Abstract
Objective:
Mechanical ventilation is an important treatment for critically ill patients. Physicians generally perform a spontaneous breathing trial (SBT) to determine whether the patients can be weaned from mechanical ventilation, but almost 17% of the patients who pass the SBT still require respiratory support. Cardiac dysfunction is an important cause of weaning failure. The use of brain natriuretic peptide or N-terminal pro-BNP is a simple method to assess cardiac function. We performed a systematic review of investigations of brain natriuretic peptide or N-terminal pro-BNP as predictors of weaning from mechanical ventilation.
Data sources:
PubMed (1950 to December 2020), Cochrane, and Embase (1974 to December 2020), and some Chinese databases for additional articles (China Biology Medicine (CBM), China Science and Technology Journal Database (CSTJ), and Wanfang Data and China National Knowledge Infrastructure (CNKI)).
Study selection:
We systematically searched observation studies investigating the predictive value of brain natriuretic peptide or N-terminal pro-brain natriuretic peptide in weaning outcome of patients with mechanical ventilation.
Data extraction:
Two independent reviewers extracted data. The differences are resolved through consultation.
Data synthesis:
We included 18 articles with 1416 patients and extracted six index tests with pooled sensitivity and specificity for each index test. For the BNP change rate predicting weaning success, the pooled sensitivity was 89% (83%–94%) and the pooled specificity was 82% (72%–89%) with the highest pooled AUC of 0.9511.
Conclusions:
The brain natriuretic peptide change rate is a reliable predictor of weaning outcome from mechanical ventilation.
Keywords: Brain natriuretic peptide, N-terminal pro-brain natriuretic peptide, ventilator weaning
Introduction
Mechanical ventilation, a method of supporting critical patients, 1 exerts important effects on global oxygen delivery and reduces the work of breathing. 2 When the respiratory muscles are unable to maintain normal pulmonary ventilation in the face of respiratory dysfunction, mechanical ventilation generally acts as a bridge to recovery. 3 However, mechanical ventilation can have life-threatening complications, such as ventilator associated pneumonia (VAP). According to the International Nosocomial Infection Control Consortium (INICC), the overall rate of VAP is 13.6 per 1000 ventilator days, the mortality associated with VAP ranges from 24% to 76%. 4 The incidence of respiratory muscle weakness and gastrointestinal bleeding increases with the duration of respiratory support.5–7 These complications have been associated with the failure to liberate from ventilator, and increased intensive care unit mortality.8,9 Thus, it should be discontinued at the earliest possible time. 10 The process of discontinuing mechanical ventilation, termed weaning, is one of the most challenging problems in intensive care. Weaning accounts for a considerable proportion of the workload of staff in an intensive care. 11 However, premature weaning may also be harmful and cause extubation failure or hymoxaemia.8,12 Thus, a simple, reasonable index to evaluate liberation from ventilator is an important issue which ICU doctors require.
The purpose of the weaning procedure is to minimize the duration of mechanical ventilation without incurring a substantial risk of failure. 13 Common weaning methods include pressure support ventilation,14,15 synchronized intermittent mandatory ventilation, 16 and a spontaneous breathing trial (SBT). 17 The SBT is the most definitive index for forecasting weaning success, 18 but the extubation failure rate remains great (15%–20%) in patients who have successfully completed SBTs. 19 Among many studies of “weaning procedures,” the pathophysiology of disengaging failure is complex,20,21 and include impaired respiratory mechanics, 22 respiratory muscle dysfunction, 23 cardiac dysfunction,24,25 cognitive dysfunction, 26 and endocrine and metabolic disorders, 27 but the comparative weight of the various implicated factors is not fully elucidated. For many reasons, cardiovascular dysfunction has been documented as a significant mechanism.20,21 During weaning, positive pressure ventilation withdrawal will appear as subclinical heart dysfunction. In critical patients, however, it is tough to determine cardiovascular dysfunction in weaning with the conventional techniques, including echocardiography, cardiac scintiscan, and pulmonary artery catheterization. They are operator-dependent, have a lack of sensitivity, are inaccessible at the bedside, or are invasive. 19
Currently, B-type natriuretic peptide (BNP) and N-terminal prohormone BNP (NT-proBNP) are reliable biomarkers for determining cardiac failure.28,29 BNP is co-secreted with the biologically inactive NT-proBNP, and they are produced by ventricles in reaction to myocardial stretch.
Removal of mechanical ventilation has physiological repercussions that reveal subclinical diastolic dysfunction and/or fluid overburden. The trimming in intrathoracic pressures increases central blood measurement,10,30 and intensifies left ventricular transmural ejection tension. Irregularities of diastolic function are often in extremely ill patients 31 and may have a part in patients being unable to wean from ventilator. Thus, BNP or NT-proBNP values may be implemented in determining cardiac dysfunction while weaning from ventilator, and may distinguish the completion of weaning from failure.10,32
BNP and NT-pro BNP were used as an evaluation index of weaning from ventilator due to cardiac dysfunction, but currently reports vary.10,19,33–48 A diagnostic test method meta-analysis is a useful tool to increase power by pooling all the published data together. In this study, we completed a diagnostic test method meta-analysis to clarify whether BNP or NT-proBNP is linked to the assessment of SBT.
Methods
PICO statement
P-patient: Adult patients were under mechanical ventilation for more than 24 h;
I-index test: BNP or NT-pro BNP was measured in all included patients;
C-complement: SBT was given to all included patients who were deemed ready to be liberated from mechanical ventilation; and
O-outcome: Efficacy of the BNP or NT-pro BNP to predict weaning outcome. Search techniques and selection criteria
This systematic review and meta-analysis has been disclosed in conformance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
We searched pertinent evaluations published PubMed (1950 to December 2020), Cochrane, and Embase (1974 to December 2020), and some Chinese databases for additional articles (CBM, CSTJ, Wanfang Data, and CNKI) without any language limitations. The search strategy is shown in attachment1.
Search of other resources
We also did a manual search for all retrieved articles and review reports published in English.
Study selection and data extraction
We selected publications that reported the sensitivity and specificity of the BNP or NT-pro BNP predicted weaning outcome. Figure 1 displays the search progress.
Figure 1.
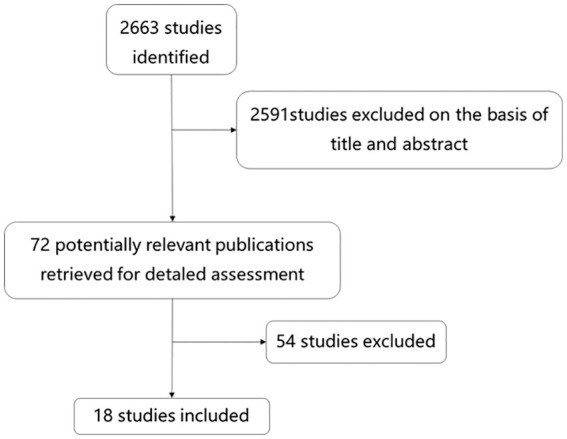
Study flow diagram 2663 articles were retrieved. Finally, 18 articles were included according to pre-set inclusion criteria.
We created an Excel spreadsheet and collected data from the articles, including: author, year of publication, country of study, sample size, sensitivity, and specificity. Numbers of true positive, true negative, false positive, and false negative values were extracted through calculations based on sensitivity, specificity, the total number of extubation successes, and extubation failures.
Assessment of risk of bias of study publications
The included studies were assessed for quality using the QUADAS-2 tool, 49 which consists of four key domains that judge bias and applicability of the reviewed studies. Based on the answers to questions from each domain, the risk of bias was judged as unclear, low, or high. A funnel plot generated by Stata 16.0 (STATA Corp, College Station, TX, USA) was used to assess publication bias, and funnel plot symmetry was assessed with Egger’s test.
The evaluation of each component for the risk of bias is detailed below: “Patient selection” domain:
Was a consecutive sample of patients enrolled? Did the study avoid inappropriate exclusions? “Index test” domain: If a threshold was used, was it pre-specified?
“Reference standard” domain:
Is the reference standard likely to correctly classify the target condition?
Were thex reference standard results interpreted without knowledge of the results of the index tests?
“Flow and timing” domain:
Was there an appropriate interval between the index test and the reference standard? Did all of the patients receive the same reference standard?
Were all of the patients included in the analysis?
Data analysis
We used meta-disc v 1.4 (Universidad Complutense, Madrid, Spain) to perform a meta-analysis in order to determine the pooled sensitivity and specificity for each diagnostic method as a predictor of weaning outcome. We used hypothesis testing to analyze the heterogeneity for each diagnostic method, Chi-square p-values, and I2 index, which is automatically calculated by meta-disc software. We interpreted the inconsistence index to be less than 50% acceptable.
To investigate a threshold effect, we plotted summary receiver operating curves (SROCs) for each diagnostic method and we also calculated the Spearman correlation coefficient between sensitivity and specificity. If the positive Spearman correlation coefficient was >0.6, we considered it to be of threshold effect.
Results
Research screening
We identified 18 studies and 1416 patients to report the predict value of brain natriuretic peptide or N-terminal pro-BNP for weaning outcome for patients who underwent mechanical ventilation. We extracted six index tests and pooled sensitivity and specificity of each index test. The characteristics of the 18 publications that met the inclusion criteria for meta-analysis are presented in Table 1. The overall quality of the included studies is shown in Figure 2 and Table 2. The source of risk in the index test arose from the threshold that was not pre-specified. However, all included studies met the review questions, so the applicability judgments were of low concern. The results of the sensitivity, specificity and small ROC of each test are shown in Figure 4.
Table 1.
Key characteristics of the meta-analyzed reports (n = 18).
| Index test | Author | Year | Country | Ref. | Cutoff value | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|
| BNP1 | Armand | 2006 | France | [10] | 275 pg/ml | 35 | 7 | 6 | 54 |
| Xu | 2013 | China | [44] | 263 pg/ml | 10 | 13 | 2 | 41 | |
| 2014 | China | [42] | 849.1 pg/ml | 26 | 9 | 8 | 80 | ||
| Zhou | 2013 | China | [46] | 204 pg/ml | 13 | 3 | 3 | 25 | |
| Ma | 2016 | China | [40] | 294.79 pg/ml | 15 | 10 | 3 | 42 | |
| He | 2013 | China | [45] | 139 pg/ml | 12 | 4 | 5 | 15 | |
| Total | 111 | 46 | 27 | 257 | |||||
| BNP2 | He | 2013 | China | [45] | 157 pg/ml | 13 | 4 | 4 | 15 |
| 2014 | China | [43] | 224.5 pg/ml | 40 | 15 | 10 | 93 | ||
| Ma | 2016 | China | [40] | 332.95 pg/ml | 16 | 6 | 2 | 46 | |
| Shereen | 2014 | Egypt | [33] | 164 pg/ml | 9 | 6 | 5 | 10 | |
| Lara | 2013 | Brazil | [37] | 299 pg/ml | 11 | 11 | 1 | 78 | |
| Total | 89 | 42 | 22 | 242 | |||||
| ΔBNP | Cheng | 2015 | China | [34] | 80 pg/ml | 31 | 12 | 2 | 11 |
| Yang | 2011 | China | [38] | 123 pg/ml | 64 | 1 | 6 | 12 | |
| He | 2013 | China | [45] | 29 pg/ml | 13 | 1 | 6 | 16 | |
| Ma | 2016 | China | [40] | 69.36 pg/ml | 48 | 2 | 4 | 16 | |
| Zhang | 2012 | China | [47] | 46 pg/ml | 44 | 2 | 2 | 10 | |
| Total | 200 | 18 | 20 | 65 | |||||
| ΔBNP% | Cheng | 2015 | China | [34] | 13.4% | 28 | 4 | 5 | 19 |
| Chien | 2008 | China | [19] | 20% | 65 | 4 | 6 | 26 | |
| Sameh | 2014 | Egypt | [35] | 20% | 23 | 2 | 2 | 13 | |
| Shereen | 2014 | Egypt | [33] | 14.9% | 13 | 5 | 3 | 9 | |
| Total | 129 | 15 | 16 | 67 | |||||
| NT-proBNP1 | Hu | 2010 | China | [48] | 3635.5 pg/ml | 41 | 16 | 13 | 90 |
| Li | 2016 | China | [39] | 715.5 pg/ml | 14 | 7 | 1 | 20 | |
| Total | 55 | 23 | 14 | 110 | |||||
| NT-proBNP2 | Gang | 2013 | China | [36] | 448 pg/ml | 6 | 7 | 1 | 15 |
| Wen | 2015 | China | [41] | 1199 pg/ml | 26 | 4 | 8 | 79 | |
| Total | 32 | 11 | 9 | 94 |
BNP1, BNP levels were measured before the preparation of SBT and the prediction of weaning failure. BNP2, BNP levels were measured at the end of SBT and the prediction of weaning failure.
ΔBNP, the change of BNP levels before and after SBT and the prediction of weaning success.
ΔBNP%, ΔBNP divided by BNP1 and the prediction of weaning success.
NT-proBNP1, NT-pro BNP levels were measured before the preparation of SBT and the prediction of weaning failure. NT-proBNP2, NT-pro BNP levels were measured at the end of the SBT and the prediction of weaning failure.
TP: true positive; FP: false positive; FN: false negative; TN: true negative.
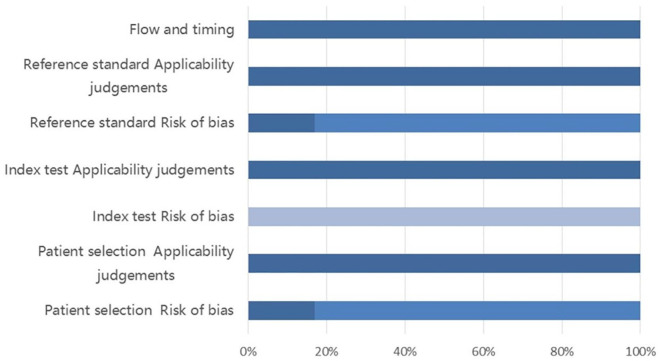
Figure 2.
QUADAS-2 results.
The results of the QUADAS-2 evaluation are provided in different color. Deep color stands for low risk, middle color stands for unclear risk and stands for light color–high risk. Because most of the literature did not report whether consecutively included sample of patients, this part of the risk of bias is higher. At present, there is no clear weaning indicator, so this part of the risk of bias is also higher.
Table 2.
QUADAS-2 results.
| Author | Year | Patient risk of bias | Selection applicability judgments | Index test risk of bias | Applicability judgments | Reference risk of bias | Standard applicability judgments | Flow and timing |
|---|---|---|---|---|---|---|---|---|
| Armand | 2006 | Low | Low | High | Low | Low | Low | Low |
| Cheng | 2015 | Unclear | Low | High | Low | Unclear | Low | Low |
| Chien | 2008 | Unclear | Low | High | Low | Low | Low | Low |
| He | 2013 | Unclear | Low | High | Low | Unclear | Low | Low |
| Hu | 2010 | Unclear | Low | High | Low | Unclear | Low | Low |
| Lara | 2013 | Unclear | Low | High | Low | Unclear | Low | Low |
| Li | 2016 | Unclear | Low | High | Low | Unclear | Low | Low |
| Ma | 2016 | Unclear | Low | High | Low | Unclear | Low | Low |
| Sameh | 2014 | Low | Low | High | Low | Unclear | Low | Low |
| Shereen | 2014 | Unclear | Low | High | Low | Unclear | Low | Low |
| Wen | 2015 | Unclear | Low | High | Low | Unclear | Low | Low |
| 2014 | Unclear | Low | High | Low | Unclear | Low | Low | |
| 2014 | Unclear | Low | High | Low | Unclear | Low | Low | |
| Xu | 2013 | Unclear | Low | High | Low | Unclear | Low | Low |
| Yang | 2011 | Unclear | Low | High | Low | Unclear | Low | Low |
| Zhang | 2012 | Unclear | Low | High | Low | Unclear | Low | Low |
| Zhou | 2013 | Unclear | Low | High | Low | Unclear | Low | Low |
| Gang | 2013 | Low | Low | High | Low | Low | Low | Low |
Figure 4.
Forest plots sensitivity, specificity and summary receiver operating characteristic (SROC) with 95% confidence interval for BNP1, BNP2, ΔBNP, ΔBNP%, NT-pro BNP1, and NT-pro BNP2.
It showed that the change of BNP levels before and after SBT was reported in five articles, the pooled sensitivity was 0.91, but the I2 values was 58.5%. ΔBNP divided by baseline BNP had the highest pooled AUC of 0. 9511. And its I2 values were 5.9% for specificity and 0% for sensitivity.
BNP1
The prediction value of BNP1 for the weaning outcome in mechanical ventilation was reported in six studies. The pooled sensitivity and 95% confidence interval for predicting weaning failure was 80% (73%–87%) and the pooled specificity was 85% (80%–89%). The I2 values were 30% for specificity and 0% for sensitivity. The pooled area under curve (AUC) was 0.8940.
BNP2
The prediction value of BNP2 for the weaning outcome in mechanical ventilation was reported in five studies. The pooled sensitivity and 95% confidence interval for predicting weaning failure was 80% (72%–87%) and the pooled specificity was 85% (81%–89%). The I2 values were 38.2% for specificity and 5.9% for sensitivity. The pooled AUC was 0.8887.
ΔBNP
The prediction value of ΔBNP for weaning outcome in mechanical ventilation was reported in five studies. The pooled sensitivity and 95% confidence interval for predicting weaning success was 91% (86%–94%) and the pooled specificity was 78% (68%–87%). The I2 values were 76.4% for specificity and 58.5% for sensitivity. The pooled AUC was 0.9486.
ΔBNP%
The prediction value of Δ BNP% for the weaning outcome in mechanical ventilation was reported in four studies. The pooled sensitivity and 95% confidence interval for predicting weaning success was 89% (83%–94%) and the pooled specificity was 82% (72%–89%). The I2 values were 5.9% for specificity and 0% for sensitivity. The pooled AUC was 0.9511.
NT-proBNP1
The prediction value of NT-proBNP1 for the weaning outcome in mechanical ventilation was reported in two studies. The pooled sensitivity and 95% confidence interval for predicting weaning failure was 80% (68%–88%) and the pooled specificity was 83% (75%–89%). The I2 values were 38.7% for specificity and 62.3% for sensitivity.
NT-proBNP2
The prediction value of NT-proBNP2 for the weaning outcome in mechanical ventilation was reported in two studies. The pooled sensitivity and 95% confidence interval for predicting weaning failure was 78% (62%–89%) and the pooled specificity was 90% (82%–95%). The I2 values were 90.8% for specificity and 0% for sensitivity.
Investigation of heterogeneity
The Spearman correlation coefficient between the logistic transformations of the true positive rate (TPR) against the logit of the false positive rate (FPR) for BNP1, BNP2, Δ BNP, Δ BNP% is 0.145 (p = 0.784), −0.900 (p = 0.037), 0.900 (p = 0.037), −0.949 (p = 0.051).
Only ΔBNP had a strong positive Spearman rank coefficient, indicating it had a threshold effect. Further, we used the Moses model to examine the changes in threshold effect, and found b (1) = −0.247, p = 0.451. The threshold was constant. The I2 statistics were 58.5% for sensitivity and 76.4% for specificity. In view of the small number of articles included, it was not possible to further analyze the heterogeneity origin of the non-threshold effects. The ROC was combined using the random effects model.
There are two articles confirming the inclusion criteria about NT-pro BNP1 and NT-pro BNP2 predicting the weaning outcome. About NT-pro BNP1, the I2 statistics were 62.3% for sensitivity and 38.7% for specificity. About NT-pro BNP2, the I2 statistics were 0% for sensitivity and 90.8% for specificity. Due to the limitation of the sample size, we could not explore the source of heterogeneity.
In addition, the funnel plot and Egger’s test were conducted to access publication bias. Both the funnel plot (Figure 3) and Egger’s test suggested no evidence of publication bias (p value = 0.3384).
Figure 3.
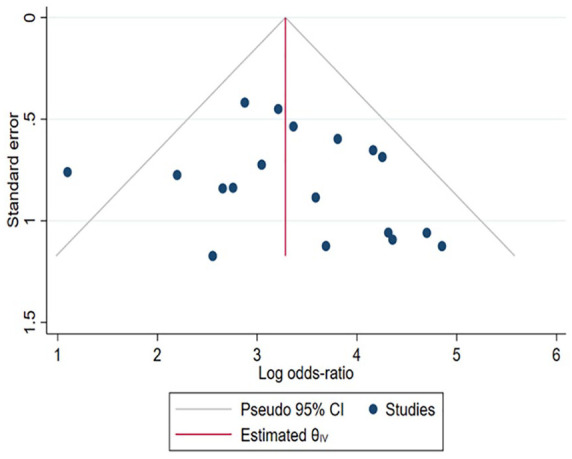
Funnel plot.
Discussion
We compared the accuracy of BNP1, BNP2, ΔBNP, ΔBNP%, NT-pro BNP1, and NT-pro BNP2 for the diagnosis of weaning outcomes from mechanical ventilation. Based on our knowledge, ours was the initial diagnostic test method meta-analysis with SROC to investigate the relationship of BNP and liberation from ventilation. In general, ΔBNP had the highest pooled sensitivity of 91%, NT-pro BNP2 had the highest pooled specificity of 90.8%, and ΔBNP% had the highest pooled AUC of 0.9511.
Brain natriuretic peptide or B-type natriuretic peptide (BNP) was found in the porcine brain in 1988, 50 and it was subsequently confirmed by the ventricular muscle cells secretion. BNP is initially present as an inactive precursor form (preBNP) in the ventricular myocyte membrane particles. When the myocytes are stimulated, they are cut into signal peptide and proBNP in the ventricular myocytes, and proBNP is released into the blood, cleaved by the enzyme furin as an inactive amino terminal fragment NT-pro BNP and biologically active carboxyl terminal fragment BNP. When the ventricular volume expands, the pressure load increases, and the BNP begins to secrete. 51
Weaning is the process of which mechanical ventilation is gradually withdrawn and the patient resumes spontaneous breathing. Unsuccessful weaning from mechanical ventilation is frequently due to cardiovascular dysfunction. Mechanical ventilation affects the cardiovascular system through the expansion of lung volume, increased alveolar pressure, and changes in intrathoracic pressure. When the patient receives mechanical ventilation, the alveoli passively expand and the lung volume increases, leading alveolar vascular resistance to increase significantly. Although alveolar pressure is partly conducted to interstitial, the total pulmonary vascular resistance increases and the total blood flow volume of the lungs decreases. The increasing lung volume compresses the heart in the mediastinum. Cardiac compliance is reduced due to this effect. Cardiac output (CO) decreases. Since the right ventricular is more compliant than the left ventricle, the right ventricle is impacted greater when pericardial pressure increases, which results in the interventricular septum moving to the left ventricle, reducing CO and blood pressure. As a result of mechanical ventilation, the intrathoracic pressure and the external environment pressure gradient increases, resulting in returned blood volume reduction. On the other hand, the central vein by the impact of pressure further limits the return of blood flow to the right ventricle. Increased intrathoracic pressure reduces left ventricular afterload, improving left ventricular function and increasing CO. When the ventilator is discontinued, it consequently increases cardiac preload and afterload. If there is the potential for cardiac insufficiency or it is present at this time, cardiovascular function will be decompensated.
In 2012, Chowdhury and co-workers 52 performed a meta-analysis that included two studies that evaluated the changes in cardiac function during an SBT. They concluded that BNP measured at the end of an SBT may predict re-intubation. However, included studies have been small and had significant limitations. At present, some new research on the relationship between BNP or NT-pro BNP and weaning outcome from mechanical ventilation has been published. In addition, some new predictors were reported. So, we performed an updated meta-analysis.
Our meta-analysis confirms the meta-analysis performed by Chowdhury and co-workers. BNP2 can effectively predict weaning failure since the area under the pooled ROC is 0.8887, which indicates a high diagnostic efficacy. The studies reported that threshold values were close to each other, which increases the reliability of the conclusion.
Armand et al. first measured the BNP1 prediction value for weaning outcome in mechanical ventilation patients; 10 however, this study did not exclude acute left heart failure patients and concluded BNP1 is higher in patients with weaning failure and correlates to weaning duration. Only one study (1/6) excluded acute left heart failure patients. The AUC is 0.8940 for BNP1, the I2 is 30% for specificity, but heterogeneity is acceptable. Therefore, BNP1 can predict weaning failure.
Yang et al. 38 and Cheng et al. 34 found the change of BNP levels before and after SBT are statistically significant; however, BNP1 has not been reported to be statistically significant. This may be because these two articles excluded patients with acute heart failure or acute myocardial infarction. The AUC is 0.9486 forΔBNP, the I2 is 58.5% for sensitivity, and 76.4% for specificity.
Heterogeneity is moderate. The AUC is close to 1, indicating a high diagnostic efficacy, but it has limited clinical use due to the moderate heterogeneity.
Lamia et al. 53 reported NT-pro BNP levels at SBT can help in the prediction of post-extubation respiratory distress. The AUC for plasma NT-pro BNP to predict post-extubation respiratory distress was 0.78 (95% CI 0.67–0.89, p = 0.0001). Yu 54 reported that NT-pro BNP1 and NT-pro BNP2 do not have a correlation with weaning outcome. At present, because there are a limited amount of studies about the relationship between NT-pro BNP and weaning outcome, we cannot further explore the topic.
ΔBNP% had the highest pooled AUC of 0.9511, the I2 is 0% for sensitivity, and 5.9% for specificity. It has the best clinical use value for predict weaning success. When the change percentage of BNP levels before and after SBT is elevated, the withdrawal of mechanical ventilation causes a sharp change in cardiac function.
Grasso et al. 55 and Zapata et al. 32 have demonstrated the usefulness of predicting and diagnosing weaning outcome of cardiac origin. Mekontso-Dessap et al. 13 reported that a BNP-guided fluid management strategy had shorter ventilator days and a higher probability of successful extubation, especially in patients with left ventricular systolic dysfunction.
Conclusion
Our study found thatΔBNP% could be used in clinical wean evaluations and to screen whether the failure of wean is associated with a cardiogenic factor. Subsequent need more diagnostic randomized controlled trials to establish the best use of this diagnostic indicator.
Attachment1
We implemented the subsequent search strategy:
PubMed
Natriuretic Peptide, Brain [Mesh]
Peptide, Brain Natriuretic [Title/Abstract]
BNP-32 [Title/Abstract]
BNP 32 [Title/Abstract]
Brain Natriuretic Peptide-32 [Title/Abstract]
Brain Natriuretic Peptide 32 [Title/Abstract]
Natriuretic Peptide-32, Brain [Title/Abstract]
Peptide-32, Brain Natriuretic [Title/Abstract]
Natriuretic Factor-32 [Title/Abstract]
Natriuretic Factor 32 [Title/Abstract]
BNP Gene Product [Title/Abstract]
Type-B Natriuretic Peptide [Title/Abstract]
Natriuretic Peptide, Type-B [Title/Abstract]
Type B Natriuretic Peptide [Title/Abstract]
Natriuretic Peptide Type-B [Title/Abstract]
Natriuretic Peptide Type B [Title/Abstract]
Nesiritide [Title/Abstract]
Brain Natriuretic Peptide [Title/Abstract]
B-Type Natriuretic Peptide [Title/Abstract]
Natriuretic Peptide, B-Type [Title/Abstract]
Ventricular Natriuretic Peptide, B-type [Title/Abstract]
Ventricular Natriuretic Peptide, B type [Title/Abstract]
Natrecor [Title/Abstract]
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
Pro-brain natriuretic peptide (1-76) [Supplementary Concept]
N-terminal pro-BNP [Title/Abstract]
ProBNP(1-76) [Title/Abstract]
NTproBNP [Title/Abstract]
N-BNP peptide [Title/Abstract]
NT-BNP [Title/Abstract]
Amino-terminal pro-brain natriuretic peptide [Title/Abstract]
Aminoterminal pro-B-type natriuretic peptide [Title/Abstract]
NT-proBNP [Title/Abstract]
Pro-brain natriuretic peptide (1-76) [Title/Abstract]
25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34
Ventilator Weaning [Mesh]
Weaning, Ventilator [Title/Abstract]
Respirator Weaning [Title/Abstract]
Weaning, Respirator [Title/Abstract]
Mechanical Ventilator Weaning [Title/Abstract]
Ventilator Weaning, Mechanical [Title/Abstract]
Weaning, Mechanical Ventilator [Title/Abstract]
36 or 37 or 38 or 39 or 40 or 41 or 42
24 or 35
43 and 44
Embase
#1 ‘artificial ventilation’/exp
#2 ‘ventilator weaning’:ab, ti
#3 ‘mechanical ventilator weaning’:ab, ti
#4 ‘respirator weaning’:ab, ti
#5 #1 OR #2 OR #3 OR #4
#6 ‘brain natriuretic peptide’/exp
#7 ‘nesiritide’:ab, ti
#8 ‘nesiritide citrate’:ab, ti
#9 ‘natrecor’: ab, ti
#10 #6 OR #7 OR #8 OR #9
#11 ‘amino terminal pro brain natriuretic peptide’/exp
#12 ‘NTproBNP’:ab, ti
#13 ‘proBNP’: ab, ti
#14'nt pro bnp':ab, ti
#15 'ntprobnp':ab, ti
#16 #11 OR #12 OR #13 OR #14 OR #15
#17 #10 OR #16
#18 #5 AND #17
Footnotes
Authors’ contributions: Jian Liu, Chuan-jiang Wang, and Fang Xu searched the scientific literature and drafted the manuscript. Fang Xu and Dan Deng performed the statistical analyzes and revised the manuscript. Chuan-jiang Wang and Shi-hui Lin participated in data interpretation and drafted the report. Jian Liu and Chuan-jiang Wang conceived of the study and contributed data. Jian Liu and Jun-huai Ran are responsible for the revision of the manuscript. Fang Xu and Yu Ma made important revisions to the study. All authors have read and approved the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Chinese Medicine Science and Technology Project of Chongqing City Health and Family Planning Committee (2020GDRC001 to FX, 2020FYYX137 to SH-L), and the High-level Medical Reserved Personnel Training Project of Chongqing (to FX), Basic Science and Cutting-Edge Technology Research Projects of Chongqing Science and Technology Commission (cstc2020jcyj-msxmX1089 to SH-L).
Ethical approval and consent to participate: This paper is a Systematic review and Meta-analysis that is not covered on the ethics of live vertebrates and higher invertebrates experiments.
ORCID iD: Fang Xu  https://orcid.org/0000-0001-8201-1595
https://orcid.org/0000-0001-8201-1595
References
- 1. Slutsky AS. Mechanical ventilation. American College of Chest Physicians’ Consensus Conference. Chest 1993; 104(6): 1833–1859. [DOI] [PubMed] [Google Scholar]
- 2. Ladeira MT, Vital FM, Andriolo RB, et al. Pressure support versus T-tube for weaning from mechanical ventilation in adults. Cochrane Database Syst Rev 2014; 2014(5): CD006056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goligher EC, Ferguson ND, Brochard LJ. Clinical challenges in mechanical ventilation. Lancet 2016; 387(10030): 1856–1866. [DOI] [PubMed] [Google Scholar]
- 4. Charles MP, Kali A, Easow JM, et al. Ventilator-associated pneumonia. Australas Med J 2014; 7(8): 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powers SK, Shanely RA, Coombes JS, et al. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 2002; 92(5): 1851–1858. [DOI] [PubMed] [Google Scholar]
- 6. Fagon J-Y, Chastre J, Domart Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation: prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Resp Dis 1989; 139(4): 877–884. [DOI] [PubMed] [Google Scholar]
- 7. Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis 1988; 137(6): 1463–1493. [DOI] [PubMed] [Google Scholar]
- 8. Combes A, Figliolini C, Trouillet JL, et al. Factors predicting ventilator-associated pneumonia recurrence. Crit Care Med 2003; 31(4): 1102–1107. [DOI] [PubMed] [Google Scholar]
- 9. Fagon JY, Chastre J, Hance AJ, et al. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med 1993; 94(3): 281–288. [DOI] [PubMed] [Google Scholar]
- 10. Mekontso-Dessap A, de Prost N, Girou E, et al. B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med 2006; 32(10): 1529–1536. [DOI] [PubMed] [Google Scholar]
- 11. Tobin MJ. Advances in mechanical ventilation. N Engl J Med 2001; 344(26): 1986–1996. [DOI] [PubMed] [Google Scholar]
- 12. Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest 1997; 112(1): 186–192. [DOI] [PubMed] [Google Scholar]
- 13. Mekontso DA, Roche-Campo F, Kouatchet A, et al. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J RespirCrit Care Med 2012; 186: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 14. Parthasarathy S, Jubran A, Tobin MJ. Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med 1998; 158(5 Pt 1): 1471–1478. [DOI] [PubMed] [Google Scholar]
- 15. Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995; 152(1): 129–136. [DOI] [PubMed] [Google Scholar]
- 16. Imsand C, Feihl F, Perret C, et al. Regulation of inspiratory neuromuscular output during synchronized intermittent mechanical ventilation. Anesthesiology 1994; 80(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 17. Esteban A, Alia I, Tobin MJ, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 1999; 159(2): 512–518. [DOI] [PubMed] [Google Scholar]
- 18. MacIntyre NR, Cook DJ, Ely EW, Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001; 120(6 Suppl): 375S–395S. [DOI] [PubMed] [Google Scholar]
- 19. Chien JY, Lin MS, Huang YC, et al. Changes in B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Crit Care Med 2008; 36(5): 1421–1426. [DOI] [PubMed] [Google Scholar]
- 20. Lemaire F, Teboul JL, Cinotti L, et al. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 1988; 69(2): 171–179. [DOI] [PubMed] [Google Scholar]
- 21. Jubran A, Mathru M, Dries D, et al. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med 1998; 158(6): 1763–1769. [DOI] [PubMed] [Google Scholar]
- 22. Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med 1998; 158(2): 378–385. [DOI] [PubMed] [Google Scholar]
- 23. Jaber S, Petrof BJ, Jung B, et al. Rapidly progressive diaphragmatic weakness and injury during mechanical ventilation in humans. Am J Respir Crit Care Med 2011; 183(3): 364–371. [DOI] [PubMed] [Google Scholar]
- 24. Dres M, Teboul JL, Monnet X. Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care 2014; 20(5): 493–498. [DOI] [PubMed] [Google Scholar]
- 25. Teboul J-L. Weaning-induced cardiac dysfunction: where are we today? Intensive Care Med 2014; 40(8): 1069–1079. [DOI] [PubMed] [Google Scholar]
- 26. van den Boogaard M, Schoonhoven L, van der Hoeven JG, et al. Incidence and short-term consequences of delirium in critically ill patients: a prospective observational cohort study. Int J Nurs Stud 2012; 49(7): 775–783. [DOI] [PubMed] [Google Scholar]
- 27. Mogensen KM, Robinson MK, Casey JD, et al. Nutritional status and mortality in the critically ill. Crit Care Med 2015; 43(12): 2605–2615. [DOI] [PubMed] [Google Scholar]
- 28. de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003; 362(9380): 316–322. [DOI] [PubMed] [Google Scholar]
- 29. Fonarow GC, Abraham WT, Albert NM, et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA 2007; 297(1): 61–70. [DOI] [PubMed] [Google Scholar]
- 30. Schmidt H, Rohr D, Bauer H, et al. Changes in intrathoracic fluid volumes during weaning from mechanical ventilation in patients after coronary artery bypass grafting. J Crit Care 1997; 12(1): 22–27. [DOI] [PubMed] [Google Scholar]
- 31. Jafri SM, Lavine S, Field BE, et al. Left ventricular diastolic function in sepsis. Crit Care Med 1990; 18(7): 709–714. [DOI] [PubMed] [Google Scholar]
- 32. Zapata L, Vera P, Roglan A, et al. B-type natriuretic peptides for prediction and diagnosis of weaning failure from cardiac origin. Intensive Care Med 2011; 37(3): 477–485. [DOI] [PubMed] [Google Scholar]
- 33. Farghaly S, Galal M, Hasan AA, et al. Brain natriuretic peptide as a predictor of weaning from mechanical ventilation in patients with respiratory illness. Aust Crit Care 2015; 28(3): 116–121. [DOI] [PubMed] [Google Scholar]
- 34. Cheng L, Jiang L, Wang M, et al. The value of changes in plasma B-type natriuretic peptide before and after spontaneous breathing trial in predicting weaning outcome in mechanically ventilated patients. Zhonghua nei ke za zhi 2015; 54(6): 486–490. [PubMed] [Google Scholar]
- 35. Maraghi SE, Hosny M, Samir M, et al. Usage of B-type natriuretic peptide for prediction of weaning outcome by spontaneous breathing trial. Egypt J Chest Dis Tuberculosis 2014; 63(3): 671–678. [Google Scholar]
- 36. Ma G, Liao W, Qiu J, et al. N-terminal prohormone B-type natriuretic peptide and weaning outcome in postoperative patients with pulmonary complications. J Int Med Res 2013; 41(5): 1612–1621. [DOI] [PubMed] [Google Scholar]
- 37. Lara TM, Hajjar LA, de Almeida JP, et al. High levels of B-type natriuretic peptide predict weaning failure from mechanical ventilation in adult patients after cardiac surgery. Clinics 2013; 68(1): 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang T-J, Zhang D-W, Lv S-J, et al. B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Chin J Respir Crit Care Med 2011; 2011(06): 590–593. [DOI] [PubMed] [Google Scholar]
- 39. Li S, Wu J, Zou Y, et al. Predictive value of plasma N·terminal-pro-brain natriuretic peptide in weaning patients from mechanical ventilation. Chin J Emerg Med 2016; 25(3): 334–337. [Google Scholar]
- 40. Ma B, Feng K, Zhao G. Guidance value of plasma BNP on invasive mechanical ventilation weaning of COPD patients. J Clin Pulmonary Med 2016; 21(9): 1563–1566. [Google Scholar]
- 41. Wen X, Cao L, Liu Y. The predictive effect of NT-proBNP on intubation events after spontaneous breathing experiment. J Clin Med 2015; 2015(09): 1665–1668. [Google Scholar]
- 42. Xing Z, Zhang X, Wang B, et al. The predictive effect of BNP in mechanical ventilation extubation of severe sepsis patients with cardiac insufficiency. Jilin Med J 2014; 2014(25): 5601–5603. [Google Scholar]
- 43. Xing B, Tan S-F, Huang S, et al. Predictive value of serum B- type natriuretic peptide on outcome of weaning mechanical ventilation in patients with COPD. Mod Med J 2014; 42(5): 494–497. [Google Scholar]
- 44. Xu J, Han X, Zhang S, et al. Predictive value of BNP in mechanical ventilation and weaning. J Nantong Univ (Med Sci) 2013; 2013(06): 533–535. [Google Scholar]
- 45. He XJ. A pilot study of plasma brain natriuretic peptide evaluated as a predictor of weaning in chronic obstructive pulmonary disease patients [D]. 2013. [Google Scholar]
- 46. Zhou P, Dong Y, Chang L, et al. Role of measurement of serum brain natriuretic peptide in prediction of mechanical ventilation weaning failure. Pract J Clin Med 2013; 10(5): 69–71. [Google Scholar]
- 47. Zhang X, He Z, Wang C. BNP for prediction of weaning failure patients with COPD and chronic corpulmonale after SBT. China Mod Doctor 2012; 2012(12): 59–60. [Google Scholar]
- 48. Hu B, Fang M, Huang W, et al. Application of receiver operating characteristic cureve in the evaluation of predictive value of serum NT-pro BNP in weaning outcome of patients with mechanical ventilation. Chin J Emerg Med 2010; 19(8): 851–854. [Google Scholar]
- 49. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155(8): 529–536. [DOI] [PubMed] [Google Scholar]
- 50. Sudoh T, Kangawa K, Minamino N, et al. A new natriuretic peptide in porcine brain. Nature 1988; 332(6159): 78–81. [DOI] [PubMed] [Google Scholar]
- 51. Dessap AM, Brochard L. B-type natriuretic peptide and weaning from mechanical ventilation. Clin Pulm Med 2009; 16(2): 89–94. [Google Scholar]
- 52. Chowdhury A, Woods CJ, Shorr AF. B-type natriuretic peptide and liberation from mechanical ventilation: a meta-analysis. Am J Respir Crit Care Med 2012; 185(1): A3092. [Google Scholar]
- 53. Ouanes-Besbes L, Dachraoui F, Ouanes I, et al. NT-proBNP levels at spontaneous breathing trial help in the prediction of post-extubation respiratory distress. Intensive Care Med 2012; 38(5): 788–795. [DOI] [PubMed] [Google Scholar]
- 54. Xin Y. The predictive value of NT-proBNP and transpulmonary thermodilution measurements for weaning outcomes of critically-ill patients with mechanical ventilation [D]. 2014. [Google Scholar]
- 55. Grasso S, Leone A, De Michele M, et al. Use of N-terminal pro-brain natriuretic peptide to detect acute cardiac dysfunction during weaning failure in difficult-to-wean patients with chronic obstructive pulmonary disease. Crit Care Med 2007; 35(1): 96–105. [DOI] [PubMed] [Google Scholar]