Abstract
Background:
There are limited data on the long-term outcomes of COVID-19 from different parts of the world.
Aims:
To determine risk factors of 90-day mortality in critically ill patients in Turkish intensive care units (ICUs), with respiratory failure.
Study design:
Retrospective, observational cohort.
Methods:
Patients with laboratory-confirmed COVID-19 and who had been followed up in the ICUs with respiratory failure for more than 24 hours were included in the study. Their demographics, clinical characteristics, laboratory variables, treatment protocols, and survival data were recorded.
Results:
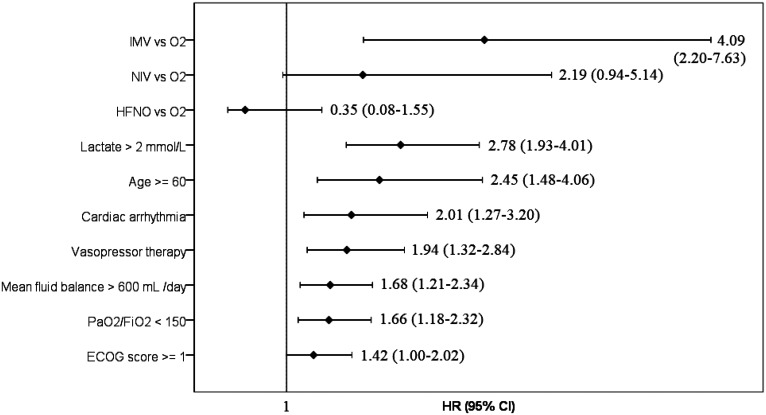
A total of 421 patients were included. The median age was 67 (IQR: 57-76) years, and 251 patients (59.6%) were men. The 90-day mortality rate was 55.1%. The factors independently associated with 90-day mortality were invasive mechanical ventilation (IMV) (HR 4.09 [95% CI: [2.20-7.63], P < .001), lactate level >2 mmol/L (2.78 [1.93-4.01], P < .001), age ≥60 years (2.45 [1.48-4.06)], P < .001), cardiac arrhythmia during ICU stay (2.01 [1.27-3.20], P = .003), vasopressor treatment (1.94 [1.32-2.84], P = .001), positive fluid balance of ≥600 mL/day (1.68 [1.21-2.34], P = .002), PaO2/FiO2 ratio of ≤150 mmHg (1.66 [1.18-2.32], P = .003), and ECOG score ≥1 (1.42 [1.00-2.02], P = .050).
Conclusion:
Long-term mortality was high in critically ill patients with COVID-19 hospitalized in intensive care units in Turkey. Invasive mechanical ventilation, lactate level, age, cardiac arrhythmia, vasopressor therapy, positive fluid balance, severe hypoxemia and ECOG score were the independent risk factors for 90-day mortality.
Keywords: COVID-19, critical care, prognosis, survival, mechanical ventilation
Introduction
The coronavirus disease-2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a global threat, causing critical illness in 5-26.4% of the affected patients.1−5 At the beginning of the pandemic, acute respiratory failure caused by severe pneumonia and acute respiratory distress syndrome (ARDS) were the predominant complications of COVID-19, whereas subsequently, it was realized that the virus damages the vascular endothelium, causing multi-system inflammatory syndrome and several complications such as kidney, cardiac, liver and brain injury,6, 7 vascular thrombosis,8 and macrophage activation syndrome.6 The short-term and long-term mortality rates of critically ill COVID-19 patients are quite high. While the intensive care unit (ICU), in-hospital, 28-day and 60-day mortality rates were reported to be up to 62%,4, 6, 9−13 mortality was reported to be even higher in patients who were mechanically ventilated (35.7-100%).11, 14−16 There are few studies reporting 90-day mortality in critically ill patients, varying between 26.9% and 31.0%.17, 18 Patient results may differ according to the geographical characteristics and income levels of countries, as well as the surge capacity of ICUs. The aim of this study was to determine the risk factors of 90-day mortality in COVID-19 patients with respiratory failure who were admitted to the Turkish ICUs.
Material and Methods
This study was performed retrospectively in 26 ICUs of 23 hospitals in Turkey between March 11, 2020 and June 11, 2020. Approvals from the Ministry of Health (2020-05-04T09_48_29) and Erciyes University Ethics Committee were obtained for the study (Date: July 22, 2020, No: 2020/401). Informed consent was waived.
Data Collection
Data were collected from the hospital electronic record systems and patient charts. Data were collected on electronic case report forms (eCRFs) by the study investigators, and then entered into the database. The investigators were trained for the study protocol, and using the database. Patient recruitment and data plausibility controls were performed daily. For data collection and management, the OpenClinica open source software 3.3 (Copyright© OpenClinica LLC and collaborators, Waltham, MA, USA, www.OpenClinica.com) was used. Independent query management, data cleaning, and source data verification were provided by Omega CRO, Ankara, Turkey, to obtain high-quality data. Patients with laboratory-confirmed COVID-19 and who had been followed up in the ICUs with respiratory failure for more than 24 hours were included in the study.19 Laboratory confirmation for SARS-CoV-2 was defined as a positive result of the real-time polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs or endotracheal aspirate, according to WHO guidelines,20 or a positive result in antibody testing with typical thorax computed tomography (CT) findings according to the Radiological Society of North America Expert Consensus Statement,21 excluding other diagnoses.
The following variables at admission, for the first 10 days of ICU follow-up, and till discharge from the hospital were collected on electronic case report forms, as follows: age, gender, Eastern Cooperative Oncology Group Performance Status (ECOG) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, co-morbidities, department of admission at the hospital, symptoms, duration from symptom onset to hospital and ICU admission, the ratio of partial arterial oxygen pressure (PaO2) to fraction of inspired oxygen (FiO2), lactate, the neutrophil to lymphocyte ratio, D-dimer, respiratory support type (conventional O2, high-flow nasal O2 (HFNO), non-invasive ventilation (NIV), or invasive ventilation (IMV)), types of treatment, use of neuromuscular blocker, vasopressor treatment, mean fluid balance per day (mean value of intake minus output per day for a 10-day follow-up period), acute kidney injury (AKI) as assessed by the Kidney Disease: Improving Global Outcomes (KDIGO) score,22 development of delirium, new-onset cardiac arrhythmia (including atrial fibrillation/flutter, sustained or non-sustained ventricular tachycardia), length of ICU and hospital stay, and the rates of ICU, 28-day, in-hospital, 60-day, and 90-day mortality. For patients who were discharged from the hospital or transferred to another hospital prior to 90 days, the national death notification system for mortality status was checked.
Statistical Analysis
The data were analyzed using the PASW Statistics for Windows, Version 18.0 (IBM SPSS Corp.; Armonk, NY, USA). Descriptive statistics were expressed as numbers and percentages (%) for categorical variables, and as median and interquartile range (IQR) for numerical variables. In the two-group and multiple group comparisons of categorical variables, the chi-square test or Fisher’s exact test was used as appropriate. For numerical variables, in two-group comparisons, the t-test or Mann−Whitney U-test was used as appropriate. Multivariate Cox regression analysis was performed by the stepwise backward likelihood ratio method, using the variables to be related with the survival in the univariate analysis. Kaplan–Meier survival curves until day 90 were computed and were compared according to respiratory support using the log-rank test. The statistical significance level was accepted as P < .05.
Results
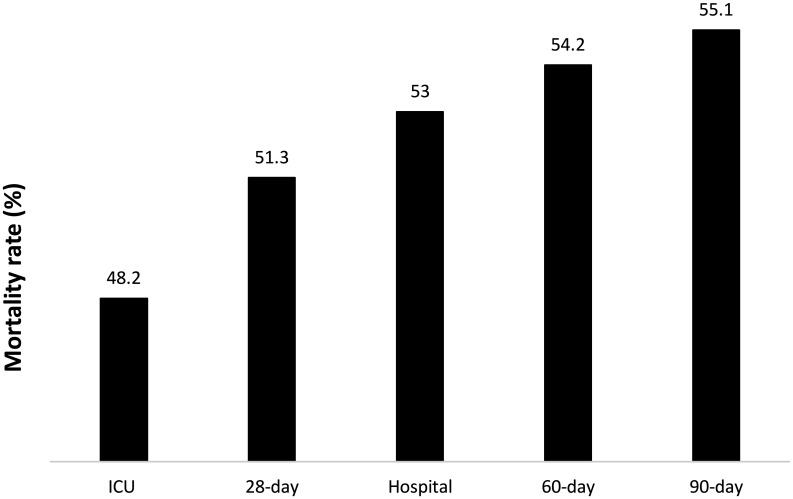
Patients were included from 26 ICUs (16 university hospitals, 10 public hospitals) in 23 hospitals (Table S1 and Figure S1). A total of 421 patients were enrolled in the study between March 11, 2020 and June 11, 2020. Of the total number of patients, 376 were PCR (+) and 45 were antibody (+), with typical thorax CT findings for COVID-19. The patients’ 90-day mortality rate was 55.1% (Figure 1).
FIG. 1.
ICU, 28-day, hospital, 60-day, and 90-day mortality rates.
The patient characteristics at admission and the 90-day survivor/non-survivor group comparisons are seen in Table 1. The median age was 67 (57-76) years, 297 (70.5%) patients being over 60 years old. The non-survivors were older than the survivors (P < .001). The median values of the APACHE II score and SOFA score were 17 (12-26) and 5 (3-8), respectively. The APACHE and SOFA scores of the non-survivor patients were higher than those of the survivors (P < .001 for both). Of the total patients, 87.6% had at least 1 comorbidity. Hypertension (51.3%) was the most common comorbidity, followed by diabetes (30.9%) and cardiac diseases (25.2%). The most common admission symptoms were shortness of breath (63.7%), cough (57.2%), and fever (55.1%). The median time from symptom onset to hospital admission was 3 (2-7) days and for ICU admission it was 7 (4-10) days. The median PaO2/FiO2 ratio was 140 (88.2-204.5) mmHg and was lower in the non-survivors (P < .001). Blood lactate (P < .001), neutrophil/lymphocyte ratio (P < .001), and D-dimer levels were higher in the non-survivor group than among the survivors (P < .01).
Table 1.
Patients’ Characteristics at ICU Admission
| All Patients, n = 421 | 90-Day Survivors, n = 189 | 90-Day Non-survivors, n = 232 | P | |
|---|---|---|---|---|
| Age, years, median (IQR) | 67 (57-76) | 58 (49-69) | 72 (64-79) | <.001 |
| Age ≥60 years, n (%) | 297 (70.5) | 89 (47.1) | 208 (89.7) | <.001 |
| Male, n (%) | 251 (59.6) | 111 (58.7) | 140 (60.3) | .74 |
| ECOG score,* n (%) | <.001 | |||
| 0 | 187 (45.6) | 114 (62.3) | 73 (32.2) | |
| 1 | 76 (18.5) | 30 (16.4) | 46 (20.3) | |
| 2 | 74 (18.0) | 24 (13.1) | 50 (22.0) | |
| 3 | 53 (12.9) | 13 (7.1) | 40 (17.6) | |
| 4 | 20 (4.9) | 2 (1.1) | 18 (7.9) | |
| APACHE II score, median (IQR) | 17 (12-26) | 13 (9-18) | 23 (16-30) | <.001 |
| APACHE II score ≥17, n (%) | 205 (49.8) | 47 (25.3) | 158 (69.9) | <.001 |
| SOFA score, median (IQR) | 5 (3-8) | 3 (2-5) | 7 (4-10) | <.001 |
| SOFA score ≥5, n (%) | 199 (51.0) | 51 (29.0) | 148 (69.2) | <.001 |
| Comorbid diseases, n (%) | 369 (87.6) | 151 (79.9) | 218 (94) | <.001 |
| Hypertension | 216 (51.3) | 80 (42.3) | 136 (58.6) | .001 |
| Diabetes mellitus | 130 (30.9) | 42 (22.2) | 88 (37.9) | <.001 |
| Cardiac disease | 106 (25.2) | 31 (16.4) | 75 (32.3) | <.001 |
| Respiratory disease | 82 (19.5) | 36 (19) | 46 (19.8) | .84 |
| Cancer | 45 (10.7) | 14 (7.4) | 31 (13.4) | .049 |
| Cerebrovascular disease | 37 (8.8) | 7 (3.7) | 30 (12.9) | .001 |
| Dementia | 26 (6.2) | 9 (4.8) | 17 (7.3) | .27 |
| End-stage renal disease | 15 (3.6) | 2 (1.1) | 13 (5.6) | .01 |
| Admission department of hospital, n (%) | ||||
| Ward | 247 (58.6) | 125 (66.1) | 122 (52.7) | |
| Emergency department | 119 (28.4) | 43 (22.8) | 76 (32.9) | .03 |
| Other | 55 (13.1) | 23 (12.1) | 31 (13.4) | |
| Admission symptoms, n (%) | ||||
| Shortness of breath | 268 (63.7) | 114 (60.3) | 154 (66.4) | .20 |
| Cough | 241 (57.2) | 120 (63.5) | 121 (52.2) | .02 |
| Fever | 232 (55.1) | 122 (64.6) | 110 (47.4) | <.001 |
| Weakness | 126 (29.9) | 57 (30.2) | 69 (29.7) | .93 |
| Myalgia | 46 (10.9) | 29 (15.3) | 17 (7.3) | <.01 |
| Diarrhea | 20 (4.8) | 10 (5.3) | 10 (4.3) | .64 |
| Chest pain | 19 (4.5) | 7 (3.7) | 12 (5.2) | .47 |
| Sore throat | 6 (1.4) | 3 (1.6) | 3 (1.3) | 1.00 |
| Duration from symptom onset to hospital admission, days, median (IQR) | 3 (2-7) | 4 (2-7) | 3 (2-5) | .02 |
| Duration from symptom onset to ICU admission, days, median (IQR) | 7 (4-10) | 7 (5-10) | 6 (3-10) | <.01 |
| Symptom onset to ICU admission of <7 days, n (%) | 192 (49.6) | 79 (43.4) | 113 (55.1) | .02 |
| PaO2/FiO2, mmHg, median (IQR) | 140 (88-205) | 170 (110-222) | 125 (75-188) | <.001 |
| PaO2/FiO2 ≤150 mmHg, n (%) | 210 (53.2) | 73 (41.0) | 137 (63.1) | <.001 |
| Lactate, mmol/L, median (IQR) | 1.4 (1-2) | 1.2 (0.9-1.6) | 1.6 (1.1-2.4) | <.001 |
| Lactate >2 mmol/L, n (%) | 98 (24.4) | 18 (10.3) | 80 (35.4) | <.001 |
| Neutrophil/lymphocyte ratio, median (IQR) | 8.0 (4.5-13.1) | 6.4 (4.0-9.8) | 9.8 (5.7-16.8) | <.001 |
| Neutrophil/lymphocyte ratio ≥8, n (%) | 192 (49.7) | 61 (33.9) | 131 (63.6) | <.001 |
| D-dimer, ng/mL, median (IQR) | 1016 (538-2186) | 820 (460-1670) | 1200 (600-2850) | <.01 |
| D-dimer >1000 ng/mL, n (%) | 123 (50.6) | 49 (43.0) | 74 (57.4) | .02 |
*ECOG score: 0, Fully active; 1, Restricted in physically strenuous activity; 2, Ambulatory and capable of all selfcare; up and about more than 50% of waking hours; 3, Capable of only limited selfcare; confined to bed or chair more than 50% of waking hours; 4, Completely disabled; totally confined to bed or chair.
The data on treatment approaches, complications, and length of stay from admission to discharge for the survivor and non-survivor groups are seen in Table 2. At admission and during 10-day follow-up, conventional oxygen support was provided to 31.6% of the patients. HFNO and NIV were provided to 8.3% and 6.2% of patients, respectively, and 53.9% of the patients were intubated and mechanically ventilated. Hydroxychloroquine (88.6%), favipiravir (82.7%), antibiotics (82.7%), azithromycin (56.8%), oseltamivir (47.7%), and corticosteroids (31.6%) were the medications most frequently administered to the patients. The mean fluid balance was 600 (275-1000) ml/per day and was higher in the non-survivor group (P < .001). The common complications observed in patients during the follow-up were delirium (9.7%) and cardiac arrhythmias (7.8%). The median values of length of stay in ICU and hospital were 7 (3-14) and 15 (9-23) days, respectively.
Table 2.
Treatment, Complications and Length of Stay from ICU Admission Till Discharge
| All Patients, n = 421 | 90-Day Survivors, n = 189 | 90-Day Non-survivors, n = 232 | P | |
|---|---|---|---|---|
| Respiratory support, n (%) | ||||
| Conventional oxygen | 133 (31.6) | 104 (55.0) | 29 (12.5) | |
| High-flow nasal oxygen | 38 (8.3) | 30 (15.9) | 5 (2.2) | <.001 |
| Non-invasive ventilation | 26 (6.2) | 15 (7.9) | 11 (4.7) | |
| Invasive ventilation | 227 (53.9) | 40 (21.2) | 187 (80.6) | |
| Treatment, n (%) | ||||
| Hydroxychloroquine | 373 (88.6) | 168 (88.9) | 205 (88.4) | .86 |
| Favipiravir | 348 (82.7) | 156 (82.5) | 192 (82.8) | .95 |
| Antibiotics | 348 (82.7) | 145 (76.7) | 203 (87.5) | <.01 |
| Azithromycin | 239 (56.8) | 112 (59.3) | 127 (54.7) | .35 |
| Oseltamivir | 201 (47.7) | 96 (50.8) | 105 (45.3) | .25 |
| Corticosteroids | 133 (31.6) | 55 (29.1) | 78 (33.6) | .32 |
| Vitamin C (more than RDA) | 131 (31.1) | 52 (27.5) | 79 (34.1) | .14 |
| Convalescent plasma | 53 (12.6) | 23 (12.2) | 30 (12.9) | .81 |
| Tocilizumab | 44 (10.5) | 17 (9.0) | 27 (11.6) | .37 |
| Zinc (more than RDA) | 35 (8.3) | 17 (9.0) | 18 (7.8) | .64 |
| Thiamine (more than RDA) | 31 (7.4) | 15 (7.9) | 16 (6.9) | .68 |
| Lopinavir/ritonavir | 21 (5.0) | 8 (4.2) | 13 (5.6) | .52 |
| Intravenous immunoglobulin | 10 (2.4) | 4 (2.1) | 6 (2.6) | 1.00 |
| Cytokine removal | 9 (2.1) | 3 (1.6) | 6 (2.6) | .73 |
| Plasmapheresis | 5 (1.2) | 2 (1.1) | 3 (1.3) | 1.00 |
| Neuromuscular blocker use, n (%) | 98 (23.5) | 17 (9) | 81 (35.4) | <.001 |
| Vasopressor therapy, n (%) | 173 (41.1) | 21 (11.1) | 152 (65.5) | <.001 |
| Mean fluid balance per day, median (IQR) | 600 (275-1000) | 393 (167-663) | 872 (400-1431) | <.001 |
| Mean fluid balance of ≥600 per day, n (%) | 202 (50.4) | 60 (33.7) | 142 (63.7) | <.001 |
| Acute kidney injury, n (%) | 155 (38.9) | 26 (14.4) | 129 (59.4) | <.001 |
| Delirium, n (%) | 41 (9.7) | 22 (11.6) | 19 (8.2) | .24 |
| Cardiac arrhythmia, n (%) | 33 (7.8) | 3 (1.6) | 30 (12.9) | <.001 |
| Length of ICU stay, days, median (IQR) | 7 (3-14) | 6 (3-13) | 8 (4-16) | .04 |
| Length of hospital stay, days, median (IQR) | 15 (9-23) | 17 (12.5-25.5) | 13 (7-22) | <.001 |
RDA, recommended daily allowance.
As seen in Figure 2, the multivariate Cox regression analysis revealed the following 8 variables relevant to 90-day mortality: invasive mechanical ventilation (IMV) (conventional oxygen as the reference), high lactate, age ≥60 years, presence of cardiac arrhythmia, vasopressor therapy, mean fluid balance ≥600 mL/day, PaO2/FiO2 ≤150 mmHg, ECOG score ≥1, adjusted for other variables SOFA ≥5 and APACHE II ≥17, presence of hypertension, diabetes mellitus, cardiac disease, cancer, cerebrovascular disease, end-stage renal disease, department of admission at the hospital, onset of symptoms to ICU admission <7 days; neutrophil/lymphocyte ratio ≥8; neuromuscular blocker use; and development of AKI.
FIG. 2.
Multivariate Cox Proportional Hazard Regression Analysis of factors associated with 90-day mortality, as a forest plot graph. IMV, invasive mechanical ventilation; O2, oxygen; NIV, non-invasive mechanical ventilation; HFNO, high-flow nasal oxygen; PaO2, partial arterial oxygen pressure; FiO2, fraction of inspired oxygen; ECOG, astern Cooperative Oncology Group Performance Status; HR, hazard ratio.
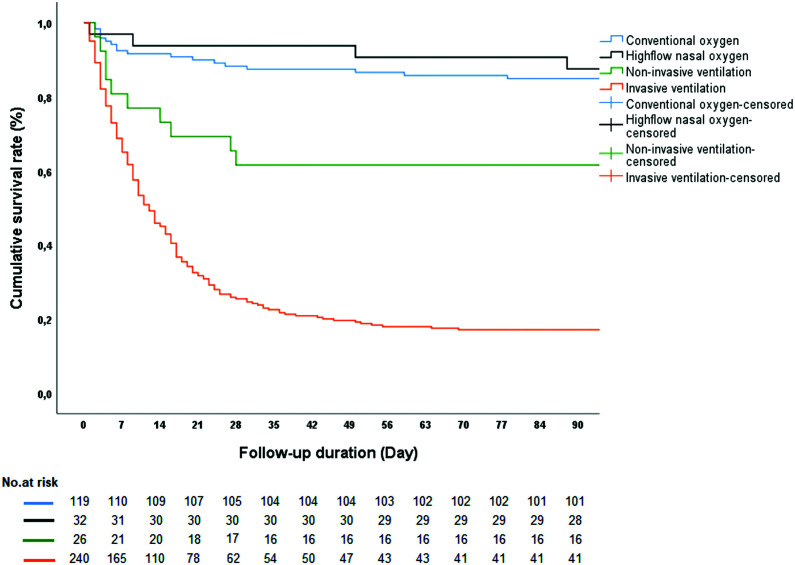
As seen in Figure 3, the Kaplan–Meier survival analysis revealed significantly lower survival in patients who had received IMV, compared to those who had received conventional O2, HFNO, and NIV (log-rank P value <.001).
FIG. 3.
Kaplan–Meier survival curves for 90-day mortality according to respiratory support. Log-rank test P < .001.
Discussion
This study, reporting the results of the first wave of the pandemic in Turkish ICUs, revealed a 90-day mortality of 55.1%. Invasive mechanical ventilation, high lactate level, age greater than 60 years, development of cardiac arrhythmia, need for vasopressor treatment, positive fluid balance, severe hypoxemia (PaO2/FiO2 ≤150), and an ECOG performance status that was not fully active were determined to be the independent risk factors for 90-day mortality.
There are few studies reporting 90-day mortality rate and the risk factors for mortality in critically ill patients with COVID-19.17, 18 In a multicenter, prospective, cohort study conducted in patients with laboratory-confirmed COVID-19 who were admitted to ICUs of 138 hospitals in France, Belgium, and Switzerland, the 90-day mortality rate was found to be 31%, and older age, immunosuppression, severe obesity, diabetes mellitus, higher renal and cardiovascular SOFA scores, lower PaO2/FiO2 ratios, and shorter time between onset to first symptoms and ICU admission were found to be independent risk factors for 90-day mortality.
Zettersten et al. performed a nationwide cohort study about the long-term outcome for COVID-19 patients in Swedish ICUs. The rate for 90-day mortality was found to be 26.9%. In Cox regression analysis, male sex, older age, comorbid diseases, and month of admission were found to be associated with mortality.18
Mortality rates in critically ill COVID-19 patients differ worldwide, with the ICU mortality rates reported from 29% to 51.8%,16, 23 hospital mortality from 42% to 60.4%,24, 25 28-day mortality at 35.4%,24 and 60-day mortality at 61.5%.13
These differences might be due to differences in disease severity, surge capacity, available resources, and other related factors. Turkey has a sufficient number of intensive care beds, reported to be more than 30 adult ICU beds per 100 000 population, with around 30% of them located in private hospitals which had limited roles in the pandemic. However, there is a severe shortage of intensive care workers, especially nurses, in Turkey. In the OECD countries, the number of physicians per 100 000 population is 348 and the number of nurses per 100 000 population is 938. In Turkey, these numbers are 348 and 301, respectively. During the COVID-19 pandemic, surge-capacities were enhanced in many countries.26−29 In a study conducted in Australia, the number of intensive care beds in the first wave of the pandemic was increased by 191%, the number of ventilators by 120%, the number of senior doctors by 240%, and the number of intensive care nurses by 249%.28 However, in Turkey, even prior to COVID-19, the number of patients per nurse could be as high as 4-5 in the third-level comprehensive units. The number of physiotherapists are still more negligible.
Acute respiratory failure is the most common cause of intensive care admission in COVID-19 patients.17, 30, 31 Most of these patients require IMV. In some studies, the frequency of IMV has been shown to vary between 12.2% and 88%.1, 13, 24, 32 In our study, 52.4% of the patients had PaO2/ FiO2 ratio below 150%, and 53.9% of the patients needed IMV at admission or during the follow-up. In this study, both the IMV and PaO2/FiO2 ≤150 were found to be risk factors determining 90-day mortality. In studies conducted in different parts of the world, the mortality rates have been shown to vary between 35.7% and 100% in invasively ventilated patients.11, 14, 16 This variety among studies might be due to differences in the standards of care, surge capacity, resources, and due to different treatment approaches in the first wave.26
Blood lactate levels are used mostly to follow-up on tissue perfusion in critically ill patients.33 Even minor increases in lactate levels are associated with higher mortality rates.34 In this study, the non-survivors had higher lactate levels compared to the survivors, and a lactate level >2 mmol/L was an independent predictor of mortality. In COVID-19 patients, the lactate levels were reported to be high in the non-survivors.17, 24, 31 However, it was not determined to be a risk factor for any of these patients, in the multivariate analysis.
In this study, vasopressor treatment, which was administered to 41.1% of the patients, was also an independent predictor of mortality. Although we have not recorded the underlying reasons for vasopressor use, this is a predictor of disease severity, and to our knowledge, there is no other study reporting vasopressor treatment as an independent risk factor for mortality, except the REVA network study revealing the cardiovascular SOFA score as being an independent risk factor for mortality.17
In this study, positive fluid balance was found to be an independent risk factor for 90-day mortality in ICU patients. It was well known that positive fluid balance increases morbidity and mortality in critically ill patients with sepsis, septic shock, and ARDS.35, 36 It had also been shown that negative fluid balance reduces respiratory failure and the need for renal replacement therapy in critically ill patients.37, 38 To our knowledge, this is the first study reporting positive fluid balance as an independent predictor of mortality. This finding necessitates meticulous control of fluid balance in critically ill COVID-19 patients.
The ECOG scoring system is used to evaluate the physical performance of patients in chronic diseases such as cancer.39 In this study, we preferred to use the ECOG score as it is easy to use and is widely known. Patients who were not fully active had poor prognosis. To our knowledge, this factor was not evaluated in other studies. However, in the REVA network-COVID-ICU study, the clinical frailty scale (CFS) was found to be higher in patients who died within 90 days,17 as in the VIP1 study, where CFS was found to be related with 30-day mortality in critically ill very elderly patients.40
In some studies, cardiac damage/arrhythmia was reported to be common in COVID-19 patients, and related with increased mortality.41, 42 We observed cardiac arrhythmia in 33 (7.8%) patients, and this was a risk factor that increased the 90-day mortality, similar to other studies. This may be due to medications and/or due to the disease itself. In the early days of the COVID-19 pandemic, hydroxychloroquine, alone or combined with azithromycin, was used frequently in some parts of the world 24, 41, 43 and in our country2 as well. In this study, hydroxychloroquine had been given to 86% and azithromycin to 56.8% of the patients.
The major strength of this study is that it has been conducted as a multicenter study in Turkish ICUs. As the pandemic is a major threat globally, data from various countries and geographic regions with different income levels and resources are needed. In addition, there are few studies reporting long-term mortality rates in COVID-19 patients, to our knowledge, with this one being the second reporting 90-day mortality. We need studies on outcomes for even longer terms. Lastly, some novel independent predictors of mortality such as positive fluid balance, baseline performance status, high admission lactate and vasopressor use have been determined, which need to be validated in future studies.
However, this study has several limitations. The number of ICUs and patients are limited due to the short study duration, and although ICUs entering the study are from different regions of Turkey, the results might not be representative of the whole country. The study was conducted during the first wave, and might not reflect the current situation. In addition, there might be other factors influencing mortality, which could not have been included in the study due to its design.
In this multicenter cohort study of critically ill adult patients with COVID-19 in Turkey, more than half of the patients died within 90 days after ICU admission. Receiving invasive mechanical ventilation, a lactate level >2 mmol/L, age ≥60 years, cardiac arrhythmia during ICU stay, receiving vasopressor treatment during ICU stay, a positive fluid balance of ≥600 mL per day during ICU follow-up, an admission PaO2/FiO2 ratio of ≤150 mmHg, and a baseline ECOG score ≥1 have been found to be independent risk factors for 90-day mortality. Some factors, such as positive fluid balance, are modifiable risk factors which need to be paid attention to during follow-up of these patients. In addition, patients should be protected from complications such as cardiac arrhythmias by close monitoring, avoiding electrolyte imbalances, and administering related medications.
Supplementary FIG. 1.
List of participant sites - —Map of Turkey.
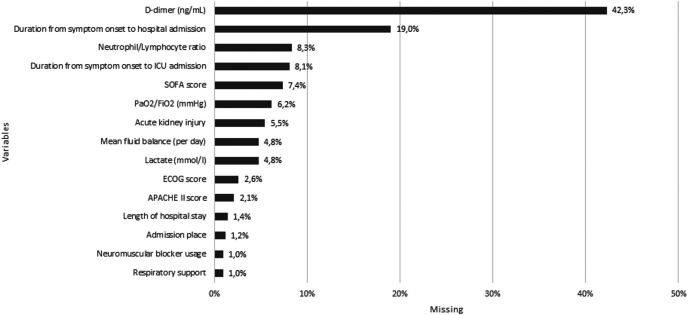
Supplementary FIG. 2.
List of missing data.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Erciyes University (Date: July 22, 2020 no: 2020/401).
Patient Consent for Publication: Written informed consent was waived.
Data-sharing Statement: After publication, data are available upon reasonable request. A proposal with a detailed description of study objectives and a statistical analysis plan will be needed for evaluation of the reasonability of requests. Additional materials might also be required during the process of evaluation.
Preprint History: Previously posted to Research Square as a preprint on January 21, 2021 (DOI:10.21203/rs.3.rs-150961/v1).
Author Contributions: Concept, design of the work, data acquisition, and analysis - KG, IHA, PH, BH, ST, and AT; Interpretation of data, the creation of new software used in the work, drafting, and revising the manuscript - KG, IHA, PH, BH, ST, ZG, KI, YB, FTB, FY, MS, RCY, EE, NDA, LT, GE, GG, IK, EA, SY, TM, SS, TA, BAC, AAA,DAO, TKS, AUE, AF, FA, MCG, AZ, AG, MT, MA, RU, JBC, CB, CK, EK, EO, EOE, SO, IAS, IHT, BC, BE, KTS, JE, UGY, NT, MS, and AT.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323(20):2052 - 2059. 10.1001/jama.2020.6775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahin Temel KG, Ulger B., Arican H. et al. Characteristics and outcomes of patients infected with SARS-CoV-2 admitted to intensive care units: Erciyes University COVID-19 center experience. Erciyes Med J. 2020. [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y.et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708 - 1720. 10.1056/NEJMoa2002032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu C, Chen X, Cai Y.et al. Risk factors associated With acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934 - 943. 10.1001/jamainternmed.2020.0994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halacli B, Kaya A, Topeli A. Critically-ill COVID-19 patient. Turk J Med Sci. 2020;50(SI-1):585 - 591. 10.3906/sag-2004-122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox SE, Akmatbekov A, Harbert JL.et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681 - 686. 10.1016/S2213-2600(20)30243-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gündoğan KTŞ, Baran Ketencioğlu B, Rabah B, Tutar N, Sungur M. Acute Kidney Injury in SARS-CoV-2 infected critically ill patients. Turk J Nephrol 2020;3:185 - 18 9. [Google Scholar]
- 8. Tutar N, Baran Ketencioglu B, Temel Ş, et al. Images in Vascular Medicine: peripheral artery thrombosis in critically ill patients with COVID-19. Vasc Med (London, England). 2020. PubMed: 1358863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petrilli CM, Jones SA, Yang J.et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York city: prospective cohort study. BMJ (Clin Res Ed). 2020;369:m1966. 10.1136/bmj.m1966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasselli G, Greco M, Zanella A.et al. Risk factors associated with mortality among patients With COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345 - 1355. 10.1001/jamainternmed.2020.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karagiannidis C, Mostert C, Hentschker C.et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853 - 862. 10.1016/S2213-2600(20)30316-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie J, Wu W, Li S.et al. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020;46(10):1863 - 1872. 10.1007/s00134-020-06211-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu J, Yang X, Yang L.et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24(1):394. 10.1186/s13054-020-03098-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auld SC, Caridi-Scheible M, Blum JM.et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48(9):e799 - e804. 10.1097/CCM.0000000000004457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou F, Yu T, Du R.et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054 - 1062. 10.1016/S0140-6736(20)30566-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ñamendys-Silva SA, Alvarado-Ávila PE, Domínguez-Cherit G.et al. Outcomes of patients with COVID-19 in the intensive care unit in Mexico: a multicenter observational study. Heart Lung. 2021;50(1):28 - 32. 10.1016/j.hrtlng.2020.10.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2020:1 - 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zettersten E, Engerström L, Bell M.et al. Long-term outcome after intensive care for COVID-19: differences between men and women-a nationwide cohort study. Crit Care. 2021;25(1):86. 10.1186/s13054-021-03511-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turkish Ministiry of health , Covid-19 guideline. (available at: https://www.ekmud.org.tr/files/uploads/files/Saglik-Bakanligi-COVID-19-rehberi-23032020.pdf); 2020:28 - 34. [Google Scholar]
- 20. World Health OrganizationClinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. January 28, 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. [Google Scholar]
- 21. Simpson S, Kay FU, Abbara S.et al. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - secondary publication. J Thorac Imaging. 2020;35(4):219 - 227. 10.1097/RTI.0000000000000524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179 - c184. 10.1159/000339789) [DOI] [PubMed] [Google Scholar]
- 23. Nachtigall I, Lenga P, Jóźwiak K.et al. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect. 2020;26(12):1663 - 1669. 10.1016/j.cmi.2020.08.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cummings MJ, Baldwin MR, Abrams D.et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York city: a prospective cohort study. Lancet. 2020;395(10239):1763 - 1770. 10.1016/S0140-6736(20)31189-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richards-Belle A, Orzechowska I, Gould DW.et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020;46(11):2035 - 2047. 10.1007/s00134-020-06267-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azoulay E, de Waele J, Ferrer R. et al. International variation in the management of severe COVID-19 patients. Crit Care. 2020;24(1):486. 10.1186/s13054-020-03194-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salluh JIF, Lisboa T, Bozza FA. Challenges for the care delivery for critically ill COVID-19 patients in developing countries: the Brazilian perspective. Crit Care. 2020;24(1):593. 10.1186/s13054-020-03278-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Litton E, Bucci T, Chavan S.et al. Surge capacity of intensive care units in case of acute increase in demand caused by COVID-19 in Australia. Med J Aust. 2020;212(10):463 - 467. 10.5694/mja2.50596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kazzaz YM, Alkhalaf H, Alharbi M.et al. Hospital preparedness and management of pediatric population during COVID-19 outbreak. Ann Thorac Med. 2020;15(3):107 - 117. 10.4103/atm.ATM_212_20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du RH, Liang LR, Yang CQ.et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5);2000524. 10.1183/13993003.00524-2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang X, Yu Y, Xu J.et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475 - 481. 10.1016/S2213-2600(20)30079-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grasselli G, Zangrillo A, Zanella A.et al. Baseline characteristics and outcomes of 1591 patients infected With SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574 - 1581. 10.1001/jama.2020.5394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726 - 1734. 10.1056/NEJMra1208943) [DOI] [PubMed] [Google Scholar]
- 34. Nichol AD, Egi M, Pettila V.et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care. 2010;14(1):R25. 10.1186/cc8888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Mourik N, Geerts BF, Binnekade JM.et al. A higher fluid balance in the days After septic shock reversal is associated with increased mortality: an observational cohort study. Crit Care Explor. 2020;2(10):e0219. 10.1097/CCE.0000000000000219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Oliveira FS, Freitas FG, Ferreira EM.et al. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30(1):97 - 101. 10.1016/j.jcrc.2014.09.002) [DOI] [PubMed] [Google Scholar]
- 37. Douglas IS, Alapat PM, Corl KA.et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest. 2020;158(4):1431 - 1445. 10.1016/j.chest.2020.04.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang YM, Zheng YJ, Chen Y.et al. Effects of fluid balance on prognosis of acute respiratory distress syndrome patients secondary to sepsis. World J Emerg Med. 2020;11(4):216 - 222. 10.5847/wjem.j.1920-8642.2020.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdel-Rahman O. ECOG performance score 0 versus 1: impact on efficacy and safety of first-line 5-FU-based chemotherapy among patients with metastatic colorectal cancer included in five randomized trials. Int J Colorectal Dis. 2019;34(12):2143 - 2150. 10.1007/s00384-019-03430-y) [DOI] [PubMed] [Google Scholar]
- 40. Flaatten H, De Lange DW, Morandi A.et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥80 years). Intensive Care Med. 2017;43(12):1820 - 1828. 10.1007/s00134-017-4940-8) [DOI] [PubMed] [Google Scholar]
- 41. Gupta S, Hayek SS, Wang W.et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436 - 1447. 10.1001/jamainternmed.2020.3596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi S, Qin M, Shen B.et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802 - 810. 10.1001/jamacardio.2020.0950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.RECOVERY Collaborative Group, Horby P, Mafham M.et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19. N Engl J Med. 2020;383(21):2030 - 2040. 10.1056/NEJMoa2022926) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a