Abstract
Background: The aim of the study was to determine whether free-living birds belonging to game species whose meat is used for human consumption can constitute a reservoir of pathogenic Campylobacter strains, spreading these bacteria to other hosts or directly contributing to human infection. Methods: A total of 91 cloacal swabs were taken from different species of wildlife waterfowl to estimate the Campylobacter prevalence, the genetic diversity of the isolates, and the presence of virulence genes and to evaluate the antimicrobial resistance. Results: The presence of Campylobacter spp. was confirmed in 32.9% of samples. Based on flaA-SVR sequencing, a total of 19 different alleles among the tested Campylobacter isolates were revealed. The virulence genes involved in adhesion were detected at high frequencies among Campylobacter isolates regardless of the host species. The highest resistance was observed for ciprofloxacin. The resistance rates to erythromycin and tetracycline were observed at the same level. Conclusions: These results suggest that wildlife waterfowl belonging to game species may constitute a reservoir of Campylobacter, spreading these bacteria to other hosts or directly contributing to human disease. The high distribution of virulence-associated genes among wildlife waterfowl Campylobacter isolates make them potentially able to induce infection in humans.
Keywords: Campylobacter, antimicrobial resistance, virulence genes, game species, wildlife waterfowl
1. Introduction
Free-living birds, including migratory species, can become vectors for a wide range of microorganisms that can be transmissible to other animals and humans [1]. In addition, bird migration provides a mechanism for the establishment of new endemic foci of disease at great distances from where an infection was acquired [2]. The intestinal tract of birds may be colonized by different bacteria, many of which are pathogenic for humans. Moreover, the close association of birds and humans in urban and agricultural settings facilitates zoonotic disease transfer [3,4]. Although bird infestations may be transmitted to other animals and humans via direct contact or inhalation of contaminated air conditioners or vents, the most common is oral transmission through food and water that has been contaminated by bird fecal material [5]. A leading worldwide foodborne zoonosis is campylobacteriosis [6]. Campylobacter spp. commonly inhabit the intestines of avian species, as their body temperature provides an optimal environment for the growth of the organism. Therefore, these bacteria are found in both poultry and wild bird feces [7,8]. Moreover, some wild bird species have successfully adapted to anthropogenic environments and routinely come into close contact with livestock, domestic animals, and people and are thus seen as a potential source of Campylobacter [9]. It is also important that numerous wildlife birds are game species whose meat is used for human consumption and can pose a potential health hazard [10]. For a better understanding of the epidemiology and transmission of Campylobacter spp., an investigation of the genetic relatedness of Campylobacter isolates is crucial. Many molecular methods have been developed to investigate the diversity within Campylobacter isolates, including a sequence analysis of the short variable region (SVR) of the flaA gene. This highly discriminatory method is widely used for a better understanding of Campylobacter population structures [11,12]. According to Hanage [13], in most pathogenic bacteria, the population is made up of multiple distinct lineages. which are associated with properties such as virulence or drug resistance. In the case of campylobacteriosis, both successful invasion and organization in host cells depend on various virulence factors linked with adhesion to intestinal mucosa, invasion of epithelial cells, toxin production, and protein secretion [14]. Among adhesion-associated markers, the following are crucial: the flaA gene, encoding the major flagellin protein (FlaA), a structural component of flagella crucial for attachment to intestinal epithelial cells and involved in autoagglutination and microcolony formation [15,16]; the cadF gene, encoding a fibronectin binding protein CadF [17]; the racR gene, encoding a DNA-binding response regulator [18]; a periplasmic cytochrome C peroxidase, encoded by docA; and the chaperone protein DnaJ, encoded by the dnaJ gene [19]. Regarding markers affecting invasion, a significant role is played by the pldA gene, encoding phospholipase A; the ciaB gene, encoding a Campylobacter invasion antigen; the virB11 gene, responsible for host cell invasion; and invasion-associated marker (iam) [20]. In addition, numerous toxins produced by Campylobacter spp. have been described, but only cytolethal distending toxin (CDT), encoded by three linked genes, namely cdtA, cdtB, and cdtC, has been well characterized [21]. Campylobacter infection in humans commonly causes gastroenteritis, but infection can also occur outside the intestines, such as polyneuropathic disorder, denominated as Guillain-Barré syndrome (GBS). The Campylobacter strains that can elicit GBS carry either wlaN or cgtB, both encoding a β-1,3-galactosyltransferase enzyme that is required for the production of sialylated lipooligosaccharide LOSSIAL, a crucial virulence factor of GBS [22].
For a better understanding of the evolution of infectious diseases, the determination of bacteria drug resistance is crucial. According to Bonnedahl and Järhult [23], wild birds should be postulated not only as reservoirs but also as potential spreaders of antibiotic resistance. Among the factors contributing to the prevalence of antibiotic resistance among wild birds, the natural preservation state, livestock, human densities, and the remoteness of an area have a significant impact [24]. Moreover, according to Skurnik et al. [25] and Allen et al. [24], the levels of resistance seem to correlate with the degree of proximity to human settlements. As wild birds seem to play a significant role as reservoirs for pathogenic enteric bacteria, they can pollute the environment with antimicrobial-resistant (AMR) bacteria and spread difficult-to-treat zoonotic diseases.
This study aimed to better understand the role of wildlife waterfowl in the transmission of Campylobacter infection among both livestock and humans. This study aimed specifically to determine (i) the prevalence rate of Campylobacter in wildlife waterfowl belonging to game species, (ii) the genetic diversity, (iii) the prevalence of virulence genes related to adherence, invasion cytotoxicity and GBS, as well as the antibiotic resistance profile in the investigated Campylobacter isolates.
2. Results
2.1. Isolation and Identification of Bacterial Strains
Out of 91 tested cloacal swabs, the presence of Campylobacter spp. was confirmed in 30 (32.9%) samples. The prevalence rate ranged from 45.5% among white-fronted geese (in 5 out of 11) to 32.8% among mallards (in 20 out of 61). None of the fecal samples from Eurasian teal were positive for Campylobacter spp. (Table 1). The majority of the obtained isolates (28/30, 93.3%) were C. jejuni, and only two (6.7%) isolates from mallards were C. coli.
Table 1.
Prevalence of Campylobacter spp. among wild birds.
| Source | No. of Samples | No. of Positive Samples (%) | ||
|---|---|---|---|---|
| Common Name | Latin Name | C. jejuni | C. coli | |
| Mallard duck | Anas platyrhynchos | 61 | 18 (29.5%) |
2 (3.3%) |
| White-fronted goose | Anser albifrons | 11 | 5 (45.5%) |
0 |
| Greylag goose | Anser anser | 8 | 3 (37.5%) |
0 |
| Eurasian teal | Anas crecca | 6 | 0 | 0 |
| Bean goose | Anser fabalis | 5 | 2 (40%) |
0 |
2.2. Detection of Virulence Genes
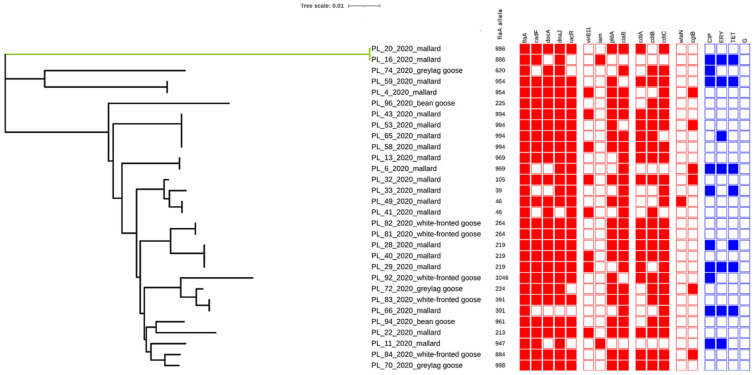
The virulence genes involved in adhesion were detected at high frequencies among Campylobacter isolates regardless of the source. All isolates originating from white-fronted geese and bean geese possessed flaA, cadF, racR, docA, and dnaJ genes. Moreover, high frequency rates were noted for these genes among isolates from mallards (100%, 80%, 85%, 75%, and 90%) and graylag geese (100%, 66.7%, 66.7%, 66.7%, and 100%, respectively). Regarding genes associated with invasion, the most prevailing were the ciaB gene (with prevalence rates ranging from 100% in bean geese and greylag geese to 80% in mallards and white-fronted geese) and the pldA gene (with prevalence rates ranging from 100% in bean geese and white-fronted geese to 60% in mallards). The virB11 and iam genes were noted only among mallard-origin isolates at the level of 40% (8/20) and 10% (2/20), respectively. Among genes associated with cytotoxicity, the most common were cdtB and cdtC genes, noted in 100% of isolates from white-fronted geese, graylag geese, and bean geese and in 55% (11/20) and 75% (15/20) of mallard-origin isolates. However, the cdtA gene was detected in 60% (12/20), 80% (4/5), and 33.3% (1/3) of Campylobacter strains isolated from mallards, white-fronted geese, and greylag geese, respectively. The cytotoxin-encoding cluster cdtABC was confirmed in 9 out 20 (45%) of mallard isolates, in four out of five (80%) of white-fronted geese isolates, and in one out of three (33.3%) greylag geese isolates. Only single isolates possessed LOSSIAL-related genes, 5% (1/20) of mallard-origin isolates were positive for the wlaN gene, and 20% (4/20), 20% (1/5), and 33.3% (1/3) of isolates recovered from mallards, white-fronted geese, and graylag geese were positive for the cgtB gene (Figure 1).
Figure 1.
Maximum likelihood tree of Campylobacter flaA-SVR allele sequences among isolates originating from wild birds. For each isolate, the following characteristics are shown: strain ID (according to the pattern: country of isolation_individual number of tested sample_year of isolation_host), flaA allele number, virulence genes, and antimicrobial resistance. The prevalence of determinants involved in virulence is indicated by red (present) and white (absent) squares. The occurrence of resistance to tested antimicrobials is indicated by blue (present) and white (absent) squares. C. coli isolates form the green cluster. The figure is visualized in the interactive tree of life (iTol).
The results showed that no Campylobacter strain was positive for all tested virulence markers, while none of the analyzed strains showed a lack of all tested genes.
2.3. Sequencing of flaA-SVR
The conducted flaA-SVR sequencing revealed a total of 19 different alleles among 30 tested Campylobacter isolates (Figure 1). The highest divergence was observed among isolates originating from greylag and bean geese, with a Simpson’s diversity index of 1.0. However, among mallards and white-fronted geese, this index was 0.932 (CI95% 0.884–0.979) and 0.9 (CI 95% 0.725–1.000), respectively.
The most commonly reported flaA-SVR alleles were 994 and 219, which were only noted among isolates originating from mallards and covering 23.3% of obtained isolates. Eleven out of 19 (57.9%) alleles occurred only once. Only the flaA-SVR allele 391 was not specific to the host and was noted among Campylobacter strains isolated from mallards and white-fronted geese.
2.4. Antimicrobial Resistance
Among the four tested antimicrobial agents, the highest resistance was observed for ciprofloxacin (in 10 out of 30 isolates, 33.3%) (Figure 1). The majority of ciprofloxacin-resistant isolates were isolated from mallards (8 out of 10, 80%). The remaining isolates originated from graylag goose (one isolate) and white-fronted goose (one isolate). The resistance rates to erythromycin and tetracycline were observed at the same level of 23.3% (in 7 out of 30 isolates), and the resistant isolates were only recovered from mallards. All Campylobacter isolates, regardless of the source, did not show resistance to gentamicin.
The most frequent resistance pattern was CIP_ERY_TET, noted in 5 out 30 (16.7%) tested isolates. None of the isolates obtained manifested resistance to the four tested antimicrobial agents. Simultaneously, sensitivity to all tested antimicrobials was observed in the majority of tested isolates with an overall rate of 63.3% (100% of bean geese, 80% of white-fronted geese, 66.7% of graylag geese, and 55% of mallards).
3. Discussion
Since campylobacteriosis has become considered an emerging foodborne disease in recent years, the majority of studies have concentrated on determining the source of Campylobacter among farm animals. However, wildlife waterfowl may play a role in the spread of campylobacteriosis through fecal contamination of the environment, feed, and surface water. Therefore, Elmberg et al. [26] emphasized the application of precautionary principles to ensure that domestic poultry does not come into contact (or share pasture or water access) with wild birds. Moreover, wildlife waterfowl may pose a risk not only to other animals but also to humans due to direct contact with birds or their feces in beaches or parks or via the consumption of vegetables infected by their feces, at least via consumption of undercooked meat from wildlife waterfowl. Wildlife waterfowl game species can also be a source of direct infection, especially when their bodies are hit by numerous pellets, which can damage the intestines and can contaminate meat with the intestinal content.
French et al. [27] suggest that feces from wildlife waterfowl in playgrounds could contribute to the occurrence of campylobacteriosis in preschool children. According to Ramonaite et al. [28], the prevalence of Campylobacter spp. among wildlife waterfowl varied from 1.4% to 72.7% depending on different countries and wild bird species. In the present study, 32.9% of wildlife waterfowl tested were positive for Campylobacter spp. The predominant species associated with human illness is Campylobacter jejuni, also described as dominant in poultry in different geographical regions [29]. Additionally, in the current study, 93.3% of isolates originating from examined birds were identified as C. jejuni. Moreover, Campylobacter spp. is described as genetically divergent, which is in accordance with the current study. The overall Simpson’s diversity index calculated for all 30 Campylobacter isolates originating from wildlife waterfowl was estimated at a value of 0.966. All flaA-SVR alleles assigned to greylag geese and bean geese isolates occurred only once, while among isolates obtained from white-fronted geese, single sequences only occurred twice. This is in contrast with the results obtained in previous studies [30], which reported that the majority of alleles co-existed among poultry (18/28, 64.3%) and human (22/34, 64.7%) isolates. According to Atterby et al. [31], strain-specific association to particular bird species is noticeable, and limited contact between wildlife waterfowl species differences in diet or feeding behavior or migration patterns can be seen as the causes of the described situation. Interestingly, studies performed by Colles et al. [32] on Campylobacter populations in wild and domesticated Mallard ducks revealed that only one sequence type was shared between the two sources, accounting for 0.9% of wild duck isolates and 5% of farmed duck isolates. In the present study, it was also noted that only a single allele was not unique to the source-flaA-SVR allele 391 was noted among mallards and white-fronted geese. An analysis of sequences deposited in the PubMLST database revealed that only a few flaA-SVR alleles (alleles 8, 219, 886, 46, 264) were found in Campylobacter strains isolated from environmental water samples, chicken meat, offal, or from human stool. Moreover, studies performed by Di Giannatale et al. [33] and Llarena et al. [34] revealed several genotypes overlapping in wild birds, farm animals, poultry, and human isolates.
For a better understanding of the epidemiology of Campylobacter infection, it is crucial not only to recognize the various sources of this pathogen but also to determine the virulence properties of bacteria since different pathogenic profiles can be identified within the species [35]. The current results have revealed the common prevalence of genes associated with adhesion (flaA, cadF, racR, docA, and dnaJ) among isolates of wildlife waterfowl origin, which is significant since every Campylobacter infection is preceded by colonization of the intestinal tract. Similar results have been previously noted by Du et al. [36] in China, Wei et al. [37] in South Korea, and Shyaka et al. [38] in Japan. These findings confirmed the strong colonization ability among strains isolated from wildlife waterfowl. Generally, the prevalence of genes involved in adhesion is common among isolates, regardless of the source and geographical region [39,40]. Regarding invasion abilities, the authors’ previous studies have revealed the common prevalence of ciaB (83.3%) and pldA (70%) genes among wildlife waterfowl, regardless of the source. The high prevalence of these genes was previously noted in wild bird isolates [37], layer poultry isolates [41], chicken meat isolates [42], and in human and cattle isolates [43]. The overall prevalence rates of two other tested virulence genes associated with invasion (virB11 and iam) were 26.7% and 6.7%, respectively. The low percentage of virB11- and iam-positive Campylobacter isolates was also detected in retail chicken meat in China [44], in Danish pigs and cattle [45], as well as in human isolates in Chile [46]. Some authors have also noted that the role of virB11 gene in pathogenesis of campylobacteriosis is still not clear [37] although Tracz et al. [47] suggested that products of pVir plasmid genes may effect a more serious course of Campylobacter infection in humans, resulting in bloody diarrhea. Similar divergent observations were described in relation to the invasion-associated marker iam. Sanad et al. [48] suggested that the use of the iam as a virulence determinant in epidemiological studies might be potentially misleading and might require reevaluation.
One of the main virulence factors related to Campylobacter spp. is cytolethal-distending toxin (CDT), encoded by the three adjacent genes cdtA, cdtB, and cdtC [49]. The carriage of cdt complex is common in isolates from poultry [49], swine [45], cattle [50], and human [30,51] isolates. In the present study, it was found that 46.7% of wildlife waterfowl isolates possessed three cdt genes.
As Campylobacter is known as one of the main etiological factors connected with the GBS occurrence in humans, it appears crucial to establish the occurrence of pathogenic genes involved in this process. It is known that Campylobacter strains carrying wlaN and cgtB genes responsible for LOSSIAL production can potentially elicit GBS due to the well-documented molecular mimicry between the LOSSIAL and the saccharide component of the human GM1 ganglioside, which is present in peripheral nerves [52]. A differential distribution of the cgtB and wlaN genes among wildlife waterfowl was noted. Only single isolates originating from wildlife waterfowl carried wlaN (3.3% of isolates) or cgtB (20% of isolates) genes. The prevalence rates of wlaN and cgtB genes were noted at similar levels in previous studies. These genes were detected in 11.3% of wildlife waterfowl isolates in South Korea [37], in 25% of human isolates in Japan [53], in 17.5% of geese carcasses in Poland [54], in 6.7% of human isolates in Argentina [55], and up to 21.9% of livestock animals in Spain [56].
In recent years, antibiotic resistance among pathogenic bacteria has become an emerging problem. The widespread use of antibiotics in industrial agriculture, mainly in animal production, has contributed to the threat of drug resistance, as the resistant bacteria in animals may directly or indirectly reach humans through food or water [57]. The increasing rates of resistance of Campylobacter isolates to fluoroquinolones and macrolides observed in recent years pose a significant risk for human health since these antimicrobial factors are commonly used in the treatment of Campylobacter infection [58]. Moreover, a significantly high percentage of tetracycline-resistant Campylobacter isolates has also been noted, which is alarming since tetracycline has been suggested as an alternative treatment choice for Campylobacter infection. In this study, the overall resistance rates to ciprofloxacin, erythromycin and tetracycline among Campylobacter isolates of wildlife waterfowl origin were 33.3%, 23.3%, and 23.3%, respectively. Interestingly, these rates were mostly mediated by isolates originating from mallards. Studies performed by Du et al. [36] in China on wildlife waterfowl from different locations and sites showed that wildlife waterfowl from urban areas have higher antibiotic resistance compared to birds from suburban areas, which might be due to contaminated environment water. These findings are in accordance with the current results since mallards, in contrast to bean goose or white-fronted goose, settle more frequently in areas of human activities, which results in different resistance levels of Campylobacter isolates obtained from wildlife waterfowl. Interestingly, the observed rates of resistance were lower than those noted among isolates of farm animals and of human origin. The previous studies revealed that 74% of Campylobacter spp. isolated from livestock, poultry processing plants, and retail meat in North Carolina were resistant to tetracycline [59], and 76% and 64% of human isolates in the study performed in Italy were resistant to ciprofloxacin and tetracycline [60]. Similar findings were noted by Marotta et al. [61], who noted that compared with farmed poultry, the incidence of AMR in the C. jejuni isolates from the other bird groups was low, confirming that the poultry are much more exposed to antimicrobials.
The current results suggest that wildlife waterfowl belonging to game species may constitute a reservoir of Campylobacter spreading these bacteria to other hosts or directly contributing to human diseases. The high distribution of virulence-associated genes among wildlife waterfowl Campylobacter isolates makes them potentially able to induce infection in humans.
4. Materials and Methods
4.1. Isolation and Identification of Bacterial Strains
In this study, a total of 91 samples of cloacal swabs from wildlife waterfowl—61 from mallard ducks (Anas platyrhynchos), 11 from white-fronted geese (Anser albifrons), eight from greylag geese (Anser anser), six from Eurasian teal (Anas crecca), and five from bean geese (Anser fabalis)—were analyzed for the presence of Campylobacter spp. The samples were taken from birds hunted mainly in northeastern Poland between August and November 2020 (Supplementary Table S1). The swabs were transported to the laboratory in Amies gel transport medium (Oxoid, Basingstoke, UK) and were subsequently transferred to 9 mL of Bolton broth (Oxoid, UK). The enrichment cultures were grown at 37 °C for 4 h and then at 41.5 °C for 44 ± 4 h under microaerobic conditions (5% O2, 10% CO2, and 85% N). A loopful of the suspension was then spread on the surface of a charcoal cefoperazone deoxycholate modified agar (mCCDA, Oxoid, UK) and agar Karmali (Oxoid, UK). After incubation under microaerobic conditions for 24–48 h, the plates were examined for morphologically typical Campylobacter colonies. Single colonies were picked up and confirmed as Campylobacter by examination of microscopic morphology, the presence of oxidase activity, motility, and lack of microaerobic growth at 25 °C. Subsequently, the isolates were subcultured only once in order to minimize changes resulting from several passages and stored at −80 °C in defibrinated horse blood (Oxoid, UK) with added glycerol (80:20 v/v).
Species identification of the isolates was carried out based on primers listed in Table 2. For this purpose, Campylobacter isolates cultured on Columbia agar supplemented with blood were suspended in 1 mL of sterile water and centrifuged at 13,000× g for 1 min. The precipitate was suspended in a Tris buffer. DNA isolation was performed using Genomic -Mini Kit (A&A Biotechnology, Gdańsk, Poland) according to the manufacturer’s instructions. The purity and concentration of the DNA were determined spectrophotometrically. The DNA was used as a template in all the PCR assays (described in detail below).
Table 2.
PCR primers used in the study.
| Target Gene | Sequences (5′–3′) | Product Size (bp) | Annealing Temperature °C | References |
|---|---|---|---|---|
|
16S rRNA for Campylobacter spp. |
F-ATCTAATGGCTTAACCATTAAAC | 857 | 58 | [62] |
| R GGACGGTAACTAGTTTAGTATT | ||||
|
mapA for C. jejuni |
F-CTATTTTATTTTTGAGTGCTTGTG | 589 | 58 | [62] |
| R-GCTTTATTTGCCATTTGTTTTATTA | ||||
|
ceuE for C. coli |
F-AATTGAAAATTGCTCCAACTATG | 462 | 58 | [62] |
| R-TGATTTTATTATTTGTAGCAGCG | ||||
| flaA-SVR | F-CTA TGG ATG AGC AAT T(AT)A AAA T | 383 | 53 | [63] |
| R-CAA G(AT)C CTG TTC C(AT)A CTG AAG | ||||
| flaA | F-AATAAAAATGCTGATAAAACAGGTG | 855 | 53 | [53] |
| R-TACCGAACCAATGTCTGCTCTGATT | ||||
| flhA | F-GGAAGCGGCACTTGGTTTGC | 735 | 53 | [64] |
| R-GCTGTGAGTGAGATTATAGCAG | ||||
| dnaJ | F-ATTGATTTTGCTGCGGGTAG | 177 | 50 | [65] |
| R-ATCCGCAAAAGCTTCAAAAA | ||||
| cadF | F-TTGAAGGTAATTTAGATATG | 400 | 45 | [66] |
| R-CTAATACCTAAAGTTGAAAC | ||||
| virB11 | F-TCTTGTGAGTTGCCTTACCCCTTTT | 494 | 53 | [53] |
| R-CCTGCGTGTCCTGTGTTATTTACCC | ||||
| docA | F-ATAAGGTGCGGTTTTGGC | 725 | 50 | [64] |
| R-GTCTTTGCAGTAGATATG | ||||
| iam | F-GCGCAAAATATTATCACCC | 518 | 52 | [67] |
| R-TTCACGACTACTATGCGG | ||||
| ciaB | F-TGCGAGATTTTTCGAGAATG | 527 | 54 | [65] |
| R-TGCCCGCCTTAGAACTTACA | ||||
| racR | F-GATGATCCTGACTTTG | 584 | 45 | [53] |
| R-TCTCCTATTTTTACCC | ||||
| pldA | F-AAGCTTATGCGTTTTT | 913 | 45 | [53] |
| R-TATAAGGCTTTCTCCA | ||||
| cdtA | F-CCTTGTGATGCAAGCAATC | 370 | 49 | [53] |
| R-ACACTCCATTTGCTTTCTG | ||||
| cdtB | F-CAGAAAGCAAATGGAGTGTT | 620 | 51 | [53] |
| R-AGCTAAAAGCGGTGGAGTAT | ||||
| cdtC | F-CGATGAGTTAAAACAAAAAGATA | 182 | 47 | [53] |
| R-TTGGCATTATAGAAAATACAGTT | ||||
| wlaN | F-TGCTGGGTATACAAAGGTTGTG | 330 | 55 | [64] |
| R-ATTTTGGATATGGGTGGGG | ||||
| cgtB | F-TAAGAGCAAGATATGAAGGTG | 561 | 52 | [68] |
| R-GCACATAGAGAACGCTACAA |
4.2. Detection of Virulence Genes
The genomic DNA was amplified by PCR to confirm the presence of genes involved in adherence (flaA, cadF, docA, dnaJ, and racR) and invasion (virB11, iam, ciaB, and pldA), responsible for the production of cytolethal distending toxin (cdtA, cdtB, and cdtC) and sialylated lipooligosaccharide (wlaN and cgtB) by using primers listed in Table 2. Amplification was performed in a 50-μL reaction mixture containing 5 μL of the PCR buffer (10 -times concentrated), 5 μL of dNTPs (final concentration of 200 µM), 0.5 μL of each primer (final concentration of 0.1 µM), 10 μL MgCl2 (final concentration of 5 mM), 2 μL (2 U) thermostable Taq polymerase (Thermo Fisher Scientific, Waltham, MA, USA), 5 μL of template DNA at the final concentration of 120 ng verified by Nano-DropTM Spectrophotometer (Thermo Fisher Scientific, USA), and DNase-and RNase-free deionized water. All PCRs were carried out using the following conditions: initial denaturation at 94 °C for 5 min followed by 30 cycles of denaturation for 1 min at 95 °C, annealing at a temperature specific to the primer pair for 1 min, and extension for 1 min at 72 °C. The final elongation step was carried out at 72 °C for 5 min. A positive control consisting of DNA extracted from C. jejuni ATCC 33291 and C. coli ATCC 43478 as well as a non-template PCR control consisting of PCR-grade water were included in each PCR run. The PCR product was run on a 2% agarose gel stained with ethidium bromide at a concentration of 5 μg/mL. The size of the amplification product was determined using the 100-bp molecular weight marker.F
4.3. Sequencing of flaA-SVR
The DNA of all isolates obtained in this study was subjected to flaA short variable region (SVR) and sequencing using the primers listed in Table 2. For PCR, the conditions were as described above. The PCR products were visualized in gel electrophoresis, purified with a Clean-Up Kit (A&A Biotechnology, Poland), and sequenced by Sanger sequencing (Genomed, Warszawa, Poland). The forward and reverse sequences were assembled using the Contig Express module in Vector NTI Express (Thermo Fisher Scientific, USA) and trimmed to a 321-bp length covering the flaA-SVR. The sequences were assigned flaA-SVR allele numbers according to the PubMLST database (http://pubmlst.org/campylobacter (accessed on 1 December 2021)), and a cluster analysis was then performed using default parameters in MEGA X v. 10.1 (http://www.megasoftware.net (accessed on 1 December 2021)). The maximum likelihood tree based on the flaA-SVR sequences was visualized in iTOL v4 (https://itol.embl.de (accessed on 1 December 2021)). The obtained sequences were submitted to the GenBank database and received the following Accession Numbers OL314289–OL314318.
The genetic diversity of Campylobacter isolates originating from wildlife waterfowl was assessed by the Simpson’s diversity index (ID) as described previously [69] using the online tool “Comparing Partitions” from the website http://www.comparingpartitions.info (accessed on 1 November 2021) [70].
4.4. Antimicrobial Resistance
Antimicrobial resistance was examined by the diffusion-disk method according to the protocol of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) for fastidious organisms. All Campylobacter isolates were suspended in a brain-heart infusion (BHI) broth to a turbidity equivalent to a 0.5 McFarland standard. Mueller–Hinton agar plates supplemented with 5% of defibrinated horse blood (Oxoid, UK), and 20 mg/L of β-Nicotinamide Adenine Dinucleotide (β-NAD) (Sigma Aldrich, Saint Louis, MO, USA) were inoculated with the prepared suspension. The selected antimicrobials were in agreement with EUCAST recommendations as crucial in the treatment of Campylobacter infection. The following antibiotic disks were placed on the surface of the dry plates: erythromycin (ERY, 15 µg), gentamicin (GEN, 10 µg), ciprofloxacin (CIP, 5 µg), and tetracycline (TET, 30 µg). The plates were incubated at 41 ± 1 °C for 24–48 h in a microaerophilic atmosphere. Zones of inhibited growth for erythromycin, ciprofloxacin and tetracycline were determined according to EUCAST breakpoints [71] and The Clinical and Laboratory Standards Institute [72] breakpoints were used for gentamicin. The results were interpreted as resistant or sensitive. The inhibition zone readings defined as intermediate were classified as resistant. The strains that showed resistance to no less than three antimicrobial classes were considered multidrug-resistant (MDR) [73].
4.5. Statistical Analysis
Statistical tests were performed using Statistica (StatSoft, version 13.3, Poland). The analyses of the presence of virulence genes and antibiotic resistance profiles were performed using a non-parametric Kruskal–Wallis one-way analysis of variance followed by a non-parametric U Mann–Whitney test for pairwise comparisons. p-Values < 0.05 were considered significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11020113/s1, Table S1: The description of Campylobacter spp. isolates obtained from wildlife waterfowl.
Author Contributions
Conceptualization, B.W. and T.S.; methodology, B.W.; formal analysis, T.S.; investigation, B.W.; writing—original draft preparation, B.W.; writing—review and editing, T.S.; visualization, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education and Science in the framework of a program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12.000.000 PLN.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to samples were taken from hunter-harvested waterfowl accordance with national regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, as they are still used for other research works.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsiodras S., Kelesidis T., Kelesidis I., Bauchinger U., Falagas M.E. Human infections associated with wild birds. J. Infect. 2008;56:83–98. doi: 10.1016/j.jinf.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed K.D., Meece J.K., Henkel J.S., Shukla S.K. Birds, migration and emerging zoonoses: West nile virus, lyme disease, influenza A and enteropathogens. Clin. Med. Res. 2003;1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKinney M.L. Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience. 2002;52:883–890. doi: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2. [DOI] [Google Scholar]
- 4.Atterby C., Ramey A.M., Hall G.G., Järhult J., Börjesson S., Bonnedahl J. Increased prevalence of antibiotic-resistant E. coli in gulls sampled in Southcentral Alaska is associated with urban environments. Infect. Ecol. Epidemiol. 2016;6:32334. doi: 10.3402/iee.v6.32334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith O.M., Snyder W.E., Owen J.P. Are we overestimating risk of enteric pathogen spillover from wild birds to humans? Biol. Rev. 2020;95:652–679. doi: 10.1111/brv.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. [(accessed on 1 November 2021)]. Available online: https://www.ecdc.europa.eu/en/campylobacteriosis.
- 7.Noormohamed A., Fakhr M.K. Prevalence and Antimicrobial Susceptibility of Campylobacter spp. in Oklahoma Conventional and Organic Retail Poultry. Open Microbiol. J. 2014;31:130–137. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon Y.K., Oh J.Y., Jeong O.M., Moon O.K., Kang M.S., Jung B.Y., An B.K., Youn S.Y., Kim H.R., Jang I., et al. Prevalence of Campylobacter species in wild birds South Korea. Avian Pathol. 2017;46:474–480. doi: 10.1080/03079457.2017.1315048. [DOI] [PubMed] [Google Scholar]
- 9.Vogt N.A., Stevens C.P., Pearl D.L., Taboada E.N., Jardine C.M. Generalizability and comparability of prevalence estimates in the wild bird literature: Methodological and epidemiological considerations. Anim. Health Res. Rev. 2020;18:1–7. doi: 10.1017/S1466252320000043. [DOI] [PubMed] [Google Scholar]
- 10.Thomas V.G., Pain D.J., Kanstrup N., Green R.E. Setting maximum levels for lead in game meat in EC regulations: An adjunct to replacement of lead ammunition. Ambio. 2020;49:2026–2037. doi: 10.1007/s13280-020-01336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacomelli M., Andrighetto C., Rossi F., Lombardi A., Rizzotti L., Martini M., Piccirillo A. Molecular characterization and genotypic antimicrobial resistance analysis of Campylobacter jejuni and Campylobacter coli isolated from broiler flocks in northern Italy. Avian Pathol. 2012;41:579–588. doi: 10.1080/03079457.2012.734915. [DOI] [PubMed] [Google Scholar]
- 12.Marotta F., Garofolo G., Di Donato G., Aprea G., Platone I., Cianciavicchia S., Alessiani A., Di Giannatale E. Population diversity of Campylobacter jejuni in poultry and its dynamic of contamination in chicken meat. BioMed Res. Int. 2015;2015:859845. doi: 10.1155/2015/859845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanage W. Attack of the clones: What causes population structure in bacteria and how can we use it?; Proceedings of the APS March Meeting 2020; Denver, CO, USA. 2–6 March 2020. [Google Scholar]
- 14.Reddy S., Zishiri O.T. Genetic characterisation of virulence genes associated with adherence, invasion and cytotoxicity in Campylobacter spp. isolated from commercial chickens and human clinical cases. Onderstepoort J. Vet. Res. 2018;85:e1–e9. doi: 10.4102/ojvr.v85i1.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerry P. Campylobacter flagella: Not just for motility. Trends Microbiol. 2007;15:456–461. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Sierra-Arguello Y.M., Perdoncini G., Rodrigues L.B., dos Santos L.R., Borges K.A., Furian T.G., Salle C.T.P., de Souza Moraes H.L., Gomes M.J.P., do Nascimento V.P. Identification of pathogenic genes in Campylobacter jejuni isolated from broiler carcasses and broiler slaughterhouses. Sci. Rep. 2021;11:4588. doi: 10.1038/s41598-021-84149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause-Gruszczynska M., van Alphen L.B., Oyarzabal O.A., Alter T., Hänel I., Schliephake A., König W., van Putten J.P.M., Konkel M.E., Backert S. Expression patterns and role of the CadF protein in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol. Lett. 2007;274:9–16. doi: 10.1111/j.1574-6968.2007.00802.x. [DOI] [PubMed] [Google Scholar]
- 18.Van der Stel A.X., van Mourik A., Łaniewski P., van Putten J.P., Jagusztyn-Krynicka E.K., Wösten M.M. The Campylobacter jejuni RacRS two-component system activates the glutamate synthesis by directly upregulating γ-glutamyltranspeptidase (GGT) Front. Microbiol. 2015;6:567. doi: 10.3389/fmicb.2015.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendrixson D.R., DiRita V.J. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 2004;52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 20.Gharbi M., Béjaoui A., Ben Hamda C., Ghedira K., Ghram A., Maaroufi A. Distribution of virulence and antibiotic resistance genes in Campylobacter jejuni and Campylobacter coli isolated from broiler chickens in Tunisia. J. Microbiol. Immunol. Infect. 2021 doi: 10.1016/j.jmii.2021.07.001. in press. [DOI] [PubMed] [Google Scholar]
- 21.Pickett C.L. Campylobacter Toxins and Their Role in Pathogenesis. In: Nachamkin I., Blaser M.J., editors. Campylobacter. American Society for Microbiology; Washington, DC, USA: 2000. pp. 179–190. [Google Scholar]
- 22.Guirado P., Paytubi S., Miró E., Iglesias-Torrens Y., Navarro F., Cerdà-Cuéllar M., Attolini C.S., Balsalobre C., Madrid C. Differential Distribution of the wlaN and cgtB Genes, Associated with Guillain-Barré Syndrome, in Campylobacter jejuni Isolates from Humans, Broiler Chickens, and wild birds. Microorganisms. 2020;26:325. doi: 10.3390/microorganisms8030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnedahl J., Järhult J.D. Antibiotic resistance in wild birds. Upsala J. Med. Sci. 2014;119:113–116. doi: 10.3109/03009734.2014.905663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen H.K., Donato J., Wang H.H., Cloud-Hansen K.A., Davies J., Handelsman J. Call of the wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010;8:251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 25.Skurnik D., Ruimy R., Andremont A., Amorin C., Rouquet P., Picard B., Denamur E. Effect of human vicinity on antimicrobial resistance and integrons in animal faecal Escherichia coli. J. Antimicrob. Chemother. 2006;57:1215–1219. doi: 10.1093/jac/dkl122. [DOI] [PubMed] [Google Scholar]
- 26.Elmberg J., Berg C., Lerner H., Waldenström J., Hessel R. Potential disease transmission from wild geese and swans to livestock, poultry and humans: A review of the scientific literature from a One Health perspective. Infect. Ecol. Epidemiol. 2017;7:1300450. doi: 10.1080/20008686.2017.1300450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.French N.P., Midwinter A., Holland B., Collins-Emerson J., Pattison R., Colles F., Carter P. Molecular epidemiology of Campylobacter jejuni isolates from wild-bird fecal material in children’s playgrounds. Appl. Environ. Microbiol. 2009;75:779–783. doi: 10.1128/AEM.01979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramonaitė S., Novoslavskij A., Zakarienė G., Aksomaitienė J., Malakauskas M. High Prevalence and Genetic Diversity of Campylobacter jejuni in Wild Crows and Pigeons. Curr. Microbiol. 2015;71:559–565. doi: 10.1007/s00284-015-0881-z. [DOI] [PubMed] [Google Scholar]
- 29.Havelaar A.H., Mangen M.-J.J., de Koeijer A.A., Bogaardt M.-J., Evers E.G., Jacobs-Reitsma W.E., van Pelt W., Wagenaar J.A., de Wit G.A., van der Zee H., et al. Effectiveness and efficiency of controlling Campylobacter on broiler chicken meat. Risk Anal. 2007;27:831–844. doi: 10.1111/j.1539-6924.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 30.Wysok B., Wojtacka J., Kivistö R. Pathogenicity of Campylobacter strains of poultry and human origin from Poland. Int. J. Food. Microbiol. 2020;334:108830. doi: 10.1016/j.ijfoodmicro.2020.108830. [DOI] [PubMed] [Google Scholar]
- 31.Atterby C., Mourkas E., Méric G., Pascoe B., Wang H., Waldenström J., Sheppard S.K., Olsen B., Järhult J.D., Ellström P. The Potential of Isolation Source to Predict Colonization in Avian Hosts: A Case Study in Campylobacter jejuni Strains from Three Bird Species. Front. Microbiol. 2018;9:591. doi: 10.3389/fmicb.2018.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colles F.M., Ali J.S., Sheppard S.K., McCarthy N.D., Maiden M.C.J. Campylobacter populations in wild and domesticated Mallard ducks (Anas platyrhynchos) Environ. Microbiol. 2011;3:574–580. doi: 10.1111/j.1758-2229.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giannatale E., Garofolo G., Alessiani A., Di Donato G., Candeloro L., Vencia W., Decastelli L., Marotta F. Tracing Back Clinical Campylobacter jejuni in the Northwest of Italy and Assessing Their Potential Source. Front. Microbiol. 2016;7:23. doi: 10.3389/fmicb.2016.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llarena A.-K., Zhang J., Vehkala M., Valimaki N., Hakkinen M., Hänninen M.-L., Roasto M., Mäesaar M., Taboada E.N., Barker D., et al. Monomorphic genotypes within a generalist lineage of Campylobacter jejuni show signs of global dispersion. Microb. Genom. 2016;2:e000088. doi: 10.1099/mgen.0.000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrlich G.D., Hiller N.L., Hu F.Z. What makes pathogens pathogenic. Genome Biol. 2008;9:225. doi: 10.1186/gb-2008-9-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J., Luo J., Huang J., Wang C., Li M., Wang B., Wang B., Chang H., Ji J., Sen K., et al. Emergence of Genetic Diversity and Multi-Drug Resistant Campylobacter jejuni From Wildlife waterfowl in Beijing, China. Front. Microbiol. 2019;29:2433. doi: 10.3389/fmicb.2019.02433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei B., Kang M., Jang H.K. Genetic characterization and epidemiological implications of Campylobacter isolates from wild birds in South Korea. Transbound. Emerg. Dis. 2019;66:56–65. doi: 10.1111/tbed.12931. [DOI] [PubMed] [Google Scholar]
- 38.Shyaka A., Kusumoto A., Asakura H., Kawamoto K. Whole-Genome Sequences of Eight Campylobacter jejuni Isolates from Wild Birds. Genome Announc. 2015;3:e00315-15. doi: 10.1128/genomeA.00315-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieczorek K., Wołkowicz T., Osek J. Antimicrobial Resistance and Virulence-Associated Traits of Campylobacter jejuni Isolated from Poultry Food Chain and Humans with Diarrhea. Front. Microbiol. 2018;9:1508. doi: 10.3389/fmicb.2018.01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wysok B., Wojtacka J. Detection of virulence genes determining the ability to adhere and invade in Campylobacter spp. from cattle and swine in Poland. Microb. Pathog. 2018;115:257–263. doi: 10.1016/j.micpath.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 41.Gahamanyi N., Song D.G., Yoon K.Y., Mboera L.E.G., Matee M.I., Mutangana D., Amachawadi R.G., Komba E.V.G., Pan C.H. Antimicrobial Resistance Profiles, Virulence Genes, and Genetic Diversity of Thermophilic Campylobacter Species Isolated from a Layer Poultry Farm in Korea. Front. Microbiol. 2021;12:554. doi: 10.3389/fmicb.2021.622275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo R.T., Nalevaiko P.C., Mendonça E.P., Borges L.W., Fonseca B.B., Beletti M.E., Rossi D.E. Campylobacter jejuni strains isolated from chicken meat harbour several virulence factors and represent a potential risk to humans. Food Control. 2013;33:227–231. doi: 10.1016/j.foodcont.2013.02.032. [DOI] [Google Scholar]
- 43.Biswas D., Hannon S.J., Townsend H.G., Potter A., Allan B.J. Genes coding for virulence determinants of Campylobacter jejuni in human clinical and cattle isolates from Alberta, Canada, and their potential role in colonization of poultry. Int. Microbiol. 2011;14:25–32. doi: 10.2436/20.1501.01.132. [DOI] [PubMed] [Google Scholar]
- 44.Zhang T., Luo Q., Chen Y., Li T., Wen G., Zhang R., Luo L., Lu Q., Ai D., Wang H., et al. Molecular epidemiology, virulence determinants and antimicrobial resistance of Campylobacter spreading in retail chicken meat in Central China. Gut Pathog. 2016;8:48. doi: 10.1186/s13099-016-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bang D.D., Nielsen E.M., Scheutz F., Pedersen K., Handberg K., Madsen M. PCR detection of seven virulence and toxin genes of Campylobacter jejuni and Campylobacter coli isolates from Danish pigs and cattle and cytolethal distending toxin production of the isolates. J. Appl. Microbiol. 2003;94:1003–1014. doi: 10.1046/j.1365-2672.2003.01926.x. [DOI] [PubMed] [Google Scholar]
- 46.González-Hein G., Huaracán B., García P., Figueroa G. Prevalence of virulence genes in strains of Campylobacter jejuni isolated from human, bovine and broiler. Braz. J. Microbiol. 2014;44:1223–1229. doi: 10.1590/S1517-83822013000400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tracz D.M., Keelan M., Ahmed-Bentley J., Gibreel A., Kowalewska-Grochowska K., Taylor D.E. pVir and bloody diarrhea in Campylobacter jejuni enteritis. Emerg. Infect. Dis. 2005;11:838–843. doi: 10.3201/eid1106.041052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanad Y.M., Kassem I.I., Abley M., Gebreyes W., LeJeune J.T., Rajashekara G. Genotypic and Phenotypic Properties of Cattle-Associated Campylobacter and Their Implications to Public Health in the USA. PLoS ONE. 2011;6:e25778. doi: 10.1371/journal.pone.0025778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carvalho A.F., Silva D.M., Azevedo S.S., Piatti R.M., Genovez M.E., Scarcelli E. Detection of CDT toxin genes in Campylobacter spp. strains isolated from broiler carcasses and vegetables in São Paulo, Brazil. Braz. J. Microbiol. 2014;44:693–699. doi: 10.1590/S1517-83822013000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wysok B., Uradziński J., Wojtacka J. Determination of the cytotoxic activity of Campylobacter strains isolated from bovine and swine carcasses in north-eastern Poland. Pol. J. Vet. Sci. 2015;18:579–586. doi: 10.1515/pjvs-2015-0075. [DOI] [PubMed] [Google Scholar]
- 51.Cho H.H., Kim S.H., Min W., Ku B.K., Kim Y.H. Prevalence of virulence and cytolethal distending toxin (CDT) genes in thermophilic Campylobacter spp. from dogs and humans in Gyeongnam and Busan, Korea. KJVR Korean J. Vet. Res. 2014;54:39–48. doi: 10.14405/kjvr.2014.54.1.39. [DOI] [Google Scholar]
- 52.Ang C.W., De Klerk M.A., Endtz H.P., Jacobs B.C., Laman J.D., van der Meche F.G.A., van Doorn P.A. Guillain-Barre syndrome- and Miller Fisher syndrome-associated Campylobacter jejuni lipopolysaccharides induce anti-GM1 and anti-GQ1b antibodies in rabbits. Infect. Immun. 2001;69:2462–2469. doi: 10.1128/IAI.69.4.2462-2469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Datta S., Niwa H., Itoh K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003;52:345–348. doi: 10.1099/jmm.0.05056-0. [DOI] [PubMed] [Google Scholar]
- 54.Wysok B., Wojtacka J., Wiszniewska-Łaszczych A., Szteyn J. Antimicrobial Resistance and Virulence Properties of Campylobacter Spp. Originating from Domestic Geese in Poland. Animals. 2020;10:742. doi: 10.3390/ani10040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casabonne C., Gonzalez A., Aquili V., Subils T., Balague C. Prevalence of Seven Virulence Genes of Campylobacter jejuni Isolated from Patients with Diarrhea in Rosario, Argentina. Int. J. Infect. 2016;3:e37727. doi: 10.17795/iji-37727. [DOI] [PubMed] [Google Scholar]
- 56.Oporto B., Juste R.A., López-Portolés J.A., Hurtado A. Genetic diversity among Campylobacter jejuni isolates from healthy livestock and their links to human isolates in Spain. Zoonoses Public Health. 2011;58:365–375. doi: 10.1111/j.1863-2378.2010.01373.x. [DOI] [PubMed] [Google Scholar]
- 57.Ma F., Xu S., Tang Z., Li Z., Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Bios. Health. 2021;3:32–38. doi: 10.1016/j.bsheal.2020.09.004. [DOI] [Google Scholar]
- 58.Wieczorek K., Osek J. Antimicrobial resistance mechanisms among Campylobacter. BioMed Res. Int. 2013;2013:340605. doi: 10.1155/2013/340605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hull D.M., Harrell E., van Vliet A.H.M., Correa M., Thakur S. Antimicrobial resistance and interspecies gene transfer in Campylobacter coli and Campylobacter jejuni isolated from food animals, poultry processing, and retail meat in North Carolina, 2018-2019. PLoS ONE. 2021;16:e0246571. doi: 10.1371/journal.pone.0246571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Fernández A., Dionisi A.M., Arena S., Iglesias-Torrens Y., Carattoli A., Luzzi I. Human Campylobacteriosis in Italy: Emergence of Multi-Drug Resistance to Ciprofloxacin, Tetracycline, and Erythromycin. Front. Microbiol. 2018;9:1906. doi: 10.3389/fmicb.2018.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marotta F., Garofolo G., Di Marcantonio L., Di Serafino G., Neri D., Romantini R., Sacchini L., Alessiani A., Di Donato G., Nuvoloni R., et al. Correction: Antimicrobial resistance genotypes and phenotypes of Campylobacter jejuni isolated in Italy from humans, birds from wild and urban habitats, and poultry. PLoS ONE. 2019;14:e0225231. doi: 10.1371/journal.pone.0225231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wieczorek K., Osek J. Multiplex PCR assays for simultaneous identification of Campylobacter jejuni and Campylobacter coli. Med. Weter. 2005;61:797–799. [Google Scholar]
- 63.Meinersmann R.J., Helsel L.O., Fields P.I., Hiett K.L. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J. Clin. Microbiol. 1997;35:2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Müller J., Schulze F., Müller W., Hänel I. PCR detection of virulence-associated genes in Campylobacter jejuni strains with differential ability to invade Caco-2 cells and to colonize the chick gut. Vet. Microbiol. 2006;13:123–129. doi: 10.1016/j.vetmic.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 65.Chansiripornchai N., Sasipreeyajan J. PCR detection of four virulence-associated genes of Campylobacter jejuni isolates from Thai broilers and their abilities of adhesion to and invasion of INT-407 cells. J. Vet. Med. Sci. 2009;71:839–844. doi: 10.1292/jvms.71.839. [DOI] [PubMed] [Google Scholar]
- 66.Konkel M.E., Kim B.J., Rivera-Amill V., Garvis S.G. Identification of proteins required for the internalization of Campylobacter jejuni into cultured mammalian cells. Adv. Exp. Med. Biol. 1999;473:215–224. doi: 10.1007/978-1-4615-4143-1_22. [DOI] [PubMed] [Google Scholar]
- 67.Carvalho A.C., Ruiz Palacios G.M., Ramos Cervantes P., Cervantes L.E., Jiang X., Pickering L.K. Molecular characterization of invasive and noninvasive Campylobacter jejuni and Campylobacter coli isolates. J. Clin. Microbiol. 2001;39:1353–1359. doi: 10.1128/JCM.39.4.1353-1359.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linton D., Gilbert M., Hitchen P.G., Dell A., Morris H.R., Wakarchuk W.W., Gregson N.A., Wren B.W. Phase variation of a beta-1,3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 2000;37:501–514. doi: 10.1046/j.1365-2958.2000.02020.x. [DOI] [PubMed] [Google Scholar]
- 69.Hunter P.R., Gaston M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpson’s index of diversity. J. Clin. Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carriço J.A., Silva-Costa C., Melo-Cristino J., Pinto F.R., de Lencastre H., Almeida J.S., Ramirez M. Illustration of a common framework for relating multiple typing methods by application to macrolide-resistant Streptococcus pyogenes. J. Clin. Microbiol. 2006;44:2524–2532. doi: 10.1128/JCM.02536-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.EUCAST European Committee on Antimicrobial Susceptibility Testing. [(accessed on 1 November 2021)]. Available online: http://www.eucast.org/clinical_breakpoints.
- 72.CLSI . Methods for Antimicrobial Dilution and Disc Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. CLSI Guideline M45-A3. [DOI] [PubMed] [Google Scholar]
- 73.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, as they are still used for other research works.