Abstract
The susceptibility of BALB/c mice to pristane-induced plasmacytomas is a complex genetic trait involving multiple loci, while DBA/2 and C57BL/6 strains are genetically resistant to the plasmacytomagenic effects of pristane. In this model system for human B-cell neoplasia, one of the BALB/c susceptibility and modifier loci, Pctr1, was mapped to a 5.7-centimorgan (cM) chromosomal region that included Cdkn2a, which encodes p16INK4a and p19ARF, and the coding sequences for the BALB/c p16INK4a and p19ARF alleles were found to be polymorphic with respect to their resistant Pctr1 counterparts in DBA/2 and C57BL/6 mice (45). In the present study, alleles of Pctr1, Cdkn2a, and D4Mit15 from a resistant strain (BALB/cDAG) carrying DBA/2 chromatin were introgressively backcrossed to the susceptible BALB/c strain. The resultant C.DAG-Pctr1 Cdkn2a D4Mit15 congenic was more resistant to plasmacytomagenesis than BALB/c, thus narrowing Pctr1 to a 1.5-cM interval. Concomitantly, resistant C57BL/6 mice, from which both gene products of the Cdkn2a gene have been eliminated, developed pristane-induced plasma cell tumors over a shorter latency period than the traditionally susceptible BALB/cAn strain. Biological assays of the p16INK4a and p19ARF alleles from BALB/c and DBA/2 indicated that the BALB/c p16INK4a allele was less active than its DBA/2 counterpart in inducing growth arrest of mouse plasmacytoma cell lines and preventing ras-induced transformation of NIH 3T3 cells, while the two p19ARF alleles displayed similar potencies in both assays. We propose that the BALB/c susceptibility/modifier locus, Pctr1, is an “efficiency” allele of the p16INK4a gene.
Susceptibility to cancer in human populations is a complex genetic trait. However, the field of cancer susceptibility has been defined largely by studying familial human cancers and targeted animal models that have sustained either gain- or loss-of-function mutations in genes, resulting in phenotypes which typically result in all-or-none responses. Therefore, the activation of oncogenes and the loss of tumor suppressor genes have dominated the field of cancer genetics as the effectors of tumor initiation, promotion, and progression. In contrast to all-or-none alleles, certain alleles of genes are partially functional, resulting in changes in the efficiencies of the proteins they encode. Due to their quantitative properties, natural alleles of genes that alter the efficiency of protein function have remained largely elusive in experimental studies of tumor susceptibility. However, putative “efficiency” alleles that modify tumor susceptibility might actually occur more frequently in people than all-or-none alleles and could make important contributions to cancer pathogenesis.
The Pctr1 (22) and Mom1 (7) loci were among the first modifiers of tumor susceptibility and resistance phenotypes identified by genome scans of restriction fragment length polymorphism and simple sequence length polymorphism markers, respectively. The Mom1 locus, occupied by the Pla2g2a gene (6, 19), was identified as a modifier of min (Apc), whose human ortholog is a major determinant of colon cancer. Mom1 modifies Apcmin mutant mice by modulating the tumor microenvironment to control the number of adenomas that develop in their intestines (6, 7, 19). Strains whose Mom1 locus contains a susceptibility allele have sustained a loss-of-function frameshift mutation.
Susceptibility to plasmacytomas in mice is a complex genetic trait, with at least five Pctr susceptibility-resistance loci determining whether mice develop hematopoietic tumors of the plasma cell lineage in response to induction with pristane, an oil which elicits a chronic inflammatory response (21, 22). The modifier loci Pctr1, -2, and -3 are linked to mouse chromosome 4 in noncontiguous portions of the chromosome (21, 22, 28, 45). The cyclin-dependent kinase inhibitor gene Cdkn2a, which encodes both p16INK4a and p19ARF products, falls within the Pctr1 region of chromosome 4, and BALB/cAn mice were found to carry a rare allele of the Cdkn2a gene (45). Until now, none of the Pctr loci have been molecularly identified, as there was no genetic evidence to directly implicate the Cdkn2a locus or the relative roles of p16INK4a and p19ARF in mouse plasmacytomagenesis.
In this report we provide in vivo evidence from congenic and knockout studies that the Cdkn2a locus can modify susceptibility to pristane-induced plasmacytomagenesis. Moreover, cell culture-based complementation studies support the conclusion that p16INK4a is functionally impaired in BALB/cAn plasma cells and can be specifically corrected by the DBA/2 allele. In contrast, the biological potencies of BALB/c and DBA/2 p19ARF were found to be identical. Correspondingly, p16INK4a, but not p19ARF, from BALB/c mice was less effective than DBA/2 p16INK4a at suppressing ras transformation in NIH 3T3 cells.
MATERIALS AND METHODS
Mice.
BALB/cDAG mice were obtained from Hillyard Festenstein (Laboratory Animal Center, Carshalton, England). They were originally created by immunizing BALB/c mice with cells bearing LY6 alloantigens from DBA/2 mice. When these mice were brought into our conventional colony, we performed a modest genome scan and identified DBA markers as passenger segments on chromosomes 3 (D3Mit21), 4 (Mtv13), 11 (D11Mit2), 15 (Ly6), and 17 (Qa). BALB/cDAG mice were found to be resistant to plasmacytomagenesis. C.DAG-Mtv13 mice were created from the BALB/cDAG mice by introgressively backcrossing DBA/2 Mtv13 and D4Lgm1 alleles onto a BALB/c background. DBA/2 alleles on chromosomes 2, 11, 15, and 17 were actively selected against, and the subsequent C.DAG-Mtv13 mouse carried BALB/c alleles on these chromosomes. This strain was found to carry approximately 6 centimorgans (cM) of genetic material from DBA/2 mice. When BALB/c mice were found to carry a rare allele of Cdkn2a, we made the C.DAG-Cdkn2a D4Mit15 N20F5 mouse by selecting for DBA/2 alleles of Cdkn2a and D4Mit15 and BALB/c alleles of Mtv13. This mouse was found to carry approximately 1.5 cM of DBA chromatin surrounding the Cdkn2a and D4Mit15 loci.
The INK4a knockout mice, with portions of exons 2 and 3 replaced by a neo cassette, were bred at Albert Einstein College of Medicine to N3F3 (36). They were transferred to a conventional mouse facility at the National Institutes of Health, where they were backcrossed onto the C57BL/6N background for an additional generation. Mice heterozygous for the knockout were intercrossed, and homozygous mice (N4F4) were tested for susceptibility and resistance to plasmacytomagenesis following two 0.5-ml injections of pristane. The N4F4 mice were examined for allelic variation at three microsatellite markers per chromosome, with special emphasis on markers linked to the Pctr1 to -4 and Pctm modifier loci. For each interval examined, 129 mice carried the same allele as resistant C57BL/6 and DBA/2 mice. Specifically, 129 mice were identical to C57BL/6 mice at the Cdkn2a locus.
Mice were examined for plasma cell tumors by examination of Wright- Giemsa-stained slides of peritoneal ascites fluid cytospins. Autopsies of congenic strains were performed as the mice developed tumors over the course of 360 days, and autopsies of knockout mice were performed at day 120 postpristane due to the significant morbidity observed in the mice. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (American HistoLabs Labs, Gaithersburg, Md.).
Construction of expression plasmids.
DBA/2N (wild-type) p16INK4a and p19ARF cDNAs were cloned into pBluescript KS. The BALB/c-derived variants A134C (H18P), G232A (V51I), and A134C+G232A of p16INK4a and BALB/c p19ARF variant G257A (R72H) were constructed by PCR-directed mutagenesis with Pfu polymerase (Stratagene) (13). The following primers generated the A134C variant: (forward) TGTGCCTGACGTGCGGGCACT and (reverse) AGTGCCCGCACGTCAGGCACA. Primers (forward) GGCAACGTTCACATAGCAGCTCTTC and (reverse) GAAGAGCTGCTATGTGAACGTTGCC generated the G232A and G257A variants of p16INK4a and p19ARF, respectively. The wild-type and variant sequences of p16INK4a and p19ARF were cloned into the pcDNA3.1 vector (Invitrogen). All p16INK4a and p19ARF plasmids were confirmed by direct sequencing of isolated plasmids (fmol DNA Sequencing System; Promega).
Cell culture and transfection.
MOPC460 and TEPC1165 cells were cultured in RPMI 1640–2 mM l-glutamine–10% fetal calf serum–50 μM β-mercaptoethanol with 3.6 ng of interleukin 6 (IL-6)/ml (40 U/ml) at 37°C in 5% CO2 to a maximum density of 5 × 105/ml. All plasmids used in transfection experiments were purified twice in cesium chloride-ethidium bromide gradients (34). The cells were pelleted and resuspended to a concentration of 107/0.25 ml; they were electroporated at room temperature with a Cell-Porator (Life Technologies, Inc., Gaithersburg, Md.) at 180 V and 1,600 μF at the low-resistance setting (3). The cells were diluted in 10 ml of medium and incubated for 48 h before fluorescence-activated cell sorter analysis. NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% penicillin and streptomycin at 37°C in 5% CO2. The cells were split into 100- by 15-mm tissue culture dishes (5 × 105 cells/dish) 5 h prior to transfection. The calcium phosphate transfection method (34) was used for NIH 3T3 cells with slight modification. All transfections were done in duplicate in each experiment, and all experiments were performed at least three times. p16INK4a protein levels were overexpressed at 4, 16, 24, and 48 h posttransfection (data not shown) as detected by standard Western blot analysis using rabbit polyclonal anti-p16 antibody (catalog no. SC-1207; Santa Cruz) followed by incubation with a sheep anti-rabbit immunoglobulin linked with horseradish peroxidase.
Cell cycle arrest analysis.
Cells were transiently cotransfected with pEGFPF (2 μg), an enhanced membrane-targeted green fluorescent protein farncsylated (EGFPF) construct (14), and one of the p16INK4a or p19ARF constructs described above (18 μg). The cells were harvested 48 h posttransfection. Dead cells were removed with Ficoll-Paque (Pharmacia Biotech), and the remaining live cells were washed twice with phosphate-buffered saline (containing 0.1% bovine serum albumin and 0.1% EDTA) and fixed in 70% ethanol for 1 h at 4°C. The fixed cells were washed once with phosphate-buffered saline containing 0.1% bovine serum albumin and 0.1% EDTA; DNA was stained with propidium iodide (50 μg/ml) containing 250 μg of RNase A/ml for 30 min at room temperature. Flow cytometry analysis was conducted with a Becton-Dickinson FACScan. EGFPF was used as a marker for analysis of transfected cells. The gate was set to select EGFPF-positive cells with a green fluorescent signal at least 40 times stronger than that of negative cells. The DNA content from at least 4,000 EGFPF-positive cells is presented in the DNA histograms. Modfit LT software (Verity Software House, Inc.) was used to analyze DNA content and determine the percentage of cells in the G1, S, and G2/M phases of the cell cycle. To analyze apoptosis in response to adriamycin treatment, 4 × 105 cells/ml were treated with adriamycin (Sigma, St. Louis, Mo.) and assayed by FACScan analysis of Annexin V-fluorescein isothiocyanate binding (Oncogene Research Products, Cambridge, Mass.) at four time points within 24 h of treatment. The percentage of specific apoptosis was calculated as 100 × (experimental apoptosis − spontaneous apoptosis)/(100 spontaneous apoptosis) (33).
Ras transformation assays.
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal calf serum, and fresh medium was added twice a week. For transfection, cells were plated in 35-mm-diameter dishes at a density of 105/plate, and transfection was performed using the standard calcium phosphate method (44). NIH 3T3 cells were transfected using a constant amount of v-ras (150 ng) expression plasmid (pPA90) and an increasing amount of DBA/2 p16 or p19 or BALB/c p16 or p19. Foci were counted 7 days after transfection.
RESULTS
Congenic strains of mice with DBA/2 alleles of genes surrounding Cdkn2a are relatively resistant to tumor induction.
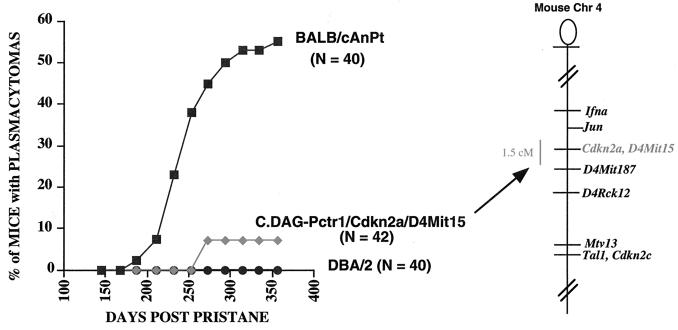
In previous experiments, congenic strains of mice carrying DBA/2 alleles on a BALB/c background had limited the location of Pctr1 to a 5.7-cM interval on mouse chromosome 4 (28). In this study, a new strain was constructed to carry an interval of 1 to 2 cM composed of DBA/2 alleles surrounding Cdkn2a and D4Mit15 on a BALB/c background. This strain was found to be significantly more resistant (Fig. 1) than BALB/c. These results served to narrow the genetic interval surrounding the Pctr1 modifier locus from a 5.7- to a 1.5-cM interval, an interval found to retain the DBA alleles of Cdkn2a and D4Mit15.
FIG. 1.
C.DAG-Pctr1 Cdkn2a D4Mit15 mice carry a 1.5-cM segment derived from DBA/2 chromatin (DBA alleles at Cdkn2a and D4Mit15; BALB/c alleles at Jun and D4Mit187) and were found to be resistant to plasmacytomagenesis relative to the susceptible BALB/cAn strain. Chr, chromosome.
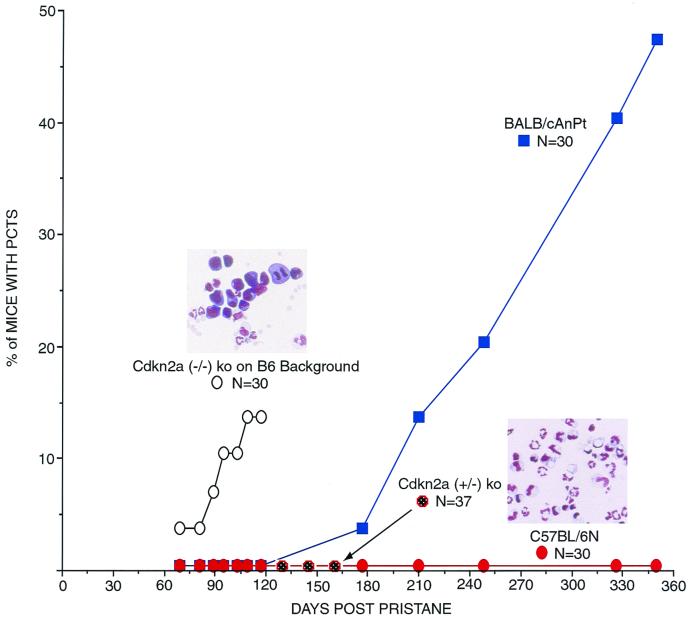
Knockout mice for the Cdkn2a locus (INK4aΔ2/3 knockouts) develop accelerated plasmacytomagenesis.
INK4aΔ2/3 mice, which were generated by deleting exons 2 and 3 of the Cdkn2a locus (36), are functional knockouts for both p16INK4a and p19ARF genes, which share exons 2 and 3 of the Cdkn2a locus. To determine if the Cdkn2a locus influences susceptibility to plasmacytoma development, homozygous and heterozygous mutant (INK4aΔ2/3) C57BL/6 mice, as well as wild-type C57BL/6 and BALB/cAn mice, were treated with pristane and examined for plasma cell tumors. The normal induction regimen for BALB/cAn involves three 0.5-ml intraperitoneal injections of pristane given at 60-day intervals, with plasmacytomas developing in 50 to 60% of the mice between 180 and 350 days after the first injection (Fig. 1 and 2). Remarkably, among 30 treated INK4aΔ2/3 knockout mice, 1 mouse developed a plasmacytoma 60 days after the first pristane injection (Fig. 2, left inset) and three additional cases were noted by 120 days after the first injection (Fig. 2). Histopathologic sections of tissues obtained at autopsy identified an additional mouse with a plasmacytoma and seven more mice with large numbers of atypical plasma cells; two of these mice also had diffuse large-cell lymphomas. Three other pristane-treated knockout mice developed histiocytic sarcomas. No plasmacytomas were observed in untreated mice or in pristane-treated C57BL/6 mice or mice heterozygous for the INK4aΔ2/3 allele (Fig. 2). Thus, the Cdkn2a locus can modify both the incidence and latency of plasma cell tumor development.
FIG. 2.
Cdkn2a (INK4aΔ2/3; p16INK4a and p19ARF) knockout (ko) mice on the C57BL/6 genetically resistant background develop plasmacytomas (PCTs) with a shorter latency period than that seen in BALB/cAnPt mice. Mice were given two 0.5-ml intraperitoneal injections of pristane at days 0 and 60. Homozygous knockout mice were autopsied at day 120 due to their significant morbidity. Insets are photomicrographs of Wright-Giemsa-stained slides of peritoneal exudate cells from knockout (left) and normal (right) mice.
As is true of Burkitt's lymphoma in humans, myc activation by translocation has been considered the hallmark lesion of mouse plasmacytomas, with approximately 90 to 95% of pristane-induced plasma cell tumors harboring t(12;15) translocations between myc and IgH sequences that are detectable by Southern blot and PCR analyses (18). Strikingly, only one of four plasma cell tumors that arose in the homozygous null INK4aΔ2/3 mice was found to harbor a t(12;15) translocation involving the myc oncogene and IgHα sequences. This tumor was the one that had arisen within the first 60 days post-pristane treatment. Northern blot analysis of the four tumors from INK4aΔ2/3 mice revealed that the Myc transcript from the tumor harboring a t(12;15) translocation was in high abundance and smaller in size, consistent with a translocation junction between exons 1 and 2. In contrast, three tumors with undetectable translocations expressed lower levels of Myc which were the same size as germ line transcripts (data not shown). The most straightforward interpretation of these results is that myc activation by translocation may not be necessary for plasmacytoma development in the Cdkn2a null background, although myc activation, when it does arise, may serve to accelerate the tumorigenic process in these mice. However, in the absence of karyotypic data, it remains possible that low-Myc-expressing tumors might have sustained a translocation that was undetectable by Southern blot and PCR analyses.
Allelic variants in the p16INK4a locus differ in their abilities to induce G1 arrest.
The susceptibility of the INK4aΔ2/3 mice to pristane-induced plasmacytomas implied that the Pctr1 modifier locus in BALB/c mice might be represented by the gene for p16INK4a or p19ARF alone or both genes together. Alleles of the Cdkn2a locus contain sequence differences in exon 1α and exon 2 of the p16INK4a gene and in exon 2 of the p19ARF gene (45). BALB/cAnPt and ABP/Le mice have rare alleles of the p16INK4a gene (encoding proline and isoleucine at positions 18 and 51, respectively) and the p19ARF gene (encoding histidine at amino acid 72) in contrast to DBA/2N and C57BL/6 mice, which carry the more common allele for these two genes (encoding histidine and valine at positions 18 and 51 in p16INK4a and arginine at position 72 in p19ARF). Therefore, the sequence divergence between the BALB/c and DBA/2 coding regions of the two genes made both genes potential candidates for the Pctr1 modifier. To determine whether one or both genes could be implicated as the modifier, we initially compared the abilities of the BALB/c and DBA/2 coding sequences of p16INK4a and p19ARF to inhibit cell growth, since several studies have shown that overexpression of either p16INK4a or p19ARF can lead to cell cycle arrest in G1 (17, 31).
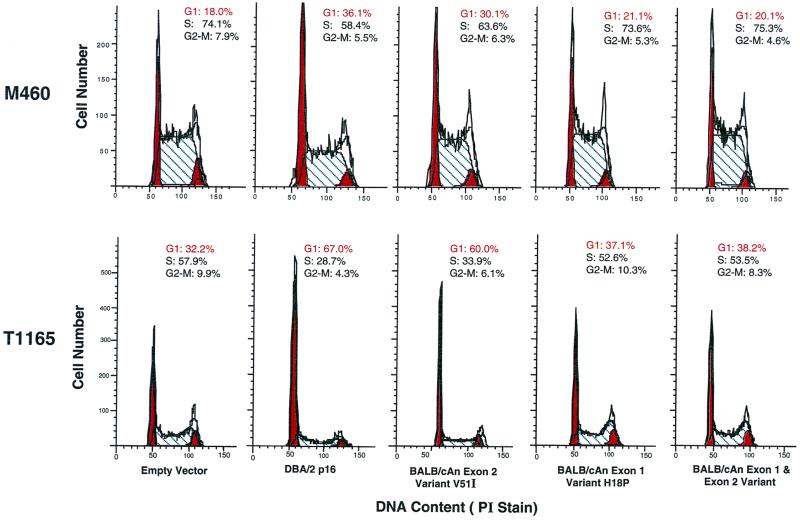
NIH 3T3 and two plasmacytoma cell lines derived from BALB/cAn mice were transiently transfected with isogenic BALB/c and DBA/2 allelic variants of these genes. Overexpression of the DBA/2 p16INK4a variant led to G1 arrest for both plasmacytoma cell lines, MOPC460 and TEPC1165 (Fig. 3), with almost twice as many cells in G1 compared to cells transfected with the empty vector. In contrast, when the BALB/c p16INK4a allele was overexpressed in the same cell lines, there was only a marginal increase (2 to 6%) in the proportion of cells in G1. Thus, the proliferative phenotype of the plasmacytoma cell lines could be rescued by overexpression of the more common (or wild-type) allele, while the BALB/c variant of p16 was much less efficient in inducing G1 arrest.
FIG. 3.
Cell cycle analysis of DBA/2 and BALB/cAn variants of p16INK4a. Overexpression of DBA/2N, but not BALB/cAnPt, p16INK4a by transient transfection can lead to G1 arrest in plasmacytoma cell lines. The inefficiency of p16INK4a function in BALB/cAn is largely attributable to the codon change seen in exon 1α. DNA content was measured by propidium iodide (PI) staining. All transfections were done in duplicate in each experiment, and all experiments were performed at least three times. The means and standard errors across experiments for the number of TEPC1165 cells in G1 are as follows: empty vector, 37 ± 3.2; DBA p16, 69 ± 3.5; BALB exon 2, 58 ± 2.2; BALB exon 1, 37 ± 0.3; BALB/c p16, 37 ± 0.7. The means and standard errors across experiments for the number of MOPC460 cells in G1 are as follows: empty vector, 20 ± 1.5; DBA p16, 40 ± 4; BALB exon 2, 30 ± 1; BALB exon 1, 22 ± 1.5; BALB/c p16, 21 ± 2.
To investigate whether one or both of the codon changes in the BALB/c p16INK4a allele was functionally relevant to the genetic inhibitory activity, constructs were engineered which contained only the exon 1α or exon 2 BALB/c variants. When expressed in the plasmacytomas, the exon 1α mutant allele was as deficient as the BALB/c variant allele (Fig. 3) while the exon 2 mutant construct was almost as active as the DBA/2 allele. The greater impairment of the exon 1α mutant correlates with its variant amino acid (proline versus histidine at residue 18) being less conservative than the exon 2 variant amino acid (isoleucine to valine at residue 51).
Overexpression of BALB/c or DBA/2 p19ARF variants leads to p53-dependent growth arrest.
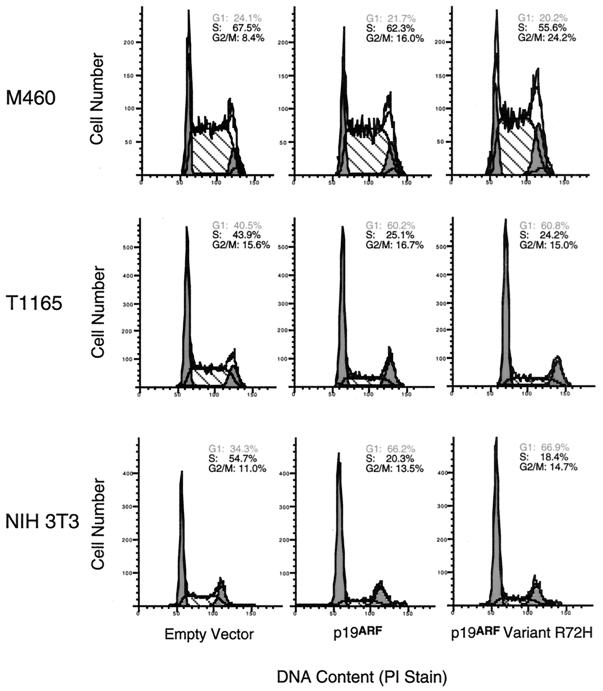
To determine whether the genetic polymorphism in exon 2 of the Cdkn2a locus affected p19ARF function, isogenic constructs containing the variants were transiently transfected into the two plasmacytoma cell lines and also into NIH 3T3 cells. Both p19ARF alleles were equally efficient at inducing G1 arrest in NIH 3T3 and TEPC1165 cells (Fig. 4), a finding in accord with the highly conservative nature of the amino acid substitution (histidine versus arginine at residue 72). Almost twice as many NIH 3T3 and TEPC1165 cells were in G1 following transfection with either construct compared with cells transfected with the empty vector. These results argue that the exon 2 sequence divergence in the BALB/c p19ARF gene does not render its product less active biologically than the DBA/2 p19ARF.
FIG. 4.
Cell cycle analysis of BALB/cAnPt and DBA/2N p19ARF allelomorphs indicated normal function of each allele. Ectopic expression of all p19ARF variants led to G1 growth arrest in NIH 3T3 fibroblasts and TEPC1165 plasmacytoma cells but not in the p53 compromised MOPC460 plasmacytoma cells. PI, propidium iodide.
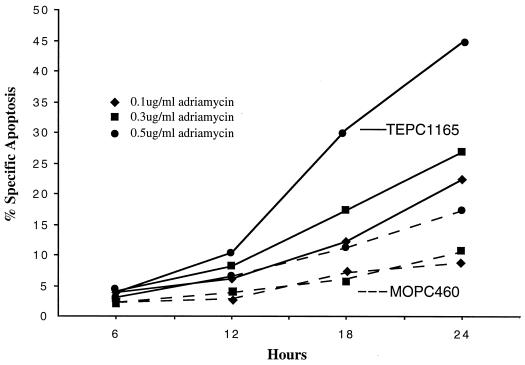
Unexpectedly, MOPC460 plasmacytoma cells did not undergo G1 arrest when either allele of the p19ARF gene was overexpressed. p19ARF is known to induce cell cycle arrest and apoptosis by blocking the ability of Mdm2 to target p53 for degradation (2, 27, 39, 46, 47), suggesting that the failure of p19ARF variants to induce G1 arrest in MOPC460 could be due to alterations in MDM2 or p53 signaling. p53 mRNA and protein levels were found to be equivalent in all cell lines except for MOPC460, which had high levels of p53 expression (data not shown). cDNA and genomic sequencing of the p53 genes in a total of five plasmacytoma cell lines revealed that four (TEPC1165, X24, TEPC2027, and MOPC265) of the five lines contained only wild-type p53 while that of MOPC460 harbored a 21-bp in-frame deletion involving the splice acceptor site of exon 5. To determine if the MOPC460 p53 gene was functionally compromised, we treated both TEPC1165 and MOPC460 cells with adriamycin, an agent that induces cell cycle arrest and apoptosis (38). At 24 h after the start of adriamycin treatment (0.5 μg/ml), almost 50% of TEPC1165 cells were arrested in G2/M and exhibited apoptosis. In contrast, approximately 90% of the MOPC460 cells were arrested in G2/M, and modest levels of apoptosis were observed (Fig. 5). Taken together, the results suggest that the p53 pathway is intact in most plasmacytoma cell lines but that it is altered in MOPC460 by the in-frame p53 deletion specific to this cell line.
FIG. 5.
TEPC1165 (wild-type p53) and MOPC460 (21-bp deletion of p53) plasmacytoma cells differed in induction of specific apoptosis induced by adriamycin treatment (0.1 to 0.5 μg/ml). MOPC460 cells exhibited attenuated apoptotic responses following treatment with adriamycin.
Inhibition of ras transformation by p16INK4a or p19ARF.
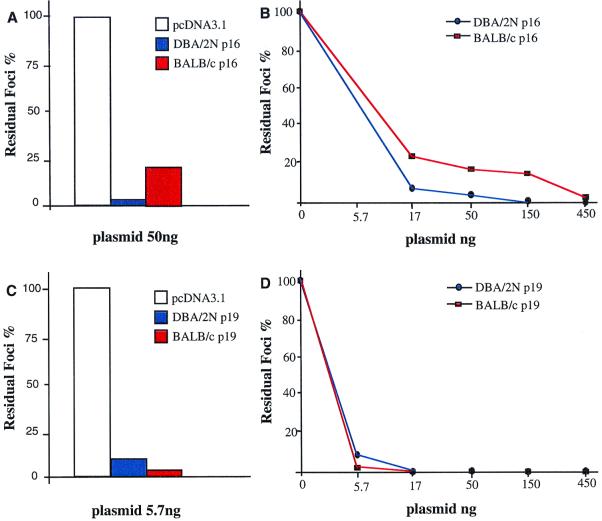
To further test the relative biological activities of the BALB/c and DBA/2 p16INK4a and p19ARF proteins, we compared their abilities to inhibit focal transformation induced by an oncogenic ras. In these experiments, NIH 3T3 cells, in which the endogenous Cdkn2a locus is mutationally inactivated (30, 31), were cotransfected with a constant amount of v-ras and increasing amounts of DBA/2 p16 or p19 or BALB/c p16 or p19 (44). Under these conditions, p16INK4a and p19ARF from either mouse strain inhibited the formation of ras-transformed foci in a dose-dependent manner (Fig. 6). However, the BALB/c p16INK4a, at any of three different doses, was less efficient than its DBA/2 counterpart, while the BALB/c p19ARF variant was as active as DBA/2 p19ARF in suppressing ras transformation. These results therefore parallel those obtained with the plasmacytoma cell lines in showing that the BALB/c p16INK4a is less active than that of DBA/2, while p19ARF proteins from both strains appear to possess similar degrees of activity. Furthermore, in contrast to the low activity of BALB/c p16INK4a in the G1 arrest assay of the plasmacytomas, its dose-dependent ability to inhibit ras-induced transformation clearly indicates that BALB/c p16INK4a retains biological activity.
FIG. 6.
Inhibition of ras-mediated focus formation. (A and B) Cotransfection of BALB/c and DBA/2 variants of p16INK4a in pcDNA3.1 with v-ras in NIH 3T3 cells. The percentages of residual foci formed relative to the foci detected on the plates transfected with ras alone are shown. (C and D) Cotransfection of BALB/c and DBA/2 variants of p19ARF in pcDNA3.1 with v-ras in NIH 3T3 cells. The transfection assays were repeated three times with similar results.
DISCUSSION
The current data provide evidence that efficiency alleles of the p16INK4a gene are likely to serve as modifiers of plasma cell tumor susceptibility among inbred strains of mice. In the present congenic mouse studies, the Pctr1 interval was narrowed from 5.7 to 1.5 cM by introgressively backcrossing resistant DBA/2 alleles from the Pctr1 interval, which includes Cdkn2a, onto a susceptible BALB/c background. Compared with wild-type BALB/cAn mice, the congenic displayed a delayed onset of plasmacytomagenesis and decreased tumor incidence. Although the DBA/2 Cdkn2a locus was present in the congenic strain, it could not be determined whether the Pctr1 modifier was the Cdkn2a locus or another tightly linked DBA/2 gene within the congenic interval. In the knockout studies, the impact of the Cdkn2a locus on plasmacytoma susceptibility is underscored by the short latency period and increased incidence of clinically apparent tumors by 120 days after pristane treatment, even though the knockout of the Cdkn2a locus was on the plasmacytoma-resistant C57BL/6 (N4) background strain. In models of accelerated plasmacytomagenesis, by the INK4aΔ2/3 knockout or by the inoculation of retroviruses harboring combinations of myc and ras or abl oncogenes (20, 43), it is plausible to hypothesize that the shorter latency period results from fewer required genetic changes. Consistent with this possibility, rearrangement of the myc gene does not occur in the retroviral systems (43) and was identified in only one out of four plasma cell tumors that arose in the INK4aΔ2/3 knockout mice by assays that can identify rearrangement of myc in more than 90% of these tumors in BALB/c mice.
Fibrosarcomas and lymphomas have been the two most common spontaneous tumor types described for the INK4aΔ2/3 knockout of the Cdkn2a locus and for p19ARF knockout mice (15, 36). These tumors developed more slowly than those in the present study and were either spontaneous or induced with two-stage carcinogenesis protocols involving the reagents DMBA and UVB commonly used to induce skin tumors. In this report, the plasma cell tumors were induced with pristane, which promotes chronic inflammation and increased production of IL-6 (29, 37). The short latency period for plasmacytoma formation in the INK4aΔ2/3 knockout mice probably results from inactivation of both p16INK4a and p19ARF, which disrupt the Rb and p53 pathways, respectively. In our present studies, BALB/c mice exhibit partially impaired p16INK4a function but appear to have an intact p19ARF-mdm2-p53 pathway in normal cells and in most plasmacytomas. The longer tumor latency period in BALB/c mice probably results from the partially functional p16INK4a gene and completely functional p19ARF gene. The development of the tumors in the knockout mice resulted from the homozygous disruption of the Cdkn2a locus, since neither the C57BL/6 mice nor the INK4aΔ2/3 heterozygotes developed plasma cell tumors. The tumor incidence studies in the Cdkn2a knockouts support the view that one or both of the genes included in the Cdkn2a locus govern susceptibility to pristane-induced plasmacytomagenesis.
Since the Cdkn2a locus includes two genes that inhibit cell growth, several biological studies were carried out to determine whether the BALB/c modifier locus was a result of p16INK4a and/or p19ARF alleles. In two different assays, the BALB/c p16INK4a protein variant was found to be less active than DBA/2 p16INK4a, while the p19ARF variants of the two strains behaved similarly. Overexpression of the BALB/c allele was not sufficient to induce G1 arrest in mouse plasma cell tumor lines. Furthermore, overexpression of the wild-type (DBA/2 and C57BL/6) allele of p16INK4a was able to complement the defect inherent in the BALB/c-derived plasma cell tumor lines. Inefficient BALB/c p16INK4a may allow phosphorylation of Rb and its release from promoter-bound E2F transcription factor, which leads to the transactivation of several genes involved in S-phase progression (24, 42); this results in cells progressing through the cell cycle and allows for sustained rounds of proliferation. Our present biological studies support the notion that BALB/c p16INK4a is less effective in inducing G1 arrest and that it could in fact contribute to continued rounds of B-cell proliferation and the possible accumulation of further genetic changes, leading to neoplasia.
In another bioassay, the abilities of the alleles to inhibit focal transformation of NIH 3T3 cells by activated ras were examined. Activation of the Ras pathway has been implicated in mouse plasmacytomas, since the combination of constitutively active ras and myc can collaborate to induce plasmacytomas (20) whose growth is dependent upon IL-6 (5). IL-6 can activate the Ras pathway (23, 26). In the in vitro ras transformation assay, we found, in four separate assays, that BALB/c p16INK4a suppressed ras-induced transformation in a dose-dependent manner, although this allele was less efficient than the DBA allele. These biological results confirm the relevance of our previous biochemical assays for BALB/c versus DBA/2 p16INK4a proteins. Taken together, these data argue that the Pctr1 modifier is encoded by the p16INK4a gene and that the BALB/c p16INK4a allele is functional, but less active, than the more common allele found in DBA/2 and C57BL/6 mice. Using the cell cycle and ras suppression bioassays, the BALB/c and DBA/2 p19ARF proteins were found to be equally active. Therefore, we have no evidence that the p19ARF gene is part of the Pctr1 modifier, although it remains possible that other assays might uncover biological differences between them.
In contrast to the Pctr1 plasmacytoma modifier described here, mouse strains which carry the recessive susceptibility allele of the Mom1 modifier have sustained a frameshift loss-of-function mutation in its encoded PLA2G2A protein (19). In the plasmacytoma system, the recessive susceptible allele (a single-base substitution) encodes a mutant full-length p16INK4a protein that is less efficient biologically and biochemically. Our data are in keeping with information derived from studies of a growing number of disease gene alleles in which point mutations account for differences in disease susceptibility (1, 4, 10, 11, 35, 41).
The molecular identification of modifier loci leading to a genetic predisposition for mouse plasmacytomas provides a powerful model for predicting genes and pathways involved in the etiologies of a variety of human B-cell neoplasias. Recently, a multiple-myeloma patient was described who was a member of a melanoma-prone family and had a germ line mutation in the Cdkn2a locus consisting of a duplication of the first eight codons of the p16INK4a gene, a lesion previously identified in other melanoma-prone families (8). Subsequent analysis of bone marrow plasma cells from the patient revealed loss of the wild-type CDKN2A allele. The results reported here should stimulate efforts to identify similar linkage in humans afflicted with plasmacytomas, Burkitt's lymphomas, and multiple myelomas. In many human and mouse tumors, it is already known that there is somatic selection against functional CDKN2A by either homozygous deletion or hypermethylation (9, 12, 16, 25, 32, 40). Relatively few tumors outside of melanoma and certain digestive cancers have shown missense mutations in the p16INK4a gene (32). Our results suggest that single-base changes in important growth-regulatory genes, particularly when they are present as several defective efficiency alleles, may be frequent predisposing events in tumorigenesis.
The majority of human cancers are not the clear result of predisposition determined by a single genetic defect. Instead, they are likely to represent the outcome of complex interactions between multiple genetic alleles and environmental factors. Mouse plasma cell tumors provide a model system for studying neoplastic processes which result from the complex interplay among several predisposing genetic factors and environmental stresses.
ACKNOWLEDGMENTS
We acknowledge Richard Nordan, who passed away during the course of these studies, and his colleague Cindy Thompson for providing us with IL-6 and valuable advice concerning transfections of plasmacytoma cells. We thank Wei Jiang for providing us with the EGFPF vector for use in our transfections and Han-Woong Lee for advice on breeding the p16 knockout mice. In addition, we thank Douglas Lowy for his insightful editorial comments and Xiaolan Qian for her involvement in the ras transformation assays.
Footnotes
We dedicate this paper to the memory of Richard P. Nordan.
REFERENCES
- 1.Ahmad N N, Barbosa de Melo M D, Singh A D, Donoso L A, Shields J A. A possible hot spot in exon 21 of the retinoblastoma gene predisposing to a low penetrant retinoblastoma phenotype? Ophthalmic Genet. 1999;20:225–231. doi: 10.1076/opge.20.4.225.2269. [DOI] [PubMed] [Google Scholar]
- 2.Bates S, Phillips A C, Clark P A, Stott F, Peters G, Ludwig R L, Vousden K H. p14ARF links the tumor suppressors RB and p53. Nature. 1998;395:124–125. doi: 10.1038/25867. [DOI] [PubMed] [Google Scholar]
- 3.Brown R T, Ades I Z, Nordan R P. An acute phase response factor/NF-κB site downstream of the junB gene that mediates responsiveness to interleukin-6 in a murine plasmacytoma. J Biol Chem. 1995;270:31129–31135. doi: 10.1074/jbc.270.52.31129. [DOI] [PubMed] [Google Scholar]
- 4.Carter A M, Catto A J, Grant P J. Association of the alpha-fibrinogen Thr312Ala polymorphism with poststroke mortality in subjects with atrial fibrillation. Circulation. 1999;99:2423–2426. doi: 10.1161/01.cir.99.18.2423. [DOI] [PubMed] [Google Scholar]
- 5.Clynes R J, Wax J, Staton L W, Smith-Gill S, Potter M, Marcu K B. Rapid induction of IgM secreting murine plasmacytomas by pristane and an IgH promoter-enhancer driven c-myc/v-Ha-ras retrovirus. Proc Natl Acad Sci USA. 1988;85:6067–6071. doi: 10.1073/pnas.85.16.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cormier R T, Hong K H, Halberg R B, Hawkins T L, Richardson P, Mulherkar R, Dove W F, Lander E S. Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat Genet. 1997;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich W F, Lander E S, Smith J S, Moser A R, Gould K A, Luongo C, Borenstein N, Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 8.Dilworth D, Liu L, Stewart A K, Berenson J R, Lassman N, Hogg D. Germline CDKN2A mutation implicated in predisposition to multiple myeloma. Blood. 1999;95:1869–1871. [PubMed] [Google Scholar]
- 9.Drexler H G. Review of alterations of the cyclin-dependent kinase inhibitor INK4 family genes p15, p16, p18 and p19 in human leukemia-lymphoma cells. Leukemia. 1998;12:845–859. doi: 10.1038/sj.leu.2401043. [DOI] [PubMed] [Google Scholar]
- 10.Farker K, Lehmann M H, Kastner R, Weber J, Janitzky V, Schubert J, Hoffmann A. Analysis of point mutation in exon 2 of CYP2E1 gene in renal cell/urothelial cancer patients in comparison with control population. Int J Clin Pharmacol Ther. 2000;38:30–34. doi: 10.5414/cpp38030. [DOI] [PubMed] [Google Scholar]
- 11.Ford M E, Whitcomb D C. Analysis of the hereditary pancreatitis-associated cationic trypsinogen gene mutations in exons 2 and 3 by enzymatic mutation detection from a single 2.2-kb polymerase chain reaction product. Mol Diagn. 1999;4:211–218. doi: 10.1016/s1084-8592(99)80024-1. [DOI] [PubMed] [Google Scholar]
- 12.Haidar M A, Cao X-B, Manshouri T, Chan L L, Glassman A, Kantarjian H M, Keating M J, Beran M S, Albitar M. p16INK4a and p15INK4b gene deletions in primary leukemias. Blood. 1995;86:311–315. [PubMed] [Google Scholar]
- 13.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W, Hunter T. Analysis of cell-cycle profiles in transfected cells using a membrane-targeted GFP. BioTechniques. 1998;24:348–354. [PubMed] [Google Scholar]
- 15.Kamijo T, Zindy F, Roussel M F, Quelle D E, Downing J R, Ashmun R A, Grosveld G, Sherr C J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–659. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 16.Klangby U, Okan I, Magnusson K P, Wendland M, Lind P, Wiman K G. p16/INK4a and p15/INK4b gene methylation and absence of p16/INK4a mRNA and protein expression in Burkitt's lymphoma. Blood. 1998;91:1680–1687. [PubMed] [Google Scholar]
- 17.Koh J, Enders G H, Dynlacht B D, Harlow E. Tumour-derived p16 alleles encoding proteins defective in cell-cycle inhibition. Nature. 1995;375:506–510. doi: 10.1038/375506a0. [DOI] [PubMed] [Google Scholar]
- 18.Kovalchuk A L, Mushinski E B, Janz S. Clonal diversification of primary BALB/c plasmacytomas harboring T(12;15) chromosomal translocations. Leukemia. 2000;14:909–921. doi: 10.1038/sj.leu.2401676. [DOI] [PubMed] [Google Scholar]
- 19.MacPhee M, Chepenik K P, Liddell R A, Nelson K K, Siracusa L D, Buchberg A M. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell. 1995;81:957–966. doi: 10.1016/0092-8674(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 20.Mock B, Wax J, Clynes R, Marcu K B, Potter M. The genetics of susceptibility to RIM-induced plasmacytomagenesis. Curr Top Microbiol Immunol. 1988;141:125–127. doi: 10.1007/978-3-642-74006-0_17. [DOI] [PubMed] [Google Scholar]
- 21.Mock B A, Hartley J, Le Tissier P, Wax J S, Potter M. The plasmacytoma resistance gene, Pctr2, delays the onset of tumorigenesis and resides in the telomeric region of Chromosome 4. Blood. 1997;90:4092–4098. [PubMed] [Google Scholar]
- 22.Mock B A, Krall M A, Dosik J K. Genetic mapping of tumor susceptibility genes involved in mouse plasmacytomagenesis. Proc Natl Acad Sci USA. 1993;90:9499–9503. doi: 10.1073/pnas.90.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann C, Zehentmaier G, Danhauser-Riedl S, Emmerich B, Hallek M. Interleukin-6 induces tyrosine phosphorylation of the Ras activating protein Shc, and its complex formation with Grb2 in the human multiple myeloma cell line LP-1. Eur J Immunol. 1996;26:379–384. doi: 10.1002/eji.1830260217. [DOI] [PubMed] [Google Scholar]
- 24.Nevins J R. Towards an understanding of the functional complexity of the E2f and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 25.Ng M H L, Chung Y F, Lo K W, Wickham N W R, Lee J C K, Huang D P. Frequent hypermethylation of p16 and p15 genes in multiple myeloma. Blood. 1997;89:2500–2506. [PubMed] [Google Scholar]
- 26.Ogata A, Chauhan D, Teoh G, Treon S P, Urashima M, Schlossman R L, Anderson K C. IL-6 triggers cell growth via the ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 27.Pomerantz J, Schreiber-Agus N, Liegeois N J, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee H-W, Cordon-Cardo C, DePinho R A. The Ink4a tumor suppressor gene product, p19ARF, interacts with MDM-2 and neutralizes MDM2's inhibition of p53. Cell. 1998;92:713–723. doi: 10.1016/s0092-8674(00)81400-2. [DOI] [PubMed] [Google Scholar]
- 28.Potter M, Mushinski E B, Wax J S, Hartley J, Mock B. Two genes on Chromosome 4 determine resistance to plasmacytoma induction in mice. Cancer Res. 1994;54:969–975. [PubMed] [Google Scholar]
- 29.Potter M, Robertson C L. Development of plasma cell neoplasms in BALB/c mice after intraperitoneal injection of paraffin-oil adjuvant, heat-killed staphylococcus mixtures. J Natl Cancer Inst. 1960;25:847–861. doi: 10.1093/jnci/25.4.847. [DOI] [PubMed] [Google Scholar]
- 30.Quelle D E, Cheng M, Ashmun R A, Sherr C J. Cancer-associated mutations at the INK4a locus cancel cell cycle arrest by p16INK4a but not by the alternative reading frame protein p19ARF. Proc Natl Acad Sci USA. 1997;94:669–673. doi: 10.1073/pnas.94.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quelle D E, Zindy F, Ashmun R A, Sherr C J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 32.Quesnel B, Preudhomme C, Fenaux P. p16INK4a gene and hematological malignancies. Leuk Lymphoma. 1996;22:11–24. doi: 10.3109/10428199609051724. [DOI] [PubMed] [Google Scholar]
- 33.Robles A I, Wang X W, Harris C C. Drug-induced apoptosis is delayed and reduced in XPD lymphoblastoid cell lines: possible role of TFIIH in p53-mediated apoptotic cell death. Oncogene. 1999;18:4681–4688. doi: 10.1038/sj.onc.1202862. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schaffner C, Stilgenbauer S, Rappold G A, Dohner H, Lichter P. Somatic ATM mutations indicate a pathogenic role of ATM in B-cell chronic lymphocytic leukemia. Blood. 1999;94:748–753. [PubMed] [Google Scholar]
- 36.Serrano M, Lee H-W, Chin L, Cordon-Cardo C, Beach D, DePinho R A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 37.Shacter E, Arzadon G K, Williams J. Elevation of interleukin-6 in response to a chronic inflammatory stimulus in mice: inhibition by indomethacin. Blood. 1992;80:194–202. [PubMed] [Google Scholar]
- 38.Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- 39.Tao W, Levine A J. p19ARF stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc Natl Acad Sci USA. 1999;96:6937–6941. doi: 10.1073/pnas.96.12.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasaka T, Berenson J, Vescio R, Hirama T, Miller C W, Nagai M, Takahara J, Koeffler H P. Analysis of the p16INK4A, p15INK4B and p18INK4C genes in multiple myeloma. Br J Haematol. 1997;96:98–102. doi: 10.1046/j.1365-2141.1997.8552482.x. [DOI] [PubMed] [Google Scholar]
- 41.Wallis Y L, Morton D G, McKeown C M, Macdonald F. Molecular analysis of the APC gene in 205 families: extended genotype-phenotype correlations in FAP and evidence for the role of APC amino acid changes in colorectal cancer predisposition. J Med Genet. 1999;36:14–20. [PMC free article] [PubMed] [Google Scholar]
- 42.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 43.Wiener F, Coleman A, Mock B A, Potter M. Nonrandom chromosomal change (trisomy 11) in murine plasmacytomas induced by an ABL-MYC retrovirus. Cancer Res. 1995;55:1181–1188. [PubMed] [Google Scholar]
- 44.Willumsen B M, Vass W C, Velu T J, Papageorge A G, Schiller J, Lowy D R. The BPV E5 oncogene can cooperate with ras: identification of a p21 amino acid segment required for transformation by c-rasH but not v-rasH. Mol Cell Biol. 1991;9:6026–6033. doi: 10.1128/mcb.11.12.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, Ramsay E S, Mock B A. Cdkn2a, the cyclin dependent kinase inhibitor encoding p16INK4a and p19ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc Natl Acad Sci USA. 1998;95:2429–2434. doi: 10.1073/pnas.95.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Xiong Y. Mutations in human ARF exon 2 disrupt its nucleolar localization and impair its ability to block nuclear export of MDM2 and p53. Mol Cell. 1999;3:579–591. doi: 10.1016/s1097-2765(00)80351-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Xiong Y, Yarbrough W G. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]