Abstract
The higher airborne microbial concentration in indoor areas might be responsible for the adverse indoor air quality, which relates well with poor respiratory and general health effects in the form of Sick building syndromes. The current study aimed to isolate and characterize the seasonal (winter and spring) levels of culturable bio-aerosols from indoor air, implicating human health by using an epidemiological health survey. Microorganisms were identified by standard macro and microbiological methods, followed by biochemical testing and molecular techniques. Sampling results revealed the bacterial and fungal aerosol concentrations ranging between (300–3650 CFU/m3) and (300–4150 CFU/m3) respectively, in different microenvironments during the winter season (December-February). However, in spring (March-May), bacterial and fungal aerosol concentrations were monitored, ranging between (450–5150 CFU/m3) and (350–5070 CFU/m3) respectively. Interestingly, Aspergillus and Cladosporium were the majorly recorded fungi whereas, Staphylococcus, Streptobacillus, and Micrococcus found predominant bacterial genera among all the sites. Taken together, the elevated levels of bioaerosols are the foremost risk factor that can lead to various respiratory and general health issues in additional analysis, the questionnaire survey indicated the headache (28%) and allergy (20%) were significant indoor health concerns. This type of approach will serve as a foundation for assisting residents in taking preventative measures to avoid exposure to dangerous bioaerosols.
1. Introduction
Indoor air quality plays a vital role in the health and well-being of people as 80–90% of the total time is spent in the indoor environment [1,2]. Typically, people inhale 10m3 air daily, which may be abundantly populated by various microorganisms in the form of colloidal suspension known as bio-aerosols [3]. Airborne microorganisms (AM) are cosmopolitan and even can be found in the highly controlled environment of operation theatres [4,5]. Fungi are the potential source of allergic and general health problems in different ways [6]. Sources of these microorganisms in indoor air may include people, organic dust, storage of various products, and circulation of air through natural and artificial ventilation systems [7] According to a previous study, roughly 30% of office workers suffer from ailments due to poor indoor air quality [8]. Exposure to these contaminants may cause allergic reactions, infections, intoxication, and various molecular reactions [6,9–13]. The importance of airborne microorganisms on health was highlighted by the World Health Organization (WHO) [14]. Moreover, respiratory and other health problems in office workers related to the indoor air quality of the built environment are termed as Sick Building Syndrome (SBS) [15].
Microbes can adapt themselves in different conditions hence, significant variation could be found in concentration in sub-areas of the same microenvironment [16]. Seasonal variations have been reported in the composition and abundance of AM, but these can also be influenced by temperature, relative humidity, and air exchange rate [17–19]. The presence of AM in pre-nursery schools may also affect the respiratory and paediatric health as well as the well-being of the children [20] In a recent study, Aspergillus, Curvularia, Penicillium, and Rhizopus were major fungal genera recorded from the different indoor areas in Kolkata, India [21]. The presence of mycotoxin-producing micromycetes in buildings plays a crucial role in causing SBS in occupants [22] Hospitals are one of the most significant indoor microenvironments for the spreading and propagation of the aero-microflora [23]. An air sampling study conducted in a Haematology hospital by Cho and co-workers reported that the Aspergillus and Penicillium were the most dominant fungal genera in the outdoors and inside buildings [24] (Fig 1).
Fig 1. Overview of the research study.
Delhi, India’s national capital, is the country’s most polluted city, drawing increased attention from the government and the general public. A few studies have been conducted in Delhi to assess the total bacterial and fungal concentration in residential areas and waste dumping sites [25–28] However, previous studies could not indicate the possible association between indoor air quality and health issues in different seasons.
The main objective of the present study is to investigate the pattern of morbidity among the people living in industrial, commercial, and residential due to poor air quality. Furthermore, biological sampling data obtained from indoor sites in different seasons will provide an idea to explain the seasonal adverse health problems. The current study’s findings may aid in the development and implementation of preventative public health programmes, as well as the creation of recommendations aimed at creating better indoor settings.
2. Methodology
2.1. Location and characterization of sampling sites
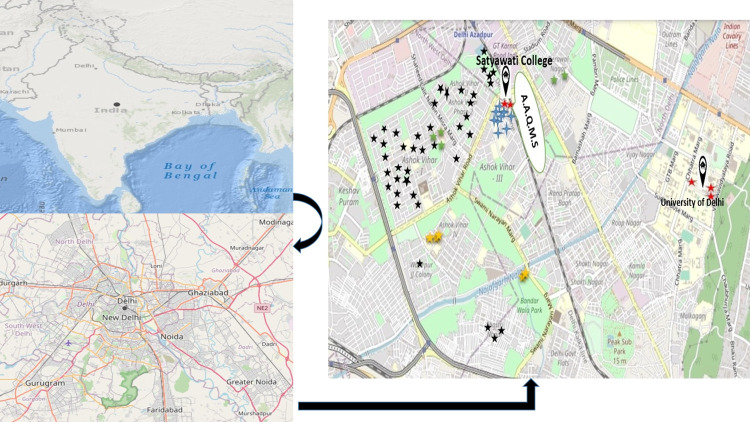
The study was carried out in selected areas of Delhi (North Delhi), the Indian capital, with a total area of 1,484 km2. Delhi is flanked on both sides by the river Yamuna, which flows from north to south and is located between 28°12’28°53’N and 76°50’77°23’E. Total culturable bacterial and fungal counts were measured in common residential microenvironments in North Delhi from December 2018- May 2019 (Fig 2).
Fig 2. Geographical location of the biological sampling areas, ambient air monitoring site (Map was generated by using USGS National Map Viewer (https://apps.nationalmap.gov/viewer) (Houses- black star, classrooms-blue star, academies-green star, laboratories-red star, and LPG godowns yellow star, (A.A.Q.M.S.)- Ambient Air Quality Monitoring System).
Residential residences, college classrooms, academies, LPG godowns, and laboratories in Satyawati College’s immediate vicinity were chosen for aerobiological sampling, whereas exact outdoor regions of sampling locations served as a control. The gas agency refers to LPG godowns where many cylinders are stored, and many workers continuously handle them for loading and unloading. Along with the indoor areas, biological sampling was conducted simultaneously with similar parameters for the same duration, and also background data was measured to check the source of contamination. Sampling localities were divided into three categories residential, commercial, and industrial.
2.2. Biological air sampling
Air samples were collected from (December 2018 to May 2019) by using the conventional settle plate method (passive gravitational method) [29]. Petri plates containing mycological medium (Sabourd Dextrose agar with Chloramphenicol and Tryptic Soy agar supplemented with cycloheximide) were used for study). All the samples were collected in triplicate and one set of agar plates was also exposed in a clean air environment as a control. Sampling was performed aseptically in each room at an average height of 1.5 m (breathing height). Petri plates were exposed for 10 minutes in case each sample.
2.3. Enumeration and identification of the culturable bioaerosols
Culturable bioaerosols from all the microenvironments were cultured at optimum conditions. The airborne microorganisms were counted using the plate count method. After sampling, bacterial samples were transferred to the laboratory aseptically and incubated for cultivation at 37°C for 24–36 hours. For fungal samples, the incubation was done at 26–28°C for 7–14 days. A digital thermometer (Checktem Digital Thermometer- HI98501) was used to assess indoor temperature and relative humidity (HTC HT-306 Temperature Humidity meter). After incubation, the bacterial and fungal colonies were analysed by standard procedures. Bacterial colonies were counted by using a colony counter. Microbial growth on agar plates was initially identified by the morphology and appearance of colonies. Furthermore, Gram’s staining was performed followed by biochemical and molecular characterization in the case of bacteria [30]. Fungal samples were visualised by using Lactophenol Cotton Blue stain [31]. Microbiological analysis of the samples were carried out and all the photographs were taken using a Nikon High-Resolution Microscope under 40X, 100X, 400X, and 1000X up to genus level. Following formula was used for calculating bioaerosol concentrations in CFU/m3 [29]
(where n is the number of colonies on the Petri plate, s is the surface of the Petri plate and t is the time of the Petri plate exposure. All the petri dishes with growth media were exposed for 10 min.)
2.4. Monitoring meteorological parameters
Meteorological data was obtained from the Delhi Pollution Control Committee (DPCC) Ambient Air Quality Monitoring System, Satyawati College Ashok Vihar Station, 110052, as well as from indoor sites. Temperature (°C), relative humidity (%), and particulate matter (PM 2.5) were measured outside and within, and their link with aerospora was identified.
2.5. Questionnaire survey
A health survey was conducted using a set of modified questionnaires based on MM 40 questionnaire [32]. Single-stage random sampling was performed for selecting subjects from different localities in the nearby areas. Apart from residential houses, students and workers from other residential sites, i.e. laboratories, classrooms, academies, and LPG godowns were also included in the questionnaire survey. The purpose of the questionnaire was to gather information on the subjects’ health and general information. The “Institutional Ethical Committee on Human Research” of Satyawati College, University of Delhi, Delhi, approved the research. The research was performed in accordance with relevant guidelines/regulations. Duly signed written consent form was obtained from all the study participants in a well-designed format. All the participants were also explained their role in the health survey and the purpose of the study. In the case of minors, consent was obtained from their parents or their guardians. A copy of the Questionnaire is attached as supplementary material (S 2 Questionnaire form in S1 File).
2.6 Data analysis
The data of bioaerosols were expressed as mean ±standard error. Statistical analysis was performed using Statistical Package for Social Sciences (SPSS 24), and Microsoft Excel 2019. All the data passed homogeneity and normality tests were evaluated by analysis of variance (ANOVA) between different groups and a probability of (p<0.05) was considered statistically significant.
3. Results
3.1. Demographics of the enrolled subjects in the study
Residents from the randomly selected sampling sites in North-west Delhi were enrolled for health survey. A total of 223 subjects signed the consent form to participate in questionnaire survey of which 153 (68.6%) were male and 70 (31.4%) females. Table 1. shows the demographic features of the participants, e.g. age, sex, and the locality.
Table 1. Demographic profile of the subjects involved in the study.
| Demographical features | Number of Subjects | % of Subjects |
|---|---|---|
| Age groups | ||
| < 18 years | 45 | 20.1 |
| 18–40 Years | 160 | 71.7 |
| > 40 years | 18 | 8.1 |
| Gender | ||
| Male | 153 | 68.6 |
| Female | 70 | 31.4 |
| Locality of enrolled subjects | ||
| Residential | 110 | 49.3 |
| Commercial | 60 | 27.0 |
| Industrial | 53 | 23.7 |
3.2 Health effects of microbial and indoor air quality on subjects
Table 2, illustrates the distribution of respiratory and general effects with indoor air quality on the health of the subjects. Data indicates that headache was the most dominant (28%) health effect followed by allergies (20%). 13% of subjects reported frequent coughing and problem of the runny nose at the time of examination. Subjects also reported about unusual thirst (11%), burning of irritated eyes (10%), sneezing attacks (8%), and as existing problems. Female subjects are more likely to suffer from the symptoms for the reason of probably spending more time in the indoor areas.
Table 2. Prevalence of the reported health effects/symptoms due to indoor air quality.
| Symptom category | Occurrence (%) |
|---|---|
| Respiratory problems | |
| Sneezing attacks | 8 |
| Nasal congestion | 8 |
| Runny nose | 13 |
| Sore or dry throat; frequent coughing | 13 |
| Tight chest; breathing difficulties (breath shortness) | 3 |
| Emphysema | 4 |
| General Health problems | |
| Chapped Lips | 5 |
| Unusual thirst | 11 |
| Allergy | 20 |
| Migraines | 3 |
| Headache | 28 |
| Multiple colds | 4 |
| Dry, itching, irritated, or watery eyes | 10 |
3.3 Variation in seasonal microbial concentration in different sampling sites
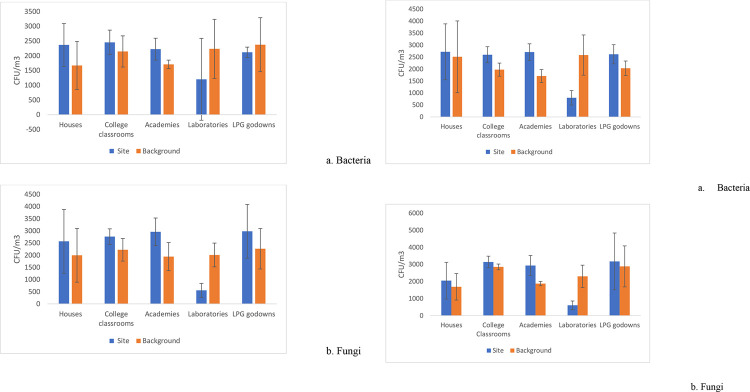
Microbial air sampling data obtained from selected indoor sites are represented in Fig 3. The average culturable microbial concentration (bacteria and fungi) in winter (December to February) and spring, (March-May) is illustrated in (Fig 3A) and (Fig 3B) respectively. During the winter season, college classrooms had the highest bacterial concentration of 2453±414 CFU/ m3, while LPG godowns had the highest fungal count of 2983±1106 CFU/ m3, followed by coaching academies with 2961±567 CFU/ m3. (Fig 3A). In contrast, in the spring, the average bacterial load in all microenvironments except laboratories was around 2600 CFU/ m3, peaking at 2719±1168 CFU/ m3 in residences. In the case of fungi, more variation has been noticed between different environments, and a higher concentration was observed in LPG godowns with 3713±1665 CFU/m3 (Fig 3B). Overall, seasonal comparison of collected air samples data from different indoor sites shows indoor microbial concentration is lower in winter than spring. Unlike bacteria, fungal communities showed significantly fluctuating results in both seasons in different microenvironments. Moreover, more variation was observed in fungal and bacterial CFU in the winter season. Despite the fact that Delhi is one of the world’s most polluted cities, fungal concentrations were found to be higher in all indoor places than outdoors, with the exception of research labs. However, the lowest concentrations of microorganisms were detected in laboratories in the case of bacteria and fungi in all the seasons. Bacterial concentration was highest in college classrooms, while the fungal load remained highest in LPG godowns. The greater bacterial and fungal load could be due to increased activities of students and staff in academic settings compared to other locations.
Fig 3.
a: Average microbial concentrations of bacteria and fungi during winter season (December-February 2018–2019) Error bars show customized standard deviation. b: Average microbial concentrations of bacteria and fungi during spring season (Mar-May 2019) Error bars show customized standard deviation.
3.4 Identification of major airborne Bacteria and Fungi present in the selected Indoor Microenvironments
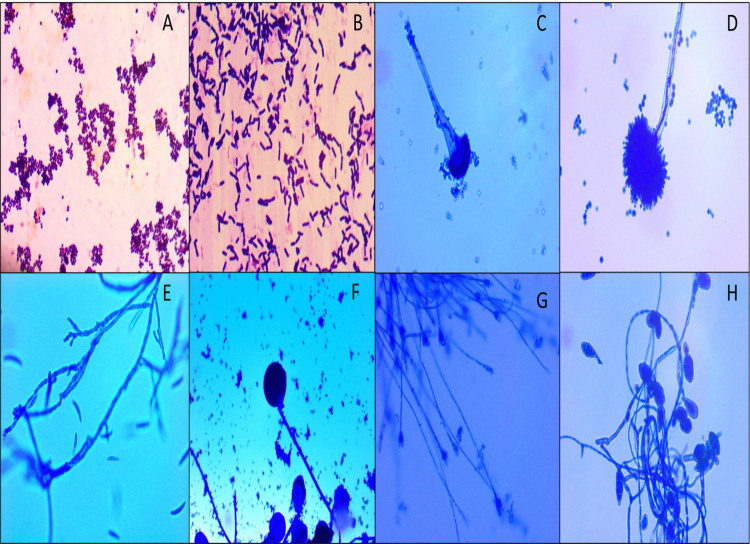
All the bacterial and fungal samples were characterized by macroscopic, microscopic and biochemical methods after being culturing in laboratory. Table 3 displays dominant culturable bacterial and fungal isolates from different indoor sampling sites. More bacterial and fungal genera were recorded from residential houses, than other sampling sites (i.e., Classrooms, LPG godowns, etc.). Of the fungal genera identified from different indoor sites shows that Aspergillus spp., Penicillium, Rhizopus, Cladosporium, Alternaria, and Candida, were present predominantly in residential houses while Cladosporium, Alternaria, and Aspergillus were also observed in all sampling sites. Aspergillus spp. and Cladosporium were seen common throughout all the sampling sites. Staphylococcus, Micrococcus, and Streptobacillus spp. were found the most dominant genera of bacteria in all the selected sites and E. coli, G+ cocci, Pseudomonas were also found in few sampling sites. Fig 4 displays the photomicrographs of the dominant microbes.
Table 3. Major fungal and bacterial isolated in different microenvironments.
| Name of the bacterial Isolates | Name of the Fungal Isolates |
|---|---|
|
Houses Staphylococcus, Micrococcus, Streptococcus, Streptobacillus, G–Bacilli, E. coli, Pseudomonas, G+ Cocci, Coaching Academies Staphylococcus, G+ Cocci, G+ Bacilli LPG godowns Micrococcus, Staphylococcus, G+ bacilli Research Laboratories Streptobacillus, Staphylococcus College classrooms Staphylococcus, G+ Cocci, G+ Bacilli Micrococcus |
Aspergillus spp. Cladosporium Penicillium Rhizopus Mucor Alternaria Candida Candida, Aspergillus spp., Fusarium Alternaria, Aspergillus spp. Penicillium, Aspergillus spp., Fusarium, Rhizopus Alternaria, Aspergillus spp. Candida, Fusarium |
Fig 4. Photomicrographs of dominant indoor microflora.
A. G+ Cocci (1000X), B. G+ Bacilli (1000X), C. Aspergillus (400X), D. Aspergillus (400X), E. Fusarium (400X), F. Rhizopus (400X), G. Penicillium (100X), H. Alternaria (100X).
3.5 Influence of meteorological parameters on microbial concentrations
The average temperature in all the sampling areas increased by passing of months from winter to spring (Table 4). December was the coldest month during sampling time, and the average temperature was highest in May. The spring season in Delhi, becomes dry and the temperature rises significantly higher in the month of April-May. Interestingly PM 2.5 concentration remained highest in December and declined to minimum in April. Relative humidity data shows fluctuation with peak in February. Indoor and outdoor microbial ratio (I/O) fluctuates in the case of bacterial and fungi (Table 5). (Table 6) represents correlation matrix (Paired T-test) of temperature and relative humidity with microbial concentrations in winter and spring.
Table 4. Description of the meteorological parameters of the sampling areas (Particulate Matter PM 2.5 Data source: Ambient Air quality monitoring system (DPCC) Ashok Vihar, Delhi).
| Season | Winter | Spring | ||||
|---|---|---|---|---|---|---|
| Month | December | January | February | March | April | May |
| Temperature(°C) | 14.1 | 18.4 | 21.9 | 23.1 | 29.9 | 30.2 |
| Relative Humidity (RH)(%) | 63.2 | 58.7 | 63.6 | 43 | 48.4 | 36.6 |
| (PM 2.5) | 414 | 362 | 290 | 190 | 187 | 249 |
Table 5. Indoor and outdoor microbial ratios (I/O ratio) in different sampling sites in both the seasons.
| Site | Winter | Spring |
|---|---|---|
| Bacteria | ||
| Houses | 1.41 | 1.08 |
| College classrooms | 1.14 | 1.31 |
| Coaching academies | 1.30 | 1.58 |
| Laboratories | 0.53 | 0.30 |
| LPG godowns | 0.89 | 1.28 |
| Fungi | ||
| Houses | 1.28 | 1.21 |
| College classrooms | 1.24 | 1.10 |
| Coaching academies | 1.52 | 1.56 |
| Laboratories | 0.27 | 0.26 |
| LPG godowns | 1.31 | 1.10 |
Table 6. Correlation matrix of meteorological parameters temperature and relative humidity with microbial concentrations in winter and spring seasons (P<0.05 means positive correlation with meteorological parameters; significant values are in boldface.).
| Site | Parameter | Bacterial CFU/m3 | Fungi CFU/m3 |
|---|---|---|---|
| Residential Houses | T | 0.022 | 0.030 |
| RH | 0.023 | 0.031 | |
| College Classrooms | T | 0.009 | 0.003 |
| RH | 0.010 | 0.004 | |
| Academies | T | 0.005 | 0.013 |
| RH | 0.012 | 0.012 | |
| Laboratories | T | 0.282 | 0.086 |
| RH | 0.290 | 0.089 | |
| LPG godowns | T | 0.002 | 0.045 |
| RH | 0.002 | 0.043 | |
| Winter | |||
| Site | Parameter | Bacterial CFU/m 3 | Fungi CFU/m 3 |
| Residential Houses | T | 0.021 | 0.004 |
| RH | 0.021 | 0.004 | |
| College Classrooms | T | 0.005 | 0.003 |
| RH | 0.005 | 0.004 | |
| Academies | T | 0.005 | 0.013 |
| RH | 0.005 | 0.013 | |
| Laboratories | T | 0.046 | 0.060 |
| RH | 0.048 | 0.061 | |
| LPG godowns | T | 0.007 | 0.003 |
| RH | 0.007 | 0.083 | |
| Spring | |||
4. Discussion
This study considered more than 65 different sampling sites (houses, classrooms, LPG godowns, laboratories, and coaching academies) for seasonal biological air sampling and health survey. Moreover, seasonal analysis of the microbes showed bacterial concentrations elevated in the houses, academies, classrooms, and LPG godowns. We observed that microbes isolated from different microenvironments belong to the opportunistic pathogens category (Table 3). Culturable bacterial spore count of indoor areas exceeds outdoor counts except for laboratories. In the case of fungi, indoor bioaerosols were higher than the outdoors in houses, academies, and LPG godowns. Interestingly, identical results were observed during the winter and spring seasons in the hospitals in Pakistan [33]. A previous study found that exposure to varied bacterial concentrations could have direct and indirect effects on human health [34].
Staphylococcus, Micrococcus, and Streptococcus were the most dominant bacterial genera in indoor than outdoors in the current study and previous studies [28,31,35]. A high abundance of Cladosporium in indoors indicate that outdoor conditions significantly affect the indoor fungal concentration. Aspergillus spp., Penicillium, Alternaria, Fusarium, and Mucor were the other dominant fungal genera observed indoors. These microbes may directly or indirectly play a role in respiratory diseases or other complications [11] In the current study Aspergillus spp. were recorded in all the microenvironments, the occurrence of fungal genera Aspergillus, Alternaria, Penicillium, and Cladosporium cause epidemiological allergy, allergic respiratory diseases in the inhabitants [6,9,11] however, the presence of Alternaria may be also responsible for asthma, skin infections, and allergic rhinitis [13]. In addition, the spore concentrations of the Aspergillus and Cladosporium were observed significantly higher in the spring season whereas, Alternaria was found considerably higher in the winter season [36]. A recent study by Srivastava and co-workers conducted the qualitative and quantitative analysis of bioaerosols near around Okhla landfill site Delhi [37]. Variation in the type of microbes might include industrial areas, dumping sites, slaughterhouses, waste decomposition, meteorological parameters, and anthropogenic activities [38]. Previous studies reported that the presence of Candida is responsible for various diseases, i.e. sinus infections, fatigue, and urinary tract infections however, presence of Alternaria and Mucor may trigger allergic reactions and asthma [9,13]. Fungi are more often released by the hair, nail, and, skin of students, which are key sources of indoor contamination of the educational buildings [39].
Previous studies confirmed that high indoor bacterial load and other physical features are responsible for the health problems in the students and staff in residential buildings [40]. A similar study conducted by Madamarandawala et al., (2019) suggested that 58% of urban and 31% of preschool students were suffering from at least one health effect related to the microbial air quality. In addition, the serum levels were elevated in children suffering from allergic reactions [41]. A similar study from Kolkata, India, also found that patients with atopic allergic history are more reactive towards fungal extracts [42]. An epidemiological study conducted in southern India, the effect of indoor air pollutants, socioeconomic, and housing characteristics on the well-being of children and women, demonstrated that these factors significantly influence respiratory health, which may increase the burden of respiratory illness [43]. Findings of the pilot study conducted in Finland to assess the indoor air problems which revealed the irritated, stuffy, or runny nose (20%), itching, burning, or irritation of the eyes (17%), and fatigue (16%) found the most common symptoms in the workers. Women had a higher proportion of indoor air problems than men, indicating the importance of the current study [12].
Few studies have been conducted in Delhi to measure biological pollution at various locations, with the majority of the studies focusing on educational institutes, libraries, dumping sites, and so on [27,28,37,44–46]. In addition, the houses of patients suffering from asthma/allergies reported (110,091) and (107,070) fungal colonies per cubic meter area in indoor and outdoor areas, respectively reported by [47]. Bacterial sampling conducted in a slum area near Jawaharlal Nehru University (South Delhi) reported a high number of bacterial aerosols between 0.43 to 3.35 x 107 CFU/m3, which is significantly greater than other similar studies [27]. In another study JNU libraries recorded the total bioaerosols with range of 911–1460 CFU/m3 and 2550–3110 CFU/m3 for bacteria and fungi respectively [26] Ambient bioaerosol concentrations measured ranging between (1740–3224 CFU/m3) for fungi and (1990–9428 CFU/m3) bacteria in different areas of Delhi. Furthermore, this study also observed the most bioaerosols in stage 4 (2.1–3.3 μm), the stage 5(2.1–2.2μm), these particles are capable of penetrating the lower parts of the lungs [45].
Subjects with high microbial concentration were more likely to suffer from respiratory and general health problems. The rare respiratory disorders connected with Pseudomonas and Bacilli found mostly in residential buildings, which is related to lower and upper respiratory tract infections, was detected in the correlation between the health consequences and isolated microorganisms [48,49]. Previously, it was also observed that presence of Bacilli is competent in causing digestive diseases and other infections [50].
Among the prominent symptoms in respondents were burning or irritation of the eyes, as well as a regular coughing problem (Table 2). Previously, a study [51] suggested the presence of microbial agents is conscientious for ophthalmic and respiratory health effects among farmers. Despite living microorganisms, spores and dead biological particles may elicit asthma and allergic reactions in the subjects [52]. According to a study conducted in south India, indoor air quality, housing conditions, and socioeconomic position are responsible for a variety of health impacts in mothers and children, including cough, runny nose, asthma, and other symptoms [43] A cross-sectional survey conducted by [53] also signified that waking up due to coughing, bronchitis, etc. were major recorded problems due to the microbial contaminated outdoor air. Similarly, the concentration of Alternaria was found higher in the houses of the subjects suffering from coughing. The previous report revealed that headache (20.6%), rhinitis (18.8%), and tiredness (22.1%) were common symptoms among the students moreover tiredness and ocular symptoms were found coordinated with Aspergillus versicolor [54]. Another cross-sectional study conducted in Australia reported the non-biological activities such as overcrowding, dust, water supply, etc., are associated with the health issues such as respiratory problems, asthma, skin problems, etc., in residents [55]. Microbial concentrations are significantly correlated with meteorological factors such as temperature and relative humidity [17,56,57]. Meteorological parameters and other growth factors are favourable in Delhi during the spring season (March-May), which could be a possible reason behind the current hypothesis. Because high temperatures are required for optimum growth, the growth of two main fungal genera, Aspergillus and Penicillium, was highest in April and May. Seasons, which are regulated by temperature, relative humidity, and exchange rates, have a substantial impact on microbial exposure [17]. The laboratories’ I/O ratios ranged from 0.26–0.53, indicating that the usage of air filters and fewer human activities on this location may be responsible for the lower indoor microbial concentrations (Table 5). High humidity and leaking conditions promote dampness, which increases the growth of the fungi on walls and other areas [58]. Additionally, hot and dry weather increase the spore buoyancy, which causes the transport of spores from outdoor regions to longer distances [59]. Although temperature has varied impacts in each season, a prior Polish study reveals a positive association between coarse particulate matters and bioaerosol concentration [57]. Ventilation plays a crucial role in maintaining the concentrations of the airborne microflora, buildings without an air conditioner (A.C.) and other anthropogenic ventilation have 1.5 times higher concentrations of bioaerosols than with AC [60]. The concentration of airborne microorganisms in the environment is influenced by vegetation and road congestion [61]. Isolation, evaluation, and characterization are essential for controlling microbial contamination in indoor environments. Human activities, poor ventilation, and waste degradation are all major contributors of these pollutants [21]. The collected data was compared to earlier research [62,63] and it was found that the current study is highly relevant since inhabitants spend a lot of time indoors and experience a variety of difficulties connected to microbiological indoor air quality. [20,42,53,64,65]. There are good clinical and experimental results associated with dampness and elevated indoor mold concentrations [14].
Culture based technique was used in present study to assess the microbial load in indoor areas. Although the culture-based studies are reliable, effective, viable, inexpensive, but have several drawbacks in the characterization of bioaerosols. Only 1–2 percent of total ambient bacteria are culturable, implying that non-viable microorganisms make up a large fraction of the isolated microflora [66]. Culture-based methods are unable to detect unculturable microbes [67] however, culture methods have other problems, such as they are time-consuming, temperature-specific, and aseptic techniques are required to avoid cross-contamination [68]. Non-culture-based or culture-independent methods can detect a hundred to thousand times more concentration than culture-based methods [69]. Viable but non-culturable may also be underestimated in the culture-dependent techniques [70]. In the case of fungi, there is no ideal medium for their growth also, the characteristics of fungi are influenced by several physical and chemical factors [71–73]. Some studies also recommended the morphological characteristics change in dimorphic fungi at certain temperatures [74].
5. Limitations of the study
The presence of high indoor bioaerosol concentrations and health effects could not be directly correlated as they may appear due to other reasons. Non-biological indoor pollutants may also cause respiratory diseases among people [75]. Volatile organic compounds emissions, CO2, PM2.5 are amongst the major pollutants responsible for health consequences [64,65]. Long term exposure to environmental tobacco smoke may act synergistically with other pollutants to cause severe respiratory health effects [76]. Furthermore, cooking methods may be a cause for concern, as it has been discovered that women who are exposed to high levels of indoor air pollution are more susceptible to illness [77]. Subjects were inquired about the cooking methods and observed that people in the sampling area only use LPG stoves for cooking which has minimal effects on human health [78]. Furthermore, investigations at multiple places over longer time periods are required to provide a thorough understanding of the airborne microbiome and the various factors that influence its ecology.
6. Conclusion
This study suggested that high concentrations of the microbes were present in indoor areas as compared to outdoors. Indoor bacterial abundance were significantly higher in spring than winter. Headache and chronic allergies were the major symptoms perceived in inhabitants. Health survey revealed that microbial abundance is positively correlated with the meteorological parameters such as temperature and relative humidity. Human activities and ventilation facility influence the diversity, abundance, and composition of bioaerosols in indoor environments. The current study would aid in the standardization of protocols to reduce the degree of biological contamination.
Supporting information
The values behind the means, standard deviations and other measures reported; The values used to build graphs; The points extracted from images for analysis are provided here.
(XLSX)
A copy of health proforma and subject consent form used for collecting the health data is provided here.
(PDF)
Acknowledgments
Authors acknowledge the Delhi Pollution Control Committee (DPCC) Delhi for providing the meteorological data.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported by Science and Engineering Research Board, Department of Science and Technology (SERB-DST), New Delhi, India (Grant No. ECR/2017/000470). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Reynolds S.J.; Black D.W.; Borin S.S.; Breuer G.; Burmeister L.F.; Fuortes L.J.; et al. Indoor environmental quality in six commercial office buildings in the midwest United States. Appl. Occup. Environ. Hyg. 2001, 16, 1065–1077. doi: 10.1080/104732201753214170 [DOI] [PubMed] [Google Scholar]
- 2.Weikl F, Tischer C, Probst AJ, Heinrich J, Markevych I, Jochner S, et al. (2016) Fungal and Bacterial Communities in Indoor Dust Follow Different Environmental Determinants. PLoS ONE 11(4): e0154131. doi: 10.1371/journal.pone.0154131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srikanth P, Sudharsanam S, Steinberg R. Bio-aerosols in indoor environment: composition, health effects and analysis. Indian J Med Microbiol. 2008. Oct-Dec;26(4):302–12. doi: 10.4103/0255-0857.43555 . [DOI] [PubMed] [Google Scholar]
- 4.Aydogdu H., Asan A. & Tatman Otkun M. Indoor and outdoor airborne bacteria in child day-care centers in Edirne City (Turkey), seasonal distribution and influence of meteorological factors. Environ Monit Assess 164, 53–66 (2010). doi: 10.1007/s10661-009-0874-0 [DOI] [PubMed] [Google Scholar]
- 5.Ghanizadeh F, Godini H. A review of the chemical and biological pollutants in indoor air in hospitals and assessing their effects on the health of patients, staff and visitors. Reviews on environmental health, 2018, 33.3: 231–245. doi: 10.1515/reveh-2018-0011 [DOI] [PubMed] [Google Scholar]
- 6.Khan H A A, Karuppayil M S. Fungal pollution of indoor environments and its management. Saudi J Biol Sci. 2012;19(4):405–426. doi: 10.1016/j.sjbs.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bragoszewska E, and Biedron I, Indoor Air Quality and Potential Health Risk Impacts of Exposure to Antibiotic Resistant Bacteria in an Office Rooms in Southern Poland, (2018. a) Int. J. Env. Res. Pub. Health, doi: 10.3390/ijerph15112604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gołofit-Szymczak M, Górny RL. Bacterial and fungal aerosols in air-conditioned office buildings in Warsaw, Poland—the winter season. Int J Occup Saf Ergon. 2010;16(4):465–76. doi: 10.1080/10803548.2010.11076861 . [DOI] [PubMed] [Google Scholar]
- 9.Baxi SN, Portnoy JM, Larenas-Linnemann D, Phipatanakul W (2016) Exposure and health effects of Fungi on humans. J Allergy Clin Immunol 4(3):396–404. doi: 10.1016/j.jaip.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung F. & Hughson W G, Health Effects of Indoor Fungal Bioaerosol Exposure. Applied Occupational and Environmental Hygiene 2001; 18:7;535–544. [DOI] [PubMed] [Google Scholar]
- 11.Husman T. Health effects of indoor-air microorganism. Scandinavian Journal of Work, Environment & Health. 1996;22(1):5–13. doi: 10.5271/sjweh.103 [DOI] [PubMed] [Google Scholar]
- 12.Kumar P., Kausar M.A., Singh A.B. et al. Biological contaminants in the indoor air environment and their impacts on human health. Air Qual Atmos Health 14, 1723–1736 (2021. a). 10.1007/s11869-021-00978-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reijula K. and Sundman-Digert C. 2004, Assessment of Indoor Air problems at Work with questionnaire. Journal of Occupational and Environmental Medicine 61,33–38. [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Guidelines for Indoor Air Quality: Dampness and Mould, World Health Organization, Copenhagen: (2009). [PubMed] [Google Scholar]
- 15.WHO. Indoor Air Quality: Biological Contaminants: Report on a Who Meeting, Rautavaara. Volume 31 World Health Organization Regional Office for Europe; Copenhagen, Denmark: 1988. [Google Scholar]
- 16.Fernstrom A, Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. J Pathog. 2013;2013:493960. doi: 10.1155/2013/493960 Epub 2013 Jan 13. ; PMCID: PMC3556854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel M, Bekö G, Timm M, Gustavsen S, Hansen E W, Anne Mette Madsen Seasonal Variations of Indoor Microbial Exposures and Their Relation to Temperature, Relative Humidity, and Air Exchange Rate. Applied and Environmental Microbiology; Nov 2012, 78 (23) 8289–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumari P., Woo C., Yamamoto N. et al. Variations in abundance, diversity and community composition of airborne fungi in swine houses across seasons. Sci Rep 6, 37929 (2016). doi: 10.1038/srep37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J., Ichijo T., Nasu M. et al. Investigation of bacterial effects of Asian dust events through comparison with seasonal variability in outdoor airborne bacterial community. Sci Rep 6, 35706 (2016). doi: 10.1038/srep35706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madamarandawala P., Weerasinghe Y., Pathiraja D. et al. Impact of microbial air quality in preschools on paediatric respiratory health. SN Appl. Sci. 1, 1280 (2019). 10.1007/s42452-019-1306-6. [DOI] [Google Scholar]
- 21.Karmakar B., Sen Gupta K., Kaur A. et al. Fungal bio-aerosol in multiple micro-environments from eastern India: source, distribution, and health hazards. SN Appl. Sci. 2, 565 (2020). 10.1007/s42452-020-2323-1. [DOI] [Google Scholar]
- 22.Švajlenka J, Kozlovská M, Pošiváková T. Assessment and biomonitoring indoor environment of buildings. Int J Environ Health Res. 2017. Oct;27(5):427–439. doi: 10.1080/09603123.2017.1373276 Epub 2017 Sep 4. . [DOI] [PubMed] [Google Scholar]
- 23.Ioana Chirca, The hospital environment and its microbial burden: challenges and solutions. Future Microbiol. (2019) 14(12), 1007–1010. [DOI] [PubMed] [Google Scholar]
- 24.Cho SY, Myong JP, Kim WB, et al. Profiles of Environmental Mold: Indoor and Outdoor Air Sampling in a Hematology Hospital in Seoul, South Korea. Int J Environ Res Public Health. 2018;15(11):2560. Published 2018 Nov 15. doi: 10.3390/ijerph15112560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Pereira B, Singh A B. Survey of airborne culturable and non-culturable fungi at different sites in Delhi metropolis. Asian Pacific journal of allergy and immunology,1993;(11)19–28. [PubMed] [Google Scholar]
- 26.Ghosh B., Lal H., Kushwaha R., et al. (2013) Estimation of bioaerosol in indoor environment in the university library of Delhi. Sustain. Environ. Res. 23:199–207. [Google Scholar]
- 27.Kumar B., Gupta G., Singh S.B., & Kulshrestha U. (2013). Study of Abundance and Characterization of Culturable Bioaerosol at Delhi, India. International Journal of Environmental Engineering and Management., 4,(3) (2013), 219–26. [Google Scholar]
- 28.Kumar P., Singh A.B. & Singh R. Seasonal variation and size distribution in the airborne indoor microbial concentration of residential houses in Delhi and its impact on health. Aerobiologia (2021b). 10.1007/s10453-021-09718-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moldoveanu A M, Biological Contamination of Air in Indoor Spaces, Current Air Quality issues 10.5772/59727, 489–514, 2015. Occupational and Environmental Medicine 2003;60:336–342. [DOI] [Google Scholar]
- 30.Bergey’s Manual of Systematic Bacteriology, 2nd ed., vol. 3, (2009) Springer-Verlag, New York, NY. [Google Scholar]
- 31.Sutton DA, Fothergill A W. Guide to Clinically Significant Fungi Paperback–Import, 1 November 1997. [Google Scholar]
- 32.Anderson K., Stridh G., Fagerlund I. amd Aslaksen W. 1993. “The MM-questionnaires–A tool when solving indoor climate problems”. Department of Occupational and Environmental Medicine, Örebro University Hospital, Örebro, Sweden. Retrieved February 24, 2020, from http://www.orebroll.se/upload/USO/YMK/Dokument/Reference3eng.pdf. [Google Scholar]
- 33.Asif A, Zeeshan M, Hashmi I et al., Microbial quality assessment of indoor air in a large hospital building during winter and spring seasons. Building and Environment 135 (2018) 68–73. [Google Scholar]
- 34.Goyer N, Lavoie J. Emissions of chemical compounds and bioaerosols during the secondary treatment of paper mill effluents. AIHAJ. 2001. May-Jun;62(3):330–41. doi: 10.1080/15298660108984635 . [DOI] [PubMed] [Google Scholar]
- 35.Shin S-K, Kim J, Ha S-m, Oh H-S, Chun J, Sohn J, et al. (2015) Metagenomic Insights into the Bioaerosols in the Indoor and Outdoor Environments of Childcare Facilities. PLoS ONE 10(5): e0126960. doi: 10.1371/journal.pone.0126960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priyamvada H, Singh RK, Akila M, Ravikrishna R, Verma RS, Gunthe SS. Seasonal variation of the dominant allergenic fungal aerosols—One year study from southern Indian region. Sci Rep. 2017. Sep 11;7(1):11171. doi: 10.1038/s41598-017-11727-7 ; PMCID: PMC5593913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srivastava A., Singh M. & Jain V.K. Identification and characterization of size-segregated bioaerosols at Jawaharlal Nehru University, New Delhi. Nat Hazards 60, 485–499 (2012). 10.1007/s11069-011-0022-3. [DOI] [Google Scholar]
- 38.Humbal C., Joshi S.K., Trivedi U.K. et al. Evaluating the colonization and distribution of fungal and bacterial bio-aerosol in Rajkot, western India using multi-proxy approach. Air Qual Atmos Health 12, 693–704 (2019). 10.1007/s11869-019-00689-6. [DOI] [Google Scholar]
- 39.Wang X, Liu W, Huang C, Cai J, Shen L, Zou Z, et al. (2016) Associations of dwelling characteristics, home dampness, and lifestyle behaviors with indoor airborne culturable fungi: on-site inspection in 454 Shanghai residences. Buil Environ 102:159–166. 10.1016/j.buildenv.2016.03.010. [DOI] [Google Scholar]
- 40.Andualem Z., Gizaw Z., Bogale L. et al. Indoor bacterial load and its correlation to physical indoor air quality parameters in public primary schools. Multidiscip Respir Med 14, 2 (2019). doi: 10.1186/s40248-018-0167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bezerra D B G.F., Haidar D.M.C., da Silva M.A.C.N. et al. IgE serum concentration against airborne fungi in children with respiratory allergies. Allergy Asthma Clin Immunol 12, 18 (2016). doi: 10.1186/s13223-016-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dey D., Ghosal K. & Bhattacharya S.G. Aerial fungal spectrum of Kolkata, India, along with their allergenic impact on the public health: a quantitative and qualitative evaluation. Aerobiologia 35, 15–25 (2019). 10.1007/s10453-018-9534-6. [DOI] [Google Scholar]
- 43.Rumchev K, Zhao Y, Spickett J. Health Risk Assessment of Indoor Air Quality, Socioeconomic and House Characteristics on Respiratory Health among Women and Children of Tirupur, South India. Int J Environ Res Public Health. 2017;14(4):429. Published 2017 Apr 17. doi: 10.3390/ijerph14040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal S, Mandal P, Srivastava A. Quantification and Characterization of Size-segregated Bioaerosols at Municipal Solid Waste Dumping Site in Delhi. Procedia Environmental Sciences 35 (2016) 400–407. [Google Scholar]
- 45.Lal H, Ghosh B, Srivastava A, Srivastava A. Identification and characterization of size-segregated bioaerosols at different sites in Delhi. Aerosol and Air Quality Research. 2017. Jun;17(6):1570–81. [Google Scholar]
- 46.Balyan P, Ghosh C, Sharma AK et al. Health effects of air pollution among residents of Delhi: a systematic review. Int J Health Sci Res. 2018; 8(1):273–282. [Google Scholar]
- 47.Sharma Rashmi et al. “Indoor fungal concentration in the homes of allergic/asthmatic children in Delhi, India.” Allergy & rhinology (Providence, R.I.) vol. 2,1 (2011): 21–32. doi: 10.2500/ar.2011.2.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baquero F, Alvarez M-E, Cantón R (1996) Bacteriologic diagnosis of respiratory tract infection. Clin Microbiol Infect 1:2S10–2S15. 10.1111/j.1469-0691.1996.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 49.Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, et al. (2010) Association of bacteria and viruses with wheezy episodes in young children:prospective birth cohort study. BMJ 341:c4978. doi: 10.1136/bmj.c4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuazon CU, Murray HW, Levy C, Solny MN, Curtin JA, Sheagren JN (1979) Serious infections from Bacillus sp. JAMA J Am Med Assoc 241:1137. 10.1001/jama.1979.03290 37004 1026. [DOI] [PubMed] [Google Scholar]
- 51.Eduard W, Heederik DJJ, Melbostad E, Mehl R, Douwes J (2001) Short term exposure to airborne microbial agents during farm work: exposure-response relations with eye and respiratory symptoms. Occup Environ Med 58:113–118. doi: 10.1136/oem.58.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burge H (1990) Bioaerosols; Prevalence and health effects in the indoor environment. J Allergy Clin Immunol 86(5):687–701. doi: 10.1016/s0091-6749(05)80170-8 [DOI] [PubMed] [Google Scholar]
- 53.Herr CE, Zur Nieden A, Jankofsky M, Stilianakis NI, Boedeker RH, Eikmann TF. Effects of bioaerosol polluted outdoor air on airways of residents: a cross sectional study. Occup Environ Med. 2003;60(5):336–342. doi: 10.1136/oem.60.5.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norbäck D, Hashim JH, Cai GH, Hashim Z, Ali F, Bloom E, et al. Rhinitis, Ocular, Throat and Dermal Symptoms, Headache and Tiredness among Students in Schools from Johor Bahru, Malaysia: Associations with Fungal DNA and Mycotoxins in Classroom Dust. PLoS One. 2016. Feb 1;11(2):e0147996. doi: 10.1371/journal.pone.0147996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melody SM, Bennett E, Clifford HD, Johnston FH, Shepherd CC, Alach Z, et al. A cross-sectional survey of environmental health in remote Aboriginal communities in Western Australia. Int J Environ Health Res. 2016. Oct-Dec;26(5–6):525–35. doi: 10.1080/09603123.2016.1194384 [DOI] [PubMed] [Google Scholar]
- 56.Mirhoseini S.H., Nikaeen M., Satoh K. and Makimur K. (2016). Assessment of Airborne Particles in Indoor Environments: Applicability of Particle Counting for Prediction of Bioaerosol Concentrations. Aerosol Air Qual. Res. 16: 1903–1910. 10.4209/aaqr.2015.08.0528. [DOI] [Google Scholar]
- 57.Brągoszewska E., Pastuszka J.S. Influence of meteorological factors on the level and characteristics of culturable bacteria in the air in Gliwice, Upper Silesia (Poland). Aerobiologia 34, 241–255 (2018. c). 10.1007/s10453-018-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heseltine E, Rosen J (eds) (2009) WHO guidelines for indoor air quality: dampness and mould. WHO Regional Office; Europe, København. [PubMed] [Google Scholar]
- 59.Kulmala M, Asmi A, Pirjola L (1999) Indoor air aerosol model: the effect of outdoor air, filtration and ventilation on indoor concentrations. Atmos Environ 33:2133–2144. 10.1016/S1352-2310(99)00070-9. [DOI] [Google Scholar]
- 60.Brągoszewska E., Biedroń I., Kozielska B. et al. Microbiological indoor air quality in an office building in Gliwice, Poland: analysis of the case study. Air Qual Atmos Health 11, 729–740 (2018). 10.1007/s11869-018-0579-z. [DOI] [Google Scholar]
- 61.Amarasekara R.W.K., Vithanage M., Samaraweera P. et al. Effect of traffic congestion and vegetation on airborne bacteria in a city of a developing country. Air Qual Atmos Health (2021). 10.1007/s11869-021-01001-1. [DOI] [Google Scholar]
- 62.Adhikari A., Kurella S., Banerjee P., & Mitra A. (2017). Aerosolized bacteria and microbial activity in dental clinics during cleaning procedures. Journal of Aerosol Science, 114, 209–218. [Google Scholar]
- 63.Chuang C.-Y., Cheng H.-C., Yang S., Fang W., Hung P.-C., & Chuang S.-Y. (2014). Investigation of the spreading characteristics of bacterial aerosol contamination during dental scaling treatment. Journal of Dental Sciences, 9(3),294–296. [Google Scholar]
- 64.Wu Y, Lu Y, Chou D C. Indoor air quality investigation of a university library based on field measurement and questionnaire survey, Int. J. Low-Carbon Technologies 2018, 13, 148–160. [Google Scholar]
- 65.Rautiainen P, Hyttinen M, Ruokolainen J, Saarinen P, Timonen J, Pasanen P. Indoor air-related symptoms and volatile organic compounds in materials and air in the hospital environment. Int J Environ Health Res. 2019. Oct;29(5):479–488. doi: 10.1080/09603123.2018.1550194 Epub 2018 Nov 26. . [DOI] [PubMed] [Google Scholar]
- 66.Sharma R., Ranjan R. Kapardar R. K. A., and Grover A. 2005. Unculturable bacterial diversity: An untapped resource. Curr. Sci. 89 (1):72–7. [Google Scholar]
- 67.Nakamura K., Iizuka R., Nishi S. et al. Culture-independent method for identification of microbial enzyme-encoding genes by activity-based single-cell sequencing using a water-in-oil microdroplet platform. Sci Rep 6, 22259 (2016). doi: 10.1038/srep22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferone M, Gowen A, Fanning S, Scannell AGM. Microbial detection and identification methods: bench top assays to omics approaches. Compr Rev Food Sci Food Saf. 2020; 19: 3106– 3129. doi: 10.1111/1541-4337.12618 [DOI] [PubMed] [Google Scholar]
- 69.Franchitti E, Pascale E, Fea E, Anedda E, Traversi D. Methods for Bioaerosol Characterization: Limits and Perspectives for Human Health Risk Assessment in Organic Waste Treatment. Atmosphere. 2020; 11(5):452. 10.3390/atmos11050452FER. [DOI] [Google Scholar]
- 70.Park J.-W., Park C. W., Lee S. H., and Hwang J. 2015. Fast monitoring of indoor bioaerosol concentrations with ATP bioluminescence assay using an electrostatic type sampler. PLoS One 10 (5):e0125251. 10.1371. doi: 10.1371/journal.pone.0125251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Keswani J., Kashon M. L., and Chen B. T. 2005. Evaluation of interference to conventional and real-time PCR for detection and quantification of fungi in dust. J. Environ. Monit. 7 (4):311–8. doi: 10.1039/b415498c [DOI] [PubMed] [Google Scholar]
- 72.Sharma G., and Pandey R. R. Influence of culture media on growth, colony character and sporulation of fungi isolated from decaying vegetable wastes. J. Yeast Fungal Res (2010). 1 (8):157–64. [Google Scholar]
- 73.King M D, Lacey R E, Pak H, Fearing A, Ramos G, Baig T, et al., Assays and enumeration of bioaerosols-traditional approaches to modern practices, Aerosol Science and Technology, (2020) 54:5, 611–633, doi: 10.1080/02786826.2020.1723789 [DOI] [Google Scholar]
- 74.Jensen P. A., and Schafer M. P. 1998. Sampling and characterization of bioaerosols. NIOSH Man. Anal. Methods 2:82–112. doi: 10.26616/nioshpub82100 [DOI] [Google Scholar]
- 75.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and Health Impacts of Air Pollution: A Review. Front Public Health. 2020;8:14. Published 2020 Feb 20. doi: 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berglund B, Brunekreef B, Knöppe H, Lindvall T, Maroni M, Mølhave L, Skov P. Effects of indoor air pollution on human health. Indoor air. 1992. Mar;2(1):2–5. [Google Scholar]
- 77.Dutta S, Banerjee S. Exposure to Indoor Air Pollution & Women Health: The Situation in Urban India. Environment and Urbanization ASIA. 2014;5(1):131–145. doi: 10.1177/0975425314521545 [DOI] [Google Scholar]
- 78.Kaur-Sidhu M, Ravindra K, Mor S, John S, Aggarwal AN. Respiratory Health Status of Rural Women Exposed to Liquefied Petroleum Gas and Solid Biomass Fuel Emissions. Air, Soil and Water Research. January 2019. doi: 10.1177/1178622119874314 [DOI] [Google Scholar]