Abstract
The objective of the present study is to evaluate the cytotoxicity of Taiwania cryptomerioides essential oil and its phytochemical on the Hep G2 cell line (human hepatocellular carcinoma). Bark essential oil has significant cytotoxicity to Hep G2 cells, and S3 fraction is the most active fraction in cytotoxicity to Hep G2 cells among the six fractions. The diterpenoid quinone, 6,7-dehydroroyleanone, was isolated from the active S3 fraction by bioassay-guided isolation. 6,7-Dehydroroyleanone exhibited significant cytotoxicity in Hep G2 cells, and the efficacy of 6,7-dehydroroyleanone was better than the positive control, etoposide. Apoptosis analysis of Hep G2 cells with different treatments was characterized via flow cytometry to confirm the cell death situation. Etoposide and 6,7-dehydroroyleanone could induce the apoptosis in Hep G2 cells using flow cytometric assay. Results revealed 6,7-dehydroroyleanone from T. cryptomerioides bark essential oil can be a potential phytochemical to develop the anticancer chemotherapeutic agent for the treatment of the human hepatocellular carcinoma.
Keywords: apoptosis, cytotoxicity, hep g2 cell line, human hepatocellular carcinoma, Taiwania cryptomerioides
1. Introduction
Taiwania cryptomerioides Hayata (Taxodaiceae) is one of the essential woody plants and distributed around Southeast Asia. T. cryptomerioides, Ginkgo biloba, Sequoiadendron giganteum, Metasequoia glyptostroboides, etc., are glacial relict plants and recognized as living fossil plants. Researchers have investigated the phytochemical and bioactivities of extracts from T. cryptomerioides heartwood and bark, which are rich in terpenoids and lignans. Previous studies have shown that natural products from T. cryptomerioides possess antibacterial, antifungal, anti-mite, mosquito larvicidal, anti-termite, antioxidant, anti-inflammatory, and antitumoral activities [1,2,3,4,5,6,7,8]. α-Cadinol, sesquiterpenoid separated from T. cryptomerioides heartwood, exhibited antiproliferative activity on Human colon adenocarcinoma cells [3]. Taiwanin A, the dibenzyl-γ-butyrolactone-type lignan, displayed superior antitumor activity against A-549 lung carcinoma and MCF-7 breast adenocarcinoma and colon adenocarcinoma cell line among the isolated lignans and sesquiterpenoids from T. cryptomerioides heartwood extract [1,9].
Hepatocellular carcinoma, primary liver cancer, is one of the main leading causes of cancer-related death in the world; major therapies are surgical resection, liver transplant, radio frequency ablation, chemotherapy, immunotherapy, etc. Medicinal drugs include sorafenib, lenvatinib, regorafenib, cabozantinib, and ramucirumab; hepatocellular carcinoma still lacks more effectual pharmacotherapies [10,11]. Many researchers are devoted to finding the promising chemicals from plant natural products for hepatocellular carcinoma treatment. Camellia ptilophylla (cocoa tea, naturally non-caffeinated tea) was found to possess anticancer and anti-obesity activities; green cocoa tea infusion could induce the apoptosis of Hep G2 cells [12]. Isoobtusilactone A, the phytochemical isolated from Cinnamomum kotoense, also presents a cytotoxic effect against Hep G2 cells [13]. Taxol (paclitaxel, a well-known chemotherapy drug derived from Taxus brevifolia) also inhibited the cytotoxicity in the HuH-7 cell line [14]. The growth of Hep G2 cells declined after being treated with β-thujaplicin in the xenograft model, and β-thujaplicin could induce both early- and late-stage apoptosis in Hep G2 cells [15]. The proliferation of Hep G2 cell line was inhibited by hinokiflavone; cell shrinkage, chromatin condensation, cell fragmentation, and apoptosis were observed in treated cell line [16].
The objectives of the present research are to evaluate the cytotoxicity and apoptosis induction of T. cryptomerioides bark essential oil in hepatocellular carcinoma cells and to find out its active phytochemical.
2. Materials and Methods
2.1. Hydrodistillation of Bark Essential Oil
Bark of T. cryptomerioides, around 40 years old, was collected from the Experimental Forest of National Taiwan University in Nantou County, Taiwan. The voucher specimen was kept in the laboratory of Chemical Utilization of Biomaterials, School of Forestry and Resource Conservation, National Taiwan University. Essential oil was extracted by hydrodistillation using the Clevenger apparatus for 8 h, the yield of bark essential oil was 0.32 mL/kg. Essential oil was stored in dark glass bottles at 4 °C [17].
2.2. Column Chromatography, Thin Layer Chromatography, and High-Performance Liquid Chromatography
Bark essential oil (8.10 g) was separated by silica gel column chromatography (CC) with the gradient elution of n-hexane and ethyl acetate of increasing polarity. Six fractions (S1–S6) were obtained by the analysis of thin layer chromatography (TLC) [18]. Yields of six fractions were S1 (4.18%), S2 (3.35%), S3 (57.48%), S4 (17.95%), S5 (13.52%), and S6 (3.52%). Active fraction, S3, were analyzed and separated by high-performance liquid chromatography (HPLC, L-2130, Hitachi, Tokyo, Japan) with a preparative Zorbax Sil column (250 mm × 9.4 mm, 5 μm). The gradient mobile phase consisted of n-hexane (A) and ethyl acetate (B). The flow rate was 2 mL/min. The elution program involved a linear gradient from 100% A for 0–3 min, 0 to 10% B in A by 3–25 min, 10 to 50% B in A by 25–35 min, and followed by 50% B in A for 10 min; the UV detector was operated at 254 nm [19,20].
2.3. Identification and Quantification of Isolated Compound
The chemical structure of isolated phytochemical was identified and characterized by spectral analyses, including UV/VIS (Ultraviolet-visible spectroscopy, V-550, Jasco, Tokyo, Japan), FTIR (Fourier transform infrared spectroscopy, FTS-40, Bio-rad, Hercules, CA, USA), and MS (mass spectroscopy, MAT-958, Finnigan, MA, USA). Additionally, 1D NMR (Nuclear magnetic resonance spectroscopy) (1H-NMR, 500 MHz; 13C-NMR, 125 MHz), and 2D NMR (HSQC, HMBC, COSY, and NOESY) were measured by the Bruker AVIII NMR spectrometer (Bruker Avance, Rheinstetten, Germany). The Oak Ridge Thermal Ellipsoid Plot (ORTEP) diagram was recorded with the X-ray single crystal diffractometer (XRD, SMART Apex CCD, Bruker, Karlsruhe, Germany) [21,22,23].
The quantification of the active compound was analyzed by a Thermo Trace GC Ultra gas chromatograph equipped with a Polaris Q MSD mass spectrometer (Thermo Fisher Scientific, Austin, TX, USA). Next, 1 μL analyte was injected into the DB-5MS capillary column (Crossbond 5% phenyl methyl polysiloxane, 30 m length × 0.25 mm i.d. × 0.25 µm film thickness). The temperature program was as follows: 60 °C initial temperature for 3 min; 3 °C/min up to 120 °C; 5 °C/min up to 240 °C and hold for 5 min. The flow rate of carrier gas, helium, was 1 mL/min, and the split ratio was 1:10. Compound was characterized by comparing the mass spectra (m/z 50–650 amu); quantification was analyzed by integrating the peak area of the chromatogram using the flame ionization detector (FID) [24,25].
2.4. Cell Culture and Cell Cytotoxicity Assay
Hep G2 cell, human hepatocellular carcinoma cell line, was provided by the Hepatitis Research Center (HRC) of the National Taiwan University Hospital (NTUH). Cell line was cultured in high-glucose DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% NEAA (non-essential amino acid) at 37 °C in a humidified atmosphere with 5% CO2. For cell cytotoxicity assay, Hep G2 cells were seeded in a 96 well plate (2 × 104 cell/well) and incubated for 24 h, then the medium was removed and treated with various concentrations of specimens in 0.2% of DMSO (dimethyl sulfoxide) for 24 h. After the medium was removed, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), 1 mg/mL) reagent was added in each well for 1 h, then the medium containing MTT was removed, and 100 µL of DMSO was added to the well to solubilize the formazan crystals. Absorbance was measured at 570 nm using an ELISA (enzyme-linked immunosorbent assay) reader (SPECTROstar Nano, BMG LABTECH, Offenburg, Germany). The percentage of cytotoxicity was calculated by the following formula: Cytotoxicity (%) = (ODcontrol − ODsample)/ODcontrol × 100. The experiments were performed in triplicate [26,27,28].
2.5. Apoptosis Analysis Using Flow Cytometric Assay
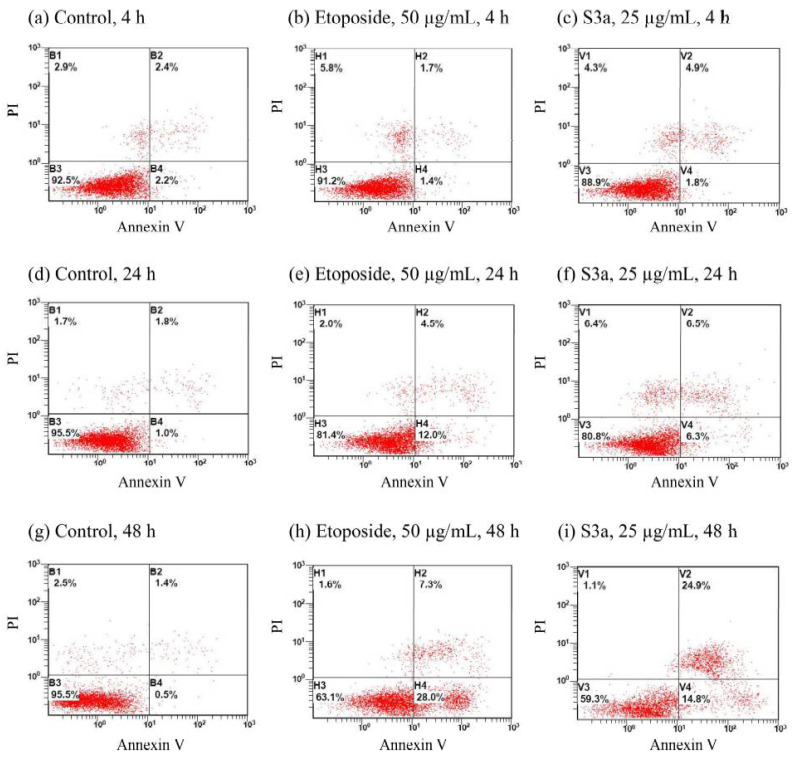
Hep G2 cells were seeded in a 6-well plate (2 × 105 cell/well) and incubated for 24 h, then the medium was removed and replaced with the medium containing various concentrations of specimens for 4, 24, and 48 h. Cells were collected and washed twice with PBS, then suspended in 500 μL of Annexin binding buffer containing 5 µL of Alexa fluor® 488 Annexin V and 5 µL of PI (propidium iodide) for 15 min in the dark. The apoptotic effect of Hep G2 cells treated with different specimens was analyzed using Cytomics FC500 flow cytometry (Beckman Coulter, Miami, FL, USA). Double immunocytochemistry labeling with FL1 (Annexin V; green fluorescence) and FL3 (propidium iodide; red fluorescence) was used to differentiate four populations (four quadrants in Figure 1), including necrotic cells (Q1, red fluorescent), late apoptotic cells (Q2, both green/red fluorescent), alive/healthy cells (Q3, no fluorescent), and early apoptotic cells (Q4, green fluorescent) [29,30].
Figure 1.
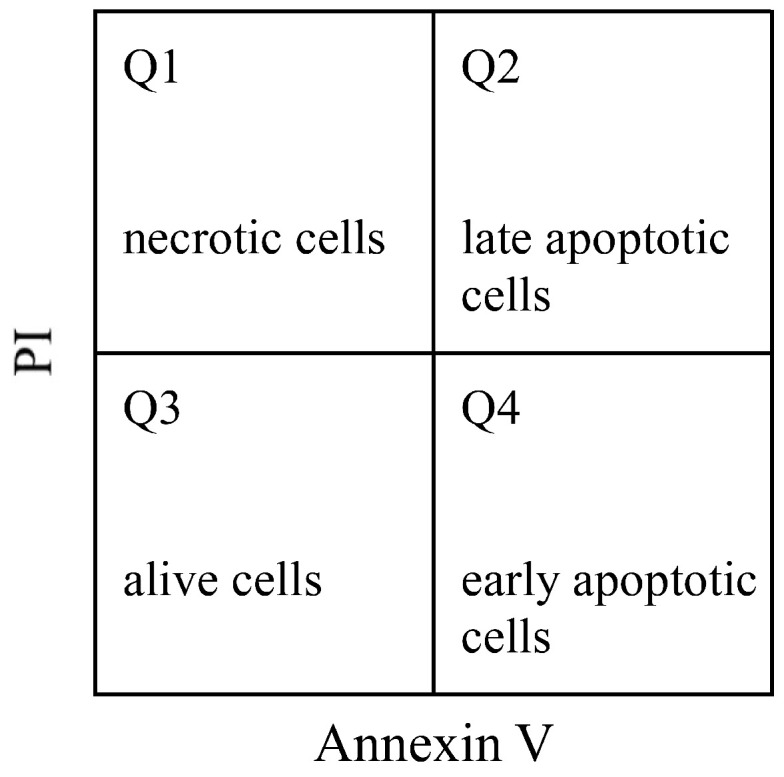
Differentiation of four cell populations by double immunocytochemistry labeling.
2.6. Statistical Analysis
The statistical analysis was performed by using SPSS (Statistical Product and Service Solutions) (Chicago, IL, USA) version 16 with Scheffe’s test, a post hoc multiple comparison method. The confidence interval was set at the 95% confidence level.
3. Results and Discussion
3.1. Cytotoxic Effect of T. cryptomerioides Bark Essential Oil and Its Fractions on Hep G2 Cells
The cytotoxicity and IC50 value of T. cryptomerioides bark essential oil and its fractions against Hep G2 cells were shown in Table 1. The antiproliferation of bark essential oil was increased with the concentration. The cytotoxicity of bark essential oil was 77.80% at a concentration of 100 µg/mL against Hep G2 cells; the IC50 value was 54.31 μg/mL for 24 h. Myint et al. investigated the cytotoxic effect of Smallanthus sonchifolius leaf extract on Hep G2 cells. The IC50 value of leaf extract was 58.2 µg/mL after 24 h treatment [31]. The green cocoa tea (Camellia ptilophylla) infusion possessed the antiproliferation of Hep G2 cells in a dose-dependent effect with an IC50 value of 292 μg/mL for 72 h [12]. Results from the MTT assay revealed that T. cryptomerioides bark essential oil had a potent cytotoxic effect on Hep G2 cells.
Table 1.
Cytotoxicity of bark essential oil and active fractions against Hep G2 cells.
| Specimen | Concentration (μg/mL) |
Cytotoxicity * (%) |
IC50 * (μg/mL) |
|---|---|---|---|
| Bark oil | 12.5 | 10.23 ± 0.98 | 54.31 ± 0.60 a |
| 25 | 22.20 ± 0.10 | ||
| 50 | 49.47 ± 0.17 | ||
| 100 | 77.80 ± 0.17 | ||
| S2 fraction | 12.5 | 18.81 ± 0.90 | 32.22 ± 4.84 b |
| 25 | 26.43 ± 9.70 | ||
| 50 | 84.44 ± 1.75 | ||
| 100 | 90.62 ± 0.33 | ||
| S3 fraction | 12.5 | 44.37 ± 4.50 | 21.17 ± 1.09 c |
| 25 | 55.98 ± 3.57 | ||
| 50 | 82.61 ± 0.70 | ||
| 100 | 93.38 ± 0.23 |
Results are mean ± SD; *: 24 h of treatment; IC50 value: half maximal inhibitory concentration. Different letters (a–c) in the Table indicate significantly different at the level of p < 0.05 according to Scheffe’s test.
Among the six fractions obtained from open column chromatography of bark essential oil, S2 fraction and S3 fraction possessed a better cytotoxic effect on Hep G2 cells. The IC50 values of the other fractions were greater than 50 μg/mL. The IC50 values of S2 fraction and S3 fraction were 32.22 and 21.17 μg/mL for 24 h, respectively (Table 1). S3 fraction showed the best cytotoxicity in Hep G2 cells with a statistical significance of p < 0.05.
3.2. Identification of Chemical and Molecular Structures of 6,7-Dehydroroyleanone
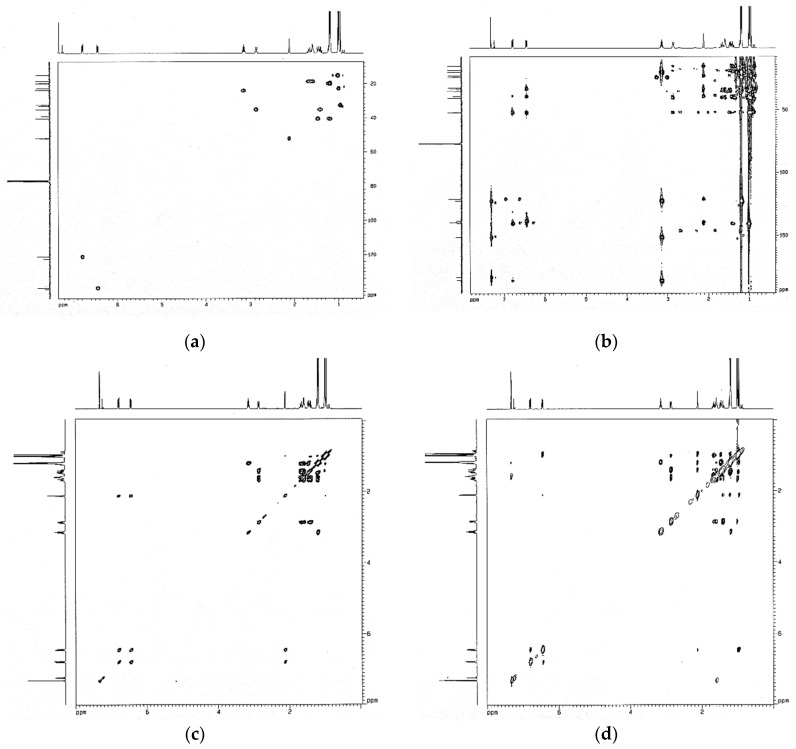
The structure of the compound from active fraction S3 is elucidated based on the 1D and 2D NMR (Figure 2), single-crystal XRD, FTIR, UV, and MS spectroscopic analyses. 6,7-Dehydroroyleanone 232 mg. Orangish red needle; mp: 168–169 °C; UV (MeOH) λmax (log ε): 221.0 (3.72), 251.0 (3.51), 333.5 (3.43), and 461.5 (2.43) nm; [α]D21.8 = −248.0° (CHCl3; c = 0.13); IR (KBr) νmax 3364, 3089, 2961, 2927, 2870, 1626, 1551, 1460, 1387, 1376, 1329, 1254, and 1165 cm−1; 1H NMR (CDCl3, 500 MHz) δ 7.32 (1H, s, OH-12), 6.78 (1H, dd, J = 9.8, 3.0 Hz, H-7), 6.44 (1H, dd, J = 9.8, 3.0 Hz, H-6), 3.14 (1H, hept, J = 7.0 Hz, H-15), 2.86 (1H, d, J = 13.4 Hz, H-3a), 2.12 (1H, t, J = 3.0 Hz, H-5), 1.67 (1H, m, H-2a), 1.59 (1H, m, H-2b), 1.47 (1H, d, J = 13.4 Hz, H-1a), 1.41 (1H, td, J = 13.4, 3.8 Hz, H-3b), 1.23 (1H, m, H-1b), 1.20 (3H, d, J = 7.0 Hz, H-17), 1.19 (3H, d, J = 7.0 Hz, H-16), 1.01 (3H, s, H-19), 0.99 (3H, s, H-18), 0.96 (3H, s, H-20). 13C NMR (CDCl3, 125 MHz) δ 186.06 (C-14), 183.44 (C-11), 151.19 (C-12), 140.51 (C-8), 139.6 (C-6), 138.5 (C-9), 122.59 (C-13), 121.10 (C-7), 52.10 (C-5), 40.52 (C-1), 39.25 (C-4), 35.15 (C-3), 33.26 (C-10), 32.60 (C-20), 24.08 (C-15), 22.80 (C-18), 20.00 (C-16), 19.80 (C-17), 18.67 (C-2), 15.17 (C-19). EI-MS m/z 314 [M]+, 299, 271, 258, 245, 232, 213, 187, 159, 141, 128, 115, 83, molecular formula C20H26O3; single-crystal XRD analysis: monoclinic crystal system; P21 space group. The chemical structure and ORTEP diagram of the identified compound are shown in Figure 3; 6,7-dehydroroyleanone is a diterpenoid quinone based on an abietane skeleton. Quantification of compound 6,7-dehydroroyleanone was 8.81 ± 0.21% using the analysis of GC.
Figure 2.
2D NMR spectra of 6,7-dehydroroyleanone. (a) HMQC; (b) HMBC; (c) COSY; (d) NOESY.
Figure 3.
Chemical and molecular structures of 6,7-dehydroroyleanone. (a) Chemical structure; (b) ORTEP diagram of the molecular structure.
6,7-Dehydroroyleanone has been isolated from Inula royleana root, Plectranthus grandidentatus, P. madagascariensis; Salvia lavandulaefolia root, Salvia jaminiana root, Taxodium distichum cone and seed, Tetradenia riparia leaf [32,33,34,35,36,37,38,39,40]. The bioactivities of 6,7-dehydroroyleanone have been reported, including anti-termite, antioxidant, antimicrobial, antileishmanial, and anti-Mycobacterium tuberculosis activities [36,37,38,41,42,43]. 6,7-Dehydroroyleanone exhibits the cytotoxicity activity against breast cancer cell lines, including MCF-7 (hormone-positive breast cancer cells), SkBr3 Her-positive, SUM159 triple-negative, and SUM159 spheres [44], and primary H7PX glioma cell lines [45].
3.3. Cytotoxic Effect of 6,7-Dehydroroyleanone on Hep G2 Cells
Etoposide, a podophyllotoxin derivative, exhibits topoisomerase II inhibition activity, thereby leading to the cytotoxicity of tumor cells [46]. Etoposide was selected as the cytotoxicity positive control in the current study. The cytotoxic activities of etoposide, bark essential oil, and 6,7-dehydroroyleanone against Hep G2 cells were presented in Table 2. The IC50 values of etoposide were 76.89 μg/mL (130.64 μM) and 29.68 μg/mL (51.84 μM) for 24 and 48 h. Bakherad et al. reported that etoposide exhibited cytotoxicity against Hep G2 cells with an IC50 value of 31.38 μM after 48 h incubation [47]; the activity of etoposide was similar to that of the present study.
Table 2.
Cytotoxicity of bark essential oil and 6,7-dehydroroyleanone against Hep G2 cells.
| Time (h) |
Specimen | IC50 (μg/mL) |
IC50 (µM) |
|---|---|---|---|
| 24 | Bark essential oil | 54.31 ± 0.60 b | - |
| 6,7-Dehydroroyleanone | 10.28 ± 0.18 d | 32.74 ± 0.57 C | |
| Etoposide * | 76.89 ± 0.34 a | 130.64 ± 0.58 A | |
| 48 | Bark essential oil | 30.27 ± 1.11 c | - |
| 6,7-Dehydroroyleanone | 5.22 ± 0.09 e | 16.62 ± 0.29 D | |
| Etoposide * | 29.68 ± 1.18 c | 50.43 ± 2.01 B |
Results are mean ± SD. *: Positive control. IC50 value: half maximal inhibitory concentration. Different letters (a–e; A–D) in the Table indicate significant difference at the level of p < 0.05 according to Scheffe’s test.
Bark essential oil exhibited cytotoxicity against Hep G2 as potent as etoposide; the IC50 values were 30.27 and 29.68 µg/mL for 48 h treatment, respectively; there were no statistically significant differences (p < 0.05) in the MTT assay. The cytotoxicity of 6,7-dehydroroyleanone was more effective than those of bark essential oil and etoposide. The IC50 values of 6,7-dehydroroyleanone were 10.28 μg/mL (32.74 μM) and 5.22 μg/mL (16.62 μM) for 24 and 48 h. Results revealed that 6,7-dehydroroyleanone is a promising phytochemical which possesses a significant superior cytotoxic effect on Hep G2 cells, of which the efficacy was superior to the positive control, etoposide.
3.4. Apoptosis Effect of 6,7-Dehydroroyleanone on Hep G2 cells
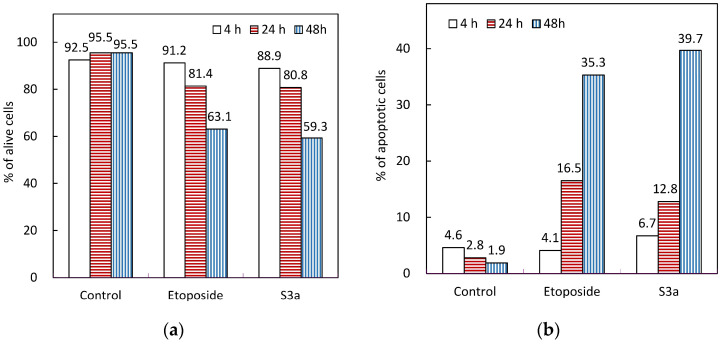
The apoptosis effects of etoposide and 6,7-dehydroroyleanone on Hep G2 cells were evaluated by using an Annexin V/propidium iodide staining method (Figure 4). The percentage of alive cells (Q2 area) of control were above 92% during the treatment period. Content of the apoptotic cells of control were kept below five percent during the 48 h of treatment. The necrotic cells (Q1 area) of the Hep G2 cell treated with etoposide was 5.8% for 4 h of treatment at a concentration of 50 μg/mL; a decrease in the necrotic process was observed after 24 and 48 h of treatment. Both Hep G2 cells exposed to etoposide and 6,7-dehydroroyleanone for 48 h had undergone the early and late apoptotic process (Q4 and Q2 areas), programmed cell death. The percentage of apoptotic cells of cells was 35.3 and 39.7% after 48 h of treatment with etoposide and 6,7-dehydroroyleanone, respectively (Figure 5). The apoptotic cells of all treated cells increased with the incubation time. Apoptosis effect of 6,7-dehydroroyleanone was confirmed through the Annexin V/PI flow cytometry assay. Sitarek et al. also reported that 6,7-dehydroroyleanone possessed the antiproliferative effect in H7PX glioma cell line by apoptosis; a similar anticancer effect was observed in this study [45]. Taiwanin A, a lignan isolated and identified from T. cryptomerioides heartwood extract, could also induce apoptosis in Hep G2 cells [6].
Figure 4.
Apoptosis analysis by Annexin V/PI flow cytometry of treated Hep G2 cells. (a) Control, 4 h; (b) etoposide, 50 µg/mL, 4 h; (c) S3a, 25 µg/mL, 4 h; (d) control, 24 h; (e) etoposide, 50 µg/mL, 24 h; (f) S3a, 25 µg/mL, 24 h; (g) control, 48 h; (h) etoposide, 50 µg/mL, 48 h; (i) S3a, 25 µg/mL, 48 h. S3a: 6,7-dehydroroyleanone.
Figure 5.
Etoposide and 6,7-dehydroroyleanone induced apoptosis in Hep G2 cells. (a) alive cells; (b) apoptotic cells. Etoposide, 50 µg/mL; S3a: 6,7-dehydroroyleanone, 25 µg/mL.
4. Conclusions
Using the colorimetric MTT assay, T. cryptomerioides bark essential oil and S3 fraction show the high cytotoxicity to Hep G2 cells. 6,7-Dehydroroyleanone was isolated from the fraction that possessed the best cytotoxicity through bioassay-guided fractionation; it is classified as a diterpenoid quinone determined by spectroscopic analyses. 6,7-Dehydroroyleanone exhibited better cytotoxicity in Hep G2 cells than etoposide, a clinical antitumor drug. The cytotoxicity of etoposide, bark essential oil, and 6,7-dehydroroyleanone was in a dose-dependent manner against the Hep G2 cells. Apoptosis analysis indicated that etoposide and 6,7-dehydroroyleanone induce apoptosis in Hep G2 cells, including early and late programmed cell death. These findings in the current study revealed 6,7-dehydroroyleanone from T. cryptomerioides bark essential oil merits further research and trials to develop as a chemotherapeutic phytochemical for human hepatocellular carcinoma.
Acknowledgments
The authors gratefully thank the Experimental Forest of National Taiwan University (NTU), Hepatitis Research Center (HRC) of the National Taiwan University Hospital (NTUH), Joint Center for Instruments and Research, College of Bioresources and Agriculture, NTU, and Shou-Ling Huang for the assistance in NMR experiments of the Instrumentation Center at NTU.
Author Contributions
Conceptualization, S.-T.C. and H.-T.C.; methodology, M.-L.C., G.-R.C. and H.-T.C.; software, G.-R.C. and Y.-T.H.; formal analysis and investigation, G.-R.C., M.-L.C. and H.-T.C.; writing—original draft preparation, H.-T.C., M.-L.C. and Y.-T.H.; writing—review and editing, H.-T.C. and S.-T.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang S.T., Wang D.S., Wu C.L., Shiah S.G., Kuo Y.H., Chang C.J. Cytotoxicity of extractives from Taiwania cryptomerioides heartwood. Phytochemistry. 2000;55:227–232. doi: 10.1016/S0031-9422(00)00275-2. [DOI] [PubMed] [Google Scholar]
- 2.Chang C.I., Chang J.Y., Kuo C.C., Pan W.Y., Kuo Y.H. Four new 6-nor5(6 → 7)abeo-abietane type diterpenes and antitumoral cytotoxic diterpene constituents from the bark of Taiwania cryptomerioides. Planta Med. 2005;71:72–76. doi: 10.1055/s-2005-837754. [DOI] [PubMed] [Google Scholar]
- 3.He K., Zeng L., Shi G., Zhao G.X., Kozlowski J.F., McLaughlin J.L. Bioactive compounds from Taiwania cryptomerioides. J. Nat. Prod. 1997;60:38–40. doi: 10.1021/np960513c. [DOI] [PubMed] [Google Scholar]
- 4.Shyur L.F., Lee S.H., Chang S.T., Lo C.P., Kuo Y.H., Wang S.Y. Taiwanin A inhibits MCF-7 cancer cell activity through induction of oxidative stress, upregulation of DNA damage checkpoint kinases, and activation of p53 and FasL/Fas signaling pathways. Phytomedicine. 2010;18:16–24. doi: 10.1016/j.phymed.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Chang S.T., Chen P.F., Wang S.Y., Wu H.H. Antimite activity of essential oils and their constituents from Taiwania Cryptomerioides. J. Med. Entomol. 2001;38:455–457. doi: 10.1603/0022-2585-38.3.455. [DOI] [PubMed] [Google Scholar]
- 6.Ho P.J., Chou C.K., Kuo Y.H., Tu L.C., Yeh S.F. Taiwanin A induced cell cycle arrest and p53-dependent apoptosis in human hepatocellular carcinoma HepG2 cells. Life Sci. 2007;80:493–503. doi: 10.1016/j.lfs.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Chang S.T., Cheng S.S., Wang S.Y. Antitermitic activity of essential oils and components from Taiwania (Taiwania cryptomerioides) J. Chem. Ecol. 2001;27:717–724. doi: 10.1023/A:1010397801826. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H.H., Kuo W.W., Day C.H., Shibu M.A., Li S.Y., Chang S.H., Shih H.N., Chen R.J., Viswanadha V.P., Kuo Y.H., et al. Taiwanin E inhibits cell migration in human LoVo colon cancer cells by suppressing MMP-2/9 expression via p38 MAPK pathway. Environ. Toxicol. 2017;32:2021–2031. doi: 10.1002/tox.22379. [DOI] [PubMed] [Google Scholar]
- 9.Harn H.J., Chuang H.M., Chang L.F., Huang A.Y., Hsieh S.T., Lin S.Z., Chou C.W., Kuo Y.H., Chiou T.W. Taiwanin A targets non-steroidal anti-inflammatory drug-activated gene-1 in human lung carcinoma. Fitoterapia. 2014;99:227–235. doi: 10.1016/j.fitote.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Viveiros P., Riaz A., Lewandowski R.J., Mahalingam D. Current state of liver-directed therapies and combinatory approaches with systemic therapy in hepatocellular carcinoma (HCC) Cancers. 2019;11:1085. doi: 10.3390/cancers11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolley D.L., Crouch A.C., Özkan A., Seeley E.H., Whitley E.M., Rylander M.N., Cressman E.N.K. Combining chemistry and engineering for hepatocellular carcinoma: Nano-scale and smaller therapies. Pharmaceutics. 2020;12:1243. doi: 10.3390/pharmaceutics12121243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X.R., Wang Y.Y., La K.K., Peng L., Song X.H., Shi X.G., Zhu X.F., Leung P.C., Ko C.H., Ye C.X. Inhibitory effects of cocoa tea (Camellia ptilophylla) in human hepatocellular carcinoma HepG2 in vitro and in vivo through apoptosis. J. Nutr. Biochem. 2012;23:1051–1057. doi: 10.1016/j.jnutbio.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Liu T.Z., Cheng J.T., Yiin S.J., Chen C.Y., Chen C.H., Wu M.J., Chern C.L. Isoobtusilactone A induces both caspase-dependent and -independent apoptosis in Hep G2 cells. Food Chem. Toxicol. 2008;46:321–327. doi: 10.1016/j.fct.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Gagandeep S., Novikoff P.M., Ott M., Gupta S. Paclitaxel shows cytotoxic activity in human hepatocellular carcinoma cell lines. Cancer Lett. 1999;136:109–118. doi: 10.1016/S0304-3835(98)00388-7. [DOI] [PubMed] [Google Scholar]
- 15.Zhang G., He J., Ye X., Zhu J., Hu X., Shen M., Ma Y., Mao Z., Song H., Chen F. β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019;10:255. doi: 10.1038/s41419-019-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mu W., Cheng X., Zhang X., Liu Y., Lv Q., Liu G., Zhang J., Li X. Hinokiflavone induces apoptosis via activating mitochondrial ROS/JNK/caspase pathway and inhibiting NF-κB activity in hepatocellular carcinoma. J. Cell. Mol. Med. 2020;24:8151–8165. doi: 10.1111/jcmm.15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C.Y., Yeh T.F., Hsu F.L., Lin C.Y., Chang S.T., Chang H.T. Xanthine oxidase inhibitory activity and thermostability of cinnamaldehyde-chemotype leaf oil of Cinnamomum osmophloeum microencapsulated with β-cyclodextrin. Molecules. 2018;23:1107. doi: 10.3390/molecules23051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starek M., Plenis A., Zagrobelna M., Dabrowska M. Assessment of lipophilicity descriptors of selected NSAIDS obtained at different TLC stationary phases. Pharmaceutics. 2021;13:440. doi: 10.3390/pharmaceutics13040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen P.L., Wu C.L., Chang S.T., Huang S.L., Chang H.T. Antioxidative lignans from phytochemical extract of Calocedrus formosana Florin. BioResources. 2012;7:4122–4131. [Google Scholar]
- 20.Santonocito D., Granata G., Geraci C., Panico A., Siciliano E.A., Raciti G., Puglia C. Carob seeds: Food waste or source of bioactive compounds? Pharmaceutics. 2020;12:1090. doi: 10.3390/pharmaceutics12111090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes A., Marto J., Gonçalves L.M., Simões S., Félix R., Ascenso A., Lopes F., Ribeiro H.M. Novel and modified neutrophil elastase inhibitor loaded in topical formulations for psoriasis management. Pharmaceutics. 2020;12:358. doi: 10.3390/pharmaceutics12040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvestre G.F.G., Lucena R.P., Oliveira G.D., Pereira H.N., Dias J.A.B., Souza I.A., Alves H.S. Anti-tumor and anti-inflammatory activity in vivo of Apodanthera congestiflora Cogn. (Cucurbitaceae) Pharmaceutics. 2021;13:743. doi: 10.3390/pharmaceutics13050743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C.Y., Liu I.H., Huang X.Z., Chen H.J., Chang S.T., Chang M.L., Ho Y.T., Chang H.T. Antimelanogenesis effects of leaf extract and phytochemicals from ceylon olive (Elaeocarpus serratus) in zebrafish model. Pharmaceutics. 2021;13:1059. doi: 10.3390/pharmaceutics13071059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. pp. 6–398. [Google Scholar]
- 25.Chang H.T., Lin C.Y., Hsu L.S., Chang S.T. Thermal degradation of linalool-chemotype Cinnamomum osmophloeum leaf essential oil and its stabilization by microencapsulation with β-cyclodextrin. Molecules. 2021;26:409. doi: 10.3390/molecules26020409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vassallo A., Armentano M.F., Miglionico R., Caddeo C., Chirollo C., Gualtieri M.J., Ostuni A., Bisaccia F., Faraone I., Milella L. Hura crepitans L. extract: Phytochemical characterization, antioxidant activity, and nanoformulation. Pharmaceutics. 2020;12:553. doi: 10.3390/pharmaceutics12060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Łapińska Z., Dębiński M., Szewczyk A., Choromańska A., Kulbacka J., Saczko J. Electrochemotherapy with calcium chloride and 17β-estradiol modulated viability and apoptosis pathway in human ovarian cancer. Pharmaceutics. 2021;13:19. doi: 10.3390/pharmaceutics13010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kryczyk-Poprawa A., Zupkó I., Bérdi P., Żmudzki P., Piotrowska J., Pękala E., Berdys A., Muszyńska B., Opoka W. Photodegradation of bexarotene and its implication for cytotoxicity. Pharmaceutics. 2021;13:1220. doi: 10.3390/pharmaceutics13081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietkiewicz S., Schmidt J.H., Lavrik I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of imaging flow cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods. 2015;423:99–103. doi: 10.1016/j.jim.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Song J., Ham J., Hong T., Song G., Lim W. Fraxetin suppresses cell proliferation and induces apoptosis through mitochondria dysfunction in human hepatocellular carcinoma cell lines Huh7 and Hep3B. Pharmaceutics. 2021;13:112. doi: 10.3390/pharmaceutics13010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myint P.P., Dao T.T.P., Kim Y.S. Anticancer activity of Smallanthus sonchifolius methanol extract against human hepatocellular carcinoma cells. Molecules. 2019;24:3054. doi: 10.3390/molecules24173054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards O.E., Feniak G., Los M. Diterpenoid quinones of Inula royleana D. C. Can. J. Chem. 1962;40:1540–1546. doi: 10.1139/v62-232. [DOI] [Google Scholar]
- 33.Michavila A., Fernández-Gadea F., Rodríguez B. Abietane diterpenoids from the root of Salvia lavandulaefolia. Phytochemistry. 1985;25:266–268. doi: 10.1016/S0031-9422(00)94547-3. [DOI] [Google Scholar]
- 34.Teixeira A.P., Batista O., Simões M.F., Nascimento J., Duarte A., de la Torre M.C., Rodríguez B. Abietane diterpenoids from Plectranthus grandidentatus. Phytochemistry. 1997;44:325–327. doi: 10.1016/S0031-9422(96)00467-0. [DOI] [Google Scholar]
- 35.Kabouche A., Boutaghane N., Kabouche Z., Seguin E., Tillequin F., Benlabed K. Components and antibacterial activity of the roots of Salvia jaminiana. Fitoterapia. 2005;76:450–452. doi: 10.1016/j.fitote.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Kusumoto N., Ashitani T., Hayasaka Y., Murayama T., Ogiyama K., Takahashi K. Antitermitic activities of abietane-type diterpenes from Taxodium distichum cones. J. Chem. Ecol. 2009;35:635–642. doi: 10.1007/s10886-009-9646-0. [DOI] [PubMed] [Google Scholar]
- 37.Gazim Z.C., Rodrigues F., Amorin A.C.L., de Rezende C.M., Soković M., Tešević V., Vučković I., Krstić G., Cortez L.E.R., Colauto N.B., et al. New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules. 2014;19:514–524. doi: 10.3390/molecules19010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demarchi I.G., Thomazella M.V., de Souza Terron M., Lopes L., Gazim Z.C., Cortez D.A., Donatti L., Aristides S.M., Silveira T.G., Lonardoni M.V. Antileishmanial activity of essential oil and 6,7-dehydroroyleanone isolated from Tetradenia riparia. Exp. Parasitol. 2015;157:128–137. doi: 10.1016/j.exppara.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Chen L., Ma X., Deng S., Yang X., Song P. Crystal structure of 6,7-de-hydro-royleanone isolated from Taxodium distichum (L.) Rich. Acta Crystallogr. E. Crystallogr. Commun. 2018;74:62–64. doi: 10.1107/S2056989017017935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia C., Silva C.O., Monteiro C.M., Nicolai M., Viana A., Andrade J.M., Barasoain I., Stankovic T., Quintana J., Hernández I., et al. Anticancer properties of the abietane diterpene 6,7-dehydroroyleanone obtained by optimized extraction. Future Med. Chem. 2018;10:1177–1189. doi: 10.4155/fmc-2017-0239. [DOI] [PubMed] [Google Scholar]
- 41.Kubínová R., Pŏrízková R., Navrátilová A., Farsa O., Hanáková Z., Băcinská A., Čížek A., Valentová M., Cížek A., Valentová M. Antimicrobial and enzyme inhibitory activities of the constituents of Plectranthus madagascariensis (Pers.) Benth. J. Enzym. Inhib. Med. Chem. 2014;6366:1–4. doi: 10.3109/14756366.2013.848204. [DOI] [PubMed] [Google Scholar]
- 42.Terron-Monich M.S., Demarchi I.G., da Silva P.R.F., Ramos-Milaré Á.C.F.H., Gazim Z.C., Silveira T.G.V., Lonardoni M.V.C. 6,7-Dehydroroyleanone diterpene derived from Tetradenia riparia essential oil modulates IL-4/IL-12 release by macrophages that are infected with Leishmania amazonensis. Parasitol Res. 2019;118:369–376. doi: 10.1007/s00436-018-6166-2. [DOI] [PubMed] [Google Scholar]
- 43.Baldin V.P., Scodro R.B.L., Lopes-Ortiz M.A., de Almeida A.L., Gazim Z.C., Ferarrese L., Faiões V.D.S., Torres-Santos E.C., Pires C.T.A. Caleffi-Ferracioli, K.R.; et al. Anti-Mycobacterium tuberculosis activity of essential oil and 6,7-dehydroroyleanone isolated from leaves of Tetradenia riparia (Hochst.) Codd (Lamiaceae) Phytomedicine. 2018;47:34–39. doi: 10.1016/j.phymed.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 44.Isca V.M.S., Sencanski M., Filipovic N., Dos Santos D.J.V.A., Čipak Gašparović A., Saraíva L., Afonso C.A.M., Rijo P., García-Sosa A.T. Activity to breast cancer cell lines of different malignancy and predicted interaction with protein kinase c isoforms of royleanones. Int. J. Mol. Sci. 2020;21:3671. doi: 10.3390/ijms21103671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sitarek P., Toma M., Ntungwe E., Kowalczyk T., Skała E., Wieczfinska J., Śliwiński T., Rijo P. Insight the biological activities of selected abietane diterpenes isolated from Plectranthus spp. Biomolecules. 2020;10:194. doi: 10.3390/biom10020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amiri F., Zarnani A.H., Zand H., Koohdani F., Jeddi-Tehrani M., Vafa M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur. J. Pharmacol. 2013;718:34–40. doi: 10.1016/j.ejphar.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Bakherad Z., Safavi M., Fassihi A., Sadeghi-Aliabadi H., Bakherad M., Rastegar H., Saeedi M., Ghasemi J.B., Saghaie L., Mahdavi M. Design and synthesis of novel cytotoxic indole-thiosemicarbazone derivatives: Biological evaluation and docking study. Chem. Biodivers. 2019;16:e1800470. doi: 10.1002/cbdv.201800470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available are available from the corresponding author on reasonable request.