Abstract
Objective:
This study aimed to determine the efficacy and acceptability of pharmacotherapies for cannabis use disorder (CUD).
Methods:
We conducted a systematic review and frequentist network meta-analysis, searching five electronic databases for randomized placebo-controlled trials of individuals diagnosed with CUD receiving pharmacotherapy with or without concomitant psychotherapy. Primary outcomes were the reduction in cannabis use and retention in treatment. Secondary outcomes were adverse events, discontinuation due to adverse events, total abstinence, withdrawal symptoms, cravings, and CUD severity. We applied a frequentist, random-effects Network Meta-Analysis model to pool effect sizes across trials using standardized mean differences (SMD, g) and rate ratios (RR) with their 95% confidence intervals.
Results:
We identified a total of 24 trials (n=1912, 74.9% male, mean age 30.2 years). Nabilone (d=−4.47 [−8.15; −0.79]), topiramate (d=−3.80 [−7.06; −0.54]), and fatty-acid amyl hydroxylase inhibitors (d=−2.30 [−4.75; 0.15]) reduced cannabis use relative to placebo. Dronabinol improved retention in treatment (RR=1.27 [1.02; 1.57]), while topiramate worsened treatment retention (RR=0.62 [0.42; 0.91]). Gabapentin reduced cannabis cravings (d=−2.42 [−3.53; −1.32], while vilazodone worsened craving severity (d=1.69 [0.71; 2.66]. Buspirone (RR=1.14 [1.00; 1.29]), venlafaxine (RR=1.78 [1.40; 2.26]), and topiramate (RR=9.10 [1.27; 65.11]) caused more adverse events, while topiramate caused more dropouts due to adverse events.
Conclusions:
Based on this review, some medications appeared to show promise for treating individual aspects of CUD. However, there is a lack of robust evidence to support any particular pharmacological treatment. There is a need for additional studies to expand the evidence base for CUD pharmacotherapy. While medication strategies may become an integral component for CUD treatment one day, psychosocial interventions should remain the first line given the limitations in the available evidence.
Keywords: Cannabinoid, Meta-analysis, Outcome assessment, Comparative effectiveness
INTRODUCTION
Cannabinoids are the most commonly used illicit recreational drugs worldwide (Brezing & Levin, 2018; United Nations Office on Drugs and Crime, 2015). In 2017, nearly 3.8% of the global adult population used cannabis (United Nations Office on Drugs and Crime, 2018). While there is increasing recognition of the adverse consequences of short and long-term cannabinoid use, there is decreasing public perception of its harms (Sherman & McRae-Clark, 2016), including cognitive and motor impairment, increased risk of motor vehicle collisions, altered judgment, chronic respiratory disorders, and psychosis (Volkow et al., 2014; National Academies of Sciences, Engineering, and Medicine et al., 2017; World Health Organization & Regional Office for Europe, 2016).
In recognition of these risks, a dependence syndrome involving cannabis has been recognized in recent decades, including a defined withdrawal syndrome that affects approximately half of those with regular or dependent use (Bahji et al., 2020; Budney & Hughes, 2006). The most recent DSM-5 defined cannabis use disorder (CUD) as a problematic pattern of cannabinoid use leading to clinically significant impairment, disability or distress (American Psychiatric Association, 2013). There were an estimated 13.1 million people with CUD globally in 2010 (0.19% [0.17–0.21%]), with peak prevalence in young males in high income (Degenhardt et al., 2013). Furthermore, it is essential to recognize that CUD can involve whole, plant-based cannabis from synthetic cannabinoids and edible preparations. While these agents bind to the same CB1 and CB2 cannabinoid receptors in the central and peripheral nervous systems, they can have very different effects and potencies (Bahji & Mazhar, 2016; Bahji & Stephenson, 2019; de Fonseca & Schneider, 2008). As well, the type of cannabinoid involved in a CUD may have important treatment implications (e.g., interventions that may show promise for smoked cannabis may not extend towards CUD involving edibles or synthetic preparations). While the risk of CUD varies among regular cannabis users, with estimates ranging from 8 to 50%, the risk is higher with daily or near-daily cannabis use, earlier age at initiation of cannabis use, more potent forms of cannabis (Hasin, 2018). To that end, there is also an increased risk of psychiatric hospitalization with cannabis use (Bahji, 2020).
Despite the high prevalence and associated consequences of cannabis use, less than one million individuals received treatment for cannabis dependence in the United States, which suggests that most of those with CUD are untreated (Sherman & McRae-Clark, 2016; Substance Abuse and Mental Health Services Administration (SAMHSA), 2015). In Europe, the EMCDDA has observed increasing numbers of individuals with CUD seeking treatment. Consequently, many countries in Europe have implemented, expanded or modified national treatment programmes to serve this population better (European Monitoring Centre for Drugs and Drug Addiction, 2015; European Monitoring Centre on Drugs and Drug Addiction, 2021).
Consequently, there has been a search for effective treatments for CUD. First-line therapies for CUD are psychosocial, including motivational enhancement treatment (MET), cognitive behavioural therapy (CBT), and contingency management (CM) (Gates et al., 2016). A recent review found moderate-quality evidence that those receiving psychosocial intervention for CUD used cannabis on fewer days than those given inactive control; however, the evidence’s overall quality and generalizability were limited (Gates et al., 2016). The most consistent evidence appears to support the use of cognitive-behavioural therapy (CBT) and motivational enhancement therapy (MET) (Gates et al., 2016). There is also some evidence that digital interventions can reduce cannabis use outside clinical settings (Hoch et al., 2016).
Regrettably, access to psychotherapy is often limited, and abstinence rates remain modest following therapy completion (Gates et al., 2016). A diverse array of psychotropic agents have shown some therapeutic potential for CUD and CWS in clinical trials, including selective serotonin reuptake inhibitors (SSRIs), bupropion, nefazodone, venlafaxine, valproic acid, lithium, and nabilone (Copeland & Swift, 2009; Marshall et al., 2014; National Academies of Sciences, Engineering, and Medicine et al., 2017; Nielsen et al., 2019). Nabilone and THC preparations appear to show promise for alleviating CWS, which often perpetuates continued cannabis use (Bahji & Mazhar, 2016). However, the extent to which any one of these medications is used in clinical practice outside of research settings is less clear and only increases the need for improving the search for effective medicines for CUD. As well, the relative merits of the pharmacological strategies used in the treatment of CUD remain unclear. Extant trials have been small, and there is a lack of head-to-head studies. Several previous reviews have attempted to uncover the comparative performance across studied agents. However, only a few have conducted meta-analyses or directly handled trial data. While traditional meta-analyses are helpful in this regard, they preclude the simultaneous comparison of multiple agents. For this reason, most previous reviews of medications for CUD have concluded that there is incomplete evidence, that the quality of evidence is low, and that there is a need for further investigation.
Fortunately, a recently-developed approach—network meta-analysis (NMA)—can potentially address these limitations (Salanti, 2012). Briefly, an NMA is a meta-analysis of multiple treatments, which was developed to extend pairwise meta-analysis to allow comparisons of more than two interventions in a single analysis (Dias & Caldwell, 2019). The advantage over traditional meta-analysis is that NMA can combine direct data within trials (drug A vs. drug B) as well as indirect data across trials with shared comparators (e.g., drug A vs. placebo in Trial X and drug B vs. placebo in Trial Y) (Rouse et al., 2017). This strategy preserves randomization and pools sample sizes across small studies to enhance the analysis’s statistical power (Salanti et al., 2008). To date, there have been no prior NMAs for the treatment of CUD. Therefore, applying NMA seems appropriate, given the lack of trials that have directly compared the efficacy of two active medications for CUD.
OBJECTIVE
The present study aimed to identify the comparative efficacy and acceptability of pharmacotherapies for CUD using NMA.
METHODS
Protocol and registration
We registered our review with the Open Science Framework (https://osf.io/yrg59/). We reported this study’s methods using the PRISMA guidelines and the NMA extension statement (Hutton et al., 2015; Liberati et al., 2009).
Eligibility criteria
We followed the population-intervention-comparison-outcomes-study design (PICOS) model for defining our eligibility criteria (Methley et al., 2014):
Population: adolescents or adults (aged 15 years or older) with a confirmed CUD diagnosis using a valid diagnostic instrument (e.g., DSM-5 cannabis use disorder).
Intervention: any pharmacotherapy delivered as monotherapy or augmentation, with or without concomitant psychotherapy.
Comparison: placebo or active comparators.
Outcomes: described below.
Study: parallel, randomized, controlled trials; we excluded human laboratory type studies, crossover trials, non-experimental designs (e.g., case reports, editorials), and studies where we could not calculate, impute, or obtain missing outcome data.
Outcome measures
For our primary outcomes, we considered the reduction in cannabis use and the retention in treatment. We defined reduction in cannabis use as the change from baseline cannabis use, as determined by individual studies, including grams/day, times/day, times/week, or other measures. We defined retention in treatment as the proportion of participants who remained in the trial until the primary study endpoint. For our secondary outcomes, we considered the frequency of adverse events, discontinuation due to adverse events, abstinence, and reduced severity of CWS, CUD, or cravings for cannabis. We defined the frequency of adverse events as the proportion who experienced a treatment-emergent adverse effect. We defined discontinuation due to adverse events as the proportion who prematurely withdrew from the study before its primary study endpoint owing to adverse events. We defined abstinence as the proportion of fully abstinent participants from cannabis by the primary study endpoint as per both self-report and an objective measure (e.g., negative urine drug screen). We considered abstinence as a complementary measure to cannabis use reduction as some trials focus on a harm-reduction model of CUD while others have concentrated on abstinence-based treatment. Finally, we defined the reduced severity of CWS, CUD, or cravings for cannabis as a change in the baseline score on validated cannabis clinical instruments, such as the Cannabis Withdrawal Scale (Allsop et al., 2011).
Information sources and search
We searched five electronic databases (PubMed, Medline, EMBASE, PsycINFO, and the Cochrane Central Register) from database inception to November 14, 2019, in consultation with an experienced health sciences research librarian (Table S1). We applied a limit for English-language records and human populations. We also searched the reference lists of included articles and previous reviews.
Study selection
Two reviewers (AB and ACM) used Covidence, a web-based review manager, to independently screen all records by title and abstract, and subsequently in full-text, against the eligibility criteria (Veritas Health Innovation, 2019). We resolved all discrepancies through consensus.
Data collection process
The same two reviewers (AB and ACM) also used Covidence to extract relevant data from included records independently. Again, we resolved all discrepancies through consensus.
Data items
Using the PICOS framework, we abstracted the following variables from each including study:
Population: sample size, age, sex, CUD diagnosis method, comorbidity status.
Intervention: type, dose, duration, and frequency of pharmacological agent, psychotherapy use.
Comparison: type, dose, duration, and frequency (e.g., placebo vs. active comparator).
Outcomes: the measure of cannabis use (e.g., grams per week, joints per day), retention in treatment, CWS and CUD severity (and relevant instruments), and other outcomes reported by individual studies.
Study design: first author, year of publication, trial location, trial setting (inpatient, outpatient, community), trial length, and follow-up duration (if applicable).
Summary measures
For continuous outcomes, such as CUD severity and reduction in cannabis use, we collected the aggregate mean and standard deviation at the end of treatment and calculated standardized mean differences (SMD) with 95% confidence intervals (CIs).
However, as the interpretation of the SMD is not straightforward, we offer an example. For cannabis reduction, the SMD (Cohen’s d) represents the difference between baseline frequency of consumption and trial-end frequency of consumption for the treatment group relative to the difference between baseline frequency of consumption and trial-end frequency of consumption for the placebo group (d=μexperimental−μcontrol/SDpooled) (Cohen, 2013).
The advantage of using an SMD over a mean difference (MD) is that it allows for the pooling of effect sizes across studies that used different instruments to measure the same outcome measure. For dichotomous outcomes, such as retention in treatment or the frequency of adverse events, we collected the number of participants with the event of interest against the total number of participants at the primary study endpoint to calculate rate ratios (RR) with their 95% CIs. While several studies have opted to use the odds ratio (OR) rather than the RR, there is no clear advantage to one measure over the other. We opted to use the RR as it is more straightforward to interpret from laypersons’ standpoint than the “odds” of an event. For trials where there were 0 events, we imputed continuity corrections by adding 0.5 to affected outcomes.
Network meta-analysis methods
We used previously-described statistical methods to conduct the present NMA (Bahji et al., 2020, 2020, 2020; Bahji, Meyyappan, et al., 2020a, 2020b). We performed all quantitative analyses on an intention-to-treat basis to preserve randomization within studies. We assumed two-sided P < .05 to indicate the statistical significance and used a frequentist NMA package (netmeta) to maintain randomized treatment comparisons across trials (Rücker et al., 2019) in R Studio version 3.5.1 (RStudio Team, 2020). We applied random-effects models due to the suspected heterogeneity across studies. We also used the R function pairwise to transform the data set into a contrast-based format for NMA. We also generated P-scores—included in all network plots—a statistical measure that indicates the certainty that one treatment is better than the other, averaged over all competing therapies; higher P-scores suggest that a particular treatment may be more helpful than others.
Assessment of transitivity
The first assumption of NMA (transitivity) is that the trials included in the network are equivalent, except for the treatment. To that end, the premise of transitivity refers to the extent of between-study heterogeneity. We first measured transitivity by comparing the trial and sample characteristics across studies to check for a balanced distribution of potential moderators, such as trial length, participant age and sex, or comorbidity status (Rouse et al., 2017). We evaluated heterogeneity crudely using forest plots and quantitatively with the τ2 and I2 statistics (Higgins & Thompson, 2002). We considered τ2 values less than 0.04, up to 0.09, and greater than 0.16 as low, moderate, and high heterogeneity, respectively (Higgins & Thompson, 2002). We considered I2 values less than 50%, up to 75%, and more than 75% as low, moderate, and high heterogeneity, respectively (Cochrane Collaboration, 2014).
Assessment of consistency
The second assumption of NMA (consistency) is that there is an agreement between direct evidence (i.e., drugs compared within a trial) and indirect evidence (i.e., drugs with shared comparators across trials). To assess for consistency, we used local and global network methods (Higgins et al., 2012; Higgins et al., 2003). We used the netsplit command to split direct and indirect evidence, and then we used the decomp.design command to determine if there was a significant design-by-treatment interaction. These commands allowed us to compare the estimates from our network to a series of pairwise meta-analyses analyzed jointly (which is a model that does not assume consistency) (Salanti, 2012). If the trade-off between model fit and complexity favours the model with assumed consistency, then the NMA model is compliant with the consistency assumption.
Risk of bias in individual studies
The same two reviewers (AB and ACM) independently appraised the risk of bias in individual trials using the Cochrane Risk of Bias Tool for randomized controlled trials. We used Cochrane’s Review Manager to generate a coloured risk of bias graph (The Cochrane Collaboration, 2014). We assigned a rating of “low,” “high,” or “unclear” risk to each of seven bias domains (randomization, allocation concealment, participant blinding, researcher blinding, selective reporting, attrition, and other risks of bias). We also created an “overall” risk of bias rating by examining the composite performance across the seven domains.
Risk of bias across studies
We assessed publication bias by evaluating funnel plots, which graphically depict effect sizes for individual agents against the pooled effect size’s standard error. When funnel plots are asymmetrical, this often results from the non-publication of small trials with negative results, which would typically have larger standard errors. To measure funnel plot asymmetry, we examined graphs crudely and a statistical battery, including the Egger’s, Begg-Mazumdar’s, and Thompson-Sharp’s tests (Egger et al., 1997). However, other factors, such as differences in trial quality or heterogeneity, can also produce asymmetry in funnel plots.
Additional analyses
As a post-hoc sensitivity analysis, we evaluated the results after removing studies with a high risk of bias.
RESULTS
Study selection
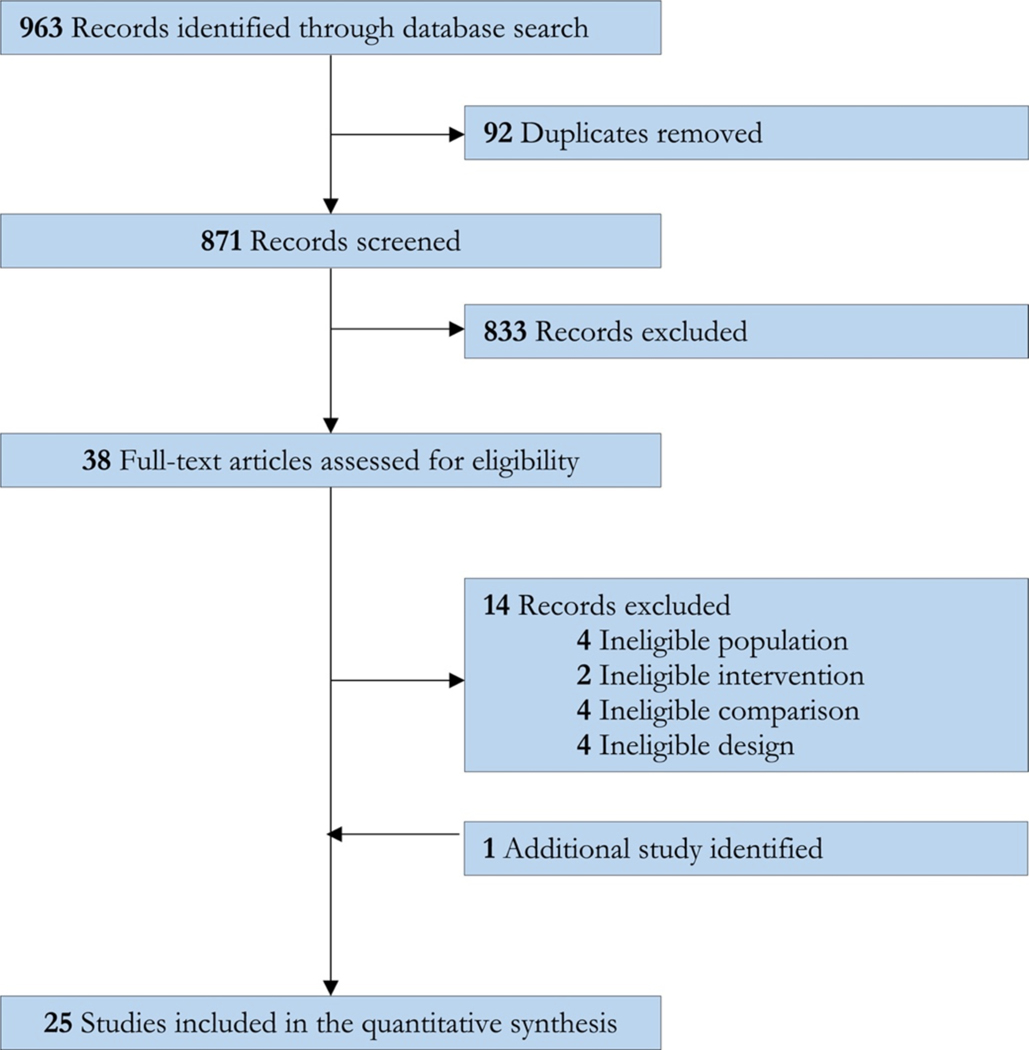
The systematic search provided a total of 945 citations (Fig. 1). We identified an additional 18 reports from two previous review articles. After adjusting for duplicates, 871 remained, from which we discarded 833 studies after the title and abstract screening. After full-text review, 24 studies met the final inclusion criteria for the systematic review (Allsop et al., 2014; Carpenter et al., 2009; Cornelius et al., 1999, 2010; D’Souza et al., 2019; Gray et al., 2012, 2017; Hill et al., 2017; Johnston et al., 2014; Levin et al., 2004; Levin et al., 2011, 2013, 2016; Lintzeris et al., 2002; Mason et al., 2012; McRae-Clark et al., 2009, 2010, 2015, 2016; Miranda et al., 2017; Penetar et al., 2012; Sherman et al., 2017; Trigo et al., 2018; Weinstein et al. 2014).
Figure 1.
PRISMA flow diagram of study selection.
Study characteristics
The 24 trials were diverse regarding study design, outcomes, population characteristics, and interventions (Table 1). The median trial duration was 12 weeks (range: 1 to 13 weeks). Three studies involved post-intervention follow-up, ranging from 4 to 13 weeks (Allsop et al., 2014; Johnston et al., 2014; Weinstein et al., 2014). Most trials occurred in outpatient settings; however, three involved inpatients (Allsop et al., 2014; Cornelius et al., 1999; Johnston et al., 2014), and one was mixed (Lintzeris et al., 2019). The most common study funders were the American National Institutes of Health (n=19) and the Australian National Health and Medical Research Council (n=3).
Table 1.
Study characteristics
| Study | Country | N | Males (%) | Age (y) | Intervention | Cannabis use at the end of treatment (SD) | Retention |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Allsop etal. (2014) | Australia | 51 | 77 | 35 | Nabiximols | Mean weekly grams cannabis use: 2.81 (5.94) vs. 5.21 (10.74) | 23/27 vs. 15/24 |
| Carpenter et al. (2009) | USA | 66 | 85 | 33 | Nefazodone | Not reported | 18/36 vs. 14/30 |
| Carpenter et al. (2009b) | USA | 70 | 76 | 34 | Bupropion SR | Not reported | 18/40 vs. 14/30 |
| Cornelius et al. (1999) | USA | 22 | 50 | 31 | Fluoxetine | Mean days cannabis use past week: 3.3 (2.6) vs. 61.3 (12.8) | 11/11 vs. 11/11 |
| Cornelius et al. (2010) | USA | 70 | 61 | 21 | Fluoxetine | Mean daily cannabis joints used: 3.88 (2.6) vs. 3.1 (2.27) | 31/34 vs. 33/36 |
| D’Souza et al. (2019) | USA | 70 | 100 | 28 | FAAH-inhibitor | Mean daily cannabis inhalations: 0.44 (0.11) vs. 1.27 (0.36) | 38/46 vs. 20/24 |
| Gray et al. (2012) | USA | 116 | 72 | 19 | N-acetylcysteine | Not reported | 37/58 vs. 33/58 |
| Grayet al. (2017) | USA | 302 | 72 | 30 | N-acetylcysteine | Not reported | 110/153 vs. 102/149 |
| Hill et al. (2017) | USA | 18 | 67 | 26 | Nabilone | Mean days cannabis use during the trial: 15 (2.55) vs. 36 (6.12) | 6/10 vs. 6/8 |
| Johnston et al. (2014) | Australia | 38 | 66 | 41 | Lithium | Mean weekly grams cannabis use: 5.22 (2.77) vs. 6.14(1.46) | 13/16 vs. 16/22 |
| Levin et al. (2004) | USA | 25 | 92 | 33 | Divalproex Sodium | Not reported | 5/13 vs. 4/12 |
| Levin et al. (2011) | USA | 156 | 82 | 38 | Dronabinol | Not reported | 61/79 vs. 47/77 |
| Levin et al. (2013) | USA | 103 | 74 | 35 | Venlafaxine XR | Not reported | 31/51 vs. 33/52 |
| Levin et al. (2016) | USA | 122 | 69 | 35 | Dronabinol/Lofexidine | Not reported | 37/61 vs. 42/61 |
| Lintzeris et al., 2019 | Australia | 128 | 77 | 35 | Nabiximols | Mean days cannabis use during the trial: 35 (32.4) vs. 53.1 (33) | 30/61 vs. 30/67 |
| Mason et al. (2012) | USA | 50 | 88 | 34 | Gabapentin | Mean weekly grams cannabis use: 0.1 (0.1) vs. 0.3 (0.3) | 7/25 vs. 11/25 |
| McRae-Clark et al. (2009) | USA | 50 | 90 | 32 | Buspirone | Not reported | 11/23 vs. 13/27 |
| McRae-Clark et al., 2010 | USA | 38 | 76 | 30 | Atomoxetine | Not reported | 9/19 vs. 7/19 |
| McRae-Clark et al. (2015) | USA | 175 | 77 | 24 | Buspirone | Not reported | 45/88 vs. 47/87 |
| McRae-Clark et al. (2016) | USA | 76 | 79 | 22 | Vilazodone | Not reported | 14/41 vs. 17/35 |
| Miranda et al. (2017) | USA | 66 | 49 | 20 | Topiramate | Mean daily grams cannabis use: 0.45 (0.03) vs. 0.62 (0.06) | 19/40 vs. 20/26 |
| Penetar et al. (2012) | USA | 22 | 55 | 31 | Bupropion SR | Not reported | 5/10 vs. 4/12 |
| Sherman et al. (2017) | USA | 16 | 63 | 26 | Oxytocin | Mean daily grams cannabis use: 0.75 (0.58) vs. 0.07 (0.54) | 6/8 vs. 7/8 |
| Trigo et al., 2018 | Canada | 40 | 73 | 33 | Nabiximols | Mean weekly grams cannabis use: 2 (1) vs. 8 (2) | 13/20 vs. 14/20 |
| Weinstein et al. (2014) | Israel | 52 | 75 | 32 | Escitalopram | Not reported | 10/26 vs. 16/26 |
Y = years; USA = United States of America; SR = sustained release; FAAH = fatty acid amyl hydroxylase; XR = extended released. For all studies, retention in treatment was defined as the proportion of randomized participants who remained in the study until its primary endpoint.
Across studies, there were 1912 individuals: 986 received active pharmacotherapy while the rest received a placebo. The majority were male (n=1432, 74.9%). The mean age was 30.2 years (SD=5.7 years). Primarily, participants received a diagnosis of DSM-IV-TR cannabis dependence. However, one study (Cornelius et al., 1999) required a DSM-III-R diagnosis of marijuana dependence, and another (Lintzeris et al., 2019) used the ICD-10 for CUD diagnosis. Although the majority of studies excluded participants with psychiatric or medical comorbidity, eight studies included individuals with co-occurring conditions, including major depressive disorder (Carpenter et al., 2009; Cornelius et al., 1999, 2010; Levin et al., 2011, 2013), ADHD (McRae-Clark et al., 2010), alcohol use disorder (Cornelius et al., 1999), and a mixture of different anxiety and mood disorders (Gray et al., 2012; Levin et al., 2004).
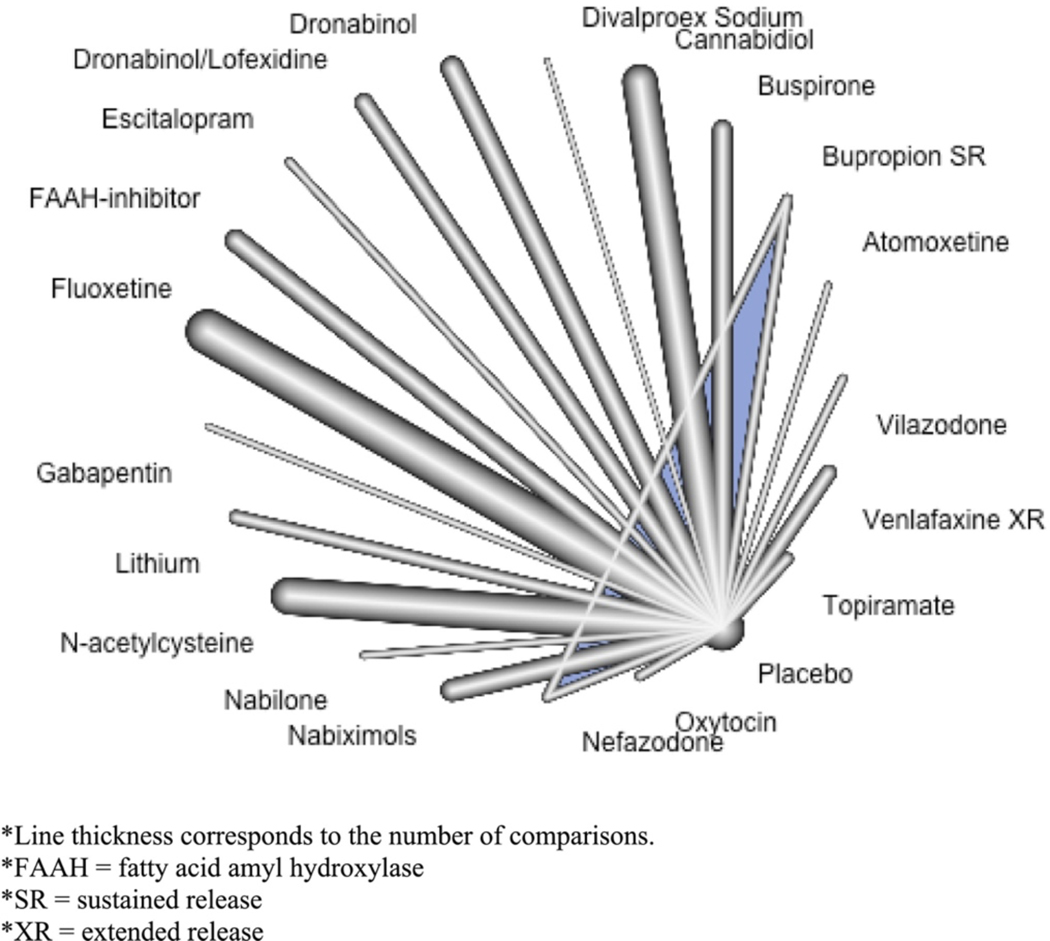
All studies involved a placebo comparator. The studied agents were atomoxetine (McRae-Clark et al., 2010), bupropion sustained-release (Carpenter et al., 2009; Penetar et al., 2012), buspirone (McRae-Clark et al., 2009, 2015), divalproex sodium (Levin et al., 2004), dronabinol (Levin et al., 2011), dronabinol with lofexidine (Levin et al., 2016), escitalopram (Weinstein et al., 2014), the fatty-acid amyl hydroxylase (FAAH) inhibitor PF-04457845 (D’Souza et al., 2019), fluoxetine (Cornelius et al., 1999, 2010), gabapentin (Mason et al., 2012), lithium (Johnston et al., 2014), N-acetylcysteine (Gray et al., 2012, 2017), nabilone (Hill et al., 2017), nabiximols (Allsop et al., 2014; Lintzeris et al., 2019; Trigo et al., 2018), nefazodone (Carpenter et al., 2009), oxytocin (Sherman et al., 2017), topiramate (Miranda et al., 2017), venlafaxine extended-release (Levin et al., 2013), and vilazodone (McRae-Clark et al., 2016) (Fig. 2). All but one study (D’Souza et al., 2019) included an adjunctive psychosocial therapy: MET (Cornelius et al., 2010; Levin et al., 2011, 2016; McRae-Clark et al., 2009, 2010, 2015, 2016; Miranda et al., 2017; Penetar et al., 2012; Sherman et al., 2017; Trigo et al., 2018; Weinstein et al., 2014), CBT (Cornelius et al., 2010; Levin et al., 2013; Weinstein et al., 2014), coping skills groups (Carpenter et al., 2009), addictions counselling (Gray et al., 2012; Johnston et al., 2014; Lintzeris et al., 2019; Mason et al., 2012), relapse prevention therapy (Johnston et al., 2014; Levin et al., 2011, 2016; Levin et al., 2004), and contingency management (Gray et al., 2012, 2017).
Figure 2.
Network plot of agents included in the network meta-analysis.
Primary outcomes (Table 2, Fig. 3)
Table 2.
Summary of network meta-analysis estimates.
| Cannabis use | Retention | Losses due to adverse events | Cannabis withdrawal severity | |
|---|---|---|---|---|
|
| ||||
| Number of studies | 11 | 24 | 15 | 12 |
| Number of treatments | 9 | 20 | 14 | 11 |
| Number of pairwise comparisons | 11 | 26 | 17 | 14 |
| Number of designs | 8 | 19 | 12 | 10 |
| τ2 | 2.5935 | 0 | 0 | 0.4507 |
| I2 | 95.5% | 0% | 0% | 84.8% |
| Total heterogeneity (Q, df, p-value) | 66.51, 3, <0.0001 | 2.68, 6, 0.8480 | 2.54, 3, 0.4684 | 19.75, 3, 0.0002 |
| Within designs (Q, df, p-value) | 66.51, 3, <0.0001 | 2.10, 5, 0.8357 | 2.54, 3, 0.4684 | 0.82, 2, 0.6626 |
| Between designs (Q, df, p-value) | 0, 0, − | 0.58, 1, 0.4455 | 0, 0, − | 18.93, 1,<0.0001 |
| Cravings | Cannabis use disorder severity | Adverse events | Abstinence | |
| Number of studies | 12 | 6 | 21 | 17 |
| Number of treatments | 10 | 7 | 19 | 16 |
| Number of pairwise comparisons | 12 | 8 | 23 | 19 |
| Number of designs | 9 | 5 | 17 | 14 |
| τ2 | 0.1741 | 0.3186 | 0 | 0.0974 |
| I2 | 73.9% | 71.2% | 0% | 32.9% |
| Total heterogeneity (Q, df, p-value) | 11.49, 3, 0.0093 | 3.47, 1, 0.0625 | 0.87, 4, 0.9293 | 4.47, 3, 0.2148 |
| Within designs (Q, df, p-value) | 11.49, 3, 0.0093 | 3.47, 1, 0.0625 | 0.87, 4, 0.9293 | 4.47, 3, 0.2148 |
| Between designs (Q, df, p-value) | 0, 0, − | 0, 0, − | 0, 0, − | 0, 0, − |
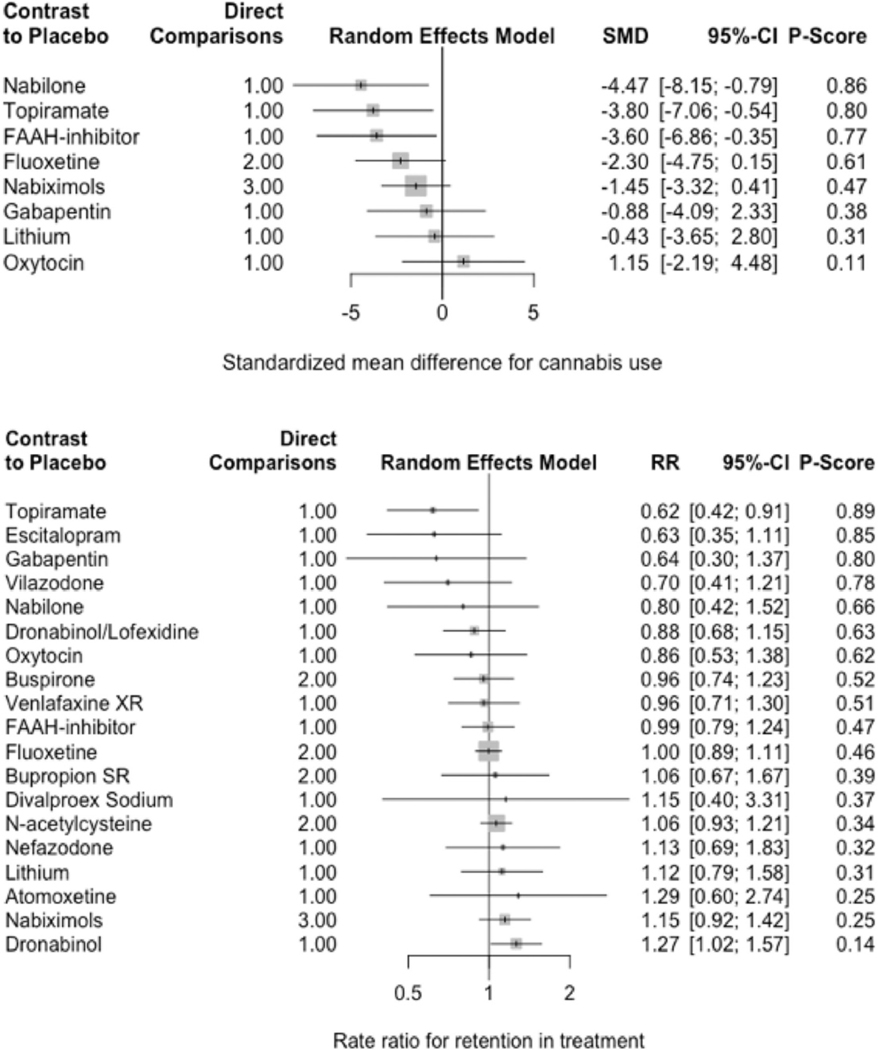
Figure 3.
Forest plots of network meta-analyses for reducing cannabis use (top) and retention in treatment (bottom).
Only nabilone (SMD = −4.47 [−8.15; −0.79]), topiramate (SMD = −3.80 [−7.06; −0.54]), and FAAH-inhibitors (SMD = −2.30 [−4.75; 0.15]) were associated with significant reductions in cannabis use. Overall, this outcome was highly heterogeneous (τ2 = 2.59; I2 = 95.5%). Dronabinol appeared to improve retention in treatment relative to placebo (RR = 1.27 [1.02; 1.57]), while topiramate worsened retention in treatment (RR = 0.62 [0.42; 0.91]). Conversely, we observed low heterogeneity in retention in treatment (τ2 = 0; I2 = 0%).
Secondary outcomes (Table 2, Table S22)
Adverse Events.
While buspirone (RR = 1.14 [1.00; 1.29]), venlafaxine extended-release (RR = 1.78 [1.40; 2.26]), and topiramate (RR = 9.10 [1.27; 65.11]) were associated with significantly more adverse events, only topiramate was associated with significantly more dropouts due to adverse events (RR = 9.10 [1.27; 65.11]). Overall, this outcome demonstrated low heterogeneity (τ2 = 0; I2 = 0%).
Abstinence.
Most trials reporting abstinence included subjective (e.g., self-reported cannabis use) and objective measures (e.g., urine drug screen for cannabinoids) of cannabis cessation. Venlafaxine extended-release was associated with worse abstinence (RR = 0.32 [0.11; 0.90]). However, no pharmacotherapies improved abstinence. Heterogeneity and inconsistency measures were low (τ2 = 0.10; I2 = 32.9%).
Cannabis withdrawal.
Instruments for measuring withdrawal included the Snaith Irritability Scale (Snaith et al., 1978), the Cannabis Withdrawal Scale (Allsop et al., 2011), and the Marijuana Withdrawal Checklist (Bonnet & Preuss, 2017). None of the investigated pharmacotherapies were significantly better than placebo at reducing the severity of cannabis withdrawal. Overall, this outcome demonstrated high heterogeneity (τ2 = 0.45 I2 = 84.8%)
Cannabis use disorder severity.
CUD severity was variably defined, using measures such as the Clinical Global Impression (Carpenter et al., 2009), the Global Assessment of Severity (Cornelius et al., 1999), the Severity of Dependence Scale (Allsop et al., 2014; Johnston et al., 2014), and the frequency of DSM criteria for either cannabis abuse or dependence diagnosis (Cornelius et al., 2010). None of the medications reduced the severity of CUD. Heterogeneity and inconsistency measures were moderate (τ2 = 0.32; I2 = 71.2%).
Cannabis cravings.
The severity of cannabis cravings was most frequently measured using the Marijuana Cravings Questionnaire (S. J. Heishman et al., 2001; Heishman et al., 2009). The only pharmacotherapy to significantly reduce cannabis cravings was gabapentin (SMD = −2.42 [−3.53; −1.32]), while vilazodone significantly worsened cravings for cannabis (SMD = 1.69 [0.71; 2.66]). Overall, this outcome demonstrated moderate heterogeneity and low inconsistency (τ2 = 0.17; I2 = 73.9%).
Risk of bias within studies
The risk of bias across rated as high for three studies, unclear for six, and low for the remainder (Table S3). Four trials (Allsop et al., 2014; D’Souza et al., 2019; Gray et al., 2012; Weinstein et al., 2014) disclosed pharmaceutical company support, primarily for study drugs; however, two trials did not report their source of funding (Cornelius et al., 2010; Levin et al., 2011). Most trials reported their trial protocols and registration details (n=14). Nearly all studies endorsed double-blinding; however, three studies did not describe their blinding methods (Hill et al., 2017; McRae-Clark et al., 2016; Sherman et al., 2017).
Risk of bias across studies
There was moderate evidence of publication bias for the reduction in cannabis use, with Egger’s test (p=0.04) and the Thompson-Sharp test (p=0.01) indicating funnel plot asymmetry (Table S4). However, there was insufficient evidence for publication bias for the remaining outcomes—retention in treatment, adverse events, cannabis withdrawal, cannabis cravings, abstinence.
Additional analyses
As a post-hoc sensitivity analysis, we evaluated the results after removing studies that had scored highly on the Cochrane Risk of Bias Tool (Table S5). The overall findings were largely unchanged for most network meta-analyses outcomes. For example, venlafaxine extended-release was still associated with worse abstinence (RR = 0.32 [0.11; 0.90]), while topiramate still led to higher adverse events (RR = 9.10 [1.27; 65.11]). As well, the same three agents—nabilone (SMD = −4.47 [−8.15; −0.79]), topiramate (SMD = −3.80 [7.06; −0.54]), and FAAH-inhibitors (SMD = −2.30 [−4.75; 0.15])—were still associated with reduction in cannabis.
DISCUSSION
To our knowledge, this is the first systematic review and NMA exploring medications for CUD. Specific agents appeared to show some benefit for particular aspects of CUD. For example, nabilone reduced cannabis use, dronabinol improved retention in treatment, and gabapentin reduced cravings. However, caution is warranted in interpreting these results as most of the investigated agents, such as dronabinol and buspirone, had very low effects, often approaching the null-effect. Conversely, some medications, like topiramate, venlafaxine, and vilazodone, worsened treatment outcomes. There were no effective agents identified for reducing CWS or overall CUD severity.
Several previous reviews have examined CUD treatments (Bahji & Mazhar, 2016; Benyamina et al., 2008; Marshall, 2008; Nielsen et al., 2019). As there appear to be multiple neurotransmitter systems involved in the neurobiology of CUD and CWS, it is not surprising that there may be a potential role for various pharmacological therapies targeting multiple receptor agonism/antagonism pathways. We have summarized some of the possible therapeutic mechanisms for the agents explored in this review as CUD treatments in Table S6. For example, CB1 and CB2 cannabinoid-receptor modulators, like nabilone and dronabinol may mitigate the extent of cannabis withdrawal. In contrast, antidepressants—like fluoxetine, venlafaxine, and vilazodone—may reduce depression and anxiety symptoms and improve sleep during cannabis withdrawal.
In the present review, cannabinoid-receptor modulators—such as dronabinol, nabilone, and nabiximols—seemed to demonstrate the most consistent evidence for both efficacy and safety-related outcomes in combination with psychosocial treatments. While we found more consistent evidence for THC-containing preparations than other pharmacotherapies—which has been a finding of previous reviews of cannabinoid replacement therapies for CUD (Bahji & Mazhar, 2016)—we must be cautious regarding these conclusions given the limited yield and quality of studies for any individual agent. As with any treatment, the potential for risks must be weighed against any potential benefit. For example, as most cannabinoid agonists produce cognitive deficits, this may limit the tolerability of THC-containing preparations to treat CUD or to augment psychosocial intervention. To that end, the utilization of cannabinoids as treatments for psychiatric disorders has been controversial, primarily because of the limited evidence. For example, while cannabinoids are frequently used as anxiolytics, there is little evidence to support their utilization in this context (Bahji, Meyyappan, et al., 2020b; Tibbo et al., 2020). While there remains a need for additional research to solidify these early findings, several recent studies appear to support our results, such as one study demonstrating that chronic dronabinol dosing can reduce cannabis self-administration among daily cannabis users and suppress cannabis withdrawal symptoms (Schlienz et al., 2018). A recent trial not included in this review found preliminary evidence that cannabidiol, at doses of 400 to 800 mg per day, led to small improvements in the number of abstinent days from cannabis (Freeman et al., 2020).
Strengths and Limitations
While our study has several strengths, including the breadth of pharmacotherapies considered and our analytic approach’s novelty, there are some limitations. The diverse ways in which trials measured outcomes impacted our analysis and results. For example, the Hill et al. (2017) trial assessed cannabis use as self-reported inhalations per day, while the Miranda et al. (2017) assessed cannabis use as grams per usage. While our analyses used standardization, this does not entirely remove the effects of different operationalization of outcomes. The relationship between the trial length and the frequency of measurement for specific outcomes, such as abstinence, could also affect the ability to compare data across trials. While the random-effects model’s application helped address these sources of heterogeneity, specific outcomes were left relatively underpowered for detecting significance.
Our study’s conclusions depend on the NMA model’s validity, contingent upon satisfying the transitivity and consistency assumptions. When trials use different medications to match different levels of CUD severity, this violates the transitivity assumption. Therefore, studies examining first-line treatment would not include combination therapies, and including combination treatments in an NMA of first-line treatments would introduce intransitivity. Currently, medication-based treatment protocols for CUD are not standardized; however, they may become standardized in this type of hierarchical, severity-based fashion. Consequently, this may inform future RCTs’ conduct, in which potential participants are screened out based on the severity of CUD to match them to one particular type of medication.
Furthermore, systematic differences in comorbidity across trials may have contributed to intransitivity. For example, participants with major depression may have been more likely to be on an antidepressant. Participants with CUD who are not depressed may have tended to do better in treatment than those with comorbid CUD and depression. If there is a systematic selection of those with comorbid CUD and MDD into the antidepressant trials and away from the cannabinoid medication trials, that would tend to “water-down” the effect size. Furthermore, the CUD diagnostic criteria for adolescent trials are less stringent than the CUD diagnostic criteria for adult trials, often because the diagnostic interview protocols are different for adolescents. To that end, this may have caused an inadvertent violation of the transitivity assumption, which may imply that separate analyses of adult and adolescent data are warranted.
For some outcomes, our NMA was potentially underpowered to detect statistical significance, such as the overall reduction in the severity of cannabis dependence, because only six studies collected data on this measure. While our eligibility criteria included non-placebo-controlled trials (e.g., trials involving an active comparator), we did not identify any such studies, which may have hindered our pooled sample sizes for specific outcome measures. For some of the comparisons for continuous outcomes, only a handful of trials contributed evidence, and often, there were discrepant findings across outcomes with the same agent. For example, topiramate reduced cannabis use but also caused more adverse effects and premature study withdrawals.
Due to the overrepresentation of young Caucasian males, our review’s findings are less generalizable to other populations. Our results are also limited to smoked, plant-based cannabinoids, as we did not find any studies where individuals had CUD involving edibles or synthetic cannabinoids. We also detected publication bias in the network estimate to reduce cannabis use, suggesting an inflated effect size. With that said, our funnel plots combined all medications, which assumes the drugs have the same (or random) effects.
Future studies
Future studies could explore functional (rather than absolute) outcomes that correlate with meaningful reductions in cannabis use. Combining clinical and biological measures could create composite CUD outcomes. More trials are needed, particularly head-to-head studies. Real-world measures of CUD treatment effectiveness might be possible through leveraging administration datasets. In parallel, research exploring the molecular mechanisms of these diverse agents may help us refine our understanding of the pathophysiology of CUD and its treatment. For example, the differential activation and involvement of CB1 and CB2 receptors may help determine which patients are better suited for particular THC-containing preparations given the inconsistencies in responses to nabilone, nabiximols, dronabinol, and FAAH-inhibitors. There is also a need to explore CUD treatment among previously understudied populations, such as women, individuals with comorbid conditions, and older adults.
CONCLUSIONS
Based on this review, some medications appeared to show promise for treating individual aspects of CUD. However, there is a lack of robust evidence to support any particular pharmacological treatment. There is a need for additional studies to expand the evidence base for CUD pharmacotherapy. While medication strategies may become an integral component for CUD treatment one day, psychosocial interventions should remain the first line given the limitations in the available evidence.
Supplementary Material
Acknowledgments
The authors would like to thank Ms. Sandra Halliday—the research librarian at Bracken Health Sciences Library—for her support in developing the systematic review strategy for this study.
Footnotes
Appendix. Supplementary materials
REFERENCES
- Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, & Sadler C. (2014). Nabiximols as an agonist replacement therapy during cannabis withdrawal: A randomized clinical trial. JAMA Psychiatry, 71(3), 281–291. [DOI] [PubMed] [Google Scholar]
- Allsop DJ, Norberg MM, Copeland J, Fu S, & Budney AJ (2011). The cannabis withdrawal scale development: Patterns and predictors of cannabis withdrawal and distress. Drug and Alcohol Dependence, 119(1), 123–129. 10.1016/j.drugalcdep.2011.06.003. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th Edition). [Google Scholar]
- Bahji A, & Mazhar MN (2016). Treatment of cannabis dependence with synthetic cannabinoids: A systematic review. Canadian Journal of Addiction, 7(4), 8. [Google Scholar]
- Bahji A, & Stephenson C. (2019). International perspectives on the implications of cannabis legalization: A systematic review & thematic analysis. International Journal of Environmental Research and Public Health, 16(17). 10.3390/ijerph16173095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, Hawken ER, & Vazquez G. (2020). Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: A systematic review and network meta-analysis. Journal of Affective Disorders, 269, 154–184. 10.1016/j.jad.2020.03.030. [DOI] [PubMed] [Google Scholar]
- Bahji A, Ermacora D, Stephenson C, Hawken ER, & Vazquez G. (2020). Comparative efficacy and tolerability of adjunctive pharmacotherapies for acute bipolar depression: A systematic review and network meta-analysis. Canadian Journal of Psychiatry. 10.1177/0706743720970857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Stephenson C, Tyo R, Hawken ER, & Seitz DP (2020). Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids: A systematic review and meta-analysis. JAMA Network Open, 3(4), Article e202370. 10.1001/jamanetworkopen.2020.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Meyyappan AC, & Hawken ER (2020a). Cannabinoids for the neuropsychiatric symptoms of dementia: A systematic review and meta-analysis. Canadian Journal of Psychiatry, 65(6), 365–376. 10.1177/0706743719892717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahji A, Meyyappan AC, & Hawken ER (2020b). Efficacy and acceptability of cannabinoids for anxiety disorders in adults: A systematic review & meta-analysis. Journal of Psychiatric Research, 129, 257–264. 10.1016/j.jpsychires.2020.07.030. [DOI] [PubMed] [Google Scholar]
- Bahji A. (2020). Incidence and correlates of cannabinoid-related psychiatric emergency care: A retrospective, multiyear cohort study. Canadian Journal of Addiction, 11(1), 14–18. 10.1097/CXA.0000000000000075. [DOI] [PubMed] [Google Scholar]
- Benyamina A, Lecacheux M, Blecha L, Reynaud M, & Lukasiewcz M. (2008). Pharmacotherapy and psychotherapy in cannabis withdrawal and dependence. Expert Review of Neurotherapeutics, 8(3), 479–491. 10.1586/14737175.8.3.479. [DOI] [PubMed] [Google Scholar]
- Bonnet U, & Preuss UW (2017). The cannabis withdrawal syndrome: Current insights. Substance Abuse and Rehabilitation, 8, 9–37. 10.2147/SAR.S109576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezing CA, & Levin FR (2018). The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology, 43(1), 173–194. 10.1038/npp.2017.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, & Hughes JR (2006). The cannabis withdrawal syndrome. Current Opinion in Psychiatry, 19(3), 233–238. 10.1097/01.yco.0000218592.00689.e5. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, & Levin FR (2009). A preliminary trial: Double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. American Journal on Addictions, 18(1), 53–64. 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration Cochrane. (2014). Cochrane Handbook: General Methods for Cochrane Reviews. Heterogeneity. https://handbook-5-1.cochrane.org/chapter_9/9_5_heterogeneity.htm [Google Scholar]
- Cohen J. (2013). Statistical power analysis for the behavioral sciences. Routledge. 10.4324/9780203771587. [DOI] [Google Scholar]
- Copeland J, & Swift W. (2009). Cannabis use disorder: Epidemiology and management. International Review of Psychiatry, 21(2), 96–103. 10.1080/09540260902782745. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Salloum IM, Haskett RF, Ehler JG, Jarrett PJ, Thase ME, & Perel JM (1999). Fluoxetine versus placebo for the marijuana use of depressed alcoholics. Addictive Behaviors, 24(1), 111–114. 10.1016/S0306-4603(98)00050-1. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, Wood DS, & Brown SJ (2010). Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug and Alcohol Dependence, 112(1–2), 39–45. 10.1016/j.drugalcdep.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, Bielen K, Surti T, Radhakrishnan R, Gupta A, Gupta S, Cahill J, Sherif MA, Makriyannis A, Morgan PT, Ranganathan M, & Skosnik PD (2019). Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: A double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry, 6(1), 35–45. 10.1016/S2215-0366(18)30427-9. [DOI] [PubMed] [Google Scholar]
- de Fonseca FR, & Schneider M. (2008). The endogenous cannabinoid system and drug addiction: 20 years after the discovery of the CB1 receptor. Addiction Biology, 13(2), 143–146. 10.1111/j.1369-1600.2008.00116.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, Flaxman AD, Engell RE, Freedman GD, Whiteford HA, & Vos T. (2013). The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: Results from the GBD 2010 study. PLOS ONE, 8(10), e76635. 10.1371/journal.pone.0076635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias S, & Caldwell DM (2019). Network meta-analysis explained. Archives of Dis-ease in Childhood. Fetal and Neonatal Edition, 104(1), F8–F12. 10.1136/archdis-child-2018-315224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction. (2015). Treatment of cannabis-related disorders in Europe. Publications Office 10.2810/621856. [DOI] [Google Scholar]
- European Monitoring Centre on Drugs and Drug Addiction. (2021). Best practice portal: Evidence database [Academic]. European Monitoring Centre on Drugs and Drug Addiction. https://www.emcdda.europa.eu/best-practice/evidence-summaries. [Google Scholar]
- Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, Freeman AM, Lees R, Craft S, Morrison PD, Bloomfield MAP, O’Ryan D, Kinghorn J, Morgan CJA, Mofeez A, & Curran HV (2020). Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry, 7(10), 865–874. 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates PJ, Sabioni P, Copeland J, Le Foll B, & Gowing L. (2016). Psychosocial interventions for cannabis use disorder. Cochrane Database of Systematic Reviews, 5, Article CD005336. 10.1002/14651858.CD005336.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, & Brady KT (2012). A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. American Journal of Psychiatry, 169(8), 805–812. 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, Carroll KM, Potter JS, Wiest K, Mooney LJ, Hasson A, Walsh SL, Lofwall MR, Babalonis S, Lindblad RW, Sparenborg S, Wahle A, King JS, Baker NL, … Levin FR. (2017). A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug and Alcohol Dependence, 177, 249–257. 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS (2018). US epidemiology of cannabis use and associated problems. Neuropsychopharmacology, 43(1), 195–212. 10.1038/npp.2017.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, & Liguori A. (2001). Marijuana craving questionnaire: Development and initial validation of a self-report instrument. Addiction, 96(7), 1023–1034. 10.1080/09652140120053084. [DOI] [PubMed] [Google Scholar]
- Heishman Stephen J., Evans RJ, Singleton EG, Levin KH, Copersino ML, & Gorelick DA. (2009). Reliability and validity of a short form of the marijuana craving questionnaire. Drug and Alcohol Dependence, 102(1–3), 35–40. 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins Julian P. T., & Thompson SG (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Higgins, Julian PT, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical Journal, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, & White IR (2012). Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Research Synthesis Methods, 3(2), 98–110. 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Palastro MD, Gruber SA, Fitzmaurice GM, Greenfield SF, Lukas SE, & Weiss RD (2017). Nabilone pharmacotherapy for cannabis dependence: A randomized, controlled pilot study. American Journal on Addictions, 26(8), 795–801. 10.1111/ajad.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch E, Preuss UW, Ferri M, & Simon R. (2016). Digital interventions for problematic cannabis users in non-clinical settings: Findings from a systematic review and meta-analysis. European Addiction Research, 22(5), 233–242. 10.1159/000445716. [DOI] [PubMed] [Google Scholar]
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JPA, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, & Moher D (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Annals of Internal Medicine, 162(11), 777–784. 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- Johnston J, Lintzeris N, Allsop D, Suraev A, Booth J, Carson D, Helliwell D, Winstock A, & McGregor I. (2014). Lithium carbonate in the management of cannabis withdrawal: A randomized placebo-controlled trial in an inpatient setting. Psychopharmacology, 231(24), 4623–4636. 10.1007/s00213-014-3611-5. [DOI] [PubMed] [Google Scholar]
- Levin, Frances Rudnick, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, & Vosburg SK (2004). Pharmacotherapy for marijuana dependence: A double-blind, placebo-controlled pilot study of divalproex sodium. American Journal on Addictions, 13(1), 21–32. 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Levin Frances R., Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, & Nunes EV. (2011). Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug and Alcohol Dependence, 116(1), 142–150. 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin Frances R., Mariani J, Brooks DJ, Pavlicova M, Nunes EV, Agosti V, Bisaga A, Sullivan MA, & Carpenter KM. (2013). A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction, 108(6), 1084–1094. 10.1111/add.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin Frances R., Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, Nunes EV, Bisaga A, Dakwar E, Carpenter KM, Sullivan MA, & Choi JC. (2016). Dronabinol and lofexidine for cannabis use disorder: A randomized, double-blind, placebo-controlled trial. Drug and Alcohol Dependence, 159, 53–60. 10.1016/j.drugalcdep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, & Moher D (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLOS Medicine, 6(7), Article e1000100. 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintzeris N, Bell J, Bammer G, Jolley DJ, & Rushworth L. (2002). A randomized controlled trial of buprenorphine in the management of short-term ambulatory heroin withdrawal. Addiction, 97(11), 1395–1404. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, Bruno R, Gugusheff J, Phung N, Montebello M, Chan T, Kirby A, Hall M, Jefferies M, Luksza J, Shanahan M, Kevin R, & Allsop D Group, for the A. R. for C. D. (ARCD) study.. (2019). Nabiximols for the treatment of cannabis dependence: A randomized clinical trial. JAMA Internal Medicine, 179(9), 1242–1253. 10.1001/jamainternmed.2019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K, Gowing L, Ali R, & Le Foll B. (2014). Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews, 12, Article CD008940. 10.1002/14651858.CD008940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P. (2008). The greatest pilgrimage: Reflections on a Baha’i frame of reference for spiritual care. Counselling and Spirituality /Counseling et Spiritualite, 27(2), 57–79. [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, Buffkins K, Kyle M, Adusumalli M, Begovic A, & Rao S. (2012). A proof-of-concept randomized controlled study of gabapentin: Effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 37(7), 1689–1698. 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, & Brady KT (2009). A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug and Alcohol Dependence, 105(1–2), 132–138. 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, & Brady KT (2010). A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. American Journal on Addictions, 19(6). 10.1111/j.1521-0391.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen TK, Wagner AM, Brady KT, DeVane CL, & Norton J. (2015). Buspirone treatment of cannabis dependence: A randomized, placebo-controlled trial. Drug and Alcohol Dependence, 156, 29–37. 10.1016/j.drugalcdep.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Gray KM, Killeen T, Hartwell KJ, & Simonian SJ (2016). Vilazodone for cannabis dependence: A randomized, controlled pilot trial. American Journal on Addictions, 25(1), 69–75. 10.1111/ajad.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methley AM, Campbell S, Chew-Graham C, McNally R, & Cheraghi-Sohi S. (2014). PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Services Research, 14. 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, Swift R, Tidey JW, & Gwaltney CJ (2017). Topiramate and motivational enhancement therapy for cannabis use among youth: A randomized placebo-controlled pilot study. Addiction Biology, 22(3), 779–790. 10.1111/adb.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, & Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. National Academies Press (US) http://www.ncbi.nlm.nih.gov/books/NBK423845/. [PubMed] [Google Scholar]
- Nielsen S, Gowing L, Sabioni P, & Le Foll B. (2019). Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews, 1, Article CD008940. 10.1002/14651858.CD008940.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar DM, Looby AR, Ryan ET, Maywalt MA, & Lukas SE (2012). Bupropion reduces some of the symptoms of marihuana withdrawal in chronic marihuana users: A pilot study. Substance Abuse: Research and Treatment, 6, 63–71. 10.4137/SART.S9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rücker G, Krahn U, König J, Efthimiou O, & Schwarzer G. (2019). Netmeta: Network meta-analysis using frequentist methods (1.1–0). [Computer software] https://CRAN.R-project.org/package=netmeta. [Google Scholar]
- Rouse B, Chaimani A, & Li T. (2017). Network meta-analysis: An introduction for clinicians. Internal and Emergency Medicine, 12(1), 103–111. 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudioTeam. (2020). RStudio: Integrated Development for R (3.5.3) [PBC]. The R Foundation for Statistical Computing. http://www.rstudio.com/. [Google Scholar]
- Salanti G, Higgins JPT, Ades AE, & Ioannidis JPA (2008). Evaluation of networks of randomized trials. Statistical Methods in Medical Research, 17(3), 279–301. 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- Salanti G. (2012). Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods, 3(2), 80–97. 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- Schlienz NJ, Lee DC, Stitzer ML, & Vandrey R. (2018). The effect of high-dose dronabinol (oral THC) maintenance on cannabis self-administration. Drug and Alcohol Dependence, 187, 254–260. doi: 10.1016/j.drugalcdep.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, & McRae-Clark AL (2016). Treatment of cannabis use disorder: Current science and future outlook. Pharmacotherapy, 36(5), 511–535. 10.1002/phar.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BJ, Baker NL, & McRae-Clark AL (2017). Effect of oxytocin pretreatment on cannabis outcomes in a brief motivational intervention. Psychiatry Research, 249, 318–320. 10.1016/j.psychres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Constantopoulos AA, Jardine MY, & McGuffin P. (1978). A clinical scale for the self-assessment of irritability. British Journal of Psychiatry: The Journal of Mental Science, 132, 164–171. 10.1192/bjp.132.2.164. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA). (2015). National Survey on Drug Use and Health (NSDUH) 2015 (NSDUH-2015-DS0001) Public-Use File Dataset[Public-Use File Dataset]. National Survey on Drug Use and Health. https://www.datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2015-nsduh-2015-ds0001-nid16894. [Google Scholar]
- The Cochrane Collaboration. (2014). Review Manager (RevMan) (5.3) [Computer software]. [Google Scholar]
- Tibbo PG, McKee KA, Meyer JH, Crocker CE, Aitchison KJ, Lam RW, & Crockford DN (2020). Are there therapeutic benefits of cannabinoid products in adult mental illness? Canadian Journal of Psychiatry 0706743720945525. 10.1177/0706743720945525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, Barnes AJ, Huestis MA, George TP, Streiner DL, Staios G, & Foll BL (2018). Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: A pilot randomized clinical trial. PLOS ONE, 13(1), Article e0190768. 10.1371/journal.pone.0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. (2015). World Drug Report 2015. Vienna, Austria: United Nations Publication. [Google Scholar]
- United Nations Office on Drugs and Crime. (2018). World Drug Report 2018. https://www.unodc.org/wdr2018/prelaunch/WDR18_Booklet_1_EXSUM.pdf [Google Scholar]
- Veritas Health Innovation. (2019). Covidence systematic review software [English]. [Google Scholar]
- Volkow ND, Baler RD, Compton WM, & Weiss SRB (2014). Adverse health effects of marijuana use. New England Journal of Medicine, 370(23), 2219–2227. 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar-Hamburger R, & Bloch M. (2014). Treatment of cannabis dependence using escitalopram in combination with cognitive-behavior therapy: A double-blind placebo-controlled study. Journal of Drug and Alcohol Abuse, 40(1), 16–22. [DOI] [PubMed] [Google Scholar]
- World Health Organization & Regional Office for Europe. (2016). The health and social effects of nomedical cannabis use. World Health Organization Stylus Publishing, LLC; [distributor. http://deslibris.ca/ID/10090267]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.