Abstract
Exosomes, key mediators of cell-cell communication, derived from Type 2 diabetes mellitus (T2DM) exhibit detrimental effects. Exercise improves endothelial function in part via secretion of exosomes into circulation. Extracellular superoxide dismutase (SOD3) is a major secretory copper (Cu) antioxidant enzyme that catalyzes the dismutation of O2•- to H2O2 whose activity requires the Cu transporter ATP7A. However, role of SOD3 in exercise-induced angiogenic effects of circulating plasma exosomes on endothelial cells (ECs) in T2DM remains unknown. Here we show that both SOD3 and ATP7A proteins were present in plasma exosomes in mice, which was significantly increased after two weeks volunteer wheel exercise. A single bout of exercise in human also showed a significant increase in SOD3 and ATP7A protein expression in plasma exosomes. Plasma exosomes from T2DM mice significantly reduced angiogenic responses in human ECs or mouse skin wound healing model, which was associated with decrease in ATP7A, but not SOD3 expression in exosomes. Exercise training in T2DM mice restored the angiogenic effects of T2DM exosomes in ECs by increasing ATP7A in exosomes, which was not observed in exercised T2DM/SOD3−/− mice. Furthermore, exosomes overexpressing SOD3 significantly enhanced angiogenesis in ECs by increasing local H2O2 levels in a heparin binding domain-dependent manner as well as restored defective wound healing and angiogenesis in T2DM or SOD3−/− mice. In conclusion, exercise improves the angiogenic potential of circulating exosomes in T2DM in a SOD3-dependent manner. Exosomal SOD3 may provide an exercise mimetic therapy that supports neovascularization and wound repair in cardiometabolic disease.
Keywords: Exosome, Exercise, Type 2 Diabetes, SOD3
1 |. INTRODUCTION
Exosomes are 50~150nm diameter nano-sized, bilayer extracellular vesicles that are secreted by various cell types. Exosomes play a crucial role in cell-cell communication by delivering biologically active cargo including proteins, lipids, mRNAs and microRNAs to recipient cells(1, 2). The inventory of cargo packaged into exosomes is regulated in a cell type specific manner and this can be further influenced by cellular conditions(3). Previous research has focused on the role of exosomes as biomarkers of disease including cardiovascular diseases, cancer, and metabolic diseases such as obesity and Type 2 diabetes mellitus (T2DM)(4, 5). Studies reveal that exosomes can have beneficial roles on cardiovascular health(6, 7) by ferrying and delivering cardioprotective factors induced by ischemic preconditioning(8) and exercise training(9, 10). In contrast, circulating exosomes-derived from cardiovascular and metabolic disease states including T2DM can have detrimental effects to promote endothelial cell (EC) dysfunction, inflammation, and impair angiogenesis (11–13).
Exercise training elicits a range of beneficial effects including endothelial function, angiogenesis and cardiovascular protection in metabolic disease such as T2DM and obesity (14). Exercise is able to stimulate the release of a variety of “exerkines” into the circulation that can be contained within exosomes(10). Exercise-derived exosomes have been shown to be released into the circulation from skeletal muscle and other cell types and mediate exercise-mediated adaptation and beneficial cardiovascular effects on distal target cells in normal and pathological conditions such as T2DM(15, 16). Thus, bioengineered modified exosomes enriched with exerkines can be used as therapeutic tools for the treatment of cardiovascular and metabolic diseases(6). However, the mechanisms by which exercise-induced exosomes stimulate systemic beneficial effects in T2DM remain poorly understood.
Extracellular superoxide dismutase (ecSOD, SOD3) is a major copper (Cu) containing antioxidant enzyme that is synthesized and secreted from vascular smooth muscle cells (VSMCs) and skeletal muscle(17, 18). SOD3 catalyzes the dismutation of superoxide (O2•-) to H2O2 (17, 19) in the vessel wall, thus preserving endothelial function in hypertension and diabetes(20–22). In addition, secreted SOD3 is anchored to the EC surface through binding to the extracellular matrix (e.g., heparin sulfate proteoglycan (HSPG)) via the C-terminal heparin binding domain (HBD) which is essential for protection of the endothelium from oxidants (17, 19). Of note, full activity of SOD3 requires the Cu transporting ATPase (ATP7A) that obtains the catalytic cofactor, Cu, from the Cu importer CTR1-Cu chaperone Atox1 axis and then incorporates Cu into SOD3 in the trans-Golgi network (TGN) (23, 24). Accumulating evidence suggests that physiological levels of H2O2 functions as a signaling molecule that participates in a number of biological functions including angiogenesis (17, 25, 26). We reported that SOD3 overexpression in human ECs promotes VEGF-induced angiogenesis by increasing H2O2 in an HBD-dependent manner (17, 25). Of note, SOD3 is epigenetically silenced in ECs and SOD3 protein must be derived from other cells (27). In fact, we and others reported that exercise increases SOD3 protein expression in vascular tissues and skeletal muscles in mice (18, 28, 29). Skeletal muscle-specific SOD3 overexpressing transgenic mice have been shown to protect against diabetic cardiomyopathy by reducing oxidative stress and inflammation in a paracrine manner (30, 31). We reported that ATP7A protein expression is downregulated in DM vascular tissues, thereby decreasing SOD3 activity which contributes to endothelial dysfunction (21, 22). However, the role of ATP7A-SOD3 axis in exercise-induced improvements in circulating exosome function of T2DM and non-diabetic conditions remains unknown.
In this study, we investigated whether SOD3 plays a role in mediating the beneficial effects of exercise especially the pro-angiogenic properties of circulating exosomes in the setting of high fat diet-induced T2DM mice and SOD3−/− mice with two weeks of voluntary wheel exercise. Here we show that SOD3 and ATP7A are present in plasma exosomes. T2DM plasma exosomes significantly reduced angiogenic effects on human ECs, which was associated with a decrease in ATP7A, but not SOD3 expression in exosomes. Exercise training in T2DM mice restored the angiogenic effects of T2DM exosomes in ECs by increasing ATP7A in exosomes, which was not observed in exercised T2DM/SOD3−/− mice. Furthermore, exosomes overexpressing SOD3 significantly enhanced angiogenesis in ECs by increasing local H2O2 levels in a HBD-dependent manner as well as restored defective wound healing and angiogenesis in T2DM or SOD3−/− mice. We provide the first evidence that exosomal SOD3 plays an important role in the angiogenic potential of circulating exosomes from exercised mice in T2DM. The SOD3 enriched exosomes could be developed for use as an exercise-mimetic therapy to restore impaired angiogenesis in T2DM.
2 |. METHODS AND MATERIALS
2.1 |. Animals and exercise protocol:
C57BL/6J mice were purchased from Jackson laboratories (Bar Harbor, ME, USA) (Stock No: 000664). SOD3−/− mice on the C57BL/6J background mice were used as previously described(20). Type 2 Diabetes Mellitus (T2DM) was induced in male and female mice (6 weeks old) by a single low dose of streptozocin (Sigma-Aldrich, St. Louis, MO, USA, Cat# S0130, 75 mg/kg BW, i.p) followed by a 60% Kcal high fat diet (HFD) (Thermofisher Scientific, Hampton, NH, USA, Cat# D12492) for at least 8 weeks. This is a well-established murine model of T2DM, which shows insulin resistance and compensatory hyperinsulinemia(21, 32, 33). The combination of low-dose streptozotocin with a HFD helps to develop a reproducible and robust T2DM model(21, 32, 33). Oral glucose tolerance tests (OGTT) were performed to confirm the induction of T2DM as we have previously reported(21). T2DM C57BL/6J and SOD3−/− mice, along with non-diabetic controls, were allowed to run freely on a voluntary mouse single activity wheel (Model 80820 from Lafayette Instrument Co., Lafayette, IN, USA). Control sedentary mice were maintained in their normal cages, while exercise mice were put each in a voluntary wheel exercise cage. The mice ran on a 5” diameter wheel for a period of 2 weeks preceded by 2 acclimatization days. Wheel rotations were measured by an I/R sensor Scurry Mouse Activity Counter (Model 86115) connected to a Scurry Interface for Animal Activity (Model 86100). Data were recorded every 15 minutes for 14 days using the Scurry Activity Monitoring Software (Model 86165) that analyzes raw activity, interval count, cumulative count, interval distance, cumulative distance, and speed. Mice ran during the nocturnal cycle while on a 12:12-h light-dark light cycle. At the end of exercise period, mice were anesthetized with intraperitoneal 125 mg/kg ketamine with 62.5 mg/kg xylazine injection at 8 am, just after finishing the exercise cycle. Blood from the mice was collected through cardiac puncture and plasma was separated. The protocol for animal use was approved by Institutional Animal Care and Use Committee at Medical College of Georgia, Augusta University.
2.2 |. Human plasma samples:
To investigate the clinical significance of findings in mice, blood samples were collected from three healthy adults (age 56±2 years, BMI 24.33±0.8) before and after 45 min of moderate intensity (50% of VO2max) treadmill exercise. Blood samples were immediately centrifuged 3,000g for 10 min at 4°C to obtain plasma. Subject characteristics with exercise training are shown in Table 1. The human protocol was approved by Institutional Review Board at Medical College of Georgia, Augusta University. Written informed consent was obtained from each subject before participation in the exercise study.
Table 1:
Subject characteristics with exercise training:
| Characteristics | Subjects(n=4) |
| Age, years, Median | 55(54–58) |
| Male, n(%) | 2(50%) |
| Female, n(%) | 2(50%) |
| Height, In (mean±SEM) | 68.24±1.28 |
| Weight, Ibs (mean±SEM) | 158.07±11.34 |
| BMI, (mean±SEM) | 23.74±0.82 |
| Fat %, (mean±SEM) | 25.22% ± 2.79 |
| SBP, mmHg (mean±SEM) | 122.5±5.545 |
| DBP, mmHg(mean±SEM) | 71.75±5.79 |
| VO2 peak (l/min)(mean±SEM) | 2.92±0.56 |
| VO2peak(ml/kg/min)(mean±SEM) | 40.57±6.12 |
| PetCO2 (mm Hg), (mean±SEM) | 30.16±1.51 |
| Peak MPH,(mean±SEM) | 4.225±0.91 |
2.3 |. Exosome isolation from mouse or human plasma:
Exosomes were isolated from both mice and human plasma by differential centrifugation, as previously reported with slight modification (6, 34). In brief, plasma was diluted with an equivalent volume of 1X PBS, followed by centrifugation at 2,000g for 30 min at 4°C. The first supernatant was collected without pellet contamination and further centrifuged at 12,000g for 45 min to remove cellular debris. The second supernatant (SN2) was collected and filtered through a 0.22 μm low protein binding filter (Millipore, Temecula, CA, Cat#SLGVR33RS) to remove larger particles including microvesicles, apoptotic bodies and cellular debris. The filtered SN2 was then finally centrifuged at 110,000g for 90 min at 4°C, the third supernatant was discarded, and exosome pellets were collected in 100 μl PBS and stored at −80° C.
2.4 |. Rat aortic smooth muscle (RASM) transduction with replication deficient adenovirus and exosome isolation from conditioned media:
RASMs, cultured at 80–90% confluency in 10% fetal bovine serum (FBS) containing Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, Waltham, MA, USA, Cat#12430112), were transduced with adenovirus expressing human SOD3 (Ad-hSOD3) or Ad-hSOD3ΔHBD or Ad-null (control) for 8 hrs in serum-free DMEM. Culture media was changed to 10% exosome-depleted FBS containing DMEM (FBS was centrifuged at 110,000 g for 2 hrs, SN was filtered through 0.22 μm filter), and conditioned media and cell lysates were collected after 2 days. Conditioned media from RASMs transduced with adenoviruses were centrifuged at 300g for 10 min to remove cell debris. The supernatant was collected and filtered through 0.22 μm low protein binding filter and ultra-centrifuged at 110,000g for 90 mins at 4°C. Exosomes from RASM conditioned media were then collected in 400 ul PBS and stored at −80°C freezer.
2.5 |. Isolation of MASM from Aorta and exosome isolation:
Vascular mouse smooth muscle cells (MASM) were isolated from both C57bl6 and SOD3 KO mice as described previously(35) (21). In brief, we isolated thoracic aorta from mouse and removed fat gently in sterile Hanks’ balanced salt solution. Next, vessels were incubated in Hanks’ solution containing 175 units/ml collagenase (Worthington Biochemical Corp, CLS-2) for 30 min at 37°C. Then we removed both adventitia and endothelium. The tissues were incubated overnight in 10% FBS in Dulbecco’s modified Eagle’s medium in a CO2 incubator. Next, vessels were incubated in Hanks’ solution containing 175 units/ml collagenase and 75 U/ml elastase (Worthington Biochemical Corp, ESFF#LS006365) for 40 min. The reaction was stopped by dilution with 10 ml of 20% calf serum in Dulbecco’s modified Eagle’s medium (DMEM) and the cells were pelleted by centrifugation and plated in dishes. MASM exosomes were isolated as described above from 80% confluent MASM cells conditioning media.
2.6 |. Exosome Characterization:
Exosomes isolated from plasma or conditioned media were negatively stained by fixing in 4% paraformaldehyde (PFA) for 1 hr, 1% Aqueous Uranyl Acetate (5 μl) was applied to the grid for 30 sec, then wicked off using Whatman filter paper. Grids were allowed to dry before imaging by transmission electron microscope (TEM) (Electron microscopy and histology core at Augusta University). Furthermore, nanoparticle tracking analysis (NTA) by Nanosight was used in characterization of exosomes, as previously described (36). Briefly, we used ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and its accompanied software (ZetaView 8.02.28) in analyzing size and concentration of exosomes by using a laser scattering video microscope in tracking exosome moving by Brownian motion. In addition, we confirmed the successful isolation of exosomes by Western blotting of exosomal markers such as CD63, CD81, Tsg101 and Alix.
2.7 |. Western blotting:
Exosomes were homogenized in 5X RIPA buffer containing 1% Triton X-100 and protease inhibitors (10 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin). Then we performed Bradford protein assay and loaded equal amount of exosomes (adjusted by 3X sample buffer) for SDS-polyacrylamide gel electrophoresis, followed by transferring to nitrocellulose membranes (Bio-Rad, Cat#1620115). Membranes were blocked 1 hr in PBS containing 5% nonfat dry milk and 0.1% Tween 20 (Thermofisher Scientific, Hampton, NH, USA, Cat#BP337), and incubated for overnight with primary antibodies. Following primary antibodies (1:500) were used: anti-eNOS (Abcam, Cambridge, UK, Cat#ab76198), anti-phospho eNOS (ThermoFisher Scientific, Hampton, NH, USA, Cat#PA5–17706), anti-Atox1 (home-made) (37), mouse/human anti-SOD3 (home-made) (38), SOD1 (Abcam, Cambridge, UK, Cat#ab13498), anti-ATP7A (LifeSpan Biosciences, Seattle, WA, USA, Cat#B8162), anti-CTR1 (home-made) (39), anti-CCS (Santa Cruz, Dallas, TX, USA, Cat#SC-55561), anti-actin (Santa Cruz, Dallas, TX, USA, Cat#SC-47778), anti-COX17 (Proteintech, Rosemont, IL, USA, Cat#11464–1-AP), anti CD63 (Santa Cruz, Dallas, TX, USA, Cat#SC-5275), anti-Alix (Cell signaling, Danvers, MA, USA, Cat#2171S), anti-Tsg101 (Santa Cruz, Dallas, TX, USA, Cat#SC-7964), anti-VEGFR2 (Cell signaling, Danvers, MA, USA, Cat#2479S), anti-phospho VEGFR2 Tyr 1175 (Cell signaling, Danvers, MA, USA, Cat#2478S), anti-AMPKα (Cell signaling, Danvers, MA, USA, Cat#2532S), anti-phospho AMPKα Thr172 (Cell signaling, Danvers, MA, USA, Cat#2535S), anti-myoglobin (Cell signaling, Danvers, MA, USA, Cat# 25919S), anti-VEGFa (Abcam, Cambridge, UK, Cat#ab46154), anti-α Tubulin (Santa Cruz, Dallas, TX, USA, Cat#SC-5286) or anti-CD81 (SBI, Palo Alto, CA, USA, Cat#EXOAB-CD81A-1). After incubation with secondary antibodies (1:2000) (Goat Anti-Rabbit IgG-HRP Conjugate, Bio-Rad, Hercules, CA, USA, Cat#1706515; Goat Anti-Mouse IgG-HRP Conjugate, Bio-Rad, Hercules, CA, USA, Cat#1706516; Peroxidase AffiniPure Donkey Anti-Chicken, Jackson immuno Research, West Grove, PA, USA, Cat#703–035-155; Peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG Light Chain Specific, Jackson immuno Research, West Grove, PA, USA, Cat#115–035-174) proteins were detected by ECL chemiluminescence.
2.8 |. Exosomes labelling with PKH67 dye:
Plasma exosomes were labelled with PKH67 green-fluorescent labeling kit (Sigma-Aldrich, St. Louis, MO, USA, Cat#MINI67–1KT) to assess their uptake by ECs. Plasma exosomes were added to an equal volume of diluent C containing 2 μM PKH67 dye for 5 min at room temperature. To stop the reaction, an equal volume of exosome-depleted serum was added and then exosomes isolated by centrifugation at 110,000g for 90 min at 4°C and collected in 200 μl of PBS. Human umbilical vein ECs (HUVECs) (ThermoFisher Scientific, Hampton, NH, USA, Cat#C0035C) were cultured on cover slips and then exposed to different concentrations of PKH67 labelled exosomes for 2 hrs. Cells were then washed twice with PBS to remove any labelled exosomes that were not taken up, followed by fixation in 4% PFA. The uptake of exosomes in HUVECs was evaluated using a fluorescence microscope (Keyence, BZ-X700) where PKH67 labelled exosomes were visualized using a green fluorescence filter and DAPI was visualized in blue. The fluorescence intensity was measured using ImageJ.
2.9 |. Endothelial cell migration (modified Boyden chamber assay) and capillary formation on Matrigel assays:
To perform cell migration and capillary formation assays, HUVECs (passages 4–7) were cultured in 60 mm dishes using 5% FBS containing EndoGRO media (Millipore, Burlington, MA, USA, Cat#SCME001 with 100 U/ml penicillin, and 100 μg/ml streptomycin). At confluency, HUVECs were starved with 0.5% exosome-depleted FBS containing EndoGRO, then treated with exosomes for 16 hrs. HUVECs were then washed, trypsinized, counted and used for the following experiments. For EC migration assays, 60K cells/well were seeded on 0.1% gelatin-coated transwell inserts with a pore size of 8 μm (Falcon, Glendale, AZ, USA, Cat#353097). To assess migration to the lower chamber, inserts were placed in a 24 well plate that contained EndoGRO medium with 0.5% exosome-depleted FBS. The inserts with HUVEC were incubated at 37°C with 5% CO2 for 6 hrs, and then cells were fixed, stained with 10% crystal violet, followed by removing non migrated cells by cotton swabs. Five representative images were taken per insert, as we reported (40). For capillary network formation assays, 30K cells/well were seeded on pre-solidified 130 ul Matrigel (Corning, Glendale, AZ, USA, Cat#354230) in 48 well plate. Five representative images were taken after 5 hrs at 37°C with 5% CO2. Numbers of branching points and tube length and number were analyzed by Image J (40).
2.10 |. Adenovirus transduction of HUVECs:
HUVECs were cultured to 90% confluency in 5% FBS containing EndoGRO. Media was changed to M199 (Sigma-Aldrich, St. Louis, MO, USA, Cat#M4530) and cells were exposed to replication deficient Ad-catalase (University of Iowa, vector core, Iowa, IA, USA, Cat#VVC-Engelhardt-3950) (0.7 ul/ml) for 1 hr, then changed back to 5% FBS containing EndoGRO for 2 days to permit overexpression of human catalase. Next, HUVECs were starved by 0.5% exosome depleted FBS containing EndoGRO and then exosomes (10 ug/ml) were added to each dish for 16 hrs.
2.11 |. DCF-DA assay for H2O2 measurement:
HUVECs were grown to 70% confluency on cover slips with 5% FBS containing EndoGRO media. Exosomes were added to HUVECs (with or without ad-Catalase transduction, as above) with either 1,000 U/ml PEG catalase (Sigma-Aldrich, St. Louis, MO, USA, Cat#C4963) or 1000 U/ml human erythrocyte Catalase (Sigma-Aldrich, St. Louis, MO, USA, Cat#9001–05-2) for 16 hrs, followed by incubation with 20 uM DCF-DA for 6 min at 37°C and then washed with PBS and fixed with 4% PFA at 37°C, as we reported previously (40). The cells were mounted using vectashield mounting medium. DCF fluorescence in cells was measured by fluorescence microscopy (Keyence, BZ-X700) and fluorescence intensity was analyzed using ImageJ.
2.12 |. Exosomes uptake by BAECs:
To assess the uptake of exosomes overexpressing human SOD3 in endothelial cells and the abundance of human SOD3 protein in ECs after treatment, we cultured bovine aortic endothelial cells (BAEC) (Sigma-Aldrich, St. Louis, MO, USA, Cat#B304–05) in Bovine Endothelial Cell Growth Medium (Sigma-Aldrich, St. Louis, MO, USA, Cat#B211–500) till 90% confluency, then added exosomes overexpressing human SOD3 isolated from (Ad-hSOD3) transduced RASM cells for 4 hrs. Next, we washed BAEC cells twice with cold PBS and collected its lysate in RIPA buffer to examine human SOD3 protein by immunoblotting.
2.13 |. Mouse skin wound healing model of angiogenesis and immunohistochemistry:
To perform the wound healing angiogenesis assays, mice were wounded on exposed skin on their backs using a 3 mm punch (Cooper surgical, Trumbull, CT, USA, Cat#DD-3MM Blue) in four different sites, as we have reported (41, 42). Exosomes (20 μg/wound) were applied at wound sites on days 0,2,4 after punching, and the wounds covered with Bioclusive Plus Transparent Film Dressing (Acelity, San Antonio, TX, USA, Cat#BIP1520). Images were taken with scale at days 0, 3, 5, 7 and wound area closure rate was analyzed using Image J. After sacrificing the mice at day 7, tissue surrounding skin wounds were harvested and fixed in 4% PFA overnight at 4°C, followed by gradual sucrose dehydration and then imbedded in OCT blocks and stored at −80°C, as we reported (41). To determine the capillary density, wound tissue sections (7 μm) were stained with anti-mouse CD31 antibody (1:300, BD, Franklin Lakes, NJ, USA, Cat#550274) followed by biotinylated anti-rat IgG antibody (1:300, Vector Laboratories, Cat#BA-4001). Next, we used R.T.U. Vectorstain Elite (Vector Laboratories, Burlingame, CA, USA, Cat#SP-8400), followed by DAB visualization (Vector Laboratories, Burlingame, CA, USA, Cat#H-1200). Five representative images were taken using 20X lens fluorescence microscope (Keyence, BZ-X700) at 5 different sites of wound area and capillary density were analyzed by Image J, as we reported (41).
2.14 |. Statistical Analysis:
Data are presented as mean ± SEM. Data were compared between groups by Students t test when one comparison was performed or by ANOVA for multiple comparisons. When significance was indicated by ANOVA, the Scheffe test for post hoc comparisons was used to specify between group differences. Values of p<0.05 was considered statistically significant. Statistical tests were performed using Prism v4 (GraphPad Software, San Diego, CA, USA).
3 |. RESULTS
3.1 |. Characterization of circulating exosomes from mice with and without exercise.
Mice were exercised by 2 weeks of voluntary wheel running (Figure 1A). C57Bl6 (wild type (WT), control) mice had free access to running wheels and running distances reached a steady-state rate within 48 hrs of access to the wheels. Average daily distance was 8.8±0.4 kilometers/day (Figure 1B, Figure S1A) with no significant impact on body weight before and after exercise (Figure S1B). We found that 2 weeks of exercise increased p-eNOS, a marker for endothelial function, in blood vessels (Figures 1C) as well as VEGF, myoglobin, or p-AMPK levels in skeletal muscles, which are involved in skeletal muscle angiogenesis (Figure S2A), confirming the effectiveness of this exercise protocol. We isolated exosomes from the plasma of exercised mice (Exe-Exosomes) and sedentary mice (Sed-Exosomes) using ultracentrifugation, as we have reported (6). The isolated plasma exosomes were analyzed with TEM, revealing the typical cup-shaped vesicles with diameters of 50 to 150 nm (Figure 1D). Exosome size was further verified by nanoparticle tracking (NTA) analysis using ZetaView (Figure 1E) and Nanosight (Figure 1F). The average diameters of Sed-Exosomes and Exe-Exosomes were 106.7 nm and 82.2 nm, respectively, indicating successful isolation of plasma exosomes (50–150nm) with and without exercise. Exercise did not significantly alter the number of exosomes isolated from the same volume of plasma (Figure 1G). Western blot analysis also verified successful isolation of exosomes as confirmed by the exosome markers CD63 and Tsg101 in samples normalized by protein content, with and without exercise (Figure 1H).
Figure 1: Characterization of plasma exosomes isolated from sedentary and voluntary wheel running exercised mice.
(A) Image of voluntary wheel exercise model. (B) Tracing of wheel activity of exercised mice (Interval distance). (C) Representative western blots for phospho-eNOS and total eNOS in mesenteric arteries at day 0,7,14,28 after exercise. (D)Transmission electron microscopy (TEM) image from sedentary and two weeks exercised mice (scale bar=100nm). (E) Exosome size and number of particles characterization from sedentary and two weeks exercised mice by Zetaview analysis (n=7). (F) Nanosight tracking of plasma exosomes. (G) Plasma exosomes concentration in sedentary and two weeks exercised mice (n=7) (H) Lysates from equal number of plasma exosomes from sedentary and exercised mice were immunoblotted (IB) with exosome markers (Tsg101 and CD63) antibodies (n=3). Results are presented as mean ± SEM.
3.2 |. Exercise enhances the angiogenic effects of plasma exosomes.
To examine whether mouse plasma exosomes influence angiogenesis in cultured ECs, we first assessed the uptake of plasma exosomes into HUVECs using exosomes labelled with PKH67, a fluorescent cell linker compound that is incorporated into cell membranes. Figure 2A shows that uptake of PKH67-labeled exosomes isolated from WT mice by HUVECs was increased in a dose-dependent manner, indicating that plasma exosomes are efficiently taken up by HUVECs. We then examined the effects of exosomes from exercised (Exe-Exosomes), sedentary mice (Sed-Exosomes), and VEGF (positive control) on angiogenic responses in serum-starved HUVECs (Figure 2B). We found that Exe-Exosomes significantly increased both EC migration (modified Boyden chamber assay) and capillary formation on Matrigel as compared to Sed-Exosomes- or control PBS-treated HUVECs (Figures 2C and 2D). Of note, Sed-Exosomes slightly but significantly increased angiogenic responses compared to the PBS-treated groups. These results suggest that exercise enhances the angiogenic function of plasma exosomes on cultured ECs.
Figure 2: Exercise enhances the angiogenic effects of plasma exosomes.
(A) Left: Representative fluorescence images to show the dose-dependent uptake of PKH67-labeled plasma exosomes (green) isolated from mice by HUVEC. Nuclei are stained with DAPI. Right: Graph shows the mean fluorescence intensity of PKH labelled exosomes in ECs. (Scale bar=20 μm, n=3). (B) Schematic diagram of plasma exosome isolation from sedentary and exercised mice, followed by their application to HUVEC to measure angiogenic responses. (C and D) Serum starved HUVECs treated with PBS, 10 ug/ml plasma exosomes from exercised (Exe-Exo) or sedentary (Sed-Exo) mice, or 20ng/ml VEGF (positive control) for 16 hrs were used to measure EC migration (modified Boyden chamber assay) (C) or capillary formation on growth factor-reduced Matrigel (D). In (C), graphs represent averaged number of migrated cells per five random fields, expressed as the fold change over PBS treated group. (n=4). In (D), graph shows averaged number of branching points (left) or tube length (right) per fields, expressed as the fold change over PBS treated group. Scale bar=50 μm. (n=4). Results are presented as mean ± SEM.
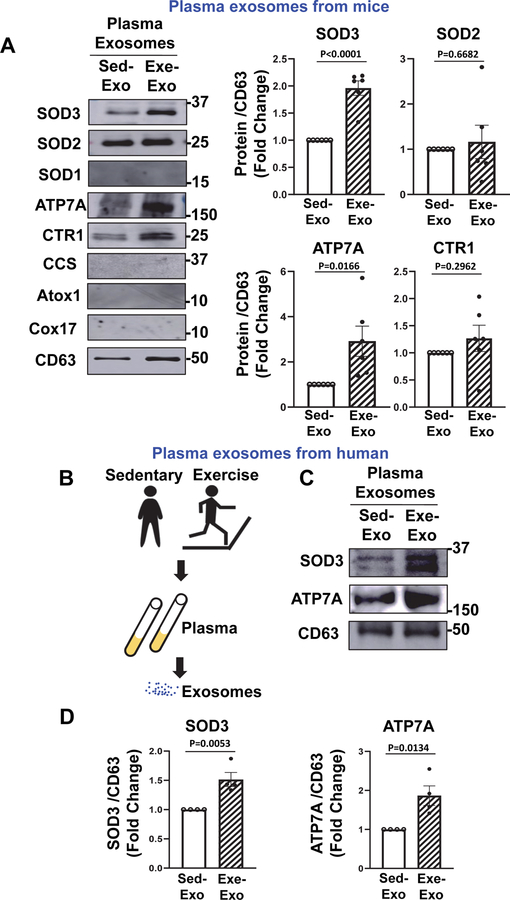
3.3 |. Exercise increases SOD3 and ATP7A proteins in plasma exosomes in both mice and humans.
We then examined the protein expression of SOD3 and ATP7A, a Cu transporter for SOD3, in Exe-Exosomes and Sed-Exosomes using Western blot analysis. Figure 3A shows that expression of SOD3 and ATP7A proteins were significantly increased in Exe-Exosomes, as compared to Sed-Exosomes. In addition, expression of other antioxidant enzymes; including SOD2 and the Cu uptake transporter CTR1 were also increased in Exe-Exosomes as compared to Sed-Exosomes. By contrast, related proteins such as SOD1, CCS (a Cu chaperone for SOD1), Cox17 or Atox1 (a Cu chaperone for ATP7A) were not detected in plasma exosomes (Figure 3A), indicating that exercise can have specific effects on the proteome of plasma exosomes. Since we found that exercise significantly increased the SOD3 protein expression in aorta and skeletal muscle (Figures S2A and S2B), these tissues may be the potential source of SOD3 in plasma exosomes increased after exercise. To assess the clinical relevance of these findings, we isolated human plasma exosomes from healthy human participants before and after a moderate bout of exercise (treadmill running for 45 min at 50% VO2max) as reported previously (43) (Figure 3B). Figures 3C and 3D show that expression of SOD3 and ATP7A protein in human plasma exosomes were significantly increased following a single bout of exercise. Taken together, these results suggest that exercise increases the expression of SOD3 and ATP7A proteins in plasma exosomes in both mice and humans.
Figure 3: Exercise increases SOD3 and ATP7A protein expression in plasma exosomes in both mice and humans.
(A) Left: Representative western blots for Cu transport proteins (ATP7A, CTR1, Atox1, CCS, Cox17) and antioxidant SODs (SOD1, SOD2, SOD3) protein expression in plasma exosomes from sedentary and exercised mice. Right: Graph shows averaged fold change normalized to CD63 exosome marker level. (n=6). (B) Schematic diagram of plasma exosome isolated from human healthy subjects before and after exercise. (C and D) Representative western blots for ATP7A and SOD3 protein expression in plasma exosomes from human participants before and after a single bout of exercise (C) and graph showing averaged fold change normalized to CD63 exosome marker level (D) (n=4). Results are presented as mean ± SEM.
3.4 |. SOD3 is required for exercise-induced pro-angiogenic effects of plasma exosomes.
To investigate the functional role of SOD3 in exercise-induced pro-angiogenic effects of plasma exosomes, we isolated plasma exosomes from WT and SOD3−/− mice with and without exercise. We confirmed that SOD3 protein was absent in SOD3−/− exosomes, while levels of the exosome marker, CD63, were not different between SOD3−/− exosomes and WT exosomes with and without exercise (Figure 4A). In addition, wheel running activity showed no difference between WT and SOD3 KO mice (Figure S1A). We found that the ability of Exe-Exosomes to promote angiogenic effects such as EC migration (Figure 4B) and capillary formation (Figure 4C) were almost completely lost in exosomes lacking SOD3. These results suggest that SOD3 is required for the pro-angiogenic effects of exercise associated plasma exosomes.
Figure 4: SOD3 is required for exercise-induced pro-angiogenic effects of plasma exosomes.
(A) Left: Representative western blots for SOD3 protein expression in plasma exosomes from sedentary (Sed-Exo) and exercised (Exe-Exo) wild type (WT) or SOD3 knockout (KO) mice. Right: Graph shows averaged fold change normalized to CD63 exosome marker level. (n=3). (B and C) Left: Serum starved HUVECs treated with 10 ug/ml plasma exosomes from sedentary or exercised WT mice (WT-Sed-Exo or WT-Exe-Exo) and SOD3 KO mice (SOD3 KO-Sed-Exo or SOD3 KO-Exe-Exo) for 16 hrs were used to measure EC migration (B) or capillary formation (C) as described above. Right: Graph shows averaged number of migrated cells per five random fields (B) or branching points or tube length per five random fields (C), expressed as the fold change from WT-Sed-Exo treated groups. (Scale bar=50 μm, n=3). Results are presented as mean ± SEM.
3.5 |. Exercise restores the angiogenic potential of T2DM plasma exosomes in vitro and in vivo.
Since exosomes derived from T2DM have detrimental effects (12, 13), we next examined if plasma exosomes isolated from T2DM mice may reduce angiogenesis in ECs and whether this can be improved by exercise (Figure 5A). We found that plasma exosomes from sedentary T2DM mice (T2DM Sed-Exosomes) significantly reduced EC migration (Figure 5B) and capillary tube formation (Figure 5C), as compared to control Sed-Exosomes. To assess the angiogenic effects of plasma exosomes in vivo, we employed a skin wound healing model by making four excisional wounds on the dorsal skin of C57Bl6 mice (Figure 5D). We found that direct application of T2DM Sed-Exosomes to the wounded tissues reduced wound healing and the number of capillary-like CD31+ ECs as compared to control Sed-Exosomes (Figures 5D and 5E). Of importance, T2DM Exe-Exosomes significantly enhanced these angiogenic responses in vitro and in vivo as compared to T2DM Sed-Exosomes. These results suggest that exercise training restores the impaired angiogenic effects of T2DM plasma exosomes in vitro and in vivo.
Figure 5: Exercise restores the angiogenic potential of T2DM plasma exosomes in vitro and in vivo.
(A) Schematic diagram of plasma exosome isolation from sedentary or exercised control or T2DM mice, followed by their application to HUVEC to measure angiogenic responses. (B and C) Left: Serum starved HUVECs treated with 10 ug/ml plasma exosomes from mice described in (A) for 16 hrs were used to measure EC migration (B) or capillary formation (C) as described. Right: Graph shows averaged number of migrated cells per five random fields (B) or averaged numbers of branching points per five random fields (C), expressed as the fold change from PBS-treated groups. (Scale bar=50 μm, n=4). (D) Control and T2DM mice were wounded on the back skin. Wound regions were applied with PBS (vehicle), 20 ug plasma exosomes from sedentary WT (Cont Sed-Exo), sedentary or exercised T2DM mice (T2DM-Sed-Exo or T2DM-Exe-Exo), and then, the wound closing rate was measured for 7 days. The wound area was expressed as percent of that measured right after the wounding. (E) Wounded skin tissues at Day 7 were used to measure CD31+ cells using CD31 antibody. Results are presented as mean ± SEM. N=3 mice per group/ 4 wounds per mouse.
3.6 |. SOD3 is required for exercise-induced restoration of impaired angiogenic effects of T2DM plasma exosomes.
We previously reported that SOD3 protein level was not changed while protein expression of ATP7A, a Cu transporter for SOD3, was reduced in diabetic vessels, which in turn decreased SOD3 specific activity, leading to endothelial dysfunction (21). To address the mechanism by which exercise improves the angiogenic effects of T2DM plasma exosomes, we measured the expression of ATP7A and SOD3 in plasma exosomes from T2DM mice with and without exercise. Figure 6A shows that protein expression of ATP7A, but not SOD3, was significantly decreased in T2DM Sed-Exosomes, and significantly restored by exercise (T2DM Exe-Exosomes). We also found that T2DM Exe-Exosomes-induced restoration of EC migration (Figure 6B) and capillary tube formation (Figure 6C) were almost completely inhibited in T2DM/SOD3−/− Exe-Exosomes. Although we could not measure SOD3 activity in isolated exosomes due to lack of enough protein, these results suggest that endogenous SOD3 is required for exercise-induced restoration of impaired angiogenic effects of T2DM Sed-Exosomes, and that increased expression of ATP7A, which transports Cu to SOD3, in exosomes might support the SOD3 activity.
Figure 6: SOD3 is required for exercise-induced restoration of impaired angiogenic effects of T2DM plasma exosomes.
(A) Left: Representative western blots for SOD3 and ATP7A protein expression in plasma exosomes from sedentary or exercised control or T2DM mice (Cont-Sed-Exo or T2DM-Sed-Exo or T2DM-Exe-Exo). Right: Graph shows averaged fold change normalized to CD63 exosome marker level (n=3). (B and C) Left: Serum starved HUVECs treated with 10 ug/ml plasma exosomes from sedentary or exercised T2DM mice (T2DM-Sed-Exo or T2DM-Exe-Exo) and SOD3 KO mice (SOD3 KO- T2DM-Sed-Exo or SOD3 KO-T2DM-Exe-Exo) for 16 hrs were used to measure EC migration (B) or capillary formation (C) as described above. Right: Graph shows averaged number of migrated cells per five random fields (B) and bottom: branching points or tube length per five random fields (C), expressed as the fold change from T2DM-Sed-Exo treated groups. (Scale bar=50 μm, n=3). Results are presented as mean ± SEM.
3.7 |. Exosomes overexpressing SOD3 promote angiogenesis in ECs via increasing H2O2 in a HBD-dependent manner.
To further investigate the mechanisms underlying the angiogenic effects of SOD3-containing exosomes, we used gain- and loss- of function exosome approach. Since SOD3 is synthesized and secreted mainly from VSMCs, we first utilized adenovirus expressing human SOD3 (Ad.hSOD3) or SOD3 lacking HBD domain (Ad.hSOD3ΔHBD) which cannot anchor to EC surface or Ad.null (control) to transduce rat aortic SMCs (RASMs)(25, 44). Of note, we used RASMs to overexpress human wild type and mutant SOD3, because endogenous rat SOD3 lacks heparin binding affinity due to dimer formation (45). We isolated exosomes from conditioned media (Figure 7A) and confirmed equivalent levels of expression of hSOD3 and hSOD3ΔHBD in isolated exosomes (Figure 7B) using antibody specific to a human SOD3 (hSOD3 Ab) which did not detect endogenous “rat” SOD3 derived from RASM. We then added exosomes overexpressing SOD3 (SOD3-Exosomes) or SOD3ΔHBD (SOD3ΔHBD-Exosomes) or control exosomes (Null-Exosomes) to serum-starved HUVECs. We found that SOD3-Exosomes, but not SOD3ΔHBD-Exosomes, significantly increased EC migration (Figure 7C) and capillary tube formation (Figure 7D) as compared to Null-Exosomes. In parallel, we confirmed incorporation of hSOD3 in bovine aortic ECs (BAECs) treated with exosomes overexpressing hSOD3 by immunoblotting with hSOD3 Ab (Figure S4A). For loss-of function exosome approach, we isolated exosomes from conditioned media of primary cultured VSMCs from WT and SOD3−/− mice and found that they significantly decreased migration and capillary tube formation in HUVECs compared to exosomes from conditioned media of VSMC from WT mice (Figure S3). These results support the critical role of exosomal SOD3 in promoting angiogenesis.
Figure 7: Exosomes overexpressing SOD3 promotes angiogenesis in ECs via increasing H2O2 in a HBD-dependent manner.
(A) Schematic diagram of exosome isolation from conditioned media from rat VSMCs infected with adenovirus expressing human SOD3 (Ad.hSOD3) or human SOD3 lacking HBD domain (Ad.hSOD3ΔHBD) or Ad.null (control), followed by their application to HUVEC to measure angiogenic responses. (B) Left: Representative western blots for human SOD3 protein expression in exosomes overexpressing SOD3 (SOD3-Exo) or SOD3ΔHBD (SOD3ΔHBD-Exo) or control exosomes (Null-Exo). Right: Graph shows averaged fold change normalized to Tsg101 exosome marker. (n=3). (C and D) Left: Serum-starved HUVECs treated with 10 ug/ml hSOD3-Exo, hSOD3ΔHBD-Exo, control Null-Exo for 16 hrs were used to measure EC migration (C) or capillary formation (D) as described above. Right: Graph shows averaged number of migrated cells per five random fields (C) or branching points or tube length per five random fields (D), expressed as the fold change from Null-Exo treated groups. (Scale bar=50 μm, n=3). (E) Dichlorofluorescein (DCF) fluorescence with DAPI staining were measured in HUVECs treated with control Null-Exo, 10 ug/ml hSOD3-Exo or hSOD3ΔHBD-Exo for 16 hrs in the presence or absence of Ad-Catalase, PEG-catalase, or human Catalase. Right panel shows the average of DCF fluorescence at 4 different fields, (n =5–8) vs. Null-Exo. (F) Serum-starved HUVECs infected with Ad-catalase or Ad-null (control) were treated with PBS (vehicle), 10 ug/ml hSOD3-Exo or control Null-Exo for 16 hrs to measure EC migration. Results are presented as mean ± SEM. (n = 3)
Since we previously reported that overexpression of SOD3, but not SOD3ΔHBD, promotes angiogenesis in ECs by increasing H2O2 (25), we next examined if SOD3-Exosomes can increase H2O2 in ECs using DCF-DA fluorescence assay (25). Figure 7E shows that SOD3-Exosomes, but not SOD3ΔHBD-Exosomes, significantly increased DCF fluorescence in HUVECs, which was inhibited not only by overexpressing catalase using adenovirus (Ad-catalase) or cell-permeable, polyethylene glycol (PEG)-conjugated-catalase, but also by exogenously applied recombinant catalase which did not enter the cells. Of note, SOD3-Exosomes-induced EC migration was almost completely inhibited in HUVECs overexpressing catalase (Figure 7F). Furthermore, applying SOD3-Exosomes to HUVECs markedly increased tyrosine phosphorylation of VEGFR2, as compared to ECs treated with control exosomes (Figure S4B). These results suggest that SOD3-Exosomes promotes angiogenic responses in ECs via increasing intracellular H2O2 that enhances VEGFR2 signaling in a HBD-dependent manner.
3.8 |. Exosomes overexpressing SOD3 restore impaired wound healing and angiogenesis in T2DM mice.
To evaluate the therapeutic potential of exosomes overexpressing SOD3 in vivo, we examined the effects of SOD3-Exosomes on impaired reparative angiogenesis in T2DM mice using a skin wound healing model (Figure 8A). As shown in Figure 8B, we made four excisional wounds on the dorsal skin in control C57Bl6 mice and T2DM mice and found that T2DM mice exhibited a marked decrease in wound closure rates as compared to control mice. Topical application of SOD3-Exosomes to the wound site significantly accelerated wound healing in T2DM mice as compared to control-Exosomes or PBS-treated groups. Figure 8C shows that capillary-like CD31+ ECs were also significantly reduced in T2DM mice as compared to control mice, which was restored by SOD3-Exosomes treatment, but not control-Exosomes or PBS. These results suggest that exosomes overexpressing SOD3 can restore impaired wound healing and angiogenesis in T2DM mice. Furthermore, consistent with results in T2DM mice, we confirmed that wound healing was significantly impaired in SOD3−/− mice as compared to WT mice (Figure S5A), and these deficits were restored by application of SOD3-Exosomes and verified by CD31+ capillary staining (Figure S5B), supporting the essential role of endogenous SOD3 for reparative angiogenesis in vivo.
Figure 8: Exosomes overexpressing SOD3 restore impaired wound healing and angiogenesis in T2DM mice.
(A) Schematic diagram of exosome isolation from conditioned media from rat VSMCs infected with adenovirus expressing human SOD3 (Ad.hSOD3)(hSOD3-Exo), Ad.null (control)(Null-Exo) or PBS, followed by their application to wounded sites on the back skin of control or T2DM mice. (B) Control and T2DM mice were wounded on the back skin. Wound regions were applied with PBS, 20 ug hSOD3-Exo, or Null-Exo, and then, the wound closing rate was measured for 7 days. The wounded area was expressed as percent of that measured right after the wounding. (C) Wounded skin tissues at day 7 were used to measure CD31+ cells using CD31 antibody. N=3 mice per group/4 wounds per mouse. Results are presented as mean ± SEM. (D) Proposed model. Application of plasma Exo from T2DM mice to cultured ECs and mouse wound healing model showed impaired angiogenesis, which was associated with decrease in expression of ATP7A, but not SOD3, in T2DM-Exo. Exercise restores angiogenic effects of T2DM plasma Exo in a SOD3 dependent manner.
4 |. DISCUSSION
Emerging evidence suggests that exercise training releases circulating exosomes that contain a unique profile of exerkines which traffic to target organs where they exert beneficial systemic effects(10, 14). Plasma exosomes from exercised mice exhibited a significantly greater angiogenic response in ECs as compared to exosomes from sedentary mice. In contrast, exosomes from T2DM lack these beneficial effects. The mechanisms underlying the different functional effects of exosomes on angiogenesis is poorly understood and in particular the role of the secretory Cu enzyme, SOD3, is entirely unknown. Here we show that protein expression of SOD3 and its Cu transporter, ATP7A were detected in plasma exosomes. Importantly, exercise increased the protein expression of SOD3 and ATP7A in plasma exosomes from both mice and healthy humans. Exosomes from mice with T2DM exhibited a decrease in the expression of ATP7A, but not SOD3 and showed impaired angiogenesis in ECs in vitro and in a mouse wound healing model. The ability of exercise training to restore the impaired angiogenic effects of T2DM-Exosomes was absent in SOD3−/− mice, suggesting a critical role of SOD3. In support of this, SOD3-Exosomes promoted angiogenesis in ECs by increasing extracellular H2O2 in an HBD-dependent manner as well as restoring defective wound healing and angiogenesis in T2DM or SOD3−/− mice. These data support an important role of exosomal SOD3 that functions as an exercise mimetic to promote neovascularization and wound repair in T2DM.
Previous studies show that exercise training increases SOD3 protein expression in vascular tissue and skeletal muscle of mice (18, 28, 29) as well as plasma in both human and mice (30, 46, 47), while in some cases, SOD3 level is not altered in serum of non-pregnant exercising mice (48). It is reported that SOD3 overexpressing mice in skeletal muscle release SOD3 into circulation to exert cardioprotective effects via a paracrine function (30, 31). We have shown that SOD3 expression is increased in ischemic muscles after hindlimb ischemia, which is required for neovascularization (49). However, role of endogenous SOD3 in mediating the exercise-induced angiogenic effects of circulating exosomes has not been reported. In the present study, exercise training did not significantly alter the number of plasma exosomes, as previously reported (16, 50) while exercise-derived circulating exosomes enhanced angiogenic responses such as EC migration and capillary formation in HUVECs as compared to exosomes from sedentary mice or PBS treated group. In contrast, sedentary plasma exosomes slightly but significantly increased EC migration without affecting capillary formation as compared to PBS group. These findings are consistent with our results that both SOD3 and its regulator ATP7A protein were present in sedentary plasma exosomes, and their expression in exosomes was further enhanced by exercise. Thus, it seems that basal SOD3 and ATP7A content in sedentary exosomes might not be sufficient to promote capillary formation, and that EC migration and capillary formation can be mediated through additional mechanisms and angiogenic factors or exerkines such as VEGF, IL-6, SDF1, and IGF1, which are shown to be contained in exosomes (10). Of note, current study showed that a moderate bout of exercise in healthy humans also increased the expression of SOD3 and ATP7A in plasma exosomes, supporting the clinical relevance of our study.
There are interconnections between the TGN secretory pathway, where essential Cu is incorporated in newly synthesized SOD3 via the actions of ATP7A, and the endosomal compartment where the biogenesis of exosomes occurs. This may explain why the secretory Cu enzyme SOD3 and Cu transporter ATP7A that delivers cofactor Cu to activate SOD3, are both packaged in exosomes as reported for other secretory proteins, such as insulin and cytokines (51, 52). In current study, we could not measure SOD3 activity directly in exosomes due to lack of enough amounts of proteins in isolated plasma exosomes required to measure its activity. However, present study using SOD3−/− mice with and without exercise reveal that SOD3 is essential for the pro-angiogenic effects of exercise-derived circulating exosomes. Since exercise significantly increased the SOD3 protein expression in blood vessels and skeletal muscle (Figure S2), these tissues may be the dominant source of exercise-derived circulating exosomes containing SOD3.
Exosomes derived from T2DM have been shown to have detrimental effects (11–13). While exosomes have been postulated to mediate the beneficial effects of exercise in T2DM (9, 10), the mechanisms remain undefined. Present study found that plasma exosomes from T2DM sedentary mice significantly decreased angiogenic responses in ECs as compared to exosomes from control sedentary mice, which were restored by exercise in T2DM mice, but not in SOD3−/− T2DM mice. Mechanistically, T2DM sedentary exosomes had a significant decrease in the expression of ATP7A, but not SOD3, as compared to control sedentary exosomes, which was rescued by exercise (T2DM Exe-exosomes). This finding is consistent with our previous report that expression of ATP7A, but not SOD3, is markedly downregulated in T2DM vessels, thereby reducing SOD3 catalytic activity, which contributes to endothelial dysfunction (21). Currently, it remains unclear regarding the mechanism by which ATP7A protein level is decreased in T2DM exosomes and why only ATP7A but not SOD3 is increased in T2DM exosomes after exercise. It is also possible that not only ATP7A-SOD3 axis reduction but also increased amounts of antiangiogenic cargo or decreased proangiogenic cargo in exosomes may impair their angiogenic effects in T2DM mice, which may be reversed by exercise. These points need to be clarified in future study by performing proteomics approaches in plasma exosomes from WT and SOD3−/− mice with and without exercise and T2DM. Despite this limitation, our findings suggest that exercise-induced increase in ATP7A protein in T2DM plasma exosomes may contribute to restoration of SOD3-dependent pro-angiogenic effects of T2DM Exe-Exosomes.
Evidence suggests that exercise-induced ROS such as H2O2 leads to the activation of multiple signaling pathways that are responsible for the beneficial effects of exercise in skeletal muscle and other tissues including ECs, which contributes to the crosstalk mediated by exerkines and exosomes (53, 54). It has been proposed that exosomes can deliver ROS generating or antioxidant enzymes to remote tissues to stimulate H2O2 signaling, leading to the widespread health benefits of exercise (55). In line with this notion, we provide the first evidence that exosomes overexpressing hSOD3 (SOD3-Exosomes), but not hSOD3-ΔHBD, significantly increased redox-sensitive DCF fluorescence and EC migration, which were abolished not only by cell permeable PEG-catalase and Ad.Catalase, but also by exogenously applied nonpegylated catalase that does not enter the cells. Mechanistically, we found that applying hSOD3-Exosomes transferred hSOD3 to ECs (Figure S4A) as well as markedly increased tyrosine phosphorylation of VEGFR2 in ECs, as compared to control exosomes (Figure S4B). These results suggest that exosomes overexpressing SOD3 plays an important role in mediating the pro-angiogenic function of exosomes by delivering SOD3 to ECs, which promotes outside-in H2O2-mediated VEGFR2 signaling in ECs via HBD-dependent mechanism (Figure 8D). To generate SOD3-Exosomes, we overexpressed human SOD3 in RASMs, as endogenous rat SOD3 lacks heparin binging affinity due to dimer formation(45), and then isolated exosomes containing SOD3 from conditioned media. One may speculate that other growth factors contained in RASM exosomes may support the angiogenic effects of hSOD3-Exosomes in ECs; however, this is unlikely given the lack of effect by hSOD3-ΔHBD expressing exosomes. The critical role of exosomal SOD3 in promoting angiogenesis was further confirmed by our finding that loss-of-function exosomes from conditioned media of primary VSMCs isolated from SOD3−/− mice significantly decreased angiogenic responses, as compared to exosomes from conditioned media of VSMCs from WT mice which have active endogenous SOD3.
The positively charged HBD located at the C-terminal end of SOD3 allows for a tight association with the negatively charged heparin sulfate within the EC extracellular matrix (56). We previously reported that HBD of SOD3 is required for the SOD3 binding to heparan sulfate proteoglycans at EC surface, providing the proximity to the VEGFR2 (25). This in turn promotes out-side in H2O2 production that enhances VEGFR2 phosphorylation via oxidative inactivation of protein tyrosine phosphatases (PTPs) at caveolae/lipid rafts, thereby enhancing angiogenic responses in ECs (25). Of note, since H2O2 is highly diffusible molecules, localizing the ROS signal at the specific subcellular compartment is essential for activating redox signaling events (57). Thus, we speculate that HBD of SOD3 is required for SOD3 transfer from exosomes to ECs surface, which may promote out-side in compartmentalized H2O2 production that enhances VEGFR2 signaling and angiogenesis. In addition, the clinical significance regarding altered disease risk in individuals expressing the SOD3 variant with decreased heparin binding affinity (R213G substitution in the HBD) in vascular disease and diabetes has been reported. For example, a R213G polymorphism in the HBD of SOD3 is associated with ischemic heart disease and heart failure in human subjects(58, 59). Lund et al reported that SOD3, not the R213G SOD3 variant, protects against endothelial dysfunction in aorta under inflammatory stress (60). Thus, it is conceivable that some of phenotype of R213G SOD3 variant might be due to loss of angiogenic properties of exosomal SOD3. Moreover, one might question why a secretory protein SOD3 is packaged in exosome. As the HBD of SOD3 is required for the angiogenic effects of SOD3-Exosomes in ECs (25), it is possible that a lipid vehicle-based delivery mechanism via exosomes may be helpful in protecting SOD3 from proteolytic removal of its HBD in an oxidative extracellular environment of T2DM (61–63). Further studies such as proteomics analysis or microRNA profiling assays in SOD3-Exosomes or ECs treated with SOD3-Exosomes will be necessary to understand how SOD3-Exosomes promote angiogenesis in ECs.
In this study, we evaluated the function of plasma exosomes from T2DM mice with or without exercise as well as SOD3-Exosomes in vivo using the mouse skin wound healing model. We found that local application of sedentary T2DM-Exosomes impaired wound healing and angiogenesis, which were restored with exercised T2DM-Exosomes. Furthermore, wound healing and angiogenesis (CD31+ ECs) were impaired in T2DM or SOD3−/− mice versus control mice, and these deficits were rescued by application of SOD3-Exosomes to wounded tissues. These results suggest that SOD3-Exosomes may function as exosomal exerkines that can improve impaired wound healing and angiogenesis in T2DM mice. We previously reported that bioengineered cardiac progenitor cell-derived exosomes transfected with pro-angiogenic miR-322 was an effective therapeutic approach to protect against myocardial infarction via the promotion of ROS-dependent angiogenesis (6). Thus, direct transfection of autologous T2DM exosomes with SOD3 may mimic exercise-derived exosomes, which can be used as a novel therapeutic approach for treatment of T2DM patients who cannot exercise.
The present study has several limitations. First, demonstrating a direct role of circulating exosomes on angiogenesis and wound healing in T2DM mice is difficult due to the lack of animal models or specific inhibitors that can alter exosomes production or secretion. Second, the present investigation utilized 2 weeks of voluntary wheel running. It will be important to investigate the effects of different durations and intensities of exercise on the angiogenic effects of circulating exosomes, because the degree of exercise may change the cellular microenvironment which regulates exosome function. For example, longer-term exercise may increase exosome concentrations of various angiogenic factors that might negate the exclusive nature of SOD3 requirement that is observed in the current conditions with two weeks of exercise. Third, since exosomes not only contain proteins but also miRNAs, the beneficial angiogenic effects of exercise plasma exosomes may be mediated through crosstalk between the ATP7A-SOD3 axis and angiogenic proteins and miRNAs (64). These experimental considerations should be clarified in future studies.
In summary, exercise training can restore the impaired pro-angiogenic function of circulating exosomes in T2DM via increased expression of ATP7A-SOD3 axis in cultured ECs and a wound healing model in vivo. Furthermore, plasma exosomes analysis from humans with a single bout of exercise showed that ATP7A and SOD3 protein levels were also upregulated, supporting the clinical significance of this study. Moreover, exosomes overexpressing SOD3 promoted angiogenic responses in ECs via increased levels of intracellular H2O2 in a HBD-dependent manner as well as restoring impaired wound healing and angiogenesis in T2DM or SOD3−/− mice. This study has uncovered a novel role of exosomal SOD3 as a key mediator of the beneficial effects of circulating exosomes from exercised animals that promotes angiogenesis in T2DM. Given that SOD3 may act as an exerkine released into the circulation in response to exercise, bioengineering exosomes expressing SOD3 may have benefits as an exercise mimetic that can promote neovascularization and wound healing in metabolic and cardiovascular diseases.
Supplementary Material
ACKNOWLEDGEMENTS AND SOURCES OF FUNDING
This study was supported by National Institute of Health grants: R01HL135584 (M.U.-F.), R01HL147550 (M.U.-F., T.F.), R01HL133613 (T.F., M.U.-F.), R01HL116976 (T.F., M.U.-F.), R01HL070187 (T.F.), R01HL134354 (Y.L.T.), Veterans Administration Merit Review Award 2I01BX001232 (T.F.), and Translational Research Award from Augusta University, Department of Medicine (K.A.).
NONSTANDARD ABBREVIATIONS
- Ad.hSOD3ΔHBD
adenoviruses expressing human SOD3 lacking heparin binding domain
- Ad-hSOD3
adenoviruses expressing human SOD3
- ATP7A
ATPase copper transporting alpha
- BAEC
bovine aortic endothelial cells
- C57BL6
wild type mouse strain
- DMEM
dulbecco’s modified eagle medium
- EC
endothelial cells
- ECSOD
extracellular superoxide dismutase
- FBS
fetal bovine serum
- HBD
heparin binding domain
- HFD
high fat diet
- HSPG
heparin sulfate proteoglycan
- HUVECs
human umbilical vein endothelial cells
- MASM
mouse aortic smooth muscle cells
- NTA
nanoparticle tracking analysis
- O2•-
superoxide
- OGTT
oral glucose tolerance test
- PFA
paraformaldehyde
- RASM
rat aortic smooth muscle cells
- ROS
reactive oxygen species
- SN
supernatant
- SOD3
extracellular superoxide dismutase
- T2DM
type two diabetes mellitus
- TEM
transmission electron microscopy
- TGN
trans-golgi network
- VSMCs
vascular smooth muscle cells
Footnotes
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Tkach M, and Thery C (2016) Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 164, 1226–1232 [DOI] [PubMed] [Google Scholar]
- 2.Lawson C, Vicencio JM, Yellon DM, and Davidson SM (2016) Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 228, R57–71 [DOI] [PubMed] [Google Scholar]
- 3.Hessvik NP, and Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75, 193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kranendonk ME, de Kleijn DP, Kalkhoven E, Kanhai DA, Uiterwaal CS, van der Graaf Y, Pasterkamp G, Visseren FL, and Group SS (2014) Extracellular vesicle markers in relation to obesity and metabolic complications in patients with manifest cardiovascular disease. Cardiovasc Diabetol 13, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, and De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youn SW, Li Y, Kim YM, Sudhahar V, Abdelsaid K, Kim HW, Liu Y, Fulton DJR, Ashraf M, Tang Y, Fukai T, and Ushio-Fukai M (2019) Modification of Cardiac Progenitor Cell-Derived Exosomes by miR-322 Provides Protection against Myocardial Infarction through Nox2-Dependent Angiogenesis. Antioxidants (Basel) 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Y, Du W, Liu J, Ma W, Zhang L, Du Z, and Cai B (2018) Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front Pharmacol 9, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, and Davidson SM (2015) Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65, 1525–1536 [DOI] [PubMed] [Google Scholar]
- 9.Li G, Liu H, Ma C, Chen Y, Wang J, and Yang Y (2019) Exosomes are the novel players involved in the beneficial effects of exercise on type 2 diabetes. J Cell Physiol [DOI] [PubMed] [Google Scholar]
- 10.Safdar A, Saleem A, and Tarnopolsky MA (2016) The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol 12, 504–517 [DOI] [PubMed] [Google Scholar]
- 11.Cheng Z, Naga Srikanth Garikipati V, Truongcao MM, Cimini M, Huang G, Wang C, Benedict C, Gonzalez C, Mallaredy V, Goukassian DA, Verma SK, and Kishore R (2021) Serum-Derived Small Extracellular Vesicles From Diabetic Mice Impair Angiogenic Property of Microvascular Endothelial Cells: Role of EZH2. J Am Heart Assoc 10, e019755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang-Doran I, Zhang CY, and Vidal-Puig A (2017) Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab 28, 3–18 [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Liu J, Qu D, Wang L, Wong CM, Lau CW, Huang Y, Wang YF, Huang H, Xia Y, Xiang L, Cai Z, Liu P, Wei Y, Yao X, Ma RCW, and Huang Y (2018) Serum exosomes mediate delivery of arginase 1 as a novel mechanism for endothelial dysfunction in diabetes. Proc Natl Acad Sci U S A 115, E6927–E6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, and Lucia A (2018) Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 15, 731–743 [DOI] [PubMed] [Google Scholar]
- 15.Fruhbeis C, Helmig S, Tug S, Simon P, and Kramer-Albers EM (2015) Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracell Vesicles 4, 28239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE, Egan CL, Cron L, Watt KI, Kuchel RP, Jayasooriah N, Estevez E, Petzold T, Suter CM, Gregorevic P, Kiens B, Richter EA, James DE, Wojtaszewski JFP, and Febbraio MA (2018) Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab 27, 237–251 e234 [DOI] [PubMed] [Google Scholar]
- 17.Fukai T, and Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15, 1583–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okutsu M, Call JA, Lira VA, Zhang M, Donet JA, French BA, Martin KS, Peirce-Cottler SM, Rembold CM, Annex BH, and Yan Z (2014) Extracellular superoxide dismutase ameliorates skeletal muscle abnormalities, cachexia, and exercise intolerance in mice with congestive heart failure. Circ Heart Fail 7, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattman CL, Schaefer LM, and Oury TD (2003) Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 35, 236–256 [DOI] [PubMed] [Google Scholar]
- 20.Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, and Harrison DG (2006) Role of extracellular superoxide dismutase in hypertension. Hypertension 48, 473–481 [DOI] [PubMed] [Google Scholar]
- 21.Sudhahar V, Okur MN, Bagi Z, O’Bryan JP, Hay N, Makino A, Patel VS, Phillips SA, Stepp D, Ushio-Fukai M, and Fukai T (2018) Akt2 (Protein Kinase B Beta) Stabilizes ATP7A, a Copper Transporter for Extracellular Superoxide Dismutase, in Vascular Smooth Muscle: Novel Mechanism to Limit Endothelial Dysfunction in Type 2 Diabetes Mellitus. Arterioscler Thromb Vasc Biol 38, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudhahar V, Urao N, Oshikawa J, McKinney RD, Llanos RM, Mercer JF, Ushio-Fukai M, and Fukai T (2013) Copper transporter ATP7A protects against endothelial dysfunction in type 1 diabetic mice by regulating extracellular superoxide dismutase. Diabetes 62, 3839–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukai T, Ushio-Fukai M, and Kaplan JH (2018) Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol 315, C186–C201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin Z, Itoh S, Jeney V, Ushio-Fukai M, and Fukai T (2006) Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. FASEB J 20, 334–336 [DOI] [PubMed] [Google Scholar]
- 25.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, and Ushio-Fukai M (2010) Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One 5, e10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, and Steinhorn RH (2011) Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal 15, 1497–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelko IN, Stepp MW, Vorst AL, and Folz RJ (2011) Histone acetylation regulates the cell-specific and interferon-gamma-inducible expression of extracellular superoxide dismutase in human pulmonary arteries. American journal of respiratory cell and molecular biology 45, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, and Harrison DG (2000) Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105, 1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hitomi Y, Watanabe S, Kizaki T, Sakurai T, Takemasa T, Haga S, Ookawara T, Suzuki K, and Ohno H (2008) Acute exercise increases expression of extracellular superoxide dismutase in skeletal muscle and the aorta. Redox Rep 13, 213–216 [DOI] [PubMed] [Google Scholar]
- 30.Call JA, Chain KH, Martin KS, Lira VA, Okutsu M, Zhang M, and Yan Z (2015) Enhanced skeletal muscle expression of extracellular superoxide dismutase mitigates streptozotocin-induced diabetic cardiomyopathy by reducing oxidative stress and aberrant cell signaling. Circ Heart Fail 8, 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Z, and Spaulding HR (2020) Extracellular superoxide dismutase, a molecular transducer of health benefits of exercise. Redox Biol 32, 101508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, and Dillmann WH (2012) Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol 303, R689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho YE, Basu A, Dai A, Heldak M, and Makino A (2013) Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice. Am J Physiol Cell Physiol 305, C1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thery C, Amigorena S, Raposo G, and Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol Chapter 3, Unit 3 22 [DOI] [PubMed] [Google Scholar]
- 35.Griendling KK, Taubman MB, Akers M, Mendlowitz M, and Alexander RW (1991) Characterization of phosphatidylinositol-specific phospholipase C from cultured vascular smooth muscle cells. J Biol Chem 266, 15498–15504 [PubMed] [Google Scholar]
- 36.Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, and Liu Y (2017) A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One 12, e0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh S, Kim HW, Nakagawa O, Ozumi K, Lessner SM, Aoki H, Akram K, McKinney RD, Ushio-Fukai M, and Fukai T (2008) Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J Biol Chem 283, 9157–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukai T, Galis ZS, Meng XP, Parthasarathy S, and Harrison DG (1998) Vascular expression of extracellular superoxide dismutase in atherosclerosis. J Clin Invest 101, 2101–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, Maryon EB, Kaplan JH, Ushio-Fukai M, and Fukai T (2010) Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, and Ushio-Fukai M (2017) ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis. Am J Physiol Cell Physiol 312, C749–C764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L, Vogt S, Yang J, Kweon J, Surenkhuu B, Ushio-Fukai M, and Fukai T (2016) Endothelial Antioxidant-1: a Key Mediator of Copper-dependent Wound Healing in vivo. Sci Rep 6, 33783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YM, Youn SW, Sudhahar V, Das A, Chandhri R, Cuervo Grajal H, Kweon J, Leanhart S, He L, Toth PT, Kitajewski J, Rehman J, Yoon Y, Cho J, Fukai T, and Ushio-Fukai M (2018) Redox Regulation of Mitochondrial Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Rep 23, 3565–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris RA, Padilla J, Hanlon KP, Rink LD, and Wallace JP (2008) The flow-mediated dilation response to acute exercise in overweight active and inactive men. Obesity (Silver Spring) 16, 578–584 [DOI] [PubMed] [Google Scholar]
- 44.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, and Heistad DD (2003) Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res 92, 461–468 [DOI] [PubMed] [Google Scholar]
- 45.Carlsson LM, Marklund SL, and Edlund T (1996) The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci U S A 93, 5219–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kander MC, Cui Y, and Liu Z (2017) Gender difference in oxidative stress: a new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 21, 1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadley AJ, Keane G, Cullen T, James L, Vautrinot J, Davies M, Hussey B, Hunter DJ, Mastana S, Holliday A, Petersen SV, Bishop NC, Lindley MR, and Coles SJ (2019) Characterization of extracellular redox enzyme concentrations in response to exercise in humans. J Appl Physiol (1985) 127, 858–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusuyama J, Alves-Wagner AB, Conlin RH, Makarewicz NS, Albertson BG, Prince NB, Kobayashi S, Kozuka C, Møller M, Bjerre M, Fuglsang J, Miele E, Middelbeek RJW, Xiudong Y, Xia Y, Garneau L, Bhattacharjee J, Aguer C, Patti ME, Hirshman MF, Jessen N, Hatta T, Ovesen PG, Adamo KB, Nozik-Grayck E, and Goodyear LJ (2021) Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab 33, 939–956.e938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim HW, Lin A, Guldberg RE, Ushio-Fukai M, and Fukai T (2007) Essential role of extracellular SOD in reparative neovascularization induced by hindlimb ischemia. Circ Res 101, 409–419 [DOI] [PubMed] [Google Scholar]
- 50.Hou Z, Qin X, Hu Y, Zhang X, Li G, Wu J, Li J, Sha J, Chen J, Xia J, Wang L, and Gao F (2019) Longterm Exercise-Derived Exosomal miR-342–5p: A Novel Exerkine for Cardioprotection. Circ Res 124, 1386–1400 [DOI] [PubMed] [Google Scholar]
- 51.Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe Ahmed M, Piemonti L, Hirosue S, Swartz MA, De Palma M, Hubbell JA, and Baekkeskov S (2017) Primary Human and Rat β-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes 66, 460–473 [DOI] [PubMed] [Google Scholar]
- 52.Cypryk W, Nyman TA, and Matikainen S (2018) From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front Immunol 9, 2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji LL (2015) Redox signaling in skeletal muscle: role of aging and exercise. Adv Physiol Educ 39, 352–359 [DOI] [PubMed] [Google Scholar]
- 54.Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP, and Louzada RA (2021) Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants (Basel) 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fortunato RS, and Louzada RA (2020) Muscle Redox Signaling: Engaged in Sickness and in Health. Antioxid Redox Signal 33, 539–541 [DOI] [PubMed] [Google Scholar]
- 56.Chu Y, Piper R, Richardson S, Watanabe Y, Patel P, and Heistad DD (2006) Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol 26, 1985–1990 [DOI] [PubMed] [Google Scholar]
- 57.Ushio-Fukai M (2006) Localizing NADPH oxidase-derived ROS. Sci STKE 2006, re8. [DOI] [PubMed] [Google Scholar]
- 58.Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, Jensen G, and Nordestgaard BG (2004) Genetically reduced antioxidative protection and increased ischemic heart disease risk: the Copenhagen City Heart Study. Circulation 109, 59–65 [DOI] [PubMed] [Google Scholar]
- 59.Kobylecki CJ, Afzal S, and Nordestgaard BG (2015) Genetically Low Antioxidant Protection and Risk of Cardiovascular Disease and Heart Failure in Diabetic Subjects. EBioMedicine 2, 2010–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lund DD, Chu Y, Brooks RM, Faraci FM, and Heistad DD (2007) Effects of a common human gene variant of extracellular superoxide dismutase on endothelial function after endotoxin in mice. J Physiol 584, 583–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan RJ, Fattman CL, Watkins SC, and Oury TD (2004) Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol (1985) 97, 2006–2013 [DOI] [PubMed] [Google Scholar]
- 62.Fattman CL, Chu CT, Kulich SM, Enghild JJ, and Oury TD (2001) Altered expression of extracellular superoxide dismutase in mouse lung after bleomycin treatment. Free Radic Biol Med 31, 1198–1207 [DOI] [PubMed] [Google Scholar]
- 63.Gao F, Kinnula VL, Myllärniemi M, and Oury TD (2008) Extracellular superoxide dismutase in pulmonary fibrosis. Antioxid Redox Signal 10, 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang TR, and Huang WQ (2020) Angiogenic Exosome-Derived microRNAs: Emerging Roles in Cardiovascular Disease. J Cardiovasc Transl Res [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.