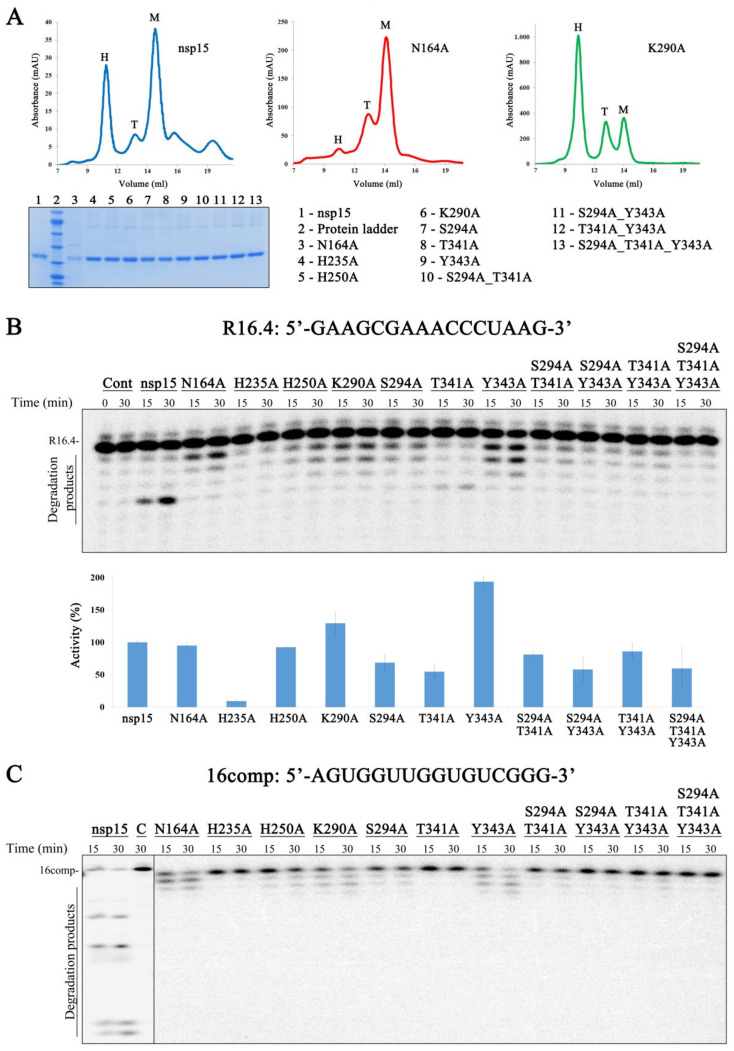
Figure 5.
Effect of nsp15 mutations on its endonucleolytic activity. (A) On the top, chromatograms obtained during nsp15 wt, N164A and K290A size exclusion chromatographies; M, monomer; T, trimer; H, hexamer; on the bottom, a SDS-PAGE gel with all hexameric fractions of the purified proteins. (B) On the top, 800 nM of either nsp15 wt or nsp15 mutant versions were incubated with 50 nM of R16.4 RNA substrate; on the bottom, quantification of nsp15 wt and mutants activity in the presence of R16.4 RNA (% of RNA substrate degraded). The activity of nsp15 wt was considered as 100%. (C) 800 nM of nsp15 wt or each mutant version were incubated with 50 nM of 16comp RNA substrate. Reactions were analyzed on 7 M urea/20% polyacrylamide gels. C, control reactions; time points are indicated in the top of each panel. All the experiments were performed at least in triplicate.