Summary
Background
Air pollution might accelerate cognitive ageing; it is unclear whether large-scale interventions, such as China’s Clean Air Act (CCAA), can mitigate cognitive deterioration. We aimed to evaluate the effect of CCAA on changes in cognitive function in older adults.
Methods
In this population-based, quasi-experimental study, we did a difference-in-differences analysis of the data collected during the 2014 and 2018 waves of the Chinese Longitudinal Healthy Longevity Survey (CLHLS). The study design used a counterfactual analysis feature by dividing CLHLS participants into two groups. The intervention group included participants who lived in areas where the provincial government set a target of reducing particulate matter (PM) by at least 5% annually from 2014 onward, whereas the control group consisted of individuals who lived in areas without a PM reduction target. Global cognitive function was measured using the Mini-Mental State Examination (MMSE). We used fixed-effects models to examine the between-group differences in MMSE score changes before and after CCAA implementation. We associated longitudinal changes in MMSE scores with changes in concentrations of PM with a diameter of less than 2·5 μm (PM2·5) concentration and other regulated pollutants. We used alternative models and sensitivity analyses to evaluate the robustness of the results from the main models.
Findings
2812 individuals participated in the 2014 and 2018 surveys (mean age 81·0 years [SD 9·3] in 2014; 1408 [50·1%] female and 1404 [49·9%] male). 2251 (80·0%) were included in the intervention group and 561 (20·0%) in the control group. After controlling for potential confounders, the intervention group had a significantly smaller decline in MMSE scores from 2014 to 2018 compared with the control group: the mean between-group difference was 2·45 points (95% CI 1·32–3·57). Interquartile increases in PM2·5 were associated with a significant MMSE score decline of 0·83 points (95% CI 0·24–1·42); similarly, increases in SO2 were also associated with a significant MMSE score decline of 0·80 points (0·32–1·29).
Interpretation
Implementing stringent clean air policies might mitigate the risk of air pollutant-associated cognitive ageing in older people.
Funding
National Natural Sciences Foundation of China, National Key R&D Program of China, China Postdoctoral Science Foundation funded project, the Duke/Duke-National University of Singapore Collaboration Pilot Project, the National Institute on Aging and Peking University-Baidu Fund, Energy Foundation, and the Fundamental Research Funds for the Central Universities.
Introduction
Poor cognitive function, a key driver of disability, contributes profoundly to health, social, and economic burdens in ageing populations.1 Global health-care expenditure for dementia reached US $1 trillion in 2018 and is expected to reach $2 trillion by 2030.2 According to the 2020 Lancet Commission on dementia prevention, around 40% of worldwide dementia cases are attributable to 12 modifiable risk factors.3 Of these, air pollution is an emerging risk factor.3 Of note, the number of people with dementia is rising more rapidly in low-income and middle-income countries (LMICs) than in high-income countries (HICs) due to more rapid population ageing and poor environmental factors.4
The associations between long-term exposure to fine particulate matter (PM) less than 2·5μm in diameter (PM2·5) and cognitive impairment, dementia, and other neurological disorders have been reported in observational studies.5–14 However, a causal inference is difficult to draw from observational studies due to residual confounding and reverse causation.15 In this regard, a quasi-experimental study leveraging air quality interventions is advantageous because an association between changes in air quality brought by an intervention—such as a clean air policy—and subsequent changes in a health outcome is more likely to be causal. This type of quasi-experimental design mimics a randomised clinical trial and therefore strengthens confidence in the putative causality of the associations derived from conventional observational studies.16,17
To our knowledge, only one study from the USA has estimated the effect of air pollution regulation between 2004 and 2013 on dementia risk.18 However, the long-term effect of the air pollution policy observed in this study might be confounded by time-varying variables, such as economic growth, changes in lifestyle, and advances in pharmaceuticals and medicines, during the long study period (>9 years). Additionally, no studies have evaluated the effect of clean air policies on cognitive health in LMICs, in which populations are ageing rapidly and are exposed to high levels of air pollution.
In 2013, the most stringent version of China’s Clean Air Act (CCAA) was introduced to address China’s widespread and severe air pollution problem.19 The 2014 and 2018 waves of the Chinese Longitudinal Healthy Longevity Survey (CLHLS) were aligned with CCAA. This provides a unique quasi-experiment opportunity to assess whether the rapid reductions in ambient concentrations of PM2·5 and other pollutants brought about by CCAA would reduce the risk of cognitive deterioration in older adults.
Methods
Study design and participants
In this population-based, quasi-experimental study, we did a difference-in-differences (DID) analysis of the data collected during the 2014 and 2018 waves of the CLHLS, which is an ongoing prospective cohort study on the determinants of healthy ageing and longevity in members of the population aged 60 years and older in China. The CLHLS is a nationwide survey on a randomly selected sample from half of the counties and cities in 23 of the 31 provinces of China, covering about 85% of the total population (figure 1). Beginning in 1998, the survey has been done every 2–3 years. To reduce attrition in numbers from death and loss to follow-up, new participants are enrolled during each follow-up. Of note, the CLHLS oversampled males older than 80 years, which shaped the age distribution of the final sample. Trained interviewers did the surveys at participants’ homes following a structured questionnaire. They collected data on sociodemographic characteristics, lifestyle, cognitive function, psychological status, and physical capacity. More details on the sampling procedure and assessment of data quality can be found in previous publications.20
Figure 1: Map of study areas with changes in annual mean of PM2·5 from 2014 to 2017.
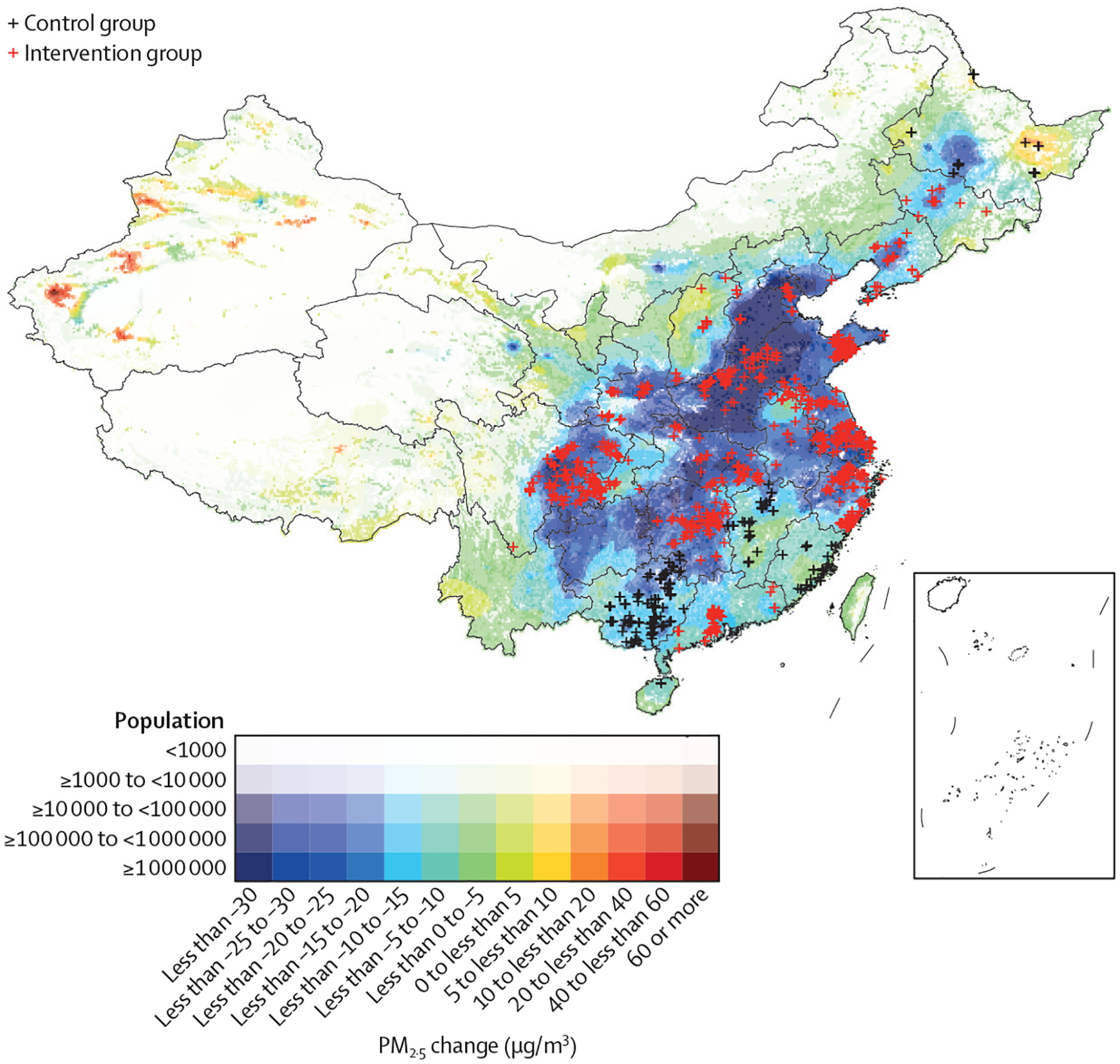
The study participants were designated into control and intervention groups according to the pre-established target of reduced concentration of PM2·5 after 2014. The intervention group were from areas with a target of annual PM2·5 reduction of 5% or more following implementation of CCAA; the control group were from areas without reduction target under CCAA. CCAA= China’s Clean Air Act. PM2·5=particulate matter with a diameter of less than 2·5 μm.
This quasi-experimental study was based on the effective ness in PM reduction through the implementation of CCAA (appendix p 3) by provincial governments. We identified 18 provinces with and five provinces without an established target of PM2·5 reduction in 2014 (appendix p 8). The study design used a counterfactual analysis by dividing CLHLS participants into two groups. We designated CLHLS participants who lived in provinces in which the Chinese Government set a target of reducing PM concentrations by more than 5% annually from 2014 onward into the intervention group and the rest of the participants into the control group (figure 1). We included participants who took part in both the 2014 and 2018 waves of the survey (ie, surveyed before and after the implementation of CCAA). Because the surveys were close in time, the potential confounding from other time-varying variables (eg, dietary patterns) was minimised. CLHLS was approved by the Biomedical Ethics Committee, Peking University, Beijing, China (IRB00001052–13074). All participants provided written informed consent before participation.
Procedures
We generated residence-specific ambient concentrations of six pollutants (PM2·5, PM10, SO2, NO2, O3, and CO) using a previously published database.21 Nationwide monitoring network observation is the gold standard for air pollution exposure assessment, but they were not available for participants who lived a long distance from the monitoring sites. Therefore, we used an atmospheric chemical transport model that described the physical and chemical processes of air pollutants based on emissions and meteorological data to estimate spatiotemporally resolved pollutant concentrations. The estimates were enhanced by combining chemical transport model simulations with monitoring data, generating hourly concentrations of the six air pollutants in a grid of 15 km by 15 km across China. Modelling inputs, algorithm, and data quality details have been reported previously.21 We coupled the original monthly average concentration data with participants survey dates and residential addresses to calculate the average concentrations of the six air pollutants during the 12 months preceding the survey dates. The time-window of exposure was determined before data analysis referring to previous studies.12 These annual average concentrations were regarded as long-term exposure estimates. We also obtained participant-specific estimates of land surface temperature from a weather-forecast research model.22 Detailed descriptions of air pollution and weather assessment are reported in the appendix (p 3).
Outcomes
The primary outcome was global cognitive function, assessed using the Mini-Mental State Examination (MMSE), a screening tool for dementia. The MMSE has been frequently used to track changes in cognitive function over time because it can reliably detect cognitive deterioration.23 The MMSE is especially useful for people unable to go through complex clinical diagnostic testing.24 The global score of MMSE ranges from 0 to 30, with a higher score indicating better cognitive function. We calculated cognitive decline by subtracting participants’ MMSE scores in 2014 from their scores in 2018 (appendix p 4).
On the basis of data from previous studies, we assessed the following factors as covariates: age, sex, education, marital status, ethnicity, place of residence (urban vs rural), occupation before retirement, survey month, alcohol consumption, smoking status, physical activity, fruit intake, vegetable intake, water quality, living conditions, and income source. Participants were encouraged to answer as many questions as possible. No proxy, such as assessment of cognitive function and physical performance, was used for objective questions.25 The detailed definition of covariates is reported in the appendix (p 4). In addition, we included model-estimated ambient temperature as a covariate.
Statistical analysis
To link MMSE scores with air pollution exposure, we used three statistical methods: the DID model to assess the effect of the clean air policy (method 1), the DID model to regress the changes in MMSE scores against the changes in air pollution concentrations (method 2), and a cross-sectional model to regress MMSE scores against air pollution concentrations (method 3). The DID models are the standard methods for a quasi-experimental design.26,27 The separate cross-sectional analyses has been commonly used in previous studies and used as a sensitivity analysis to check the robustness of our findings.
Method 1 evaluated the effect of CCAA. We calculated within-individual differences between the MMSE scores in 2014 and 2018. Then we tested the null hypothesis that the mean within-individual difference of the intervention group (ie, target >5% from 2013 to 2017) was equal to that of the control group (ie, target ≤5% from 2013 to 2017). The ability to compare the intervention group with the control group might be undermined due to the differences in baseline demographic characteristics, long-term trends of outcome variables, and changes in longitudinal risk factors during the study period. To control for these differences we applied inverse probability weighting (IPW) to match the two groups in terms of baseline population characteristics including age, education, sex, residence, ethnicity, occupation before retirement, alcohol drinking status, smoking status, physical labour, and living conditions.28 Weighting was used to allow the two groups to be compared in terms of the previously highlighted variables (appendix p 10). We used within-individual MMSE score changes from the 2011 survey to the 2014 survey as a variable to approximate the longer-term individual-level MMSE score trends independent of policy-related reductions in pollutants. We then adjusted for temporal changes between 2011 and 2014 in the longitudinal risk factors for cognitive change, including survey month, marital status, alcohol drinking, smoking, physical activity, fruit intake, vegetable intake, water quality, living conditions, and income source.
Method 2 assessed the association between MMSE scores and air pollutant exposure. We used a similar approach to the DID analysis used in method 1, in which intervention or control was determined by a binary policy indicator. In method 2, we replaced the policy indicator variable with a continuous variable of changes in exposure to a single pollutant. Using PM2·5 changes as an example, individuals living in places with smaller PM2·5 reductions acted as controls in comparisons with those who lived in the places with larger PM2·5 reductions. The DID model can be described in a fixed effect regression (equation 1).
i denotes participant index; t denotes index of CLHLS survey and is equalled to 0 (the 2014 wave) or 1 (2018 wave); MMSEi,t denotes the MMSE score for the participant in the survey; ei,t denotes the random error; xi,t denotes air pollutant exposure; β is the change in MMSE per unit change in exposure; zi,t denotes the selected controlled covariates (described previously); γ is the coefficient for a specific covariate; ηi denotes the participant-specific fixed effect; and Δt denotes the effect of temporal trend. ηi models the effects of all individual variables that are invariant between the two surveys. For instance, ηi not only controls for MMSE baseline scores but also the air pollutant exposure at baseline. Equation 1 can also be used to express method 1, when replacing xi,t by the binary indicator for intervention (equation 2).
To control for seasonal variation in MMSE, we included an index of survey month in the main models. We also used an alternative approach by replacing the survey month with a spline term of monthly temperature (three degrees of freedom).
In method 3, we did cross-sectional analyses of the 2014 or 2018 data, using linear regression models controlling for selected covariates. The cross-sectional model for the 2014 survey correlated the MMSE baseline scores with air pollutant exposure at baseline; the model for 2018 survey re-evaluated the correlation after CCAA intervention. If all confounders were captured by the cross-sectional models, two estimates for the same pollutant should be statistically comparable. If the cross-sectional estimates for the same pollutant were inconsistent, they were regarded as unreliable due to uncontrolled biases.
We also did sensitivity and effect modification analyses for methods 1 and 2. First, we analysed whether cognitive decline and PM2·5 exposure had a different linear trend in the intervention group versus the control group before CCAA implementation using the 2011 and 2014 CLHLS datasets (appendix p 11). Our data also showed that in the control group, the MMSE varied in an expected pattern, which could be explained by age-related changes in cognitive function (appendix p 11). Second, we applied different stricter cutoffs of the particulate matter reduction targets (10%, 12%, and 15%) to define intervention versus control groups. Third, we repeated the analysis without IPW and without offsetting the longer-term MMSE trend. Fourth, we examined the interaction between CCAA indicator pollutant exposure and subpopulation indicator to assess whether exposure-outcome relationships were different between different subgroups. Statistical significance of the interaction term was evaluated by the Wald test. Fifth, we explored the non-linear exposure-response curves by replacing the linear term (xi,t) by its penalised then-plate spline expansions in equation 1. Finally, we derived two-pollutant non-linear models by parametrising the exposure term using a two-dimensional spline function. The two-pollutant exposure-response associations were used to explore how temporal variation in a pollutant was linked with MMSE score change, given the scenario in which the other modelled pollutant was unchanged (appendix p 5).
All statistical analyses were done using R (version 3.3.2). Imputation was done with the mice package (version 3.12.0). The linear mixed-effects models were inferred using the lme4 package (version 1.1–23), and fixed-effects models were inferred with the plm package (version 2.2–4). IPWs were calculated using the IPW package (version 1.0–11). Penalised spline functions were parameterised using the mgcv package (version 1.8–31), and inference of the non-linear mixed-effects models was done with the gamm4 package (version 1.2–6).
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
2812 individuals participated in both the 2014 and 2018 surveys and were included in our analysis, according to several exclusion criteria (appendix p 2). In the 2014 wave, the mean age of participants was 81·0 years (SD 9·3). 1408 (50·1%) were women and 1621 (57·6%) were from rural areas (table 1). 2251 (80·0%) participants were in the intervention group and 561 (20·0%) were in the control group. Participants in the intervention and control groups had similar age and sex distributions (table 1). We observed significant between-group differences for area of residence (p=0·0011) and ethnicity (p<0·0001). Therefore, we applied inverse probability weighting to control for these differences in our statistical models.
Table 1:
Characteristics of study participants
| Total population (n=28l2) | Control group (n=56l) | Intervention group (n=2251) | ||||
|---|---|---|---|---|---|---|
| 2014 | 2018 | 2014 | 2018 | 2014 | 2018 | |
| Age (year) | 81·0 (9·3) | 84·7 (9·4) | 81·4 (9·7) | 85·3 (9·7) | 80·9 (9·2) | 84·5 (9·3) |
| Education (year) | 2·8 (3·6) | 2·8 (3·6) | 2·9 (3·8) | 2·9 (3·8) | 2·8 (3·6) | 2·8 (3·6) |
| Sex | ||||||
| Female | 1408 (50·1%) | 1408 (50·1%) | 294 (52·4%) | 294 (52·4%) | 1114 (49·5%) | 1114 (49·5%) |
| Male | 1404 (49·9%) | 1404 (49·9%) | 267 (47·6%) | 267 (47·6%) | 1137 (50·5%) | 1137 (50·5%) |
| Area of residence | ||||||
| Urban | 318 (11·3%) | 318 (11·3%) | 48 (8·6%) | 48 (8·6%) | 270 (12·0%) | 270 (12·0%) |
| Suburb | 873 (31·0%) | 873 (31·0%) | 220 (39·2%) | 220 (39·2%) | 653 (29·0%) | 653 (29·0%) |
| Rural | 1621 (57·6%) | 1621 (57·6%) | 293 (52·2%) | 293 (52·2%) | 1328 (59·0%) | 1328 (59·0%) |
| Ethnicity | ||||||
| Han | 2623 (93·3%) | 2623 (93·3%) | 502 (89·5%) | 502 (89·5%) | 2121 (94·2%) | 2121 (94·2%) |
| Other | 189 (6·7%) | 189 (6·7%) | 59 (10·5%) | 59 (10·5%) | 130 (5·8%) | 130 (5·8%) |
| Occupation before retirement | ||||||
| Agriculture | 1115 (39·7%) | 1115 (39·7%) | 241 (43·0%) | 241 (43·0%) | 874 (38·8%) | 874 (38·8%) |
| Employee | 486 (17·3%) | 486 (17·3%) | 75 (13·4%) | 75 (13·4%) | 411 (18·3%) | 411 (18·3%) |
| Other | 1211 (43·1%) | 1211 (43·1%) | 245 (43·7%) | 245 (43·7%) | 966 (42·9%) | 966 (42·9%) |
| MMSE score | 25·8 (5·2) | 24·0 (7·1) | 26·4 (4·8) | 24·3 (6·8) | 25·7 (5·3) | 23·9 (7·2) |
| PM2·5, μg/m3 | 58·2 (14·8) | 41·5 (11·3) | 42·2 (14·3) | 31·3 (8·4) | 62·1 (12·0) | 44·1 (10·4) |
| PM10, μg/m3 | 84·2 (25·7) | 70·2 (22·0) | 54·6 (15·5) | 47·0 (10·8) | 91·6 (22·2) | 76·0 (20·2) |
| SO2, μg/m3 | 25·5 (11·2) | 12·8 (3·8) | 14·6 (8·0) | 10·6 (3·4) | 28·2 (10·2) | 13·4 (3·6) |
| NO2, μg/m3 | 26·6 (11·7) | 25·5 (9·6) | 14·8 (7·9) | 14·6 (6·1) | 29·5 (10·6) | 28·2 (8·3) |
| CO, mg/m3 | 0·86 (0·30) | 0·78 (0·17) | 0·61 (0·14) | 0·66 (0·14) | 0·919 (0·30) | 0·81 (0·17) |
| Max 8 hr O3, μg/m3 | 85·5 (11·2) | 94·5 (12·4) | 79·7 (15·7) | 80·3 (5·5) | 86·9 (9·2) | 98·0 (11·0) |
| Temperature, °C | 24·6 (5·1) | 24·4 (4·7) | 24·9 (5·8) | 24·7 (6·1) | 24·3 (5·4) | 24·2 (3·8) |
| Married and living with spouse | 1580 (56·2%) | 1376 (48·9%) | 344 (61·3%) | 321 (57·2%) | 1236 (54·9%) | 1055 (46·9%) |
| Regular physical activity | 884 (31·4%) | 809 (28·8%) | 153 (27·3%) | 154 (27·5%) | 731 (32·5%) | 655 (29·1%) |
| Current alcohol drinker | 498 (17·7%) | 430 (15·3%) | 97 (17·3%) | 87 (15·5%) | 401 (17·8%) | 343 (15·2%) |
| Current smoker | 518 (18·4%) | 450 (16·0%) | 106 (18·9%) | 92 (16·4%) | 412 (18·3%) | 358 (15·9%) |
| Fruit intake | ||||||
| Very often* | 408 (14·5%) | 484 (17·2%) | 75 (13·4%) | 78 (13·9%) | 333 (14·8%) | 379 (16·8%) |
| Often† | 835 (29·7%) | 720 (25·6%) | 161 (28·7%) | 176 (31·4%) | 674 (29·9%) | 590 (26·2%) |
| Sometimes‡ | 950 (33·8%) | 904 (32·1%) | 197 (35·1%) | 194 (34·6%) | 753 (33·5%) | 714 (31·7%) |
| Rarely§ | 619 (21·9%) | 674 (24·0%) | 128 (22·8%) | 113 (20·1%) | 491 (21·8%) | 568 (25·2%) |
| Vegetable intake | ||||||
| Very often* | 1701 (60·5%) | 1691 (60·1%) | 345 (61·5%) | 377 (67·2%) | 1356 (60·2%) | 1314 (58·4%) |
| Often† | 884 (31·4%) | 854 (30·4%) | 144 (25·7%) | 121 (21·6%) | 740 (32·9%) | 733 (32·6%) |
| Sometimes‡ | 174 (6·2%) | 200 (7·1%) | 50 (8·9%) | 41 (7·3%) | 124 (5·5%) | 159 (7·1%) |
| Rarely§ | 53 (1·9%) | 67 (2·4%) | 22 (3·9%) | 22 (3·9%) | 31 (1·4%) | 45 (2·0%) |
| Water quality | ||||||
| Tap water | 1896 (67·4%) | 2091 (74·4%) | 325 (57·9%) | 364 (64·9%) | 1571 (69·8%) | 1727 (76·7%) |
| Natural water (eg, stream) | 226 (8·1%) | 208 (7·4%) | 83 (14·8%) | 44 (7·8%) | 143 (6·4%) | 164 (7·3%) |
| Well water | 690 (24·5%) | 513 (18·2%) | 153 (27·3%) | 153 (27·3%) | 537 (23·9%) | 360 (16·0%) |
| Living situation | ||||||
| Living with family members | 2159 (76·8%) | 2159 (76·8%) | 421 (75·0%) | 451 (80·4%) | 1738 (77·2%) | 1708 (75·9%) |
| Living alone | 622 (22·1%) | 607 (21·6%) | 133 (23·7%) | 90 (16·0%) | 489 (21·7%) | 503 (22·3%) |
| Residential care home | 31 (1·1%) | 46 (1·6%) | 7 (1·2%) | 6 (1·1%) | 24 (1·1%) | 40 (1·8%) |
| Income source | ||||||
| Family support | 1352 (48·1%) | 1422 (50·6%) | 317 (56·5%) | 314 (56·0%) | 1035 (46·0%) | 1108 (49·2%) |
| Retirement pension | 608 (21·6%) | 620 (22·0%) | 113 (20·1%) | 113 (20·1%) | 495 (22·0%) | 507 (22·5%) |
| Social insurance | 235 (8·4%) | 244 (8·7%) | 71 (12·7%) | 59 (10·5%) | 164 (7·3%) | 185 (8·2%) |
| Working payment | 413 (14·7%) | 264 (9·4%) | 46 (8·2%) | 26 (4·6%) | 367 (16·3%) | 238 (10·6%) |
| Other | 204 (7·3%) | 262 (9·3%) | 14 (2·5%) | 49 (8·7%) | 190 (8·4%) | 213 (9·5%) |
Data are mean (SD) or n (%), unless otherwise specified. Max 8 h O3=maximum 8 h daily average O3 measurements. MMSE= Mini-Mental State Examination.
Almost everyday.
At least once a week.
At least once a month.
No habit.
Across all the participants, mean MMSE scores declined from 25·8 (SD 5·2) in 2014 to 24·0 (7·1) in 2018 (table 1). Mean MMSE scores reduced from 26·4 (4·8) in 2014 to 24·3 (6·8) in 2018 in the control group and from 25·7 (5·3) in 2014 to 23·9 (7·2) in 2018 in the intervention group. Long-term exposure to average annual concentrations of PM2·5, PM10, SO2, NO2, and CO across all participants was lower in 2018 than in 2014 (table 1). By contrast, long-term O3 exposure was higher in 2018 than in 2014 (table 1). The reduction in PM2·5, PM10, and SO2 or the increase in O3 was larger in the intervention group than in the control group; no group difference was observed for NO2 or CO (appendix p 12)
The effects of CCAA on MMSE scores, estimated in the DID models with different covariates, are reported in table 2. When unadjusted (method 1), the intervention group had a smaller decline in MMSE scores than the control group, which suggested that CCAA was associated with an incremental improvement in MMSE scores of 1·36 (95% CI 0·47–2·25; p=0·0027). In the fully adjusted model, the difference in MMSE scores between the intervention and control groups was larger (2·45 [95% CI 1·32–3·57]) compared with the unadjusted model. In a sensitivity analysis, excluding the IPW weights and the offset of MMSE trend, the estimates were generally consistent with results from the fully adjusted models (appendix p 17). Additionally, MMSE scores were not transformed in the analysis because the residuals were normally distributed (appendix p 17). Moreover, statistically significant differences between the intervention and control groups were consistently robust when the 5% or more reduction target was replaced with three larger reduction targets. When using the 10% target, 1027 (35·7%) participants were included in the control group and 1785 (63·5%) were included in the intervention group; for the 12% target, 1223 (43·5%) participants were included in the control group and 1589 (56·5%) were included in the intervention group; and for the 15% target, 1800 (64·0%) participants were included in the control group and 1012 (36·0%) were in the intervention group (appendix p 13). The estimated effect on improvement of MMSE scores was slightly lower for the model based on a larger reduction target (appendix p 13).
Table 2:
The estimated effect of the CCAA on changes in MMSE score by difference-in-differences models.
| Effect estimate (95% CI), MMSE score | p value | |
|---|---|---|
| Target ≥ 5% | ||
| Model 1 | 1·36 (0·47–2·25) | 0·0032 |
| Model 2 | 2·38 (1·25–3·50) | <0·0001 |
| Model 3 | 2·35 (1·22–3·48) | <0·0001 |
| Model 4 | 2·45 (1·32–3·57) | <0·0001 |
| Target ≥10%* | 1·39 (0·52–2·27) | 0·0023 |
| Target ≥12%* | 1·27 (0·45–2·09) | 0·0031 |
| Target ≥15%* | 1·07 (0·22–1·92) | 0·014 |
Model 1 is adjusted for inverse probability weighting from baseline and offset of MMSE trend. Model 2 is adjusted for survey month based on model 1. Model 3 is adjusted for marital status, alcohol drinking, smoking, and physical activity based on model 2. Model 4 is based on model 3, but is also adjusted for intake of fruit and vegetables, water quality, living condition, and income source. Effect estimates were also provided using different targets of the annual particulate matter reduction targets (≥10%, ≥12%, and ≥15%). CCAA=China’s Clean Air Act. MMSE=Mini-Mental State Examination.
assessed using model 4.
We also examined potential effect modifications (appendix p 14). We found that effect estimates were modified by age (adjusted MMSE score 3·18 [95% CI 1·66–4·70] for participants aged 75 years or younger; 2·97 [1·76–4·17] for 76–85-year-olds; and 1·66 [0·45–2·88] for participants aged 86 years and older), ethnicity (5·33 [3·51–7.16] for participants of non-Han ethnicity and 2·26 [1·10–3·35] for people of Han ethnicity), education (1·66 [0·47–2·86] for participants who had received no education; 2·99 [1·69–4·29] for those who had completed elementary school; and 3·41 [2·09–4·73] for those who completed junior high school or higher levels of education), marital status (2·02 [0·84–3·21] for individuals who were widowed or not married and 2·96 [1·75–4·17] for those who were married), and physical activity (3·32 [1·99–4.66] for those who did regular physical exercise (at least once a week) and 2·07 [0·91–3·23] for those who did not take regular physical exercise).
Figure 2 shows MMSE score changes associated with per unit increase in pollutant exposure, adjusted for all longitudinal covariates (marital status, survey month, alcohol drinking, smoking, physical activity, fruit intake, vegetable intake, water quality, living condition, and income source). We found significantly negative associations between MMSE score changes and PM2·5 and SO2 exposures and significantly positive associations for O3. The estimates for the other pollutants were not significantly different between 2014 and 2018. Adjusting for ambient temperature did not change these associations (appendix p 15). Additionally, the DID analyses for subgroups stratified by age, sex, ethnicity, rural versus urban area of residence, marital status, and level of education suggest an effect modification similar to that found in analysis of pollutant concentrations (appendix p 16). Furthermore, pollutant exposure changes were also associated with MMSE score changes using non-linear models (figure 3), which yielded results similar to the effects observed in the linear models. The non-linear models consistently showed an inverse association of MMSE scores with PM2·5 and SO2 and a positive association with O3. The positive association between MMSE changes and O3 changes should not be interpreted as a protective effect, due to a strong inverse correlation between PM2·5 and O3. To illustrate this issue, in a sensitivity analysis, we linked MMSE changes with PM2·5 and O3 changes jointly. The estimate for O3 was statistically non-significant when conditioned on the PM2·5 level (appendix p 17). The two-pollutant models also suggested SO2 had a significant effect on MMSE change after controlling for O3 and PM2·5 on a constant level.
Figure 2: Changes in MMSE scores associated with an IQR increase in pollutant exposure.
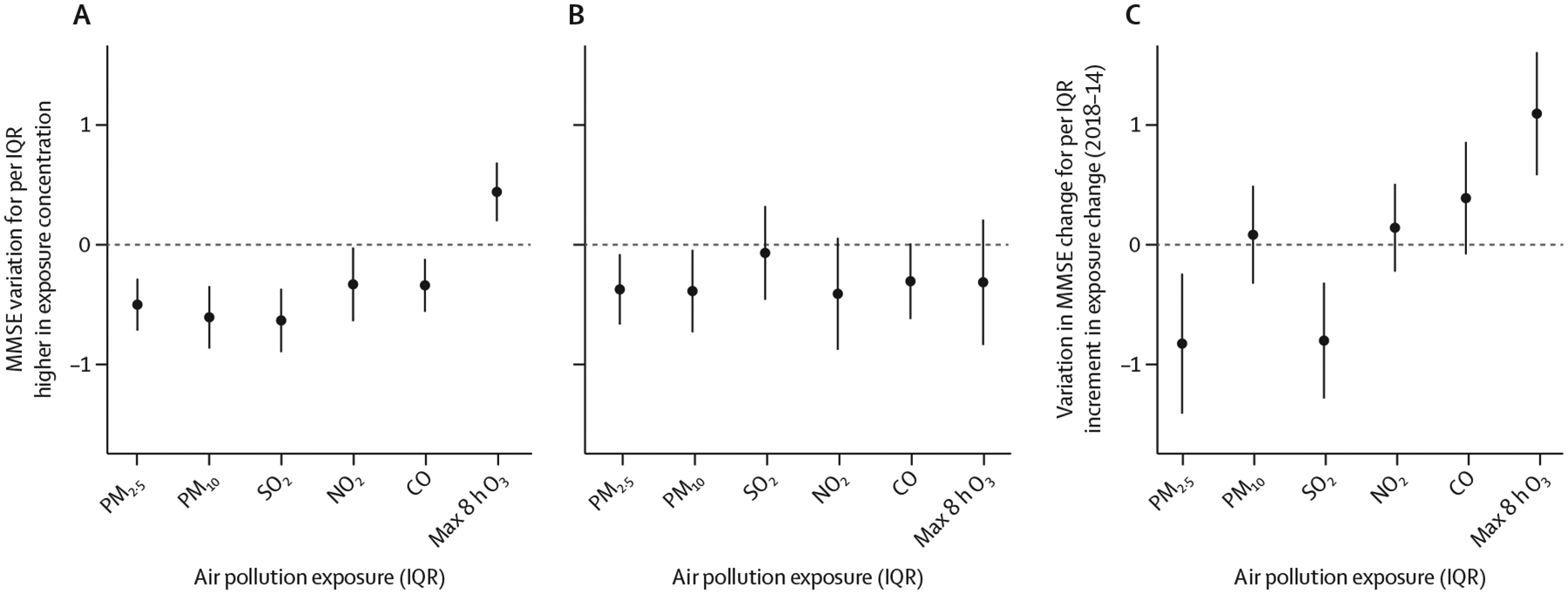
(A) 2014 cross-sectional analysis. (B) 2018 cross-sectional analysis. (C) Difference-in-difference analysis. In the cross-sectional analyses, MMSE scores were regressed against pollutant exposure across all participants in 2014 and 2018. In the temporal (2018 vs 2014) change analysis, within-person temporal changes in MMSE scores were regressed against temporal changes in pollutant exposure. In the difference-in-difference analysis marital status, survey month, alcohol drinking, smoking, physical activity, fruit intake, vegetable intake, water quality, living condition, and income source were adjusted for. The cross-sectional analysis was also adjusted for the time-invariant covariates (age in 2014, sex, education, ethnicity, place of residence, and occupation before retirement). Error bars are 95% CI. Max 8 h O3=maximum 8 h daily average O3 measurements. MMSE=Mini-Mental State Examination. PM2·5= particulate matter with a diameter of less than 2·5 μm. PM10=particulate matter with a diameter of less than 10 μm.
Figure 3: Non-linear estimates for the associations between changes in PM2·5 (A), PM10 (B), SO2 (C), NO2 (D), CO (E), and max 8 h O3 (F) and MMSE score changes by difference-in-differences models.
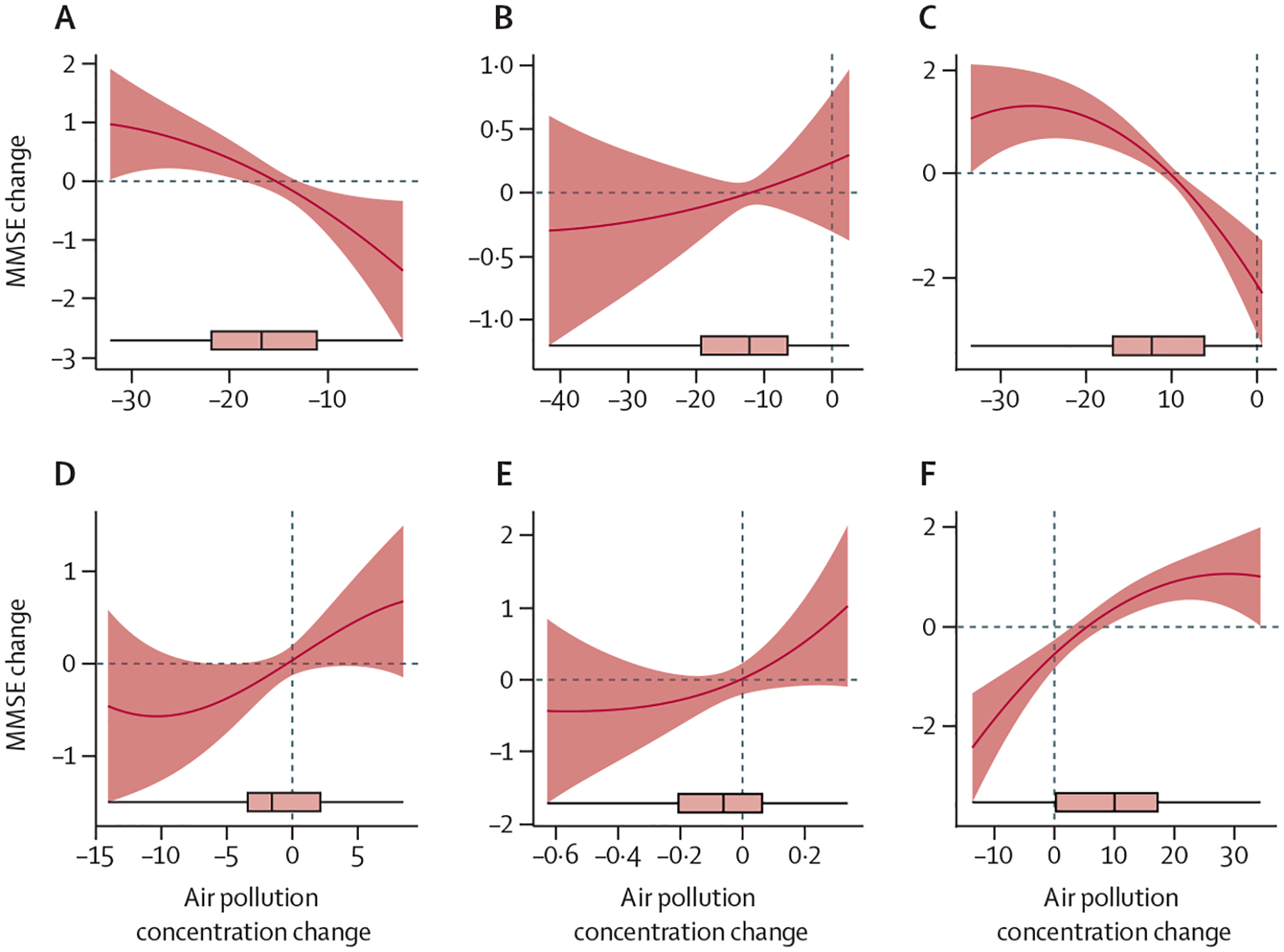
Solid lines are fitted estimates of the associations between air pollution changes and MMSE score changes. Shaded areas are 95% CI. The boxplots present the distributions of changes in air pollutants before and after the intervention. Max 8 h O3=maximum 8 h daily average O3 measurements. MMSE=Mini-Mental State Examination. PM2·5=particulate matter with a diameter of less than 2·5 μm. PM10=particulate matter with a diameter of less than 10 μm.
For all pollutants, except O3, Separate cross-sectional analyses of the 2014 and the 2018 data (method 3) generated results that consistently indicated negative associations between MMSE scores and exposure levels according to the point-estimates (figure 2). For O3, the general linear models show cross-sectional associations in opposite directions, suggesting a failure to control for confounders. According to the non-linear two-pollutant model (appendix pp 7–19), PM2·5 might be the confounder that had been ignored by the single pollutant models of O3.
Discussion
In this quasi-experimental study of a nationwide sample of older adults in China, we observed a beneficial effect of a national clean air policy on cognitive function. Specifically, when adjusted for covariates, the mean MMSE score decline from 2014 to 2018 was less in the policy intervention group than in the control group. Using annual average ambient pollutant concentrations at the residence level as exposure estimates, we found that increased PM2·5 exposure was associated with decreased MMSE scores across all participants in both the 2014 and the 2018 waves. Of note, we found that smaller MMSE score reductions from 2014 to 2018 were associated with larger PM2·5 reductions over the same period. Taken together, these findings support a potential causal role of PM2·5 in accelerating cognitive decline in older adults, contributing to the emerging evidence, reported in previous observational studies, that long-term PM2·5 exposure is a risk factor of cognitive decline.5–13
To the best of our knowledge, our study is the first to assess the effectiveness of a national clean air policy in attenuating ageing-driven cognitive decline. By integrating CCAA targets for PM2·5 between 2013 and 2017 with the CLHLS survey waves done before the policy implementation and after the implementation, we had the unique opportunity to design this quasi-experimental study to evaluate the effect of the clean air policy. In our DID analysis approach, we leveraged an experimental design consisting of an intervention group and control group based on differences in PM2·5 reduction targets. This allowed us to obtain an appropriate counterfactual framework to estimate a causal effect. A strength of the DID approach is that an individual is compared with themselves at different time points; therefore, potential confounders—such as genetic factors—are partly controlled. Similar to previous DID designs, we controlled for slow-changing, individual-level variables, including sociodemographic factors (eg, age or marital status), lifestyle (eg, smoking status), and socioeconomic status (eg, dietary habits).29,30 We also controlled for annual temperature, which might have varied with time. These adjustments reduced potential confounding of the effects of CCAA and PM2·5 observed in our study.
The DID analysis approach has been used in environmental interventional studies of other health outcomes.16 Previous studies have focused primarily on mortality associated with particulate matter.15,30 In addition, our previous studies found that clean air actions in China are associated with a decrease in household medical expenditures26 and reduced prevalence of depressive symptoms.27 However, to date, only one study in the USA has assessed and reported the effect of air pollution regulations on dementia diagnosis in people older than 75 years.18 This study investigated the association between reductions in annual average PM2·5 concentrations from 2004 to 2013 and reductions in dementia incidence rate in a population-level analysis, but it did not control for individual-level data—such as lifestyle, socioeconomic status, health conditions, and other individual-level factors. These individual-level variables might have changed substantially during the 9-year evaluation period, which might confound the effect of the policy. In our study, we did a series of sensitivity analyses that considered individual-level variables and their changes, confirming the robustness of the beneficial effect on cognitive function of PM2·5 exposure reduction brought by CCAA.
A previous study showed that a within-individual change in MMSE scores of at least 2–4 points can be classified as a true or reliable change in cognitive function and a minimum change of at least 1·4 points as clinically important. Although our findings refer to between-group differences, it might be possible that the difference between the intervention and control groups over 4 years of 2·45 (95% CI 1·32–3·57) reflects a clinically meaningful difference, on the basis of these previous findings.31,32 Our finding is consistent with previous epidemiological evidence on the detrimental effects of air pollutants on brain health.5–13 A metaanalysis of 31 studies, including cross-sectional and longitudinal analyses, provided compelling evidence that air pollution exposure is associated with accelerated cognitive decline across the life course.23 Our study, through a design mimicking a randomised clinical trial, strengthens the confidence in the putative causality of similar results from existing observational studies.17 These epidemiological findings are supported by the biological mechanisms through which fine particle matter impairs cognitive function. Experimental research has shown that fine and especially ultrafine particles (both components of PM2·5) can enter the brain either via circulation or intranasally by direct translocation through the olfactory bulb, leading to detrimental and toxic effects in the brain.33 In a post-mortem study of 19 people aged 34–83 years, who had died of non-neurological causes, expression of cyclooxygenase-2, an inflammatory mediator, and accumulation of the 42-amino acid form of β-amyloid, a cause of neuronal dysfunction, were higher in those who had lived in severely polluted cities in Mexico than in those who had lived in less polluted cities.34 A study published in 2020 showed an association between air pollution and local gyrification index, a marker of local brain atrophy in the ageing brain, suggesting that chronic exposure to PM2·5 might influence the physiological ageing process of the brain.35
A strength of this study is the quasi-experimental design to examine the potential causal link between CCAA and cognitive decline, by taking advantage of the rapid reduction of air pollution over 4 years. The causal inference is strengthened by including a counterfactual analysis feature (ie, the intervention group of participants who lived in an area with a PM2·5 reduction exceeding a target [eg, 5% annually]) compared with the control group (people who lived in an area with no target PM2·5 reduction). A previous study has shown that about 90% of PM2·5 variations during 2014 and 2017 in China were attributable to CCAA implementation.19 Therefore, PM2·5 reduction could serve as a discriminative indicator for CCAA effectiveness in our DID models. A significant intervention effect suggested by our group comparison is strengthened by a significant exposure-response association between MMSE score change and PM2·5 change from 2014 to 2018. Another strength is our ability to use residence-level air pollution estimates (as opposed to city-level or post code-level estimates widely used in previous studies) and individual-level sociodemographic and behavioural data. Moreover, we did a nationwide, prospective cohort study with neuropsychological data and individual characteristics in a diverse population in China, increasing the generalisability of our findings. Because China is one of the largest LMICs in the world, our findings might have policy implications for dementia prevention in other LMICs.
This study has some limitations. First, its quasi-experimental design does not eliminate the possibility of residual confounding. Although we found improvement in cognitive decline after the implementation of CCAA, we cannot rule out the possibility that some other changes during this time period or lifestyle alterations as a result of the policy (eg, more physical activity or social participation after the air quality improves) might have slowed or delayed cognitive decline. However, our methodology, including the use of time-fixed and policy-fixed effects and a time-varying variable, helped mitigate this limitation. Second, model-based residence-level exposure estimates cannot be verified with measured data for all areas, although the models have been well validated.36 Third, the participants in our control group included those living in poorer provinces with a range of other risk factors, such as higher rates of cigarette smoking and poverty. We used sensitivity analyses to evaluate the effect of some of these potential confounding factors; however, we could not control the effect of unmeasured potential confounders (eg, residual confounding), such as different PM2·5 compositions between northern and southern provinces. Fourth, we were not able to control for some potential confounders (eg, indoor air quality) due to a scarcity of relevant data. Fifth, a substantial proportion of the participants dropped out during the follow-up period mainly due to either death or refusal to participate; therefore, our results might be affected by selective survival bias, which is often the case in longitudinal studies of older populations. Finally, the uncertainties inherent in estimations of concentrations of air pollutants might cause exposure misclassification, which might lead to unstable or biased associations. For instance, the counterintuitive positive point-estimate for the effect of NO2 and CO might be related to the relatively low accuracy of the estimated concentrations for these two pollutants.
Findings from this study have public health policy implications for ageing societies. Dementia burden is projected to rapidly increase globally, especially in LMICs. Controlling any preventable risk factors is of paramount importance for a healthy ageing society. The evidence generated from our study, with a unique strength of causal inference, supports the need for implementing well designed clean air policies to reduce population and individual exposure to air pollutants, especially PM2·5. This need is particularly urgent considering the already known global disease burden due to air pollution. Policy-oriented interventions offer widespread protection and enhanced health with less reliance on conventional individual-centred lifestyle modifications.37 We also found that some subpopulations (eg, people from non-Han ethnic groups and physically active people) might be more susceptible to the effect of air pollution than the others. However, whether factors related to subpopulations (eg, environmental factors, lifestyles, or genetic susceptibility) might potentially modify the effect of air pollution needs to be investigated. One of the potential implications for governmental policy could be to encourage and support extensive research regarding the greater effect of air pollution on cognitive health in people from minority ethnic populations in China. Our findings can act as preliminary evidence to promote localised stringent air quality standards for those areas where susceptible individuals are clustered.
In conclusion, this quasi-experimental study adds additional evidence that PM2·5 exposure accelerates cognitive ageing in older people. Considering the rapid population ageing and increasing global disease burden of dementia, implementing policy-oriented interventions—such as clean air policies—is of paramount importance, especially in LMICs, for heathy brain ageing.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, Web of Science, and Google Scholar for studies in English published from database inception of each database until Oct 30, 2020, that examined the associations of air pollution or clean air policy with cognitive function. We used the combination of search terms: (“PM2·5” OR “fine particulate matters” OR “fine particles” OR “air pollution” OR “air pollutants”) OR (“clean air” OR “air act” OR “air policy” OR “air pollution regulation”) AND (“cognition” OR “cognitive” OR “dementia”). To date, published studies have used conventional statistical approaches, including cross-sectional and longitudinal designs, to study the association between exposure to air pollutants and cognitive deterioration in humans. One ecological study in the USA has estimated the reduction of particulate matter (PM) less than 2·5 μm in diameter (PM2·5) resulting from the effect of clean air acts during 2004 and 2013 in relation to dementia diagnoses at a state level. None of the previous studies have evaluated the effect of air pollution reduction interventions—such as the Chinese Clean Air Act—on cognitive disorders or cognitive function at the individual level. Furthermore, no study to date has examined the association between air pollutants and cognitive impairment using causal inference approaches with quasi-experimental designs.
Added value of this study
To our knowledge, this is the first quasi-experimental study done in a nationwide diverse sample of 2812 older adults (aged ≥65 years) from 23 provinces across China to study the effect of PM-reduction policies on cognitive ageing. We used a difference-in-differences analysis to compare longitudinal changes in cognitive function between participants who live in areas that had PM reductions resulting from policy interventions and participants who live in areas with no PM reductions. The evidence generated from this study strengthens the causal inference of the detrimental effect of PM on cognitive function and especially on accelerated cognitive ageing. Our results confirmed that air pollution is one of the modifiable risk factors for dementia, identified by the 2020 Lancet Commission on dementia prevention.
Implications of all the available evidence
Dementia burden is projected to rapidly increase globally and especially in low-income and middle-income countries. Taken together, the evidence generated from this study suggest that long-term exposure to PM2·5 might play a causal role in accelerating cognitive decline in older adults. The evidence supports the need for implementing adequate clean air policies to reduce population and individual exposure to air pollutants, especially PM2·5. This need is particularly urgent considering the already known global disease burden due to air pollution. The policy-oriented interventions offer widespread risk reduction with less reliance on conventional individual-centred lifestyle modifications.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (81903392, 4217050142, and 81941021), National Key Research and Development Program of China (2018YFC2000400 and 2017YFC1311100), China Postdoctoral Science Foundation funded project (2019M650359), the US National Institute on Aging (P01AG031719), the Duke/Duke-National University of Singapore Collaboration Pilot Project, and Peking University-Baidu Fund (2020BD031), Energy Foundation (G-2107-33169 and R-2109-33379), and the Fundamental Research Funds for the Central Universities (BMU2021YJ042). We are grateful to the CLHLS participants for providing the data for this research. The CLHLS was supported by funds from the US National Institute on Aging, National Institutes of Health, the Duke/Duke-NUS Collaboration Pilot Project, the National Natural Science Foundation of China, the China Social Science Foundation, and the UN Fund for Population Activities. The CLHLS was managed by the Center for Healthy Aging and Development Studies, Peking University. We also thank the support from the Healthy Aging Consortium of the China Cohort Consortium. We would like to thank Hongkai Li, Luke Parsons, Lei Hou, and Bin Han for their thoughtful comments that helped improve this paper.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
The data that support the findings of this study are available from Center for Healthy Aging and Development Studies, Peking University, Beijing, China, but restrictions apply to the availability of residential data. The residential data of each participant in the Chinese Longitudinal Healthy Longevity Survey (CLHLS) were sensitive information and was used under license and authorisation, so they are not publicly available. However, data are available from the authors upon reasonable request and with permission of Yi Zeng (zengyi@nsd.pku.edu.cn).
See Online for appendix
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013; 9: 63–75.e2. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017; 13: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396: 413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Chen X, Zhang X. The impact of exposure to air pollution on cognitive performance. Proc Natl Acad Sci USA 2018; 115: 9193–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schikowski T, Altuğ H. The role of air pollution in cognitive impairment and decline. Neurochem Int 2020; 136: 104708. [DOI] [PubMed] [Google Scholar]
- 7.Kulick ER, Wellenius GA, Boehme AK, et al. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 2020; 94: e1782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzivian L, Dlugaj M, Winkler A, et al. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf Recall Study. Environ Health Perspect 2016; 124: 1361–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 2012; 172: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 2020; 77: 801–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tallon LA, Manjourides J, Pun VC, Salhi C, Suh H. Cognitive impacts of ambient air pollution in the National Social Health and Aging Project (NSHAP) cohort. Environ Int 2017; 104: 102–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi L, Wu X, Danesh Yazdi M, et al. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 2020; 4: e557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Li T, Lv Y, et al. Fine particulate matter and poor cognitive function among Chinese older adults: evidence from a community-based, 12-year prospective cohort study. Environ Health Perspect 2020; 128: 67013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Coull B, Lin X, et al. Short-term air pollution, cognitive performance, and nonsteroidal anti-inflammatory drug use in the Veterans Affairs normative aging study. Nat Aging 2021; 1: 430–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Braun D, Schwartz J, Kioumourtzoglou MA, Dominici F. Evaluating the impact of long-term exposure to fine particulate matter on mortality among the elderly. Sci Adv 2020; 6: eaba5692. 10.1126/sciadv.aba5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bind MA. Causal modeling in environmental health. Annu Rev Public Health 2019; 40: 23–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman GT, Dominici F. Don’t abandon evidence and process on air pollution policy. Science 2019; 363: 1398–400. [DOI] [PubMed] [Google Scholar]
- 18.Bishop KC, Ketcham JD, Kuminoff NV. Hazed and confused: the effect of air pollution on dementia. National Bureau of Economic Research, 2018. https://www.nber.org/papers/w24970 (accessed Aug 30, 2020). [Google Scholar]
- 19.Zhang Q, Zheng Y, Tong D, et al. Drivers of improved PM2.5 air quality in China from 2013 to 2017. Proc Natl Acad Sci USA 2019; 116: 24463–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet 2017; 389: 1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao T, Lei K, Jiang Z, et al. Data from a six-year long high-resolution air quality reanalysis dataset over China from 2013 to 2018. Science Data Bank 2020; published online April 21. 10.11922/sciencedb.00053 [DOI] [Google Scholar]
- 22.Xue T, Zhu T, Zheng YX, Zhang Q. Declines in mental health associated with air pollution and temperature variability in China. Nat Commun 2019; 10: 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clifford A, Lang L, Chen R, Anstey KJ, Seaton A. Exposure to air pollution and cognitive functioning across the life course—a systematic literature review. Environ Res 2016; 147: 383–98. [DOI] [PubMed] [Google Scholar]
- 24.Pezzotti P, Scalmana S, Mastromattei A, Di Lallo D. The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: a prospective observational study. BMC Fam Pract 2008; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Poston DL, Vlosky DA, Gu D. Healthy longevity in China: demographic, socioeconomic, and psychological dimensions. Berlin: Springer Science & Business Media, 2008. [Google Scholar]
- 26.Xue T, Zhu T, Peng W, et al. Clean air actions in China, PM2.5 exposure, and household medical expenditures: a quasi-experimental study. PLoS Med 2021; 18: e1003480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue T, Guan T, Zheng Y, et al. Long-term PM2.5 exposure and depressive symptoms in China: a quasi-experimental study. Lancet Reg Health West Pac 2020; 6: 100079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansournia MA, Altman DG. Inverse probability weighting. BMJ 2016; 352: i189. [DOI] [PubMed] [Google Scholar]
- 29.Yu W, Guo Y, Shi L, Li S. The association between long-term exposure to low-level PM2.5 and mortality in the state of Queensland, Australia: a modelling study with the difference-in-differences approach. PLoS Med 2020; 17: e1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Kloog I, Coull BA, Kosheleva A, Zanobetti A, Schwartz JD. Estimating causal effects of long-term PM2.5 exposure on mortality in New Jersey. Environ Health Perspect 2016; 124: 1182–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry 2007; 78: 1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howard R, Phillips P, Johnson T, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. Int J Geriatr Psychiatry 2011; 26: 812–17. [DOI] [PubMed] [Google Scholar]
- 33.Oberdörster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract--and beyond? Environ Health Perspect 2002; 110: A440–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderón-Garcidueñas L, Reed W, Maronpot RR, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol 2004; 32: 650–58. [DOI] [PubMed] [Google Scholar]
- 35.Nußbaum R, Lucht S, Jockwitz C, et al. Associations of air pollution and noise with local brain structure in a cohort of older adults. Environ Health Perspect 2020; 128: 67012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YL, Batterman S. Residence location as a measure of environmental exposure: a review of air pollution epidemiology studies. J Expo Anal Environ Epidemiol 2000; 10: 66–85. [DOI] [PubMed] [Google Scholar]
- 37.Susser M, Susser E. Choosing a future for epidemiology: I. Eras and paradigms. Am J Public Health 1996; 86: 668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


