Abstract
Background
Anoxic brain injuries represent the main determinant of poor outcome after cardiac arrest (CA). Large animal models have been described to investigate new treatments during CA and post‐resuscitation phase, but a detailed model that includes extensive neuromonitoring is lacking.
Method
Before an electrically‐induced 10‐minute CA and resuscitation, 46 adult pigs underwent neurosurgery for placement of a multifunctional probe (intracranial pressure or ICP, tissue oxygen tension or PbtO2 and cerebral temperature) and a bolt‐based technique for the placement and securing of a regional blood flow probe and two sEEG electrodes; two modified cerebral microdialysis (CMD) probes were also inserted in the frontal lobes and accidental misplacement was prevented using a perforated head support.
Result
42 animals underwent the CA procedure and 41 achieved the return of spontaneous circulation (ROSC). In 4 cases (8.6%) an adverse event took place during preparation, but only in two cases (4.3%) this was related to the neurosurgery. In 6 animals (13.3%) the minor complications that occurred resolved after probe repositioning.
Conclusion
Herein we provide a detailed comprehensive neuromonitoring approach in a large animal model of CA that might help future research.
Keywords: anoxic injury, heart arrest, ischemiareperfusion, post‐arrest, resuscitation
The figures displays multiple neuromonitoring devices in place to allow simultaneous measurements of multiple brain‐derived parameters during cardiac arrest and post‐resuscitation period.
1. INTRODUCTION
CA remains a leading cause of death and morbidity worldwide 1 and brain injurie remains responsible for most deaths among survivors. 2 Many treatments have been proposed, 3 , 4 but the discrepancies between experimental results and their translation into clinical practice reinforce the necessity for better preclinical models. 5
Pigs are appropriate as CA models because of anatomic similarity between cardiovascular systems, the possibility of applying standardized resuscitation maneuvers, and their tolerability for invasive procedures and frequent blood sampling. 6 , 7 , 8 Similarly, they possess a gyrencephalic brain with regional susceptibility to ischemia‐reperfusion comparable to humans. Also, their brain is large enough to allow the placement of multiple human monitoring devices.
Various CA models are reported in the literature 9 , 10 , 11 but no multimodal neuromonitoring approach has been described in detail so far. Thus this manuscript aims to provide a model of prolonged CA in swine that includes multiple neuromonitoring devices.
2. METHODS
The Institutional Review Board for Animal Care of the Free University of Brussels (Belgium) approved all experimental procedures (Ethical Committee approval: 704N), in compliance with ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. Care and handling of the animals were in accord with National Institutes of Health guidelines (Institute of Laboratory Animal Resources).
2.1. General preparatory phase
On the day of the experiment the animal (Sus Scrofa Domesticus, 6 months old, both sexes) were fasted for 12 hours and were sedated with an intramuscular injection of midazolam (1 mg/kg) and ketamine (10 mg/kg) in the neck. Electrocardiogram (ECG) monitoring was initiated and after cannulation of a marginal ear vein, a continuous infusion of sufentanyl‐citrate (0.2–1 µg/kg/h) was started.
A 4.5 Fr catheter was inserted in the femoral artery and connected to a pressure transducer (Edwards). After the injection of 1 mg atropine‐sulfate, 3 µg/kg of sufentanyl‐citrate and 1.2 mg/kg of rocuronium, an 8 mm inside diameter endotracheal tube (Medtronic) was placed and mechanical ventilation initiated in volume‐controlled mode (Drägerwerk) with a tidal volume of 8 ml/kg, 5 cmH2O of PEEP, and FiO2 of 1, and inspired sevofluorane was started. Ventilation parameters were adjusted to achieve an end‐tidal CO2 of 35–45 mmHg and a PaO2 >70 mmHg with the minimum FiO2. A rocuronium (1–4 mg/kg/h) and sufentanyl‐citrate (3.5 µg/kg/h) infusion was maintained, and balanced crystalloids (Plasmalyte) started.
After 2g of amoxicillin‐clavulanate, a 14 Fr Foley catheter was surgically placed in the bladder thorough a midline incision; a triple lumen central venous catheter (Arrow) was placed in the external jugular vein and the drug infusion was transferred to it. A 6 Fr introducer (TERUMO) was placed proximally to the triple‐lumen catheter to allow introduction of a pacing wire. Lastly, an 8.5 Fr introducer was placed contralaterally and a continuous cardiac output Swan‐Ganz (Edwards) catheter was advanced into the pulmonary artery. The animal was proned and the bed angled to a 30° anti‐Trendelenburg position.
2.2. Neurosurgical procedure
The forehead of the animal was shaved and disinfected with povidone‐iodine. At approximately 0.5 cm from the midline, an inverted mirrored F incision was executed on both sides, and two cutaneous flaps were made to expose the frontal bones (Figure 1). Using an electric drill (Ruijin Medical), six burr holes were placed in the skull. In the rostral butt holes, a 10‐mm length microdialysis catheter (CMA‐microdialysis) was inserted after cutting the fixation flaps to allow penetration of the hole engulfed in bone wax (Figure 2), and then tunneled into the flap, and a continuous perfusion (CNS fluid, CMA‐microdialysis) at the rate of 0.3 µl/min was started. In the remaining holes, four bolts were placed (Bolt CH5, Raumedic‐AG). A bolt consists of a screw with seal and a fixing cap and once secured to the skull, it allows the insertion of a custom‐built needle to open the dura mater with minimal traumatism. After positioning the probe, the screwing of the fixing cap narrows the sealing component, securing the probe at the desired depth. On one side, a probe allowing the simultaneous measurement of cerebral temperature, ICP and PbtO2 (Neurovent PTO2, Raumedic‐AG) was inserted and connected to its monitor (Raumedic‐AG); on the other side, a laser‐Doppler flowmetry probe was placed (Oxyflow4000, Oxford Optronic). In each of the caudal bolts, a 5‐contact intracerebral stereoelectroencephalography (sEEG) wire (Dixi medical) was inserted (Figure 3). The animal was turned supine, and the table angled to 0 degrees. To prevent damage to the probes, the head was held beyond the edge of the table, and the spine was kept aligned via a perforated massage table headrest (Figure 4), which prevented compression of the probes while providing support for the head.
FIGURE 1.
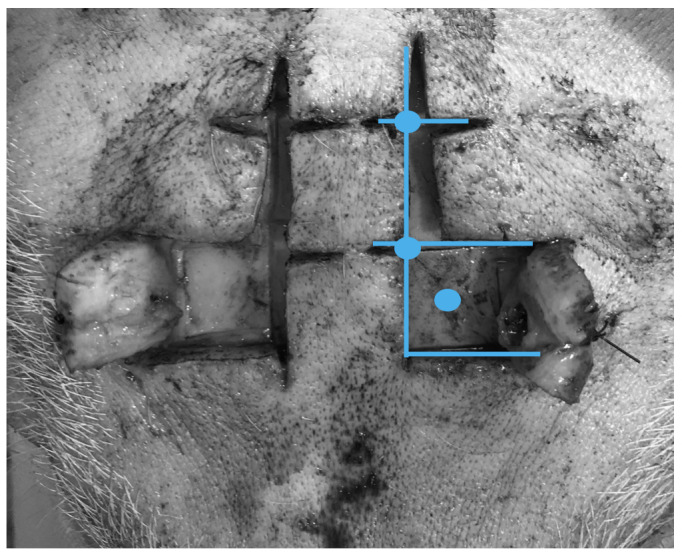
Inverted F incision in the scalp with flaps. Blue lines represent incision lines; blue circles represent areas for skull drilling
FIGURE 2.
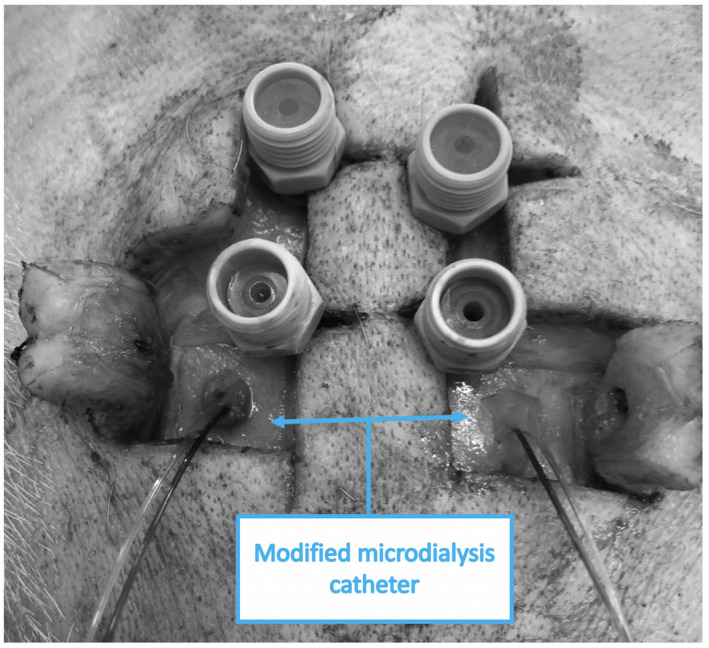
Bolts and modified microdialysis catheters after intracranial placement, before connection to the CNS perfusion pump
FIGURE 3.
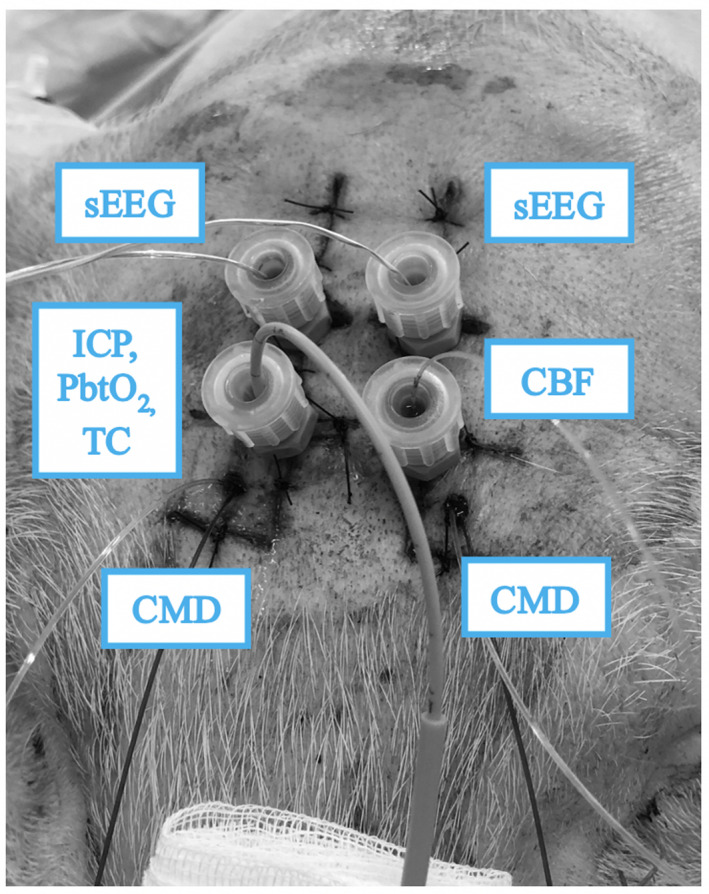
All neuromonitoring probes in place before the start of the experiment. Microdialysis catheters are tunneled in the scalp and four probes are secured to the bolts. sEEG, stereoelectroencephalography; CMD, cerebral microdialysis catheter; CBF, cerebral blood flow probe; ICP, intracranial pressure; Tc, brain temperature; PbtO2, brain oxygen tension
FIGURE 4.
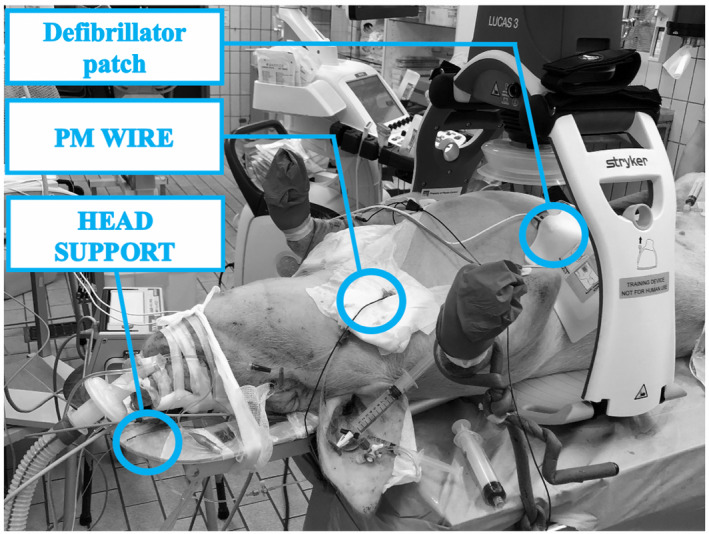
Animal in position before CA induction. PM, pace maker
2.3. Cardiac arrest induction
After placing the automatic chest compressor (LucasIII, Jolife AB/Stryker), two latero‐lateral defibrillating pads (Stad‐padzII, Zoll Medical) were connected to a defibrillator (Zoll M series, Zoll Medical). A pacing wire was advanced through the 6 Fr introducer until it reached the endocardium and then connected to a 9V battery to induce ventricular fibrillation (VF), which was confirmed by the ECG waveform and the decrease in arterial pressure. The ventilation was stopped, and the animal left untreated for 10 minutes.
2.4. Resuscitation maneuvers
Chest compressions were started at 100/minute for 5 minutes and ventilation was resumed with a FiO2 of 1. After one minute, an intravenous injection of epinephrine (30 µg/kg) was administered, and the anesthetic gas resumed. At the end of the 5 minutes, a 4J/kg biphasic countershock was delivered. In the case of unsuccessful resuscitation, CPR was continued for an additional minute, and another shock was delivered. CPR was stopped if ROSC wasn't achieved within 10 shocks.
2.5. Post‐resuscitation care
After ROSC, the animals were turned to the prone position and treated with target temperature management using an external feedback device (Arctic‐Sun 5000, Bard). At the end of the observation period, the animals were sacrificed under deep anesthesia with an injection of potassium‐chloride. Death was confirmed by VF ECG pattern and the drop of arterial blood pressure.
3. RESULTS
Forty‐six animals were eligible for CA. In 4 animals (8.6%), the protocol couldn't be completed due to traumatic neurosurgery (2 animals), a pulmonary catheter‐triggered ventricular arrythmia (1 animal) and in another case the animal was excluded because of refractory supraventricular arrythmia while inducing VF.
Of the remaining 42 animals that underwent both neurosurgery and CA, 41 achieved ROSC (97.6%) and were observed for 12 hours. Among those, the weight was 49 kg (IQRs 45–53), and 24 were males (58.5%). CPR lasted for 300 seconds (IQR 300–360) and 1 shock (IQR 1–2) was necessary to achieve ROSC. Minor complications after ROSC occurred in 6 (14.6%) animals: in three cases (7.3%) they were related to the position of the brain probes resulting in a poor signal whereas in the others the measurement was considered not reliable. Apart from those, in the 12 hours post‐ROSC all monitoring devices resulted in valid measurements.
4. DISCUSSION
In the post‐resuscitation phase, there is growing evidence that multimodal neuromonitoring might have a therapeutic benefit, since episodes of cerebral hypoxia appear to be frequent and ICP values are heterogeneous. 12 , 13 , 14
Three elements characterize our approach: (1) the use of a bolt‐based mini‐invasive technique extended to several cerebral probes, (2) the systematic use of a perforated head support during CPR to preserve the neuromonitoring tools from CPR‐related damage, and (3) the modification of the microdialysis catheter by cutting the fixation flaps to allow its insertion in the burr‐hole engulfed in bone wax.
Other groups have previously reported the use of invasive neuromonitoring in large animal models of CA, but either did not report multiple measurements such as CMD, EEG, brain temperature 15 or the probes were placed via a craniotomy 16 , 17 , 18 or were limited to one multifunctional probe. 17 A multimodal approach has been described with measurement of ICP, PbtO2 and CMD, but all the probes were placed in one burr hole with the help of a neurosurgeon. 19 , 20 Moreover, it is not clear if and how the probes were secured during CPR and complication rates were not reported. Our model has some limitations: we did not revive animals, which would have allowed for neurobehavioral tests; we included young healthy animals with no comorbidities; and the prone position used is not suited for studies requiring repeated echocardiographic evaluations.
In conclusion, we have described here few improvements to a large animal CA model that allow for multimodal neuromonitoring and could serve in future research.
CONFLICT OF INTEREST
The Authors have no conflict of interests to declare that compromise the quality of this article.
AUTHOR CONTRIBUTIONS
FA, GB, FST, JC and FS conceived the experiment; FA, FS, LAH, LP, BG, AH and AK conducted the experiments; FA wrote the first draft of the manuscript; all authors substantially contributed to the final draft of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank for their support Raumedic, via its Belgian subsidiary Rembrant Medical, for the free loan of the MPR2 monitor and assistance; Jolife AB/Stryker Lund, Sweden for the free loan of Lucas III device; Bard Medical for the free loan of the Arctic Sun device.
Annoni F, Peluso L, Hirai LA, et al. A comprehensive neuromonitoring approach in a large animal model of cardiac arrest. Anim Models Exp Med. 2022;5:56–60. doi: 10.1002/ame2.12200
Funding information
Dr Annoni F. has been supported by the "Fonds Erasme pour la Recherche Médicale" for the entire length of the project.
REFERENCES
- 1. Gräsner J‐T, Herlitz J, Tjelmeland IBM, et al. European resuscitation council guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61‐79. [DOI] [PubMed] [Google Scholar]
- 2. Lemiale V, Dumas F, Mongardon N, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972‐1980. [DOI] [PubMed] [Google Scholar]
- 3. Taccone FS, Crippa IA, Dell’Anna AM, Scolletta S. Neuroprotective strategies and neuroprognostication after cardiac arrest. Best Pract Res Clin Anaesthesiol. 2015;29:451‐464. [DOI] [PubMed] [Google Scholar]
- 4. Lascarrou JB, Merdji H, Le Gouge A, et al. Target temperature management for cardiac arrest with no shockable rhythm. N Engl J Med. 2019;381(24):2327‐2337. [DOI] [PubMed] [Google Scholar]
- 5. Olai H, Thornéus G, Watson H, et al. Meta‐analysis of targeted temperature management in animal models of cardiac arrest. Intensive Care Med Exp. 2020;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherry BH, Nguyen AQ, Hollrah RA, Olivencia‐Yurvati AH, Mallet RT. Modeling cardiac arrest and resuscitation in the domestic pig. World J Crit Care Med. 2015;4(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xanthos T, Lelovas P, Vlachos I, et al. Cardiopulmonary arrest and resuscitation in Landrace/Large White swine: a research model. Lab Anim. 2007;41:353‐362. [DOI] [PubMed] [Google Scholar]
- 8. Walters EM, Agca Y, Ganjam V, Evans T. Animal models got you puzzled?: think pig. Ann N Y Acad Sci. 2011;1245:63‐64. [DOI] [PubMed] [Google Scholar]
- 9. Wang J, Weil MH, Tang W, Chang YT, Huang L. A comparison of electrically induced cardiac arrest with cardiac arrest produced by coronary occlusion. Resuscitation. 2007;72:477‐483. [DOI] [PubMed] [Google Scholar]
- 10. Wu CJ, Li CS, Zhang Y, Yang J, Yin Q, Hang CC. Differences of postresuscitation myocardial dysfunction in ventricular fibrillation versus asphyxiation. Am J Emerg Med. 2013;31:1690‐1696. [DOI] [PubMed] [Google Scholar]
- 11. Babini G, Grassi L, Russo I, et al. Duration of untreated cardiac arrest and clinical relevance of animal experiments: the relationship between the "no‐flow" duration and the severity of post‐cardiac arrest syndrome in a porcine model. SHOCK. 2018;49:205‐212. [DOI] [PubMed] [Google Scholar]
- 12. Sekhon MS, Griesdale DE. Individualized perfusion targets in hypoxic ischemic brain injury after cardiac arrest. Crit Care. 2017;21:259‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sekhon MS, Gooderham P, Menon DK, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest. Crit Care Med. 2019;47(7):960‐969. [DOI] [PubMed] [Google Scholar]
- 14. Sekhon MS, Griesdale DE, Ainslie PN, et al. Intracranial pressure and compliance in hypoxic ischemic brain injury patients after cardiac arrest. Resuscitation. 2019;141:96‐103. [DOI] [PubMed] [Google Scholar]
- 15. Cavus E, Bein B, Dörges V, et al. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2006;71:97‐106. [DOI] [PubMed] [Google Scholar]
- 16. Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35(9):2145‐2149. [DOI] [PubMed] [Google Scholar]
- 17. Nelskylä A, Nurmi J, Jousi M, et al. The effect of 50% compared to 100% inspired oxygen fraction on brain oxygenation and post cardiac arrest mitochondrial function in experimental cardiac arrest. Resuscitation. 2017;116:1‐7. [DOI] [PubMed] [Google Scholar]
- 18. Bahlmann L, Klaus S, Baumeier W, et al. Brain metabolism during cardiopulmonary resuscitation assessed with microdialysis. Resuscitation. 2003;59:255‐260. [DOI] [PubMed] [Google Scholar]
- 19. Putzer G, Braun P, Martini J, et al. Effects of head‐up vs. supine CPR on cerebral oxygenation and cerebral metabolism – a prospective, randomized porcine study. Resuscitation. 2018;128:51‐55. [DOI] [PubMed] [Google Scholar]
- 20. Putzer G, Martini J, Spraider P, et al. Effects of different adrenaline doses on cerebral oxygenation and cerebral metabolism during cardiopulmonary resuscitation in pigs. Resuscitation. 2020;156:223‐229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


