Abstract
Influenza is a contagious respiratory disease that causes severe illness and death, particularly in elderly populations. Two enhanced formulations of quadrivalent influenza vaccine (QIV) are available in Spain. Adjuvanted QIV (aQIV) is available for those aged 65+ and high-dose QIV (HD-QIV) for those aged 60+. In this study, we used a health economic model to assess the costs and outcomes associated with using aQIV or HD-QIV in subjects aged 65+. Using aQIV instead of HD-QIV to vaccinate an estimated 5,126,343 elderly people results in reductions of 5405 symptomatic cases, 760 primary care visits, 171 emergency room visits, 442 hospitalizations, and 26 deaths in Spain each year. Life-years (LYs) and quality-adjusted LYs (QALYs) increases by 260 and 206, respectively, each year. Savings from a direct medical payer perspective are EUR 63.6 million, driven by the lower aQIV vaccine price and a minor advantage in effectiveness. From a societal perspective, savings increase to EUR 64.2 million. Results are supported by scenario and sensitivity analyses. When vaccine prices are assumed equal, aQIV remains dominant compared to HD-QIV. Potential savings are estimated at over EUR 61 million in vaccine costs alone. Therefore, aQIV provides a highly cost-effective alternative to HD-QIV for people aged 65+ in Spain.
Keywords: influenza, vaccination, Spain, cost-effectiveness, adjuvanted, high dose, burden of illness
1. Introduction
Seasonal influenza is an acute respiratory infection caused by influenza viruses, which circulate in all parts of the world. It is characterized by a sudden onset of fever, cough, headache, muscle and joint pain, severe malaise, sore throat, and a runny nose. Whilst most people quickly recover without requiring medical attention, worldwide influenza is estimated to cause three to five million cases of severe illness and 290,000 to 650,000 respiratory deaths each year [1]. An analysis of the EuroMOMO network estimated 152,000 deaths (150,000 to 155,000) in the 2017/2018 influenza season in Europe [2].
Influenza spreads rapidly during the winter months resulting in epidemics that lead to high demand for healthcare resources and substantial economic burden. The average incidence in Spain is estimated at 2000 cases per 100,000 inhabitants, with associated costs due to primary care, hospital care, treatments, and absences from work of EUR 1 billion per year [3]. A substantial proportion of this burden is associated with patients aged 65 years and older (65+) and, as a consequence, many countries, including Spain, recommend routine annual vaccination against influenza in people aged 65+ [4].
Four types of influenza viruses (A to D) are currently in circulation, with influenza A and B as the main ones responsible for seasonal epidemics in humans. Influenza A is classified into subtypes based on combinations of hemagglutinin (HA) and neuraminidase (NA). Currently circulating influenza A virus subtypes are A/H1N1 (also known as A/H1N1 pdm09) and A/H3N2. The commonly circulating strains for influenza B are B/Yamagata or B/Victoria [5]. Quadrivalent influenza vaccines (QIVs) are designed to provide protection against all four of these subtypes [1].
There are a number of QIVs available in Spain and two enhanced QIVs that have been recently licensed [6,7]. Adjuvanted QIV (aQIV), available for people aged 65+, combines MF59 adjuvant (an oil-in-water emulsion of squalene oil) and a standard dose of antigen, and is designed to produce stronger, broader, and longer immune responses against the selected influenza vaccine strains [8]. HD-QIV contains a higher concentration of antigen than the standard-dose influenza vaccines and is designed to produce stronger immune responses against the selected influenza vaccine strains [9]. Both have been developed to provide improved protection among older age groups in whom immune responses with regular standard-dose QIVs can be suboptimal. The objective of this study was to determine the cost-effectiveness and burden of disease associated with vaccinating the population aged 65+ with either aQIV or HD-QIV in Spain.
2. Materials and Methods
2.1. Model Structure
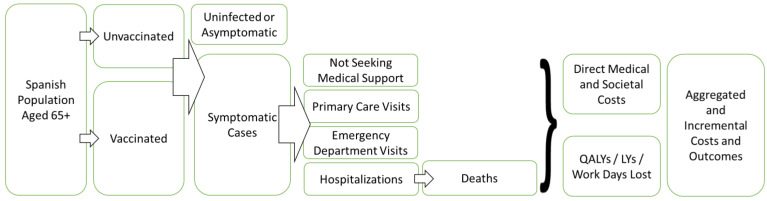
A health economic model simulating the costs, benefits, and burden of disease for the Spanish population aged 65+ vaccinated with either aQIV or HD-QIV over a single influenza season was developed. The model was based on the static, decision-tree model developed by Ruiz-Aragón et al. for the Spanish setting [10]. This structure has been used extensively in other influenza cost-effectiveness analyses [11,12,13,14,15], and the analysis was designed in line with Spanish best practices for health economic modeling [16]. A schematic of the economic is shown in Figure 1.
Figure 1.
Schematic of the health-economic model. Abbreviations: LYs = life years; QALYs = quality-adjusted life years.
In the model, the Spanish population aged 65+ can be either vaccinated or unvaccinated. Vaccinated people in one comparator arm receive aQIV and in the other arm they receive HD-QIV. People from both the vaccinated and unvaccinated populations then, over the course of the one-year time-horizon (which represents one influenza season), enter one of the following disease states: uninfected or asymptomatic; symptomatic cases not seeking medical support; or symptomatic cases requiring either a primary care visit, emergency department visit, or hospitalization. Patients hospitalized then have a probability of death. Each state has a fixed cost and disutility associated with it. Costs and outcomes are finally aggregated across the different states to calculate the totals for each cohort. Two cost perspectives are included: direct medical payer and societal. All costs and outcomes are calculated for an entire influenza season, except for productivity loss due to death and quality-adjusted life year (QALY) loss due to death. These are calculated over a lifetime horizon and discounted at 3% per year, following Spanish cost-effectiveness guidelines [16]. This discount rate is applied to both costs and QALYs.
2.2. Epidemiology
Vaccine coverage, population size, and life expectancy for the 65+ population was taken from national 2021 Spanish statistics [17,18,19]. Vaccine coverage was 54.7% [17], life expectancy was 9.8 years [18], and the population size was 9,371,743 [19].
2.3. Rates of Clinical Outcomes
The rates per 100,000 for the different clinical outcomes are shown in Table 1. These are based on the influenza seasons from 2017 to 2018, 2018 to 2019, and 2019 to 2020. Incidence of clinically reported influenza cases in the Spanish population was taken from surveillance reports from the sentinel general practitioners of the Sistema Centinela de Vigilancia de Gripe in Spain (ScVGE) [20,21,22]. Patients were split into those that visit a primary care physician (81.67%) and those that visit an emergency department (18.33%) [23]. The distribution of hospitalizations were also taken from Spanish public reports [20,21,22]. The death rate was based on the calculated mortality rates of 6% per hospitalization from Crepey et al. [24]. The average across these three influenza seasons were used to estimate baseline incidence rates in the model base case.
Table 1.
Rates of different clinical events per 100,000 population aged 65+.
| Season | Symptomatic Cases | Primary Care Visits | Emergency Department Visits | Hospitalizations | Deaths |
|---|---|---|---|---|---|
| 2017–2018 | 22,530 | 950 | 213 | 668 | 40 |
| 2018–2019 | 13,697 | 545 | 122 | 489 | 29 |
| 2019–2020 | 10,636 | 445 | 100 | 324 | 19 |
The outcomes in the model from the Spanish sentinel surveys are based on a mix of vaccinated and unvaccinated people. We assume that this population is vaccinated with standard-dose QIV (SD-QIV). We assume the relative vaccine efficacy for HD-QIV vs. SD-QIV is 24% [25,26] which we use in the base-case for the model.
2.4. Vaccine Effectiveness
Studies reporting the relative vaccine effectiveness of adjuvanted trivalent and quadrivalent influenza vaccines (aTIV or aQIV) compared to high-dose trivalent and quadrivalent influenza vaccines (HD-TIV and HD-QIV) for the prevention of influenza-related hospitalizations (or composite outcomes including influenza-related hospital admissions) were identified from a systematic review that covered publications from 1997 (first licensure of aTIV) to 15 July, 2020 [27]. Additionally, a targeted non-systematic review was conducted by a single reviewer by searching in PubMed in July 2021 to identify potential additional relevant studies published between July 2020 and July 2021. The PRISMA checklist for the additional searches is provided in the Supplementary Table S1 Information. Data were extracted into a structured data template that captured the study design, season of study, intervention, comparator, outcome definitions, and effect estimates/confidence intervals. The quadrivalent formulations for the adjuvanted and high-dose seasonal influenza vaccines were first available during the 2020–2021 influenza season and therefore only publications evaluating the relative vaccine effectiveness of their trivalent predecessors were identified in the review. It is assumed that the relative vaccine effectiveness of the two quadrivalent vaccines would be equivalent to the relative vaccine effectiveness of the two trivalent vaccines.
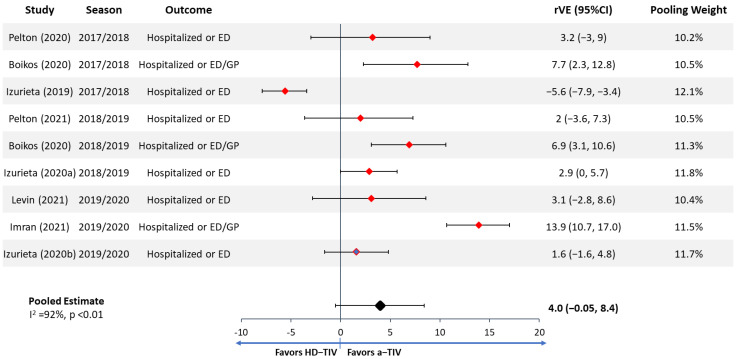
A total of eight publications were included in the meta-analysis, one of which reported separate effect estimates for two seasons and was therefore included twice in the meta-analysis. Four studies [28,29,30,31] were identified from the published systematic review. The remaining four studies/effect estimates [32,33,34,35] were identified via the targeted review, two of which were not yet published as of the time of the analysis. As the relative vaccine effectiveness of aTIV vs. HD-TIV may be expected to vary based on the characteristics of the study (e.g., influenza season, population included, outcome definition, etc.), a random-effects model was used for the meta-analysis [36]. The meta-analysis was conducted using the R [37] package meta [38] in R 4.0.2. The forest plots of the data and relative effectiveness estimates are shown in Figure 2.
Figure 2.
Meta-analysis of effect estimates from identified studies reporting the relative vaccine effectiveness of aTIV vs. HD-TIV for prevention of influenza-related hospitalizations (or composite outcomes including influenza-related hospital admissions). Study pooling weights were calculated based on DerSimonian and Laird random-effects meta-analysis [41]. Abbreviations: CI = confidence interval; ED = emergency department; GP = general practitioner; rVE = relative vaccine effectiveness.
Identified publications were all retrospective cohort studies and reported outcomes based on incidence rate ratios (IRR) and odds ratios (OR), which were included in the same meta-analysis based on the assumption that ORs would approximate IRRs due to influenza hospitalization being rare outcome. Vaccine effectiveness was back calculated to IRR/OR for synthesis and then converted back to vaccine effectiveness. Identified publications reported relevant data for the three US influenza seasons from 2017 to 2020. During those three seasons, the trivalent formulation of influenza vaccines contained B-Victoria [39] and not B-Yamagata. During the 2017-2018 influenza season in the United States [40], approximately 24% of circulating viruses among patients aged ≥65 were B-Yamagata while the same was true for only about 1% of the circulating viruses during the 2018–2019 and 2019–2020 seasons.
The pooled estimated of the relative vaccine effectiveness of aTIV compared to HD-TIV was 4.0% (95% CI: −0.05 to 8.4), indicating that the point estimate favored aTIV over HD-TIV for prevention of influenza-related hospitalizations (or composite outcomes including influenza-related hospital admissions), but the difference was not statistically significant. There was high heterogeneity (I2 = 92%, p <0.01) due to variability in effect estimates between studies. Between study heterogeneity may be due to differences in study design/outcome selection, characteristics of the underlying study populations, and characteristics of the influenza season studied.
2.5. Utilities
Health-related quality of life (HRQoL) for clinical events was taken from Hollmann et al. [42]. These values were from a longitudinal study of Spanish patients from major hospitals. Patients reported their HRQoL using the EQ-5D-3L instrument for the influenza period and the week before. The disutility was calculated as the difference between EQ-5D-3L prior to the influenza episode and during it. Estimated duration of disutility for inpatients and outpatients was 21 and seven days, respectively. Disutility for inpatients was 0.60 and for outpatients was 0.33. Disutility for symptomatic cases was from Dolk et al. and estimated at 0.32 for seven days [43]. Baseline utility for the cohort of people aged 65+ was 0.65 [23].
2.6. Costs
The model was run using tender prices for vaccines, which were EUR 13 for aQIV and EUR 25 for HD-QIV [44]. These were used in the base case, with a scenario analysis using the list prices, which are EUR 23 for aQIV and EUR 32 for HD-QIV [45]. The resource unit costs were collected from official Spanish sources, including three bulletins of the Autonomous Communities: Andalucía, Murcia, and País Vasco. The middle value of them was selected for the model, with the cost of a primary-care physician visits at EUR 59 and emergency department visits at EUR 183 [46,47,48]. The hospitalization weighted average cost was calculated for relevant complications from 2019 APR-DRG statistical data published by the ministry of health and inflated to 2021 euros, and also included an intensive care unit stay for 9 days for 7.5% of admissions, at EUR 4467 [47,49]. All costs are for 2021. Patients with symptomatic disease who did not attend a primary care physician visit, emergency department visits or have an in-patient hospitalization were conservatively assumed to have no public payer or societal costs. A comedication cost of EUR 3.21 for the primary care visit was taken from the publication of Perez-Rubio and Eiros [50]. Administration and transportation costs were not included as they are expected to be the same across vaccines.
The societal perspective includes productivity losses due to direct illness, calculated using the discounted human capital approach and based on working days lost multiplied by the probability of being employed [51]. This was 1.2% for those aged 65 to 69 years and 0.3% for those aged 70+ [52]. Productivity loss per hour was EUR 17.44 [53]. The time spent caring for influenza patients at 5.4% for those aged 65 to 69 years and 14% for those aged 70+ was also included [54]. Productivity losses were assumed to be five working days for outpatients and 15 working days for inpatients [10].
2.7. Analysis
The outputs from the model include burden of illness, economic cost, and incremental analysis. The burden of illness outcomes included the number of symptomatic cases, primary care visits, emergency department visits, hospitalizations, and deaths when aQIV or HD-QIV is used to vaccinate the population aged 65+. Public payer costs and discounted societal costs were calculated as well as total discounted QALYs. The public payer costs were not discounted as they were only calculated over one year, whereas societal costs and QALY losses due to death were calculated based on life expectancy and discounted accordingly. Incremental cost-effectiveness ratios (ICERs) were calculated for aQIV vs. HD-QIV from a direct medical payer and societal perspective.
A series of scenario analyses were conducted to test the impact of the model assumptions on the ICERs. The impact of input uncertainty was evaluated through one-way deterministic sensitivity analysis (DSA). In addition, a probabilistic sensitivity analysis (PSA) was conducted by varying parameters based on their confidence intervals during 10,000 iterations of the model to assess the effect of uncertainty on the ICERs. The ICERs were compared against a willingness-to-pay threshold of EUR 25,000 per QALY gained. This is the willingness-to-pay threshold recently identified as the range used by the National Health Service in Spain [55,56]. A summary of the values and references for the parameters sourced for the model are shown in Table 2.
Table 2.
Summary of parameters sourced for the model.
| Parameter | Value | Source |
|---|---|---|
| Percentage of 65+ population vaccinated | 54.7% | [17] |
| Life expectancy for 65+ population | 9.8 years | [18] |
| 65+ population size | 9,371,743 | [19] |
| aQIV tender price | EUR 13 | [44] |
| aQIV list price | EUR 23 | [45] |
| HD-QIV tender price | EUR 25 | [44] |
| HD-QIV list price | EUR 32 | [45] |
| Primary-care physician visits cost | EUR 59 | [46,47,48] |
| Emergency department visit cost | EUR 183 | [46,47,48] |
| Hospitalization cost | EUR 4467 | [47,49] |
| Comedication cost | EUR 3.21 | [50] |
| Probability of being employed (65–69 years old) | 1.2% | [52] |
| Probability of being employed (75+ years old) | 0.3% | [52] |
| Productivity loss per hour | EUR 17.44 | [53] |
| Probability of requiring care at home (65–69 years old) | 5.4% | [54] |
| Probability of requiring care at home (75+ years old) | 14% | [54] |
| Baseline utility for 65+ | 0.65 | [23] |
| Disutility value for symptomatic patients | 0.32 | [43] |
| Disutility value for outpatients | 0.33 | [42] |
| Disutility value for inpatients | 0.6 | [42] |
| Disutility duration for symptomatic patients | 7 days | [43] |
| Disutility duration for outpatients | 7 days | [42] |
| Disutility duration for inpatients | 21 days | [42] |
| Hospital mortality rate for 65+ population | 6% | [24] |
| Discount rates for costs and outcomes | 3% | [16] |
3. Results
The total number of people vaccinated in the model with aQIV or HD-QIV in the simulation was 5,126,343, based on the coverage and population shown above. The rest of the cohort remained unvaccinated. The results show that using aQIV instead of HD-QIV results in a reduction of 5405 symptomatic cases. This includes 760 primary care visits, 171 emergency room visits, 442 hospitalizations, and 26 deaths.
Incremental costs and outcomes are shown in Table 3. The incremental costs for the clinical events are lower for aQIV compared with HD-QIV. From a direct medical payer perspective, using aQIV results in a net saving of EUR 63.6 million and from a societal perspective EUR 64.2 million.
Table 3.
Total and incremental costs and outcomes associated with aQIV and HD-QIV when used to vaccine people aged 65+ in Spain.
| Category | Clinical Events | Costs (EUR Millions) | ||||
|---|---|---|---|---|---|---|
| Vaccine | HD-QIV | aQIV | Difference | HD-QIV | aQIV | Difference |
| Primary care visits | 54,946 | 54,186 | −760 | 3.42 | 3.37 | −0.05 |
| Emergency department visits | 12,332 | 12,161 | −171 | 2.26 | 2.23 | −0.03 |
| Hospitalization | 47,371 | 46,930 | −442 | 212.7 | 210.7 | −1.98 |
| Deaths | 2842 | 2816 | −26 | 0.0 | 0.0 | 0.0 |
| Vaccine cost * | 261.1 | 199.6 | −61.5 | |||
| Productivity loss | 60.7 | 60.1 | −0.6 | |||
| QALY loss | 21,040 | 20,833 | −206 | |||
| LYs lost | 27,940 | 27,679 | −260 | |||
| Total costs (public payer) | 479.5 | 415.9 | −63.6 | |||
| Total costs (societal) | 540.2 | 476.0 | −64.2 | |||
* Excludes administration costs which are equal across both vaccines. Abbreviations: aQIV = advanced QIV; HD-QIV = high-dose QIV; LY = life year; QALY = quality-adjusted life year.
HD-QIV is dominated by aQIV as it is both more expensive and less effective, from both the societal and direct medical payer perspective. Whilst there are small savings associated with the reduction in clinical event and productivity loses the overwhelming driver is the difference in vaccine costs, which result in a net saving of EUR 61.5 million.
A series of scenario analyses were run to test the impact of the model assumptions on the ICER. These are shown in Table 4.
Table 4.
Scenario analysis for aQIV compared to HD-QIV.
| Parameter | Parameter Change | Original Value | QALY Gain | Incremental Costs | ICER | Reference |
|---|---|---|---|---|---|---|
| Base Case | 206 | EUR –63.6 million | aQIV dominates HD-QIV | |||
| Lower95% rVE | −0.05% | 4.00% | −2.6 | EUR –61.5 million | EUR 23,875,227 * | Figure 2 |
| Upper95% rVE | 8.40% | 4.00% | 433 | EUR –65.8 million | aQIV dominates HD-QIV | Figure 2 |
| Colean et al. rVE | 3.20% | 4.00% | 165 | EUR –63.2 million | aQIV dominates HD-QIV | Coleman et al. [27] |
| List prices | EUR 32 for HD-QIV and EUR 25 for aQIV | EUR 25 for HD-QIV and EUR 13 for aQIV | 206 | EUR –37.9 million | aQIV dominates HD-QIV | Vademecum [45] |
* ICER for HD-QIV vs. aQIV. HD-QIV is not cost-effective vs. aQIV. Abbreviations: aQIV = advanced QIV; HD-QIV = high-dose QIV; ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life year; rVE = relative vaccine efficacy.
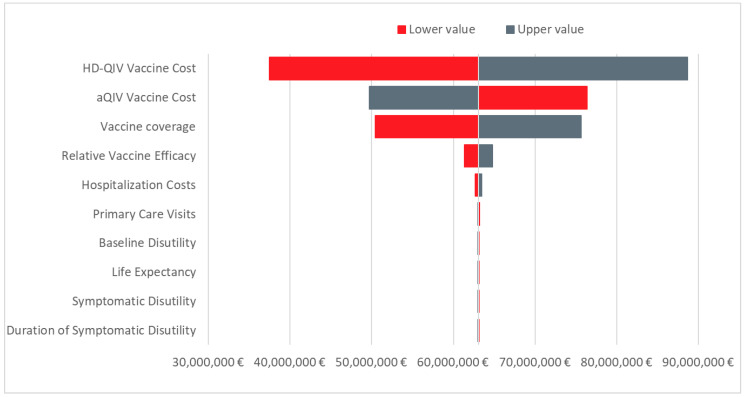
A DSA was conducted with the tornado plot summarizing the 10 most influential parameters for the ICER presented in Figure 3. Vaccine costs are the most influential parameters in the model, followed by vaccine coverage. Other inputs have a relatively low impact on the cost-effectiveness.
Figure 3.
Tornado diagram showing the incremental net monetary benefit for aQIV vs. HD-QIV at a willingness-to-pay threshold of EUR 25,000 per QALY. Abbreviations: aQIV = adjuvanted QIV; HD-QIV = high-dose QIV.
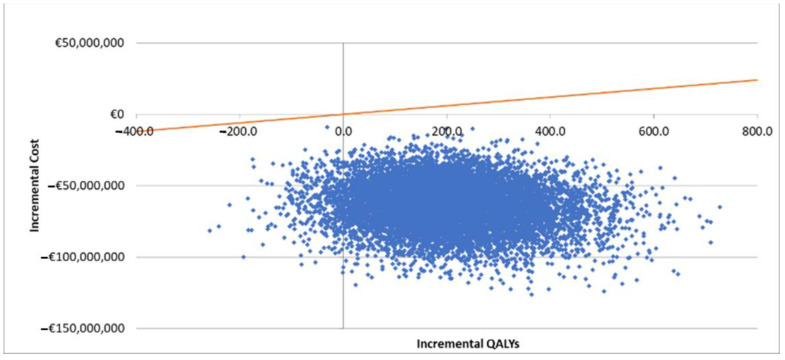
A PSA was also conducted with the scatter plots on the cost-effectiveness plane shown in Figure 4. All of the iterations fall below the EUR 25,000 per QALY willingness-to-pay threshold, which means aQIV was cost-effective in 100% of iterations. In addition, as 96% of all iterations fell within the southeast quadrant, aQIV was dominant 96% of the time.
Figure 4.
Cost-effectiveness plane for aQIV vs. HD-QIV. Abbreviation: QALY = quality-adjusted life year. Orange line represents the willingness-to-pay threshold in Spain of EUR 25,000 per QALY [55,56].
4. Conclusions
Cost-effectiveness analysis is frequently used to assess the value of new vaccines, with a number of influenza models being published recently for Spain [10,23,24,57]. They enable healthcare providers to make informed decision around optimum vaccination strategies based on best available evidence. In the Spanish setting, aQIV has yet to be compared the HD-QIV in the population aged 65+. This analysis demonstrates that, largely driven by the economic benefits associated with vaccinating a large population with a less expensive vaccine with comparable effectiveness, aQIV is cost-saving compared to HD-QIV from both a direct medical payer and societal perspective. The results from Spain reflect those for the UK, Germany, and Italy, which also compared aQIV to HD-QIV [58]. Here the outcomes were considered similar with the key driver behind cost-effectiveness being cost of vaccines and the comparable effectiveness between these two enhanced vaccines.
The improvement in outcomes is driven by a non-significant improvement in vaccine effectiveness of aQIV vs. HD-QIV. This is based on a meta-analysis that involved TIV which is a limitation of the analysis. As time progresses and more data becomes available, this type of analysis can be revisited and reviewed. Moreover, the effectiveness data is being applied across all outcomes (e.g., symptomatic cases, primary care visits, emergency department visits, hospitalizations, and deaths) whilst it is primarily derived from emergency department visits and hospitalizations. This assumption is a further limitation to the analysis. However, the DSA demonstrates that these are not key drivers as the difference in vaccine costs and coverage drives the cost-effectiveness results.
Most of the data used in the analysis, such as incidence, general practitioner visits, emergency room visits, hospitalization, demographic data, resource use, costs, vaccine coverage, mortality, and some utility data are from Spain. Data from Dolk et al. [43] from the UK and Belgium though were used in previous Spanish influenza models [10]. These analyses and the estimates presented here are based on data from previously published studies. The time horizon for the model is one year, which may limit the effectiveness of the vaccine if there is cross immunity or effect across years. The assumption that the vaccine currently used in Spain is SD-QIV is conservative, as it is likely to be a mix of TIV and SD-QIV and, therefore, less effective than SD-QIV alone. Additionally, the model is static rather than dynamic, meaning herd immunity is not accounted for. However, this is a conservative assumption and likely to have no impact on the conclusions given that a small proportion of the total Spanish population is vaccinated, the number vaccinated in each cohort is the same, and vaccine effectiveness differences between the two vaccines is comparable. We also estimate incidence rates over the previous three years. Although this is an area of great uncertainty, these are unlikely to affect the conclusions, given the value drivers between the two vaccines are differences in effectiveness and price.
The impact of influenza in the population aged 65+ can be very severe and, therefore, it is vital that they are protected. There is currently a trend towards using more effective QIV vaccines that are currently replacing TIV and SD-QIV. These results should be considered during the local regional tenders in the Spanish regions, especially to provide improved healthcare with considerable savings. Given the ever-present pressures on health care budgets, aQIV offers both an affordable and cost-saving alternative to HD-QIV for this relevant group in Spain.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines10020176/s1, Table S1: Publications were identified via a previously published systematic review and meta-analysis and a subsequent structured non-systematic review. The methods and PRISMA checklist for the previously published systematic re-view can be found in the corresponding publication [27]. The PRISMA checklist below [59] corresponds to the subse-quent structured non-systematic review conducted for this study.
Author Contributions
Conceptualization, methodology, writing, and data collection were developed by J.R.-A., S.M.-P., R.G., P.A. and R.G.-L. Software used was Microsoft Excel®. Validation and formal analysis were conducted by R.G. and R.G.-L., J.R.-A., S.M.-P., R.G., P.A. and R.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by Seqirus, the manufacturers of the adjuvanted quadrivalent influenza vaccine.
Conflicts of Interest
Ray Gani, Richard Guerrero-Luduena, and Piedad Alvarez are salaried employees of Evidera and are not allowed to accept remuneration from any clients for their services. Jesús Ruiz-Aragón and Sergio Márquez received consultancy fees from Evidera to conduct the study and develop this manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Influenza (Seasonal) 2018. [(accessed on 1 November 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 2.Nielsen J., Vestergaard L.S., Richter L., Schmid D., Bustos N., Asikainen T., Trebbien R., Denissov G., Innos K., Virtanen M.J., et al. European all-cause excess and influenza-attributable mortality in the 2017/18 season: Should the burden of influenza B be reconsidered? Clin. Microbiol. Infect. 2019;25:1266–1276. doi: 10.1016/j.cmi.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Rubio A., Platero L., Eiros Bouza J.M. Seasonal influenza in Spain: Clinical and economic burden and vaccination programmes. Med. Clin. Barc. 2019;153:16–27. doi: 10.1016/j.medcle.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Ministerio de Sanidad Consumo y Bienestar Social Recomendaciones de Vacunación Frente a la Gripe. 2021–2022. [(accessed on 1 November 2021)]. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/programasDeVacunacion/docs/Recomendaciones_vacunacion_gripe.pdf.
- 5.World Health Organization Recommended Composition of Influenza Virus Vaccines for Use in the 2022 Southern Hemisphere Influenza Season. 2021. [(accessed on 1 November 2021)]. Available online: https://cdn.who.int/media/docs/default-source/influenza/who-influenza-recommendations/vcm-southern-hemisphere-recommendation-2022/202109_recommendation.pdf?sfvrsn=698a54b9_12&download=true.
- 6.European Medicines Agency Fluad Tetra. 2021. [(accessed on 1 November 2021)]. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fluad-tetra#product-information-section.
- 7.Efluelda Suspension Inyectable en Jeringa Precargada Spanish Agency of Medicines and Medical Devices. [(accessed on 1 November 2021)]. Available online: http://cima.aemps.es/cima/publico/detalle.html?nregistro=85068.
- 8.Agencia Española de Medicamentos y Productos Sanitarios Ficha Tecnica Fluad Tetra Suspension Inyectable en Jering Pregarcada. 2020. [(accessed on 1 November 2021)]. Available online: https://cima.aemps.es/cima/dochtml/ft/1201433001/FT_1201433001.html.
- 9.Chang L.J., Meng Y., Janosczyk H., Landolfi V., Talbot H.K., Group QHDS Safety and immunogenicity of high-dose quadrivalent influenza vaccine in adults >/= 65 years of age: A phase 3 randomized clinical trial. Vaccine. 2019;37:5825–5834. doi: 10.1016/j.vaccine.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Aragon J., Gani R., Marquez S., Alvarez P. Estimated cost-effectiveness and burden of disease associated with quadrivalent cell-based and egg-based influenza vaccines in Spain. Hum. Vaccines Immunother. 2020;16:2238–2244. doi: 10.1080/21645515.2020.1712935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chit A., Roiz J., Aballea S. An Assessment of the Expected Cost-Effectiveness of Quadrivalent Influenza Vaccines in Ontario, Canada Using a Static Model. PLoS ONE. 2015;10:e0133606. doi: 10.1371/journal.pone.0133606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed C., Meltzer M.I., Finelli L., Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–1998. doi: 10.1016/j.vaccine.2011.12.098. [DOI] [PubMed] [Google Scholar]
- 13.Uhart M., Bricout H., Clay E., Largeron N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Hum. Vaccines Immunother. 2016;12:2259–2268. doi: 10.1080/21645515.2016.1180490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamotte A., Chong C.F., Manton A., Macabeo B., Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16:630. doi: 10.1186/s12889-016-3297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mennini F.S., Bini C., Marcellusi A., Rinaldi A., Franco E. Cost-effectiveness of switching from trivalent to quadrivalent inactivated influenza vaccines for the at-risk population in Italy. Hum. Vaccines Immunother. 2018;14:1867–1873. doi: 10.1080/21645515.2018.1469368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.López-Bastida J., Oliva-Moreno J., Antoñanzas F., García-Altés A., Gisbert R., Mar J., Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur. J. Health Econ. 2010;11:513–520. doi: 10.1007/s10198-010-0244-4. [DOI] [PubMed] [Google Scholar]
- 17.Ministerio de Sanidad Consumo y Bienestar Social Tabla 13. 2020–2021. Tabla13.pdf. [(accessed on 1 November 2021)]. Available online: https://www.mscbs.gob.es.
- 18.Instituto National de Estadistica Tablas de mortalidad por año, sexo, edad y funciones. 2019. [(accessed on 1 November 2021)]. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=27153.
- 19.Instituto Nacional de Estadistica Población Residente por Fecha, Sexo y Edad. 2021. [(accessed on 1 November 2021)]. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=31304.
- 20.ISCIII Informe de Vigilancia de la Gripe en España Temporada 2018–2019. Instituto de Salud Carlos III. Sistema de Vigilancia de la Gripe en España. 2017–2018. [(accessed on 1 November 2021)]. Available online: https://vgripe.isciii.es/documentos/20172018/InformesAnuales/Informe_Vigilancia_GRIPE_2017-2018_22julio2018.pdf.
- 21.ISCIII Informe de Vigilancia de la Gripe en España Temporada 2018–2019. Instituto de Salud Carlos III. Sistema de Vigilancia de la Gripe en España. 2018–2019. [(accessed on 1 November 2021)]. Available online: https://vgripe.isciii.es/documentos/20182019/InformesAnuales/Informe_Vigilancia_GRIPE_2018-2019_22julio2019.pdf.
- 22.ISCIII Informe de Vigilancia de la Gripe en España Temporada 2019–2020. Instituto de Salud Carlos III. Sistema de Vigilancia de la Gripe en España. 2019–2020. [(accessed on 1 November 2021)]. Available online: https://vgripe.isciii.es/documentos/20192020/InformesAnuales/Informe_Vigilancia_GRIPE_2019-2020_03092020.pdf.
- 23.Garcia A., Ortiz de Lejarazu R., Reina J., Callejo D., Cuervo J., Morano Larragueta R. Cost-effectiveness analysis of quadrivalent influenza vaccine in Spain. Hum. Vaccines Immunother. 2016;12:2269–2277. doi: 10.1080/21645515.2016.1182275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crépey P., Redondo E., Diez-Domingo J., De Lejarazu R.O., Martinón-Torres F., Gil De Miguel Á., López-Belmonte J.L., Alvarez F.P., Bricout H., Solozabal M. From trivalent to quadrivalent influenza vaccines: Public health and economic burden for different immunization strategies in Spain. PLoS ONE. 2020;15:e0233526. doi: 10.1371/journal.pone.0233526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz Granados C.A., Dunning A.J., Kimmel M., Kirby D., Treanor J., Collins A., Pollak R., Christoff J., Earl J., Landolfi V., et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N. Engl. J. Med. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.K.H., Lam G.K.L., Shin T., Kim J., Krishnan A., Greenberg D.P., Chit A. Efficacy and effectiveness of high-dose versus standard-dose influenza vaccination for older adults: A systematic review and meta-analysis. Expert Rev. Vaccines. 2018;17:435–443. doi: 10.1080/14760584.2018.1471989. [DOI] [PubMed] [Google Scholar]
- 27.Coleman B.L., Sanderson R., Haag M.D.M., McGovern I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influ. Other Respir. Viruses. 2021;15:813–823. doi: 10.1111/irv.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izurieta H.S., Chillarige Y., Kelman J., Wei Y., Lu Y., Xu W., Lu M., Pratt D., Chu S., Wernecke M., et al. Relative Effectiveness of Cell-Cultured and Egg-Based Influenza Vaccines Among Elderly Persons in the United States, 2017–2018. J. Infect. Dis. 2019;220:1255–1264. doi: 10.1093/infdis/jiy716. [DOI] [PubMed] [Google Scholar]
- 29.Izurieta H.S., Chillarige Y., Kelman J., Wei Y., Lu Y., Xu W., Lu M., Pratt D., Wernecke M., MaCurdy T., et al. Relative Effectiveness of Influenza Vaccines Among the United States Elderly, 2018–2019. J. Infect. Dis. 2020;222:278–287. doi: 10.1093/infdis/jiaa080. [DOI] [PubMed] [Google Scholar]
- 30.Pelton S.I., Divino V., Shah D., Mould-Quevedo J., Dekoven M., Krishnarajah G., Postma M.J. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017–2018 Influenza Season. Vaccines. 2020;8:446. doi: 10.3390/vaccines8030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boikos C., Fischer L., O’Brien D., Vasey J., Sylvester G., Mansi J. Relative Effectiveness of aTIV versus TIVe, QIVe and HD-TIV in Preventing Influenza-Related Medical Encounters during the 2017–2018 and 2018–2019 Influenza Seasons in the US; Proceedings of the National Foundation for Infectious Diseases; Virtual. 18–19 June 2020. [Google Scholar]
- 32.Pelton S.I., Divino V., Postma M.J., Shah D., Mould-Quevedo J., DeKoven M., Krishnarajah G. A retrospective cohort study assessing relative effectiveness of adjuvanted versus high-dose trivalent influenza vaccines among older adults in the United States during the 2018–19 influenza season. Vaccine. 2021;39:2396–2407. doi: 10.1016/j.vaccine.2021.03.054. [DOI] [PubMed] [Google Scholar]
- 33.Izurieta H.S., Lu M., Kelman J., Galante M., Ferrer M., Dominguez A. Comparative effectiveness of influenza vaccines among U.S. Medicare beneficiaries ages 65 years and older during the 2019–20 season. Clin. Infect. Dis. 2020;73:e4251–e4259. doi: 10.1093/cid/ciaa1727. [DOI] [PubMed] [Google Scholar]
- 34.Levin M.J., Divino V., Shah D., DeKoven M., Mould-Quevedo J., Pelton S.I., Postma M.J. Comparing the Clinical and Economic Outcomes Associated with Adjuvanted versus High-Dose Trivalent Influenza Vaccine among Adults Aged ≥ 65 Years in the US during the 2019–20 Influenza Season—A Retrospective Cohort Analysis. Vaccines. 2021;9:1146. doi: 10.3390/vaccines9101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imran M., Puig-Barbera J., Ortiz J., Divino V., Postma M.J., Shah D. Relative Effectiveness of MF59-Adjuvanted, Trivalent Influenza Vaccine vs Quadrivalent Influenza Vaccine and High-Dose Trivalent Influenza Vaccine in Preventing Influenza-Related Medical Encounters in Adults ≥ 65 Years of Age during the 2019–2020 Influenza Season in the United States; Proceedings of the European Society of Clinical Microbiology and Infectious Diseases Conference on Vaccines (ECCMID); Vienna, Austria. 9–7 July 2021. [Google Scholar]
- 36.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 37.R Foundation for Statistical Computing A Language and Environment for Statistical Computing. 2020. [(accessed on 1 November 2021)]. Available online: https://www.R-project.org/
- 38.Balduzzi S., Rücker G., Schwarzer G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Évid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization Candidate Vaccine Viruses and Potency Testing Reagents. 2021. [(accessed on 1 November 2021)]. Available online: https://www.who.int/teams/global-influenza-programme/vaccines/who-recommendations/candidate-vaccine-viruses.
- 40.CDC Fluview Interactive Age Group Distribution of Influenza Positive Specimens Reported by Public Health Laboratories, National Summary, 2021–2022, Influenza Season through the Week Ending 6 November 2021. [(accessed on 1 November 2021)];2021 Available online: https://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html.
- 41.Marquez-Pelaez S.G.R., Alvarez P., Divino V., Postma M.J., Shah D. An Economic Evaluation of Enhanced Influenza Vaccines for the Elderly in Spain. The Adjuvanted Quadrivalent Influenza Vaccine versus High-Dose Quadrivalent Influenza Vaccine; progressing of the 8th European Scientific Working Group on Influenza (ESWI); virtual. 4–7 December 2021; #176. Poster Presentation. [Google Scholar]
- 42.Hollmann M., Garin O., Galante M., Ferrer M., Dominguez A., Alonso J. Impact of influenza on health-related quality of life among confirmed (H1N1)2009 patients. PLoS ONE. 2013;8:e60477. doi: 10.1371/journal.pone.0060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolk C., Eichner M., Welte R., Anastassopoulou A., Van Bellinghen L.-A., Nautrup B.P., Van Vlaenderen I., Schmidt-Ott R., Schwehm M., Postma M. Cost-Utility of Quadrivalent Versus Trivalent Influenza Vaccine in Germany, Using an Individual-Based Dynamic Transmission Model. Pharmacoeconomics. 2016;34:1299–1308. doi: 10.1007/s40273-016-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ministerio de Sanidad Consumo y Bienestar Social Acuerdo Marco para la Seleccion de Suministradores de Vacunas Frente a la Gripe Estacional (INGESA y Ciudades de Ceuta y Melilla y Varias Comunidades Autonomas) 2021. [(accessed on 1 November 2021)]. Available online: https://contrataciondelestado.es/wps/wcm/connect/7c41cd41-00c8-4c07-be3d-272d29585268/DOC20210419131140PCAP+Gripe+2021-2025.pdf?MOD=AJPERES.
- 45.Vademecum Efluelda Suspension Injectable in Pre-Loaded Syringe. 2021. [(accessed on 2 November 2021)]. Available online: https://www.vademecum.es/medicamento-efluelda_49277.
- 46.País Vasco Tarifas para Facturación de Servicios Sanitarios y Docentes de Osakidetza para el Año 2021 (From KOL) 2020. [(accessed on 1 November 2021)]. Available online: https://www.osakidetza.euskadi.eus/contenidos/informacion/osk_servic_para_empresas/es_def/adjuntos/LIBRO-DE-TARIFAS_2020_osakidetza.pdf.
- 47.Junta de Andalucía Precios públicos de servicios sanitarios prestados en el SSPA. 2019. [(accessed on 1 November 2021)]. Available online: https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/profesionales/recursos-para-profesionales/precios-publicos.
- 48.Boletin Oficial de la Region de Murcia Comunidad Autónoma. 2019. [(accessed on 1 November 2021)]. Available online: https://www.borm.es/services/anuncio/ano/2019/numero/1263/pdf.
- 49.Ministerio de Sanidad Consumo y Bienestar Social Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud. CMBD. Norma Estatal. [(accessed on 1 November 2021)];2019 Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbd.htm.
- 50.Perez-Rubio A., Eiros J.M. Economic and Health impact of influenza vaccination with adjuvant MF59 in population over 64 years in Spain. Rev. Esp. Quimioter. 2018;31:43–52. [PMC free article] [PubMed] [Google Scholar]
- 51.Kirch W., editor. Encyclopedia of Public Health. Springer; Dordrecht, The Netherlands: 2008. Human Capital Approach; pp. 697–698. [Google Scholar]
- 52.Instituto Nacional de Estadistica Ocupados por Sexo y Grupo de Edad. Valores Absolutos y Porcentajes Respecto del Total de Cada Sexo. 2021. [(accessed on 1 November 2021)]. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=4076.
- 53.Instituto Nacional de Estadistica Encuesta Anual de Coste Laboral (EACL) Año 2019. 2021. [(accessed on 1 November 2021)]. Available online: https://www.ine.es/prensa/eacl_2019.pdf.
- 54.Instituto Nacional de Estadistica Población por Sexo y Grupo de Edad. Valores Absolutos y Porcentajes Respecto del Total de Cada Sexo. 2021. [(accessed on 1 November 2021)]. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=4028.
- 55.Vallejo-Torres L., Garcia-Lorenzo B., Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27:746–761. doi: 10.1002/hec.3633. [DOI] [PubMed] [Google Scholar]
- 56.Sacristan J.A., Oliva J., Campillo-Artero C., Piung-Junoi J., Pinto-Prades J.L., Dilla T., Rubio-Terrés C., Ortún V. What is an efficient health intervention in Spain in 2020? Gac. Sanit. 2020;34:189–193. doi: 10.1016/j.gaceta.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Redondo E., Drago G., López-Belmonte J.L., Guillén J.M., Bricout H., Alvarez F.P., Callejo D., Gil de Miguel Á. Cost-utility analysis of influenza vaccination in a population aged 65 years or older in Spain with a high-dose vaccine versus an adjuvanted vaccine. Vaccine. 2021;39:5138–5145. doi: 10.1016/j.vaccine.2021.07.048. [DOI] [PubMed] [Google Scholar]
- 58.Kohli M.A., Maschio M., Mould-Quevedo J.F., Drummond M., Weinstein M.C. The cost-effectiveness of an adjuvanted quadrivalent influenza vaccine in the United Kingdom. Hum. Vaccines Immunother. 2021;17:4603–4610. doi: 10.1080/21645515.2021.1971017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page M.J., McKenzie J.E., Bossuyt P.M., López-Belmonte J.L., Guillén J.M., Bricout H. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.