Abstract
Manual proteomic sample preparation methods limit sample throughput and often lead to poor data quality when thousands of samples must be analyzed. Automated liquid handler systems are increasingly used to overcome these issues for many of the sample preparation steps. Here, we detail a step-by-step protocol to prepare samples for bottom-up proteomic analysis for Gram-negative bacterial and fungal cells. The full modular protocol consists of three optimized protocols to: (A) lyse Gram-negative bacteria and fungal cells; (B) quantify the amount of protein extracted; and (C) normalize the amount of protein and set up tryptic digestion. These protocols have been developed to facilitate rapid, low variance sample preparation of hundreds of samples, be easily implemented on widely-available Beckman-Coulter Biomek automated liquid handlers, and allow flexibility for future protocol development. By using this workflow 50 micrograms of protein from 96 samples can be prepared for tryptic digestion in under an hour. We validate these protocols by analyzing 47 Pseudomonas putida and Rhodosporidium toruloides samples and show that this modular workflow provides robust, reproducible proteomic samples for high-throughput applications. The expected results from these protocols are 94 peptide samples from Gram-negative bacterial and fungal cells prepared for bottom-up quantitative proteomic analysis without the need for desalting column cleanup and with protein relative quantity variance (CV%) below 15%.
Introduction
Proteomic sample preparation protocols consist of many liquid transfer steps that are well suited for automation with liquid handler systems. As the number of proteomic samples for biotechnological and clinical applications increases, automated solutions will be required to minimize human error, save time and resources, and improve the data quality. There have been a number of automated sample preparation protocols developed for both mammalian and bacterial cells that reduce processing time, variability, and overall cost [1–12]. Most of these methods automate the sample cleanup and tryptic digestion portions of the workflow whereas a few automate the entire workflow from cell lysis to digestion [5, 6, 11]. These automation methods show significant improvement in variability and time-savings over manual sample preparation methods. Additionally, high-quality, low variance results can be achieved by researchers without extensive experience in proteomic sample preparation. While automation methods for the full workflow are powerful and convenient they are not as flexible, consequently, when proteomic research projects incorporate new organisms, different amounts of cells, or other variations the entire automated process must be modified. The three protocols described here separate the steps of the fully automated protocol described in Chen et al. [6] to enable flexibility for changing research directions and needs. The modular protocols are much simpler to operate, enable flexible methods development, and process samples in half the time (<1 hour) of the fully-automated protocol due to manual intervention at various steps, such as centrifugation and protein resuspension. Furthermore, the modular automation protocols offer greater flexibility and adaptability without highly-specialized liquid handler systems.
These protocols detail three optimized step-by-step methods to: (A) lyse Gram-negative bacteria and fungal cells; (B) quantify the amount of protein extracted; and (C) normalize the amount of protein and set up tryptic digestion. Importantly, samples prepared through these protocols do not include salts that must be removed prior to LC-MS analysis, thus minimizing sample handling and the associated variance. These protocols have been developed to facilitate rapid, low variance sample preparation of hundreds of samples, be easily implemented on widely-available Beckman-Coulter Biomek automated liquid handlers that use disposable pipet tips, and allow flexibility for future protocol development. By using this modular workflow 96 samples can be prepared for tryptic digestion in under an hour. The tryptic digestion step can be optimized for the given application with many high-throughput digestion protocols such as microwave, elevated temperature, and ultrasonic methods [13, 14] or traditional overnight digestion.
Materials and methods
The protocol described in this peer-reviewed article is published on protocols.io (dx.doi.org/10.17504/protocols.io.b3gxqjxn) and is included for printing as S1 File with this article.” The individual protocols are published on protocols.io (Cell lysis: dx.doi.org/10.17504/protocols.io.b3gsqjwe, Protein quantification: dx.doi.org/10.17504/protocols.io.b3grqjv6, Protein normalization: dx.doi.org/10.17504/protocols.io.b3gtqjwn) and are included for printing as S2–S4 Files with this article.
Expected results
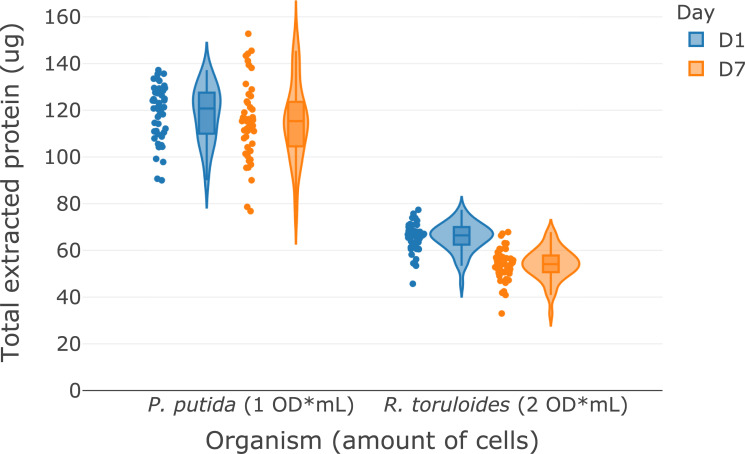
The modular bottom-up proteomic sample preparation automation protocol (S1 File) is composed of three protocols that detail: (A) cell lysis, protein extraction, protein precipitation; (B) protein quantification; and (C) protein normalization and tryptic digestion. Using the chloroform-methanol protein extraction protocol (S2 File) described, we obtained median amounts of over 115 μg and 50 μg of protein from one OD*mLs (~1 x 109 cells) of P. putida and two OD*mLs R. toruloides, respectively (Fig 1). To demonstrate the inter-day variability of the protocol, a single overnight cell culture of P. putida and R. toruloides was harvested and distribute into two 96 deep well plates and the protocol was repeated on two separate days (Day 1 and Day 7) to demonstrate the reproducibility of the method. The protocol takes 20 minutes to process one 96-well plate, including centrifugation steps. The amount of protein scaled with the starting amount of biomass which provides flexibility for the desired application. This amount of protein is sufficient for typical nano- and standard-flow LC-MS data acquisition methods and can easily be adjusted for applications requiring larger amounts of protein. The upper limit on the amount of biomass that can be processed with this protocol is limited by the amount of chloroform and methanol that can be added to the PCR plate (~125 μL). For applications that require larger amounts of protein, such as multi-dimensional chromatography, the protocol can easily be adapted to extractions in 96 deep-well plates with more chloroform-methanol. The protocol can also be scaled down to lower cell amounts, but the amount of protein extracted becomes increasingly variable as the amount of cells decreases, so increasing the number of replicates would be advisable. Sample types other than microbial cell pellets, such as tissues and complex biofluids, haven’t been tested with this protocol and may need additional preparation steps. Proteins resulting from these samples however are readily suitable for the following two protocols in the workflow.
Fig 1. Violin plots with data points showing the total protein extracted by using the modular automated protocol on P. putida and R. toruloides from different amounts of biomass (n = 47).
D1 and D7 samples correspond to repeat analysis of a single culture of each organism seven days apart to demonstrate the inter-day variability of the protocol.
The protein quantification protocol (S3 File) takes 15 minutes and produces concentration data for two replicates of the samples in a 96-well plate by using the DC protein assay (Bio-Rad), a modified Lowry protein quantification method [15]. The protocol uses a total of 3 μL of each sample and requires aliquoting known concentrations of a BSA standard in a separate plate for calibration curve generation. Duplicate protein quantification was chosen based on previous experience as a balance between sample consumption and accurate concentration measurement. When protein samples are processed by the protein extraction protocol above, the concentration measured by this method falls within a calibration range of 0.125 to 2 μg/μL. For larger or smaller amounts of cells, the concentration may fall outside the calibration range described here, consequently, the dilution factor may need to be adjusted. Once the concentrations of the samples are known the third protocol (S4 File) described here is used to normalize the amount of protein for tryptic digestion and subsequent LC-MS analysis. This protocol takes 20 minutes on the Biomek NX-S8 liquid handler system because the concentration of each well must be adjusted individually. Trypsin, iodoacetic acid, and tris(2-carboxyethyl)phosphine (TCEP) are then added via the Biomek NX-S8 or multi-channel pipette. These protocols are being used for proteomic analysis of metabolically engineered bacteria and fungi. The expected quantitative proteomic results from samples prepared by the modular protocol is demonstrated in Figs 2 and 3 by using an Agilent 1290 UHPLC coupled to a Thermo Orbitrap Exploris 480 system operating in data-dependent acquisition (DDA) mode [16]. The LC-MS/MS method (15 minute total run time) identified over 900 proteins (>6000 peptides) from 14 μg load of P. putida protein digest and over 1000 proteins (>4500 peptides) from 10 μg load of R. toruloides protein digest. To demonstrate the inter-day variability of the protocol, a single overnight cell culture of P. putida and R. toruloides was harvested and distribute into two 96 deep well plates at a total cell amount of 1 OD* mL and 2 OD*mL per well, respectively, and subsequently processed via the modular automation workflow on different days. We used the MS1 ion intensity method with Skyline [17] to quantify over 900 proteins from P. putida and over 1000 proteins from R. toruloides samples. The median protein variance for the samples were between 18 and 22% CV on two separate days from automated sample preparation protocol (Figs 2 and 3). The high-throughput modular automated protocol enables one researcher to prepare thousands of bottom-up proteomic samples per week. Supporting publications and other organisms are under development.
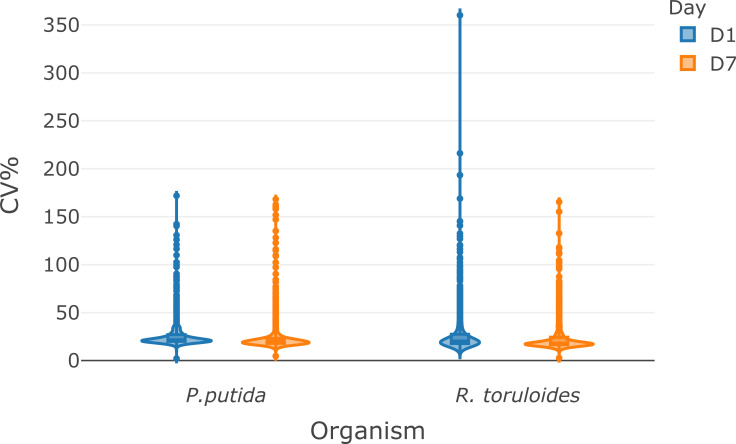
Fig 2. Reproducibility of the modular automated sample preparation workflow as measured by label-free LC-MS/MS shotgun proteomics analysis.
(A) Violin plots showing the coefficient of variation of MS1 ion intensity quantification for over 900 and 1000 proteins from P. putida and R. toruloides, respectively (n = 47). The violin plots display the kernel density estimation of the CV and inside each violin plot is a box plot summarizing ranges (IQR, whiskers, outlier points) and individual medians (solid lines). The LCMS analysis raw data have been deposited to the ProteomeXchange Consortium data depository at http://www.proteomexchange.org/. They are publicly accessible with the dataset identifier PXD029122 and 10.6019/PXD029122.
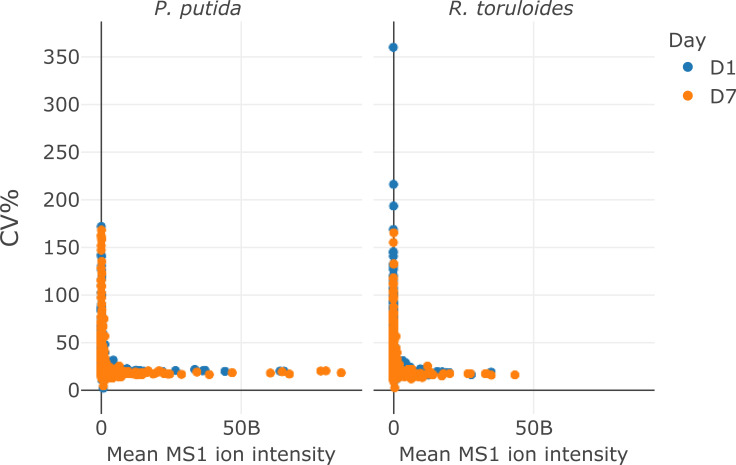
Fig 3. Scatter plot display of the CV% for each protein (y-axis) vs the mean MS1 ion intensity detected for each protein (x-axis).
Supporting information
Also available on protocols.io.
(PDF)
Also available on protocols.io.
(PDF)
Also available on protocols.io.
(PDF)
Also available on protocols.io.
(PDF)
Acknowledgments
The authors thank Kristin Burnum-Johnson, Yuzian Gao, and Nathalie Muñoz for helpful discussions about the protocols and Stephen Tan for help with instrumentation.
Data Availability
All proteomic data are available via ProteomeXchange with identifier PXD029122 and 10.6019/PXD029122.
Funding Statement
The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. The proof-of-concept work and resources were part of the Joint BioEnergy Institute (JBEI; http://www.jbei.org) and extension of the procedure and identification of the sources of error were part of the Agile BioFoundry (ABF; http://agilebiofoundry.org) supported through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U. S. Department of Energy.
References
- 1.Fu Q, Kowalski MP, Mastali M, Parker SJ, Sobhani K, van den Broek I, et al. Highly reproducible automated proteomics sample preparation workflow for quantitative mass spectrometry. J Proteome Res. 2018;17: 420–428. doi: 10.1021/acs.jproteome.7b00623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu X, Wang Z, Gao Y, Chen W, Wang L, Huang P, et al. Autoproteome chip system for fully automated and integrated proteomics sample preparation and peptide fractionation. Anal Chem. 2020. doi: 10.1021/acs.analchem.0c00752 [DOI] [PubMed] [Google Scholar]
- 3.Dayon L, Núñez Galindo A, Corthésy J, Cominetti O, Kussmann M. Comprehensive and Scalable Highly Automated MS-Based Proteomic Workflow for Clinical Biomarker Discovery in Human Plasma. J Proteome Res. 2014;13: 3837–3845. doi: 10.1021/pr500635f [DOI] [PubMed] [Google Scholar]
- 4.Arul A-B, Byambadorj M, Han N-Y, Park JM, Lee H. Development of an Automated, High-throughput Sample Preparation Protocol for Proteomics Analysis. Bull Korean Chem Soc. 2015;36: 1791–1798. doi: 10.1002/bkcs.10338 [DOI] [Google Scholar]
- 5.Müller T, Kalxdorf M, Longuespée R, Kazdal DN, Stenzinger A, Krijgsveld J. Automated sample preparation with SP3 for low-input clinical proteomics. Mol Syst Biol. 2020;16: e9111. doi: 10.15252/msb.20199111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Guenther JM, Gin JW, Chan LJG, Costello Z, Ogorzalek TL, et al. Automated “Cells-To-Peptides” Sample Preparation Workflow for High-Throughput, Quantitative Proteomic Assays of Microbes. J Proteome Res. 2019;18: 3752–3761. doi: 10.1021/acs.jproteome.9b00455 [DOI] [PubMed] [Google Scholar]
- 7.Liang Y, Acor H, McCown MA, Nwosu AJ, Boekweg H, Axtell NB, et al. Fully Automated Sample Processing and Analysis Workflow for Low-Input Proteome Profiling. Anal Chem. 2020. doi: 10.1021/acs.analchem.0c04240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Specht H, Harmange G, Perlman DH, Emmott E, Niziolek Z, Budnik B, et al. Automated sample preparation for high-throughput single-cell proteomics. BioRxiv. 2018. doi: 10.1101/399774 [DOI] [Google Scholar]
- 9.Lee J, Kim H, Sohn A, Yeo I, Kim Y. Cost-Effective Automated Preparation of Serum Samples for Reproducible Quantitative Clinical Proteomics. J Proteome Res. 2019;18: 2337–2345. doi: 10.1021/acs.jproteome.9b00023 [DOI] [PubMed] [Google Scholar]
- 10.Fu Q, Johnson CW, Wijayawardena BK, Kowalski MP, Kheradmand M, Van Eyk JE. A Plasma Sample Preparation for Mass Spectrometry using an Automated Workstation. J Vis Exp. 2020. doi: 10.3791/59842 [DOI] [PubMed] [Google Scholar]
- 11.Switzar L, van Angeren J, Pinkse M, Kool J, Niessen WMA. A high-throughput sample preparation method for cellular proteomics using 96-well filter plates. Proteomics. 2013;13: 2980–2983. doi: 10.1002/pmic.201300080 [DOI] [PubMed] [Google Scholar]
- 12.Potriquet J, Laohaviroj M, Bethony JM, Mulvenna J. A modified FASP protocol for high-throughput preparation of protein samples for mass spectrometry. PLoS ONE. 2017;12: e0175967. doi: 10.1371/journal.pone.0175967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Ferrer D, Capelo JL, Vázquez J. Ultra fast trypsin digestion of proteins by high intensity focused ultrasound. J Proteome Res. 2005;4: 1569–1574. doi: 10.1021/pr050112v [DOI] [PubMed] [Google Scholar]
- 14.Zheng YZ, DeMarco ML. Manipulating trypsin digestion conditions to accelerate proteolysis and simplify digestion workflows in development of protein mass spectrometric assays for the clinical laboratory. Clinical Mass Spectrometry. 2017. doi: 10.1016/j.clinms.2017.10.001 [DOI] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193: 265–275. doi: 10.1016/S0021-9258(19)52451-6 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Gin JW, Petzold CJ. Discovery proteomic (DDA) LC-MS/MS data acquisition and analysis. In: protocols.io [Internet]. 7 May 2021. [cited 11 Aug 2021]. Available: 10.17504/protocols.io.buthnwj6 [DOI] [Google Scholar]
- 17.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26: 966–968. doi: 10.1093/bioinformatics/btq054 [DOI] [PMC free article] [PubMed] [Google Scholar]