Abstract
Purpose
miR-500a-3p has been extensively reported to be implicated in the development and progression in several human cancer types. This study aimed to investigate the diagnostic and prognostic significance of miR-500a-3p as a biomarker in hepatocellular carcinoma (HCC).
Methods
miR-500a-3p expression was evaluated by in situ hybridization (ISH) and real-time PCR in 10 adjacent normal tissues (ANT), 21 liver fibrosis tissues, and 110 HCC tissues. Statistical analysis was used to investigate the correlation of miR-500a-3p expression with clinicopathological features in HCC patients. Kaplan–Meier survival analysis was performed to evaluate the prognostic significance of miR-500a-3p in overall survival and recurrence-free survival in HCC patients.
Results
In this study, we found that expression levels of miR-500a-3p were enhanced in HCC tissues. High miR-500a-3p levels were positively correlated with multiple clinicopathological features, including advanced clinical stage, distant metastatic status, increased AFP levels and poor tumor differentiation degree. More importantly, high miR-500a-3p levels predicted poor overall survival and early recurrence in HCC patients. Finally, a strong and positive correlation of miR-500a-3p mRNA expression with ISH staining scores was observed in clinical HCC tissues.
Conclusion
Our findings suggest that miR-500a-3p might be used as a novel biomarker to facilitate early diagnosis and predict prognosis in HCC patients.
Keywords: miR-500a-3p, diagnosis and prognosis, biomarker, hepatocellular carcinoma
Introduction
Liver cancer is one of the most common digestive system malignancies,1 and hepatocellular carcinoma (HCC) is the most common subtype of liver cancer, accounting for approximately 90% of all HCC cases.2 Despite great improvement regarding patient prognosis after surgical resection or liver transplantation in the past decades, the prognosis is still dismal due to the fact that diagnosis is often made at a late stage in the development of disease. Therefore, it is significantly necessary to identify potential novel biomarkers for diagnosis and prognosis of HCC.
MicroRNAs (miRNAs) are a family of small, non-coding RNA molecules that regulate expression of target genes at the post-transcriptional level by binding to specific sequences in the 3′ untranslated region (3′UTR) of downstream target genes, leading to mRNA degradation and/or translational inhibition.3 MiRNAs have been reported to play an important role in a variety of biological processes, such as apoptosis, cell cycle and proliferation, cell differentiation and immune response.3,4 Numerous literatures have demonstrated that aberrant expression of miRNAs is extensively reported to be implicated in the tumorigenesis, progression and metastasis in a variety of human cancer types.5–9 Furthermore, the potential clinical applicable value of miRNAs as biomarker for early detection and intervention of cancer seizes our great momentum, including HCC. Liu et al reported that combined miR-15b and miR-130b expression in the serum of HCC patients were used demonstrated as a classifier for early HCC detection, and the sensitivity and specificity were 98.2% sensitivity and 91.5% specificity, respectively, with a receiver operating characteristic curve area of 0.98. Importantly, the combined miR-15b and miR-130b also identified early-stage HCC cases that could not be detected by AFP.10 In addition, a panel of seven miRNAs, including miR-801, miR-27a, miR-26a, miR-223, miR-21, miR-192 and miR-122, has been shown to have a high diagnostic accuracy in the early diagnosis of HBV-related HCC with AUC of 0.864 and 0.888 for training and validation data set, respectively. The microRNA panel can also differentiate HCC from healthy (AUC = 0.941), chronic hepatitis B (AUC = 0.842), and cirrhosis (AUC = 0.884), respectively.11 Therefore, these findings suggest that miRNAs may serve as a novel molecular biomarker for early diagnosis of HCC.
As one of the originally identified miRNAs, miR-500a was found to be upregulated in benign disease, such as endometriosis,12 and malignant cancer, for example, chronic lymphocytic leukemia, breast cancer.13,14 Our previous study has shown that miR-500a-3p was overexpressed in HCC tissue samples, as indicated by our own HCC tissues and multiple independent publicly available HCC datasets, including The Cancer Genome Atlas (TCGA), E-GEOD-21362 and E-GEOD-31384, which was significantly correlated with poorer overall and relapse-free survival in HCC patients,15 as well as Guo’s study.16 As miR-500a-3p expression is examined and analyzed using real-time PCR in the previous studies, a more reliable and commonly used clinical technique is required to further determine the clinical significance of miR-500a-3p in HCC patients.
In this study, our results demonstrated that the ISH staining scores of miR-500a-3p were remarkably upregulated in HCC tissues using in situ hybridization technique, which was positively associated with miR-500a-3p mRNA expression levels by real-time PCR. High levels of miR-500a-3p were positively associated with advanced clinical stage, distant metastatic status, increased AFP levels and poor tumor differentiation degree. More importantly, high miR-500a-3p levels predicted poor overall survival and early recurrence in HCC patients. Collectively, these findings imply that miR-500a-3p may hold a promising value as a novel biomarker for early detection and poor prognosis in HCC patients.
Materials and Methods
Patients and Tumor Tissues
In total, 110 paraffin embedded, archived HCC tissues were obtained during surgery at the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China) between 2006 and 2010, and the clinicopathological characteristics of HCC patients are summarized in Table 1. Patients were diagnosed based on clinical and pathological evidence, and the specimens were immediately snap-frozen and stored in liquid nitrogen tanks. For the use of these clinical materials for research purposes, prior patients’ consents and approval from the Institutional Ethics Committee for Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University, and the ethical approval number was [2020]514. This study was conducted in accordance with the Declaration of Helsinki.
Table 1.
The Clinicopathological Characteristics in 110 Hepatocellular Carcinoma Patients
| Parameters | Number of Cases | Parameters | Number of Cases |
|---|---|---|---|
| Gender | Age (years) | ||
| Female | 37 | <60 | 51 |
| Male | 73 | ≥60 | 59 |
| AFP (ng/mL) | Differentiation | ||
| <400 | 46 | High/moderate | 43 |
| ≥400 | 64 | Poor | 67 |
| Clinical stage | T stage | ||
| I | 23 | T1–T2 | 58 |
| II–IV | 87 | T3–T4 | 52 |
| N stage | M stage | ||
| N0 | 41 | M0 | 53 |
| N1 | 69 | M1 | 57 |
| Survival status | Tumor size (cm) | ||
| Alive | 29 | <5 | 47 |
| Dead | 69 | ≥5 | 63 |
| Venous invasion | Fibrotic or cirrhotic conditions | ||
| Negative | 53 | Negative | 2 |
| Positive | 57 | Positive | 108 |
Abbreviation: AFP, alpha fetoprotein.
In situ Hybridization (ISH)
ISH was performed on HCC tumors using locked nucleic acid (LNA) probes for miR-500a-3p (Exiqon, Vedbaek, Denmark) as described previously.17 Briefly, the slides of frozen section were digested with proteinase K (20 μg/mL) (Cat. sc-473603, Santa Cruz) for 10 min at 37°C, fixed in 4% paraformaldehyde (Cat. sc-478134, Santa Cruz) for 10 min at room temperature, and then dehydrated by immersion in an ethanol gradient and air dried, slides were hybridized with the locked nucleic acid (LNA) probes for miR-500a-3p (Exiqon, Vedbaek, Denmark) overnight, denatured in hybridization buffer (Cat. 786–558, G-Biosciences) at 77°C for 5 min and chilled on ice immediately. Each slide was covered with 20–50 μL diluted probe and incubated in a humidified hybridization chamber at 37°C overnight. Slides were washed twice in 2×SSC/0.1% Tween-20 at 37°C for 15 min. Then, the slides were rinsed and incubated with anti-digoxigenin (Cat. sc-57583, Santa Cruz), a horseradish peroxidase (HRP)-linked antibody (Cat. ab181658, Abcam) for 2 hr. After washing three times in PBS/0.05% Tween-20, the slides were stained with 3,3′-Diaminobenzidine (DAB) Enhanced Liquid Substrate System (Cat. D8001, Sigma-Aldrich, USA) for 3 min, counterstained with hematoxylin for 1 min, dehydrated by immersion in an ethanol gradient and sealed by cover slip.
The ISH scores of all the tissues were analyzed and calculated by the two independent investigators based on their experience by evaluating each slide through a microscope by naked eyes. Each investigator gave one score for “staining intensity” and one for “the proportion of positive tumor cells”. Then, the final ISH score of each tissue was calculated as the product of staining intensity score and the proportion of positive tumor cells. Tumor cell proportion was scored as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10–35% positive tumor cells), 3 (35–70% positive tumor cells), and 4 (>70% positive tumor cells). The staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). Using this method of assessment, we evaluated miR-500a-3p expression in HCC samples by determining the staining intensity (SI), with scores of 0, 1, 2, 3, 4, 6, 8, 9, or 12. High and low expression of miR-500a-3p were stratified by the following criteria: The SI <4 was used to define tumors with low expression of miR-500a-3p, and SI score of ≥4 as tumors with high expression of miR-500a-3p.
RNA Extraction, Reverse Transcription, and Real-Time RT-PCR
Total RNA from tissues was extracted using the RNA Isolation Kit (Qiagen, USA) according to the manufacturer’s instructions. cDNA was synthesized from total RNA with specific stem-loop primers and the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems; Life Technologies). The detailed procedure of real-time RT-PCR was performed as described previously.18 Primers for U6 and miR-500a-3p were synthesized and purified by RiboBio (Guangzhou, China). U6 was used as the endogenous controls. Relative fold expressions were calculated with the comparative threshold cycle (2-ΔΔCt) method.
Statistical Analysis
All statistical analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Associations between miR-500a-3p score and clinicopathological characteristics of the patients were analyzed using the Chi-squared test. Survival curves were plotted using the Kaplan–Meier method and compared using the Log rank test. P < 0.05 was considered significant.
Results
miR-500a-3p is Upregulated in Hepatocellular Carcinoma
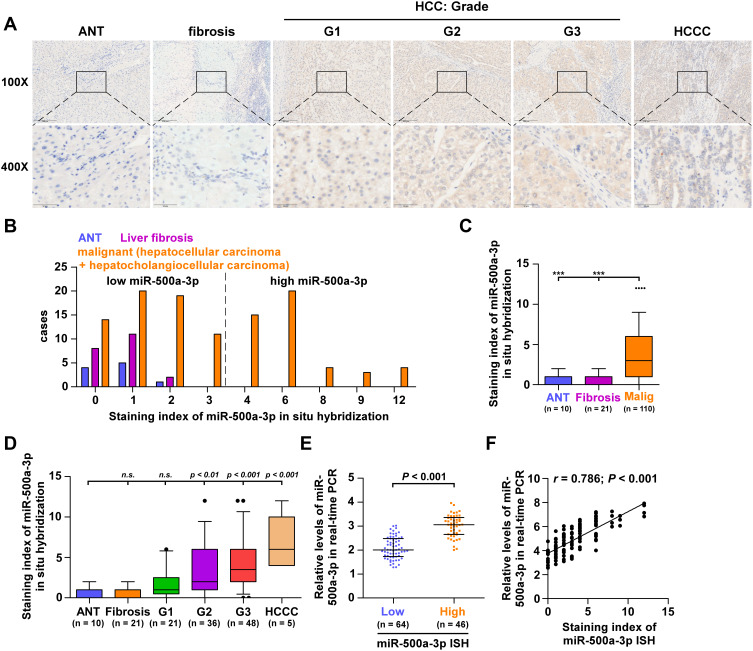
To investigate the potential prognostic significance of miR-500a-3p as a tumor biomarker, we first collected 10 adjacent normal tissues (ANT), 21 liver fibrosis tissues, and 110 HCC tissues, including 5 hepatocholangiocellular carcinoma (HCCC), 48 grade III HCC tissues, 36 grade II HCC tissues, 21 grade I HCC tissues, as assessed by H&E staining (Figure 1). Then, we further examined miR-500a-3p expression levels in these tissues using in situ hybridization (ISH). The results showed that miR-500a-3p was primarily observed in the cytoplasma (Figure 2A), and the staining intensity of miR-500a-3p in HCC tissues was robustly higher than that in ANT or liver fibrosis tissues (Figure 2B). Furthermore, the staining scores of miR-500a-3p in 110 malignant tumor tissues were obviously upregulated compared with that in 10 ANT and 21 liver fibrosis tissues (Figure 2C). Then, we further analyzed the expression levels of miR-500a-3p in 10 ANT, 21 liver fibrosis tissues, 21 grade I HCC tissues, 36 grade II HCC tissues, 48 grade III HCC tissues and 5 hepatocholangiocellular carcinoma respectively, and found that there was no significant difference of miR-500a-3p expression between ANT and liver fibrosis tissues, and ANT and grade I HCC tissues (Figure 2D); whereas miR-500a-3p expression was slightly increased in grade II HCC tissues and dramatically upregulated in grade III HCC tissues and hepatocholangiocellular carcinoma tissues (Figure 2D). Consistently, HCC tissues with low ISH staining scores of miR-500a-3p presented lower miR-500a-3p levels by real-time PCR; conversely, HCC tissues with high ISH staining scores of miR-500a-3p showed higher miR-500a-3p levels (Figure 2E). Furthermore, there was a positive linear correlation observed between ISH staining scores of miR-500a-3p and miR-500a-3p mRNA levels (Figure 2F). Overall, the aforementioned findings indicated that miR-500a-3p is significantly upregulated in clinical HCC tissues.
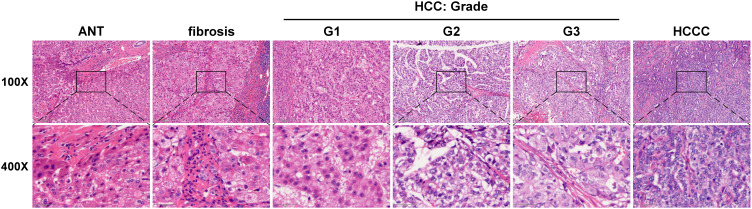
Figure 1.
Representative images of low-power (100×, top) and high-power (400×, bottom) fields by H&E staining in adjacent normal tissue (ANT, n = 10), liver fibrosis tissues (n = 21), HCC tissues with grade I (G1, n = 21), HCC tissues with grade II (G2, n = 36), HCC tissues with grade III (G3, n = 48) and hepatocholangiocellular carcinoma tissues (HCCC, n =5). Scale bars, 200 mm for 100× magnification and 50 mm for 400× magnification.
Figure 2.
miR-500a-3p is upregulated in hepatocellular carcinoma. (A) Representative images of low-power (100×, top) and high-power (400×, bottom) fields of miR-500a-3p expression by in situ hybridization (ISH). Scale bars, 200 mm for 100× magnification and 50 mm for 400× magnification. (B) The number of ANT, liver fibrosis tissues and malignant HCC tissues in different staining index groups of ISH. (C) Staining index of miR-500a-3p in 10 ANT, 21 liver fibrosis tissues, and 110 HCC tissues by ISH. “•” indicates HCC tissues with very high miR-500a-3p level above the upper standard deviation of the average miR-500a-3p level in all HCC tissues. ***Indicates P < 0.001. (D) Staining index of miR-500a-3p by ISH in 10 ANT, 21 liver fibrosis tissues, 21 HCC tissues with grade I, 36 HCC tissues with grade II, 48 HCC tissues with grade III and 5 hepatocholangiocellular carcinoma tissues. “••” indicates HCC tissues with very high miR-500a-3p level above the upper standard deviation of the average miR-500a-3p level in all HCC tissues. (E) Real-time PCR analysis of miR-500a-3p in 46 HCC tissues with high ISH scores compared with 64 HCC tissues with low ISH scores. The median ISH score in HCC tissues was used to stratify low and high ISH scores. (F) Correlation of miR-500a-3p expression level with ISH scores with expression levels of miR-500a-3p by real-time PCR in HCC tissues.
High Levels of miR-500a-3p Correlates with Advanced Clinicopathological Features in HCC Patients
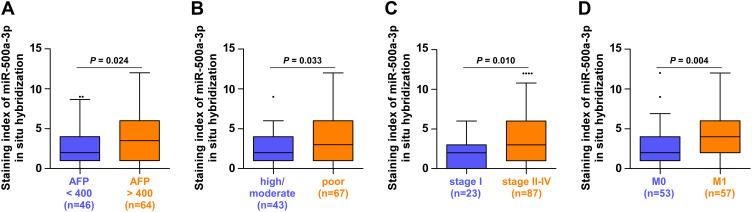
Then, we further analyzed the correlation of miR-500a-3p expression with clinicopathological features in HCC patients. As shown in Table 2, overexpression of miR-500a-3p was positively correlated with advanced clinical stage, early distant metastatic status, increased AFP levels and poor tumor differentiation in HCC patients. Further investigation showed that staining index of miR-500a-3p in ISH in HCC tissues with advanced clinical stage, distant metastasis, high AFP levels or poor tumor differentiation was higher than that in HCC tissues with early clinical stage, no distant metastasis, low AFP levels or high/moderate tumor differentiation (Figure 3A–D). Therefore, our results indicate that high levels of miR-500a-3p correlates with multiple clinicopathological features, including clinical stage, distant metastatic status, AFP levels and tumor differentiation degree, in HCC patients.
Table 2.
The Correlation Between miR-500a-3p and Clinicopathological Characteristics in 110 Patients with Hepatocellular Carcinoma
| Parameters | Number of Cases | miR-500a-3p ISH | P values | |
|---|---|---|---|---|
| Low | High | |||
| Gender | ||||
| Female | 37 | 24 | 13 | 0.312 |
| Male | 73 | 40 | 33 | |
| Age (years) | ||||
| <60 | 51 | 30 | 21 | 0.899 |
| ≥60 | 59 | 34 | 25 | |
| AFP (ng/mL) | ||||
| <400 | 46 | 34 | 12 | 0.005* |
| ≥400 | 64 | 30 | 34 | |
| Differentiation | ||||
| High/moderate | 43 | 31 | 12 | 0.018* |
| Poor | 67 | 33 | 34 | |
| T stage | ||||
| T1–T2 | 58 | 33 | 25 | 0.773 |
| T3–T4 | 52 | 31 | 21 | |
| N stage | ||||
| N0 | 41 | 26 | 15 | 0.391 |
| N1 | 69 | 38 | 31 | |
| M stage | ||||
| M0 | 53 | 39 | 14 | 0.002* |
| M1 | 57 | 25 | 32 | |
| Clinical stage | ||||
| I | 23 | 19 | 4 | 0.008* |
| II–IV | 87 | 45 | 42 | |
| Tumor size (cm) | ||||
| <5 | 47 | 31 | 16 | 0.175 |
| ≥5 | 63 | 33 | 30 | |
| Venous invasion | ||||
| Negative | 53 | 35 | 18 | 0.107 |
| Positive | 57 | 29 | 28 | |
Note: “*” indicates P < 0.05.
Abbreviation: AFP, alpha fetoprotein.
Figure 3.
High levels of miR-500a-3p correlates with advanced clinicopathological features in HCC patients. (A) ISH staining index of miR-500a-3p in HCC tissues with low (n = 46) and high (n = 64) levels of AFP. (B) ISH staining index of miR-500a-3p in HCC tissues with high/moderate (n = 43) and poor (n = 67) differentiation status. (C) ISH staining index of miR-500a-3p in HCC tissues with clinical stage I (n = 23) and clinical stage II–IV (n = 87). (D) ISH staining index of miR-500a-3p in HCC tissues with distant metastasis (n = 57) and without metastasis (n = 53). •, ••, ••••indicate HCC tissues with very high miR-500a-3p level above the upper standard deviation of the average miR-500a-3p levelin all HCC tissues.
High Levels of miR-500a-3p Predict Poor Prognosis and Early Recurrence in HCC Patients
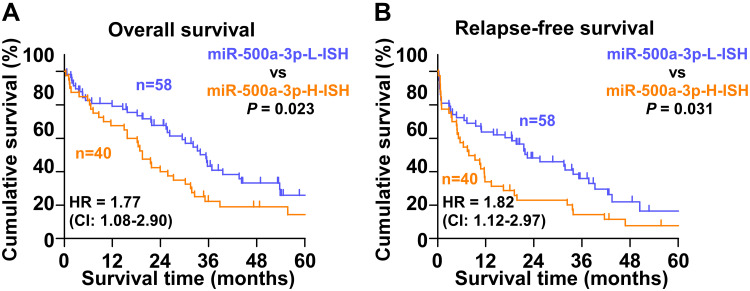
The prognostic significance of miR-500a-3p was further investigated. First, the expression levels of miR-500a-3p were examined in all HCC tissues using ISH (Figure 2). Then, the median staining index (SI = 3) of miR-500a-3p in all HCC tissues was used as the cutoff value to stratify high and low expression of miR-500a-3p. Based on this criteria, 58 HCC tissues were defined as low-ISH score tissues with SI < 4 and 40 were defined as high-ISH score tissues with SI ≥ 4. Kaplan–Meier survival analysis revealed that HCC patients with high miR-500a-3p scores presented poor overall survival compared with low scores of miR-500a-3p in HCC patients (Figure 4A). In addition, high scores of miR-500a-3p were significantly correlated with early recurrence compared with low miR-500a-3p scores in HCC patients (Figure 4B). Collectively, our results indicate that overexpression of miR-500a-3p predicts poor prognosis and early recurrence in HCC patients.
Figure 4.
High levels of miR-500a-3p predict poor prognosis and early recurrence in HCC patients. (A) Kaplan–Meier analysis of overall curves of patients with HCC in high miR-500a-3p ISH (n=40) and low miR-500a-3p ISH (n=58). P < 0.01, Log rank test. The data shown in scatter plot and bar graph were determined by the median with interquartile range and median with standard deviation. (B) Kaplan–Meier analysis of relapse-free curves of patients with HCC in high miR-500a-3p ISH (n=40) and low miR-500a-3p ISH (n=58). P =0.014, Log rank test. The data shown in scatter plot and bar graph were determined by the median with interquartile range and median with standard deviation.
Discussion
In the present study, our results revealed that miR-500a-3p expression was increased in HCC tissues by in situ hybridization, which positively predicted with advanced clinical stage, early distant metastatic status, increased AFP levels and poor tumor differentiation in HCC patients. Kaplan–Meier survival analysis showed that high miR-500a-3p levels predicted poorer overall survival and early recurrence-free survival compared with those with low miR-500a-3p levels. Therefore, our results imply that miR-500a-3p may hold the potential as a novel tumor biomarker to facilitate early diagnosis and predict prognosis of HCC.
Several lines of evidence have reported that miR-500a-3p expression was upregulated in numerous human cancer types, including chronic lymphocytic leukemia,13 gastric cancer,19 breast cancer.14,20 Conversely, miR-500a-3p was found to be reduced in non-small cell lung cancer and function as a tumor-suppressive miRNA.21 These findings supported the notion that miR-500a-3p could play an oncogenic or tumor-suppressive miRNA dependent on cancer types. In this study, expression levels of miR-500a-3p were further examined in 110 HCC tissues using in situ hybridization technique. The results demonstrated that miR-500a-3p expression was dramatically upregulated in HCC tissues compared with that in ANT and liver fibrosis tissues. Statistical analysis further revealed that overexpression of miR-500a-3p was positively correlated with advanced clinical stage, early distant metastatic status, increased AFP levels and poor tumor differentiation in HCC patients. Kaplan–Meier survival analysis showed that high miR-500a-3p levels predicted poorer overall survival and early recurrence-free survival compared with those with low miR-500a-3p levels. Collectively, our results indicate that miR-500a-3p may serve as a potential clinical diagnostic or prognostic biomarker to facilitate early detection and monitor the recurrence of HCC.
Accumulating studies have shown that miRNAs present favorable prospects in early detection and prognosis monitoring of cancer as novel tumor biomarker, especially HCC. The study from Tomimaru et al found that increased level of miR-21 in the serum of HCC patients, which could distinguish HCC from healthy controls and patients with chronic hepatitis, the sensitivity and specificity even superior to that of AFP as a biomarker for early diagnosis and detection of HCC.22 In addition, Han et al have reported that HCC patients with high levels of miR-155 exhibited poorer overall survival (P < 0.001) and recurrence-free survival (P < 0.001).23 These findings suggest that miRNAs may serve as a novel molecular biomarker for early diagnosis or prognosis monitoring of HCC. In the current study, our results demonstrated that miR-500a-3p was remarkably overexpression in HCC tissues, and overexpression of miR-500a-3p was associated with poor clinicopathological characteristics in HCC patients. Importantly, patient with high miR-500a-3p levels had shorter overall survival and early recurrence-free survival compared with those with low miR-500a-3p levels. Notably, miR-500a level was found to be higher in HCC patients than healthy controls or those with chronic hepatitis B, but statistical difference between healthy controls and patients with chronic hepatitis B was not observed, suggesting that serum miR-500a might hold a promising potential for the diagnosis of HCC as a tumor biomarker.24 Collectively, our findings in combination with others support the potential clinical applicable value for early detection and diagnosis of HCC as a novel tumor biomarker.
Development of metastatic cancer has been reported to contribute to the majority of cancer-related deaths.25,26 Intrahepatic metastases and distant metastasis to common target organs, such as lung, bone and brain, contribute to poorer prognosis in HCC,27 which is commonly reported in this type of tumor due to the abundant blood flow in liver anatomy.28 In the present study, our results demonstrated that high miR-500a-3p levels predicted poor prognosis in HCC patients. Notably, our results further revealed that high level of miR-500a-3p was significantly correlated with distant metastasis (M stage), as well as venous invasion. This finding suggested that miR-500a-3p may play an important role in distant metastasis of HCC, which may further contribute to poor prognosis in HCC patients. However, the biological role and clinical significance of miR-500a-3p in distant metastasis of HCC are still to be further investigated in the following work.
Notably, the levels of miR-500a-3p in HCCC were dramatically higher than those in HCC tissues, even than the worst HCC stage. This finding supported the notion that miR-500a-3p can be also increased in patients who only have cholangiocarcinoma, which may reduce the specific and applicable value of miR-500a-3p as a diagnostic biomarker in HCC, as well as in cholangiocarcinoma or HCCC. However, since limited HCCC samples (only five) were analyzed in the current study, more solid conclusion regarding the specific diagnostic significance of miR-500a-3p in HCC, cholangiocarcinoma or both is definitely worth further investigation in larger samples of the following study.
In summary, our results in this study indicate that miR-500a-3p is upregulated in HCC tissues, and high levels of miR-500a-3p predict poor prognosis and early relapse in HCC patients. Therefore, our results suggest that miR-500a-3p might serve as a potential biomarker to facilitate early diagnosis and predict prognosis in HCC patients.
Funding Statement
This study was supported by the Natural Science Foundation of Guangdong Province (No. 2018A0303130165; No. 2017A030313792); the Science and Technology Program of Guangzhou (No. 201904010046); the National Natural Science Foundation of China (No. 81874227).
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Qiu Z, Li H, Zhang Z, et al. A pharmacogenomic landscape in human liver cancers. Cancer Cell. 2019;36(2):179–193 e111. doi: 10.1016/j.ccell.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 4.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997 [DOI] [PubMed] [Google Scholar]
- 5.Dai Y, Wu Z, Lang C, et al. Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-beta signaling. Theranostics. 2019;9(21):6063–6079. doi: 10.7150/thno.36735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wa Q, Li L, Lin H, et al. Downregulation of miR19a3p promotes invasion, migration and bone metastasis via activating TGFbeta signaling in prostate cancer. Oncol Rep. 2017. doi: 10.3892/or.2017.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren D, Lin B, Zhang X, et al. Maintenance of cancer stemness by miR-196b-5p contributes to chemoresistance of colorectal cancer cells via activating STAT3 signaling pathway. Oncotarget. 2017;8(30):49807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang C, Yu M, Xie X, et al. miR-217 targeting DKK1 promotes cancer stem cell properties via activation of the Wnt signaling pathway in hepatocellular carcinoma. Oncol Rep. 2017;38(4):2351–2359. doi: 10.3892/or.2017.5924 [DOI] [PubMed] [Google Scholar]
- 9.Ren D, Yang Q, Dai Y, et al. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-kappaB signaling pathway. Mol Cancer. 2017;16(1):117. doi: 10.1186/s12943-017-0688-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu AM, Yao TJ, Wang W, et al. Circulating miR-15b and miR-130b in serum as potential markers for detecting hepatocellular carcinoma: a retrospective cohort study. BMJ Open. 2012;2(2):e000825. doi: 10.1136/bmjopen-2012-000825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Yu L, Gao X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29(36):4781–4788. doi: 10.1200/JCO.2011.38.2697 [DOI] [PubMed] [Google Scholar]
- 12.Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: a comprehensive array-based analysis. Fertil Steril. 2016;106(2):402–409. doi: 10.1016/j.fertnstert.2016.04.013 [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Lafuente N, Alcaraz-Garcia MJ, Sebastian-Ruiz S, et al. IL-4 up-regulates MiR-21 and the MiRNAs hosted in the CLCN5 gene in chronic lymphocytic leukemia. PLoS One. 2015;10(4):e0124936. doi: 10.1371/journal.pone.0124936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YS, Park SJ, Lee YS, Kong HK, Park JH. miRNAs involved in LY6K and estrogen receptor alpha contribute to tamoxifen-susceptibility in breast cancer. Oncotarget. 2016;7(27):42261–42273. doi: 10.18632/oncotarget.9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang C, Long J, Liu B, et al. miR-500a-3p promotes cancer stem cells properties via STAT3 pathway in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2017;36(1):99. doi: 10.1186/s13046-017-0568-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Chen L, Sun C, Yu C. MicroRNA-500a promotes migration and invasion in hepatocellular carcinoma by activating the Wnt/beta-catenin signaling pathway. Biomed Pharmacother. 2017;91:13–20. doi: 10.1016/j.biopha.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Ren D, Wu X, et al. miR-1266 contributes to pancreatic cancer progression and chemoresistance by the STAT3 and NF-kappaB signaling pathways. Mol Ther Nucleic Acids. 2018;11::142–158. doi: 10.1016/j.omtn.2018.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edens BM, Vissers C, Su J, et al. FMRP modulates neural differentiation through m(6)A-dependentmRNA nuclear export. Cell Rep. 2019;28(4):845–854 e845. doi: 10.1016/j.celrep.2019.06.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin H, Zhang L, Zhang C, Liu P. Exosomal MiR-500a-3p promotes cisplatin resistance and stemness via negatively regulating FBXW7 in gastric cancer. J Cell Mol Med. 2020;24(16):8930–8941. doi: 10.1111/jcmm.15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oztemur Islakoglu Y, Noyan S, Aydos A, Gur Dedeoglu B. Meta-microRNA biomarker signatures to classify breast cancer subtypes. OMICS. 2018;22(11):709–716. doi: 10.1089/omi.2018.0157 [DOI] [PubMed] [Google Scholar]
- 21.Liao XH, Xie Z, Guan CN. MiRNA-500a-3p inhibits cell proliferation and invasion by targeting lymphocyte antigen 6 complex locus K (LY6K) in human non-small cell lung cancer. Neoplasma. 2018;65(5):673–682. doi: 10.4149/neo_2018_170516N355 [DOI] [PubMed] [Google Scholar]
- 22.Tomimaru Y, Eguchi H, Nagano H, et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J Hepatol. 2012;56(1):167–175. doi: 10.1016/j.jhep.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 23.Han ZB, Chen HY, Fan JW, Wu JY, Tang HM, Peng ZH. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol. 2012;138(1):153–161. doi: 10.1007/s00432-011-1076-z [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Ge S, Wang X, et al. Serum miR-483-5p as a potential biomarker to detect hepatocellular carcinoma. Hepatol Int. 2013;7(1):199–207. doi: 10.1007/s12072-012-9341-z [DOI] [PubMed] [Google Scholar]
- 25.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370 [DOI] [PubMed] [Google Scholar]
- 26.Ren D, Dai Y, Yang Q, et al. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J Exp Med. 2019;216(2):428–449. doi: 10.1084/jem.20180661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15(3):137–151. doi: 10.1038/nrgastro.2017.169 [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Feng L, Dougherty CA, et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials. 2016;104::361–371. doi: 10.1016/j.biomaterials.2016.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]