Abstract
We present a case of a 55-year-old man with a heart transplant who acquired Invasive Aspergillosis by Aspergillus fumigatus with the focus in the kidney. During about two years of antifungal treatment, most of the time with voriconazole, it was possible to obtain nine isolates of A. fumigatus, with the same genotypic characteristics, but with an increase in MIC for several azoles. The two last isolates presented high MICs for Voriconazole (>8 μg/mL>). Sequencing of the CYP51A gene showed G448S amino acid substitution in the same two isolates. In long-term treatments with antifungals, it would be important to regularly evaluate the susceptibility of isolated strains, as resistance to azoles has been increasingly described around the world.
Keywords: Aspergillus fumigatus, Invasive aspergillosis, Azole-resistance, Brazil
1. Introduction
Solid organ or bone marrow transplantation have come to play an important role in extending the estimated life span due medicine advances. The significant increase in the number of transplants placed the opportunistic fungus, Aspergillus spp., in a prominent place as a causative agent of invasive infections in immunocompromised patients [1]. Aspergillus species are associated with a wide variety of infections, being Aspergillus fumigatus the most isolated species and also responsible for the highest rate of Invasive Aspergillosis (IA) [2]. Invasive aspergillosis usually requires a long-term antifungal therapy. The first-line therapy are azole antifungals such as Voriconazole (VRC), however, these treatments often cause several side effects and may induce the selection of resistant isolates [2,3]. Resistance is usually attributed to mutations in the CYP51A gene, which encodes cytochrome P450 14-α-lanosterol demethylase [2,3]. The University Hospital of Campinas (HC-UNICAMP) performs an average of 400 transplants every year, therefore receiving many patients susceptible to opportunistic infections, such as IA. This manuscript reports the case of a patient who developed IA after heart transplantation. During two years of antifungal treatment, especially with VRC, it was possible to obtain nine A. fumigatus isolates, with the same genotypic characteristics in microsatellites analyses, but with distinguished Minimal Inhibitory Concentration (MICs) for several azoles, resulting in the detection of the G448S mutation in the CYP51A gene.
2. Case presentation
In 2018 a 55-year-old man was admitted to the HC-UNICAMP with intense pain in the right iliac fossa, and pain on deep palpation of the abdomen. The patient had a medical history of hypertension, diabetes mellitus, and heart transplant performed in 2015. He also presented multiple rejections episodes which demanded pulse therapy with methylprednisone and thymoglobulin. After physical examination, he underwent abdominal computed tomography (CT), which detected an abscess in the right kidney (Fig. 1). The first day of hospital admission was defined as “day 0” in this report. On day +12 since the first admission the abscess was punctured, and the aspirate material was sent to the Microbiology Laboratory. Results of culture, macroscopic microscopic and Spectrometric analysis (Maldi-Tof) identified Aspergillus fumigatus. The antifungal treatment was initiated using intravenous (IV) Micafungin (MCF) 100mg for 14 days and continued with IV VRC (200mg/12h) for 30 days. On day +43, the patient underwent a partial nephrectomy of the right kidney. The abscess content was submitted to new culture and A. fumigatus has identified again. On days +49, +56, +65, +73, blood galactomannan tests were performed and 1.27, 1.34, 0.99 and 0.03 OD values were obtained, respectively.
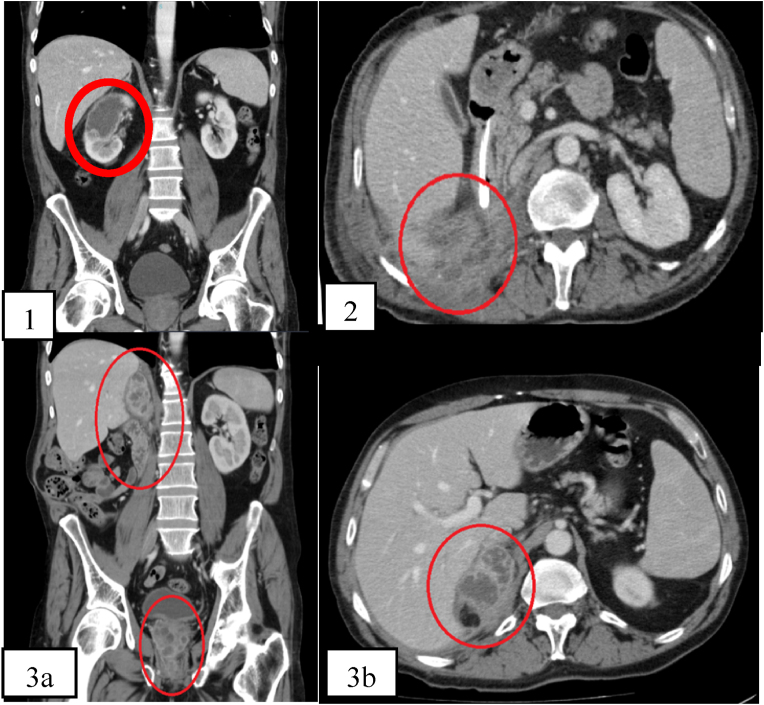
Fig. 1.
(1) Sagittal image from abdominal CT on day 0 showing in the right kidney a cystic image, compatible with renal abscess. (2) Axial image from CT on day +183 indicates the presence of stores with chronic-looking collections (no change from the previous exam), extending to the soft parts of the right flank and right adrenal gland, with suspected enteric fistula. (3a) Axial and (3b.) sagittal CT images of the abdomen on day +456, showing abscesses in the right nephrectomy pocket and prostate.
Another kidney puncture was performed, and A. fumigatus was identified. Under the intravenous administration of VRC 200mg twice a day, there was a significant improvement in the patient's condition, and he was discharged from the hospital. The continuous administration of oral VRC (200mg/12h) was maintained. On day +81 after the first admission, due to difficulty in acquiring VRC, it was necessary to change to oral Itraconazole (ITC) (200mg/12h) for 102 days. On day +183, the patient returned to the hospital complaining about dysuria, decreased urinary stream, and abdominal pain. Hospitalized again, an abdominal CT showed new abscesses in the right kidney and prostate (Fig. 1), the galactomannan marker was positive (1.71 OD). Transurethral resection of the prostate was performed. With the worsening of the condition with the use of oral ITC 200mg/12h, the treatment was substituted by oral VRC (200mg/12h).
As susceptibility testing is not routinely performed in HC-UNICAMP, a special request was made and broth microdilution tests for A. fumigatus isolated from the previous clinical specimens were performed (Fig. 1). Treatment with IV amphotericin B (AMB) lipid complex (1mg/kg/day) was initiated on day +456. During this period, the patient underwent a radical prostatectomy on day +489. Once again, the prostate material was positive for A. fumigatus and, to control the treatment, galactomannan was performed, showing positive (3.44 OD) higher results.
The patient evolved with clinical improvement. The blood serum level of galactomannan was negative (0.02 OD). Due to the impossibility of performing the AMB lipid complex at home, the patient was discharged from the hospital +605 days after the first hospitalization with a prescription of oral (20mg/kg/day) Posaconazole (POS). On +702 day, the patient was hospitalized again due to a pulmonary IA involvement, with right pleural effusion. The POS was then replaced by IV AMB lipid complex (1mg/kg/day). He underwent thoracentesis and the pleural fluid was sent to the laboratory, confirming A. fumigatus pulmonary infection. Pulmonary drainage and subsequent pleurotomy were performed. After using the AMB lipid complex, the patient showed a significant improvement and he was discharged from the hospital on +809 day, presenting negative galactomannan (0.04).
3. Discussion
All nine A. fumigatus isolates recovered from clinical samples were analyzed. These isolates were submitted to molecular identification using beta-tubulin (β-tubulin 2A/B) primers [4]. The gold standard susceptibility test (broth microdilution) was performed [5] evidencing two resistant isolates, LIF3546 (MZ673598) and LIF3608 (MZ673599), presenting MICs: VRC>8 μg/mL. The CYP51A gene mutations search [6] showed both resistant isolates carrying G1413A mutation, which is responsible for a G448S amino acid substitution. Microsatellite genotyping analysis [[6], [7]], performed at the Laboratory of Molecular Epidemiology of Infectious Diseases (LEMDI-UNICAMP), demonstrated the same microsatellite pattern in all isolates.
Invasive Aspergillosis is the second leading cause of morbidity and mortality among transplant recipients [8]. In heart transplant recipients, in addition to rejection problems, this kind of infection remains one of the main complications reported. The infection causes approximately 20% of deaths in the first year after transplantation and Aspergillus spp. has been reported as the most frequent invasive fungal infection, causing pneumonia with high attributable mortality, ranging from 53% to 78% in transplant patients [[9], [10], [11]].
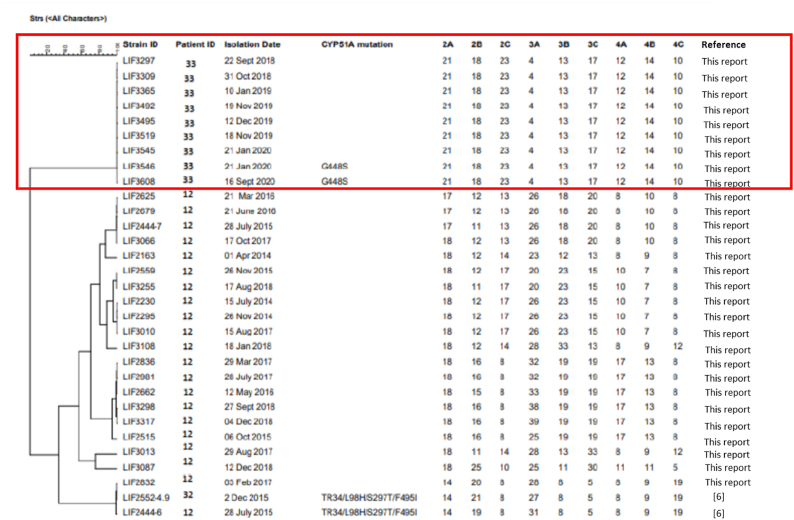
In recent years, mutations found in the CYP51A gene became a concern in all diagnostic centers worldwide [[12], [13], [14]]. In Brazil, until now, there is only one study attributing azole resistance to the TR48/L98H/S297T/F495I mutation in the CYP51A gene. Our research group identified two A. fumigatus isolates carrying this mutation and presenting resistance to ITC. Although those isolates had been obtained from inpatients, they were considered only colonizers [6]. Fig. 2 presents a dendrogram comparing the microsatellite characteristics of the present case (patient ID 3) and those isolates from the previously published case (patient ID 12 and 32) presenting the mutation (TR48/L98H/S297T/F495I) and other susceptible isolates not previously described of patient 12. In the dendrogram, it is possible to observe that there is no genetic correlation among the isolates.
Fig. 2.
Genotypic relationship among Aspergillus fumigatus isolates from patient 33 (described in this case report, in red), isolates from patient 12, and the resistant isolate, already published, from patient 32 (LIF 2444-6 and LIF 2552-4.9) [6]. The dendrogram is based on a categorical analysis of nine microsatellite markers in combination with the arithmetic mean unweighted pair group clustering method (UPGMA) using Phyloviz 2.0a. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
This case report has some limitations, therapeutic drug monitoring (TDM) for VRC was not performed. Unfortunately, HC-UNICAMP does not perform TDM for VRC, preventing to obtain actual serum levels of VRC in the patient and therefore low doses may have been administered, leading to progressive IA and resistance to the treatment. Another important limitation is the lack of other national publications to compare the genotypic characteristics of our Aspergillus fumigatus isolates.
The patient had continuous isolates recovered from the renal abscess since the second week of hospitalization. The non-effectiveness of VRC and ITC led A. fumigatus spread to the prostate and then to the lungs.
In this manuscript, we report the finding of the G448S mutation in A. fumigatus isolated in the renal capsule. Kidney infections caused by Aspergillus spp. are rare and is interesting that after about two years of antifungal treatment, especially with VRC, it was possible to obtain nine isolates of A. fumigatus, with the same microsatellites’ genotypic characteristics, but with an increase in azoles MICs until the detection of the G448S mutation in the CYP51A gene. The G448S substitution was found in the second last (LIF 3546) and in the last (LIF 3608) isolates recovered respectively after +489 and + 740 days. LIF 3545 (recovered from the prostate) and LIF 3546 (recovered from the renal capsule) were isolated on the same date (+489), but from different body sites, which suggests that the microorganism developed the mutation in the host, or the resistant ones were selected there due to a high antifungal concentration. Invasive prostatic aspergillosis (IPRA) is a rare condition with a few cases in the literature. Moreover, the prostate is considered a reservoir of microorganisms, where higher doses of VRC are recommended for treatment [15,16]. Table 1 shows increasing MICs for VRC according to the isolates date. Aspergillus fumigatus carrying G448S mutation usually presents high MIC for VRC (>8 μg/mL) [20].
Table 1.
In vitro antifungal susceptibility test results and presence of mutation detected on CYP51A gene sequence of the nine isolates analyzed.
| Isolate | Days after first hospitalization | Clinical specimen | CLSI MIC (μg/mL) |
CYP51A mutation | |||||
|---|---|---|---|---|---|---|---|---|---|
| MCFG | CPFG | AMPH-B | ITC | VRC | POS | ||||
| LIF 3297 | +12 | Renal abscess | 0.03 | 0.25 | 1 | 0.5 | 2 | 0.25 | None |
| LIF 3309 | +43 | Renal abscess | 0.015 | 0.12 | 2 | 0.5 | 2 | 0.25 | None |
| LIF 3365 | +114 | Renal abscess | 0.015 | 0.5 | 2 | 0.5 | 4 | 0.5 | None |
| LIF 3492 | +426 | Prostate | 0.015 | 0.25 | 2 | 0.5 | 2 | 0.25 | None |
| LIF 3519 | +427 | Prostate | 0.015 | 0.12 | 2 | 0.5 | 1 | 0.25 | None |
| LIF 3495 | +456 | Prostate | 0.015 | 0.25 | 1 | 0.5 | 1 | 0.25 | None |
| LIF 3545 | +489 | Prostate | 0.015 | 0.12 | 1 | 1 | 4 | 0.25 | None |
| LIF 3546 | +489 | Renal capsule | 0.015 | 0.12 | 2 | 2 | >8 | 0.5 | G448S |
| LIF 3608 | +740 | Pleural fluid | 0.015 | 0.25 | 2 | 2 | >8 | 1 | G448S |
MICs were determined using CLSI method M38-A2. MCFG, micafungin; CPFG, caspofungin; AMPH-B, amphotericin B; ITC, itraconazole; VRC, voriconazole; POS, posaconazole.
The G448S is considered a clinical mutation acquired after a long time of VRC exposure [17,18]. This substitution has already been reported in clinical cases of A. fumigatus infection [19,20]. G448 is conserved in all P450 cytochromes encoded by ERG11/CYP51 of yeasts and filamentous fungi. In Candida albicans and Cryptococcus neoformans, this substitution is related to Fluconazole resistance [21,22].
To our knowledge, this is the first case report of an A. fumigatus with G448S substitution causing IA in an immunocompromised patient in our institution. It is possible to confirm that the resistance to VRC observed in vitro led to therapeutic failure. This case report suggests that in all long-term treatments with antifungals, especially azoles, it would be important to regularly evaluate the susceptibility of isolated strains and to perform TDM of antifungal levels monitoring, although this practice is not common, and is also not available in our institution. The data presented here confirms the role of this mutation in resistance to VRC, since genotyping results of all isolates have demonstrated to be equal from the first to the last isolate. A. fumigatus azole resistance surveillance has become essential within the clinical laboratory, as resistance to azoles is increasingly detected around the world.
Ethical form
The present study was approved by the local Ethics Committee (CAAE51794615.0.0000.54.
Medical Mycology Case Reports requires full disclosure of all sources of funding and potential conflicts of interest. The journal also requires a declaration that the author(s) have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
If you have nothing to declare in any of these categories then this should be stated. Funding Source.
All sources of funding should be acknowledged and you should declare any extra funding you have received for academic research of this work. If there are none state ‘there are none’.
Please state any competing interests
None.
Consent
Please declare that you have obtained written and signed consent to publish the case report from the patient or legal guardian(s).
Please state that consent has been obtained from the patient or legal guardian(s)
Written informed consent was obtained from the patient or legal guardian(s) for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
As corresponding author, I hereby declare that I sign this document on behalf of all the authors of the above mentioned manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) Finance Code 001 (LP Scholarship); AMED (Japan Agency for Medical Research and Development, under Grant Number JP21jm0110015)/JICA (Japan International Cooperation Agency), SATREPS (Science and Technology Research Partnership for Sustainable Development), and University of Campinas, Brazil, International Cooperation Project 02-P-9427/2018; São Paulo Research Foundation (FAPESP) no. 2015/25035-7.
Declaration of competing interest
Please declare any financial or personal interests that might be potentially viewed to influence the work presented. Interests could include consultancies, honoraria, patent ownership or other. If there are none state ‘there are none’.
Acknowledgments
We thank E.M.P, R.F, and T.C.L. for helping with the clinical data provided.
References
- 1.Pagano L., Caira M., Candoni A., Offidani M., Fianchi L., Martino B., et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 2.Walsh T.J., Anaissie E.J., Denning D.W., Herbrecht R., Kontoyiannis D.P., Marr K.A., et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 3.Patterson T.F., Thompson G.R., 3rd, Denning D.W., Fishman J.A., Hadley S., Herbrecht R., et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious Diseases Society of America. Clin. Infect. Dis. 2016;63(4):e1–e60. doi: 10.1093/cid/ciw326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balajee S.A., Kano R., Baddley J.W., Moser S.A., Marr K.A., Alexander B.D., et al. Molecular identification of Aspergillus species collected for the transplant-associated infection surveillance network. J. Clin. Microbiol. 2009;47(10):3138–3141. doi: 10.1128/JCM.01070-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI . 2008. M38-A2 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi Approved Standard—Second Edition; p. 13. [Google Scholar]
- 6.Pontes L., Beraquet C.A.G., Arai T., Pigolli G.L., Lyra L., Watanabe A., et al. Aspergillus fumigatus clinical isolates carrying CYP51A with TR34/L98H/S297T/F495I substitutions detected after four-year retrospective azole resistance screening in Brazil. Antimicrob. Agents Chemother. 2020;64(3):e02059–19. doi: 10.1128/AAC.02059-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Li Z., Han X., Tian S., Zhao J., Chen F., et al. Elevated MIC values of imidazole drugs against Aspergillus fumigatus isolates with TR34/L98H/S297T/F495I mutation. Antimicrob. Agents Chemother. 2018;62(5):e01549–19. doi: 10.1128/AAC.01549-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas P.G., Alexander B.D., Andes D.R., Hadley S., Kauffman C.A., Freifeld A., et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin. Infect. Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 9.Flores-Umanzor E., Ivey-Miranda J.B., Pujol-Lopez M., Cepas-Guillen P., Fernandez-Valledor A., Caldentey G., et al. Invasive pulmonary aspergillosis in heart transplant recipients: is mortality decreasing? Rev. Port. Cardiol. 2019;38(7):497–501. doi: 10.1016/j.repc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Montoya J.G., Chaparro S.V., Celis D., Cortés J.A., Leung A.N., Robbins R.C., et al. Invasive aspergillosis in the setting of cardiac transplantation. Clin. Infect. Dis. 2003;37(Suppl 3):S281–S292. doi: 10.1086/376527. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz P., Vena A., Cerón I., Valerio M., Palomo J., Guinea J., et al. Invasive pulmonary aspergillosis in heart transplant recipients: two radiologic patterns with a different prognosis. J. Heart Lung Transplant. 2014;33(10):1034–1040. doi: 10.1016/j.healun.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 12.van der Linden J.W., Camps S.M., Kampinga G.A., Arends J.P., Debets-Ossenkopp Y.J., Haas P.J., et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 2013;57(4):513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 13.Howard S.J., Cerar D., Anderson M.J., Albarrag A., Fisher M.C., Pasqualotto A.C., et al. Frequency and evolution of Azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 2009;15(7):1068–1076. doi: 10.3201/eid1507.090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snelders E., van der Lee H.A., Kuijpers J., Rijs A.J., Varga J., Samson R.A., et al. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008;5(11):e219. doi: 10.1371/journal.pmed.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valerio M., Fernandez-Cruz A., Fernández-Yañez J., Palomo J., Guinea J., Durán R., et al. Prostatic aspergillosis in a heart transplant recipient: case report and review. J. Heart Lung Transplant. 2009;28(6):638–646. doi: 10.1016/j.healun.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Khawand N., Jones G., Edson M. Aspergillosis of prostate. Urology. 1989;34(2):100–101. doi: 10.1016/0090-4295(89)90173-8. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan Natesan S., Wu W., Cutright J.L., Chandrasekar P.H. In vitro-in vivo correlation of voriconazole resistance due to G448S mutation (cyp51A gene) in Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 2012;74(3):272–277. doi: 10.1016/j.diagmicrobio.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Wiederhold N.P., Verweij P.E. Aspergillus fumigatus and pan-azole resistance: who should be concerned? Curr. Opin. Infect. Dis. 2020;33(4):290–297. doi: 10.1097/QCO.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 19.Bellete B., Raberin H., Morel J., Flori P., Hafid J., Manhsung R.T. Acquired resistance to voriconazole and itraconazole in a patient with pulmonary aspergilloma. Med. Mycol. 2010;48(1):197–200. doi: 10.3109/13693780902717018. [DOI] [PubMed] [Google Scholar]
- 20.Pelaez T., Gijón P., Bunsow E., Bouza E., Sánchez-Yebra W., Valerio M., et al. Resistance to voriconazole due to a G448S substitution in Aspergillus fumigatus in a patient with cerebral aspergillosis. J. Clin. Microbiol. 2012;50(7):2531–2534. doi: 10.1128/JCM.00329-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanglard D., Ischer F., Koymans L., Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 1998;42(2):241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodero L., Mellado E., Rodriguez A.C., Salve A., Guelfand L., Cahn P., et al. G484S amino acid substitution in lanosterol 14-alpha demethylase (ERG11) is related to fluconazole resistance in a recurrent Cryptococcus neoformans clinical isolate. Antimicrob. Agents Chemother. 2003;47(11):3653–3656. doi: 10.1128/AAC.47.11.3653-3656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]