Abstract
The most widely distributed members of the family of insulin receptor substrate (IRS) proteins are IRS-1 and IRS-2. These proteins participate in insulin and insulin-like growth factor 1 signaling, as well as the actions of some cytokines, growth hormone, and prolactin. To more precisely define the specific role of IRS-1 in adipocyte biology, we established brown adipocyte cell lines from wild-type and IRS-1 knockout (KO) animals. Using differentiation protocols, both with and without insulin, preadipocyte cell lines derived from IRS-1 KO mice exhibited a marked decrease in differentiation and lipid accumulation (10 to 40%) compared to wild-type cells (90 to 100%). Furthermore, IRS-1 KO cells showed decreased expression of adipogenic marker proteins, such as peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer-binding protein alpha (C/EBPα), fatty acid synthase, uncoupling protein-1, and glucose transporter 4. The differentiation deficit in the KO cells could be reversed almost completely by retrovirus-mediated reexpression of IRS-1, PPARγ, or C/EBPα but not the thiazolidinedione troglitazone. Phosphatidylinositol 3-kinase (PI 3-kinase) assays performed at various stages of the differentiation process revealed a strong and transient activation in IRS-1, IRS-2, and phosphotyrosine-associated PI 3-kinase in the wild-type cells, whereas the IRS-1 KO cells showed impaired phosphotyrosine-associated PI 3-kinase activation, all of which was associated with IRS-2. Akt phosphorylation was reduced in parallel with the total PI 3-kinase activity. Inhibition of PI 3-kinase with LY294002 blocked differentiation of wild-type cells. Thus, IRS-1 appears to be an important mediator of brown adipocyte maturation. Furthermore, this signaling molecule appears to exert its unique role in the differentiation process via activation of PI 3-kinase and its downstream target, Akt, and is upstream of the effects of PPARγ and C/EBPα.
Adipocytes play a central role in lipid homeostasis and the maintenance of energy balance in vertebrates (18). White adipose tissue is the primary site of storage of triglycerides and release of fatty acids in response to changing energy needs (12). Brown adipocytes, on the other hand, store smaller amounts of triglycerides and account for much of the basal thermogenic energy expenditure through the expression of uncoupling protein-1 (UCP-1) (19). Obesity, an excessive accumulation of white adipose tissue, occurs when energy intake by an individual exceeds the rate of energy expenditure, whereas brown adipocyte mass is highest in young mammals and disorders such as pheochromocytoma (27).
Characterization of cell lines that progress from an undifferentiated progenitor state to mature white adipocytes has led to a good understanding of the factors involved in the adipogenic program. Among these factors, the transcription factors peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding proteins (C/EBPs) appear to play a central role. PPARγ is highly enriched in adipose tissue, and its expression is upregulated early during differentiation of preadipocytes into adipocytes (30, 31). Ectopic expression and activation of PPARγ in fibroblasts has been shown to promote their conversion into adipocytes (31). Of the members of the C/EBP family, C/EBPβ and -δ are induced very early and have been shown to activate PPARγ, thereby initiating the differentiation program of preadipocytes (29, 34, 36, 39). In contrast, C/EBPα is activated after PPARγ but precedes the synthesis of a number of proteins characteristic of a fully differentiated phenotype, such as fatty acid synthase (FAS) or glucose transporter 4 (Glut4) (37). Overexpression of C/EBPα in fibroblasts has been shown to induce their differentiation into mature adipocytes, similar to PPARγ (10). Furthermore, C/EBP- and PPARγ-binding sites have been described in the promoters of a number of adipogenic genes (5, 14, 22, 26, 28).
The upstream signals regulating induction and expression of these transcription factors during adipogenic differentiation are poorly understood. Activation of the phosphatidylinositol 3-kinase (PI 3-kinase) pathway occurs during differentiation and has been demonstrated to be necessary for complete differentiation of white preadipocytes (25). Furthermore, it has been shown that binding of insulin receptor substrate 1 (IRS-1) and IRS-2 to PI 3-kinase is transiently increased during differentiation of preadipocyte cell lines into adipocytes (25); however, the role of either of these proteins in adipocyte differentiation is unclear.
In the present study, we have investigated the role of IRS-1 in differentiation by establishing immortalized brown preadipocytes from IRS-1 KO mice and their wild-type counterparts. We find that differentiation of preadipocytes into adipocytes is severely impaired in cells lacking IRS-1. Furthermore, retrovirus-mediated reexpression of IRS-1, PPARγ, or C/EBPα is able to reconstitute differentiation capacity almost to the level of wild-type cells. Signaling studies suggest that decreased IRS-1-associated and total PI 3-kinase, as well as decreased Akt activation in the KO cells, might be responsible for the lack of differentiation observed.
MATERIALS AND METHODS
Materials.
Antibodies used for immunoprecipitation and immunoblotting included anti-IRS-1, anti-IRS-2, and antiphosphotyrosine 4G10 (kindly provided by Morris White, Joslin Diabetes Center, Boston, Mass.); anti-insulin receptor (kindly provided by Bentley Cheatham, Joslin Diabetes Center); anti-UCP-1 (Alpha Diagnostic International, San Antonio, Tex.); anti-phospho-specific-Akt (New England Biolabs, Beverly, Mass.); anti-Akt, anti-C/EBPδ, anti-PPARγ, and anti-C/EBPα (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.); and anti-Glut4 (Chemicon International, Inc., Temecula, Calif.). The anti-PI 3-kinase p85 antibody (Upstate Biotechnology, Inc., Lake Placid, N.Y.) recognizes p85α, p55α, and p50α equally well, with p85α being the predominant isoform expressed in brown adipocytes (data not shown). Protein A-Sepharose and protein G-Sepharose were purchased from Pharmacia, Inc. (Piscataway, N.J.), and [γ-32P]ATP was from NEN Life Science Products (Boston, Mass.). Phosphoinositol was obtained from Avanti Polar Lipids (Alabaster, Ala.), nitrocellulose was from Schleicher & Schuell, Inc. (Keene, N.H.), thin-layer chromatography plates were from VWR (Bridgeport, N.J.), and electrophoresis supplies were from Bio-Rad Laboratories (Hercules, Calif.). All other supplies were from Sigma Chemical Co. (St. Louis, Mo.).
Cell isolation and culture.
Brown adipocytes and their precursor cells were isolated from newborn wild-type and IRS-1 KO mice by collagenase digestion as described previously (16). Preadipocytes were immortalized by infection with the retroviral vector pBabe, encoding SV40T antigen (kindly provided by J. DeCaprio, Dana Farber Cancer Institute, Boston, Mass.) and selected with puromycin (1 μg/ml). Preadipocytes were grown to confluence in culture medium supplemented with 20 nM insulin and 1 nM T3 (differentiation medium) (day 0). Adipocyte differentiation was induced by treating confluent cells for 48 h in differentiation medium further supplemented with 0.5 mM isobutylmethylxanthine, 0.5 μM dexamethasone, and 0.125 mM indomethacin. After this induction period (day 2), cells were changed back to differentiation medium, which was then changed every second day.
Plasmids and retroviral infection of cells.
The C/EBPα viral expression vector pWZL-C/EBPα-Hyg was obtained from Sven Freytag (35). Full-length human IRS-1 and PPARγ, described previously (3, 23), were cloned into the pBabe-bleo vector (20). Viral ΦNX-packaging cells (9) were transfected at 70% confluence by calcium phosphate coprecipitation with 15 μg of retroviral vectors, and viral supernatants were harvested 48 h after transfection. IRS-1 KO cells were infected at 60% confluence with Polybrene (4 μg/ml)-supplemented virus overnight. Selection with 250 μg of the bleomycin analogue Zeocin/ml or 200 μg of hygromycin (Invitrogen, Carlsbad, Calif.) per ml was started 48 h after infection.
Oil red O staining.
Dishes were washed twice with phosphate-buffered saline and fixed with 10% buffered formalin for at least 1 h at room temperature. Cells were then stained for 2 h at room temperature with a filtered oil red O solution (0.5 g of oil red O in 100 ml of isopropyl alcohol), washed twice with water, and visualized.
Immunoprecipitation and Western and Northern blot analysis.
Cells were harvested in lysis buffer (50 mM HEPES, 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Na4P2O7, 10 mM NaF, 2 mM EDTA, 10% glycerol, 1% Igepal CA-630, 2 mM vanadate, 10 μg of leupeptin/ml, 10 μg of aprotinin/ml, 2 mM phenylmethylsulfonyl fluoride; pH 7.4). After lysates were clarified by centrifugation at 12,000 × g for 10 min at 4°C, the protein amount in the supernatants was determined by the Bradford method (2). Equal amounts of protein (100 and 500 μg, respectively) were either directly solubilized in Laemmli sample buffer (LSB) or immunoprecipitated with the indicated antibodies for at least 2 h at 4°C. Immune complexes were collected with protein A-Sepharose and protein G-Sepharose, washed in lysis buffer, and solubilized in LSB. Lysates or immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Membranes were blocked for 30 min and incubated with the appropriate antibody for 2 h. Specifically bound primary antibodies were detected with peroxidase-coupled secondary antibody and enhanced chemiluminescence.
Total cellular RNA was isolated using RNA Stat-60 (Tel-Test B, Inc., Friendswood, Tex.). Twenty micrograms of RNA was then subjected to Northern analysis with various probes as described previously (16).
PI 3-kinase assays.
Cell lysates were obtained as described above, and supernatants containing 500 μg of protein were subjected to immunoprecipitation with the indicated antibodies for 2 h at 4°C. Immune complexes were collected with protein A-Sepharose, washed twice with phosphate-buffered saline containing 1% Igepal CA-630, twice with 0.5 M LiCl in 0.1 M Tris (pH 7.5), and twice in reaction buffer (10 mM Tris [pH 7.5], 100 mM NaCl, 1 mM EDTA). Sepharose beads were resuspended in a mixture containing 50 μl of reaction buffer, 10 μl of 100 mM MgCl2, and 10 μl of phosphatidylinositol (2 μg/μl). Reactions were initiated by adding 5 μl of reaction mixture (880 μM ATP, 20 mM MgCl2, and 10 μCi of [γ-32P]ATP [3,000 Ci/mmol]) per tube and stopped after 10 min by addition of 20 μl of 8 N HCl and 160 μl of CHCl3-methanol (1:1). The samples were briefly centrifuged, and 50 μl of the lower organic phase was spotted on a silica gel thin-layer chromatography plate. The plate was developed in CHCl3-methanol-H2O-NH4OH (120:94:23:2.4), dried, exposed to a PhosphorImager screen, and quantitated with a Molecular Dynamics densitometer.
Glucose uptake assays.
Glucose transport activity was determined essentially as described previously (21). Briefly, differentiated monolayers of brown adipocytes were treated with insulin for 30 min, after which 50 μl of 2-deoxy-[3H]glucose (0.5 μCi/ml, final concentration) was added for an additional 3 min. The incorporated radioactivity was determined by liquid scintillation counting.
RESULTS
Differentiation of immortalized IRS-1 KO preadipocytes is impaired.
Wild-type and IRS-1-deficient preadipocytes were differentiated into brown adipocytes using insulin, T3, isobutylmethylxanthine, dexamethasone, and indomethacin as described in Materials and Methods. At different days of the differentiation protocol, cells from both genotypes were stained with oil red O, a fat-specific dye (Fig. 1A). Confluent, noninduced cells from both genotypes showed no significant fat staining (Fig. 1A). After induction, wild-type preadipocytes rapidly accumulated fat, and a fully differentiated phenotype with 90 to 100% of the cells containing multilocular fat droplets could be observed by 6 days after induction (Fig. 1A). In contrast, only 10 to 40% of the IRS-1-deficient preadipocytes treated with the same differentiation protocol as the wild-type cells were able to accumulate fat (Fig. 1A). This impaired differentiation capacity of IRS-1-deficient preadipocytes was observed in eight independent cell lines derived from eight different KO animals (data not shown).
FIG. 1.
IRS-1 KO preadipocytes show impaired differentiation. Brown adipose precursor cells isolated from newborn wild-type (WT) and IRS-1 KO (KO) mice were grown to confluence. At confluence (day 0), differentiation was induced as described in Materials and Methods. At the indicated days, cells were stained with oil red O (A) or prepared for Northern blot (B) and Western blot (C) analysis of various adipogenic markers, as shown. Results are representative of at least two independent experiments.
This defect in fat accumulation in the IRS-1 KO cells was accompanied by differences in mRNA and protein content of various differentiation markers (Fig. 1B and C). As previously noted in white adipocytes, during differentiation there is an increase in the level of a number of transcription factors and adipocyte differentiation-dependent proteins, including C/EBPδ, PPARγ, C/EBPα, FAS, UCP-1, and the insulin-sensitive Glut4. Protein levels of C/EBPδ, PPARγ, C/EBPα, FAS, UCP-1, and Glut4 were significantly decreased in the IRS-1-deficient cells compared to levels in wild-type adipocytes (Fig. 1C). Interestingly, at the mRNA level a somewhat different pattern was observed. Thus, C/EBPδ mRNA was upregulated, whereas PPARγ mRNA was decreased in IRS-1-deficient cells compared to that in their wild-type counterparts (Fig. 1B). FAS mRNA as well as both transcripts of C/EBPα were expressed to a similar extent in both cell lines during differentiation (Fig. 1B).
Reexpression of IRS-1 in IRS-1-deficient cells reconstitutes differentiation.
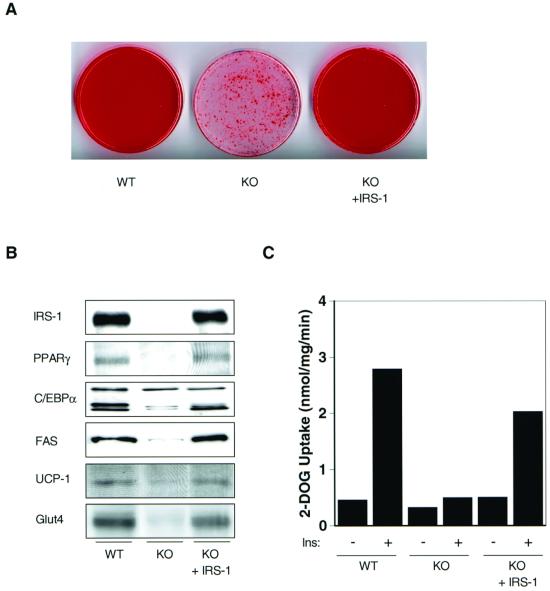
To confirm that the lack of IRS-1 was responsible for impaired differentiation in the KO cells, IRS-1-deficient preadipocytes were infected with a retrovirus expressing full-length human IRS-1, as described in Materials and Methods. Following retroviral-mediated gene transfer, the level of IRS-1 reexpression in the KO cells was about 70% of that seen in wild-type adipocytes (Fig. 2B). IRS-1-deficient cells reexpressing IRS-1 showed significantly increased accumulation of multilocular fat compared to the KO cells, with fat droplets detectable in 70 to 95% of the cells by day 6 of differentiation (Fig. 2A). Furthermore, reexpression of IRS-1 in the KO cells was accompanied by an increased protein content of adipogenic markers such as PPARγ, p30 C/EBPα, FAS, UCP-1, and Glut4 compared to the levels in KO cells (Fig. 2B). The effects of IRS-1 deficiency on Glut4 expression were paralleled by changes in basal and insulin-stimulated 2-deoxyglucose uptake. Thus, there was a 30% decrease in basal and a >80% decrease in insulin-stimulated glucose transport activity in IRS-1-deficient adipocytes compared to wild-type cells (Fig. 2C). When expressed in terms of the stimulation index, adipocytes derived from wild-type animals showed a sixfold increase in glucose uptake upon insulin stimulation, whereas glucose transport in IRS-1-deficient cells was increased only 1.5-fold (Fig. 2C). This decrease in insulin-induced glucose uptake in the KO cells was reconstituted to about 70% of the wild-type level by reexpression of IRS-1, which was consistent with the level of reconstitution of the IRS-1 protein (Fig. 2C).
FIG. 2.
Reexpression of IRS-1 in IRS-1 KO cells reconstitutes differentiation. Wild-type (WT) and IRS-1-deficient (KO) adipocytes, as well as KO cells reexpressing IRS-1 (KO + IRS-1), were either stained with oil red O (A) or prepared for Western blot analysis of several adipogenic marker proteins as shown (B) at day 6 of the differentiation protocol. (C) Adipocytes were serum starved for 16 to 18 h, after which 100 nM insulin (Ins) was added for 30 min prior to exposing cells to 2-deoxy-[3H]glucose (2-DOG) for 3 min. Data are expressed as nanomoles of glucose uptake per milligram of cell protein per minute.
Activation of IR and IRS-1 but not IRS-2 during differentiation is impaired in IRS-1-deficient cells.
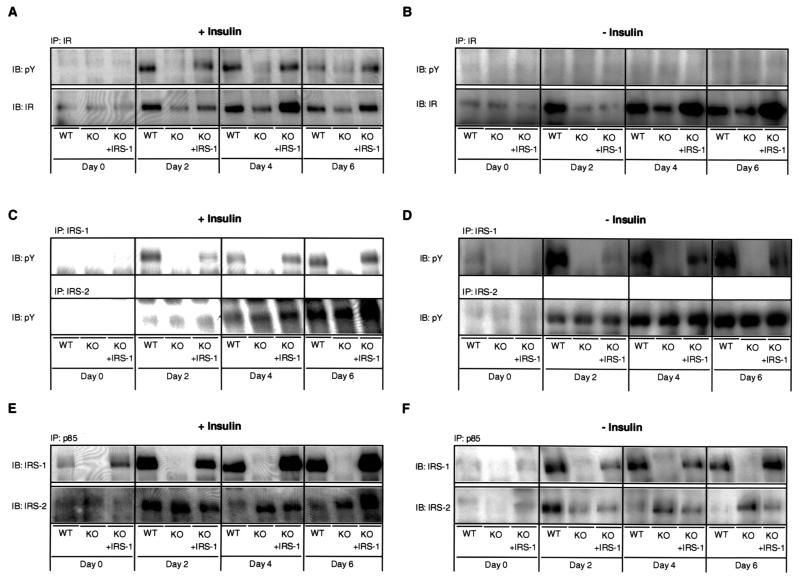
The specific upstream signals that trigger adipocyte differentiation remain unknown. To determine the role of the IRS proteins in this process, we examined whether changes in IR, IRS-1, and IRS-2 tyrosine phosphorylation occur during differentiation of wild-type, KO, and IRS-1-reconstituted KO cells. At day 0, no differences in IR content could be detected between the three cell lines and no tyrosine phosphorylation of the IR was observed in any of the different cells (Fig. 3A). However, following induction of differentiation, IR tyrosine phosphorylation increased up to 20-fold at day 2, remained elevated at day 4, and then declined to about 30% of its maximum by day 6 in the wild-type cells (Fig. 3A). This was accompanied by a fourfold increase in IR content, which remained stable from day 2 to 6 (Fig. 3A). The apparently higher extent of induction of IR tyrosine phosphorylation compared to IR content was mainly due to the very low level of IR tyrosine phosphorylation at day 0 (essentially undetectable). In contrast, there was only a modest twofold increase in IR content and a less-than-fourfold increase in IR tyrosine phosphorylation in IRS-1-deficient cells throughout the time course of differentiation (Fig. 3A). The IRS-1-reconstituted KO cells demonstrated a pattern of IR expression and phosphorylation very similar to that in the wild-type cells (Fig. 3A).
FIG. 3.
Differentiation-dependent activation of IR and IRS-1 is impaired in IRS-1 KO cells. Cell lines designated in Fig. 2 were differentiated in insulin-containing (A, C, and E) or non-insulin-containing (B, D, and F) medium as described in Materials and Methods, and lysates were prepared at the indicated days of differentiation. (A, B) Protein lysates were subjected to immunoprecipitation (IP) with IR β-subunit-specific antibody followed by immunoblotting (IB) with a pY (upper panel) or IR (lower panel) antibody. (C, D) IRS-1 (upper panel) and IRS-2 (lower panel) were immunoprecipitated with specific antibodies, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting using pY antibodies. (E, F) Immunoprecipitations with a p85-specific antibody were performed and immunoblots were prepared using IRS-1-specific (upper panel) or IRS-2-specific (lower panel) antibodies. The representative immunoblots in panel E were exposed to film for a longer period of time than those in panel F. A representative blot of at least two independent experiments is shown.
No significant IRS-1 tyrosine phosphorylation was detectable in the three cell lines at day 0 (Fig. 3C). After induction, IRS-1 tyrosine phosphorylation significantly increased 22-fold in the wild-type cells at day 2 and remained elevated until day 6 (Fig. 3C). As expected, no tyrosine phosphorylation was apparent in IRS-1 KO cells (Fig. 3C). IRS-1 tyrosine phosphorylation in KO cells reconstituted with IRS-1 was similar to that in wild-type cells, with an 18-fold increase detectable at day 6 (Fig. 3C). In contrast, tyrosine phosphorylation of IRS-2 was increased to a similar extent in all three cell lines, with maximal induction occurring at day 6 of the differentiation protocol (Fig. 3C).
To determine the role of insulin itself in these changing patterns of protein expression and phosphorylation, the wild-type, KO, and IRS-1-reconstituted KO cells were differentiated in the absence of insulin. Although differentiation of all three cell lines cultured in insulin-free medium was somewhat decreased and delayed, even in the absence of insulin, 80 to 90% of wild-type cells and 60 to 80% of IRS-1-reconstituted KO cells differentiated, compared to only 5 to 20% of the KO cells (data not shown). Under these conditions no increase in IR tyrosine phosphorylation was apparent in the three cell lines during differentiation, whereas the change in IR content was comparable to the change observed in cells cultured in insulin-containing medium (Fig. 3B). Furthermore, the changes in IRS-1 and IRS-2 tyrosine phosphorylation were similar to that observed in cells differentiated in the presence of insulin (Fig. 3D).
Activation of PI 3-kinase during differentiation is decreased in IRS-1 KO cells.
As both IRS-1 and IRS-2 tyrosine phosphorylation increased during differentiation, we determined whether this increase was accompanied by enhanced binding of each IRS to the p85 regulatory subunit of PI 3-kinase. The amount of IRS-1 bound to p85 increased maximally sevenfold at day 2 in the wild-type cells whereas, as expected, no binding was detectable in the KO cells (Fig. 3E). Similar to wild-type cells, IRS-1-reconstituted KO adipocytes showed a fourfold increase in IRS-1 binding to p85 at day 4 of differentiation (Fig. 3E). On the other hand, binding of IRS-2 to p85 increased uniformly by a factor of three in all three cell lines at day 2, with sustained levels in the KO and IRS-1-reconstituted KO cell lines at days 4 and 6 of differentiation and decreasing levels in the wild-type adipocytes (Fig. 3E). Furthermore, a similar pattern of IRS-1 and IRS-2 binding to p85 could be detected in cells differentiated in the absence of insulin (Fig. 3F).
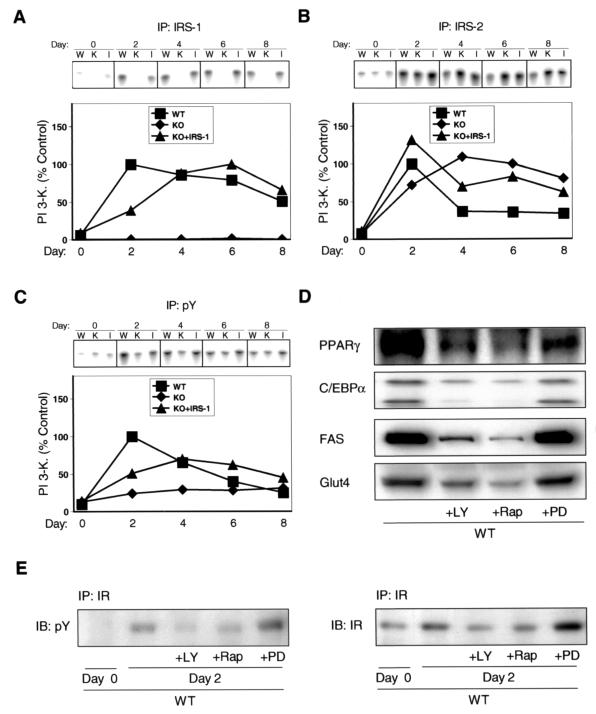
PI 3-kinase activity assays paralleled the results for association of IRS-1 and IRS-2 with p85. Thus, a strong 20-fold increase in IRS-1-associated PI 3-kinase activity could be observed in the wild-type cells at day 2 of the differentiation protocol, with PI 3-kinase decreasing to 50% of maximal level at day 8 (Fig. 4A). No significant IRS-1-associated PI 3-kinase activity could be observed in IRS-1 KO cells during the differentiation process, whereas a maximal 12-fold increase was detected in KO cells reexpressing IRS-1 (Fig. 4A). IRS-2-associated PI 3-kinase activity was maximally 13-fold increased in the wild-type cells at day 2 of the differentiation protocol and rapidly declined to 35% of maximal level between days 4 and 8 (Fig. 4B). IRS-1-deficient cells showed a strong sustained increase in IRS-2-associated PI 3-kinase activity with a maximal 16-fold activation occurring at day 4 of differentiation (Fig. 4B). IRS-2-associated PI 3-kinase activity in KO cells reexpressing IRS-1 was increased 13-fold at day 2 and decreased to 50% of maximal level at day 8 (Fig. 4B).
FIG. 4.
Differentiation-dependent PI 3-kinase activation is impaired in IRS-1-deficient cells. Cell lines designated in Fig. 2 were differentiated and at the indicated days lysates were subjected to immunoprecipitation with antibodies against IRS-1 (A), IRS-2 (B), or pY (C). PI 3-kinase activity in these immunoprecipitates was measured as described in Materials and Methods. (A, B, and C) A representative experiment (upper panel) and a graph (lower panel) representing the average of at least two independent experiments are shown. W, WT; K, KO; I, KO + IRS-1. (D) Confluent wild-type cells (day 0) were induced in the presence or absence of the PI 3-kinase inhibitor LY294002 (LY; 10 μM), the p70S6 kinase inhibitor rapamycin (Rap; 1 μM), or the MEK inhibitor PD098059 (PD; 50 μM). At day 6 of differentiation, cell lysates were prepared and adipogenic marker proteins were determined as indicated. (E) Wild-type cells were induced as described for panel D and lysates were prepared at days 0 and 2 of differentiation. Protein lysates were subjected to immunoprecipitation with IR β-subunit-specific antibody followed by immunoblotting with a pY (left panel) or IR (right panel) antibody.
To assess the impact of altered IRS-1- versus IRS-2-associated PI 3-kinase activity at the cellular level, we determined total PI 3-kinase activity in phosphotyrosine (pY) immunoprecipitates at various days of the differentiation protocol. Confluent noninduced wild-type and KO cells and KO cells reexpressing IRS-1 (day 0) showed comparable levels of pY-associated PI 3-kinase activity (Fig. 4C). A 10-fold increase in pY-associated PI 3-kinase activity could be observed at day 2 of the differentiation protocol in wild-type cells (Fig. 4C). Between day 2 and day 8 total PI 3-kinase activity in these cells continuously decreased to 25% of maximum activation (Fig. 4C). In contrast, pY-associated PI 3-kinase activity in IRS-1 KO cells was increased only about 2.5-fold and no apparent peak of activity was detectable (Fig. 4C). IRS-1-deficient cells reexpressing IRS-1 showed a maximal sixfold activation of total PI 3-kinase occurring at day 4 of differentiation (Fig. 4C).
PI 3-kinase activation has been shown to play a role in differentiation of white adipose tissue (25). To determine if the same was true for brown fat, we tested whether pharmacological inhibition of PI 3-kinase would affect differentiation of wild-type cells. Treatment of adipocytes with the PI 3-kinase inhibitor LY294002 during differentiation significantly decreased protein amounts of the adipogenic markers PPARγ, C/EBPα, FAS, and Glut4 (Fig. 4D) and inhibited cellular accumulation of fat, compared to results in nontreated cells (data not shown). Furthermore, pharmacological inhibition of p70S6 kinase by rapamycin also inhibited differentiation of wild-type cells, supporting previous results that indicated a role for this kinase in white adipocyte differentiation (1, 38) (Fig. 4D). In contrast, inhibition of mitogen-activated protein (MAP) kinase with the MEK inhibitor PD098059 did not significantly affect adipogenic marker protein expression (Fig. 4D). We further determined whether inhibition of PI 3-kinase, p70S6 kinase and MAP kinase would affect the strong induction in IR tyrosine phosphorylation and content observed in wild-type cells between days 0 and 2 of differentiation. Treatment of cells with LY294002 and rapamycin led to impaired induction of IR tyrosine phosphorylation and content, compared to that in nontreated cells, whereas IR phosphorylation and content were slightly increased in wild-type cells treated with the MEK inhibitor PD098059 (Fig. 4E).
IRS-1 is the predominant tyrosine-phosphorylated protein bound to p85 during differentiation of wild-type cells.
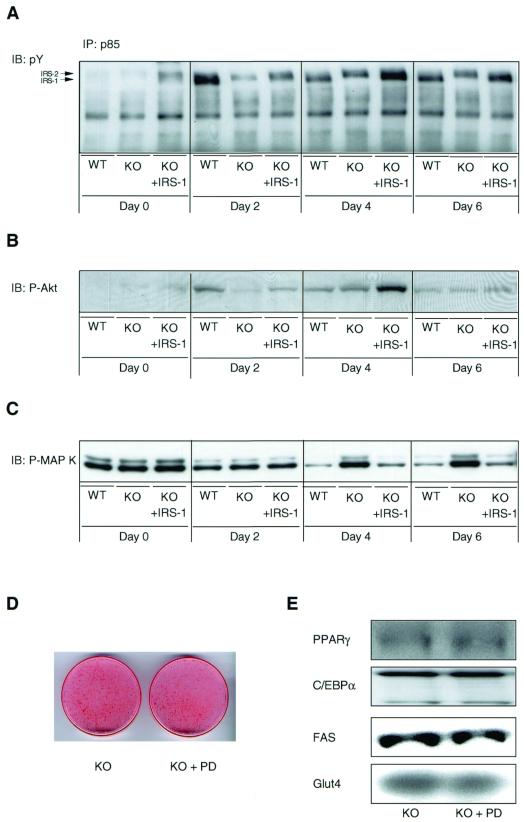
Our data above suggest a more important role of IRS-1- versus IRS-2-associated PI 3-kinase activity in the differentiation process. To assess which tyrosine-phosphorylated IRS is predominantly bound to the p85 regulatory subunit of PI 3-kinase during differentiation, we immunoprecipitated cell lysates with a p85-specific antibody followed by immunoblotting with an antiphosphotyrosine antibody. In confluent, noninduced wild-type, KO, and IRS-1-reconstituted KO cells (day 0), the slightly higher migrating tyrosine-phosphorylated IRS-2 was the predominant protein associated with p85 (Fig. 5A). At day 2 of the differentiation protocol wild-type cells showed significantly increased binding of both IRS-1 and IRS-2 to p85, with the lower migrating IRS-1 being predominant (Fig. 5A). At days 4 and 6 significant binding to p85 could only be detected for IRS-1 in these cells (Fig. 5A). As expected, IRS-1-deficient cells showed only an increase in tyrosine-phosphorylated IRS-2 protein bound to p85 during differentiation (Fig. 5A). In IRS-1-reconstituted KO cells, IRS-2 was the predominant tyrosine-phosphorylated protein bound to p85 on day 2, whereas IRS-1 was more prominent on days 4 and 6 (Fig. 5A). Protein levels of p85 slightly increased upon induction of differentiation but were comparable between the three different cell lines (data not shown).
FIG. 5.
Differentiation-dependent Akt and MAP kinase activation is altered in IRS-1 KO cells. The three different cell lines (designated as in Fig. 2) were differentiated as described in Materials and Methods. (A) Lysates were subjected to immunoprecipitation (IP) with a p85-specific antibody followed by immunoblotting (IB) with a pY antibody. Alternatively, lysates were immunoblotted with a phospho-specific Akt (B) or a phospho-specific MAP kinase antibody (C). (D, E) Confluent IRS-1-deficient cells (day 0) were induced in the presence or absence of the MEK inhibitor PD098059 (PD; 50 μM). At day 6 of differentiation, cells were either stained with oil red O (D) or prepared for Western blot analysis of several adipogenic marker proteins as shown (E). A representative experiment of at least two independent experiments is shown.
Transient activation of Akt during differentiation is decreased in IRS-1 KO cells.
The serine/threonine kinase Akt is a downstream target of PI 3-kinase and has been implicated in adipogenic and myogenic differentiation. No apparent differences in Akt activity between the three cell lines could be detected in confluent cells (day 0) as determined by immunoblotting lysates with a phospho-specific antibody against the activated form of Akt (Fig. 5B). A rapid and transient 12-fold increase in Akt phosphorylation at day 2 of the differentiation protocol was apparent in the wild-type cells (Fig. 5B). IRS-1-deficient cells showed a maximal 7-fold increase in Akt phosphorylation at day 4, whereas a 15-fold increase could be detected in KO cells reexpressing IRS-1 (Fig. 5B). The peak of Akt phosphorylation in the wild-type and IRS-1-reconstituted KO cell lines correlated with the maximum of tyrosine-phosphorylated IRS-1 bound to p85 (Fig. 5A and B). At day 6 of the differentiation protocol, comparable levels of Akt phosphorylation were detectable in the three different cell lines. No significant change of Akt content could be observed between the different cell lines during differentiation (data not shown).
MAP kinase phosphorylation decreases during differentiation in wild-type and IRS-1-reconstituted KO adipocytes, but not IRS-1-deficient cells.
MAP kinase has been shown to phosphorylate PPARγ (11) and thus potentially act as a negative regulator of adipogenesis. Phosphorylation of this kinase was comparable between the three cell lines in confluent noninduced cells (day 0) as determined by immunoblotting with a phospho-specific antibody against the activated isoforms p42 and p44 (Fig. 5C). On day 2, MAP kinase phosphorylation uniformly decreased in the three different cell lines (Fig. 5C). Phosphorylation of this kinase further decreased in wild-type and IRS-1-reconstituted KO adipocytes at days 4 and 6 of differentiation, whereas it was rather increased in the KO cells (Fig. 5C). This is consistent with the finding that inhibition of MAP kinase by PD098059 in wild-type cells did not significantly affect the adipogenic process (see above). Likewise, pharmacological inhibition of MAP kinase did not affect the differentiation capacity of IRS-1 KO cells, as determined by oil red O staining (Fig. 5D) and Western blot analysis of various adipogenic markers (Fig. 5E). Amounts of MAP kinase protein were comparable in the three different cell lines throughout differentiation (data not shown).
Overexpression of PPARγ or C/EBPα increases differentiation capacity of IRS-1-deficient cells.
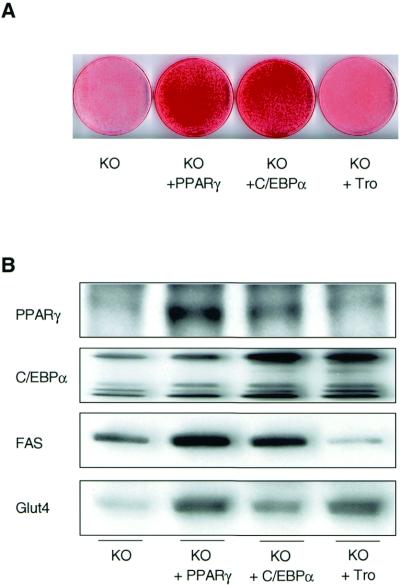
Since both PPARγ and C/EBPα have been shown to play important roles in adipogenesis of white fat, we determined the influence of retrovirus-mediated overexpression of either protein, as well as activation of PPARγ by troglitazone, on the differentiation capacity of IRS-1-deficient cells. Following retroviral infection, IRS-1-deficient cells overexpressed PPARγ about eightfold and C/EBPα about fivefold, compared to control KO cells (Fig. 6B). On day 6 of the differentiation protocol, both the PPARγ- and the C/EBPα-overexpressing KO cell lines showed increased fat accumulation compared to that in IRS-1-deficient cells (Fig. 6A). Furthermore, compared to the KO cells a significant increase in FAS and Glut4 protein content could be observed in PPARγ- and, to a lesser extent, C/EBPα-overexpressing cells (Fig. 6B). In contrast, treatment of IRS-1-deficient cells with troglitazone did not significantly change total fat accumulation (Fig. 6A) or the level of expression of PPARγ and FAS (Fig. 6B). On the other hand, troglitazone treatment of the IRS-1 KO cells significantly upregulated the protein amounts of C/EBPα and Glut4, creating a dissociation in those differentiation markers from the adipogenic process (Fig. 6B).
FIG. 6.
Differentiation capacity of IRS-1-deficient cells is improved by overexpression of PPARγ or C/EBPα. IRS-1 KO cell lines overexpressing PPARγ (KO + PPARγ) or C/EBPα (KO + C/EBPα) were prepared as described in Materials and Methods. Furthermore, IRS-1-deficient cells were treated with 50 μM troglitazone (KO + Tro) throughout the differentiation process. At day 6 of differentiation, cells were either stained with oil red O (A) or prepared for Western blot analysis of various adipogenic markers as shown (B). Results are representative of at least two independent experiments.
DISCUSSION
In the current study, we have utilized SV40T antigen-immortalized brown preadipocytes isolated from wild-type and IRS-1 KO mice to examine the role of IRS-1 in adipocyte differentiation. These cells provide an attractive model to study differentiation processes for several reasons. We have recently shown that, similar to other white preadipocyte cell lines such as 3T3-L1 cells, brown preadipocytes can be differentiated into mature adipocytes, with accumulation of multilocular fat droplets and expression of adipogenic and thermogenic markers, including FAS and UCP-1 (16). The cells also contain the major elements of the insulin signaling system, including the insulin receptor itself, IRS-1, and IRS-2 (32). Furthermore, immortalized brown preadipocytes can be established using a single newborn or late fetal mouse, thereby allowing creation of metabolically active, insulin-responsive cells from different animal models of diabetes and obesity, including knockout and transgenic mice.
Using this system, we found that the percentage of preadipocytes accumulating fat droplets during differentiation is dramatically decreased in IRS-1-deficient cells compared to their wild-type counterparts. Consistent with the diminished number of fat-accumulating cells, the protein content of the adipogenic markers C/EBPδ, PPARγ, C/EBPα, FAS, UCP-1, and Glut4 is significantly decreased in IRS-1 KO cells. Interestingly, this appears to be due to alterations at the posttranscriptional as well as the transcriptional level. Thus, C/EBPδ mRNA is actually increased in IRS-1-deficient cells, whereas PPARγ mRNA is decreased and levels of C/EBPα and FAS mRNA are similar between both genotypes, despite the difference in expression at the protein level. Both transcriptional and posttranscriptional regulation of adipogenesis have been described (25, 31, 35). In the IRS-1 KO cells, reexpression of IRS-1 at physiological levels is sufficient to almost completely reverse the differentiation deficit, emphasizing a specific role of IRS-1 in the differentiation process.
The upstream signals leading to adipocyte differentiation remain poorly understood. Since our results suggested that IRS-1 signaling was important in adipocyte differentiation, we determined whether this was mediated via changes in IRS tyrosine phosphorylation and activation of subsequent SH2-mediated signals such as PI 3-kinase and MAP kinase. In fact, tyrosine phosphorylation of both IRS-1 and IRS-2 is strongly and rapidly increased after induction of confluent preadipocytes to differentiate. This is similar to previous findings showing that both IRS-1 and IRS-2 are activated during differentiation of white adipocytes (25). However, the stimuli inducing the phosphorylation of both IRS proteins during differentiation are unclear. Thus, the tyrosine phosphorylation of the IRS proteins is observed over the entire 6 days of differentiation and can be seen whether or not insulin is present in the differentiation medium. During differentiation, the expression of the IR is increased and this is associated with increased IR phosphorylation when cells are differentiated in insulin-containing differentiation medium. However, differentiation is only slightly decreased and delayed in wild-type cells cultured in the absence of insulin, and under these conditions the enhanced phosphorylation of the IRS proteins is preserved despite an absence of IR tyrosine phosphorylation. Whether the nonligand IR is sufficient to induce this phosphorylation or some other tyrosine kinase is involved in this process will require further study.
Tyrosine-phosphorylated IRS proteins have been shown to bind the p85 regulatory subunit of PI 3-kinase, thereby activating the p110 catalytic subunit of the enzyme (4). During differentiation of wild-type brown adipocytes, there is an increase in binding of both IRS proteins to p85, and IRS-1- and IRS-2-associated PI 3-kinase activities are rapidly increased. Further experiments using p85 immunoprecipitation followed by pY immunoblotting suggested that of these two substrates, IRS-1 is the predominant signaling protein during differentiation. These results are in agreement with findings in white adipocytes (25). In contrast to the essential role of IRS-1 in differentiation of brown adipocytes, cells lacking IRS-2 do not show any differentiation deficit (7). Furthermore, while IRS-1-deficient cells do not show any of the IRS-1-associated changes, IRS-2-associated PI 3-kinase activity in the KO cells exceeds the levels observed in wild-type and IRS-1-reconstituted KO cells, especially between days 4 and 8 of differentiation. Since IRS-1 is the predominant IRS protein bound to PI 3-kinase during differentiation, it is not surprising that IRS-1-deficient cells show a 60 to 70% decrease in total PI 3-kinase activation compared to that in wild-type and IRS-1-reconstituted KO adipocytes. Thus, the lack of IRS-1-associated PI 3-kinase activation in the KO cells during differentiation cannot be compensated for by IRS-2.
Consistent with findings in white adipocytes (25) and skeletal muscle cells in culture (13, 15), PI 3-kinase activity appears to be necessary for brown adipocyte differentiation. Wild-type cells treated with the pharmacological PI 3-kinase inhibitor LY294002, but not the MEK inhibitor PD98059, show decreased differentiation and decreased expression of adipogenic marker proteins. Taken together with the data above, it appears that IRS-1 mediates differentiation-dependent signals through PI 3-kinase and that the decrease in PI 3-kinase activity in IRS-1-deficient cells might well be linked to the observed differentiation deficit. This defect in PI 3-kinase activation in the IRS-1 KO cells is associated with an ∼50% decrease in phosphorylation of Akt, a protein serine/threonine kinase that serves as a major downstream effector of PI 3-kinase (33). It has been demonstrated that the positive effect of PI 3-kinase on L6 myocyte differentiation is also mediated via Akt (13) and that overexpression of a constitutively active Akt can increase the differentiation capacity of 3T3-L1 preadipocytes (17). However, in preliminary experiments we have not been able to successfully infect brown adipocytes with adenoviruses encoding either dominant negative or constitutively active membrane-targeted Akt. Thus, defining the exact role of Akt in brown adipocyte differentiation will require further study. Furthermore, whether a 50% decrease in Akt phosphorylation is sufficient to limit differentiation remains to be determined.
It has been shown that IRS-1 signals are also mediated through MAP kinase activation (33). Interestingly, phosphorylation of this signaling intermediate decreased during the differentiation period in the wild-type and IRS-1-reconstituted KO cells, whereas it was sustained in IRS-1-deficient cells. Thus, it appears that increased differentiation of brown adipocytes correlates with decreased MAP kinase phosphorylation. In fact, MAP kinase has been proposed as a negative effector of adipocyte differentiation (8). Furthermore, MAP kinase has been shown to phosphorylate PPARγ, thereby blocking the ability of this transcription factor to activate transcriptional events necessary for complete adipocyte differentiation (11). However, as pharmacological inhibition of MAP kinase in IRS-1 KO adipocytes does not improve differentiation capacity, the sustained activation of this signaling intermediate does not appear to be the primary reason for the differentiation deficit observed in cells lacking IRS-1.
In recent years evidence has been emerging that the activation of PPARγ and C/EBPα transcription is essential for differentiation of white adipocytes (27). In IRS-1 KO cells, overexpression of either PPARγ or C/EBPα could overcome most of the differentiation blockade, suggesting that the effect of IRS-1 deficiency is upstream of these factors. Whatever the exact mechanism, these results suggest that downregulation of both PPARγ and C/EBPα is an essential part of the impaired differentiation phenotype observed in the KO cells. Interestingly, treatment of IRS-1-deficient cells with the PPARγ activator troglitazone reversed some features of the differentiation deficit (C/EBPα and Glut4 expression) of IRS-1-deficient cells, while other features were unchanged (fat accumulation and PPARγ and FAS expression). These results suggest an effect of PPARγ in differentiation, and pharmacological activation of this pathway may be able to be, at least in part, separated, and this could occur as a result of modification of IRS-1-mediated signaling. This observation could have important implications in the use of thiazolidinediones as therapeutics in obese and diabetic states. Furthermore, as overexpression of PPARγ as well as activation of this protein by troglitazone leads to increased synthesis of C/EBPα, and C/EBPα overexpression results in increased amounts of PPARγ, our data support prior studies suggesting a positive feedback loop between these two transcription factors (24, 35).
Finally, IRS-1 KO mice show a 50% reduction in white and brown adipose tissue mass compared to their wild-type counterparts, which corresponds to the 50% decrease in whole body weight observed in these animals (6). Further studies have demonstrated that the number, but not the size, of adipocytes derived from IRS-1 KO mice is decreased (6). Recently IRS-1/IRS-3 double KO mice have been shown to have a 90% reduction in white adipose tissue mass, supporting a role in vivo for IRS-1 in concert with IRS-3 in white adipocyte development (6). Interestingly, brown adipose tissue mass in both of these animals is decreased only to the same extent as whole body mass (6). This suggests that in vivo other factors may act to modify our in vitro findings.
In summary, we have demonstrated a critical role for IRS-1 in brown adipocyte differentiation. We find that a lack of IRS-1 results in a severe differentiation deficit that appears to be mediated via decreases in PI 3-kinase and Akt activation and is upstream of transcriptional regulators such as PPARγ and C/EBPα. Further work will be needed to define the exact upstream signaling events responsible for IRS-1 and IRS-2 activation during differentiation, as well as the link of the various adipogenic signals to each other during the differentiation process.
ACKNOWLEDGMENTS
M. Fasshauer and J. Klein contributed equally to this work.
This work was supported by NIH grants DK 5545, DK 33201, and DK 36836 (Joslin's Diabetes and Endocrinology Research Center grant). M.F. was supported by a grant from the Studienstiftung des deutschen Volkes. J.K. was supported by a grant from the Deutsche Forschungsgemeinschaft.
We thankfully acknowledge James DeCaprio (Dana Farber Cancer Institute, Boston, Mass.) and Sven Freytag (Henry Ford Health System, Detroit, Mich.) for providing us with the retroviral vectors coding for SV40T and C/EBPα, respectively. We are indebted to Terri-Lyn Azar and Jennifer Konigsberg for excellent secretarial assistance.
REFERENCES
- 1.Bell A, Grunder L, Sorisky A. Rapamycin inhibits human adipocyte differentiation in primary culture. Obes Res. 2000;8:249–254. doi: 10.1038/oby.2000.29. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brüning J C, Winnay J, Cheatham B, Kahn C R. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheatham B, Kahn C R. Insulin action and the insulin signaling network. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 5.Christy R J, Yang V W, Ntambi J M, Geiman D E, Landschulz W H, Friedman A D, Nakabeppu Y, Kelly T J, Lane M D. Differentiation-induced gene expression in 3T3–L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- 6.Curtis S E, Michael M D, Crute B E, Keller S R, Lienhard G E. Double knockout of IRS proteins reveals critical roles of IRS-1 and IRS-3 in the maintenance of glucose homeostasis. Diabetes Suppl. 2000;49:A5. [Google Scholar]
- 7.Fasshauer M, Klein J, Ueki K, Kriauciunas K M, Benito M, White M F, Kahn C R. Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem. 2000;275:25494–25501. doi: 10.1074/jbc.M004046200. [DOI] [PubMed] [Google Scholar]
- 8.Font de Mora J, Porras A, Ahn N, Santos E. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3–L1 adipocytic differentiation. Mol Cell Biol. 1997;17:6068–6075. doi: 10.1128/mcb.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Force W R, Cheung T C, Ware C F. Dominant negative mutants of TRAF3 reveal an important role for the coiled coil domains in cell death signaling by the lymphotoxin-beta receptor. J Biol Chem. 1997;272:30835–30840. doi: 10.1074/jbc.272.49.30835. [DOI] [PubMed] [Google Scholar]
- 10.Freytag S O, Paielli D L, Gilbert J D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- 11.Hu E, Kim J B, Sarraf P, Spiegelman B M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 12.Hwang C S, Loftus T M, Mandrup S, Lane M D. Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol. 1997;13:231–259. doi: 10.1146/annurev.cellbio.13.1.231. [DOI] [PubMed] [Google Scholar]
- 13.Jiang B H, Aoki M, Zheng J Z, Li J, Vogt P K. Myogenic signaling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc Natl Acad Sci USA. 1999;96:2077–2081. doi: 10.1073/pnas.96.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaestner K H, Christy R J, Lane M D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci USA. 1993;87:251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaliman P, Vinals F, Testar X, Palacin M, Zorzano A. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J Biol Chem. 1996;271:19146–19151. doi: 10.1074/jbc.271.32.19146. [DOI] [PubMed] [Google Scholar]
- 16.Klein J, Fasshauer M, Ito M, Lowell B B, Benito M, Kahn C R. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 17.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3/L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 18.Kraus S. Functional differentiation of white and brown adipocytes. Bioessays. 1996;19:215–223. doi: 10.1002/bies.950190307. [DOI] [PubMed] [Google Scholar]
- 19.Lowell B B, Flier J S. Brown adipose tissue, β3-adrenergic receptors, and obesity. Annu Rev Med. 1997;48:307–316. doi: 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- 20.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titer retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyers J S, Bilan P J, Reynet C, Kahn C R. Overexpression of Rad inhibits glucose uptake in cultured muscle and fat cells. J Biol Chem. 1996;271:23111–23116. doi: 10.1074/jbc.271.38.23111. [DOI] [PubMed] [Google Scholar]
- 22.Park E A, Roesler W J, Liu J, Klemm D J, Gurney A L, Thatcher J D, Shuman J, Friedman A, Hanson R W. The role of the CCAAT/enhancer-binding protein in the transcriptional regulation of the gene for phosphoenolpyruvate carboxykinase (GTP) Mol Cell Biol. 1990;10:6264–6272. doi: 10.1128/mcb.10.12.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn C R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 24.Rosen E D, Sarraf P, Troy A E, Bradwin G, Moore K, Milstone D S, Spiegelman B M, Mortensen R M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 25.Sakaue H, Ogawa W, Matsumoto M, Kuroda S, Takata M, Sugimoto T, Spiegelman B M, Kasuga M. Posttranscriptional control of adipocyte differentiation through activation of phosphoinositide 3-kinase. J Biol Chem. 1998;273:28945–28952. doi: 10.1074/jbc.273.44.28945. [DOI] [PubMed] [Google Scholar]
- 26.Schoonjans K, Peinado-Onsurbe J, Lefebvre A M, Heyman R A, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegelman B M, Flier J S. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 28.Tai T A C, Jennermann C, Brown K K, Oliver B B, MacGinnitie M A, Wilkison W O, Brown H R, Lehmann J M, Kliewer S A, Morris D C, Graves R A. Activation of the nuclear receptor peroxisome proliferator-activated receptor gamma promotes brown adipocyte differentiation. J Biol Chem. 1996;271:29909–29914. doi: 10.1074/jbc.271.47.29909. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tontonoz P, Hu E, Granes R A, Budavari A I, Spiegelman B M. MPPARχ: tissue specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 31.Tontonoz P, Hu E, Spiegelman B M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 32.Valverde A M, Kahn C R, Benito M. Insulin signaling in insulin receptor substrate (IRS)-1-deficient adipocytes: requirement of IRS-1 for lipid synthesis. Diabetes. 1999;48:2122–2131. doi: 10.2337/diabetes.48.11.2122. [DOI] [PubMed] [Google Scholar]
- 33.White M F. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 34.Wu Z, Bucher N L R, Farmer S R. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Rosen E D, Brun R, Hauser S, Adelmant G, Troy A E, McKeon C, Darlington G J, Spiegelman B M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Xie Y, Bucher N L, Farmer S R. Conditional ectopic expression of C/EBP beta in NIH-3T3 cells induces PPAR gamma and stimulates adipogenesis. Genes Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Xie Y, Morrison R F, Bucher N L, Farmer S R. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. J Clin Investig. 1998;101:22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh W C, Bierer B E, McKnight S L. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3–L1 cells. Proc Natl Acad Sci USA. 1995;92:11086–11090. doi: 10.1073/pnas.92.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeh W C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]