Abstract
Due to the increasing health and environmental risks associated with the use of fungicides in agriculture, alternatives—such as using plant growth-promoting bacteria (PGPB) to suppress phytopathogens—that simultaneously improve plant yield, are important. This study evaluated the biocontrol efficiency of Bacillus velezensis CE100 against Macrophomina phaseolina and Fusarium oxysporum f. sp. fragariae, the respective causal agents for charcoal rot and fusarium wilt diseases in strawberry, and its potential to enhance strawberry growth and fruit production. B. velezensis CE 100 produced fungal cell wall-degrading enzymes, chitinases, and β-1,3-glucanases; and inhibited the mycelial growth of M. phaseolina and F. oxysporum f. sp. fragariae by 64.7% and 55.2%, respectively. The mycelia of both phytopathogenic fungi showed severe swelling and rupturing of the hyphae compared to the smooth, normal growth in the control group. Moreover, B. velezensis CE100 produced up to 2.8 units/mL of indole-3-acetic acid (IAA) during incubation and enhanced root biomass in strawberries. Consequently, B. velezensis CE 100 not only increased the fruit yield of strawberries by controlling the fungal diseases but also through enhancing plant growth. The findings of this study indicate that B. velezensis CE100 could be a safe, ecofriendly biocontrol alternative to chemical fungicides in strawberry production.
Keywords: biocontrol agent, lytic enzymes, phytopathogenic fungi, auxin, strawberry production
1. Introduction
Strawberry (Fragaria × ananassa Duch.) is an important commercial fruit crop with rich organoleptic qualities such as a pleasant flavor, texture, and taste, and numerous health benefits [1,2]. Strawberries are widely consumed as fresh fruit or processed into various food products [1,2]. Based on the most recent statistics, global strawberry production has increased by 41% in the last decade; and China, the United States of America (USA), the European Union (EU), Mexico, Turkey, Egypt, and South Korea are the leading producers [1,3]. In 2019, the annual strawberry yields in the USA and China were 1,021,490 t from 18,130 ha (563,425 hg/ha) and 3,221,557 t from 126,126 ha (255,424 hg/ha), respectively [1,3]. The increase in strawberry productivity over the past decade could be attributed to scientific innovations in the areas of breeding, improving cultural practices, and pest and disease management [4,5,6].
However, strawberry production is heavily prone to field and post-harvest losses due to high susceptibility to fungal diseases such as root rot, crown rot, charcoal rot, and fusarium wilt disease [7,8,9,10]. Some of the most notable soil-borne fungal pathogens, such as Macrophomina phaseolina and Fusarium oxysporum f. sp. Fragariae, have great longevity and exhibit highly competitive saprophytic ability [10,11]. Specifically, M. phaseolina is a cosmopolitan fungal pathogen with a wide host range of approximately 500 plant species [12]. It causes crown and charcoal rot diseases which are characterized by the browning of the root’s vascular and cortical tissues and can lead to tremendous losses in strawberry production [12,13]. M. phaseolina produces microsclerotia, which are highly resilient and resistant structures that are heat tolerant, making them extremely difficult to control [14]. The microsclerotia enable prolonged survival and overwintering of M. phaseolina and are usually the main form of inoculum in subsequent infections [10,14]. In addition, F. oxysporum is also a notorious soil-borne pathogen that penetrates strawberry plants through the root system and causes root rot and vascular discoloration of the crown tissues [11,15]. It causes rapid wilting of strawberry plants and reduced yields. It can lead to devastating losses, especially when exacerbated by heat and water stress [8].
To reduce losses caused by phytopathogenic fungal diseases in strawberry production, farmers used to rely on methyl bromide (MB), a soil fumigant [7,10,11]. However, MB soil fumigants are currently restricted in many countries due to their destructive nature to the ozone layer [7,16,17]. Several alternatives to MB, such as iodomethane, methyl iodide, chloropicrin, 1,3-dichloropropene, 1,3-D-methyl isothiocyanate, and metam-sodium/potassium are being used in various jurisdictions [18,19,20]. Soil fumigants such as chloropicrin, and chloropicrin in combination with iodomethane, have demonstrated the ability to protect strawberries from pests and pathogens and can increase yield by 100% [18]. Even so, exposure to soil fumigants, mainly by inhalation, causes toxicity to humans and animals, such as the disruption of fetal growth [16,17,20]. Moreover, excessive application of chemical fungicides causes constant exposure of the phytopathogens to sub-lethal levels of the fungicide, which could potentially lead to the development of fungicide resistance [21]. In addition, quality assurance standards regarding consumer safety from the risk of chronic illness due to the persistence of chemicals in the food, and the environmental protection concerns regarding the effects of chemical fungicides on non-target organisms, are increasingly driving consumers’ preferences, especially in developed countries [22,23,24]. Certainly, future trends in consumers’ preferences will increasingly favor organic over conventionally produced foods, and the premium prices for organic products will gradually cause a shift in food production methods.
Biological control alternatives such as the use of plant growth-promoting bacteria (PGPB) with antagonistic activity against phytopathogenic fungi have been widely reported [25,26,27,28]. Among the PGPB, Bacillus spp. are among the most important biocontrol agents that have been widely exploited in the control of plant fungal pathogens [27,28,29,30]. PGPB such as Bacillus velezensis control plant diseases by direct competition and inhibition of phytopathogens using antibiotic compounds such as cyclic tetrapeptides, bacteriocins such as colicin-like bacteriocins, and hydrolytic enzymes such as chitinases and β-1,3-glucanases [26,27,28,29,31]. However, a previous study indicated that antifungal compounds obtained from B. velezensis (cyclic tetrapeptides) were produced in very low concentrations that cannot effectively control fungal pathogens under field conditions [26]. The fungal and oomycete cell walls which are mainly composed of chitin and β-glucan polymers play a vital role in their survival, multiplication, and host infection [32,33]. Thus, the cell wall could be a suitable target site for the biomolecules used in the control of phytopathogenic fungi and oomycetes [34,35,36]. Thus, cell wall-degrading enzymes produced by PGPB, such as chitinases and β-1,3-glucanases, are valuable alternatives for the biocontrol of plant diseases caused by phytopathogenic fungi and oomycetes [27,29,36].
In addition, PGPB can also enhance plant growth through the secretion of plant growth hormones such as indole acetic acid (IAA) auxin, which enhances root hair growth and lateral root development in plants [27,29,37,38,39]. The improved root architecture increases the rate of nutrient uptake from the soil, which leads to enhanced plant growth and fruit yield [39]. The use of PGPB such as B. velezensis CE 100 has also been reported to enhance the growth of forest seedlings mainly through the production of IAA, and increasing nitrogen and phosphorus availability in the soil through ammonium production and phosphate solubilization activity, respectively [27,29,40]. Previous studies have highlighted the multiple functional properties of Bacillus spp., including plant disease management and growth promotion [27,28,29,30]. Bacillus spp. have previously demonstrated biocontrol potential against various phytopathogens that cause diseases of economic importance in strawberries [6,9,41]. These include M. phaseolina which causes charcoal rot, F. oxysporum f. sp. fragariae that cause fusarium wilt, and Botrytis cinerea, which causes grey mold in strawberries [6,9,41,42]. The biocontrol and bio-stimulation potential of Bacillus spp. are attributed to their functional properties, such as the production of antibiotics, lytic enzymes, IAA, ammonia-N, and phosphate solubilization [29,41,42].
However, the potential for simultaneous biocontrol and bio-stimulation potential of PGPB is still a subject of research. Due to the increase in demand for safe, chemical-free food production systems, new research innovations about the use of PGPB to control plant disease and to enhance crop yield are important. Therefore, this study aimed to examine the potential use of B. velezensis CE 100 as a biocontrol agent against fungal pathogens of the strawberry, M. phaseolina, and F. oxysporum f. sp. fragariae, while simultaneously enhancing plant growth and fruit yields.
2. Materials and Methods
2.1. Growth of Bacillus velezensis CE 100
The bacterial strain B. velezensis CE 100 used in this study was obtained from Purne Inc. (Jangseong, Korea). Bacillus velezensis CE 100 was originally isolated from tomato cultivated soil in Korea as previously described [43]. To examine the growth pattern of B. velezensis CE 100, the bacterial strain was obtained from Purne Inc. (Purne Inc., Jangseong, Korea) and inoculated in 2 g/L of pink broth (PB) medium (Purne Inc., Jangseong, Korea) [29]. The medium was supplemented with 3 g/L sucrose (Fisher Scientific, Fair Lawn, NJ, USA) and 3 g/L chemical fertilizer (N:P:K = 20:20:20, Integrated Agribusiness Professionals, Fresno, CA, USA), and incubated at 50 °C in a shaking incubator at 130 rpm for 10 days. During the incubation period, 1 mL of the bacterial culture was sampled daily to enumerate the colony-forming unit (CFUs) using the standard plate count method on nutrient agar (NA) media. In addition, 1 mL of the bacterial culture was sampled daily and used for the analysis of lytic enzyme activity produced by B. velezensis CE 100. The experiment was conducted with three replications.
2.2. Production of Chitinase and β-1,3-Glucanase by Bacillus velezensis CE 100
For the chitinase and β-1,3-glucanase assays, 1 mL bacterial cultures sampled daily during the incubation period (as indicated above) were centrifuged at 10,000 rpm for 15 min. The supernatants were used for the analysis of the lytic enzyme activity of chitinase and β-1,3-glucanase.
Chitinase activity was determined by estimating the amount of reducing sugars produced per hour, from the hydrolysis of chitin by the enzymatic solution using a modified method described by Moon et al. [44]. Briefly, 50 µL of the enzymatic solution (bacterial supernatant) was mixed with a 500 µL substrate of 0.5% colloidal chitin. The mixture was then mixed with 450 µL of 50 mM sodium acetate buffer (pH 5.0). The contents were incubated at 37 °C for 1 h using Eppendorf tubes submerged in a water bath (Temperature-Controlled Bater Bath 1660504EDU, Bio-Rad Laboratories, inc., Inc., Hercules, CA, USA). The reaction was terminated by adding 200 µL of 1 N NaOH followed by centrifugation at 10,000 rpm for 10 min at 4 °C. The concentration of reducing sugars was estimated calorimetrically based on the loss of color after reacting 750 µL of the solution with 1 mL of Schales’ reagent (0.5 M sodium carbonate and 0.5 g/L potassium ferricyanide in water). The solution was diluted with250 µL of deionized distilled water and boiled at 100 °C for 15 min. Then the optic density was measured at 420 nm (OD420) using a UV spectrophotometer (UV-1650PC, Shimadzu, Kyoto, Japan). One unit of chitinase activity was defined as the reducing activity that released 1 µmol of N-acetyl-glucosamine per hour at 37 °C, which is equivalent to a decline of approximately 0.1287 OD420.
The activity of the β-1,3-glucanase enzyme was determined by estimating the amount of glucose produced per hour from the hydrolysis of laminarin using a modified method described by Choub et al. [29]. The reaction mixture contained 50 μL of enzyme solution (bacterial supernatant), 50 μL of 1% laminarin solution, and 400 μL of 50 mM sodium acetate buffer (pH 5.0). The solution was incubated at 37 °C for 1 h and the reaction was terminated with 1.5 mL of 3,5-dinitrosalicylic acid (DNS) reagent and boiled in a water bath for 5 min. The increase in optical density at 550 nm was then measured using a UV spectrophotometer (UV-1650PC, Shimadzu, Kyoto, Japan) to determine the concentration of reducing sugars produced. One unit of β-1,3-glucanase activity was defined as the amount of enzyme that catalyzed the release of 1 µmol of glucose per hour at 37 °C, which is equivalent to an increase of approximately 0.7354 OD550.
2.3. Antagonistic Activity of Bacillus velezensis CE 100 against Fungal Pathogens
The phytopathogenic fungi M. phaseolina isolate GL1310 and F. oxysporum f. sp. fragariae isolate GL1080 [45] were provided by T.R. Gordon Laboratory from the Department of Plant Pathology, University of California, Davis. The phytopathogenic fungi were sub-cultured at 25 °C for seven days using potato dextrose agar (PDA), obtained from Difco Laboratories Inc. (Detroit, MI, USA). The antagonistic activity of B. velezensis CE 100 against M. phaseolina and F. oxysporum f. sp. fragariae was examined on PDA plates, using the dual culture method. Briefly, a 6-mm plug of each phytopathogenic fungus from the 7-day-old culture was placed on the left-hand side of the PDA plate using a sterile cork-borer. Then a loop of two-day bacterial culture was streaked at a distance of 4 cm from the phytopathogenic fungal plug, on the right-hand side of the same plate. The control group was prepared by inoculating the phytopathogenic fungi on the PDA plates without B. velezensis CE 100. Nine replicates of each group were incubated at 25 °C for 7 days for M. phaseolina and 11 days for F. oxysporum f. sp. fragariae. The inhibition of fungal growth by B. velezensis CE 100 was determined using the following formula: inhibition (%) = ((X − Y)/X) × 100, where X represents the radial growth of the phytopathogenic fungi in the control group and Y is the radial growth of the phytopathogenic fungi in the dual culture group [46]. Then small pieces of the phytopathogenic fungal mycelia were gently picked from the fungal growth border in both dual culture and control groups to examine the morphological alterations of the hyphae. The fungal specimens were wet-mounted on glass slides, covered with glass coverslips, and the morphological deformations of M. phaseolina and F. oxysporum f. sp. fragariae were examined at a magnification of 200× using a light microscope (BX41, Olympus, Tokyo, Japan). The mycelial specimens obtained from the dual culture plates were picked from the fungal growth border next to the bacterial streak. All experiments for morphological observation of the mycelia were performed in triplicate.
2.4. Quantitative Analysis of Indole-3-Acetic Acid (IAA) Produced by Bacillus velezensis CE 100
The concentration of IAA produced by B. velezensis CE 100 was determined calorimetrically based on its oxidation reaction by peroxidase. The amount of IAA oxidized was estimated from the intensity of pink coloration develop by reacting the samples with Salkowski’s reagent, using a modified method described by Choub et al. [29]. The bacterial culture was prepared in a sterilized PB medium amended with 0.6 g/L of yeast extract and 0.1 g/L of L-tryptophan and incubated for 10 days in an H1012 Incu-Shaker set at 30 °C and 140 rpm. For the control, no bacterial inoculation was done. During the incubation, 2 mL of broth cultures were collected daily and centrifuged at 10,000 rpm for 20 min. Then 1 mL of each supernatant was transferred into a 5 mL test tube and mixed with 2 mL of Salkowski’s reagent followed by two drops of phosphoric acid. Salkowski’s reagent was prepared on the same day (about 15 min before use) by adding 1 mL of 0.5 M FeCl3 (freshly prepared in deionized water) into 50 mL of 35% perchloride acid at room temperature. The solutions were then incubated at room temperature, under dark conditions for 25 min, and the absorbance was immediately measured at 530 nm using a UV spectrophotometer (UV-1650PC, Shimadzu, Kyoto, Japan). IAA production was calculated based on the increase in the intensity of the pink coloration using an equation obtained from the calibration curve for indole-3-acetic acid standard (Sigma-Aldrich®, Darmstadt, Germany).
2.5. Growth of Strawberry Seedlings under Greenhouse Experimental Conditions
Albion and Sequoia strawberry seedlings were obtained from Lemuria nursery located in Dixon, CA, USA, in April 2020 and used for the greenhouse experiment. To determine their susceptibility to phytopathogenic fungi, a seedling from each cultivar was pre-infected with either M. phaseolina or F. oxysporum f. sp. fragariae. The disease symptoms were more severe in Albion cultivars infected with M. phaseolina and Sequoia cultivars infected with F. oxysporum f. sp. fragariae. On the other hand, both Albion cultivars infected with F. oxysporum f. sp. fragariae and Sequoia cultivars infected with M. phaseolina only exhibited mild symptoms. Consequently, Albion cultivars were used for M. phaseolina infection, and Sequoia cultivars were used for F. oxysporum f. sp. fragariae infection; both experiments were conducted concurrently. Each strawberry plant was transferred into a 1 L pot filled with premixed soil, and the experiment was performed in a greenhouse located at the University of California (UC), Davis, CA, USA. The greenhouse temperature was maintained in the range of 20 to 25 °C. Plants were grown under natural light and the water was supplied daily by overhead irrigation.
For each experimental group (M. phaseolina or F. oxysporum f. sp. fragariae infection), three treatments were applied: (i) non-inoculated control (without phytopathogenic fungi or bacteria), (ii) inoculation with only M. phaseolina or F. oxysporum f. sp. fragariae, and (iii) co-inoculation of B. velezensis CE 100 and either M. phaseolina or F. oxysporum f. sp. fragariae. The seedlings were transplanted at the end of April 2020. All plants were maintained under similar greenhouse experimental conditions for the first two weeks before treatment application.
The phytopathogenic fungi M. phaseolina and F. oxysporum f. sp. fragariae were separately cultured on PDA plates amended with 0.01% tetracycline hydrochloride (Fisher Scientific, Fair Lawn, NJ, USA) (PDA-t). Both M. phaseolina and F. oxysporum f. sp. fragariae plates were incubated at 25 °C for five and seven days, respectively. For phytopathogenic fungal infection, two separate inoculations were done in the rhizosphere of each seedling on 4 May and 11 May 2020, respectively. For the first inoculation, two 6-mm plugs of M. phaseolina or F. oxysporum f. sp. fragariae were buried 5 cm under the soil adjacent to the seedlings in the respective treatment groups. When the color of the leaves did not change, then four 6-mm plugs of the phytopathogenic fungi were re-inoculated after a week. For the non-inoculated control group, two 6-mm plugs followed by four 6-mm plugs of PDA-t without phytopathogenic fungi were buried 5 cm in the rhizosphere of the seedlings on 4 and 11 May 2020, respectively.
The bacterial strain was inoculated in 2 g/L of pink broth (PB) medium (Purne Inc., Jangseong, Korea). The media was supplemented with 3 g/L sucrose (Fisher Scientific, Fair Lawn, NJ, USA), 3 g/L chemical fertilizer (N:P:K = 20:20:20, Integrated Agribusiness Professionals, Fresno, CA, USA), and incubated at 50 °C for 5 days. Then, the bacterial cultures were diluted with tap water (1:1 v/v) and 200 mL of the diluted cultures were poured onto the rhizosphere of each in the co-inoculation treatment group. For the non-inoculated control group and the treatment group inoculated with only M. phaseolina or F. oxysporum f. sp. fragariae, an equal volume (200 mL) of tap water was applied at the same rate with the co-inoculation treatment group. The treatments were applied at a 7-day interval, 8 times from May to June 2020. The experiment was conducted using a completely randomized block design with 3 replications of 5 plants each and a total of 90 seedlings were used.
2.6. Measurement of Strawberry Growth and Fruit Yield
To determine the growth characteristics, all seedlings in each treatment group were collected at the end of June 2020 and cleaned of all the media and debris. The seedlings were separated into shoots and roots and oven-dried at 80 °C for 72 h. Then, the biomass of shoots and roots was measured and recorded.
Fruits were harvested from each pot through May and June 2020, when fruits were fresh and ripe, to determine the fruit yield. The total weight of fruits per plant was recorded and summed up for the study period.
2.7. Statistical Analysis
Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences), version 25 (Armonk, NY, USA). Plant biomass and fruit yield data were subjected to an analysis of variance (ANOVA). The mean values were compared using Fisher’s least significant difference (LSD) test at p = 0.01.
3. Results
3.1. Inhibitory Effect of Bacillus velezensis CE 100 against Strawberry Fungal Pathogens
3.1.1. Cell Growth of Bacillus velezensis CE 100
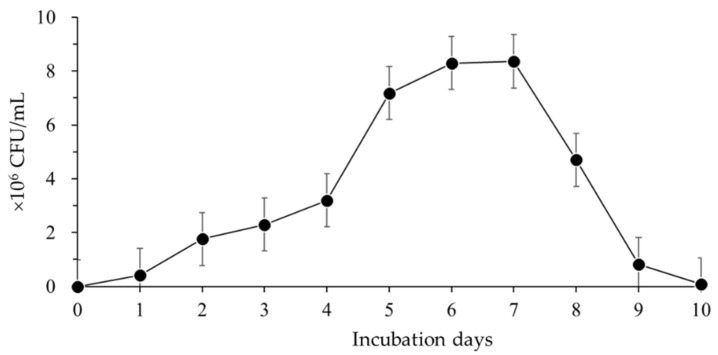
Bacillus velezensis CE 100 showed a gradual increase in cell growth until four days after incubation (Figure 1). The rate of cell growth then rapidly increased from 3.2 × 106 to 7.18 × 106 CFU/mL between 4 and 5 days of incubation. Thereafter, the growth of B. velezensis CE 100 increased until 7 days after inoculation, when the growth rate reached a maximum value of 8.4 × 106 CFU/mL. The growth of B. velezensis CE 100 sharply declined from 8 days post-inoculation until the end of the experimental period, as the bacteria depleted the nutrients supplied in the media, and cell growth was negligible from 9 days to 10 days after inoculation (Figure 1).
Figure 1.
Colony-forming units (CFUs) of Bacillus velezensis CE 100 during the incubation period. Error bars represent standard deviation (n = 3).
3.1.2. Chitinase and β-1,3-Glucanase Activity of Bacillus velezensis CE 100
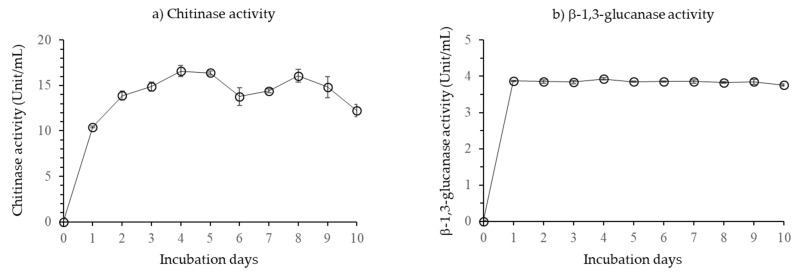
When cultured in the liquid media supplemented with sucrose as an energy source and other essential nutrients, B. velezensis CE100 produced strong hydrolytic enzyme activity during growth. Specifically, the chitinase activity produced by B. velezensis CE100 rapidly increased in the first day after inoculation to 10.5 unit/mL, and then attained the maximum value of 16.6 unit/mL after 5 days (Figure 2a). Chitinase activity then slightly fluctuated between 5 to 8 days after inoculation, before gradually decreasing towards the end of the experiment, which is consistent with the decline in cell growth (Figure 2a).
Figure 2.
Changes in the activity of chitinase (a) and β-1,3-glucanase (b) secreted by Bacillus velezensis CE 100 during the incubation period. Error bars represent the standard deviation (n = 3).
For β-1,3-glucanase enzyme activity, B. velezensis CE 100 exhibited a rapid increase from zero at the start of the experiment to 3.93 unit/mL, after 1 day after incubation (Figure 2b). Then B. velezensis CE 100 maintained relatively constant production of β-1,3-glucanase enzyme from the first day after inoculation throughout the experiment, regardless of the changes in cell growth (Figure 2b).
3.1.3. Antagonistic Activity of Bacillus velezensis CE 100 against Fungal Pathogens
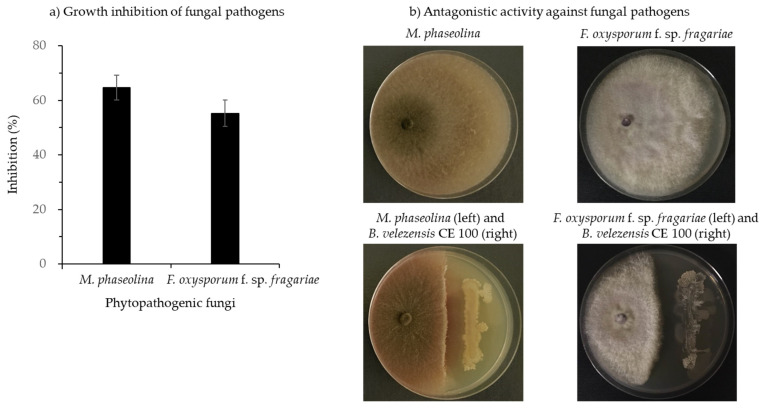
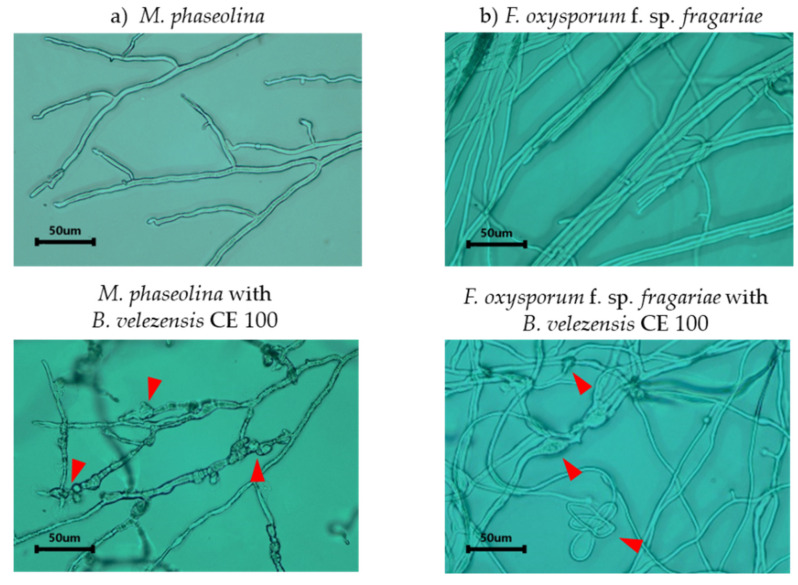
B. velezensis CE 100 caused 64.7% and 55.2% growth inhibition against M. phaseolina and F. oxysporum f. sp. fragariae, respectively (Figure 3). When observed on a light microscope, the hyphal morphologies of both phytopathogenic fungi, M. phaseolina, and F. oxysporum f. sp. fragariae in dual culture with B. velezensis CE 100 showed striking differences compared to the control group, grown as a pure culture without bacterial antagonism (Figure 4). The treatment groups for both fungal pathogens showed mycelial abnormalities, such as degradation, deformation, and cell wall lysis of the hyphae compared to normal hyphae structure in the control group (Figure 4).
Figure 3.
Inhibition of the mycelial growth Macrophomina phaseolina and Fusarium oxysporum f. sp. fragariae by Bacillus velezensis CE 100 (a) and antagonistic activity of B. velezensis CE 100 against M. phaseolina and F. oxysporum f. sp. fragariae (b), using the dual culture method on PDA medium. Error bars represent the standard deviation (n = 3).
Figure 4.
Inhibition effect of Bacillus velezensis CE 100 on phytopathogenic hyphal morphologies of Macrophomina phaseolina (a) and Fusarium oxysporum f. sp. fragariae (b), observed via light microscopy. On the top, the normal morphology in the control groups, and at the bottom, the corresponding effect of B. velezensis CE 100. Arrows indicate hyphal alterations with swelling and deformation of structures caused by B. velezensis CE 100.
In the greenhouse experiment, co-inoculation of B. velezensis CE 100 with M. phaseolina or F. oxysporum f. sp. fragariae suppressed the disease symptoms and improved the growth vigor in both Albion and Sequoia strawberry cultivars. However, strawberry plants in the non-inoculated control (without phytopathogenic fungi or bacteria) and plants inoculated with only M. phaseolina or F. oxysporum f. sp. fragariae exhibited diseases symptoms such as reddish-brown necrosis in older leaves and leaf chlorosis (turning from green to pale-green). These disease symptoms were consistent with crown-rot pathogen infections and fusarium wilt disease (Figure 5a,b). Except for the treatment groups co-inoculated with bacterial and fungal pathogens, all others plants exhibited disease symptoms such as stunted growth, leaf chlorosis, and premature leaf death. The older leaves initially turned reddish-brown along the margins followed by wilting. Thus, the spread of M. phaseolina and F. oxysporum f. sp. fragariae occurred throughout all the treatment groups including the non-inoculated control groups, mainly due to water splashes during irrigation. Moreover, the phytopathogenic fungi M. phaseolina or F. oxysporum f. sp. fragariae were successfully recovered (re-isolated) from strawberry plants in both the non-inoculated control groups and the groups inoculated with only phytopathogenic fungi. However, the recovery of both phytopathogens was not successful in the treatment groups co-inoculation of B. velezensis CE 100 (Figure 5c,d).
Figure 5.
Effects of different treatments on growth and fruit production in Albion strawberries infected with Macrophomina phaseolina (a) and Sequoia strawberries inoculated with only Fusarium oxysporum f. sp. fragariae (b). Isolation of M. phaseolina (c) and F. oxysporum f. sp. fragariae (d) from infected crowns of the Albion and Sequoia strawberry plants, respectively.
3.2. Production of Indole-3-Acetic Acid (IAA) by Bacillus velezensis CE 100
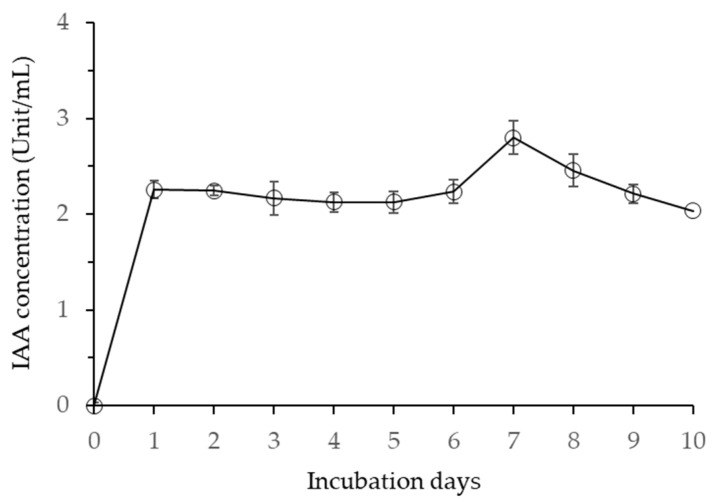
The concentration of IAA secreted by B. velezensis CE 100 increased rapidly from 0.0 to 2.3 units/mL on the first day of incubation (Figure 6). The concentration of IAA in the media remained relatively stable for six days before showing a sharp increase to reach the maximum value of 2.8 units/mL after 7 days of incubation.
Figure 6.
Indole-3-acetic acid (IAA) production by Bacillus velezensis CE 100 at 30 °C for 10 days. Error bars represent the standard deviation (n = 3).
The concentration of IAA then started to decline from 2.8 unit/mL on day 7 to the lowest value of 2.0 unit/mL after 10 days of incubation (Figure 6). The highest IAA concentration in the broth culture of B. velezensis CE 100 at 7 days after inoculation also corresponded to the time of maximum cell growth, as observed in Figure 1. Similarly, the decline in IAA concentration from 7 days to 10 days after inoculation was also consistent with the pattern of decline in the viable cells of B. velezensis CE 100 (Figure 1).
3.3. Effect of Bacillus velezensis CE 100 on Fruit Yield
For the non-inoculated control plants and plants infected with either of the phytopathogenic fungi without bacterial treatment, the total plant dry mass was significantly lower compared to the group inoculated with B. velezensis CE 100 (Table 1). Moreover, the non-inoculated control plants and plants infected with either of the phytopathogenic fungi had significantly lower fruit yields compared to those receiving bacterial treatment (Table 1).
Table 1.
Effects of different treatments on plant biomass and fruit yield in strawberry plants after inoculation with Macrophomina phaseolina or Fusarium oxysporum f. sp. fragariae.
| Treatments | Plant Biomass (g/Plant) | Fruit Yield (g/Plant) |
||
|---|---|---|---|---|
| Shoot | Root | Total | ||
| Control | 1.1 ± 0.2 b | 1.2 ± 0.5 b | 2.4 ± 0.6 b | 1.6 ± 1.9 b |
| M. phaseolina | 1.4 ± 0.5 b | 1.5 ± 0.6 b | 2.9 ± 1.1 b | 2.2 ± 4.8 b |
| M. phaseolina with B. velezensis CE 100 | 8.7 ± 2.7 a | 2.1 ± 0.5 a | 10.8 ± 3.2 a | 32.3 ± 13.5 a |
| Control | 1.0 ± 0.3 b | 1.1 ± 0.4 b | 2.1 ± 0.6 b | 1.6 ± 2.0 b |
| F. oxysporum f. sp. fragariae | 1.1 ± 0.4 b | 1.4 ± 0.5 b | 2.5 ± 0.6 b | 5.2 ± 3.2 b |
| F. oxysporum f. sp. fragariae with B. velezensis CE 100 | 10.2 ± 3.9 a | 1.9 ± 0.4 a | 12.2 ± 4.0 a | 25.3 ± 7.7 a |
Means with different superscripts a and b in the same column indicate significantly different values, at p = 0.01 when compared using the LSD test.
Co-inoculation with B. velezensis CE 100 with M. phaseolina or F. oxysporum f. sp. fragariae in strawberry plants resulted in a significant increase in plant dry mass compared to both the non-inoculated control groups and the groups inoculated with only phytopathogenic fungi without bacterial treatment (Table 1). Specifically, co-inoculation with B. velezensis CE 100 in strawberry plants infected with M. phaseolina or F. oxysporum f. sp. fragariae resulted in an increase in shoot dry mass by 6.2-fold or 9.2-fold, respectively. In addition, co-inoculation with B. velezensis CE 100 in strawberry plants infected with M. phaseolina or F. oxysporum f. sp. fragariae also increased shoot dry mass by 7.9-fold or 10.2-fold compared to the non-inoculated control groups.
Moreover, co-inoculation with B. velezensis CE 100 significantly increased the fruit yield compared to non-inoculated control plants and plants only inoculated with fungal pathogens (Table 1). The co-inoculation with B. velezensis CE 100 in the group infected with M. phaseolina or F. oxysporum f. sp. fragariae increased strawberry productivity compared to only fungal inoculation by 10.6-fold or 4.9-fold, respectively. Co-inoculation with B. velezensis CE 100 in plants infected with M. phaseolina or F. oxysporum f. sp. fragariae also improved strawberry yield compared to the non-inoculated control group, by 14.7-fold or 15.8-fold respectively.
4. Discussion
4.1. Antagonism of Macrophomina phaseolina and Fusarium oxysporum by Cell Wall-Degrading Enzymes Produced by Bacillus velezensis CE 100
Bacillus spp. are a versatile group of PGPB that have exhibited strong biocontrol activity against various fungal, bacterial, and viral plant pathogens [41,42,47,48,49]. Specifically, Bacillus spp. have been reported to control various plant fungal pathogens of economic importance in strawberry production, such as Botrytis cinerea, F. oxysporum f. sp. fragariae, and M. phaseolina [6,9,41]. Bacillus spp. control plant phytopathogenic fungi through various mechanisms, including the production of secondary metabolites such as antibiotics and hydrolytic enzymes that degrade the fungal cell wall [26,27,29,50,51,52]. Specifically, B. velezensis CE 100 produces antifungal biomolecules such as cyclic tetrapeptides which inhibit phytopathogenic fungal growth and sporulation [26]. However, the antifungal cyclic tetrapeptides from B. velezensis CE 100 are produced at very low concentrations that do not effectively cause inhibitory activity under field conditions. For instance, cyclic tetrapeptide only caused 18.8% mycelial growth inhibition of Colletotrichum gloeosporioides at 1000 µg/mL, and no inhibition was observed at 500 µg/mL [26]. In addition, the concentration of cyclic tetrapeptide from B. velezensis CE 100 culture broth was determined to be only 330 µg/mL, which is too low to cause an antifungal effect. Therefore, the biocontrol activity of B. velezensis CE 100 against plant phytopathogens under field conditions was demonstrated to be the effect of cell wall-degrading enzymes [27,29,50]. For instance, B. velezensis secretes lytic enzymes such chitinase and β-1,3-glucanase which degrade the chitin and β-glucan polymers, which are major structural components of the cell wall matrix in phytopathogenic fungi/oomycetes, leading to hyphal deformation and mycelial growth inhibition [29,32,50,52]. Some biocontrol agents have been reported to antagonize the growth of M. phaseolina and F. oxysporum f. sp. fragariae, and thus could potentially reduce the incidence of crown rot and fusarium wilt infections in strawberries [6,9]. In the present study, B. velezensis CE 100 produced high levels of chitinase and β-1,3-glucanase (Figure 2). These lytic enzymes could be involved in the antagonistic activity of B. velezensis CE 100 against soil-borne pathogens, such as M. phaseolina and F. oxysporum f. sp. fragariae (Figure 3). From the results of the dual culture assays of B. velezensis CE 100 with M. phaseolina and F. oxysporum f. sp. fragariae, the mycelia of both fungi showed severe abnormalities, such as swelling, deformation, and degradation of the hyphae, which indicates the disintegration of the cell wall matrix (Figure 4). In the greenhouse experiment, co-inoculation of B. velezensis CE 100 culture with M. phaseolina or F. oxysporum f. sp. fragariae suppressed the disease symptoms and enhanced the growth vigor of strawberry plants (Figure 5a,b). However, strawberry plants in both the non-inoculated control groups and the groups inoculated with only the phytopathogenic fungi without bacterial inoculation exhibited severe symptoms which were consistent with charcoal rot and fusarium wilt diseases. The symptoms included reddish-brown discoloration along the margins of old leaves followed by wilting and pre-mature leaf death. The infected plants also exhibited severe chlorosis with leaves turning from green to pale green, stunted growth, and reduced yield (Figure 5a,b). The occurrence of disease symptoms in the non-inoculated control groups indicated the spreading of phytopathogens from the treatment groups due to water splashes during irrigation [53]. Thus, the effects of M. phaseolina and F. oxysporum f. sp. fragariae infection, such as leaf discoloration, stunted growth, and yield losses, were relatively similar in both treatment groups (groups inoculated with only the phytopathogenic fungi, and the non-inoculated control groups). Leaf discoloration could be a result of impeded nutrient and mineral transport through the vascular system due to the damage caused by the phytopathogenic fungal infection [12,13]. Co-inoculation of B. velezensis CE 100 prevented infection by both M. phaseolina and F. oxysporum f. sp. fragariae in strawberry plants. This was mainly due to the role of cell wall-degrading enzymes such as chitinase and β-1,3-glucanase which antagonize the fungal cell wall activities that are vital for survival, growth, and pathogenicity of fungal pathogens [32,50,51,52].
4.2. Effects of Bacillus velezensis CE 100 on Strawberry Growth and Fruit Production
Co-inoculation with B. velezensis CE 100 culture not only protected the plants infected with M. phaseolina or F. oxysporum f. sp. fragariae from severe disease symptoms but also enhanced the growth and fruit yield of strawberries. The symptoms of charcoal rot and fusarium wilt disease include chlorosis, reddish-brown necrosis on leaf margins, wilting, and premature loss of leaves. These fungi attack the root cortical and vascular tissues, thereby impeding water and nutrient transport in strawberries [6,7,11]. The reduced transport of water and mineral nutrients in the plant tissues reduces the rate of photosynthesis [6,7,8]. Such symptoms could substantially lower biomass production in strawberry plants. Thus, infected plants can exhibit severe chlorosis, wilting of leaves, stunted growth, and drastic losses in yield by more than 50%, especially under conditions of water stress [7,11,12,15]. In this study, the symptoms of charcoal rot and fusarium wilt disease were prevalent in both the non-inoculated control groups and the groups inoculated with only phytopathogenic fungi without bacterial treatment (Figure 5a,b). Consequently, the non-inoculated control groups and the groups only inoculated with the phytopathogenic fungi exhibited stunted growth, low biomass, and low fruit yield due to infections (Table 1). On the other hand, co-inoculation with B. velezensis CE 100 suppressed the disease symptoms, which could have consequently improved the photosynthetic rate and nutrient transport through the vascular tissues, resulting in a higher growth rate and fruit yield (Table 1).
Moreover, PGPB can also produce indole-3-acetic acid (IAA), which promotes the development of root hairs, lateral roots, and adventitious roots in plants [39,40,54]. In this study, B. velezensis CE 100 produced IAA auxin during its growth (Figure 6). The IAA produced by B. velezensis CE 100 enhanced the growth and development of root hairs and lateral roots, which led to an increase in root biomass (Table 1). The enhanced root development increases the root surface area, which improves the absorption of nutrients from the soil and consequently enhances plant growth and productivity [27,29,50]. Moreover, B. velezensis CE 100 has been reported to produce ammonium during its growth phase [29]. Ammonium is a precursor for nitrate in the soil, and thus its release into the rhizosphere increases nitrogen availability for plant uptake [29,50]. This consequently improves the photosynthetic rate and enhances plant growth and productivity [29,40,50]. In addition, B. velezensis CE 100 has been reported to exhibit the potential for phosphate solubilization [29]. The solubilization of insoluble phosphate increases the availability of phosphorus in the soil and thus enhances the root architecture and nutrient absorption by plants [29,50]. The present study showed significant increases in strawberry growth and fruit production in the treatment groups co-inoculated with B. velezensis CE 100 (Table 1).
M.phaseolina and F. oxysporum f. sp. fragariae infections can cause devastating losses in strawberry production [6,7,11]. Indeed, the non-inoculated control and the treatment group inoculated with only phytopathogenic fungi exhibited extremely low biomass and fruit yield due to phytopathogenic fungal infections (Table 1). On the other hand, co-inoculation of phytopathogenic fungi with B. velezensis CE 100 suppressed the fungal disease symptoms through the lytic enzyme activity of chitinase and β-1,3-glucanase. Moreover, B. velezensis CE 100 exhibits several plant growth-promoting traits, such as IAA and ammonium production and phosphate solubilization. Therefore, B. velezensis CE 100 is not only an effective biocontrol agent against strawberry diseases caused by M. phaseolina and F. oxysporum f. sp. fragariae but also releases plant growth factors that enhance the growth and fruit yield of strawberries.
5. Conclusions
The findings of this study demonstrate the antagonistic effect of B. velezensis CE 100 against plant phytopathogenic fungi and its potential to enhance strawberry production. B. velezensis CE 100 produced chitinase and β-1,3-glucanase enzymes, which degraded the fungal cell walls and effectively inhibited mycelial growth of M. phaseolina and F. oxysporum f. sp. fragariae fungi, the causal agents of charcoal rot and fusarium wilt diseases in strawberry, respectively. Co-inoculation of B. velezensis CE 100 with the phytopathogenic fungi effectively suppressed the symptoms associated with charcoal rot and fusarium wilt diseases in strawberries. In addition, B. velezensis CE 100 produced IAA, which enhances root hair growth and lateral root development. The production of IAA not only improves plant nutrient uptake, but also facilitates cell division and differentiation. Our results confirmed that inoculation of B. velezensis CE 100 leads to increased plant growth, biomass production, and fruit yield in strawberries. Therefore, this study demonstrates the potential of B. velezensis CE 100 as a biocontrol alternative to chemical fungicides in the management of fungal diseases caused by M. phaseolina and F. oxysporum f. sp. fragariae, and a bio-stimulant to enhance strawberry growth and fruit yield.
Acknowledgments
The Department of Plant Pathology, University of California, Davis, CA, USA provided the facility for this experiment.
Author Contributions
Conceptualization, funding acquisition, and project administration, Y.S.A.; investigation, S.H., T.Y.K., S.-J.W., J.-H.M. and K.Y.K.; data analysis, S.H., T.Y.K., S.-J.W., J.-H.M. and H.B.A.; original draft preparation, S.H., H.B.A., K.Y.K. and Y.S.A.; review and editing, H.B.A. and Y.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, funded by the Ministry of Education, Science and Technology (number 2018R1D1AB07050052). Additionally, this research was supported by a grant (number 2021R1I1A305423811) from the National Research Foundation (NRF) of Korea under the Basic Science Research Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data relevant to this manuscript is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The founder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish results of the study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Simpson D. The Economic Importance of Strawberry Crops. In: Hytönen T., Graham J., Harrison R., editors. The Genomes of Rosaceous Berries and Their Wild Relatives. Compendium of Plant Genomes. Volume 43. Springer Nature Switzerland AG; Cham, Switzerland: 2018. pp. 1–7. [DOI] [Google Scholar]
- 2.Afrin S., Gasparrini M., Forbes-Hernandez T.Y., Reboredo-Rodriguez P., Mezzetti B., Varela-López A., Giampieri F., Battino M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016;64:4435–4449. doi: 10.1021/acs.jafc.6b00857. [DOI] [PubMed] [Google Scholar]
- 3.FAOSTAT Food and Agriculture Data. [(accessed on 7 October 2021)]. Available online: http://www.fao.org/faostat/en/#data/QC/visualize.
- 4.Lee W.S. Production of ever-bearing strawberry and production technology in Korea. Acta Hortic. 2014;1049:561–564. doi: 10.17660/ActaHortic.2014.1049.87. [DOI] [Google Scholar]
- 5.Ruan J., Lee Y.H., Yeoung Y.R. Flowering and fruiting of day-neutral and ever-bearing strawberry cultivars in high-elevation for summer and autumn fruit production in Korea. Hortic. Environ. Biotechnol. 2013;54:109–120. doi: 10.1007/s13580-013-0185-9. [DOI] [Google Scholar]
- 6.Pastrana A.M., Basallote-Ureba M.J., Aguado A., Akdi K., Capote N. Biological control of strawberry soil-borne pathogens Macrophomina phaseolina and Fusarium solani, using Trichoderma asperellum and Bacillus spp. Phytopathol. Mediterr. 2016;55:109–120. doi: 10.14601/Phytopathol_Mediterr-16363. [DOI] [Google Scholar]
- 7.Hutton D.G., Gomez A.O., Mattner S.W. Macrophomina phaseolina and its association with strawberry crown rot in Australia. Int. J. Fruit Sci. 2013;13:149–155. doi: 10.1080/15538362.2012.698143. [DOI] [Google Scholar]
- 8.Koike S.T., Gordon T.R. Management of fusarium wilt of strawberry. Crop Prot. 2015;73:67–72. doi: 10.1016/j.cropro.2015.02.003. [DOI] [Google Scholar]
- 9.Nam M.-H., Park M.-S., Kim H.-G., Yoo S.-J. Biological control of strawberry fusarium wilt caused by Fusarium oxysporum f. sp. fragariae using Bacillus velezensis BS87 and RK1 formulation. J. Microbiol. Biotechnol. 2009;19:520–524. doi: 10.4014/jmb.0805.333. [DOI] [PubMed] [Google Scholar]
- 10.Zveibil A., Mor N., Gnayem N., Freeman S. Survival, host-pathogen interaction, and management of Macrophomina phaseolina on strawberry in Israel. Plant Dis. 2012;96:265–272. doi: 10.1094/PDIS-04-11-0299. [DOI] [PubMed] [Google Scholar]
- 11.Koike S.T., Gordon T.R., Daugovish O., Ajwa H., Bolda M., Subbarao K. Recent developments on strawberry plant collapse problems in California caused by Fusarium and Macrophomina. Int. J. Fruit Sci. 2013;13:76–83. doi: 10.1080/15538362.2012.697000. [DOI] [Google Scholar]
- 12.Kaur S., Dhillon G.S., Brar S.K., Vallad G.E., Chand R., Chauhan V.B. Emerging phytopathogen Macrophomina phaseolina: Biology, economic importance, and current diagnostic trends. Crit. Rev. Microbiol. 2012;38:136–151. doi: 10.3109/1040841X.2011.640977. [DOI] [PubMed] [Google Scholar]
- 13.Koike S.T. Crown rot of strawberry caused by Macrophomina phaseolina in California. Plant Dis. 2008;92:1253. doi: 10.1094/PDIS-92-8-1253B. [DOI] [PubMed] [Google Scholar]
- 14.Baggio J.S., Cordova L.G., Peres N.A. Sources of inoculum and survival of Macrophomina phaseolina in Florida strawberry fields. Plant Dis. 2019;103:2417–2424. doi: 10.1094/PDIS-03-19-0510-RE. [DOI] [PubMed] [Google Scholar]
- 15.Fang X., Kuo J., You M.P., Finnegan P.M., Barbetti M.J. Comparative root colonization of strawberry cultivars Camarosa and Festival by Fusarium oxysporum f. sp. fragariae. Plant Soil. 2012;358:75–89. doi: 10.1007/s11104-012-1205-8. [DOI] [Google Scholar]
- 16.Rosskopf E.N., Chellemi D.O., Kokalis-Burelle N., Church G.T. Alternatives to methyl bromide: A Florida perspective. Plant Health Prog. 2005;6:19. doi: 10.1094/PHP-2005-1027-01-RV. [DOI] [Google Scholar]
- 17.Gemmill A., Gunier R.B., Bradman A., Eskenazi B., Harley K.G. Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environ. Health Perspect. 2013;121:737–743. doi: 10.1289/ehp.1205682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajwa H.A., Klose S., Nelson S.D., Minuto A., Gullino M.L., Lamberti F., Lopez-Aranda J.M. Alternatives to methyl bromide in strawberry production in the United States of America and the Mediterranean region. Phytopathol. Mediterr. 2003;42:220–244. [Google Scholar]
- 19.Ruzo L.O. Physical, chemical and environmental properties of selected chemical alternatives for the pre-plant use of methyl bromide as a soil fumigant. Pest Manag. Sci. 2006;62:99–113. doi: 10.1002/ps.1135. [DOI] [PubMed] [Google Scholar]
- 20.Ajwa H., Ntow W.J., Qin R., Gao S. Properties of soil fumigants and their fate in the environment. In: Krieger R., editor. Hayes’ Handbook of Pesticide Toxicology. 3rd ed. Elsevier; San Diego, CA, USA: 2010. pp. 315–330. Chapter 9. [DOI] [Google Scholar]
- 21.Deising H.B., Reimann S., Pascholati S.F. Mechanisms, and significance of fungicide resistance. Braz. J. Microbiol. 2008;39:286–295. doi: 10.1590/S1517-83822008000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbey J.A., Percival D., Abbey L., Asiedu S.K., Prithiviraj B., Schilder A. Bio fungicides as an alternative to synthetic fungicide control of grey mold (Botrytis cinerea): Prospects and challenges. Biocontrol Sci. Technol. 2019;29:207–228. doi: 10.1080/09583157.2018.1548574. [DOI] [Google Scholar]
- 23.Annunziata A., Vecchio R. Organic farming and sustainability in food choices: An analysis of consumer preference in Southern Italy. Agric. Agric. Sci. Procedia. 2016;8:193–200. doi: 10.1016/j.aaspro.2016.02.093. [DOI] [Google Scholar]
- 24.Yiridoe E.K., Bonti-Ankomah S., Martin R.C. Comparison of consumer perceptions and preference toward organic versus conventionally produced foods: A review and update of the literature. Renew. Agric. Food Syst. 2005;20:193–205. doi: 10.1079/RAF2005113. [DOI] [Google Scholar]
- 25.Kwon J.-H., Won S.-J., Moon J.-H., Lee U., Park Y.-S., Maung C.E.H., Ajuna H.B., Ahn Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases the production of jujube fruit under field conditions. Horticulturae. 2021;7:49. doi: 10.3390/horticulturae7030049. [DOI] [Google Scholar]
- 26.Choub V., Maung C.E.H., Won S.-J., Moon J.-H., Kim K.Y., Han Y.S., Cho J.-Y., Ahn Y.S. Antifungal activity of cyclic tetrapeptide from Bacillus velezensis CE 100 against plant pathogen Colletotrichum gloeosporioides. Pathogens. 2021;10:209. doi: 10.3390/pathogens10020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon J.-H., Won S.-J., Maung C.E.H., Choi J.-H., Choi S.-I., Ajuna H.B., Ahn Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes the growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms. 2021;9:821. doi: 10.3390/microorganisms9040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won S.-J., Kwon J.-H., Kim D.-H., Ahn Y.S. The effect of Bacillus licheniformis MH48 on control of foliar fungal diseases and growth promotion of Camellia oleifera seedlings in the coastal reclaimed land of Korea. Pathogens. 2019;8:6. doi: 10.3390/pathogens8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choub V., Ajuna H.B., Won S.-J., Moon J.-H., Choi S.-I., Maung C.E.H., Kim C.-W., Ahn Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021;22:10438. doi: 10.3390/ijms221910438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Won S.-J., Choub V., Kwon J.-H., Kim D.-H., Ahn Y.S. The control of fusarium root rot and development of coastal pine (Pinus thunbergii Parl.) seedlings in a container nursery by use of Bacillus licheniformis MH48. Forests. 2019;10:6. doi: 10.3390/f10010006. [DOI] [Google Scholar]
- 31.Grinter R., Milner J., Walker D. Bacteriocins active against plant pathogenic bacteria. Biochem. Soc. Trans. 2012;40:1498–1502. doi: 10.1042/BST20120206. [DOI] [PubMed] [Google Scholar]
- 32.Free S.J. Fungal cell wall organization and biosynthesis. Adv. Genet. 2013;81:33–82. doi: 10.1016/B978-0-12-407677-8.00002-6. [DOI] [PubMed] [Google Scholar]
- 33.Geoghegan I., Steinberg G., Gurr S. The role of the fungal cell wall in the infection of plants. Trends Microbiol. 2017;25:957–967. doi: 10.1016/j.tim.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Cortés J.C.G., Curto M.-Á., Carvalho V.S., Pérez P., Ribas J.C. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 2019;37:107352. doi: 10.1016/j.biotechadv.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Georgopapadakou N.H. Update on antifungals targeted to the cell wall: Focus on β-1, 3-glucan synthase inhibitors. Expert Opin. Investig. Drugs. 2001;10:269–280. doi: 10.1517/13543784.10.2.269. [DOI] [PubMed] [Google Scholar]
- 36.Schönbichler A., Díaz-Moreno S.M., Srivastava V., McKee L.S. Exploring the potential for fungal antagonism and cell wall attack by Bacillus subtilis natto. Front. Microbiol. 2020;11:521. doi: 10.3389/fmicb.2020.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng Q., Jiang H., Hao J.J. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol. Control. 2016;98:18–26. doi: 10.1016/j.biocontrol.2016.03.010. [DOI] [Google Scholar]
- 38.Sturz A.V., Christie B.R., Nowak J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000;19:1–30. doi: 10.1080/07352680091139169. [DOI] [Google Scholar]
- 39.Cho H.-T., Lee R.D.W. Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front. Plant Sci. 2013;4:448. doi: 10.3389/fpls.2013.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park H.-G., Lee Y.-S., Kim K.-Y., Park Y.-S., Park K.-H., Han T.-H., Park C.-M., Ahn Y.S. Inoculation with Bacillus licheniformis MH48 promotes nutrient uptake in seedlings of the ornamental plant Camellia japonica grown in Korean reclaimed coastal lands. Hortic. Sci Technol. 2017;35:11–20. doi: 10.12972/kjhst.20170002. [DOI] [Google Scholar]
- 41.Chen X., Wang Y., Gao Y., Gao T., Zhang D. Inhibitory abilities of Bacillus isolates and their culture filtrates against the gray mold caused by Botrytis cinerea on postharvest fruit. Plant Pathol. J. 2019;35:425. doi: 10.5423/PPJ.OA.03.2019.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joly P., Calteau A., Wauquier A., Dumas R., Beuvin M., Vallenet D., Crovadore J., Cochard B., Lefort F., Berthon J.-Y. From strain characterization to field authorization: Highlights on Bacillus velezensis strain B25 beneficial properties for plants and its activities on phytopathogenic fungi. Microorganisms. 2021;9:1924. doi: 10.3390/microorganisms9091924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi T.G., Maung C.E.H., Lee D.R., Ajuna H.B., Lee Y.S., Kim K.Y. Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot nematode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol Sci. Technol. 2020;30:685–700. doi: 10.1080/09583157.2020.1765980. [DOI] [Google Scholar]
- 44.Moon J.-H., Won S.-J., Maung C.E.H., Choi J.-H., Choi S.-I., Ajuna H.B., Ahn Y.S., Jo Y.H. The Role of Lysobacter antibioticus HS124 on the control of fall webworm (Hyphantria cunea Drury) and growth promotion of Canadian poplar (Populus canadensis Moench) at Saemangeum reclaimed land in Korea. Microorganisms. 2021;9:1580. doi: 10.3390/microorganisms9081580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry P.M., Kirkpatrick S.C., Islas C.M., Pastrana A.M., Yoshisato J.A., Koike S.T., Daugovish O., Gordon T.R. The population of Fusarium oxysporum f. sp. fragariae, cause of fusarium wilt of strawberry, in California. Plant Dis. 2017;101:550–556. doi: 10.1094/PDIS-07-16-1058-RE. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y.C., Jung H., Kim K.Y., Park S.K. An effective biocontrol bioformulation against Phytophthora blight of pepper using growth mixtures of combined chitinolytic bacteria under different field conditions. Eur. J. Plant Pathol. 2008;120:373–382. doi: 10.1007/s10658-007-9227-4. [DOI] [Google Scholar]
- 47.Nakkeeran S., Saranya N., Senthilraja C., Renukadevi P., Krishnamoorthy A., El Enshasy H.A., Eldawi H., Malathi V., Salmen S.H., Ansari M. Mining the genome of Bacillus velezensis VB7 (CP047587) for MAMP genes and non-ribosomal peptide synthetase gene clusters conferring antiviral and antifungal activity. Microorganisms. 2021;9:2511. doi: 10.3390/microorganisms9122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L., Wu H., Chen L., Yu X., Borriss R., Gao X. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 2015;5:12975. doi: 10.1038/srep12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang F., Xiao J., Zhang Y., Li R., Liu L., Deng J. Biocontrol ability and action mechanism of Bacillus halotolerans against Botrytis cinerea causing grey mold in postharvest strawberry fruit. Postharvest Biol. Technol. 2021;174:111456. doi: 10.1016/j.postharvbio.2020.111456. [DOI] [Google Scholar]
- 50.Won S.-J., Moon J.-H., Ajuna H.B., Choi S.-I., Maung C.E.H., Lee S., Ahn Y.S. Biological control of leaf blight disease caused by Pestalotiopsis maculans and growth promotion of Quercus acutissima Carruth container seedlings using Bacillus velezensis CE 100. Int. J. Mol. Sci. 2021;22:11296. doi: 10.3390/ijms222011296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim I.P., Chung K.-C. Production of an antifungal protein for control of Colletotrichum lagenarium by Bacillus amyloliquefaciens MET0908. FEMS Microbiol. Lett. 2004;234:177–183. doi: 10.1111/j.1574-6968.2004.tb09530.x. [DOI] [PubMed] [Google Scholar]
- 52.Dahiya N., Tewari R., Hoondal G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006;71:773–782. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 53.Café-Filho A.C., Lopes C.A., Rossato M. Management of Plant Disease Epidemics with Irrigation Practices. In: Ondrasek G., editor. Irrigation in Agroecosystems. Agroecology. Volume 1. Intech Open; London, UK: 2019. pp. 123–139. [DOI] [Google Scholar]
- 54.Enders T.A., Strader L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015;102:180–196. doi: 10.3732/ajb.1400285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this manuscript is available on request from the corresponding author.