Abstract
The transmission of coronavirus disease 2019 (COVID-19) in workplaces has been a persistent issue throughout the pandemic. In response, a not-for-profit initiative emerged to mitigate COVID-19 workplace transmission in Canada. We report the process for establishing a workplace frequent rapid antigen test (RAT) program. The screening program identified 473 asymptomatic individuals who tested positive on the RAT and confirmed positive by a nasopharyngeal polymerase chain reaction (PCR) diagnostic test. One in 4300 RATs was presumptive positive but later tested PCR negative, and thus, false positives did not meaningfully disrupt workplace operations. Most employers rated the program highly and felt strongly that the program contributed to workplace and community safety. The findings describe a sustained and scalable implementation plan for establishing a frequent workplace testing program. High-frequency testing programs offer the potential to break chains of transmission and act as an extra layer of protection in a comprehensive public health response.
We present a guide to developing a large-scale workplace screening program for COVID-19.
INTRODUCTION
Regular, frequent rapid antigen testing has been proposed as a scalable method to control coronavirus disease 2019 (COVID-19) transmission through asymptomatic screening (1, 2). A recent systematic review of rapid antigen tests (RATs) has demonstrated the effectiveness in detecting most cases early in the course of infection (3), and recent studies continue to support the finding that antigen tests are accurate (4). One of the reasons employees in workplaces are at risk is that we lack information on those who are asymptomatic and infectious and could transmit to others within workplaces or their homes (5). Instead, the protocols in place typically target those who are symptomatic and have gone for diagnostic testing, which can take several days, thereby missing the opportunity to quickly contain transmission before the onset of clear symptoms. Individuals who are infectious but asymptomatic (mostly because they are presymptomatic, paucisymptomatic, or have mild, vague symptoms) are important contributors to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission, particularly in the early stages of their infection (6, 7). Newer variants also may be more likely to be asymptomatic or have shorter infectious windows, making it more important to test before waiting for symptom onset (8). RATs have continued to have the ability to detect new variants of concern that have emerged over the pandemic (9). Without asymptomatic testing, there are no other ways to detect these infectious individuals until they become symptomatic, after which spread could have already occurred, and the ability to test-trace-isolate is substantially hindered (6, 7). The larger consequence of this information gap is that these infectious cases contribute to spread, uncontrolled transmission, and workplace shutdowns (5, 10–13). Rapid antigen screening for COVID-19 provides useful information on who is likely to be infectious (11), and information can be gained in real time to proactively manage infectious individuals, particularly when serial screening (e.g., at least two times weekly) is one of several layers of protection alongside others such as vaccination, masking, physical distancing, and ventilation (1).
While some asymptomatic screening programs have been described in the literature (12–15), they have been limited in size and scope or have been focused on contextually different settings outside of workplaces (16), including clinical settings (17). Furthermore, other large-scale screening programs that have a serial design (i.e., regular, frequent screening rather than ad hoc or one-time testing) have not had their implementation process systematically described, data collected, nor even suggestive evidence of their efficacy. Furthermore, public health officials often cite concerns regarding logistics or unintended consequences of RAT-based screening. However, no studies of this scale report on the experience of individuals and organizations participating in the program to adequately justify those concerns. The Creative Destruction Lab Rapid Screening Consortium (CDL RSC) is a not-for-profit initiative formed in Canada in August 2020 to help workplaces manage the COVID-19 crisis. The system was designed to be sector agnostic and applied to organizations of every size (from two or more employees up to hundreds of thousands). The guiding principle of the CDL RSC has been a comprehensive framing of the health crisis, specifically that the management of the COVID-19 pandemic is an information problem (5). This study, being the largest known implementation of a standardized routine asymptomatic screening program in a range of workplaces, with standardized data collection, fills many of these gaps.
The goal of this paper is to provide a comprehensive description of a real-world, large-scale implementation of a frequent antigen screening program implemented in hundreds of organizations across Canada. We describe the process and data on adoption and positive cases identified and report on experiences using survey data from participants and organizations.
RESULTS
The CDL RSC process
The CDL RSC is a coordinating entity that developed standard operating procedures (SOPs) to guide implementation in organizations and adherence to public health guidelines, a data system, and support for implementing organizations.
Standard operating procedures
An SOP is a process to be followed routinely in a designated situation. To enable a rapid antigen screening program at a wide variety of organizations, we developed a detailed playbook to the process that allows organizations to get up and running quickly. This guide is aimed at the people running the screening program for the individual organizations (“site leads”) and describes various aspects of implementing a rapid screening system that together represent the CDL RSC program. This guide is the central aspect of our screening program. It summarizes the process developed with the initial pilot partners in consultation with public health authorities. It provides participants with the knowledge they need to set up their own workplace screening. Table 1 briefly describes the SOP used in this program. See text S1 for complete information and links to documents.
Table 1. Elements of the SOPs followed by participants in the CDL RSC.
PCR, polymerase chain reaction.
| Step | Description |
| Preparation | The preparation requires leadership and (if applicable) union buy-in and a clear assignment of responsibility. Key decisions include who will administer the RATs (a health care professional, a trained professional, or self-screening by the individual employees) and whether screening will be mandatory or voluntary. |
| Forms | There are a variety of mandatory requirements and forms included in the process: the letter of agreement, software licensing agreement, contact registration, team member registration, an invitation to the CDL RSC community Slack workplace, new screening site, screen preorder, certificate of inventory, and regular questionnaires on RATs used, cases detected, and operational challenges. These elements are critical to ensure program fidelity and quality. |
| RATs and supplies | Site leads need a process for calculating the number of screens that RATs required (number of participants × 2 per week × number of weeks × 1.05 to allow for spoilage and training). There may also be distinct types of equipment needed, including tables, chairs, timers, gloves, gowns, and biohazard waste bags. The Canadian government provides RATs. RAT orders were tracked through a CDL RSC form; CDL RSC coordinated directly with the supply chain managers in the federal and provincial governments to support shipment fulfillment. For on-site screening programs, inventory control requires daily checks and a full weekly inventory of all supplies. |
| Screening station layout and setup where applicable |
While there is some flexibility, there are several requirements for screening stations if they are on-site. Waiting areas, traffic flow, and signage also need to be specified. Waiting areas, for example, require floor markings to allow people to be safely distanced. |
| Communications and registration | The program strongly encourages initial communication from the organization’s leadership. The goal is to provide sufficient information so that employees can understand the program’s purpose, make an informed decision about participation if voluntary, and give employees the opportunity to ask questions. A standard consent form is required for all participants (see text S2). |
| Screening frequency and booking | The program is anchored around regular screening, defined as at least twice every 7 days, as screening more frequently is needed with a less sensitive test to reduce transmission. It is continually emphasized that more frequent screening with a rapid turnaround time (i.e., isolating can happen right away) is as effective as less frequent screening with a more sensitive test. The days that testing is available need to be selected (e.g., Monday and Thursday) and communicated to employees. |
| Training | The program provides written materials and video guides that provide the necessary training for self- or supervised specimen collection. In addition, each screening kit comes with positive and negative control swabs. These control swabs validate that the screening devices are working correctly and that the administrator can perform and interpret the tests correctly. |
| Communicating results | Employees are immediately informed of results or declare if self-screening. This is automatic if using an application. It is recommended to collect another swab and repeat the RAT if inconclusive. To maintain confidentiality, any in-person or phone conversations must be in a private area where others cannot overhear. Furthermore, the participant should be able to ask questions. The employee should be told the next steps, including collecting their belongings, going home to self-isolate, and arranging a confirmatory diagnostic PCR test at a local assessment center. The organization also should begin their plan for internal contact tracing and notification processes once the confirmatory PCR test result is provided. |
| Data | To keep track of whether individuals are screened at least twice every 7 days, it is necessary to keep some identifying information for use only by the employer. A consent form and clear privacy policy are therefore required. In addition, data collected are deidentified before being sent to the CDL RSC central data infrastructure. This central data infrastructure is used to improve operations, prepare aggregated reports with public health authorities, and conduct evaluations. |
Engagement with public health authorities
Given that rapid antigen workplace screening is a new public health tool, we worked closely with public health authorities to ensure that the system adhered to public health guidelines while preserving its flexibility and operational feasibility. In particular, we repeatedly engaged in conversations and shared data that we had gathered in an effort to balance safety guidelines with the benefits of a procedure against the costs of not having that procedure available. As the CDL RSC was starting up, the regulatory environment in Canada was limited, with few screening technologies approved, and those that were approved required health care professionals to administer them. The situation was dynamic, with accommodations and updating needed to the administration throughout. We worked with public health authorities in most provinces and federally to describe the challenges in bringing rapid screening to workplaces of all sizes. Central to this process was relationship building through frequent communication and interaction both provincially and federally.
Regular screening
An important aspect of the CDL RSC requirement is for regular serial screening of employees, (i.e., at least two times weekly) rather than tracking the number of RATs used. Regular screening, which we define as two times per 7-day period, has been shown to reduce transmission (2). We selected twice per 7-day period on the basis of modeling studies that demonstrate the impact of screening every 3 days and the 3- to 5-day incubation period of the virus. Therefore, screening at least twice per week should detect most asymptomatic cases before they are able to infect others at the workplace, given the properties of the virus that were circulating at the time. There is also the benefit of the rapid turnaround of the test in more quickly being able to isolate immediately (18). Regular screening adds substantially to the operational burden of a rapid screening system because it requires a privacy-compliant data system to keep track of individual workers. Data privacy and consent, data collection and sharing, and the central data infrastructure aspects of the SOPs are all necessary to ensure that employees are regularly screened and not simply tested in an ad hoc manner.
Peer support
The CDL RSC provided training materials on how to set up the system with an existing organization, including a resource plan, job descriptions, and an organizational chart. To implement quickly and reduce errors, the program emphasizes information sharing and peer support through regular “town hall” meetings and through an organization-to-organization buddy system. The peer support system enables shared learning so that successful innovation can be scaled quickly and so that mistakes are not repeated across organizations.
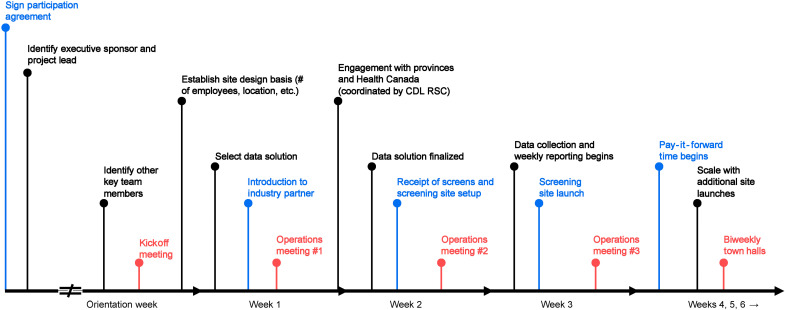
Figure 1 shows the onboarding process for the screening program. Once the participation agreement is signed and the team members are identified, the meetings begin. The kickoff meeting involves a public health orientation, a description of the playbook and actions that need to be taken, and an open discussion. Subsequent meetings allocate half the time to updates from the participating organizations and key public health updates, actions to be taken, the data system, and an open discussion. Toward the end, “pay-it-forward” time begins, in which participating organizations mentor an incoming cohort. Weekly meetings are designed and delivered in a specific manner to optimize an efficient transfer of knowledge across all organizations. The CDL RSC team engages with the community to encourage positivity, collaboration, and openness to extract anecdotes from participating organizations to build relationships, reinforce lessons learned, and ultimately develop best practices.
Fig. 1. The onboarding process for organizations enrolled in the RSC.
This timeline figure represents the starting point from which the organization decides to participate (far left), followed by the different stages of the program indicated by lines in the timeline. All organizations follow this process and ensure the integrity of the program. The arrow to the far right indicates that engagement through town halls continues as long as organizations are screening in the program.
Outcomes of the rapid screening system
We describe two types of results. First, we provide data from the CDL RSC data system on the number of organizations, number screened, and the number of positive cases identified. Second, we report the results of a survey of participating organizations.
Adoption, RATs, and cases
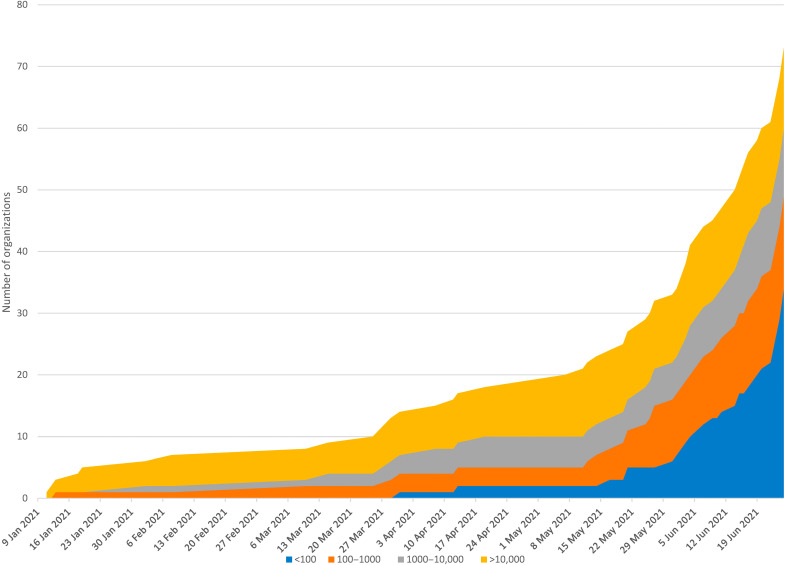
Figure 2 shows the number of distinct organizations in the CDL RSC over time. Organizations are considered active in the week that they record their first screen. Growth was slow at first and dominated by large organizations with more than 10,000 employees. In March and April, organizations between 100 and 10,000 employees joined. Starting in mid-May, most new organizations were under 100 employees.
Fig. 2. Total number of organizations over time by the number of employees between 11 January 2021 and 25 June 2021.
The number of organizations is on the y axis, and the calendar date is on the x axis. The graph represents the cumulative number across the program with colors indicating the size of the organization according to the number of employees, with blue representing organizations with less than 100 employees, orange indicating organizations with between 100 and 1000 employees, gray representing organizations with 1000 to 10,000 employees, and yellow representing those with >10,000 employees.
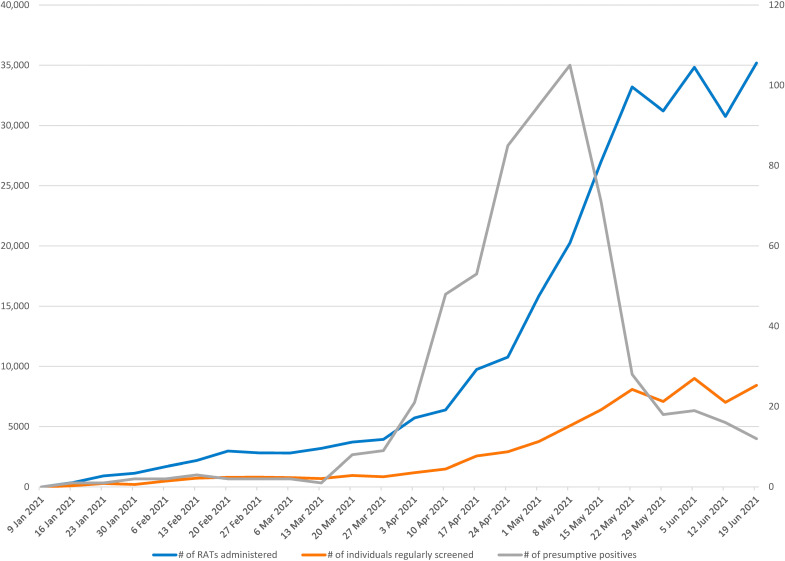
Figure 3 shows the weekly values for the number of RATs deployed, the number of people regularly screened (twice in 7 days), and the number of positives. The figure shows a sharp increase in the number of RATs and the number regularly screened during Canada’s third wave, in April and May 2021. After the wave, the number of people screened flattened out, even as the number of organizations continued to increase (as evidenced in Fig. 2). This means that the number of people screened per organization declined. The number of positive cases grew sharply in April and peaked at 105 during the week starting on 8 May 2021. Discussions with the organizations suggest that this decline in the number screened was due to vaccinated people opting out of the voluntary screening. As one site leader put it in the Slack channel on 7 May 2021, “People are thinking with vaccinations ramping up, rapid testing/screening is no longer required.”
Fig. 3. Total number of RATs administered, the number of employees regularly screening, and the number of presumptive positives (positive on RAT) over time between 11 January 2021 and 25 June 2021.
The total number of RATs and the number of employees regularly screened are counted on the left y axis, and the number of presumptive positives is counted on the right y axis. The calendar date is on the x axis. The lines on the graph represent the number of RATs (blue line), the number of employees that were regularly screening at least twice per week (orange line), and the number of presumptive positives (gray line).
In total, Table 2 shows that we identified 604 presumptive positive cases between the launch of the program on 11 January 2021 and 25 June 2021, from 321,905 total RATs used (see Table 1). Of these, 473 were confirmed by polymerase chain reaction (PCR), 75 were false positives, and we did not receive data for the remaining 56. Assuming that the ratio of 75 confirmed negatives to 473 confirmed positives holds for the unconfirmed, then approximately 1 in 4300 RATs comes back as false positive. Using nonparametric bounds for the missing data, between 1 in 2457 and 1 in 4292 were false positives (19). In contrast, if the ratio holds, then approximately 1 in 600 RATs identified cases that were confirmed positive by PCR.
Table 2. Total number of RATs deployed, presumptive positives, and reported PCR confirmation results in the program between 11 January 2021 and 25 June 2021.
| Variable | Total |
| RATs deployed | 321,905 |
| Number of organizations | 73 |
| Presumptive positive RATs | 604 |
| Confirmed positive by PCR | 473 |
| False positive (PCR negative) | 75 |
| No PCR data available | 56 |
| Abbott Panbio RATs | 285,465 |
| Abbott Panbio false positive (PCR negative) |
54 |
| BD Veritor RATs | 36,440 |
| BD Veritor false positive (PCR negative) |
21 |
The program used two different types of RATs: Abbott Panbio and BD Veritor. For Abbott Panbio, the rate confirmed by PCR false positive was approximately 1 in 5285 RATs. For BD Veritor, it was approximately 1 in 1735. In the literature, real-world estimates suggest that the Panbio is 73 to 98% sensitive and 100% specific (20–22) and that the BD Veritor is 84 to 96% sensitive and 99 to 100% specific (23). Overall, by taking at least 473 cases out of the workplace, regular workplace rapid antigen screening likely substantially reduced the prevalence of COVID-19 in workplaces that remained open.
Survey data
The survey results are provided as shown in fig. S1. We sent the survey to all 163 individuals identified as project leads and received 116 responses for a 71% response rate. In terms of satisfaction, 68% of participants were very satisfied with the program, and an additional 31% were satisfied. One participant was dissatisfied, although they remained in the program. This participant found the onboarding process more difficult than expected. In the unlikely scenario that the nonresponses represented the dissatisfied, the lower bound on satisfaction with the program is 70%.
In terms of recommendations, 73% strongly agree that they would recommend to others. Of these, 79% have already done so. An additional 23% agree, and 78% of these have already done so. The remaining 4% neither agree nor disagree, although 1 of them (25% of 4) has already recommended the program to others. Overall, 69% of respondents have already recommended the program to others.
The survey also examined the four aspects of the program described above.
1) SOPs: A total of 96% agreed or strongly agreed with the statement, “I had access to all the information needed to set up and operate my screening site.” One respondent strongly disagreed, and no respondents disagreed. A total of 89% agreed or strongly agreed with the statement, “When I was unsure what to do, I relied on the CDL RSC resources such as the playbook, Slack, and standard operating procedures.” A total of 3% disagreed or strongly disagreed. A total of 78% agreed or strongly agreed with the statement, “I found the data system valuable.” A total of 3% disagreed or strongly disagreed.
2) Engagement with public health authorities: A total of 69% agreed or strongly agreed with the statement, “The CDL RSC enabled communication with government and public health authorities.” A total of 28% neither agreed nor disagreed. A total of 3% disagreed or strongly disagreed.
3) Regular screening: A total of 89% of respondents agreed or strongly agreed with the statement, “Screening at least two times weekly is vital to the CDL RSC’s success.” A total of 3% disagreed or strongly disagreed. This high level of agreement suggests that the public health emphasis on safe workplaces through regular screening was understood by respondents.
4) Peer support: A total of 94% agreed or strongly agreed with the statement, “I felt there was a strong sense of community within the CDL RSC.” No one disagreed or strongly disagreed. A total of 77% agreed or strongly agreed with the statement, “The weekly operations meetings and biweekly town halls were a good use of my time.” A total of 10% disagreed or strongly disagreed. A total of 54% agreed or strongly agreed with the statement, “When I was unsure what to do, I relied on my industry partner/buddy.” A total of 20% disagreed or strongly disagreed. This suggests that peer support was useful for some, but many found that the SOPs and weekly meetings were more useful.
In addition, we sought feedback from employees in a wide range of roles, which have been collated in table S1. Employees reported feeling safe and more comfortable and confident in the workplace. Several commented on the importance of this program for keeping their families safe. Many described feeling initially apprehensive but remarked how easy the process was once established. Employees recognized that the program contributed to the safety of everyone around them, which resulted in a very positive experience.
DISCUSSION
Identifying and isolating COVID-19 cases are critical to the prevention and management of the pandemic. However, to date, testing has been overwhelmingly used for diagnostic purposes versus as an approach to contribute to control. We describe the first widespread implementation of a high-frequency antigen screening program. We detail the process for establishing and deploying a rapid screening program across multiple regulatory jurisdictions within a country. We further describe how implementation barriers were overcome, the experience of the users in the program, and the number of asymptomatic infected cases detected through the regular screening system.
Overall, we identify several aspects of the program including SOPs, regular communication with public health authorities, emphasis on regular screening, and peer support as important elements to overcome operational limitations often stated as a reason not to implement such programs. Throughout the program, we worked through several barriers to implementation, such as the additional logistics and cost of having health care professionals administer and the benefits of home screening (so that people did not come into the workplace setting with COVID-19). Public health officials, provincially and federally, were a critical part of the process in quickly responding to new information and addressing issues that would have severely hindered operations and ability to scale. One important feature of the system is the overwhelmingly positive feedback from participants from a range of programs. Participants emphasized the value of the CDL RSC as a forum for sharing best practices. Participants also understand the importance of biweekly screening to control transmission.
One of the most important findings is the 473 antigen-detected and PCR-confirmed asymptomatic and infectious cases that were identified by this screening program. As a result of screening, these infectious individuals did not enter the workplace and were able to be quickly isolated and traced. With the lags in time for symptoms to show up and turnaround time for diagnostic tests, had these cases not been screen-detected, they may have meaningfully contributed to COVID-19 transmission both within workplaces and within the community, particularly during high-growth periods. Related to this point, we have found RATs to be useful in breaking chains of transmission in workplaces regardless of vaccination status. This aligns with the current guidance that, for the purpose of detecting individuals who are infectious, RATs are useful regardless of symptom and vaccination status (24).
One important aspect of the interpretation of this study is the emphasis that these RATs were not meant to replace diagnostic tests or to loosen other public health measures. This message was consistently emphasized throughout the program, and the participants showed a good understanding of frequent screening as an extra layer of protection to control transmission.
Costs for a program such as this include the RATs, coordination of the delivery and program, confirmatory PCRs (many of which would be required eventually), and follow-up. However, on the benefit side, if the positive cases were not picked up sooner, then there may be an outbreak in the organization, and the outbreak may also be larger. These outbreaks result in high costs to the organization in the form of shutdowns and additional costs for the health system and society that come with preventing transmission early, as has been shown in cost-effectiveness studies (25).
There are several important limitations to acknowledge. First, this study describes the real-world experience of the program and does not include validation data that would have been included in other screening program assessments. That is, only those that screened presumptive positive were eligible for a confirmatory PCR. Nevertheless, we found the false-positive rate to be low, which is expected given several other studies that report the high specificity of these tests for detecting infectious individuals, including among asymptomatic individuals (17, 26, 27). Second, there is a possibility that those who did not respond to the survey had a different experience, potentially introducing some response bias to the results. However, we did have a high response rate, thus minimizing the impact of this. Third, the organizations opted to sign up, and thus, it is possible that the experience, when implemented outside those willing to implement such a program, would be different. However, we note that we had more than 600 organizations that varied widely in size and scope, thus representing a very broad cross section of organizations. Last, we cannot comment on this workplace antigen screening program’s impact on COVID-19 in the communities more broadly, requiring a different design and control data. We envision that this type of work may be possible in the future with more comprehensive data on the extent of the pandemic over time and in communities.
In summary, we demonstrate that it is feasible to implement a large-scale frequent rapid antigen screening program. The program successfully allowed for the easy and quick identification of asymptomatic infectious individuals, thereby contributing to further transmission. The learning from this real-world implementation has several implications for considering the implementation of a frequent, large-scale antigen-based screening in settings globally. Protection must be in place in workplaces to ensure that sick pay or other subsidy is in place to ensure that employees who test positive do not experience negative financial or other consequences. In addition, with increasing recognition of the importance of layered public health response to COVID-19, this study can be used to inform other broad-scale efforts that support rapid identification and isolation of infectious cases as part of a comprehensive response to control COVID-19 spread.
MATERIALS AND METHODS
We organized our study through two modalities. First, we provide descriptive statistics from the CDL RSC data system on the number of organizations, screenings, and positive cases identified. The number of employees was self-reported upon registration.
Second, we report the results of a survey of participating organizations as well as a brief qualitative employee survey. Each organization was requested to ask each employee to complete a form. The employees were prompted with the following questions: How did it feel to get screened? Why should other people get screened before going to their workplace? How do you feel about your organization implementing rapid screening systems? We received few responses to the employee survey. For the organization-level survey, we sent it to all 163 individuals identified as project leads and operators representing the 141 active companies on the day the survey was sent (12 July 2021). We received 116 responses from 94 different companies for a response rate of 71% at the individual level and 67% at the company level. Results are reported for all responses, including multiple individuals from the same company if applicable. The survey and survey results are available in the Supplementary Materials. The data were collected to inform the operational requirements of deploying rapid antigen screens in workplaces. This study was approved by the University of Toronto Research Ethics Board (protocol no. 42015).
Acknowledgments
We thank A. Kaur, K. Robinson, M. Vertolli, D. Elke and A. Burnett for help with the manuscript.
Funding: CDL RSC is a not-for-profit organization founded in November 2020 through the financial contributions of 12 corporations: Air Canada, CPP Investments, Genpact, Loblaw Companies Limited, Magna, MDA, MLSE, Nutrien, Rogers, Scotiabank, Shoppers Drug Mart, and Suncor. In April 2021, CDL RSC received funding via the Safe Restart Agreement (SRA) from the Government of Canada (Health Canada).
Author contributions: Conceptualization: A.A., J.G., and L.C.R. Process development: S.S., L.C.R., A.A., J.G., A.G., and J.S. Implementation: S.S., L.C.R., J.G., and J.S. Data analysis: A.G. Writing—original draft: A.G. Writing—review and editing: L.C.R., A.A., J.G., A.G., S.S., and J.S.
Competing interests: All authors comprise the steering committee of the CDL RSC, and they receive compensation for services to the CDL RSC. All authors have received research grants from the Government of Canada unrelated to this project. A.G.’s full disclosure statement is available at www.avigoldfarb.com/disclosure. J.G. has written the books Economics in the Age of Covid-19, The Pandemic Information Gap, and The Pandemic Information Solution on which he receives royalties. J.S. is a senior strategic advisor to Digital Public Square, a not-for-profit that works on the uses of technology for community development. The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Texts S1 and S2
Fig. S1
Table S1
REFERENCES AND NOTES
- 1.Mina M. J., Andersen K. G., COVID-19 testing: One size does not fit all. Science 371, 126–127 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Larremore D. B., Wilder B., Lester E., Shehata S., Burke J. M., Hay J. A., Tambe M., Mina M. J., Parker R., Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 7, eabd5393 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brümmer L. E., Katzenschlager S., Gaeddert M., Erdmann C., Schmitz S., Bota M., Grilli M., Larmann J., Weigand M. A., Pollock N. R., Macé A., Carmona S., Ongarello S., Sacks J. A., Denkinger C. M., Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 18, e1003735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui Z. K., Chaudhary M., Robinson M., McCall A. B., Peralta R., Esteve R., Callahan C. W., Manabe Y. C., Campbell J. D., Johnson K. J., Elhabashy M., Kantsiper M.; CONQUER COVID Consortium, Ficke J. R., Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol. Spectr. 9, e01008-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.J. Gans, The Pandemic Information Gap: The Brutal Economics of COVID-19 (MIT Press, 2020). [Google Scholar]
- 6.Sun K., Wang W., Gao L., Wang Y., Luo K., Ren L., Zhan Z., Chen X., Zhao S., Huang Y., Sun Q., Liu Z., Litvinova M., Vespignani A., Ajelli M., Viboud C., Yu H., Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 371, eabe2424 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansson M. A., Quandelacy T. M., Kada S., Prasad P. V., Steele M., Brooks J. T., Slayton R. B., Biggerstaff M., Butler J. C., SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw. Open 4, e2035057 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B., Deng A., Li K., Hu Y., Li Z., Xiong Q., Liu Z., Guo Q., Zou L., Zhang H., Zhang M., Ouyang F., Su J., Su W., Xu J., Lin H., Sun J., Peng J., Jiang H., Zhou P., Hu T., Luo M., Zhang Y., Zheng H., Xiao J., Liu T., Che R., Zeng H., Zheng Z., Huang Y., Yu J., Yi L., Wu J., Chen J., Zhong H., Deng X., Kang M., Pybus O. G., Hall M., Lythgoe K. A., Li Y., Yuan J., He J., Lu J., Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. medRxiv, 10.1101/2021.07.07.21260122 , (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekliz M., Adea K., Essaidi-Laziosi M., Sacks J. A., Escadafal C., Kaiser L., Eckerle I., SARS-CoV-2 antigen-detecting rapid tests for the delta variant. Lancet Microbe, 10.1016/S2666-5247(21)00302-5 , (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.P. Romer, A. M. Garber, Will Our Economy Die from Coronavirus? New York Times (23 March 2020).
- 11.Hellewell J., Abbott S., Gimma A., Bosse N., Jarvis C., Russell T., Munday J., Kucharski A., Edmunds W. J.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group, Funk S., Eggo R., Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health 8, E488–E496 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mboma O., Rieke E., Ahmad-Nejad P., Wirth S., Aydin M., Diagnostic performance of SARS-CoV-2 rapid antigen test in a large, German cohort. Children 8, 682 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D. P. Mancini, C. Cookson, Aggressive Testing Helps Italian Town Cut New Coronavirus Cases to Zero, Financial Times (17 March 2020).
- 14.G. Pisano, R. Sadun, M. Zanini, Lessons from Italy’s Response to the Coronavirus, Harvard Business Review (online edition) (27 March 2020).
- 15.Shaw J. L. V., Deslandes V., Smith J., Desjardins M., Evaluation of the Abbott PanbioTM COVID-19 Ag rapid antigen test for the detection of SARS-CoV-2 in asymptomatic Canadians. Diagn. Microbiol. Infect. Dis. 101, 115514 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albert E., Torres I., Bueno F., Huntley D., Molla E., Fernández-Fuentes M. A., Martínez M., Poujois S., Forqué L., Valdivia A., Solano de la Asunción C., Ferrer J., Colomina J., Navarro D., Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 27, 472.e7–472.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmon A., Chang C., Salcedo N., Sena B., Brooke Herrera B., Bosch I., Holberger L. E., Validation of an at-home direct antigen rapid test for COVID-19. JAMA Netw. Open 4, e2126931 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.J. Gans, The Pandemic Information Solution: Overcoming the Brutal Economics of Covid-19, (Endeavor Literary Press, 2021). [Google Scholar]
- 19.C. F. Manski, Patient Care Under Uncertainty (Princeton Press, 2018). [Google Scholar]
- 20.Ng Nsoga M. T., Kronig I., Perez Rodriguez F. J., Sattonnet-Roche P., Da Silva D., Helbling J., Sacks J. A., de Vos M., Boehm E., Gayet-Ageron A., Berger A., Jacquerioz-Bausch F., Chappuis F., Kaiser L., Schibler M., Renzoni A., Eckerle I., Diagnostic accuracy of Panbio rapid antigen tests on oropharyngeal swabs for detection of SARS-CoV-2. PLOS ONE 16, e0253321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gremmels H., Winkel B. W. F., Schuurman R., Rosingh A., Rigter N. A. M., Rodriguez O., Ubijaan J., Wensing A. M. J., Bonten M. J. M., Hofstra L. M., Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine 31, 100677 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linares M., Pérez-Tanoira R., Carrero A., Romanyk J., Pérez-García F., Gómez-Herruz P., Arroyo T., Cuadros J., Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 133, 104659 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pekosz A., Parvu V., Li M., Andrews J. C., Manabe Y. C., Kodsi S., Gary D. S., Roger-Dalbert C., Leitch J., Cooper C. K., Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin. Infect. Dis. 73, e2861–e2866 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention, Self-Testing, Updates December 6, 2021; www.cdc.gov/coronavirus/2019-ncov/testing/self-testing.html [accessed 11 December 2021].
- 25.Du Z., Pandey A., Bai Y., Fitzpatrick M. C., Chinazzi M., Pastore Y Piontti A., Lachmann M., Vespignani A., Cowling B. J., Galvani A. P., Meyers L. A., Comparative cost-effectiveness of SARS-CoV-2 testing strategies in the USA: A modelling study. Lancet Public Health 6, e184–e191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbasi J., Antigen testing every 3 days is highly sensitive for SARS-CoV-2. JAMA 326, 473 (2021). [DOI] [PubMed] [Google Scholar]
- 27.L. Ford, M. J. Whaley, M. M. Shah, P. P. Salvatore, H. E. Segaloff, A. Delaney, D. W. Currie, L. Boyle-Estheimer, M. O’Hegarty, C. N. Morgan, J. Meece, L. Ivacic, N. J. Thornburg, A. Tamin, J. L. Harcourt, J. M. Folster, M. Medrzycki, S. Jain, P. Wong, K. Goffard, D. Gieryn, J. Kahrs, K. Langolf, T. Zochert, J. E. Tate, C. H. Hsu, H. L. Kirking. Characteristics of children and antigen test performance at a SARS-CoV-2 community testing site. (medRxiv, 2021); www.medrxiv.org/content/10.1101/2021.07.06.21259792v1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Texts S1 and S2
Fig. S1
Table S1