Abstract
The development of new antibiotics to treat multidrug-resistant (MDR) bacteria or possess broad-spectrum activity is one of the challenging tasks. Unfortunately, there are not many new antibiotics in clinical trials. So, the molecular hybridization approach could be an effective strategy to develop potential drug candidates using the known scaffolds. We synthesized a total of 31 diverse linezolid conjugates 3, 5, 7, 9, 11, 13, and 15 using our established benzotriazole chemistry with good yield and purity. Some of the synthesized conjugates exhibited promising antibacterial properties against different strains of bacteria. Among all the synthesized compounds, 5d is the most promising antibacterial agent with MIC 4.5 µM against S. aureus and 2.25 µM against B. subtilis. Using our experimental data pool, we developed a robust QSAR (R2 = 0.926, 0.935; R2cvOO = 0.898, 0.915; R2cvMO = 0.903, 0.916 for the S. aureus and B. subtilis models, respectively) and 3D-pharmacophore models. We have also determined the drug-like properties of the synthesized conjugates using computational tools. Our findings provide valuable insight into the possible linezolid-based antibiotic drug candidates.
Keywords: linezolid, conjugates, antibacterial, QSAR, pharmacophore
1. Introduction
Antibiotics are among the most clinically used drugs for the treatment of various serious diseases in humans [1,2]. Due to overuse and abuse of antibiotics under the influence of various man-made and external factors, the drug resistance for both Gram-positive and Gram-negative bacteria is increasing at an alarming rate [3,4,5]. Today, MDR has attracted increasing attention because of the recent statistics reported by the World Health Organization (WHO). According to the WHO, around 50,000 patients die from infectious diseases every day worldwide [6]. Oxazolidinones are a new class of antimicrobial agents that have a unique structure and show good activity against different pathogenic bacteria, including methicillin- and vancomycin-resistant staphylococci, vancomycin-resistant enterococci, penicillin-resistant pneumococci, and anaerobes [7]. Oxazolidinone antibacterial agents are new synthetic antibacterial agents that have been explored after sulfonamides and quinolones [8]. 5-(Aminomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one (Linezolid) can be considered as the first member of the class of oxazolidinone antibiotics. Linezolid’s mode of action is to prevent the synthesis of bacterial protein via binding to rRNA on both the 30S and 50S ribosomal subunits [9]. It inhibits the formation of the initiation complex, which can reduce the length of the developed peptide chains and decrease the rate of the translation reaction [9]. Because of the unique site of inhibition, cross-resistance to other protein synthesis inhibitors has not yet been demonstrated [10]. Linezolid may also prevent the expression of virulence elements, leading to decreased toxins produced by Gram-positive pathogens [11].
Although linezolid was approved by the US Food and Drug Administration in 2000 as an antibiotic, oxazolidinones are considered a potential building block in the development of drug candidates for the last 40 years [12,13]. Despite extensive efforts that have been undertaken to synthesize more potential and safer analogs, linezolid remained the only FDA-approved antibiotic in this class for more than 20 years. Currently, there are several oxazolidinones in the process of clinical trials [14].
The ideal goal is to develop a new potential antibiotic that should show broad-spectrum effect, enhanced efficacy, a better safety profile, and the possibility for use by multiple routes of administration. To achieve the desired target, molecular hybridization is one of the attractive strategies.
Molecular hybridization is about modifying the parent structural motifs to derive an array of significantly potent candidates with minimal toxic or side effects and to avoid any possible drug resistance development. This approach seemed attractive because it does not require the discovery of new antibacterial scaffolds or validation of novel biological targets, which has proven to be an extremely difficult and time-consuming task.
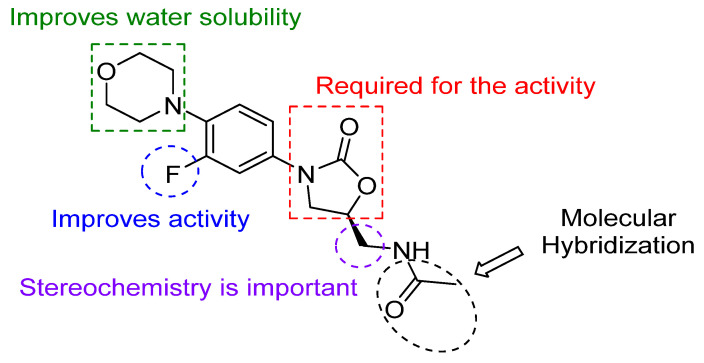
In continuation to our current efforts in drug development [15,16,17,18,19,20], we utilized a molecular modification approach for enhancing the drug-like properties of linezolid; herein, we synthesized a series of novel linezolid conjugates via NH2 acylation of de-acetyl linezolid 1 using a series of different benzotriazole-activated molecules. Recently, Rahman and coworkers reported the synthesis of linezolid analogs with different C5-acylamino substituents using EDC and DMAP as coupling reagents [21]. The reported method gave moderate yield after HPLC purification. Our method of N-acylation using benzotriazole chemistry [22] has several advantages, such as quantitative yield and high purity without the use of column chromatography. In this present work, we did not make any change to the essential components of the linezolid structure (Figure 1). We introduced amino acids as part of the conjugates, as amino acids are well known for improving the antibacterial property and cell permeability [23,24,25,26].
Figure 1.
Structure of Linezolid and its important scaffolds.
Moreover, acylation using different benzotriazole-activated aromatic acids and alkylation using a series of substituted benzyl halides, and 3-nitroxyl propyl bromide have been carried out to study the structure-activity relationship. On the other hand, the reaction of deacetyl linezolid 1 with 3-bromopropyl nitrate is supposed to produce a NO-releasing compound, which acts as a delivery system of NO that plays an integral role in defending against a wide range of pathogens [27] and results in synergizing the deacetyl linezolid antimicrobial effect.
2. Results and Discussion
2.1. Chemistry
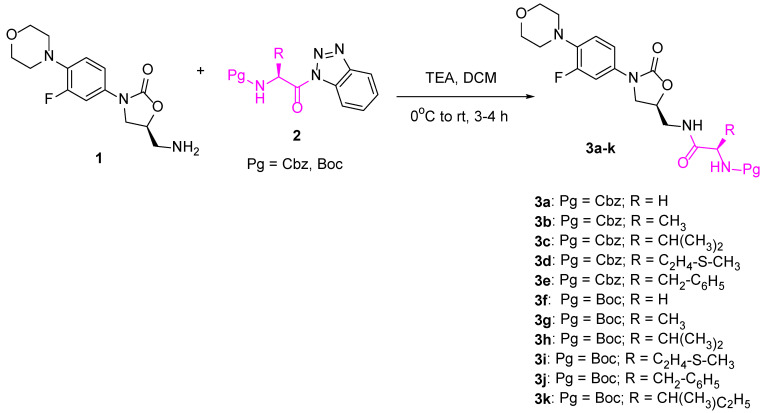
The current study is focused on synthesizing some linezolid conjugates 3a–k by reacting linezolid 1 with benzotriazole-activated protected amino acids 2a–k in dichloromethane (DCM) containing triethylamine at 0 °C for 3–4 h (Scheme 1).
Scheme 1.
Synthesis of linezolid-amino acid conjugates 3a–k.
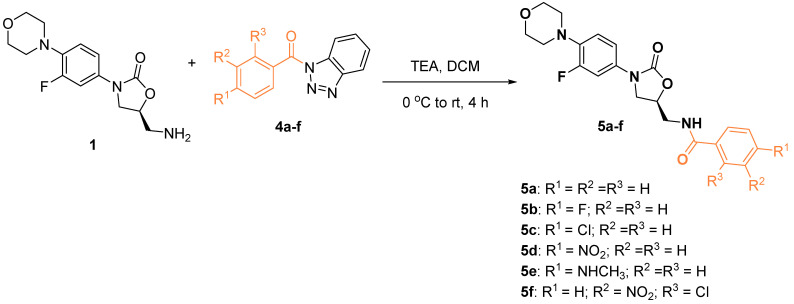
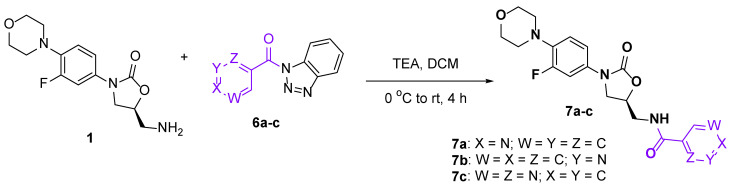
To diversify the pool of conjugates and study the structure-activity relationship, we have prepared the linezolid conjugates with substituted aromatic and heteroaromatic acids using our optimized benzotriazole chemistry (Scheme 2 and Scheme 3).
Scheme 2.
Synthesis of linezolid-aromatic acid conjugates 5a–f.
Scheme 3.
Synthesis of linezolid-heteroaromatic acid conjugates 7a–c.
Compound 5a was prepared from benzoyl benzotriazole in an excellent yield (98%) compared with the reported yield of the same compound (54%) using benzoyl chloride [28]. Moreover, compound 5c was prepared in yield 30% using 4-chlorobenzoyl chloride, which reflects the advantage of utilizing benzotriazole chemistry in obtaining pure products in high yields.
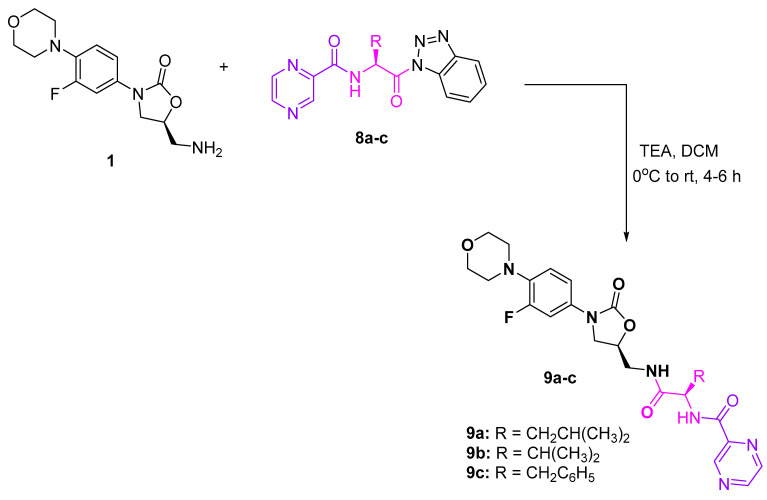
To synthesize a set of hybrid conjugates of linezolid, amino acid, and heteroaromatic acid, we treated benzotriazole-activated pyrazinoic acid-amino acid conjugate 8a–c (which we reported in our previous report [19]) in DCM in the presence of TEA at 0 °C for 4–6 h (Scheme 4).
Scheme 4.
Synthesis of linezolid hybrid conjugates 9a–c.
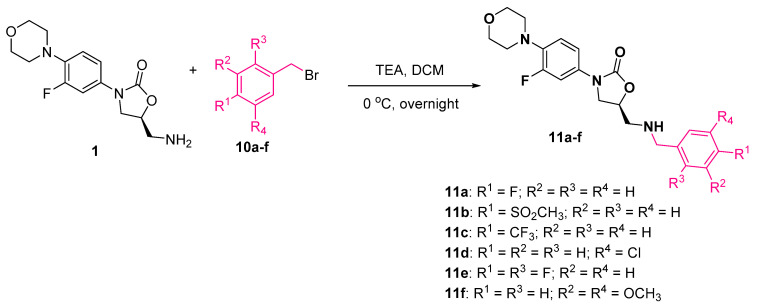
Further, we treated de-acetyl linezolid 1 with substituted aryl bromides 10a–f in DCM in presence of TEA at 0 °C overnight. The desired conjugates 11a–f were isolated in good yields after purification using column chromatography (Scheme 5).
Scheme 5.
Synthesis of linezolid conjugates 11a–f.
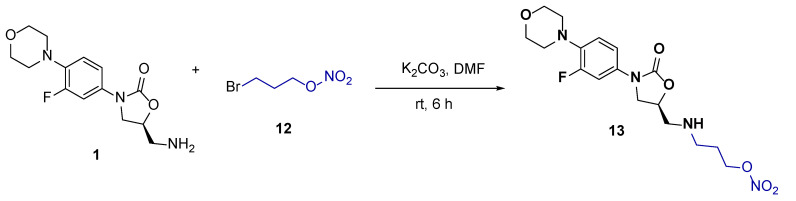
To introduce the nitrogen-releasing component in the linezolid conjugate, we reacted linezolid 1 with 3-bromopropyl nitrate 12 in DMF in the presence of potassium carbonate (K2CO3) at room temperature for 6 h (Scheme 6).
Scheme 6.
Synthesis of linezolid conjugates with 3-bromopropyl nitrate.
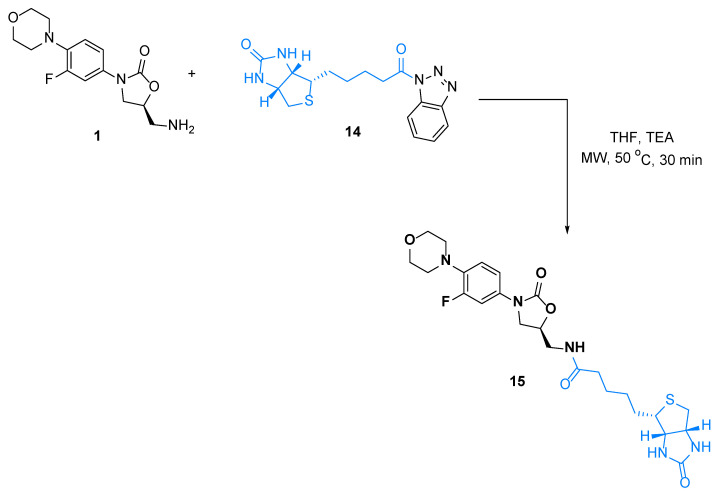
Linezolid is known as a highly tolerated antibiotic and is used for various complicated infections. This drug has lots of potentials; to better understand the role of this drug in various treatments, we coupled linezolid with biotin. Biotin is usually used as a marker to unfold the mode of action of drugs. We treated de-acetyl linezolid 1 with benzotriazole activated biotin 14 [23] in THF under microwave irradiation in the presence of TEA at 50 °C for 30 min to obtain the desired conjugate 15 in good yield (Scheme 7). We tried this reaction both in conventional heating and microwave heating, we found that the use of microwave gave us better yield with high purity.
Scheme 7.
Synthesis of linezolid conjugates with biotin.
2.2. Biological Studies
2.2.1. Antimicrobial Studies
The synthesized linezolid conjugates (3a–k, 5a–f, 7a–c, 9a–c, 11a–f, 13, and 15) were subjected for antimicrobial properties determination against Gram-positive (Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6633) and Gram-negative (Pseudomonas aeruginosa ATCC 15692, Escherichia coli ATCC 47076) bacteria by the standard technique [29,30]. From the antimicrobial properties revealed (Table 1), it is noticeable that the synthesized agents still possess better activity towards the tested Gram-positive bacteria than the Gram-negative ones in similar behavior to their parent antibiotic (standard reference, linezolid). It has also been noticed that compound 5d (R1 = NO2, R2 = R3 = H) is the most effective agent prepared with comparable potency against S. aureus relative to the parent antibiotic (MIC = 4.5, 5.929 μM for 5d and linezolid, respectively). Considerable antimicrobial properties were also noticed by compounds 5a, 5b, and 5e against S. aureus (MIC = 9.336–10.015 μM).
Table 1.
Antimicrobial properties of the tested compounds.
| Entry | Compd. | MIC, μg/mL ± SE (μmol) | |||
|---|---|---|---|---|---|
| S. Aureus (ATCC 6538) | B. Subtilis (ATCC 6633) | P. Aeruginosa (ATCC 15692) | E. Coli (ATCC 47076) | ||
| 1 | 3a | 16 ± 2.1 (32.888) | 4 ± 0.3 (8.222) | 64 ± 1.4 (131.552) | 32 ± 1.0 (65.776) |
| 2 | 3b | 32 ± 1.5 (63.932) | 32 ± 2.1 (63.932) | 64 ± 1.9 (127.864) | 32 ± 2.5 (63.932) |
| 3 | 3c | 32 ± 1.1 (60.540) | 32 ± 2.7 (60.540) | 64 ± 2.9 (121.079) | 32 ± 1.6 (60.540) |
| 4 | 3d | 32 ± 1.6 (57.078) | 32 ± 2. 4 (57.078) | 64 ± 3.1(114.155) | 32 ± 2.0 (57.078) |
| 5 | 3e | 32 ± 2.2 (55.495) | 32 ± 1.9 (55.495) | 64 ± 2.1 (110.990) | 32 ± 1.0 (55.495) |
| 6 | 3f | 32 ± 1.4 (70.721) | 8 ± 0.4 (17.680) | 32 ± 1.6 (70.721) | 32 ± 2.8 (70.721) |
| 7 | 3g | 32 ± 1.5 (68.594) | 32 ± 2.4 (68.594) | 32 ± 1.2 (68.594) | 32 ± 2.7 (68.594) |
| 8 | 3h | 32 ± 1.3 (64.704) | 32 ± 0.7 (64.704) | 32 ± 0.5 (64.704) | 32 ± 1.1 (64.704) |
| 9 | 3i | 32 ± 2.1 (60.765) | 32 ± 1.4 (60.765) | 32 ± 0.8 (60.765) | 32 ± 0.5 (60.765) |
| 10 | 3j | 64 ± 2.5 (117.948) | 32 ± 1.3 (58.974) | 64 ± 1.8 (117.948) | 32 ± 0.9 (58.974) |
| 11 | 3k | 32 ± 2.1 (62.919) | 32 ± 1.3 (62.919) | 32 ± 1.3 (62.919) | 32 ± 2.1 (62.919) |
| 12 | 5a | 4 ± 0.2 (10.015) | 1 ± 0.1 (2.504) | 32 ± 0.8 (80.116) | 32 ± 1.6 (80.116) |
| 13 | 5b | 4 ± 0.1 (9.583) | 1 ± 0.1 (2.396) | 32 ± 1.2 (76.663) | 32 ± 2.4 (76.663) |
| 14 | 5c | 8 ± 0.3 (18.439) | 2 ± 0.1 (4.610) | 32 ± 1.9 (73.757 | 32 ± 0.7 (73.757) |
| 15 | 5d | 2 ± 0.1 (4.500) | 1 ± 0.1 (2.250) | 32 ± 1.6 (72.004) | 32 ± 2.2 (72.004) |
| 16 | 5e | 4 ± 0.2 (9.336) | 1 ± 0.1 (2.334) | 32 ± 2.2 (74.686) | 32 ± 1.6 (74.686) |
| 17 | 5f | 8 ± 0.2 (16.706) | 4 ± 0.3 (8.353) | 32 ± 1.4 (66.825) | 32 ± 0.6 (66.825) |
| 18 | 7a | 8 ± 0.3 (19.980) | 2 ± 0.2 (4.995) | 8 ± 0.1 (19.980) | 32 ± 0.9 (79.918) |
| 19 | 7b | 8 ± 0.2 (19.980) | 2 ± 0.1 (4.995) | 32 ± 1.2 (79.918) | 32 ± 1.0 (79.918) |
| 20 | 7c | 8 ± 0.1 (19.930) | 2 ± 0.1 (4.983) | 32 ± 1.1 (79.721) | 32 ± 0.7 (79.721) |
| 21 | 9a | 32 ± 0.4 (62.189) | 32 ± 2.0 (62.189) | 32 ± 1.3 (62.189) | 32 ± 1.6 (62.189) |
| 22 | 9b | 32 ± 1.7 (63.932) | 32 ± 1.3 (63.932) | 32 ± 2.5 (63.932) | 32 ± 3.4 (63.932) |
| 23 | 9c | 32 ± 0.9 (58.332) | 32 ± 0.5 (58.332) | 32 ± 0.8 (58.332) | 32 ± 1.0 (58.332) |
| 24 | 11a | 32 ± 2.7 (79.320) | 32 ± 1.2 (79.320) | 32 ± 2.2 (79.320) | 32 ± 2.5 (79.320) |
| 25 | 11b | 32 ± 1.2 (69.037) | 32 ± 1.4 (69.037) | 64 ± 2.8 (138.074) | 32 ± 1.1 (69.037) |
| 26 | 11c | 32 ± 0.8 (70.572) | 16 ± 0.4 (35.286) | 64 ± 1.5 (141.143) | 32 ± 0.9 (70.572) |
| 27 | 11d | 32 ± 1.0 (76.212) | 32 ± 1.2 (76.212) | 32 ± 1.7 (76.212) | 32 ± 2.1 (76.212) |
| 28 | 11e | 32 ± 0.2 (75.934) | 32 ± 1.3 (75.934) | 32 ± 0.5 (75.934) | 32 ± 0.3 (75.934) |
| 29 | 11f | 32 ± 1.7 (71.831) | 32 ± 0.3 (71.831) | 32 ± 1.9 (71.831) | 32 ± 0.8 (71.831) |
| 30 | 13 | 32 ± 1.0 (80.323) | 32 ± 0.9 (80.323) | 32 ± 1.5 (80.323) | 32 ± 0.6 (80.323) |
| 31 | 15 | 32 ± 0.4 (61.349) | 32 ± 0.2 (61.349) | 32 ± 0.3 (61.349) | 32 ± 0.7 (61.349) |
| 32 | Linezolid | 2 ± 0.1 (5.929) | 0.25 ± 0.01 (0.741) | 32 ± 0.7 (94.857) | 8 ± 0.2 (23.714) |
| 33 | Ciprofloxacin [24] | 1250.0 ± 9.6 (3772.4) | NT | 4.8 ± 0.5 (14.5) | NT |
| 34 | Norfloxacin [24] | 1250.0 ± 14.2 (3914.3) | NT | 4.8 ± 0.2 (15.0) | NT |
NT = not tested.
Compound 5d also exhibited promising antimicrobial properties against B. subtilis (MIC = 2.25 μM). Similar observations were also noticed by compounds 5a, 5b and 5e (MIC = 2.334–2.504 μM). Compounds 5c, 7a–c also showed considerable antimicrobial properties against B. subtilis (MIC = 4.61–4.995 μM).
Linezolid is an effective antibiotic against Gram-positive bacteria; however, some of the synthesized agents exhibited antimicrobial properties against P. aeruginosa (Gram-negative bacteria), with slightly enhanced efficacy compared to that of the parent. Compound 7a was the most effective agent prepared with moderate antimicrobial properties revealed against P. aeruginosa (MIC = 19.98, 94.857 μM for 7a and linezolid, respectively). Most of the synthesized agents reveal mild properties against P. aeruginosa (MIC = 58.332–80.323 μM).
Based on the antimicrobial properties observed, some SAR (structure-activity relationships) were noticed. Generally, the synthesized agents 5 and 7 were the most effective conjugates synthesized against the Gram-positive bacteria tested. In other words, attachment of either aromatic or heteroaromatic acids with linezolid can afford a promising antimicrobial agent. Attachment of an electron-withdrawing group to the aroyl fragment enhanced the antimicrobial properties observed, as shown by compounds 5d and 5b “possessing p-nitro and p-fluorobenzoyl substituent, respectively” (MIC = 4.5, 9.583; 2.25, 2.396 μM for compounds 5d and 5b against S. aureus and B. subtilis, respectively). The π-deficient heterocycle (pyridinyl and pyrazinyl) “which possessed electron-withdrawing effects” also enhanced the revealed antimicrobial properties against the Gram-positive bacteria, as revealed by compounds 7a–c (MIC = 19.93–19.98; 4.983–4.995 μM for compounds 7a–c against S. aureus and B. subtilis, respectively).
2.2.2. Cytotoxicity Studies
Safety profile against the RPE1 (human immortalized retinal pigment epithelial cell line) was investigated, along with the antiproliferation activity studies of the synthesized compounds by following the standard MTT bioassay. None of the synthesized agents showed any signs of toxicity towards the tested cell line up to 100 µM (Supplementary Figure S1).
2.3. Computational Studies
2.3.1. QSAR Studies
Physico-chemical properties (descriptors) can determine the correlation between the biological/antimicrobial properties and chemical entities quantitatively in mathematical equations expressed in the QSAR model, which can determine the most important features needed for the antimicrobial properties [31,32].
2.3.2. S. Aureus
The four-descriptor model describes the antimicrobial properties of the tested compounds against S. aureus with a broad range of biological observations expressed in log (MIC, μM) values “observed log (MIC, μM) = 0.653–2.072, predicted log (MIC, μM) = 0.793–2.057 i.e., observed (MIC, μM) = 4.5–117.948, predicted (MIC, μM) = 6.212–114.088”. The correlation coefficient values support the accuracy/goodness of the model (R2 = 0.926, R2cvOO = 0.898, R2cvMO = 0.903) (Supplementary Tables S1–S3, Figure S2). The partial surface area for atom H is a charge-related descriptor with a high criterion value among the other model’s descriptors (t = 7.89) with a high mathematically coefficient value (3.29174). This is an indication of the low antimicrobial efficacy of an agent with a high mathematical descriptor value and vice versa, as shown by compounds 5d and 11f (descriptor value = 0.465, 0.640; predicted MIC = 6.212, 114.088 μM for 5d and 11f, respectively). The partial positively (PPSA1)/negatively (PNSA1) charged surface area can be determined by the Equations (1) and (2) [33].
| (1) |
| (2) |
SA is either the positively or negatively charged solvent accessible surface area.
The difference (DPSA) between the total partial charged positive and negative surface areas is also a charge-related descriptor (t = 6.954). Although its low coefficient value (0.00087934) and its high mathematical value affords capability for controlling the estimated antimicrobial observations as exhibited in compounds 3e and 5d (descriptor value = 1308.602, 847.5723 corresponding to estimated MIC = 49.005, 6.212 μM for compounds 3e and 5d, respectively). The descriptor value can be calculated by Equation (3) [33].
| (3) |
PPSA2 and PNSA2 stand for the total weighted partial charge of the positively and negatively charged surface areas, respectively.
The highest occupied molecular orbital (HOMO) energy is also a semi-empirical descriptor with a negative coefficient value (−0.426925). Due to the negative mathematical value of the descriptor energy, the synthesized agent with high mathematical descriptor value turns a potent antimicrobial property and was revealed by compounds 5d and 7a (descriptor value = −8.532, −9.02 corresponding to an estimated MIC = 6.212, 19.693 μM for compounds 3e and 5d, respectively). The descriptor value can be calculated by Equation (4) [33].
| (4) |
since stands for the Fock operator.
The maximum e-e repulsion for bond C-N is also a semi-empirical descriptor with a negative coefficient value (−0.475568). This is why the synthesized agent with a high mathematical descriptor value optimizes a potent antimicrobial active agent, as shown in compounds 3d and 5e (descriptor value = 165.9557, 166.9239 corresponding to an estimated MIC = 84.064, 9.498 μM for compounds 3d and 5e, respectively). The descriptor value can be calculated by Equation (5) [33].
| (5) |
since A and B are two different atomic species. The Pμν, Pλσ are the density matrix elements over the atomic basis {μνλσ}.
The ⟨μν│λσ⟩ are the electron repulsion integrals on the atomic basis {μνλσ}.
2.3.3. B. Subtilis
The three-descriptor model describes the broad-range antimicrobial properties of the synthesis agents against B. subtilis (observed log (MIC, μM) = 0.352–1.905, predicted log (MIC, μM) = 0.47–1.99, i.e., observed (MIC, μM) = 2.25–80.323, predicted (MIC, μM) = 2.953–97.751). The coefficient values support the QSAR goodness (R2 = 0.935, R2cvOO = 0.915, R2cvMO = 0.916) (Supplementary Tables S4–S6, Figure S3). The semi-empirical descriptor and minimum total interaction for the H-C (t = −3.867) bond appeared with a negative sign in the QSAR model (coefficient = −2.09499). This explains the high estimated antimicrobial properties of the compounds with a high mathematical descriptor value, as revealed in compounds 3d and 5b (descriptor value = 11.8739, 12.0794, corresponding to an estimated MIC = 97.751, 2.953 μM for compounds 3d and 5b, respectively). Equation (6) can calculate the total interaction energy of two atoms [33].
| (6) |
where A and B are the two different atoms. The EC (AB) is electrostatic interaction energy between the two atomic species A and B. The Eexc (AB) is electronic exchange energy between the two atomic species A and B.
The minimum total interaction for bond C-C is also a semi-empirical descriptor with a negative coefficient value (−2.13915). Again, the higher the mathematical descriptor value, the higher potency of the constructed agent, as shown in compounds 3d and 5b (descriptor value = 13.2499, 13.6499 corresponding to an estimated MIC = 97.751, 2.953 μM for compounds 3d and 5b, respectively). Equation (6) can also calculate the descriptor value [33].
The semi-empirical descriptor maximum e-e repulsion for N also appeared with a negative sign in the QSAR model. Similar to the aforementioned, the high antimicrobial properties of 3d over 5b can be justified (descriptor value = 143.4363, 144.2946 corresponding to estimated MIC = 97.751, 2.953 μM for compounds 3d and 5b, respectively). Equation (7) can calculate the electron–electron repulsion energy of an atom [33].
| (7) |
since A and B are two different atomic species. The Pμν, Pλσ are the density matrix elements over the atomic basis {μνλσ}.
The ⟨μν│λσ⟩ are the electron repulsion integrals on the atomic basis {μνλσ}.
The comparable predicted antimicrobial properties relative to the observed, especially for the potent analogs, is a good indication for the accuracy of the QSAR models. The statistical parameters, including the Fisher criteria (F) and standard deviation (s), are also good indications for the QSAR goodness (F = 81.973, 129.818; s2 = 0.010, 0.024 for the S. aureus and B. subtilis models, respectively). The comparable values of leave-one-out and leave-many-out modifications to the QSAR model coefficient value are also good evidence for the accuracy of the models (R2 = 0.926, 0.935; R2cvOO = 0.898, 0.915; R2cvMO = 0.903, 0.916 for the S. aureus and B. subtilis models, respectively).
2.4. Pharmacophoric Modeling
Pharmacophoric modeling is an accessible technique in medicinal chemistry that is usually expressed in various steric or electrostatic features (positive/negative ionizable, hydrogen bonding donor/acceptor, and hydrophobic) [34,35].
2.4.1. S. Aureus
Three chemical feature models were observed for the 3D-pharmacophoric model of the synthesized agents with variable antimicrobial properties against S. aureus, including a hydrogen-bonding donor and two hydrophobics (Supplementary Table S7, Figures S4 and S5). It is noticeable that the estimated antimicrobial properties were comparable with the observed properties, preserving the potency, especially for the highly effective agents discovered. It has also been noticed that all the synthesized agents (compound 5e is an exception) show the alignment of the morpholinyl and phenyl group attached to the oxazolidinyl N with the hydrophobics. However, compound 5e shows an alignment of the methylphenyl group with the hydrophobic, while the hydrogen bonding acceptor function is aligned with the exocyclic amidic carbonyl. It is also noticeable that all the potent, and most of the mild, antimicrobial agents synthesized reveal the alignment of the oxazolidinyl carbonyl with the hydrogen-bonding acceptor function. However, the low antimicrobial agents show the alignment of the hydrogen-bonding function with the exocyclic amidic carbonyl. In other words, the fitness of the proper group in the hydrogen bonding acceptor function can predicate the potency of the constructed agent. This supports the aforementioned SAR describing the role of phenyl substituted with the electron-withdrawing group and the π-deficient heterocycles in enhancing the antimicrobial properties observed. This is rationalized due to the electronic effect transfer giving rise to better accessibility to the oxazolidinyl carbonyl for interacting/alignment with the pharmacophoric hydrogen-bonding acceptor function.
2.4.2. B. Subtilis
Four chemical features were observed by the pharmacophoric model of the antimicrobial agents against B. subtilis, comprising three hydrogen-bonding acceptors and a hydrophobic. All the tested agents show the alignment of the morpholinyl oxygen and oxazolidinyl carbonyl with hydrogen-bonding acceptors. Additionally, the phenyl group attached to the oxazolidinyl N is aligned with the hydrophobic. These observations are similar to what was revealed in the pharmacophoric model by most of the tested agents against S. aureus. It is noticeable that the hydrogen bonding acceptor (HBA-1) is aligned with the group linked to the oxazolindinyl C-5. Variation of this group, due to the diversity in chemical function utilized, led to variable fitness and, consequently, variability in estimated bio-properties. This, again, can support the aforementioned SAR (Supplementary Table S8, Figures S6 and S7).
2.4.3. ADMET
Computational ADMET (absorption, distribution, metabolism, excretion, and toxicity) “Discovery Studio 2.5 software” [36] exhibits that the aqueous solubility of the synthesized agents ranged from good to low level (aqueous solubility level: 2–3). This is due to the heterocyclic scaffold of the synthesized agents. Most of the synthesized agents revealed good intestinal absorption (intestinal absorption level = 0). Additionally, all the synthesized conjugates 5a–f and 7a–c, show promising antimicrobial properties that exhibit high plasma protein binding (PPB level = 2). Compounds 11a–f also show a high PPB level. All the synthesized conjugates were non-hepatotoxic agents. The ADMET descriptor values seemed promising, especially for the potent agents discovered, and can be considered for future studies targeting the development of promising hits/leads (Table 2).
Table 2.
Computational ADMET descriptor values for the synthesized agents.
| Entry | Compd. | Aqueous Solubility | Intestinal Absorption | PPB | Hepatotoxicity |
|---|---|---|---|---|---|
| 1 | 3a | 3 | 0 | 0 | 0 |
| 2 | 3b | 3 | 0 | 0 | 0 |
| 3 | 3c | 2 | 0 | 0 | 0 |
| 4 | 3d | 3 | 0 | 0 | 0 |
| 5 | 3e | 2 | 1 | 1 | 0 |
| 5 | 3f | 3 | 0 | 0 | 0 |
| 7 | 3g | 3 | 0 | 0 | 0 |
| 8 | 3h | 3 | 0 | 0 | 0 |
| 9 | 3i | 3 | 0 | 0 | 0 |
| 10 | 3j | 2 | 0 | 0 | 0 |
| 11 | 3k | 2 | 0 | 0 | 0 |
| 12 | 5a | 3 | 0 | 2 | 0 |
| 13 | 5b | 2 | 0 | 2 | 0 |
| 14 | 5c | 2 | 0 | 2 | 0 |
| 15 | 5d | 3 | 0 | 2 | 0 |
| 16 | 5e | 3 | 0 | 2 | 0 |
| 17 | 5f | 2 | 0 | 2 | 0 |
| 18 | 7a | 3 | 0 | 2 | 0 |
| 19 | 7b | 3 | 0 | 2 | 0 |
| 20 | 7c | 3 | 0 | 2 | 0 |
| 21 | 9a | 3 | 0 | 0 | 0 |
| 22 | 9b | 3 | 0 | 0 | 0 |
| 23 | 9c | 3 | 0 | 0 | 0 |
| 24 | 11a | 2 | 0 | 2 | 0 |
| 25 | 11b | 3 | 0 | 2 | 0 |
| 26 | 11c | 2 | 0 | 2 | 0 |
| 27 | 11d | 2 | 0 | 2 | 0 |
| 28 | 11e | 2 | 0 | 2 | 0 |
| 29 | 11f | 2 | 0 | 2 | 0 |
| 30 | 13 | 3 | 0 | 0 | 1 |
| 31 | 15 | 3 | 0 | 0 | 0 |
Aqueous solubility level: 0, extremely low; 1, very low; 2, low; 3, good; 4; optimal; 5, too soluble; 6, unknown. Intestinal absorption level: 0, good; 1, moderate; 2, poor; 4, very poor. Plasma protein binding (PPB) level: 0, <90%; 1, >90%; 2, >95%. Hepatotoxicity level: 0, non toxic; 1, toxic.
3. Materials and Methods
3.1. Chemistry
Melting points were determined on a capillary point apparatus equipped with a digital thermometer and were uncorrected. NMR spectra were recorded in CDCl3 or DMSO-d6 on a Bruker spectrometer operating at 500 MHz for 1H (with TMS as an internal standard) and 125 MHz for 13C using the NMR facility at the Department of Chemistry and Physics, Faculty of Science and Mathematics, Augusta University, Augusta, GA, USA. IR spectra (KBr, cm−1) were recorded on a Thermo Fisher Scientific, Waltham, MA, USA (Nicolet iS5) spectrophotometer at the Department of Chemistry and Physics, Faculty of Science and Mathematics, Augusta University, Augusta, GA, USA. HRMS were measured using Agilent Technologies 6545 Q-TOF LC/MS. TLC was performed on precoated silica gel (Merck 60 F254); spots were visualized by iodine vapors or irradiation with UV light (254 nm).
3.1.1. General Method for Preparation of Compounds 3a–k, 5a–f, 7a–c, and 9a–c
A round bottom flask (50 mL) containing a small stir bar was charged with a series of benzotriazole-activated acids (2a–k, 4a–f, 6a–c, and 8a–c) (1.1 eq.) and 5-(aminomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one (1) (500 mg, 1.0 eq.) dissolved in DCM (15 mL), along with TEA (1.5 eq.). The reaction mixture was stirred starting at 0 °C and continuing until at room temperature; the progress of the reaction was monitored by TLC. After completion of the reaction, the solvent was evaporated under reduced pressure and the residue was treated three times with 10 mL 20% Na2CO3 cold solution. The precipitate formed was filtered out, washed with water, and dried under vacuum to get the desired products.
3.1.2. Benzyl (S)-(2-(((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-2-oxoethyl)carbamate (3a)
White solid, mp: 153–155 °C, yield: 63% (0.57 g). IR: νmax/cm−1; 3336 (NH), 3296 (NH), 3065 (CH, aromatic), 2931, 2846 (CH, aliphatic), 1728, 1697, 1672 (C = O), 1517 (C = C), 1291 (C-N), 1238 (C-O), 1110 (C-F); 1H NMR (CDCl3) δ: 7.46 (s, 1H, NH), 7.43 (s, 1H, NH), 7.33–7.30 (m, 5H, Ar–H), 7.05 (s, 2H, Ar–H), 6.78 (s, 1H, Ar–H), 5.37 (s, 1H, CH), 5.05 (s, 2H, CH2), 4.72 (s, 1H, CH2), 3.96 (s, 1H, CH2), 3.87 [s, 5H, (1H, CH2) + (4H, 2CH2)], 3.72 (s, 1H, CH2), 3.65 (s, 2H, CH2), 3.06 (s, 4H, 2CH2); 13C NMR (CDCl3) δ: 170.4, 156.9, 154.7, 154.5, 136.1, 128.8, 128.5, 128.4, 114.2, 107.8, 71.9, 67.6, 66.7, 51.5, 47.7, 44.8, 41.9, 31.2; HRMS: m/z for C24H27FN4O6 [M+Na]+ Calcd.: 509.1807, Found: 509.1807.
3.1.3. Benzyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-1-oxopropan-2-yl)carbamate (3b)
White solid, mp: 186–188 °C, yield: 51% (0.48 g). IR: νmax/cm−1; 3319 (NH), 3040, 3001 (CH, aromatic), 2928, 2845 (CH, aliphatic), 1726, 1690 (C = O), 1518 (C = C), 1254 (C-N), 1238 (C-O), 1110 (C-F); 1H NMR (CDCl3) δ: 7.50 (s, 1H, NH), 7.47 (s, 1H, NH), 7.32–7.29 (m, 5H, Ar–H), 7.06 (s, 2H, Ar–H), 6.78 (s, 1H, Ar–H), 5.12 (s, 1H, CH), 5.05 (d, J = 11.1 Hz, 1H, CH2), 4.94 (d, J = 11.0 Hz, 1H, CH2), 4.72 (s, 1H, CH), 4.17 (s, 1H, CH2), 3.90 (s, 4H, 2CH2), 3.77 (s, 1H, CH2), 3.72–3.70 (m, 1H, CH2), 3.61–3.58 (m, 1H, CH2), 3.08 (s, 4H, 2CH2), 1.32 (s, 3H, CH3); 13C NMR (CDCl3) δ: 173.8, 156.7, 154.4, 136.1, 128.8, 128.5, 128.4, 114.2, 108.0, 107.8, 72.0, 67.5, 66.5, 51.7, 51.0, 47.7, 41.8, 18.2; HRMS: m/z for C25H29FN4O6 [M+H]+ Calcd.: 501.2144, Found: 501.2132.
3.1.4. Benzyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-3-methyl-1-oxobutan-2-yl)carbamate (3c)
White solid, mp: 173–175 °C, yield: 60% (0.59 g). IR: νmax/cm−1; 3298 (NH), 3058 (CH, aromatic), 2958, 2854 (CH, aliphatic), 1744, 1692 (C = O), 1518 (C = C), 1257 (C-N), 1240 (C-O), 1115 (C-F); 1H NMR (CDCl3) δ: 7.51 (s, 1H, NH), 7.48 (s, 1H, NH), 7.32–7.30 (m, 5H, Ar–H), 7.17 (s, 1H, Ar–H), 7.05 (s, 1H, Ar–H), 6.60 (s, 1H, Ar–H), 5.20 (s, 1H, CH), 5.04 (d, J = 11.6 Hz, 1H, CH2), 4.95 (d, J = 11.8 Hz, 1H, CH2), 4.71 (s, 1H, CH), 3.95 (s, 1H, CH2), 3.91 (s, 4H, 2CH2), 3.74 (s, 1H, CH2), 3.65 (s, 2H, CH2), 3.10 (s, 4H, 2CH2), 2.15 (s, 1H, CH), 0.93 (s, 3H, CH3) 0.84 (s, 3H, CH3); 13C NMR (CDCl3) δ: 186.8, 157.2, 156.7, 145.1, 128.9, 128.8, 128.5, 128.3, 120.7, 114.2, 72.0, 67.4, 66.4, 61.0, 51.8, 51.7, 47.7, 42.0, 30.7, 19.5; HRMS: m/z for C27H33FN4O6 [M+Na]+ Calcd.: 551.2276, Found: 551.2284.
3.1.5. Benzyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-4-(methylthio)-1-oxobutan-2-yl)carbamate (3d)
White solid, mp: 165–167 °C, yield: 84% (0.88 g). IR: νmax/cm−1; 3271 (NH), 3020 (CH, aromatic), 2915, 2850 (CH, aliphatic), 1744, 1706 (C = O), 1516 (C = C), 1258 (C-N), 1226 (C-O), 1118 (C-F); 1H NMR (CDCl3) δ: 7.52 (s, 1H, NH), 7.49 (s, 1H, NH), 7.31–7.29 (m, 5H, Ar–H), 7.18 (s, 1H, Ar–H), 7.05 (s, 1H, Ar–H), 6.97 (s, 1H, Ar–H), 5.45 (s, 1H, CH), 5.04 (d, J = 11.4 Hz, 1H, CH2), 4.94 (d, J = 11.6 Hz, 1H, CH2), 4.72 (s, 1H, CH), 4.30 (s, 1H, CH2), 3.91 [s, 5H, (1H, CH2) + (4H, 2CH2)], 3.76–3.68 (m, 1H, CH2), 3.61–3.59 (m, 1H, CH2), 3.10 (s, 4H, 2CH2), 2.49 (s, 2H, CH2), 2.03 (s, 3H, CH3), 1.89 (s, 2H, CH2); 13C NMR (CDCl3) δ: 172.7, 156.8, 154.4, 136.1, 128.8, 128.5, 128.3, 114.1, 108.0, 107.8, 79.9, 71.9, 67.5, 66.4, 54.3, 51.8, 47.7, 41.9, 30.4, 15.5; HRMS: m/z for C27H33FN4O6S [M+Na]+ Calcd.: 583.1997, Found: 583.1994.
3.1.6. Benzyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-1-oxo-3-phenylpropan-2-yl)carbamate (3e)
White solid, mp: 120–122 °C, yield: 85% (0.91 g). IR: νmax/cm−1; 3308 (NH), 3025 (CH, aromatic), 2951, 2923, 2854 (CH, aliphatic), 1739, 1687 (C = O), 1515 (C = C), 1285 (C-N), 1237 (C-O), 1121 (C-F); 1H NMR (DMSO-d6) δ: 8.44 (s, 1H, NH), 7.91 (s, 1H, NH), 7.54–7.46 (m, 2H, Ar–H), 7.31–7.04 (m, 10H, Ar–H), 7.03 (s, 1H, Ar–H), 4.91–4.84 (m, 2H, CH2), 4.72 (s, 1H, CH), 4.23 (s, 1H, CH), 4.03 (s, 1H, CH2), 3.72 (s, 5H, (1H, CH2) + (4H, 2CH2)), 3.60 (s, 1H, CH2), 3.47 (s, 1H, CH2), 2.92 (s, 4H, 2CH2), 2.89 (s, 1H, CH2), 2.77–2.72 (m, 1H, CH2); 13C NMR (DMSO-d6) δ: 172.5, 155.8, 155.5, 154.0, 138.0, 136.9, 135.4, 133.4, 129.1, 128.2, 128.0, 127.4, 126.2, 119.1, 114.2, 106.9, 78.9, 71.5, 67.0, 66.1, 65.1, 56.3, 50.7, 47.0, 41.0, 37.4; HRMS: m/z for C31H33FN4O6 [M+H]+ Calcd.: 577.2457, Found: 577.2445.
3.1.7. Tert-butyl (S)-(2-(((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino)-2-oxoethyl)carbamate (3f)
Off white solid, mp: 54–56 °C, yield: 92% (0.70 g). IR: νmax/cm−1; 3305 (NH), 3016 (CH, aromatic), 2968, 2875 (CH, aliphatic), 1748, 1681 (C = O), 1515 (C = C), 1218 (C-N), 1116 (C-O), 1049 (C-F); 1H NMR (DMSO-d6) δ: 8.18 (s, 1H, NH), 7.52–7.43 (m, 1H, Ar–H), 7.17 (d, J = 7.5 Hz, 1H, Ar–H), 7.04 (s, 1H, NH), 6.97 (s, 1H, Ar–H), 4.71 (s, 1H, CH), 4.05–4.03 (m, 1H, CH2), 3.73 (s, 5H, (1H, CH2) + (4H, 2CH2)), 3.54 (s, 2H, CH2), 3.44 (s, 2H, CH2), 2.95 (s, 4H, 2CH2), 1.36 (s, 9H, 3CH3); 13C NMR (DMSO-d6) δ: 170.2, 155.8, 154.0, 153.6, 135.6, 133.5, 119.2, 114.2, 106.6, 78.0, 71.4, 66.1, 50.7, 47.2, 43.2, 41.1, 28.2; HRMS: m/z for C21H29FN4O6 [M+H]+ Calcd.: 453.2144, Found: 453.2146.
3.1.8. Tert-butyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl) amino)-1-oxopropan-2-yl)carbamate (3g)
Yellowish white solid, mp: 71–72 °C, yield: 78% (0.62 g). IR: νmax/cm−1; 3306 (NH), 3050 (CH, aromatic), 2972, 2857 (CH, aliphatic), 1737, 1673 (C = O), 1514 (C = C), 1227 (C-N), 1168 (C-O), 1114 (C-F); 1H NMR (DMSO-d6) δ: 8.18 (s, 1H, NH), 7.53–7.44 (m, 1H, Ar–H), 7.21–7.14 (m, 1H, Ar–H), 7.05 (s, 1H, NH), 6.92 (s, 1H, Ar–H), 4.72 (s, 1H, CH), 4.04–4.03 (m, 1H, CH), 3.92–3.82 (m, 1H, CH2), 3.73 [s, 5H, (1H, CH2) + (4H, 2CH2)], 2.95 [s, 5H, (1H, CH2) + (4H, 2CH2)], 2.86–2.77 (m, 1H, CH2), 1.34 (s, 9H, 3CH3), 1.18–1.07 (m, 3H, CH3); 13C NMR (DMSO-d6) δ: 173.9, 155.0, 154.4, 154.1, 135.5, 133.4, 119.2, 113.9, 106.6, 77.9, 71.6, 66.1, 64.9, 50.7, 49.8, 47.0, 44.1, 28.2, 15.2; HRMS: m/z for C22H31FN4O6 [M+H]+ Calcd.: 467.2300, Found: 467.2329.
3.1.9. Tert-butyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl) amino)-3-methyl-1-oxobutan-2-yl)carbamate (3h)
Off white solid, mp: 61–63 °C, yield: 87% (0.73 g). IR: νmax/cm−1; 3306 (NH), 3045 (CH, aromatic), 2965 (CH, aliphatic), 1747 (C = O), 1514 (C = C), 1224 (C-N), 1167 (C-O), 1115 (C-F); 1H NMR (DMSO-d6) δ: 8.25 (s, 1H, NH), 7.52–7.44 (m, 1H, Ar–H), 7.15 (s, 1H, NH), 7.04 (s, 1H, Ar–H), 6.70 (d, J = 5.1 Hz, 1H, Ar–H), 4.73 (s, 1H, CH), 4.05–4.03 (m, 1H, CH), 3.73 (s, 6H, 3CH2), 3.51–3.45 (m, 2H, CH2), 2.95 (s, 4H, 2CH2), 1.87 (s, 1H, CH), 1.34 (s, 9H, 3CH3), 0.81 (s, 6H, 2CH3); 13C NMR (DMSO-d6) δ: 172.3, 155.4, 154.0, 153.6, 135.5, 133.5, 125.2, 119.2, 114.2, 106.6, 77.9, 71.5, 66.1, 59.9, 50.7, 46.9, 40.9, 30.2, 28.1, 19.2, 18.3; HRMS: m/z for C24H35FN4O6 [M+H]+ Calcd.: 495.2613, Found: 495.2607.
3.1.10. Tert-butyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl) amino)-4-(methyl thio)-1-oxobutan-2-yl)carbamate (3i)
White solid, mp: 56–58 °C, yield: 64% (0.57 g). IR: νmax/cm−1; 3304 (NH), 3086 (CH, aromatic), 2970, 2856 (CH, aliphatic), 1745, 1708 (C = O), 1514 (C = C), 1225 (C-N), 1166 (C-O), 1114 (C-F); 1H NMR (DMSO-d6) δ: 8.23 (s, 1H, NH), 7.52–7.43 (m, 1H, Ar–H), 7.21–7.13 (m, 1H, Ar–H), 7.06–6.98 (m, 2H, NH + Ar–H), 4.73 (s, 1H, CH), 4.08–4.02 (m, 1H, CH), 3.96 (s, 1H, CH2), 3.73 [s, 5H, (1H, CH2) + (4H, 2CH2)], 2.95 [s, 5H, (1H, CH2) + (4H, 2CH2)], 2.85–2.77 (m, 1H, CH2), 2.42–2.36 (m, 2H, CH2), 2.04–1.95 (m, 3H, CH3), 1.74–1.64 (m, 2H, CH2), 1.35 (s, 9H, 3CH3); 13C NMR (DMSO-d6) δ: 176.0, 155.4, 154.1, 153.6, 135.4, 133.4, 119.2, 113.9, 106.7, 78.0, 74.0, 71.5, 66.2, 53.8, 50.7, 47.1, 44.2, 29.7, 28.8, 28.1, 14.5; HRMS: m/z for C24H35FN4O6S [M+H]+ Calcd.: 527.2334, Found: 527.2373.
3.1.11. Tert-butyl ((S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl) amino)-1-oxo-3-phenylpropan-2-yl)carbamate (3j)
Off white solid, mp: 69–71 °C, yield: 74% (0.75 g). IR: νmax/cm−1; 3294 (NH), 3040 (CH, aromatic), 2975 (CH, aliphatic), 1748 (C = O), 1514 (C = C), 1225 (C-N), 1166 (C-O), 1115 (C-F); 1H NMR (CDCl3) δ: 7.94 (s, 1H, NH), 7.49–7.28 (m, 6H, Ar–H), 7.16 (s, 1H, Ar–H), 6.97 (s, 1H, Ar–H), 5.43 (s, 1H, CH), 4.77–4.71 (m, 1H, CH), 4.54 (s, 1H, CH2), 3.99 (s, 1H, CH2), 3.93 (s, 4H, 2CH2), 3.68 (s, 2H, CH2), 3.15 (s, 1H, CH2), 3.10 (s, 4H, 2CH2), 3.02 (s, 1H, CH2), 1.43 (s, 9H, 3CH3); 13C NMR (CDCl3) δ: 173.0, 156.4, 155.5, 154.5, 136.6, 136.4, 133.1, 129.3, 128.6, 127.0, 118.9, 114.2, 114.0, 107.6, 107.4, 80.1, 72.0, 71.8, 67.0, 60.5, 55.9, 51.0, 47.7, 41.7, 38.7, 28.3; HRMS: m/z for C28H35FN4O6 [M+Na]+ Calcd.: 565.2433, Found: 565.2439.
3.1.12. Tert-butyl ((2S,3S)-1-((((S)-3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl)amino)-3-methyl-1-oxopentan-2-yl)carbamate (3k)
White solid, mp: 62–64 °C, yield: 77% (0.67 g). IR: νmax/cm−1; 3306 (NH), 3075 (CH, aromatic), 2966 (CH, aliphatic), 1747 (C = O), 1514 (C = C), 1225 (C-N), 1168 (C-O), 1115 (C-F); 1H NMR (DMSO-d6) δ: 8.23 (s, 1H, NH), 7.53–7.45 (m, 1H, Ar–H), 7.22–7.15 (m, 1H, Ar–H), 7.05 (s, 1H, NH), 6.84–6.75 (m, 1H, Ar–H), 4.73 (s, 1H, CH), 4.07–4.03 (m, 1H, CH), 3.78 (s, 1H, CH2), 3.73 [s, 5H, (1H, CH2) + (4H, 2CH2)], 3.45–3.37 (m, 2H, CH2), 2.95 (s, 4H, 2CH2),1.62 (s, 1H, CH), 1.33 (s, 9H, 3CH3), 1.09–1.04 (m, 2H, CH2), 0.75 (s, 6H, 2CH3); 13C NMR (DMSO-d6) δ: 172.3, 155.5, 155.3, 154.0, 135.5, 133.5, 119.2, 114.2, 106.6, 77.9, 71.5, 66.1, 58.9, 50.7, 46.9, 44.1, 36.2, 28.1, 24.5, 15.3, 10.9; HRMS: m/z for C25H37FN4O6 [M+H]+ Calcd.: 509.2770, Found: 509.2800.
3.1.13. General Method for Synthesis of Compounds 4a–f
1H-Benzotriazole (4.0 eq.) was dissolved in anhydrous THF. Thionyl chloride (1.5 eq.) was added and the mixture was stirred for 30 min. The corresponding acid (1.0 eq.) was then added and the reaction mixture was stirred for another 2–3 h at room temperature. The reaction was monitored by TLC and, upon its completion, the solvent was evaporated, a few crystals of ice were added and the formed residue was then washed 3 times with 15 mL 20% Na2CO3 solution. After filtration, the collected precipitate was crystallized from diethyl ether to yield 4e and 4f in pure form [22].
3.1.14. (1H-Benzo[d][1,2,3] triazol-1-yl)(4-(methylamino)phenyl)methanone (4e)
Shiny yellow microcrystals, mp: 179–181 °C, yield: 85% (0.71 g). IR: νmax/cm−1; 3364 (NH streching), 3001 (CH, aromatic), 2875, 2783 (CH, aliphatic), 1680 (C = O), 1595 (NH bending), 1530 (C = C), 1286 (C-N); 1H NMR (CDCl3) δ: 8.32 (d, J = 8.3 Hz, 1H, Ar–H), 8.22–8.19 (m, 2H, Ar–H), 8.12 (d, J = 8.3 Hz, 1H, Ar–H), 7.63 (t, J = 8.1 Hz, 1H, Ar–H), 7.48 (t, J = 8.1 Hz, 1H, Ar–H), 6.70–6.67 (m, 2H, Ar–H), 2.94 (s, 3H, CH3); 13C NMR (CDCl3) δ: 165.6, 153.7, 145.8, 134.9, 133.0, 130.0, 126.0, 120.1, 115.0, 111.8, 30.5; HRMS: m/z for C14H12N4O [M+] Calcd.: 252.1011, Found: 252.1019.
3.1.15. (1H-Benzo[d][1,2,3] triazol-1-yl)(2-chloro-3-nitrophenyl)methanone (4f)
White solid, mp: 135–137 °C, yield: 73% (0.55 g). IR: νmax/cm−1; 3029 (CH, aromatic), 1723 (C = O), 1533 (C = C), 1290 (C-N), 748 (C-Cl); 1H NMR (CDCl3) δ: 8.39 (d, J = 8.3 Hz, 1H, Ar–H), 8.16 (d, J = 8.3 Hz, 1H, Ar–H), 8.06 (dd, J = 8.2, 1.6 Hz, 1H, Ar–H), 7.81 (dd, J = 7.7, 1.6 Hz, 1H, Ar–H), 7.78–7.74 (m, 1H, Ar–H), 7.64–7.58 (m, 2H, Ar–H); 13C NMR (CDCl3) δ: 164.1, 148.9, 146.6, 136.3, 133.0, 131.4, 131.2, 128.0, 127.8, 127.4, 125.6, 120.9, 114.6; HRMS: m/z for C13H7ClN4O3 [M+H]+ Calcd.: 303.0279, Found: 303.0281.
3.1.16. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)benzamide (5a)
White microcrystals, mp: 174–176 °C, yield: 98% (0.66 g). IR: νmax/cm−1; 3280 (NH), 3062 (CH, aromatic), 2854 (CH, aliphatic), 1720 (C = O), 1517 (C = C), 1223 (C-N), 1193 (C-O), 1117 (C-F); 1H NMR (CDCl3) δ: 7.75–7.73 (m, 2H, Ar–H), 7.52–7.40 (m, 4H, Ar–H), 7.04 (s, 1H, Ar–H), 7.71 (t, J = 6.1 Hz, 1H, Ar–H), 4.89–4.84 (m, 1H, CH), 4.06 (t, J = 9.0 Hz, 1H, CH2), 3.94–3.90 (m, 1H, CH2), 3.88 (t, J = 4.3 Hz, 4H, 2CH2) =, 3.84–3.77 (m, 2H, CH2), 3.07 (t, J = 4.4 Hz, 4H, 2CH2); 13C NMR (CDCl3) δ: 168.5, 156.7, 154.8, 154.5, 133.7, 132.2, 128.9, 127.3, 114.2, 108.0, 107.7, 72.3, 66.7, 51.6, 48.0, 42.7; HRMS: m/z for C21H22FN3O4 [M+H]+ Calcd.: 400.1667, Found: 400.1679.
3.1.17. (S)-4-Fluoro-N-((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) benzamide (5b)
White microcrystals, mp: 173–175 °C, yield: 96% (0.68 g). IR: νmax/cm−1; 3363 (NH), 3058 (CH, aromatic), 2932, 2867 (CH, aliphatic), 1745 (C = O), 1503 (C = C), 1220 (C-N), 1196 (C-O), 1120 (C-F); 1H NMR (DMSO-d6) δ: 8.84 (t, J = 5.7 Hz, 1H, Ar–H), 7.93–7.90 (m, 2H, NH + Ar–H), 7.47 (dd, J = 15.0, 2.6 Hz, 1H, Ar–H), 7.32–7.28 (m, 2H, Ar–H), 7.18 (dd, J = 8.8, 2.2 Hz, 1H, Ar–H), 7.05 (t, J = 9.2 Hz, 1H, Ar–H), 4.87–4.82 (m, 1H, CH), 4.14 (t, J = 9.0 Hz, 1H, CH2), 3.83 (q, J = 6.0 Hz, 1H, CH2), 3.73 (t, J = 4.5 Hz, 4H, 2CH2), 3.67–3.57 (m, 2H, CH2), 2.95 (t, J = 4.6 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 165.9, 164.9, 162.9, 155.5, 154.1, 153.6, 135.5, 133.4, 130.5, 130.0, 119.2, 115.3, 114.1, 106.7, 71.3, 66.1, 50.7, 47.5, 42.3; HRMS: m/z for C21H21F2N3O4 [M+Na]+ Calcd.: 440.1392, Found: 440.1405.
3.1.18. (S)-4-Chloro-N-((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) benzamide (5c)
Off white microcrystals, mp: 213–215 °C, yield: 30% (0.22 g). IR: νmax/cm−1; 3349 (NH), 3085 (CH, aromatic), 2955 (CH, aliphatic), 1732 (C = O), 1515 (C = C), 1226 (C-N), 1197 (C-O), 1112 (C-F), 757 (C-Cl); 1H NMR (DMSO-d6) δ: 8.91 (t, J = 5.7 Hz, 1H, Ar–H), 7.88–7.85 (m, 2H, NH + Ar–H), 7.57–7.54 (m, 2H, Ar–H), 7.48 (dd, J = 15.0, 2.5 Hz, 1H, Ar–H), 7.19 (dd, J = 8.8, 2.1 Hz, 1H, Ar–H), 7.06 (t, J = 9.1 Hz, 1H, Ar–H), 4.88–4.83 (m, 1H, CH), 4.15 (t, J = 9.0 Hz, 1H, CH2), 3.84 (q, J = 6.0 Hz, 1H, CH2), 3.74 (t, J = 4.5 Hz, 4H, 2CH2), 3.68–3.58 (m, 2H, CH2), 2.96 (t, J = 4.6 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 166.0, 155.5, 154.1, 153.6, 136.3, 135.5, 133.5, 132.7, 129.2, 128.4, 119.2, 114.2, 106.8, 71.3, 66.2, 50.7, 47.6, 42.4; HRMS: m/z for C21H21ClFN3O4 [M+H]+ Calcd.: 434.1277, Found: 434.1285.
3.1.19. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)-4-nitro benzamide (5d)
Yellow microcrystals, mp: 218–220 °C, yield: 97% (0.73 g). IR: νmax/cm−1; 3370 (NH), 3076 (CH, aromatic), 2850 (CH, aliphatic), 1734 (C = O), 1518 (C = C), 1237 (C-N), 1210 (C-O), 1113 (C-F); 1H NMR (DMSO-d6) δ: 9.16 (t, J = 5.7 Hz, 1H, Ar–H), 8.33–8.30 (m, 2H, NH + Ar–H), 8.08–8.05 (m, 2H, Ar–H), 7.47 (dd, J = 15.0, 2.5 Hz, 1H, Ar–H), 7.19 (dd, J = 8.8, 2.1 Hz, 1H, Ar–H), 7.05 (t, J = 9.2 Hz, 1H, Ar–H), 4.90–4.85 (m, 1H, CH), 4.16 (t, J = 9.0 Hz, 1H, CH2), 3.84 (q, J = 6.0 Hz, 1H, CH2), 3.73 (t, J = 4.5 Hz, 4H, 2CH2), 3.69–3.63 (m, 2H, CH2), 2.95 (t, J = 4.6 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 165.4, 155.5, 154.0, 153.6, 149.1, 139.6, 135.6, 133.3, 128.8, 123.5, 119.2, 114.1, 106.8, 71.2, 66.1, 50.7, 47.5, 42.5; HRMS: m/z for C21H21FN4O6 [M+H]+ Calcd.: 445.1518, Found: 445.1523.
3.1.20. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)-4-(methyl amino) benzamide (5e)
The crude product was purified through column chromatography using 1% MeOH in DCM to obtain shiny white crystals, 5e in pure form, mp: 195–197 °C, yield: 40% (0.29 g). IR: νmax/cm−1; 3373 (NH), 3062 (CH, aromatic), 2918, 2812 (CH, aliphatic), 1738 (C = O), 1506 (C = C), 1226 (C-N), 1188 (C-O), 1119 (C-F); 1H NMR (DMSO-d6) δ: 8.36 (t, J = 5.8 Hz, 1H, Ar–H), 7.66 (s, 1H, NH), 7.64 (s, 1H, NH), 7.48 (dd, J = 15.0, 2.6 Hz, 1H, Ar–H), 7.18 (dd, J = 8.8, 2.1 Hz, 1H, Ar–H), 7.04 (t, J = 9.2 Hz, 1H, Ar–H), 6.53 (s, 1H, Ar–H), 6.51 (s, 1H, Ar–H), 6.22–6.19 (m, 1H, Ar–H), 4.84–4.79 (m, 1H, CH), 4.12 (t, J = 9.0 Hz, 1H, CH2), 3.83 (q, J = 6.0 Hz, 1H, CH2), 3.73 (t, J = 4.5 Hz, 4H, 2CH2), 3.62–3.51 (m, 2H, CH2), 2.95 (t, J = 4.6 Hz, 4H, 2CH2), 2.70 (d, J = 4.8 Hz, 3H, CH3); 13C NMR (DMSO-d6) δ: 166.9, 155.5, 154.1, 153.6, 152.4, 135.5, 133.5, 128.8, 120.3, 119.2, 114.1, 110.3, 106.7, 71.5, 66.1, 50.7, 47.5, 42.1, 29.3; HRMS: m/z for C22H25FN4O4 [M+] Calcd.: 428.1860, Found: 428.1864.
3.1.21. (S)-2-Chloro-N-((3-(3-fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methy-l)-3-nitrobenzamide (5f)
Fine yellow microcrystals, mp: 200–202 °C, yield: 91% (0.74 g). IR: νmax/cm−1; 3290 (NH), 3054 (CH, aromatic), 2938, 2856 (CH, aliphatic), 1720 (C = O), 1514 (C = C), 1239 (C-N), 1146 (C-O), 1115 (C-F), 716 (C-Cl); 1H NMR (DMSO-d6) δ: 9.09 (t, J = 5.8 Hz, 1H, Ar–H), 8.08 (dd, J = 7.9, 1.8 Hz, 1H, Ar–H), 7.68–7.62 (m, 2H, NH + Ar–H), 7.50 (dd, J = 15.0, 2.5 Hz, 1H, Ar–H), 7.19 (dd, J = 8.8, 2.2 Hz, 1H, Ar–H), 7.07 (t, J = 9.2 Hz, 1H, Ar–H), 4.90–4.85 (m, 1H, CH), 4.17 (t, J = 9.1 Hz, 1H, CH2), 3.84 (q, J = 6.4 Hz, 1H, CH2), 3.73 (t, J = 4.5 Hz, 4H, 2CH2), 3.69–3.62 (m, 2H, CH2), 2.96 (t, J = 4.6 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 165.6, 155.5, 154.0, 148.5, 139.1, 135.5, 133.4, 131.9, 128.6, 125.5, 121.4, 119.3, 114.0, 106.6, 71.2, 66.1, 50.7, 47.2, 41.7; HRMS: m/z for C21H20ClFN4O6 [M+] Calcd.: 478.1055, Found: 478.1070.
3.1.22. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)isonicotin-amide (7a)
White solid, mp: 198–200 °C, yield: 90% (0.61 g). IR: νmax/cm−1; 3303 (NH), 3031 (CH, aromatic), 2953 (CH, aliphatic), 1746 (C = O), 1647 (C = N), 1513 (C = C), 1223 (C-N), 1118 (C-F); 1H NMR (DMSO-d6) δ: 9.12 (t, J = 5.5 Hz, 1H, Ar–H), 8.73 (s, 1H, NH), 8.72 (s, 1H, Ar–H), 7.74 (s, 1H, Ar–H), 7.73 (s, 1H, Ar–H), 7.47 (dd, J = 14.9, 1.8 Hz, 1H, Ar–H), 7.18 (d, J = 8.5 Hz, 1H, Ar–H), 7.05 (t, J = 9.2 Hz, 1H, Ar–H), 4.89–4.84 (m, 1H, CH), 4.15 (t, J = 9.0 Hz, 1H, CH2), 3.84 (q, J = 6.2 Hz, 1H, CH2), 3.73 (t, J = 3.8 Hz, 4H, 2CH2), 3.68–3.62 (m, 2H, CH2), 2.95 (t, J = 3.8 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 165.5, 155.5, 154.0, 153.6, 150.2, 140.9, 135.5, 133.4, 121.2, 119.2, 114.1, 106.8, 71.2, 66.1, 50.7, 47.5, 42.4; HRMS: m/z for C20H21FN4O4 [M+H]+ Calcd.: 401.1620, Found: 401.1617.
3.1.23. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)nicotinamide (7b)
White solid, mp: 182–184 °C, yield: 86% (0.59 g). IR: νmax/cm−1; 3286 (NH), 3031 (CH, aromatic), 2938, 2872 (CH, aliphatic), 1737 (C = O), 1651 (C = N), 1511 (C = C), 1219 (C-N), 1195 (C-O), 1109 (C-F); 1H NMR (DMSO-d6) δ: 9.02 (t, J = 5.5 Hz, 1H, Ar–H), 8.98 (s, 1H, NH), 8.71 (d, J = 3.9 Hz, 1H, Ar–H), 8.17 (d, J = 8.0 Hz, 1H, Ar–H), 7.52–7.49 (m, 1H, Ar–H), 7.46 (d, J = 2.1 Hz, 1H, Ar–H), 7.19 (dd, J = 8.9, 1.2 Hz, 1H, Ar–H), 7.05 (t, J = 9.2 Hz, 1H, Ar–H), 4.89–4.84 (m, 1H, CH), 4.15 (t, J = 9.0 Hz, 1H, CH2), 3.85 (q, J = 6.1 Hz, 1H, CH2), 3.73 (t, J = 4.1 Hz, 4H, 2CH2), 3.68–3.62 (m, 2H, CH2), 2.95 (t, J = 4.2 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 165.6, 155.5, 154.0, 152.0, 148.4, 135.6, 135.0, 133.4, 129.5, 123.4, 119.2, 114.1, 106.7, 71.2, 66.1, 50.7, 47.5, 42.2; HRMS: m/z for C20H21FN4O4 [M+H]+ Calcd.: 401.1620, Found: 401.1617.
3.1.24. (S)-N-((3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)pyrazine-2-carboxamide (7c)
White solid, mp: 190–192 °C, yield: 92% (0.63 g). IR: νmax/cm−1; 3389 (NH), 3004 (CH, aromatic), 2906 (CH, aliphatic), 1741, 1681 (C = O), 1603 (C = N), 1514 (C = C), 1224 (C-N), 1192 (C-O), 1115 (C-F); 1H NMR (DMSO-d6) δ: 9.22–9.20 (m, 2H, Ar–H), 8.89 (d, J = 2.0 Hz, 1H, Ar–H), 8.75 (s, 1H, NH), 7.46 (dd, J = 14.9, 1.7 Hz, 1H, Ar–H), 7.19 (d, J = 8.7 Hz, 1H, Ar–H), 7.05 (t, J = 9.3 Hz, 1H, Ar–H), 4.91–4.86 (m, 1H, CH), 4.13 (t, J = 8.9 Hz, 1H, CH2), 3.89 (q, J = 5.9 Hz, 1H, CH2), 3.73 (t, J = 3.9 Hz, 4H, 2CH2), 3.69–3.62 (m, 2H, CH2), 2.96 (t, J = 4.0 Hz, 4H, 2CH2); 13C NMR (DMSO-d6) δ: 163.6, 155.5, 154.0, 147.7, 144.4, 143.6, 143.4, 135.6, 133.4, 119.2, 114.2, 106.8, 71.0, 66.1, 50.7, 47.6, 42.0; HRMS: m/z for C19H20FN5O4 [M+H]+ Calcd.: 402.1572, Found: 402.1580.
3.1.25. N-((S)-1-((((S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino) -4-methyl-1-oxopentan-2-yl)pyrazine-2-carboxamide (9a)
The crude product was purified through column chromatography using 1% MeOH in DCM to obtain shiny yellowish white microcrystals, 9a in pure form, mp: 90–92 °C, yield: 38% (0.33 g). IR: νmax/cm−1; 3306 (NH), 3062 (CH, aromatic), 2957 (CH, aliphatic), 1747 (C = O), 1662 (C = N), 1514 (C = C), 1224 (C-N), 1171 (C-O), 1115 (C-F); 1H NMR (CDCl3) δ: 9.13 (s, 1H, NH), 8.69–8.68 (m, 1H, Ar–H), 8.43 (s, 1H, NH), 8.01 (d, J = 8.0 Hz, 1H, Ar–H), 7.39 (d, J = 14.4 Hz, 1H, Ar–H), 7.33–7.29 (m, 1H, Ar–H), 7.04–7.03 (m, 1H, Ar–H), 6.96–6.95 (m, 1H, Ar–H), 4.76–4.72 (m, 1H, CH), 4.62–4.58 (m, 1H, CH), 3.90 (s, 4H, 2CH2), 3.82–3.71 (m, 3H, (2H, CH2) + (1H, CH2)), 3.57–3.52 (m, 1H, CH2), 3.12 (s, 4H, 2CH2), 1.78–1.72 (m, 1H, CH), 1.68–1.62 (m, 2H, CH2), 0.91 (d, J = 6.3 Hz, 3H, CH3), 0.93 (d, J = 6.3 Hz, 3H, CH3); 13C NMR (CDCl3) δ: 173.1, 163.5, 156.6, 154.4, 147.8, 144.5, 143.7, 142.8, 134.6, 128.5, 120.1, 114.0, 107.8, 72.0, 66.6, 52.3, 51.6, 47.5, 41.6, 40.8, 25.1, 23.1, 22.1; HRMS: m/z for C25H31FN6O5 [M+H]+ Calcd.: 515.2413, Found: 515.2418.
3.1.26. N-((S)-1-((((S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino) -3-methyl-1-oxobutan-2-yl)pyrazine-2-carboxamide (9b)
The crude product was purified through column chromatography using 1% MeOH in DCM to obtain shiny offwhite microcrystals, 9b in pure form, mp: 94–96 °C, yield: 78% (0.66 g). IR: νmax/cm−1; 3305 (NH), 3062 (CH, aromatic), 2963 (CH, aliphatic), 1747 (C = O), 1660 (C = N), 1514 (C = C), 1224 (C-N), 1171 (C-O), 1115 (C-F); 1H NMR (CDCl3) δ: 9.18 (s, 1H, NH), 8.69 (d, J = 2.4 Hz, 1H, Ar–H), 8.46 (s, 1H, NH), 8.23 (d, J = 8.7 Hz, 1H, Ar–H), 7.51 (t, J = 6.0 Hz, 1H, Ar–H), 7.34 (dd, J = 14.4, 2.3 Hz, 1H, Ar–H), 6.99 (dd, J = 8.8, 2.2 Hz, 1H, Ar–H), 6.89 (t, J = 9.0 Hz, 1H, Ar–H), 4.76–4.71 (m, 1H, CH), 4.46 (q, J = 6.9 Hz, 1H, CH), 3.91 (t, J = 9.0 Hz, 1H, CH2), 3.85 (t, J = 4.4 Hz, 4H, 2CH2), 3.78–3.69 (m, 2H, (1H, CH2) + (1H, CH2)), 3.62–3.57 (m, 1H, CH2), 3.04 (t, J = 4.4 Hz, 4H, 2CH2), 2.28–2.21 (m, 1H, CH), 0.97 (d, J = 6.8 Hz, 3H, CH3), 0.93 (d, J = 6.8 Hz, 3H, CH3); 13C NMR (CDCl3) δ: 172.3, 163.4, 156.5, 154.6, 147.7, 144.5, 144.0, 142.8, 135.2, 133.9, 119.6, 114.1, 107.8, 72.1, 66.8, 59.0, 53.6, 51.4, 47.7, 41.8, 31.1, 19.6, 18.3; HRMS: m/z for C24H29FN6O5 [M+H]+ Calcd.: 501.2256, Found: 501.2269.
3.1.27. N-((S)-1-((((S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl) amino) -1-oxo-3-phenylpropan-2-yl)pyrazine-2-carboxamide (9c)
The crude product was purified through column chromatography using 1% MeOH in DCM to obtain a fine pale brown solid, 9c in pure form, mp: 172–174 °C, yield: 52% (0.49 g). IR: νmax/cm−1; 3306 (NH), 3032 (CH, aromatic), 2858 (CH, aliphatic), 1743, 1681 (C = O), 1665 (C = N), 1514 (C = C), 1227 (C-N), 1146 (C-O), 1115 (C-F); 1H NMR (CDCl3) δ: 9.14 (s, 1H, Ar–H), 8.70–8.68 (m, 1H, Ar–H), 8.42 (s, 1H, NH), 8.22 (d, J = 8.0 Hz, 1H, Ar–H), 7.41–7.35 (m, 1H, Ar–H), 7.22–7.17 (m, 4H, Ar–H), 7.13–6.97 (m, 3H, Ar–H), 4.91–4.81 (m, 1H, CH), 4.69–4.59 (m, 1H, CH), 3.89–3.84 [m, 5H, (4H, 2CH2) + (1H, CH2)], 3.73–3.50 [m, 3H, (1H, CH2) + (2H, CH2)], 3.22–3.18 (m, 1H, CH2), 3.13–3.08 (m, 5H, (1H, CH2) + (4H, 2CH2)); 13C NMR (CDCl3) δ: 171.9, 163.3, 156.6, 154.3, 147.8, 144.5, 143.7, 142.9, 136.3, 129.4, 129.3, 128.9, 127.4, 114.1, 107.6, 71.9, 66.6, 55.0, 51.6, 47.6, 41.7, 38.2; HRMS: m/z for C28H29FN6O5 [M+H]+ Calcd.: 549.2256, Found: 549.2254.
3.1.28. General Method for Preparation of Compounds 11a–f
Different substituted benzyl halides 10a–f (1.2 eq.) were added to a solution of deacetyl linezolid 1 (500 mg, 1.0 eq.) in anhydrous DCM (10 mL), in the presence of TEA (2.5 eq.) and chilled to 0 °C. The reaction mixture was stirred overnight, allowing the temperature to become room temperature; TLC monitored the progress of the reaction. After completion of the reaction, the solvent was evaporated under reduced pressure and the residue was treated with a few crystals of ice and 10 mL of 10% NaOH solution. The crude product was extracted with ethyl acetate (15 mL) three times and was then purified by column chromatography (1% MeOH in DCM) to get the desired products 11a–f in pure form.
3.1.29. (S)-3-(3-Fluoro-4-morpholinophenyl)-5-[{(4-fluorobenzyl) amino} methyl] oxazolidin-2-one (11a)
Yellow oil, yield: 28% (0.19 g). IR: νmax/cm−1; 3334 (NH), 3074 (CH, aromatic), 2938, 2853 (CH, aliphatic), 1743 (C = O), 1602 (NH bending), 1510 (C = C), 1218 (C-N), 1114 (C-F); 1HNMR (CDCl3) δ: 1H NMR (CDCl3) δ: 8.00 (s, 1H, NH), 7.41 (dd, J = 14.4, 2.6 Hz, 1H, Ar–H), 7.27–7.24 (m, 2H, Ar–H), 7.09 (dd, J = 8.8, 2.2 Hz, 1H, Ar–H), 6.99 (t, J = 8.7 Hz, 2H, Ar–H), 6.90 (t, J = 9.1 Hz, 1H, Ar–H), 4.76–4.70 (m, 1H, CH), 3.96 (t, J = 8.7 Hz, 1H, CH2), 3.85 [t, J = 4.6 Hz, 5H, (1H, CH) + (4H, 2CH2)], 3.82–3.76 (m, 2H, CH2), 3.03 (t, J = 4.6 Hz, 4H, 2CH2), 2.97 (dd, J = 13.0, 4.1 Hz, 1H, CH2), 2.87–2.83 (m, 1H, CH2); 13C NMR (CDCl3) δ: 161.3, 156.7, 154.7, 136.6, 135.3, 133.6, 130.0, 129.9, 119.1, 115.7, 115.5, 114.0, 107.7, 72.4, 67.2, 53.2, 51.6, 51.2, 48.4; HRMS: m/z for C21H23F2N3O3 [M+] Calcd.: 403.1707, Found: 403.1728.
3.1.30. (S)-3-(3-Fluoro-4-morpholinophenyl)-5-[{(4-(methylsulfonyl) benzyl} amino] methyl) oxazolidin-2-one (11b)
White paste, yield: 32% (0.25 g). IR: νmax/cm−1; 3270 (NH), 3064 (CH, aromatic), 2941, 2850 (CH, aliphatic), 1732 (C = O), 1647 (NH bending), 1515 (C = C), 1228 (C-N), 1195 (C-O), 1114 (C-F); 1H NMR (CDCl3) δ: 7.96 (s, 1H, NH), 7.94–7.87 (m, 2H, Ar–H), 7.52 (s, 1H, Ar–H), 7.50 (s, 1H, Ar–H), 7.42 (dd, J = 14.4, 2.5 Hz, 1H, Ar–H), 7.09 (dd, J = 8.8, 2.1 Hz, 1H, Ar–H), 6.91 (t, J = 9.1 Hz, 1H, Ar–H), 4.77–4.72 (m, 1H, CH), 3.98 (t, J = 8.6 Hz, 1H, CH2), 3.93 (d, J = 3.4 Hz, 2H, CH2), 3.85 (t, J = 4.5 Hz, 4H, 2CH2), 3.79 (q, J = 7.0 Hz, 1H, CH2), 3.04–3.03 [m, 5H, (1H, CH2) + (4H, 2CH2)), 3.03 (s, 3H, CH3], 2.85 (q, J = 7.1 Hz, 1H, CH2); 13C NMR (CDCl3) δ: 156.7, 154.6, 146.4, 139.7, 136.6, 133.4, 130.1, 129.1, 128.5, 127.9, 119.1, 115.7, 114.0, 107.7, 72.4, 67.2, 53.4, 51.8, 51.3, 48.3, 44.8; HRMS: m/z for C22H26FN3O5S [M+] Calcd.: 463.1577, Found: 463.1591.
3.1.31. (S)-3-(3-Fluoro-4-morpholinophenyl)-5-[{(4-(trifluoromethyl) benzyl} amino] methyl) oxazolidin-2-one (11c)
Yellow paste, yield: 31% (0.24 g). IR: νmax/cm−1; 3271 (NH), 3062 (CH, aromatic), 2925, 2853 (CH, aliphatic), 1746 (C = O), 1515 (C = C), 1226 (C-N), 1112 (C-F); 1H NMR (CDCl3) δ: 7.98 (s, 1H, NH), 7.56 (s, 1H, Ar–H), 7.55 (s, 1H, Ar–H), 7.43–7.40 (m, 3H, Ar–H), 7.09 (dd, J = 8.8, 1.8 Hz, 1H, Ar–H), 6.90 (t, J = 9.1 Hz, 1H, Ar–H), 4.75–4.70 (m, 1H, CH), 3.96 (t, J = 8.7 Hz, 1H, CH2), 3.89 (d, J = 5.1 Hz, 2H, CH2), 3.84 (t, J = 4.6 Hz, 4H, 2CH2), 3.78 (q, J = 6.8 Hz, 1H, CH2), 3.02 (t, J = 4.7 Hz, 4H, 2CH2), 2.97 (dd, J = 13.0, 4.0 Hz, 1H, CH2), 2.84 (q, J = 5.9 Hz, 1H, CH2); 13C NMR (CDCl3) δ: 156.7, 154.6, 143.9, 136.5, 133.6, 129.8, 129.6, 129.3, 128.5, 125.6, 123.3, 119.0, 114.0, 107.7, 72.4, 67.1, 53.4, 51.7, 51.2, 48.3; HRMS: m/z for C22H23F4N3O3 [M+H]+ Calcd.: 454.1748, Found: 454.1759.
3.1.32. (S)-5-[{(3-Chlorobenzyl) amino} methyl]-3-(3-fluoro-4-morpholinophenyl) oxazolidin-2-one (11d)
Yellow oil, yield: 26% (0.18 g). IR: νmax/cm−1; 3269 (NH), 3059 (CH, aromatic), 2957, 2853 (CH, aliphatic), 1746 (C = O), 1514 (C = C), 1224 (C-N), 1194 (C-O), 1115 (C-F), 732 (C-Cl); 1H NMR (CDCl3) δ: 7.99 (s, 1H, NH), 7.42 (dd, J = 14.4, 2.5 Hz, 1H, Ar–H), 7.30–7.28 (m, 1H, Ar–H), 7.25–7.20 (m, 2H, Ar–H), 7.17–7.16 (m, 1H, Ar–H), 7.10 (dd, J = 8.8, 1.8 Hz, 1H, Ar–H), 6.90 (t, J = 9.1 Hz, 1H, Ar–H), 4.74–4.69 (m, 1H, CH), 3.95 (t, J = 8.7 Hz, 1H, CH2), 3.84 (t, J = 4.6 Hz, 4H, 2CH2), 3.81–3.77 (m, 3H, (1H, CH2) + (2H, CH2)), 3.02 (t, J = 4.6 Hz, 4H, 2CH2), 2.96 (dd, J = 13.0, 4.1 Hz, 1H, CH2), 2.83 (dd, J = 13.2, 6.0 Hz, 1H, CH2); 13C NMR (CDCl3) δ: 156.7, 154.6, 142.0, 136.5, 134.6, 133.6, 130.0, 128.4, 127.6, 126.4, 119.0, 114.0, 107.7, 72.4, 67.2, 53.4, 51.7, 51.2, 48.3; HRMS: m/z for C21H23ClFN3O3 [M+] Calcd.: 419.1412, Found: 419.1425.
3.1.33. (S)-5-[{(2,4-Difluorobenzyl) amino} methyl]-3-(3-fluoro-4-morpholinophenyl) oxazolid-ine-2-one (11e)
Shiny white microcrystals obtained after purification of the crude product prepared either by the above method or using anhydrous K2CO3 (3 eq.) in DMF, mp: 74–76 °C, yield: 42% (0.30 g). IR: νmax/cm−1; 3250 (NH), 3068 (CH, aromatic), 2936, 2850 (CH, aliphatic), 1733 (C = O), 1509 (C = C), 1226 (C-N), 1194 (C-O), 1112 (C-F); 1H NMR (DMSO-d6) δ: 7.95 (s, 1H, NH), 7.52–7.47 (m, 2H, Ar–H), 7.20–7.14 (m, 2H, Ar–H), 7.07–7.02 (m, 2H, Ar–H), 4.75–4.70 (m, 1H, CH), 4.05 (t, J = 8.9 Hz, 1H, CH2), 3.79–3.76 [m, 3H, (1H, CH2) + (2H, CH2)], 3.73 (t, J = 4.7 Hz, 4H, 2CH2), 2.96 (t, J = 4.7 Hz, 4H, 2CH2), 2.80 (d, J = 5.2 Hz, 2H, CH2); 13C NMR (DMSO-d6) δ: 161.3, 159.4, 155.5, 154.2, 135.3, 133.5, 131.3, 123.7, 119.2, 114.0, 111.2, 106.6, 103.4, 72.4, 66.1, 50.9, 50.7, 47.7, 45.4; HRMS: m/z for C21H22F3N3O3 [M+] Calcd.: 421.1613, Found: 421.1628.
3.1.34. (S)-5-[{(3,5-Dimethoxybenzyl) amino} methyl]-3-(3-fluoro-4-morpholinophenyl) oxazo-lidine-2-one (11f)
Yellow oil, yield: 24% (0.18 g). IR: νmax/cm−1; 3343 (NH), 3031 (CH, aromatic), 2917, 2837 (CH, aliphatic), 1743 (C = O), 1595 (NH bending), 1514 (C = C), 1223 (C-N), 1200 (C-O), 1114 (C-F); 1H NMR (CDCl3) δ: 8.04 (s, 1H, NH), 7.45 (dd, J = 14.4, 2.6 Hz, 1H, Ar–H), 7.13 (dd, J = 8.8, 1.8 Hz, 1H, Ar–H), 6.94 (t, J = 9.1 Hz, 1H, Ar–H), 6.54 (d, J = 2.2 Hz, 2H, Ar–H), 6.39 (t, J = 2.2 Hz, 1H, Ar–H), 4.88–4.83 (m, 1H, CH), 4.02 (t, J = 8.7 Hz, 1H, CH2), 3.90–3.88 [m, 6H, (2H, CH2) + (4H, 2CH2)], 3.84–3.81 (m, 1H, CH2), 3.80 (s, 6H, 2OCH3), 3.08–3.04 (m, 5H, (4H, 2CH2) + (1H, CH2)), 2.98–2.90 (m, 1H, CH2); 13C NMR (CDCl3) δ: 161.3, 156.7, 154.5, 136.7, 136.6, 133.4, 133.3, 119.0, 114.2, 107.8, 106.7, 100.0, 71.7, 67.2, 55.6, 53.4, 51.2, 50.9, 48.5; HRMS: m/z for C23H28FN3O5 [M+Na]+ Calcd.: 468.1905, Found: 468.1925.
3.1.35. General Procedure for Preparation of Compound 13
A round bottom flask (50 mL) containing a small stir bar was charged with deacetyl-linezolid 1 (500 mg, 1.0 eq.) and a solution of 3-nitrooxypropyl bromide 12, prepared as reported [29] (1.2 eq.) in DMF (20 mL) along with anhydrous K2CO3 (414 mg, 3.0 eq.). The reaction mixture was stirred at room temperature; TLC monitored the progress of the reaction. After completion of the reaction, the reaction mixture was poured on iced water and extracted with ethyl acetate (15 mL) three times; then, the solvent was evaporated and the residue was purified by column chromatography to get the desired product 13.
3.1.36. (S)-3-[{(3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl) methyl} amino] propyl nitrate (13)
White crystals from ethanol, mp: 183–185 °C, yield: 37% (0.25 g). IR:νmax/cm−1; 3052 (CH, aromatic), 2950, 2857 (CH, aliphatic), 1732 (C = O), 1604 (NH bending), 1514 (C = C), 1220 (C-N), 1195 (C-O), 1114 (C-F); 1H NMR (DMSO-d6) δ: 7.49 (dd, J = 15.0, 2.4 Hz, 1H, Ar–H), 7.20 (dd, J = 8.8, 1.8 Hz, 1H, Ar–H), 7.07 (t, J = 9.3 Hz, 1H, Ar–H), 4.92–4.86 (m, 1H, CH), 4.23–4.16 (m, 2H, CH2), 4.12 (t, J = 9.0 Hz, 1H, CH2), 3.77 (q, J = 6.5 Hz, 1H, CH2), 3.73 (t, J = 4.4 Hz, 4H, 2CH2), 3.63 (dd, J = 14.7, 4.0 Hz, 1H, CH2), 3.55 (dd, J = 14.6, 7.3 Hz, 1H, CH2), 3.45–3.39 (m, 2H, CH2), 2.96 (t, J = 4.5 Hz, 4H, 2CH2), 1.97–1.93 (m, 2H, CH2); 13C NMR (DMSO-d6) δ: 155.5, 153.9, 135.6, 133.4, 119.2, 114.2, 106.8, 71.3, 66.6, 66.1, 51.8, 50.7, 47.5, 46.6, 21.8; HRMS: m/z for C17H23FN4O6 [M+H]+ Calcd.: 399.1674, Found: 399.1667.
3.1.37. General Procedure for Preparation of Compound 15
A dried heavy-walled Pyrex tube containing a small stir bar was charged with a solution of benzotriazole activated biotin 14 (1.1 eq.) in THF (3 mL) and de-acetyl linezolid 1 (1.0 eq.) along with triethylamine (1.5 eq.). The reaction mixture was exposed to microwave irradiation (20 W) at 50 °C for 30 min. After completion of the reaction, the solvent was evaporated under reduced pressure and the residue was treated three times with 5 mL of cold 20% Na2CO3 solution. The precipitate formed was filtered, washed with de-ionized water (10 mL) and dried under vacuum to get the desired product 15 in very good yield.
3.1.38. N-(((S)-3-(3-Fluoro-4-morpholinophenyl)-2-oxooxazolidin-5-yl)methyl)-5-((3aS, 4S, 6aR)-2-oxohexahydro-1H-thieno [3,4-d] imidazol-4-yl)pentanamide (15)
White solid, mp: 222–224 °C, yield: 88% (0.77 g). IR: νmax/cm−1; 3442, 3282, 3268 (NH streching), 3062 (CH, aromatic), 2941 (CH, aliphatic), 1731, 1700 (C = O), 1648 (NH bending), 1517 (C = C), 1230 (C-N), 1194 (C-O), 1115 (C-F); 1H NMR (DMSO-d6) δ: 8.19 (s, 1H, NH), 7.48 (dd, J = 15.0, 2.4 Hz, 1H, Ar–H), 7.16 (dd, J = 8.8 Hz, 2.1 Hz, 1H, Ar–H), 7.06 (t, J = 9.2 Hz, 1H, Ar–H), 6.38 (s, 1H, NH), 6.34 (s, 1H, NH), 4.74–4.69 (m, 1H, CH), 4.28 (t, J = 5.4 Hz, 1H, CH), 4.09–4.05 [m, 2H, (1H, CH) + (1H, CH2)], 3.73 (t, J = 4.4 Hz, 4H, 2CH2), 3.71–3.69 (m, 1H, CH2), 3.47–3.36 (m, 2H, CH2), 3.03–2.99 (m, 1H, CH2), 2.96 (t, J = 4.5 Hz, 4H, 2CH2), 2.79 (q, J = 5.1 Hz, 1H, CH2), 2.56 (d, J = 12.4 Hz, 1H, CH), 2.11–2.08 (m, 2H, CH2), 1.59–1.37 (m, 4H, 2CH2), 1.31–1.17 (m, 2H, CH2); 13C NMR (DMSO-d6) δ: 173.0, 162.7, 155.5, 154.0, 135.5, 133.4, 119.2, 114.0, 106.6, 71.5, 66.1, 60.9, 59.2, 55.3, 50.7, 47.2, 41.2, 35.0, 30.7, 28.0, 27.9, 25.3; HRMS: m/z for C24H32FN5O5S [M+H]+ Calcd.: 522.2181, Found: 522.2192.
3.2. Biological Studies
Antibacterial properties of the synthesized compounds (3a–k, 5a–f, 7a–c, 9a–c, 11a–f, 13 and 15) were screened against Gram-positive (Staphylococcus aureus ATCC 6538, Bacillus subtilis ATCC 6633) and Gram-negative (Pseudomonas aeruginosa ATCC 15,692 and Escherichia coli ATCC 47076) bacteria utilizing the standard technique [30]. Linezolid was used as a standard reference/drug in the current study. The tested bacterial strains were obtained from MERCIN Center, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
To determine the antibacterial activity, stock solutions of the tested compounds and standard reference were prepared with up to 100% DMSO and stored at 20 °C. If necessary, the solutions were heated to 40–60 °C before testing to facilitate complete dissolution. Double distilled water was used in all dilutions prepared. The final concentration of DMSO in the test series was <1% and did not affect the assay results. A microdilution susceptibility test was used for MIC determination (Mueller–Hinton broth in 96-well plates) according to the CLSI (the Clinical and Laboratory Standards Institute, Malvern, PA, USA). The bacterial inoculum was adjusted to approximately 2.5–3 × 104 cells/mL in Mueller–Hinton broth and incubated in a ratio of 1:1 with the test derivatives in polystyrene 96-well flat-bottom microplates. Positive growth control (without test derivatives) and negative control (without bacteria) were included. The microplates were placed in an incubator set to 37 °C for 24 h. Absorbance was measured at 620 nm at the end of the incubation period. The cut-off level for follow-up studies was defined as a minimum of 90% inhibition of growth compared to the untreated sample. Experiments were conducted once in triplicate. For selected compounds, minimum inhibitory concentration (MIC) values were determined by dose–response experiments. Experiments were performed at least twice, in triplicate per concentration tested.
4. Conclusions
In summary, we synthesized a total of 31 diverse linezolid conjugates in good yields using benzotriazole chemistry. The synthesized conjugates were screened against four different strains of bacteria following the in vitro standard procedure. Some of the synthesized conjugates show promising antibacterial activity and have drug-like properties. All the biological data were validated by QSAR and 3D-pharmacophore studies. We believe the developed SAR, robust BMLR-QSAR model, and 3D-pharmacophore data could be utilized as useful tools for the development of potential drug candidates.
Acknowledgments
We thank the Augusta University Provost’s office, and the Translational Research Program of the Department of Medicine, Medical College of Georgia at Augusta University for their support.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15020191/s1, 1H NMR, 13C NMR, HRMS and IR spectra of all compounds; Table S1: Descriptors of the QSAR model for the tested agents against S. aureus; Table S2: Observed and estimated antimicrobial properties for the tested compounds agents against S. aureus according to the BMLR-QSAR model; Table S3: Molecular descriptor values of the QSAR model for the tested compounds against S. aureus; Table S4: Descriptors of the QSAR model for the tested agents against B. subtilis; Table S5: Observed and estimated antimicrobial properties for the tested compounds agents against B. subtilis according to the BMLR-QSAR model; Table S6: Molecular descriptor values of the QSAR model for the tested compounds against B. subtilis; Table S7. Observed and estimated activity values for the tested compounds against S. aureus according to the 3D-pharmacophore model; Table S8. Observed and estimated activity values for the tested compounds against B. subtilis according to the 3D-pharmacophore model; Figure: S1. Dose-response curve for the tested compounds against RPE1 (retinal pigment epithelium) cell line; Figure S2: QSAR plot representing the observed versus predicted log(MIC, μM) for the tested compounds against S. aureus; Figure S3: QSAR plot representing the observed versus predicted log(MIC, μM) for the tested compounds against B. subtilis; Figure S4: (A) Constraint distances “H-1 – H-2 = 3.696, H-1 – HBA = 3.768, H-2 – HBA = 6.837 Å”, (B) Constraint angle “H-2 – H-1 – HBA = 132.68 °” of the generated 3D-pharmacophore for the tested compounds against S. aureus; Figure S5: 3D-pharmacophore model mapped on the tested compounds against S. aureus; Figure S6: (A) Constraint distances “HBA-1 – HBA-2 = 10.738, HBA-1 – HBA-3 = 3.833, HBA-2 – HBA-3 = 8.105, HBA-1 – H = 6.626, HBA-2 – H = 5.232, HBA-3 – H = 3.905 Å”, (B) Constraint angle “HBA-1 – HBA-2 – HBA-3 = 17.17, HBA-1 – HBA-2 – H = 28.48, HBA-1 – HBA-3 – H = 117.82, HBA-2 – HBA-2 – H = 32.21 °” of the generated 3D-pharmacophore for the tested compounds against B. subtilis; Figure S7: 3D-pharmacophore model mapped on the tested compounds against B. subtilis.
Author Contributions
Conceptualization, A.S.G. and S.S.P.; Methodology, R.M.B., F.R., E.S.N., R.F.B., A.S.G. and S.S.P.; Software, A.S.G. and S.S.P.; Validation, A.S.G. and S.S.P.; Formal analysis, R.M.B., A.S.G., T.S.I., F.R., E.S.N., R.F.B., R.S. and S.S.P.; Software, A.S.G. and S.S.P.; Investigation, R.M.B., T.S.I., A.S.G. and S.S.P.; Resources, A.S.G., T.S.I. and S.S.P.; Data curation, R.M.B., T.S.I., A.S.G. and S.S.P.; Writing—original draft preparation, R.M.B., A.S.G. and S.S.P.; Writing—review and editing, R.M.B., A.S.G., T.S.I., F.R., E.S.N., R.F.B., E.H.A.-A., A.M.M.A.-M. and S.S.P.; Visualization, A.S.G. and S.S.P.; Supervision, A.S.G., T.S.I., E.H.A.-A., A.M.M.A.-M. and S.S.P.; Project administration, A.S.G. and S.S.P.; Funding acquisition, T.S.I. and S.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project under grant No. (RG-18-166-41). The authors, therefore, gratefully acknowledge DSR technical and financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hutchings M.I., Truman A.W., Wilkinson B. Antibiotics: Past, present, and future. Curr. Opin. Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Tomasz A. Microbiology. Weapons of microbial drug resistance abound in soil flora. Science. 2006;311:342–343. doi: 10.1126/science.1123982. [DOI] [PubMed] [Google Scholar]
- 3.Gaston M.H., Verter J.I., Woods G., Pegelow C., Kelleher J., Presbury G., Zarkowsky H., Vichinsky E., Iyer R., Lobel J.S., et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N. Engl. J. Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Guidelines for Treatment of Drug-Susceptible Tuberculosis and Patient Care, update. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 5.Alós J.-I. Antibiotic resistance: A global crisis. Enferm. Infecc. Microbiol. Clin. 2015;33:692–699. doi: 10.1016/j.eimc.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Lepage P., Blumental S. Specialty grand challenge in pediatric infectious diseases. Front. Pediatr. 2017;5:185. doi: 10.3389/fped.2017.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pigrau C., Almirante B. Oxazolidinonas, glucopéptidos y lipopéptidos cíclicos. Enferm. Infecc. Microbiol. Clin. 2009;27:236–246. doi: 10.1016/j.eimc.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Zurenko G.E., Yagi B.H., Schaadt R.D., Allison J.W., Kilburn J.O., Glickman S.E., Hutchinson D.K., Barbachyn M.R., Brickner S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996;40:839–845. doi: 10.1128/AAC.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batts D.H. Linezolid-a new option for treating Gram-positive infections. Oncology. 2000;14:23–29. [PubMed] [Google Scholar]
- 10.Ament P.W., Jamshed N., Horne J.P. Linezolid: Its role in the treatment of Gram-positive, drug-resistant bacterial infections. Am. Fam. Phys. 2002;65:663–670. [PubMed] [Google Scholar]
- 11.Zurenko G.E., Gibson J.K., Shinabarger D.L., Aristoff P.A., Ford C.W., Tarpley W.G. Oxazolidinones: A new class of antibacterials. Curr. Opin. Pharmacol. 2001;1:470–476. doi: 10.1016/S1471-4892(01)00082-0. [DOI] [PubMed] [Google Scholar]
- 12.Brickner S.J., Barbachyn M.R., Hutchinson D.K., Manninen P.R. Linezolid (ZYVOX), the first member of a completely new class of antibacterial agents for treatment of serious gram-positive infections. J. Med. Chem. 2008;51:1981–1990. doi: 10.1021/jm800038g. [DOI] [PubMed] [Google Scholar]
- 13.Perry C.M., Jarvis B. Linezolid: A review of its use in the management of serious Gram-positive infections. Drugs. 2001;61:525–551. doi: 10.2165/00003495-200161040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Locke J.B., Finn J., Hilgers M., Morales G., Rahawi S., Kedar G.C., Picazo J.J., Lm W., Shaw K.J., Stein J.L. Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob. Agents Chemother. 2010;54:5337–5343. doi: 10.1128/AAC.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faidallah H.M., Girgis A.S., Tiwari A.D., Honkanadavar H.H., Thomas S.J., Samir A., Panda S.S. Synthesis, antibacterial properties and 2D-QSAR studies of quinolone-triazole conjugates. Eur. J. Med. Chem. 2018;143:1524–1534. doi: 10.1016/j.ejmech.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Panda S.S., Girgis A.S., Honkanadavar H.H., George R.F., Srour A.M. Synthesis of new ibuprofen hybrid conjugates as potential anti-inflammatory and analgesic agents. Future Med. Chem. 2020;12:1369–1386. doi: 10.4155/fmc-2020-0109. [DOI] [PubMed] [Google Scholar]
- 17.Panda S.S., Girgis A.S., Thomas S.J., Capito J.E., George R.F., Salman A., El-Manawaty M.A., Samir A. Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates. Eur. J. Med. Chem. 2020;196:112293. doi: 10.1016/j.ejmech.2020.112293. [DOI] [PubMed] [Google Scholar]
- 18.Seliem I.A., Panda S.S., Girgis A.S., Nagy Y.I., George R.F., Fayad W., Fawzy N.G., Ibrahim T.S., Al-Mahmoudy A.M.M., Sakhuja R., et al. Design, synthesis, antimicrobial, and DNA gyrase inhibitory properties of fluoroquinolone-dichloroacetic acid hybrids. Chem. Biol. Drug Des. 2020;95:248–259. doi: 10.1111/cbdd.13638. [DOI] [PubMed] [Google Scholar]
- 19.Panda S.S., Girgis A.S., Mishra B.B., Elagawany M., Devarapalli V., Littlefield W.F., Samir A., Fawzy N.G., Srour A.M., Bokhtia R.M. Novel pyrazinoic acid-isoniazid conjugates with amino acid linker: Microwave assisted synthesis, anti-infective properties, and molecular modeling studies. RSC Adv. 2019;9:20450–20462. doi: 10.1039/C9RA03380G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bokhtia R.M., Panda S.S., Girgis A.S., Honkanadavar H.H., Ibrahim T.S., Al-Mahmoudy A.M.M., George R.F., Kashef M.T., Fayad W., Sakhuja R., et al. Fluoroquinolone-3-carboxamide amino acid conjugates: Synthesis, antibacterial properties and molecular modeling studies. Med. Chem. 2021;17:71–84. doi: 10.2174/1573406415666190904143852. [DOI] [PubMed] [Google Scholar]
- 21.Matsingos C., Al-Adhami T., Jamshidi S., Hind C., Clifford M., Sutton J.M., Rahman K.M. Synthesis, microbiological evaluation and structure activity relationship analysis of linezolid analogues with different C5-acylamino substituents. Bioorg. Med. Chem. 2021;49:116397. doi: 10.1016/j.bmc.2021.116397. [DOI] [PubMed] [Google Scholar]
- 22.Panda S.S., Hall C.D., Scriven E., Katritzky A.R. Aminoacyl benzotriazolides: Versatile reagents for the preparation of peptides, their mimetics and conjugates. Aldrichimica Acta. 2013;46:43–55. [Google Scholar]
- 23.Panda S.S., Naumov R.N., Asiri A.M., Katritzky A.R. Microwave-assisted synthesis of biotin conjugates with quinolone antibiotics via amino acids. Synthesis. 2014;46:1511–1517. doi: 10.1002/chin.201448043. [DOI] [Google Scholar]
- 24.Panda S.S., Detistov O.S., Girgis A.S., Mohapatra P.P., Samir A., Katritzky A.R. Synthesis and molecular modeling of antimicrobial active fluoroquinolone-pyrazine conjugates with amino acid linkers. Bioorg. Med. Chem. Lett. 2016;26:2198–2205. doi: 10.1016/j.bmcl.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim M.A., Panda S.S., Birs A.S., Juan C., Serrano J.C., Gonzalez C.F., Katritzky A.R. Synthesis and antibacterial evaluation of amino acid-antibiotic conjugates. Bioorg. Med. Chem. Lett. 2014;24:1856–1861. doi: 10.1016/j.bmcl.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 26.Panda S.S., Liaqat S., Girgis A.S., Samir A., Hall C.D., Katritzky A.R. Novel antibacterial active quinolone-fluoroquinolone conjugates and 2D-QSAR studies. Bioorg. Med. Chem. Lett. 2015;25:3816–3821. doi: 10.1016/j.bmcl.2015.07.077. [DOI] [PubMed] [Google Scholar]
- 27.Fang F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy P.K., Mukkanti K., Rao D.M. Synthesis of antibiotic linezolid analogues. Asian J. Chem. 2012;24:3479–3482. [Google Scholar]
- 29.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 9th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. CLSI Document M07-A9. [Google Scholar]
- 30.Seliem I.A., Panda S.S., Girgis A.S., Moatasim Y., Kandeil A., Mostafa A., Ali M.A., Nossier E.S., Rasslan F., Srour A.M., et al. New quinoline-triazole conjugates: Synthesis, and antiviral properties against SARS-CoV-2. Bioorg. Chem. 2021;114:105117. doi: 10.1016/j.bioorg.2021.105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girgis A.S., Tala S.R., Oliferenko P.V., Oliferenko A.A., Katritzky A.R. Computer-assisted rational design, synthesis, and bioassay of nonsteroidal anti-inflammatory agents. Eur. J. Med. Chem. 2012;50:1–8. doi: 10.1016/j.ejmech.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 32.Ghanim A.M., Girgis A.S., Kariuki B.M., Samir N., Said M.F., Abdelnaser A., Nasr S., Bekheit M.S., Abdelhameed M.F., Almalki A.J., et al. Design and synthesis of ibuprofen-quinoline conjugates as potential anti-inflammatory and analgesic drug candidates. Bioorg. Chem. 2022;119:105557. doi: 10.1016/j.bioorg.2021.105557. [DOI] [PubMed] [Google Scholar]
- 33.CodessaPRO, User’s Manual. [(accessed on 27 December 2021)]. Available online: http://www.codessa-pro.com/manuals/manual.htm.
- 34.Youssef M.A., Panda S.S., El-Shiekh R.A., Shalaby E.M., Aboshouk D.R., Fayad W., Fawzy N.G., Girgis A.S. Synthesis and molecular modeling studies of cholinesterase inhibitor dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines] RSC Adv. 2020;10:21830–21838. doi: 10.1039/D0RA03064C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz M.N., Panda S.S., Shalaby E.M., Fawzy N.G., Girgis A.S. Facile synthetic approach towards vasorelaxant active 4-hydroxyquinazoline-4-carboxamides. RSC Adv. 2019;9:28534–28540. doi: 10.1039/C9RA04321G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shan R., Velazquez C., Knaus E.E. Syntheses, Calcium Channel Agonist–Antagonist Modulation Activities, and Nitric Oxide Release Studies of Nitrooxyalkyl 1,4- Dihydro-2,6-dimethyl-3-nitro-4-(2,1,3-benzoxadiazol-4-yl)pyridine-5-carboxylate Racemates, Enantiomers, and Diastereomers. J. Med. Chem. 2004;4:254–261. doi: 10.1021/jm030333h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or supplementary material.