evaluating the combination of niraparib with nivolumab in patients with advanced pancreatic cancer who have no disease progression with at least 16 weeks of platinum. Other combination trials such as the DAPPER [60] trial evaluating durvalumab (anti-PD-L1) with olaparib and NCT03637491 [56] trial evaluating avelumab (anti-PD-L1) with talazoparib are currently under investigation. Combination with targeted therapy is currently under evaluation with multiple agents. Trials in combination with olaparib, such as NCT03682289 [38] with AZD6738 (ATR kinase inhibitor), NCT03205176 [40] with AZD5153 (bivalent BRD4/BET bromodomain inhibitor), and NCT02498613 [39] with cediranib maleate (VEGF-TKI) are under investigation. The safety of such combinations could be a predominant issue, as seen in NCT02711137 [54], evaluating BET inhibitor as a single agent and in combination with multiple interventions, including rucaparib, currently terminated in view of safety issues.
Multiple resistance mechanisms have been hypothesized, leading to the failure of PARP inhibitors such as secondary mutations in BRCA genes resulting in the restoration of DNA repair mechanism, efflux pumps resulting in reduced intracellular PARP concentrations, epigenetic modifications, and loss of PARP expression, to mention a few [61]. Multiple newer PARP inhibitors are currently under development to overcome such resistance mechanisms. Other strategies, such as combination and sequential therapies, are more likely to improve the efficacy of PARP inhibitors.
Conclusions
Pancreatic cancer has an abysmal prognosis and chemotherapy has been the mainstay of treatment with limited improvement in survival. PARP inhibitors, which work by targeting the DDR mechanism, especially patients with BRCA mutations, are currently approved for maintenance therapy in pancreatic cancer. Beyond maintenance therapy, it is very encouraging to see PARP inhibitors emerging as a new therapeutic option as monotherapy in combination with chemotherapy, immunotherapy, and targeted therapies. Multiple trials are also evaluating the response to therapy beyond germline BRCA mutation patients. Such clinical trials on the application of PARP inhibitors and their efficacy with combination therapy will be critical to improving patient outcomes.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics, 2019. Siegel RL, Miller KD, Jemal A. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. Lowery MA, Wong W, Jordan EJ, et al. J Natl Cancer Inst. 2018;110:1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. Shindo K, Yu J, Suenaga M, et al. J Clin Oncol. 2017;35:3382–3390. doi: 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. Genet Med. 2019;21:213–223. doi: 10.1038/s41436-018-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevalence of germline mutations in cancer predisposition genes in patients with pancreatic cancer. Grant RC, Selander I, Connor AA, et al. Gastroenterology. 2015;148:556–564. doi: 10.1053/j.gastro.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BRCA2 germline mutations in familial pancreatic carcinoma. Hahn SA, Greenhalf B, Ellis I, et al. J Natl Cancer Inst. 2003;95:214–221. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 8.Genetic susceptibility to pancreatic cancer. Klein AP. Mol Carcinog. 2012;51:14–24. doi: 10.1002/mc.20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. Mandelker D, Zhang L, Kemel Y, et al. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. Holter S, Borgida A, Dodd A, et al. J Clin Oncol. 2015;33:3124–3129. doi: 10.1200/JCO.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 11.Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 12.Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, et al. J Med Genet. 2005;42:711–719. doi: 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Axilbund JE, Argani P, Kamiyama M, et al. Cancer Biol Ther. 2009;8:131–135. doi: 10.4161/cbt.8.2.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Schneider R, Slater EP, Sina M, et al. Fam Cancer. 2011;10:323–330. doi: 10.1007/s10689-010-9414-x. [DOI] [PubMed] [Google Scholar]
- 15.PALB2 mutations in familial breast and pancreatic cancer. Hofstatter EW, Domchek SM, Miron A, et al. Fam Cancer. 2011;10:225–231. doi: 10.1007/s10689-011-9426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ATM mutations in patients with hereditary pancreatic cancer. Roberts NJ, Jiao Y, Yu J, et al. Cancer Discov. 2012;2:41–46. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevalence of pathogenic mutations in cancer predisposition genes among pancreatic cancer patients. Hu C, Hart SN, Bamlet WR, et al. Cancer Epidemiol Biomarkers Prev. 2016;25:207–211. doi: 10.1158/1055-9965.EPI-15-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDKN2A germline mutations in familial pancreatic cancer. Bartsch DK, Sina-Frey M, Lang S, et al. Ann Surg. 2002;236:730–737. doi: 10.1097/00000658-200212000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. de Snoo FA, Bishop DT, Bergman W, et al. Clin Cancer Res. 2008;14:7151–7157. doi: 10.1158/1078-0432.CCR-08-0403. [DOI] [PubMed] [Google Scholar]
- 20.Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. https://doi.org/10.1002/1097-0215(20000915)87:6<809::AID-IJC8>3.0.CO;2-U. Int J Cancer. 2000;87:809–811. [PubMed] [Google Scholar]
- 21.Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Bryant HE, Schultz N, Thomas HD, et al. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 22.Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Farmer H, McCabe N, Lord CJ, et al. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 23.Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Dietlein F, Thelen L, Reinhardt HC. Trends Genet. 2014;30:326–339. doi: 10.1016/j.tig.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 24.A decade of clinical development of PARP inhibitors in perspective. Mateo J, Lord CJ, Serra V, et al. Ann Oncol. 2019;30:1437–1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Golan T, Kanji ZS, Epelbaum R, et al. Br J Cancer. 2014;111:1132–1138. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. Robson M, Im SA, Senkus E, et al. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 27.Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. Moore K, Colombo N, Scambia G, et al. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 28.Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Mateo J, Porta N, Bianchini D, et al. Lancet Oncol. 2020;21:162–174. doi: 10.1016/S1470-2045(19)30684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pancreatic adenocarcinoma. [ Feb; 2022 ];https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf 2019
- 30.Abstract 1651: BGB-290, a novel PARP inhibitor with unique brain penetration ability, demonstrated strong synergism with temozolomide in subcutaneous and intracranial xenograft models. Tang Z, Jiang B, Shi Z, et al. Cancer Res. 2015;75:1651. [Google Scholar]
- 31.Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Murai J, Huang SY, Renaud A, et al. Mol Cancer Ther. 2014;13:433–443. doi: 10.1158/1535-7163.MCT-13-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Hopkins TA, Shi Y, Rodriguez LE, et al. Mol Cancer Res. 2015;13:1465–1477. doi: 10.1158/1541-7786.MCR-15-0191-T. [DOI] [PubMed] [Google Scholar]
- 33.Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. Golan T, Hammel P, Reni M, et al. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phase II study of olaparib for BRCAness phenotype in pancreatic cancer. Golan T, Varadhachary GR, Sela T, et al. J Clin Oncol. 2018;36:297. [Google Scholar]
- 35.Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Domchek SM, Aghajanian C, Shapira-Frommer R, et al. Gynecol Oncol. 2016;140:199–203. doi: 10.1016/j.ygyno.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Bendell J, O'Reilly EM, Middleton MR, et al. Ann Oncol. 2015;26:804–811. doi: 10.1093/annonc/mdu581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olaparib in combination with irinotecan, cisplatin, and mitomycin C in patients with advanced pancreatic cancer. Yarchoan M, Myzak MC, Johnson BA 3rd, et al. Oncotarget. 2017;8:44073–44081. doi: 10.18632/oncotarget.17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phase II trial of AZD6738 alone and in combination with olaparib. [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT03682289 2022
- 39.Clinical activity and safety of cediranib and olaparib combination in patients with metastatic pancreatic ductal adenocarcinoma without BRCA mutation. Kim JW, Cardin DB, Vaishampayan UN, et al. Oncologist. 2021;26:0–9. doi: 10.1002/onco.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First-in-human study of AZD5153, a small molecule inhibitor of bromodomain protein 4 (BRD4), in patients (pts) with relapsed/refractory (RR) malignant solid tumor and lymphoma: preliminary data. Wang JS, Vita SD, Karlix JL, et al. J Clin Oncol. 2019;37:3085. [Google Scholar]
- 41.Targeted PARP or MEK/ERK inhibition in patients with pancreatic cancer. [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT04005690 2019
- 42.Basket studies: redefining clinical trials in the era of genome-driven oncology. Tao JJ, Schram AM, Hyman DM. https://doi.org/10.1146/annurev-med-062016-050343. Annu Rev Med. 2018;69:319–331. doi: 10.1146/annurev-med-062016-050343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Outcome of BRCA 1/2-mutated (BRCA+) and triple-negative, BRCA wild type (BRCA-wt) breast cancer patients in a phase I study of single-agent veliparib (V) Pahuja S, Beumer JH, Appleman LJ, et al. J Clin Oncol. 2014;32:135. [Google Scholar]
- 44.A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. Tuli R, Shiao SL, Nissen N, et al. EBioMedicine. 2019;40:375–381. doi: 10.1016/j.ebiom.2018.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Final report of a phase I/II study of veliparib (Vel) in combination with 5-FU and oxaliplatin (FOLFOX) in patients (pts) with metastatic pancreatic cancer (mPDAC) Pishvaian MJ, Wang H, Parenti S, et al. J Clin Oncol. 2019;37:4015. [Google Scholar]
- 46.Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. O'Reilly EM, Lee JW, Zalupski M, et al. J Clin Oncol. 2020;38:1378–1388. doi: 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randomized phase II study of second-line modified FOLFIRI with PARP inhibitor ABT-888 (Veliparib) (NSC-737664) versus FOLFIRI in metastatic pancreatic cancer (mPC): SWOG S1513. Chiorean EG, Guthrie KA, Philip PA, et al. J Clin Oncol. 2019;37:4014. doi: 10.1158/1078-0432.CCR-21-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.A phase I study of veliparib (Vel) in combination with oxaliplatin (Ox) and capecitabine (Cap) in advanced solid tumors. Turk AA, Deming DA, Lubner SJ, et al. J Clin Oncol. 2018;36:314. [Google Scholar]
- 49.Phase I safety, pharmacokinetic, and pharmacodynamic study of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. LoRusso PM, Li J, Burger A, et al. Clin Cancer Res. 2016;22:3227–3237. doi: 10.1158/1078-0432.CCR-15-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abstract CT234: a Phase II, single arm study of maintenance rucaparib in patients with platinum-sensitive advanced pancreatic cancer and a pathogenic germline or somatic mutation in BRCA1, BRCA2 or PALB2. Binder KA, Mick R, O'Hara M, et al. Cancer Res. 2019;79:234. doi: 10.1200/JCO.21.00003. [DOI] [PubMed] [Google Scholar]
- 51.Rucaparib monotherapy in patients with pancreatic cancer and a known deleterious BRCA mutation. Shroff RT, Hendifar A, McWilliams RR, et al. JCO Precis Oncol. 2018;2018 doi: 10.1200/PO.17.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liposomal irinotecan, fluorouracil, leucovorin calcium, and rucaparib in treating patients with metastatic pancreatic, colorectal, gastroesophageal, or biliary cancer. [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT03337087 Liposomal Irinotecan, Fluorouracil, Leucovorin Calcium, and Rucaparib in Treating Patients With Metastatic Pancreatic, Colorectal, Gastroesophageal, or Biliary Cancer. 2017
- 53.A Study to Evaluate Rucaparib in Patients With Solid Tumors and With Deleterious Mutations in HRR Genes (LODESTAR) [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT04171700 2019
- 54.Development of 2 bromodomain and extraterminal inhibitors with distinct pharmacokinetic and pharmacodynamic profiles for the treatment of advanced malignancies. Falchook G, Rosen S, LoRusso P, et al. Clin Cancer Res. 2020;26:1247–1257. doi: 10.1158/1078-0432.CCR-18-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. de Bono J, Ramanathan RK, Mina L, et al. Cancer Discov. 2017;7:620–629. doi: 10.1158/2159-8290.CD-16-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Systems approach to rational combination therapy: PARP inhibitors. Sun C, Fang Y, Labrie M, Li X, Mills GB. Biochem Soc Trans. 2020;48:1101–1108. doi: 10.1042/BST20191092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niraparib in patients with pancreatic cancer. [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT03601923 2018
- 58.Niraparib in metastatic pancreatic cancer after previous chemotherapy (NIRA-PANC): a phase 2 trial. Kasi A, Chalise P, Williamson SK, et al. J Clin Oncol. 2019;37:4168. [Google Scholar]
- 59.A randomized phase II trial of niraparib plus either nivolumab or ipilimumab in patients with advanced pancreatic cancer whose cancer has not progressed on platinum-based therapy. Reiss KA, Mick R, O'Hara MH, et al. J Clin Oncol. 2019;37:4161. [Google Scholar]
- 60.Basket combination study of inhibitors of DNA damage response, angiogenesis and programmed death ligand 1 in patients with advanced solid tumors (DAPPER) [ Feb; 2022 ];https://clinicaltrials.gov/ct2/show/NCT03851614 2019
- 61.Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Lord CJ, Ashworth A. Nat Med. 2013;19:1381–1388. doi: 10.1038/nm.3369. [DOI] [PubMed] [Google Scholar]