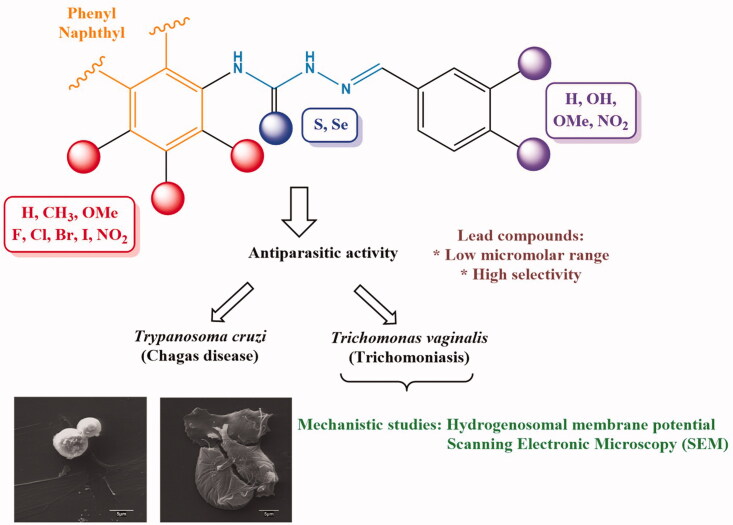
Abstract
Herein, we report the preparation of a panel of Schiff bases analogues as antiprotozoal agents by modification of the stereoelectronic effects of the substituents on N-1 and N-4 and the nature of the chalcogen atom (S, Se). These compounds were evaluated towards Trypanosoma cruzi and Trichomonas vaginalis. Thiosemicarbazide 31 showed the best trypanocidal profile (epimastigotes), similar to benznidazole (BZ): IC50 (31)=28.72 μM (CL-B5 strain) and 33.65 μM (Y strain), IC50 (BZ)=25.31 μM (CL-B5) and 22.73 μM (Y); it lacked toxicity over mammalian cells (CC50 > 256 µM). Thiosemicarbazones 49, 51 and 63 showed remarkable trichomonacidal effects (IC50 =16.39, 14.84 and 14.89 µM) and no unspecific cytotoxicity towards Vero cells (CC50 ≥ 275 µM). Selenoisosters 74 and 75 presented a slightly enhanced activity (IC50=11.10 and 11.02 µM, respectively). Hydrogenosome membrane potential and structural changes were analysed to get more insight into the trichomonacidal mechanism.
Keywords: Trypanosoma cruzi, Trichomonas vaginalis, antiprotozoal agents, thio(seleno)semicarbazones, unspecific cytotoxicity, MoA
Graphical Abstract
1. Introduction
Protozoa are responsible for a large number of severe parasitic diseases in humans, livestock and pets, causing important morbidity and mortality worldwide1. This constitutes a burden for health systems in low-medium income tropical and subtropical countries from Africa, Asia and Latin America, where some of these protozooses are endemic2. Despite affecting more than one billion people, almost one sixth of the human population, and causing roughly half million deaths per year, many of these diseases are largely ignored, and indeed, some of them are categorised into the so-called Neglected Tropical Diseases (NTDs) group3.
Management of diseases caused by pathogenic protozoa is not a simple task4. On the one hand, development of successful vaccines is a hitherto unachieved goal5; on the other hand, the chemotherapeutic arsenal available so far suffers from important drawbacks: most of them are old drugs that are developing chemoresistance6, and are endowed with severe side-effects7 and low efficiency8. Moreover, their high prices, and complex administration protocols make them unaffordable for underdeveloped countries9. Accordingly, the development of new antiprotozoal agents is a hot topic in current Medicinal Chemistry research10,11.
Herein, we have focussed our attention on Chagas disease and trichomoniasis. On the one hand, Chagas disease (aka American trypanosomiasis), discovered by the Brazilian physician Carlos R.J. das Chagas in 1909, is endemic in Latin America, where it affects roughly 7 million people12; nevertheless, it is also being spread to USA, Canada, Europe and Australia, because of human migrations13, and therefore, becoming a global health problem14. Chagas disease is caused by the haemoflagellate protozoan Trypanosoma cruzi, whose transmission to humans naturally takes place by contact with faeces or urine of infected triatomine insects. The infection can also be transmitted by non-vectorial routes, such as the iatrogenic and the congenital one12. The life-cycle of the parasite involves three stages: epimastigotes (extracellular and replicative form found in the intestine of the vector), amastigotes (intracellular and proliferative form of the vertebrate host) and trypomastigotes (extracellular and non-replicative state found in the bloodstream)15,16. Currently, there are only two available drugs for the specific treatment of Chagas disease: benznidazole (BZ), a nitroimidazole, and nifurtimox, a nitrofurane both showing some disadvantages (e.g., low efficacy in the chronic phase, adverse effects and parasite drug resistance) that constitute one of the main drawbacks of this parasitosis17.
Cruzipain, the main papain-like cysteine peptidase in T. cruzi is currently considered as a validated therapeutic target against Chagas disease18, and thus compounds inhibiting such enzyme constitute an interesting alternative to classical antichagasic drugs. Some thio-19‒21 and selenosemicarbazones22 have been reported to be good inhibitors of such enzymes.
On the other hand, regarding the four curable sexually-transmitted infections (STI), three of them are caused by bacteria (chlamydia, gonorrhoea and syphilis), whereas the fourth one (trichomoniasis) is caused by a flagellated protozoan. In this context, a recent report has indicated23 that within population ranging from 15 to 49 years, 376.4 million new cases of STI were estimated to appear each year, among which, 156.0 million cases of urogenital trichomoniasis are found. Human trichomoniasis, recently categorised as a neglected parasitic infection (NPI)24, not only accounts for more than 40% of curable STI, but also represents a severe health risk25, as it increases the susceptibility to HIV, human papilloma virus (HPV), or herpes simplex virus (HSV) infections, as well as cervical and prostate cancers26. In this sense, some authors have demonstrated the positive impact of trichomoniasis treatment on the prevention of HIV transmission27. Therefore, as the development of these neoplasia seems to be associated with the inflammatory response induced by the parasite26, its diagnosis and treatment could also reduce the risk of their subsequent development. Moreover, and due to the fact that trichomoniasis is not a notifiable STI, with a high number of asymptomatic patients, epidemiologic data might be underestimated28. Currently, there are only two drugs available for the treatment of trichomoniasis29, both of them being nitroimidazole derivatives: metronidazole (MTZ), discovered in 1959, and still the first drug of choice, and tinidazole, approved in 2004. Due to the scarce number of commercialised drugs against trichomoniasis, when drug resistance, side-effects or hipersensibility to 5-nitroimidazoles emerge, no alternative treatments are available29.
Herein, we have accomplished the preparation of an ample panel of thiosemicarbazones with the aim of developing antiprotozoal agents with a different mechanism of action than that exhibited by nitroheterocyclic derivatives. This type of Schiff bases analogues has been reported to be endowed with a broad spectrum of relevant biological properties, like inhibitors of aldose reductase30, tyrosinase31, urease32 cholinesterases33, or β-amyloid aggregation34, and also as antimicrobial35, or anticancer agents36‒38, the latter being the most widely studied. Although there are some reports on the use of thiosemicarbazones as antiprotozoal agents against Toxoplasma gondii39‒41 or T. cruzi42‒45, studies on T. vaginalis are very scarce and limited to nitrofurane derivatives and bisthiosemicarbazones46–49.
2. Materials and methods
2.1. General procedures
TLCs were performed using aluminium-coated sheets (Merck 60 F254), 0.25 mm gel thickness. Each eluant is indicated in the experimental procedures. Spots were visualised by UV light (λ = 254 nm), and by charring with 10% ethanolic vainillin containing 1% H2SO4, or with 5% ethanolic phosphomolibdic acid.
Column chromatography purifications were performed using silica gel stationary phase (Merck 60, particle size 40‒63 µm), eluting by gravity, or with a mild pressure. Eluants are indicated in each case.
NMR spectra were registered in the Centro de Investigación, Tecnología e Innovación de la Universidad de Sevilla (CITIUS), using Bruker Avance III 300 spectrometer (300.1 MHz for 1H, 75.5 MHz for 13 C), using the solvent indicated in each case. Chemical shifts (δ) are expressed in ppm, and coupling constants (J) in Hz. Residual signals from the solvent are used as internal references for the calibration. Mass spectra were registered using a QExactive™ spectrometer, using Electrospray Ionisation (ESI), and calibrated using the PierceTM LTQ Velos ESI Positive Ion Calibration Solution (ThermoFisher Scientific).
2.2. Biological assessments
General procedures for the biological assays accomplished herein can be found in the Supporting Information.
2.3. Chemistry
General procedures for the preparation of isothiocyanates 11‒20, thiosemicarbazides 22‒30, thiosemicarbazones 36‒65 and selenosemicarbazones 74, 75, together with spectroscopic characterisation of new compounds can be found in the Supporting Information.
3. Results and discussion
3.1. Chemistry
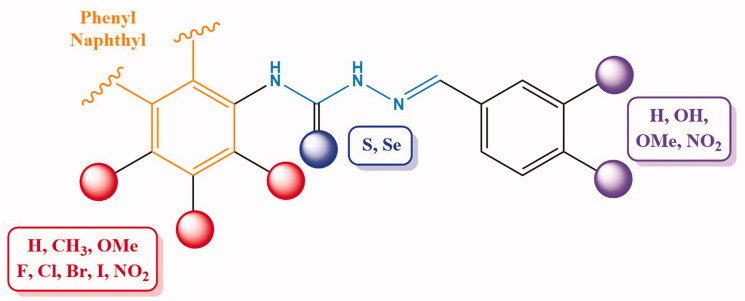
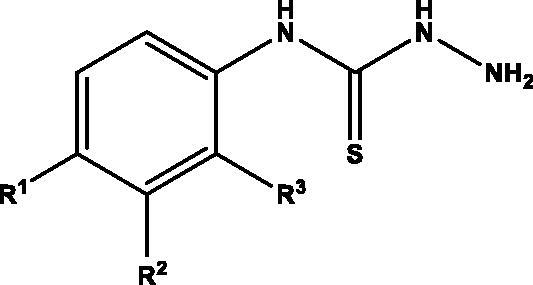
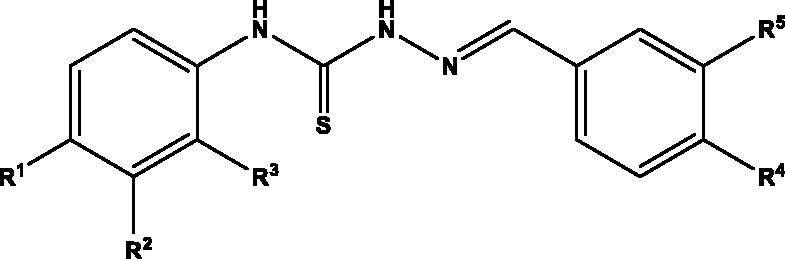
Considering the variety of biological properties exhibited by thiosemicarbazones, including some antiprotozoal activities, we have accomplished the preparation of an ample panel of Schiff bases analogues with the aim of targeting protozoal-mediated diseases like Chagas disease, or trichomoniasis. For this purpose, we envisioned the general structure depicted in Figure 1: an aromatic residue (phenyl, naphthyl) is located on position N-4 of the thiosemicarbazone, which in turn incorporates substituents with different stereoelectronic properties, like methyl, methoxy, halogens, or NO2. Moreover, the second aromatic motif, on position N-1 of the Schiff base is decorated with a different number of substituents, including free and masked phenolics, and NO2, which can modulate both the bioavailability and activity of the compounds.
Figure 1.
General structure for the ntiprotozoal thio(seleno)semicarbazones prepared herein.
We have also considered the isosteric replacement of sulphur with selenium; our group has previously prepared a plethora of organoselenium derivatives exhibiting interesting redox properties50,51, together with strong antiproliferative activities37,38,52‒55.
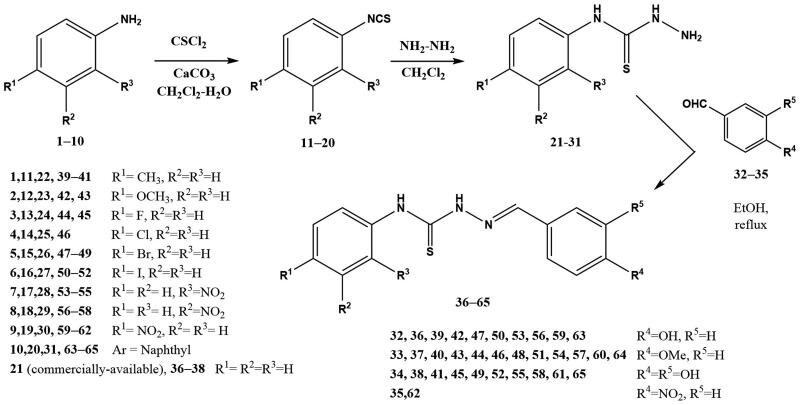
The synthetic approach used for accessing thiosemicarbazones is depicted in Scheme 1. Thus, treatment of commercially available anilines 1‒10 with thiophosgene as the thionating agent afforded isothiocyanates 11‒20, which in turn were transformed into target thiosemicarbazones 36‒65 upon subsequent treatment with hydrazine, followed by condensation of intermediate thiosemicarbazides 21‒31 with the corresponding aldehydes 32‒35. Final compounds precipitated in the medium; 13 C-NMR resonances at 175‒177 ppm (C = S) and at 143‒146 ppm (C = N) confirmed their structures.
Scheme 1.
Preparation of thiosemicarbazones 36‒65.
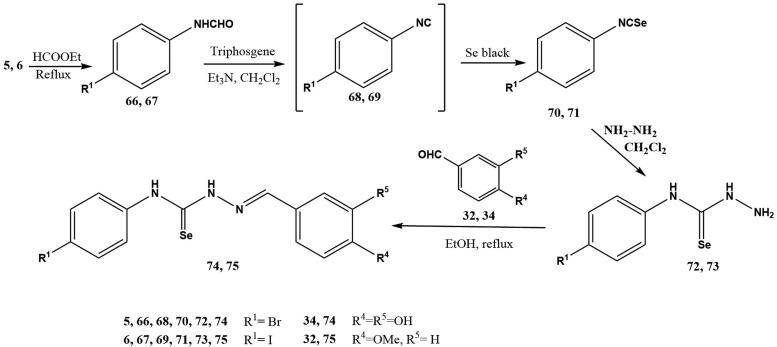
Selenosemicarbazones 74, 75 were obtained using a similar synthetic pathway (Scheme 2); the main difference is the access to the key isoselenocyanates 70, 71. They were obtained using a methodology developed in our group56,57 for the preparation of alkyl- and aryl isoselenocyanates, using triphosgene as a safe substitute for hazardous phosgene in the dehydration of formamides 66, 67 to furnish transient isocyanides 68, 69, which underwent addition of elemental black selenium.
Scheme 2.
Preparation of selenosemicarbazones 72, 73.
3.2. Biological assays
3.2.1. In vitro evaluation of anti-T. cruzi activity
Trypanocidal activity was evaluated by following a sequential screening procedure in which all compounds were primarily tested over T. cruzi epimastigotes of the drug-sensitive CL-B5 lacZ strain (DTU TcVI)58,59. Then, compounds whose trypanocidal profiles were similar to that of the reference drug BZ, were assayed over such an extracellular form of the moderately drug-resistant Y strain (DTU TcII)60. Only those compounds with selectivity indexes (SI) on epimastigotes similar or higher than that of BZ, were moved to a more specific assay against the replicative and intracellular forms of the parasite (amastigotes)58,59. Table 1 depicts the results of trypanocidal activity shown by parent thiosemicarbazides (23‒25, 27‒31) and the corresponding thiosemicarbazones (36‒65).
Table 1.
In vitro activity against Trypanosoma cruzi CL-B5 (DTU TcVI) and Y (DTU TcII) parasites and unspecific cytotoxicity on L929 and J774 cells, expressed as IC50 and CC50 values, respectively. Selectivity indexes (SI) for each strain and form are also calculated.
| Compound | IC50 (µM)a CL-B5 epimastigotes |
CC50 (µM)a L929 cells |
SIb CL-B5 epi |
IC50 (µM)a Y epimastigotes |
CC50 (µM)a J774 cells |
SIb Y epi |
IC50 (µM)a CL-B5 amastigotes |
SIb CL-B5 ama |
|---|---|---|---|---|---|---|---|---|
| ||||||||
|
23 (R1=OMe, R2=R3=H) |
53.51 ± 12.98 | >256 | >4.78 | 59.91 ± 10.43 | >256 | >4.27 | − | − |
|
24 (R1=F, R2=R3=H) |
58.80 ± 8.77 | >256 | >4.35 | 97.26 ± 3.45 | >256 | >2.63 | − | − |
|
25 (R1=Cl, R2=R3=H) |
44.17 ± 13.61 | 67.66 ± 3.16 | 1.53 | − | − | − | − | − |
|
27 (R1=I, R2=R3=H) |
49.76 ± 14.25 | 50.99 ± 8.88 | 1.02 | − | − | − | − | − |
|
28 (R1=R2=H, R3=NO2) |
44.15 ± 5.38 | 210.34 ± 15.34 | 4.76 | − | − | − | − | − |
|
29 (R1=R3=H, R2=NO2) |
79.86 ± 15.40 | 205.76 ± 7.25 | 2.58 | − | − | − | − | − |
|
30 (R1=NO2, R2=R3=H) |
72.49 ± 1.23 | 23.58 ± 2.84 | 0.33 | − | − | − | − | − |
|
31 (Ar = Naphthyl) |
28.72 ± 4.61 | >256 | >8.91 | 33.65 ± 5.72 | >256 | >7.61 | 39.66 ± 9.91 | >6.45 |
| ||||||||
| R1=R2= R3=H | ||||||||
|
36 R4=OH, R5=H |
70.88 ± 10.96 | 46.29 ± 0.15 | 0.65 | − | − | − | − | − |
|
37 R4=OMe R5=H |
39.79 ± 6,43 | >256 | >6.43 | 85.10 ± 17.15 | >256 | >3.01 | − | − |
|
38 R4=R5=OH |
15.34 ± 2.22 | 45.19 ± 0.58 | 2.95 | − | − | − | − | − |
| R1=Me, R2=R3=H | ||||||||
|
39 R4=OH, R5=H |
39.58 ± 7.08 | 60.99 ± 3.72 | 1.54 | − | − | − | − | − |
|
40 R4=OMe R5=H |
>256 | >256 | NDc | − | − | − | − | − |
|
41 R4=R5=OH |
118.61 ± 7.68 | 20.02 ± 1.87 | 0.17 | − | − | − | − | − |
| R1=OMe, R2=R3=H | ||||||||
|
42 R4=OH, R5=H |
49.19 ± 9.97 | 43.31 ± 16.61 | 0.88 | − | − | − | − | − |
|
43 R4=OMe R5=H |
26.75 ± 2.68 | >256 | >9.57 | 55.57 ± 16.30 | >256 | >4.61 | 39.04 ± 14.33 | >6.56 |
| R1=F, R2=R3=H | ||||||||
|
44 R4=OMe R5=H |
>256 | >256 | ND | − | − | − | − | − |
|
45 R4=R5=OH |
97.38 ± 5.33 | 30.47 ± 3.93 | 0.31 | − | − | − | − | − |
| R1=Cl, R2=R3=H | ||||||||
|
46 R4=OMe R5=H |
30.53 ± 4.10 | 49.72 ± 3.76 | 1.63 | − | − | − | − | − |
| R1=Br, R2=R3=H | ||||||||
|
47 R4=OH, R5=H |
42.79 ± 12.01 | 66.96 ± 5.43 | 1.56 | − | − | − | − | − |
|
48 R4=OMe R5=H |
>256 | >256 | ND | − | − | − | − | − |
|
49 R4=R5=OH |
104.5 ± 15.71 | 16.71 ± 0.70 | 0.16 | − | − | − | − | − |
| R1=I, R2=R3=H | ||||||||
|
50 R4=OH, R5=H |
22.80 ± 1.61 | 41.44 ± 8.36 | 1.82 | − | − | − | − | − |
|
51 R4=OMe R5=H |
>256 | >256 | NDc | − | − | − | − | − |
|
52 R4=R5=OH |
69.37 ± 4.73 | 20.82 ± 7.92 | 0.30 | − | − | − | − | − |
| R1=R2=HR3=NO2 | ||||||||
|
53 R4=OH, R5=H |
21.77 ± 2.98 | 34.89 ± 8.65 | 1.60 | − | − | − | − | − |
|
54 R4=OMe R5=H |
210.40 ± 14.54 | >256 | >1.22 | − | − | − | − | − |
|
55 R4=R5=OH |
41.99 ± 9.06 | 21.40 ± 4.12 | 0.51 | − | − | − | − | − |
| R1=R3=HR2=NO2 | ||||||||
|
56 R4=OH, R5=H |
36.85 ± 3.84 | 28.81 ± 3.25 | 0.78 | − | − | − | − | − |
|
57 R4=OMe R5=H |
22.96 ± 6.33 | >256 | >11.15 | >256 | >256 | ND | 22.27 ± 4.20 | >11.49 |
|
58 R4=R5=OH |
33.79 ± 7.59 | 11.98 ± 1.42 | 0.35 | − | − | − | − | − |
| R1=NO2 R2=R3=H | ||||||||
|
59 R4=OH, R5=H |
76.37 ± 9.91 | 29.44 ± 2.52 | 0.39 | − | − | − | − | − |
|
60 R4=OMe R5=H |
>256 | >256 | ND | − | − | − | − | − |
|
61 R4=R5=OH |
110.56 ± 14.83 | 5.37 ± 0.30 | 0.05 | − | − | − | − | − |
|
62 R4=NO2 R5=H |
>256 | 53.50 ± 4.82 | <0.21 | − | − | − | − | − |
| Ar = Naphthyl | ||||||||
|
63 R4=OH R5=H |
53.65 ± 11.28 | 31.14 ± 9.21 | 0.58 | − | − | − | − | − |
|
64 R4=OMe R5=H |
>256 | >256 | ND | − | − | − | − | − |
|
65 R4=R5=OH |
70.23 ± 6.99 | 28.79 ± 7.77 | 0.41 | − | − | − | − | − |
| Benznidazole | 25.31 ± 1.63 | >256 | >10.11 | 22.73 ± 1.82 | >256 | >11.26 | 0.47 ± 0.09 | >544.68 |
aResults are expressed as the mean ± SD of three independent experiments (n = 3).
bSI CL-B5 epi = CC50 L929/IC50 CL lacZ epimastigotes, SI Y epi = CC50 J774/IC50 Y epimastigotes and SI CL-B5 ama = CC50 L929/IC50 CL lacZ amastigotes.
cND: not determined.
As it can be seen, thiosemicarbazides 23 and 31 and thiosemicarbazones 37, 43 and 57 were active on CL-B5 epimastigotes, showing selectivity indexes (SI) ranging from >4.78 to >11.15. According to these results, they were tested on Y strain epimastigotes: three of them displayed slightly lower activity against moderately drug-resistant parasites, and only 23 and 31 showed similar trypanocidal profiles over both DTU TcVI and TcII strains. As depicted in Table 1, none of these molecules triggered toxic effects on mammalian cells, either phagocytic or non-phagocytic (CC50 >256 µM). In fact, previous studies introduce the capability of different series of thiosemicarbazone-based compounds to inhibit epimastigotes growth, proposing the interaction of these molecules with parasite proteases (i.e., cruzipain, one validated target for Chagas disease) as responsible of such an effect19‒21. Improved inhibition of cruzipain (low nanomolar range) was reported22 for the isosteric replacement of sulphur with selenium, what was claimed to be responsible for the antiparasitic activity of such selenosemicarbazones (epimastigotes and intracellular amastigotes).
Regarding the activity on CL-B5 amastigotes, compounds 31, 43 and 57 were the only derivatives assayed against the replicative and intracellular form of the parasite, according to their SI on CL-B5 epimastigotes: SI 31 > 8.91, SI 43 > 9.57 and SI 57 > 11.15. Unfortunately, none of these derivatives were as active as BZ, with IC50 values on amastigotes ranging from 22.27 to 39.66 µM. This reduction in the parasite burden of infected cells could also occur because of cruzipain inhibition: the cysteine protease, also present in the intracellular form of T. cruzi, has been usually proposed as putative target of thiosemicarbazones61,62.
3.2.2. In vitro evaluation of anti-Trichomonas vaginalis activity
Activity against T. vaginalis was evaluated following the sequential flow chart protocol of drug screening in which the most active molecules against the parasite are subsequently evaluated against Vero cells to determine their selectivity indexes (SI)63. The antiparasitic activity of thiosemicarbazides 23‒25, 27‒31 and thiosemicarbazones 36‒65 is shown in Table 2. Activity of all the compounds was lower than that of the reference drug MTZ (IC50=2.68 µM). However, almost 37% of all the synthetic molecules exhibited from moderate to good trichomonacidal activity with an IC50≤50 µM and were screened against mammalian cell lines.
Table 2.
In vitro activity against Trichomonas vaginalis (IC50), unspecific cytotoxicity of the most promising molecules against Vero cells (CC50) and selectivity indexes (SI).
| Compound | IC50 (µM) a | CC50 (µM) | SI b |
|---|---|---|---|
| |||
|
23 (R1=OMe, R2=R3=H) |
255.70 [205.26–348.10] | – | – |
|
24 (R1=F, R2=R3=H) |
200.49 [134.86–366.51] | – | – |
|
25 (R1=Cl, R2=R3=H) |
49.19 [30.46–77.32] | >300 | >6.10 |
|
27 (R1=I, R2=R3=H) |
75.69 [57.77–102.71] | – | – |
|
28 (R1=R2=H, R3=NO2) |
>300 | – | – |
|
29 (R1=R3=H, R2=NO2) |
19.14 [14.92–23.52] | >300 | >15.67 |
|
30 (R1=NO2, R2=R3=H) |
105.92 [63.13–236.25] | – | – |
|
31 (Ar = Naphthyl) |
30.20 [11.76–55.08] | >300 | >9.93 |
| |||
| R1=R2= R3=H | |||
|
36 R4=OH, R5=H |
74.01 [60.18–92.68] | – | – |
|
37 R4=OMe R5=H |
167.29 [116.44–283.25] | – | – |
|
38 R4=R5=OH |
88.59 [65.64–125.65] | – | – |
| R1=Me, R2=R3=H | |||
|
39 R4=OH, R5=H |
66.10 [43.85–103.72] | – | – |
|
40 R4=OMe R5=H |
225.96 [118.97–1084.50] | – | – |
|
41 R4=R5=OH |
80.61 [69.32–94.63] | – | – |
| R1=OMe, R2=R3=H | |||
|
42 R4=OH, R5=H |
64.79 [55.04–76.69] | – | – |
|
43 R4=OMe R5=H |
257.50 [141.94–834.94] | – | – |
| R1=F, R2=R3=H | |||
|
44 R4=OMe R5=H |
>300 | – | – |
|
45 R4=R5=OH |
226.35 [192.65–275.96] | – | – |
| R1=Cl, R2=R3=H | |||
|
46 R4=OMe R5=H |
32.27 [21.71–47.99] | >300 | >9.30 |
| R1=Br, R2=R3=H | |||
|
47 R4=OH, R5=H |
52.36 [35.6–77.09] | – | – |
|
48 R4=OMe R5=H |
55.14 [47.61–63.92] | – | – |
|
49 R4=R5=OH |
16.39 [0.08–28.72] | >300 | >18.30 |
| R1=I, R2=R3=H | |||
|
50 R4=OH, R5=H |
42.25 [36.30–48.52] | >300 | >7.10 |
|
51 R4=OMe R5=H |
14.85 [5.94–24.24] | >300 | >20.20 |
|
52 R4=R5=OH |
24.89 [11.95–41.25] | >300 | >12.05 |
| R1=R2=HR3=NO2 | |||
|
53 R4=OH, R5=H |
92.80 [81.78–103.30] | – | – |
|
54 R4=OMe, R5=H |
34.20 [29.47–39.39] | >300 | >8.77 |
|
55 R4=R5=OH |
132.56 [120.93–146.15] | – | – |
| R1=R3=HR2=NO2 | |||
|
56 R4=OH, R5=H |
24.28 [12.49–38.45] | 168.93 [127.03–239–12] | 7.00 |
|
57 R4=OMe R5=H |
51.91 [31.66–85.57] | – | – |
|
58 R4=R5=OH |
73.41 [50.52–110.14] | – | – |
| R1=NO2 R2=R3=H | |||
|
59 R4=OH, R5=H |
35.95 [29.98–42.63] | 254.15 [170.16–725.83] | 7.07 |
|
60 R4=OMe R5=H |
155.17 [93.43–395.10] | – | – |
|
61 R4=R5=OH |
127.98 [77.11–294.93] | – | – |
|
62 R4=NO2 R5=H |
151.81 [112.95–209.64] | – | – |
| Ar = Naphthyl | |||
|
63 R4=OH R5=H |
14.89 [11.51–18.30] | 274.75 [193.19–562.70] | 18.45 |
|
64 R4=OMe R5=H |
30.46 [17.60–46.35] | >300 | >9.85 |
|
65 R4=R5=OH |
20.14 [16.37–24.03] | 174.81 [110.58–371.96] | >8.68 |
| Metronidazole | 2.68 [2.37–3.03] | >300 | >111 |
IC50 and CC50 were calculated with growth inhibition values showing a standard deviation of less than 10%.
–: Not evaluated against Vero cells owing to the low trichomonacidal activity.
aResults in brackets refer to 95% confidence interval.
bSelectivity indexes SI = CC50 Vero cells/IC50 T. vaginalis.
In particular, four molecules displayed remarkable activity against T. vaginalis, namely thiosemicarbazide 29, and thiosemicarbazones 49, 51 and 63, with IC50 values ranging from 14.85‒19.14 µM, and SI > 15.7.
It is important to highlight that most of the thiosemicarbazones synthetised lacked unspecific cytotoxicity at the highest concentration evaluated towards Vero cells. These results are in agreement with the phenylthiosemicarbazones evaluated by Gomes et al.64 against T. gondii, which show cytotoxic effect on Vero cells between 300 to 700 µM. Also, the results published by Merlino et al.65 refute the low cytotoxic profile of this scaffold showing a low non-specific cytotoxicity on human red blood cells and J-774 mouse macrophages.
In this context, due to the promising trichomonacidal profile and the absence of unspecific cytotoxicity of the most potent derivatives, these molecules can be considered as excellent candidates for further studies. According to this, two selenium compounds (i.e., 74 and 75) were prepared as the isosters of the two best trichomonacidal compounds evaluated previously (derivatives 49 and 51, respectively). The activity of these selenosemicarbazones on T. vaginalis was slightly enhanced in comparison with that of their sulphur counterparts. Curiously, both derivatives exhibit a similar trichomonacidal effect: IC50 49 = 11.10 µM and IC50 51 =11.02 µM.
Although thiosemicarbazone derivatives 49 and 51 did not exhibit unspecific cytotoxicity, their selenium analogue 74 presented a low cytotoxicity effect at the highest concentrations evaluated against Vero cells (CC50 = 114.50 µM), while 75 did not present toxic effects at the maximum concentration tested. Moreover, SI observed in both compounds continues being higher than 10, which demonstrates their specific antiparasitic profile.
According to the trichomonacidal profile, only a few in vitro studies based thiosemicarbazones of 5-nitrothiophene-2-carboxaldehyde46, or bis (thiosemicarbazone) and bis(4-methylthiosemicarbazone)49 have been evaluated against T. vaginalis. However, the great structural difference between these molecules and the thio(seleno)semicarbazones prepared herein, makes it not possible to compare the antiparasitic effects.
3.2.2.1. Mechanistic study of the anti-trichomonas activity
In order to get more insight into the anti-trichomonas mode of action (MoA) of title thiosemicarbazones, we have analysed the alteration of the hydrogenosome membrane potential and structural changes in the protozoan; the two more promising compounds (thiosemicarbazones 51 and 63) were included in this study.
Thus, to determine if the hydrogenosome could be involved in their mode of action, alterations in the membrane potential of this organelle were studied. Membrane potential is indicative of the correct functioning of the organelle, which is essential for parasite survival and also a key aspect for the activation of 5-nitroimidazoles. For this purpose, JC-1 dye (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide) was used; this compound aggregates inside the mitochondria or hydrogenosomes in healthy cells, being detected by fluorescence at 590 nm. However, alterations in the membrane potential of the organelle preclude the agglutination of JC-1, which remains as a monomer in the cytoplasm with fluorescence emission at 535 nm.
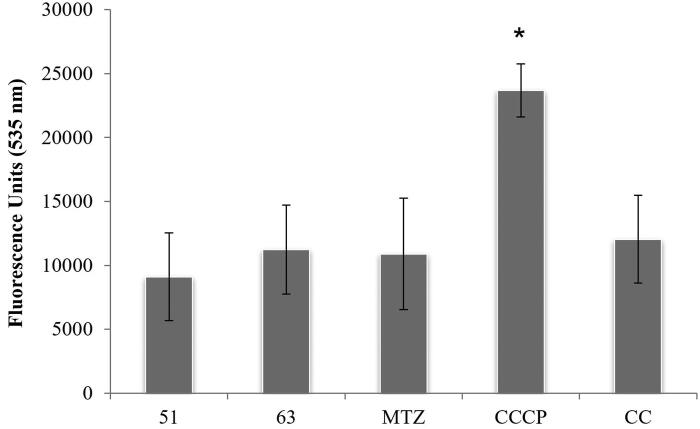
Figure 2 depicts results of this assay after 4 h of incubation with derivatives 51 and 63 at 100 µM concentration; MTZ (24 µM) was included for comparison.
Figure 2.
Plots of fluorescence units at 535 nm after addition of JC-1. MTZ (metronidazole), CCCP (m-chlorocarbonylcyanide phenylhydrazone) and CC (culture control). Data indicate mean ± SD of three independent experiments (n = 3). Data that are significantly different from control experiment are marked with an asterisk (*) (ρ < 0.05).
Results demonstrate that neither the tested thiosemicarbazides nor the reference drug alter the hydrogenosome membrane potential of the parasite. Only CCCP (positive control) showed a significant difference (t-Student) with the others, suggesting a charge deregulation and thus, disturbance in the hydrogenosome.
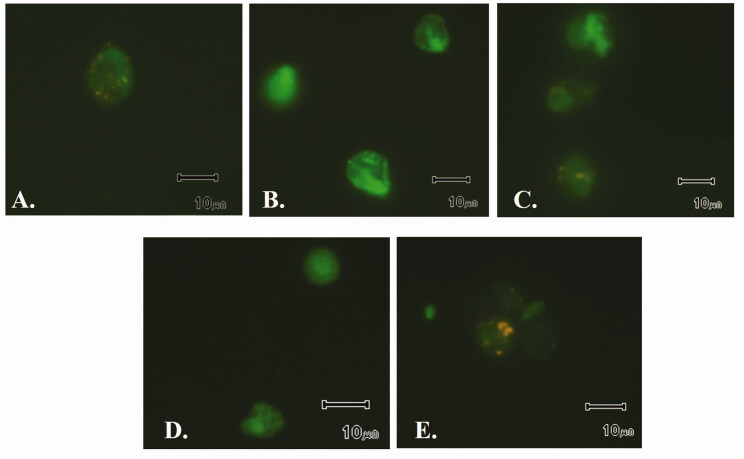
These data are in agreement with images obtained from fluorescence microscopy. Figure 3 shows that uncoupler agent CCCP (Figure 3(B)) provokes a disturbance in the hydrogenosome membrane potential, as indicated by fluorescence distribution throughout the trophozoite body, which is much more intense and delocalised. However, for the rest of images − control (Figure 3(A)), and T. vaginalis treated with MTZ (Figure 3(C)), compound 51 (Figure 3(D)) and 63 (Figure 3(E)) − fluorescence restricted to certain regions of the parasite is observed. In other words, the good physiological condition of this organelle is demonstrated with the fluorescence results observed in Figure 2 and corroborated in the microscope. The accumulation of JC-1 in these organelles is clearly observed in growth controls and trophozoites treated with both compounds as reflects Figure 3.
Figure 3.
Fluorescence emitted by JC-1 in hydrogenosome. A. Control. B. Positive control (treatment with the uncoupler agent CCCP). C. Treatment with MTZ. D. Treatment with 51. E. Treatment with 63.
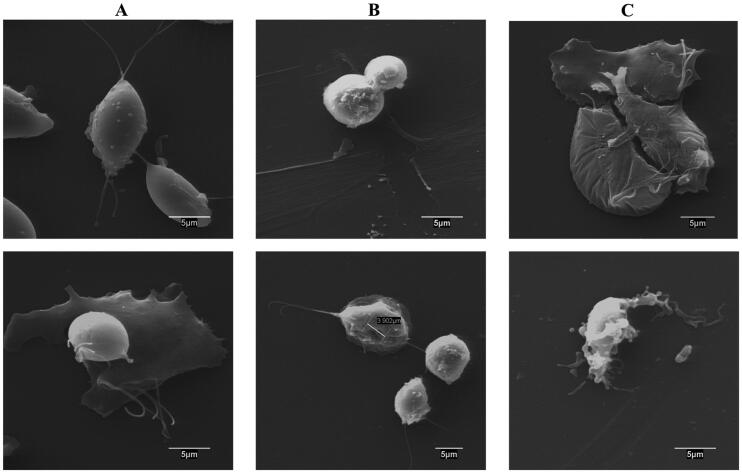
With the aim of studying any structural alteration in the parasite triggered by these thiosemicarbazones, scanning electronic microscopy (SEM) was used to observe surface disturbances upon 24 h treatment at 15 µM concentration (Figure 4).
Figure 4.
Scanning electron microscopy images of T. vaginalis cultures. A. Control. B. Treatment with compound 51. C. Treatment with compound 63.
Control experiment (Figure 4(A)) shows trophozoites with no alterations in the plasmatic membrane, with pear-shaped or even amoeboid cells. Interestingly, treatment with thiosemicarbazone 51 induced a clear disturbance in the trophozoite surface (Figure 4(B)), showing parasites with a more rounded shape and even pseudocyst forms; some trophozoites were found to exhibit invaginations and pores in the surface; structural abnormalities have therefore an apoptotic aspect. It has been reported that T. vaginalis adopts this rounded shape, with internalised flagella in endocytic vesicles upon stress situation or lack of nutrients66. Regarding derivative 63, it induced amoeboid cells in most of the culture with smooth disturbances in the cytoplasmatic surface and showing a wrinkled shape in many trophozoites, as depicted in Figure 4(C).
Therefore, these MoA experiments show that both thiosemicarbazones provoke a trichomonacidal effect in a hydrogenosome-independent mechanism inducing the trophozoites death with a clear disruption of the parasite surface.
4. Conclusions
In the present work we have accomplished the preparation of an ample number of thio(seleno)semicarbazones as potential antiparasitic agents against T. cruzi (responsible for Chagas disease) and T. vaginalis (responsible for trichomoniasis) with a mode of action different to that of classical nitroheterocyclic compounds, the current only available drugs for treating both parasitic infections. We have carried out an extensive analysis of SARs upon modification of the stereoelectronic effects of the aromatic substituents, together with the nature of the chalcogen atom (S vs. Se). Thiosemicarbazide 31, bearing a naphthyl residue on N-4, exhibited the best trypanocidal activity at the epimastigote stage of two different parasitic strains, with similar profile to that of the reference drug benznidazole.
Moreover, halogenated thiosemicarbazones 49, 51 and naphthyl-containing 63 exhibited a remarkable trichomonacidal profile, with activities within the low micromolar range, and excellent selectivities. Their selenium isosters afforded a slightly enhancement of the activity.
Analysis of the hydrogenosome membrane potential, and structural changes through scanning electronic microscopy (51, 63) afforded valuable information concerning their mechanisms of action.
Supplementary Material
Acknowledgements
M.M.-M. thanks CONACYT for the award of a predoctoral grant. We would also like to thank the Servicio de Resonancia Magnética Nuclear, CITIUS (University of Seville) for the performance of NMR experiments and the Microscopy Unit – CAI Medicina y Biología de la Universidad de Alcalá – for assistance with scanning electron microscopy.
Funding Statement
The present work was financially supported by Grant PID2020-116460RB-I00, funded by MCIN/AEI/10.13039/501100011033, Junta de Andalucía (FQM-134), UCM Research Group ref. 911120 Epidemiología, Diagnóstico y Terapia antiparasitaria (PARADET) and the Mexican CONACYT (CB-2015/257465).
Disclosure statement
The authors report no conflict of interest.
References
- 1.1. Sutrave S, Richter MH.. The Truman Show for protozoan parasites: a review of in vitro cultivation platforms. PLoS Negl Trop Dis 2021;15:e0009668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varikuti S, Jha BK, Volpedo G, et al. Host-directed drug therapies for neglected tropical diseases caused by protozoan parasites. Front Microbiol 2018;9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molyneux DH, Savioli L, Engels D.. Neglected tropical diseases: progress towards addressing the chronic pandemic. Lancet 2017;389:312–25. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro V, Dia N, Paiva T, et al. Current trends in the pharmacological management of Chagas disease. Int J Parasitol Drugs Drug Resist 2020;12:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatriz Palatnik-de-Sousa CN. The delay in the licensing of protozoal vaccines: a comparative history. Front Immunol 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capela R, Moreira R, Lopes F.. An overview of drug resistance in protozoal diseases. Int J Mol Sci 2019;20:5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro JA, Montalto de Mecca M, Bartel LC.. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis). Hum Exp Toxicol 2006;25:471–9. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Padilla J, Cortés-Serra N, Pinazo MJ, et al. Strategies to enhance access to diagnosis and treatment for Chagas disease patients in Latin America. Expert Rev Anti Infect Ther 2019;17:145–57. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser M, Mäser P, Tadoori LP, et al. Antiprotozoal activity profiling of approved drugs: a starting point toward drug repositioning. PloS One 2015;10:e0135556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel OPS, Jesumoroti OJ, Legoabe LJ, Beteck RM.. Metronidazole-conjugates: a comprehensive review of recent developments towards synthesis and medicinal perspective. Eur J Med Chem 2021;210:112994. [DOI] [PubMed] [Google Scholar]
- 11.Nocentini A, Cadoni R, Dumy P, et al. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J Enzyme Inhib Med Chem 2018;33:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(ntiprot-trypanosomiasis) (last accessed 18th December 2021).
- 13.Choudhury SD. Nano-medicines a hope for Chagas disease! Front Mol Biosci 2021;8:655435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmunis GA, Yadon ZE.. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 2010;115:14–21. [DOI] [PubMed] [Google Scholar]
- 15.Brindha J, Balamurali MM, Kaushik C.. An overview on the therapeutics of neglected infectious diseases—Leishmaniasis and Chagas diseases. Front Chem 2021;9:622286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira DE, Benchimol M, Crepaldi PH, de Souza W.. Interactive multimedia to teach the life cycle of Trypanosoma cruzi, the causative agent of Chagas disease. PloS Negl Trop Dis 2012;6:e1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Huertas P, Cardona-Castro N.. Advances in the treatment of Chagas disease: promising new drugs, plants and targets. Biomed Pharmacother 2021;142:112020. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Sarduy E, Landaburu LU, Karpiak J, et al. Novel scaffolds for inhibition of Cruzipain identified from high-throughput screening of anti-kinetoplastid chemical boxes. Sci Rep 2017;7:12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leite ACL, de Lima RS, Moreira DRM, et al. Synthesis, docking, and in vitro activity of thiosemicarbazones, aminoacyl-thiosemicarbazides and acyl-thiazolidones against Trypanosoma cruzi. Bioorg Med Chem 2006;14:3749–57. [DOI] [PubMed] [Google Scholar]
- 20.Caputto ME, Fabian LE, Benítez D, et al. Thiosemicarbazones derived from 1-indanones as new anti-Trypanosoma cruzi agents. Bioorg Med Chem 2011;19:6818–26. [DOI] [PubMed] [Google Scholar]
- 21.Moreno-Rodríguez A, Salazar-Schettino PM, Bautista JL, et al. In vitro antiparasitic activity of new thiosemicarbazones in strains of Trypanosoma cruzi. Eur J Med Chem 2014;87:23–9. [DOI] [PubMed] [Google Scholar]
- 22.Pizzo C, Faral-Tello P, Salinas G, et al. Selenosemicarbazones as potent cruzipain inhibitors and their antiparasitic properties against Trypanosoma cruzi. MedChemComm 2012;3:362–8. [Google Scholar]
- 23.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Org 2019;97:548–562P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielczarek E, Blaszkowska J.. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection 2016;44:447–58. [DOI] [PubMed] [Google Scholar]
- 25.Menezes CB, Piccoli Frasson A, Tasca T.. Trichomoniasis – Are we giving the deserved attention to the most common non-viral sexually transmitted disease worldwide? Microb Cell 2016;3:404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark JR, Judson G, Alderete JF, et al. Prospective study of Trichomonas vaginalis infection and prostate cancer incidence and mortality: physicians’ health study. J Natl Cancer Inst 2009;101:1406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis 2009;36:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 2015;15:305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cudmore SL, Delgaty KL, Hayward-McClelland SF, et al. Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev 2004;17:783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shehzad MT, Khan A, Halim SA, et al. Synthesis of indole-substituted thiosemicarbazones as an aldose reductase inhibitor: an in vitro, selectivity and in silico study. Future Med Chem 2021;13:1185–201. [DOI] [PubMed] [Google Scholar]
- 31.Cheng R, Shi W, Yuan Q, et al. 5-Substituted isatin thiosemicarbazones as inhibitors of tyrosinase: insights of substituent effects. Spectrochim Acta A Mol Biomol Spectrosc 2021;255:119669. [DOI] [PubMed] [Google Scholar]
- 32.Islam M, Khan A, Shehzad MT, et al. Therapeutic potential of N4-substituted thiosemicarbazones as new urease inhibitors: biochemical and in silico approach. Bioorg Chem 2021;109:104691. [DOI] [PubMed] [Google Scholar]
- 33.Jawaria R, Hussain M, Ahmad HB, et al. Probing ferrocene-based thiosemicarbazones and their transition metal complexes as cholinesterase inhibitors. Inorg Chim Acta 2020;508:119658. [Google Scholar]
- 34.Sagnou M, Mavroidi B, Kaminari A, et al. Novel isatin thiosemicarbazone derivatives as potent inhibitors of β-amyloid peptide aggregation and toxicity. ACS Chem Neurosci 2020;11:2266–76. [DOI] [PubMed] [Google Scholar]
- 35.Sevinçli ZŞ, Duran GN, Özbil M, Karalı N.. Synthesis, molecular modeling and antiviral activity of novel 5-fluoro-1H-indole-2,3-dione 3-thiosemicarbazones. Bioorg Chem 2020;104:104202. [DOI] [PubMed] [Google Scholar]
- 36.He Z-X, Huo J-L, Gong Y-P, et al. Design, synthesis and biological evaluation of novel thiosemicarbazone-indole derivatives targeting prostate cancer cells. Eur J Med Chem 2021;210:112970. [DOI] [PubMed] [Google Scholar]
- 37.Fuentes-Aguilar A, Romero-Hernández LL, Arenas-González A, et al. New selenosteroids as antiproliferative agents. Org Biomol Chem 2017;15:5041–54. [DOI] [PubMed] [Google Scholar]
- 38.Calcatierra V, López Ó, Fernández-Bolaños JG, et al. Phenolic thio- and selenosemicarbazones as multi-target drugs. Eur J Med Chem 2015;94:63–72. [DOI] [PubMed] [Google Scholar]
- 39.Ansari M, Montazeri M, Daryani A, et al. Synthesis and in vitro anti-Toxoplasma gondii activity of a new series of aryloxyacetophenone thiosemicarbazones. Mol Divers 2020;24:1223–34. [DOI] [PubMed] [Google Scholar]
- 40.Gomes MAGB, Carvalho LP, Rocha BS, et al. Evaluating anti-Toxoplasma gondii activity of new series of phenylsemicarbazone and phenylthiosemicarbazones in vitro. Med Chem Res 2013;22:3574–80. [Google Scholar]
- 41.de Aquino TM, Liesen AP, da Silva REA, et al. Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg Med Chem 2008;16:446–56. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues de Assis DR, Oliveira AA, Porto SL, et al. 4-Chlorophenylthioacetone-derived thiosemicarbazones as potent antitrypanosomal drug candidates: investigations on the mode of action. Bioorg Chem 2021;113:105018. [DOI] [PubMed] [Google Scholar]
- 43.da Silva A, Maia PidS, Lopes CD, et al. Synthesis, characterization and antichagasic evaluation of thiosemicarbazones prepared from chalcones and dibenzalacetones. J Mol Struct 2021;1232:130014. [Google Scholar]
- 44.Aguilera E, Perdomo C, Espindola A, et al. A nature-inspired design yields a new class of steroids against trypanosomatids. Molecules 2019;24:3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Oliveira Cardoso MV, de Oliveira Filho GB, Pessoa de Siqueira LR, et al. 2-(Phenylthio)ethylidene derivatives as anti-Trypanosoma cruzi compounds: structural design, synthesis and antiparasitic activity. Eur J Med Chem 2019;180:191–203. [DOI] [PubMed] [Google Scholar]
- 46.Bharti N, Husain K, González Garza MT, et al. Synthesis and in vitro antiprotozoal activity of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazone derivatives. Bioorg Med Chem Lett 2002;12:3475–8. [DOI] [PubMed] [Google Scholar]
- 47.Coombs GH, Clackson TE.. Antitrichomonal activity of compounds that affect DNA and its repair. J Antimicrob Chemother 1983;11:191–4. [DOI] [PubMed] [Google Scholar]
- 48.Barrett PA, Beveridge E, Bradley PL, et al. Biological activities of some alpha-dithiosemicarbazones. Nature 1965;206:1340–1. [DOI] [PubMed] [Google Scholar]
- 49.Michaels RM, Peterson JL, Stahl GL.. The activity of substituted thiosemicarbazones against Trichomonas vaginalis and Trichomonas foetus in vitro and in experimental animals. J Parasitol 1962;48:891–7. [PubMed] [Google Scholar]
- 50.Merino-Montiel P, Maza S, Martos S, et al. Synthesis and antioxidant activity of O-alkyl selenocarbamates, selenoureas and selenohydantoins. Eur J Pharm Sci 2013;48:582–92. [DOI] [PubMed] [Google Scholar]
- 51.Merino-Montiel P, López Ó, Fernández-Bolaños JG.. l-Isofucoselenofagomine and derivatives: dual activities as antioxidants and as glycosidase inhibitors. Tetrahedron 2012;68:3591–5. [Google Scholar]
- 52.Fonseca-Berzal C, Ibáñez-Escribano A, Reviriego F, et al. Antichagasic and trichomonacidal activity of 1-substituted 2-benzyl-5-nitroindazolin-3-ones and 3-alkoxy-2-benzyl-5-nitro-2H-indazoles. Eur J Med Chem 2016;115:295–310. [DOI] [PubMed] [Google Scholar]
- 53.Fonseca-Berzal C, Ibáñez-Escribano A, Vela N, et al. Antichagasic, leishmanicidal, and trichomonacidal activity of 2-benzyl-5-nitroindazole-derived amines. ChemMedChem 2018;13:1246–59. [DOI] [PubMed] [Google Scholar]
- 54.Begines P, Sevilla-Horrillo L, Puerta A, et al. Masked phenolic–selenium conjugates: potent and selective antiproliferative agents overcoming P-gp resistance. Pharmaceuticals 2020;13:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lagunes I, Begines P, Silva A, et al. Selenocoumarins as new multitarget antiproliferative agents: synthesis, biological evaluation and in silico calculations. Eur J Med Chem 2019;179:493–501. [DOI] [PubMed] [Google Scholar]
- 56.Begines P, Oliete A, López Ó, et al. Chalcogen-containing phenolics as antiproliferative agents. Future Med Chem 2018;10:319–34. [DOI] [PubMed] [Google Scholar]
- 57.Romero-Hernández LL, Merino-Montiel P, Montiel-Smith S, et al. Diosgenin-based thio(seleno)ureas and triazolyl glycoconjugates as hybrid drugs. Antioxidant and antiproliferative profile. Eur J Med Chem 2015;99:67–81. [DOI] [PubMed] [Google Scholar]
- 58.Fernández-Bolaños JG, López Ó, Ulgar V, et al. Synthesis of O-unprotected glycosyl selenoureas. A new access to bicyclic sugar isoureas. Tetrahedron Lett 2004;45:4081–4. [Google Scholar]
- 59.López Ó, Maza S, Ulgar V, et al. Synthesis of sugar-derived isoselenocyanates, selenoureas, and selenazoles. Tetrahedron 2009;65:2556–66. [Google Scholar]
- 60.Fonseca-Berzal C, da Silva CF, Batista DGJ, et al. Activity profile of two 5-nitroindazole derivatives over the moderately drug-resistant Trypanosoma cruzi Y strain (DTU TcII): in vitro and in vivo studies. Parasitology 2020;147:1216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espíndola JWP, Cardoso MVO, Filho GBO, et al. Synthesis and structure–activity relationship study of a new series of antiparasitic aryloxyl thiosemicarbazones inhibiting Trypanosoma cruzi cruzain. Eur J Med Chem 2015;101:818–35. [DOI] [PubMed] [Google Scholar]
- 62.Rettondin AR, Carneiro ZA, Gonçalves ACR, et al. Gold(III) complexes with ONS-Tridentate thiosemicarbazones: toward selective trypanocidal drugs. Eur J Med Chem 2016;120:217–26. [DOI] [PubMed] [Google Scholar]
- 63.Ibáñez-Escribano A, Meneses-Marcel A, Marrero-Ponce Y, et al. A sequential procedure for rapid and accurate identification of putative trichomonacidal agents. J Microbiol Methods 2014;105:162–7. [DOI] [PubMed] [Google Scholar]
- 64.Gomes MAG, Carvalho LP, Rocha BS, et al. Evaluating anti-Toxoplasma gondii activity of new serie of phenylsemicarbazone and phenylthiosemicarbazones in vitro. Med Chem Res 2013;22:3574–80. [Google Scholar]
- 65.Merlino A, Benítez D, Chávez S, et al. Development of second generation amidinohydrazones, thio-and semicarbazones as Trypanosoma cruzi-inhibitors bearing benzofuroxan and benzimidazole 1,3dioxide core scaffolds. Med Chem Commun 2010;1:216–28. [Google Scholar]
- 66.Pereira-Neves A, Ribeiro KC, Benchimol M.. Pseudocysts in trichomonads-new insights. Protist 2003;154:313–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.