Letter to the Editor:
Deep brain stimulation (DBS) of the anterior limb of the internal capsule (ALIC) has shown promise as a therapy for refractory obsessive-compulsive disorder (OCD) [1,2] and major depressive disorder (MDD) [3]. DBS currently is ‘open-loop’ with stimulation parameters remaining fixed over time. In contrast, ‘closed-loop’ DBS delivers stimulation only when a biomarker of the target symptom state indicates it is needed, decreasing stimulation time- thereby potentially reducing the risk of side effects, prolonging battery life, and potentially improving efficacy by mitigating neural adaptation. This method has shown efficacy in treating epilepsy [4] and promise in treating Parkinson’s disease [5]. One challenge to the development of closed-loop DBS for psychiatric indications is the dearth of biomarkers of symptom state [6]. Here, we report identification of an electrophysiological depression biomarker in a patient receiving DBS in the ALIC and nearby bed nucleus of the stria terminalis (BNST). This case represents a proof-of-concept that personalized biomarkers can be detected using existing commercially available DBS devices, marking a step towards the development of closed-loop systems.
The patient is a 51-year-old man with a history of severe, treatment-refractory OCD (Yale-Brown Obsessive Compulsive Scale: Y-BOCS of 31) and co-morbid severe MDD. The patient’s harm-based OCD began in his teenage years and was formally diagnosed at the age of 28 years. His OCD and depression did not adequately respond to trials of 5 SSRIs, 1 SNRI, augmentation strategies (2 antipsychotics, clomipramine, stimulant, IV ketamine), and transcranial magnetic stimulation. The patient had extensive trials of exposure-response prevention and psychodynamic therapy with limited benefit. Given this history of treatment refractoriness and significant functional impairment related to severe OCD and MDD, the patient was deemed an appropriate candidate for DBS targeting the ALIC, which has been shown to be helpful for both disorders [1–3].
DBS leads (3387; Medtronic, Minneapolis, MN) were stereotactically implanted within the ALIC and surrounding BNST bilaterally and were connected to an Activa PC (Medtronic, Minneapolis, MN) internal pulse generator. The patient underwent standard stimulation programming with partial response in OCD symptoms (reduction in Y-BOCS from 36 to 25; 31% improvement) and notable improvement in depression (reduction in PHQ-9 from 25 to 15; 40% improvement) from pre-op to one year follow up. Subsequently, the patient underwent IPG exchange due to battery depletion with the Percept PC (Medtronic, Minneapolis, MN) at approximately 15 months post-implantation. This new device is the first clinically approved device to have the ability to perform local field potential (LFP) recordings from the DBS leads in addition to stimulation. Ultimately, stimulation from the right lead was discontinued given lack of benefit, and the patient was primarily treated with DBS from the left ALIC contacts, while recordings were performed from the right contacts.
We localized electrode placement using pre- and post-operative MRI and found the right dorsal three contacts were in the BNST while all contacts on the stimulating lead were located in the ventral ALIC (Figure 1A, supplemental methods) [7]. Pre-operative diffusion-tensor imaging was used to determine putative fiber tracts being modulated by DBS from the left, stimulating lead. (Figure 1B, 1C) and identified prominent connections to the frontal cortex, temporal lobe, and contralateral BNST, which are tracts that have been associated with mood improvements in prior studies [8].
Figure 1: Chronic Intracranial BNST Recordings Demonstrate Gamma Correlates with Depression Symptoms.
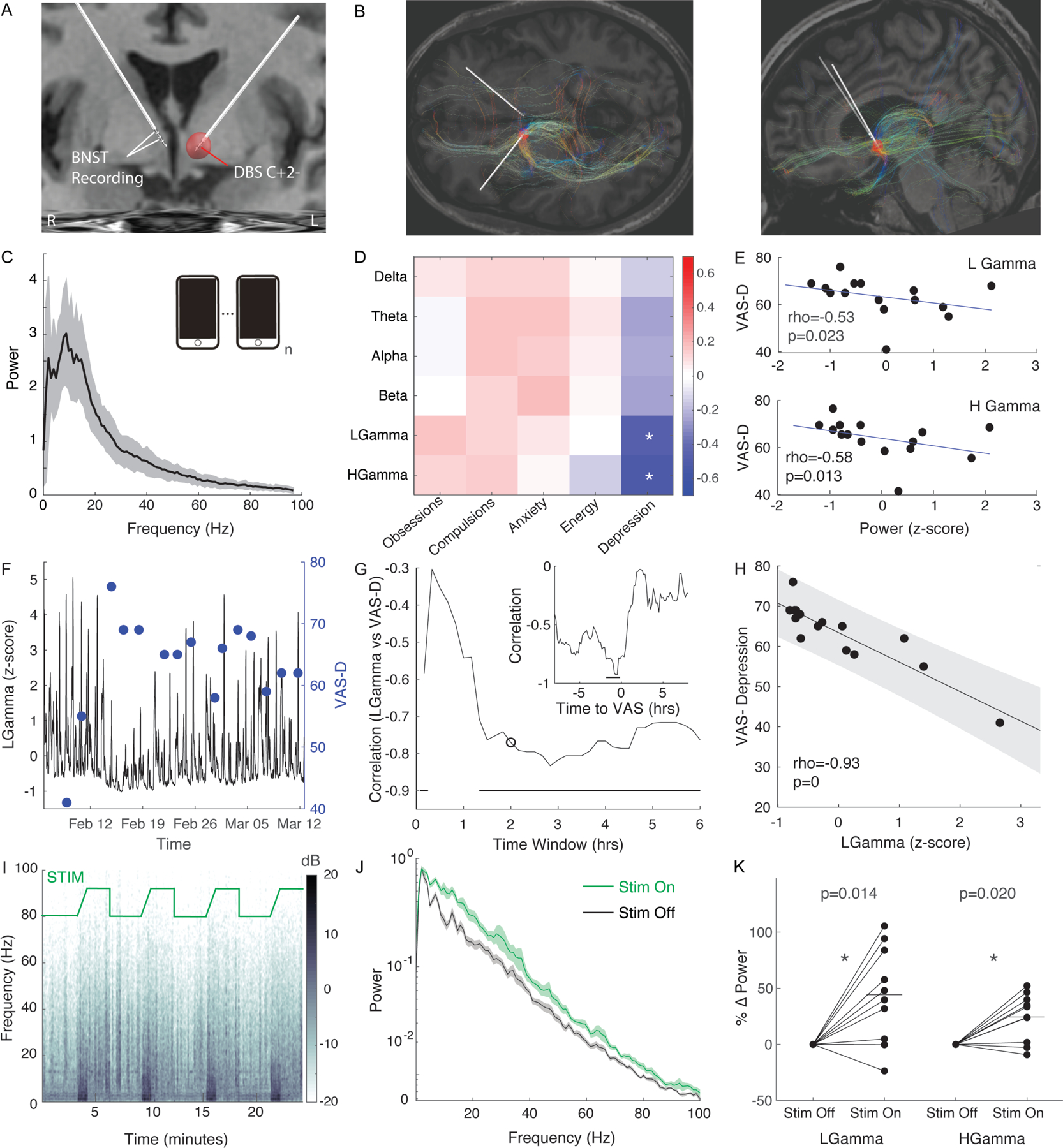
A) Coronal MRI with reconstruction of DBS quadripolar electrode placement. Active monopolar contact is contact 2 on left lead. Recording in bipolar configuration on right lead contacts 3 and 1. Red sphere represents estimated volume of tissue activation.
B) Fiber tracts from estimated volume of tissue activation based upon diffusion tensor imaging (DTI) in the horizontal (left panel) and sagittal (right panel) plane
C) Average power spectra of 15 patient triggered events. Shaded region represents S.E.M.
D) Spearman correlation coefficients of power bands vs visual analogue scales (VAS) of symptoms.
E) Plot of power for low gamma (25–50Hz) (top panel) and high gamma (50–100Hz) (bottom panel) vs VAS-Depression. Rho represents Spearman correlation. P-values derived from permutation test.
F) Sliding average of low gamma (25±2.5Hz) (black) activity averaged over 2hr bins and VAS-Depression self-reports across time
G) Spearman correlation of low gamma (25±2.5Hz) vs VAS-Depression binned across various size time windows. Circle demarcates correlation for 2hr time window. Line indicates time windows in which correlation is significant based upon Bonferroni corrected permutation test. Inset demonstrates Spearman correlation of low gamma vs VAS-D for various times relative to VAS surveys. Dark line represents time lags in which correlation was significant based upon a bootstrap test.
H) Plot of low gamma (25±2.5Hz) power within a 2hr window ending 30min prior to survey vs self-reported VAS-Depression. Line represents linear fit and shaded region represents 95% confidence interval. P-value represents results of permutation test.
I) Spectrogram while stimulation cycles from Off (3 min) to On (3min). There is a ramp in stimulation amplitude at the onset of the stimulation trial.
J) Power spectra during rest period when stimulation is On (green) and Off (black). Shaded region represents S.E.M.
K) Percent change in power from stimulation Off to On. Line represent average percent change with stimulation On. * indicates p<0.05 based upon a sign-rank test.
We tracked the patient’s symptom state using validated visual analogue scales (VAS) across several domains (obsessions, compulsions, anxiety, energy, and depression). LFP recordings were simultaneously acquired from the patient’s right-sided sensing lead using the Percept IPG’s patient-triggered power spectra recording mode. We collected a set of power spectral density (PSD) recordings derived from 30 sec of LFP (Figure 1C). Power in 6 frequency bands (delta: 1–4Hz; theta: 4–8Hz; alpha: 8–12Hz; beta: 12–25Hz; low gamma: 25–50Hz; high gamma: 50–97Hz) was correlated with VAS scores of symptom state. We found a significant inverse correlation between low and high gamma power and VAS-Depression ratings (Figure 1D, 1E).
We then monitored power in a low gamma range (25Hz±2.5Hz) continuously over ~6 weeks using the timeline mode (Figure 1F) in parallel with the VAS symptom ratings. We found that low gamma power preceding VAS depression scoring was again inversely correlated with VAS depression ratings (Figure 1G, 1H), and this correlation became stronger by averaging over a longer time window (Figure 1G). The strength of the low gamma correlation with VAS-D was strongest immediately prior to the rating, demonstrating a strong temporal relationship between the biomarker and depressive symptoms (Fig 1G, inset). These results were validated using leave-on-out cross-validation analysis (R2=0.72, p=5.8×10−5).
To test whether the putative biomarker of depression severity was responsive to stimulation, we also recorded streamed LFP traces from the right lead while DBS of the left ALIC was cycled on and off in-office. We found that left DBS stimulation in the left ALIC generated a broadband increase in power in the right BNST (Figure 1I, J, supplemental methods), including significant increases in low and high gamma power (Figure 1K) accompanied by a general improvement in symptoms (see supplemental video).
In summary, we describe a case where we identified BNST gamma power to be a putative personalized biomarker that is both associated with depression symptoms and responsive to therapeutic DBS. Gamma enhancement with stimulation may be mediated by structural connectivity between our stimulation contacts in the left ALIC and our recording contact in the right BNST (Fig 1B). The correlation between BNST gamma activity and mood is consistent with studies demonstrating the BNSTs role in reward motivation pathways [9,10]. We utilized a personalized method for discovering biomarkers of symptom state by correlating spectral features from intracranial recordings with longitudinal self-reports. The interindividual variability of this marker across subjects remains unknown. However, we speculate that different biomarkers may be unique to individuals, disorders, and brain locations, highlighting the importance of developing individualized closed-loop DBS that can adapt to patients’ symptoms in real-time. Further work will be needed to extend this work other patients with OCD and co-morbid severe MDD; patients with MDD without OCD; and to ultimately implement and test closed-loop DBS therapy targeting depression.
Supplementary Material
Acknowledgements and Disclosures:
We thank the subject who volunteered to be a part of this study. We also thank Andrew Krystal, who provided helpful comments on the manuscript.
Funding:
This work is supported by the National Institute of Mental Health (Grant No. K23MH125018), P&S Fund from the Brain and Behavior Foundation (Grant No. A136828), and Foundation for OCD Research (Grant No. P0548058).
Footnotes
Declarations of Interest: none
References:
- [1].Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry 2016;21:1272–80. 10.1038/mp.2015.124. [DOI] [PubMed] [Google Scholar]
- [2].Mosley PE, Windels F, Morris J, Coyne T, Marsh R, Giorni A, et al. A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder. Transl Psychiatry 2021;11. 10.1038/s41398-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Notten P, Van Laarhoven J, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression. JAMA Psychiatry 2016;73:456–64. 10.1001/jamapsychiatry.2016.0152. [DOI] [PubMed] [Google Scholar]
- [4].Morrell MJ RNS System in Epilepsy Study Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011;77:1295–304. 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- [5].Little S, Pogosyan A, Neal S, Zavala B, Zrinzo L, Hariz M, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol 2013;74:449–57. 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sani OG, Yang Y, Lee MB, Dawes HE, Chang EF, Shanechi MM. Mood variations decoded from multi-site intracranial human brain activity. Nat Biotechnol 2018;36:954. 10.1038/nbt.4200. [DOI] [PubMed] [Google Scholar]
- [7].Horn A, Li N, Dembek TA, Kappel A, Boulay C, Ewert S, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293–316. 10.1016/j.neuroimage.2018.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, et al. Connectivity Profile Predictive of Effective Deep Brain Stimulation in Obsessive-Compulsive Disorder. Biol Psychiatry 2019;85:735–43. 10.1016/j.biopsych.2018.12.019. [DOI] [PubMed] [Google Scholar]
- [9].Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, et al. Distinct extended amygdala circuits for divergent motivational states. Nature 2013;496:224–8. 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Giardino WJ, Eban-Rothschild A, Christoffel DJ, Bin Li S, Malenka RC, de Lecea L. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci 2018;21:1084–95. 10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


