Highlights
-
•
PCI for LS-SCLC patients has become more controversial.
-
•
Literature search on PCI impact on overall survival in LS-SCLC yielded 28 studies.
-
•
Meta-analysis of adjusted HRs revealed pooled HR of 0.62 (95% CI: 0.57–0.69).
-
•
Findings support PCI in current practice while awaiting prospective trial results.
Abstract
Background
Prophylactic cranial irradiation (PCI) for limited-stage small-cell lung cancer (LS-SCLC) patients has become more controversial. Since the publication of the systematic review by Aupérin et al. in 1999, no randomized controlled trials regarding PCI in LS-SCLC have been completed. The aim of this study was to systematically review and meta-analyze the effect of PCI on overall survival (OS) in patients with LS-SCLC.
Methods
A systematic search was conducted in the databases of MEDLINE (PubMed), Embase and the Cochrane library. Only studies that reported an adjusted hazard ratio (aHR), indicating the effect of PCI versus no PCI on OS (adjusted for confounders) in patients with LS-SCLC were included for critical appraisal and meta-analysis. A pooled aHR estimate was calculated using a random-effects model.
Results
Pooling of 28 retrospective studies including a total of 18,575 patients demonstrated a significant beneficial effect of PCI versus no PCI on OS with a pooled aHR of 0.62 (95% CI: 0.57–0.69). Substantial heterogeneity of reported aHRs among studies was observed (I2 = 65.9%). Subgroup analyses revealed that this heterogeneity could partly be explained by study sample size. The pooled aHR among 7 versus 21 studies with a sample size of > 300 versus ≤ 300 patients was 0.79 (95% CI: 0.64–0.97) versus 0.56 (95% CI: 0.46–0.69; p < 0.001), respectively.
Conclusions
This meta-analysis demonstrates a significant beneficial effect of PCI on OS in patients with LS-SCLC. Larger studies reported a milder beneficial effect, possibly due to a decreased risk of model overfitting. Serious risk of selection and confounding bias were of concern due to the lack of prospective trials. These results support the role of PCI in standard clinical practice in patients with LS-SCLC while awaiting results of prospective trials on alternative strategies.
Introduction
Lung cancer is the second most frequent cancer worldwide and the most common cause of cancer-related death [1]. Small cell lung cancer (SCLC) represents about 15% of all lung cancer cases [2]. At diagnosis, 37% of patients is classified as having limited-stage SCLC (LS-SCLC) with no distant metastases (M0) according to the TNM-staging system (8th edition) [3], [4]. Patients with very limited LS-SCLC can be treated with surgery (followed by adjuvant chemotherapy), but the majority of LS-SCLC is treated by concurrent chemoradiotherapy (CRT). If no progression of disease is observed after completion of local and systemic therapy, prophylactic cranial irradiation (PCI) is recommended for the prevention of clinical or radiological manifestation of brain metastases [5].
The meta-analysis based on individual patient data of 7 prospective studies (published between 1983 and 1998) conducted by Aupérin et al. still represents the major foundation of international guidelines recommending PCI in LS-SCLC [6]. This meta-analysis demonstrated a beneficial effect of PCI on overall survival (OS) in patients with LS-SCLC who had a complete response on a chest X-ray after chemotherapy with or without thoracic radiotherapy. However, limitations of these data in the light of contemporary practice include the use of outdated imaging (e.g. poor resolution CT, unavailability of PET-CT, no or poor brain imaging), patient selection criteria, chemotherapy, supportive care, and radiotherapy techniques in the included studies [6], [7], [8], [9], [10], [11].
PCI for LS-SCLC patients has become more controversial for several reasons, including the lack of new randomized studies since the review of Aupérin et al. [6], the increased quality and availability of brain imaging in contemporary practice, and the increasing knowledge and awareness of neurocognitive side effects of radiotherapy to the brain [12]. After 1999, several non-randomized retrospective studies have reported improved OS after PCI in LS-SCLC [13], [14], [15], but this could not be confirmed by other studies [16], [17], [18]. In addition, a recent randomized study in extensive-stage SCLC (ES-SCLC) without brain metastases suggested equivalence in OS after brain MRI surveillance instead of PCI [19]. In order to overcome current controversies and shortcoming of individual studies, the aim of this study was to perform a systematic review and meta-analysis based on published data of the effect of PCI on OS in patients with LS-SCLC.
Materials and methods
The study protocol was registered in the PROSPERO international database (CRD42021224656, available at http://www.crd.york.ac.uk/prospero). Reporting was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20].
Search strategy
A systematic search was conducted in the databases of MEDLINE (PubMed), Embase and the Cochrane library. The search was last updated November 6, 2021. To identify all studies reporting on the use of PCI in patients with LS-SCLC the terms ‘LS-SCLC’, ‘prophylactic cranial irradiation’ and ‘survival’ in combination were searched, with synonyms and related MeSH terms (Supplementary Table 1).
Study selection
After deduplication conducted with Mendeley, titles and abstracts were independently screened for eligibility by 3 authors using Rayyan QCRI. Only studies in English, Dutch and German language were included. Studies published before the key systematic review and meta-analysis of Aupérin et al. in 1999 were excluded [6]. Any disagreements during the study selection process were solved by reaching consensus. Studies using different types of databases were eligible for inclusion (e.g. SEER database, single-center or multi-center databases). Only studies reporting an adjusted hazard ratio (aHR) with 95% confidence interval (CI), indicating the effect of PCI on OS (adjusted for confounders) in patients with LS-SCLC were included for critical appraisal and meta-analysis. The aHR was chosen as primary outcome measure because this represents the least biased within-study estimate of the survival impact of PCI (in contrast to unadjusted HR or crude survival point estimates). Through application of further inclusion criteria (SCLC, PCI) and exclusion criteria (no treatment with chemotherapy, no comparative group of no-PCI, ES-SCLC only, reviews, case-reports or conference abstracts, no full-text available, overlapping publication with the same cohort, or median follow-up < 1 year), the eligibility of the studies was determined by 3 authors independently.
Data extraction and risk of bias assessment
Data from individual studies was extracted to create an overview of study characteristics (i.e. year of publication, country, study design, primary study determinant, number of patients, age, treatment for primary tumor therapy, PCI dose, use of brain MRI at baseline, and follow-up). By means of the Risk of Bias in Non-randomized Studies or Interventions (ROBINS-I) tool, a risk of bias assessment of the methodological quality was conducted [21]. For each study, 7 domains of bias (i.e. confounding, selection, classification of intervention, deviation from intended intervention, missing data, measurement of outcome, selection of reported results) were graded as having a low, moderate or severe risk of bias. Bias due to confounding was considered as serious if the HRs were adjusted for < 5 parameters. Selection bias was scored as serious when studies unevenly divided partial/complete responders after induction therapy in the PCI group and non-responders in the no-PCI group or if the response to induction therapy was not reported. Two authors performed the risk of bias assessment independently, whereafter consensus was reached.
Statistical analysis
A meta-analysis of the available aHRs indicating the independent association between PCI and OS was conducted using a random-effects model, resulting in a pooled aHR estimate. For determination of heterogeneity among reported aHRs the I2 statistic was calculated. An I2 between 50 and 90% was considered as substantial heterogeneity in accordance with the Cochrane Handbook for Systematic Reviews [22].
Subgroup analyses were performed with study-level covariates using meta-regression random-effects models to study the relation of specific patient-, tumor-, treatment-, and study-related characteristics with the prognostic value of PCI on OS. Cut-off values for subgroups were determined so that each subgroup had a sufficient number of studies. A stratified pooled aHR for each subgroup was calculated. The R2 statistic was calculated for each subgroup analysis in order to quantify the amount of overall heterogeneity explained by the subgroup differentiation. Additional meta-regression analysis was performed to study potential differences in reported aHRs between studies that performed HR adjustment (versus studies that did not) for age, gender, performance status, tumor size or T-stage, and response to chemotherapy. Analyses were performed using R 4.0.3 software (The R Foundation for Statistical Computing, Vienna, Austria; ‘metafor’ package) and a p-value of < 0.05 was considered statistically significant.
Results
Identification of studies
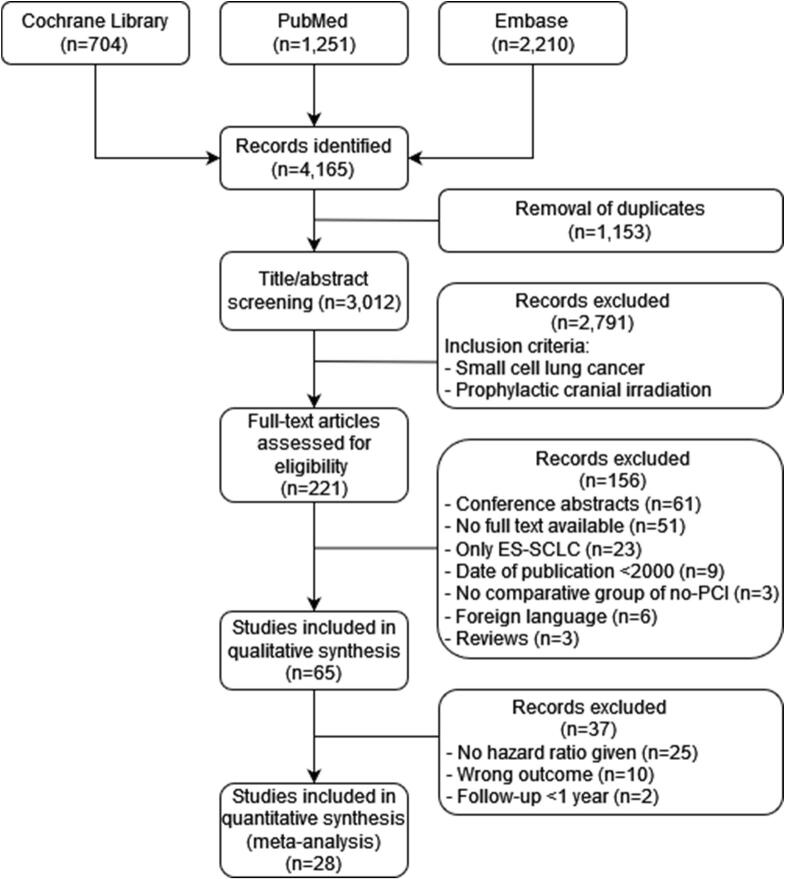
A total of 4,165 studies were identified after the systematic search, of which 221 met the inclusion criteria (SCLC, PCI) and these were included for full-text screening. After application of the predefined exclusion criteria, 28 studies including a total of 18,575 patients remained eligible for critical appraisal and meta-analysis (Fig. 1).
Fig. 1.
Flowchart summarizing search results and study selection.
Study characteristics
The extracted study characteristics from the 28 included studies are presented in Table 1. Of the 18,575 patients, 3,633 (20%) underwent PCI and 14,942 (80%) were not treated with PCI. All studies were retrospective by design and most were recent with 16 studies (57%) published in or after 2018. Seven studies (25%) had a sample size of > 300 study participants. Mean age of included patients was ≤ 65 years in 15 studies (54%). The majority (64%) of studies originated from Western countries. Twelve (43%) of the studies reported standard acquisition of a brain MRI before considering PCI. The EQD2 (biologically equivalent dose in 2-Gy equivalents) of PCI treatment was > 26 Gy in 6 (21%) of all studies, with the most commonly reported dose regimen being 25 Gy in 10 fractions (46%).
Table 1.
Study characteristics of studies comparing PCI to no-PCI in patients with limited-stage small-cell lung cancer.
| Study,year | Country | Study design | Primary study determinant | PCI (n) | No-PCI (n) | Age (mean) | Primary tumor therapy | PCI dose EQD2 (Gy) | Baseline brain MRI | Median follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ng, 2007 [31] | Australia | Retro | Both | 46 | 44 | 65 | CRT | 36 | No | 50 |
| Patel, 2009 [13] | USA | Retro* | PCI | 670 | 7,325 | 67 | NR | NR | NR | 13 |
| Giuliani, 2010 [24] | Canada | Retro | PCI | 127 | 80 | 65.7 | CRT | 26 | NR | 19 |
| Bettington, 2013 [32] | Australia | Retro | TRT | 37 | 42 | 63.8 | CRT | 26 or 30 or 36 | NR | NR |
| Eaton, 2013 [15] | USA | Retro* | PCI | 138 | 1,788 | 74.5 | CRT | NR | NR | >100 |
| Zhu, 2014 [33] | China | Retro | PCI | 67 | 126 | 56 | Surgery | 26 | Yes | NR |
| Xu, 2016 [34] | China | Retro | PCI | 114 | 234 | 60 | Surgery | NR | NR | NR |
| Yang, 2016 [35] | USA | Retro | PCI | 104 | 850 | 66.8 | Surgery | NR | NR | 43 |
| Eze, 2017 [36] | Germany | Retro | PCI | 71 | 113 | 63 | CRT | 30 | Yes | NR |
| Farooqi, 2017 [14] | USA | Retro | PCI | 364 | 294 | 62 | CRT | 26 | Yes | 21 |
| Wu, 2017 [37] | USA | Retro | Both | 116 | 167 | NR | Both | NR | NR | NR |
| Zhang, 2017 [38] | China | Retro | TRT | 94 | 76 | 58 | CRT | 26 | NR | 30 |
| Nakamura, 2018 [39] | Japan | Retro | PCI | 93 | 69 | 67.5 | CRT | 26 | NR | 38 |
| Sas-Korczynska, 2018 [40] | Poland | Retro | PCI | 167 | 104 | 60.5 | CRT | 30 | Yes | 33.2 |
| Yin, 2018 [41] | China | Retro | PCI | 88 | 52 | <60 | Both | 30 or 32.5 | Yes | NR |
| Chen, 2019 [42] | China | Retro | TRT | 69 | 69 | <60 | Both | 26 | NR | 66 |
| Kim, 2019 [43] | South-Korea | Retro | PCI | 139 | 95 | 61 | CRT | 26 | Yes | 22 |
| Kou, 2019 [44] | USA | Retro* | PCI | 394 | 2,178 | <65 | NR | NR | NR | NR |
| Resio, 2019 [45] | USA | Retro | PCI | 202 | 657 | 66 | Surgery | NR | NR | NR |
| Elegbede, 2020 [46] | Canada | Retro | Both | 60 | 60 | 66 | CRT | NR | NR | NR |
| Jeong, 2020 [47] | South-Korea | Retro | TRT | 45 | 56 | 64 | CRT | 26 | Yes | 27 |
| Lou, 2020 [48] | China | Retro | PCI | 46 | 100 | 63 | Surgery | NR | Yes | 28 |
| Pezzi, 2020 [16] | USA | Retro | PCI | 84 | 84 | 66 | CRT | 26 or 30 | Yes | 84 |
| Ghanta, 2021 [49] | USA | Retro | PCI | 63 | 50 | 66 | CRT | 26 | Yes | 21.3 |
| Li, 2021 [50] | China | Retro | PCI | 70 | 43 | <70 | CRT | 26 | Yes | 17.8 |
| Held, 2021 [51] | Denmark | Retro | PCI | 52 | 27 | 63.8 | CRT | 26 | Yes | 23 |
| Yan, 2021 [52] | Canada | Retro | PCI | 70 | 38 | 65.6 | CRT | 26 | No | 22.3 |
| Zhou, 2021 [53] | USA | Retro | PCI | 43 | 121 | 68 | Surgery | 26 | No | NR |
| CRT: chemoradiotherapy. NR: not reported. PCI: prophylactic cranial irradiation. Retro: retrospective. TRT: thoracic radiotherapy. USA: United States of America. *: SEER database studies. | ||||||||||
PCI was the primary study determinant in 21 studies (75%). In the remaining 7 studies (25%), the association of PCI with OS was reported as secondary outcome. In 17 studies (61%) only patients who underwent CRT for the primary tumor were included, whereas 6 other studies (21%) included surgical patients only, 3 studies (11%) included both surgical and non-surgical patients and 2 studies (7%) lacked reporting on the primary tumor treatment. Among CRT studies, 15 (54% of total) included only patients with complete or partial response to chemotherapy. The median follow-up was > 30 months in 7 studies (25%).
Quality assessment
An overall moderate to serious risk of bias was observed in the included studies (Table 3). Serious risk of confounding (n = 10, 36%) and selection bias (n = 21, 75%) were observed as a result of the prescription of PCI in patients with partial/complete response to chemotherapy only while including patients with no response to chemotherapy or disease progression in the no-PCI group. Deviation from intended interventions bias was observed in 7 studies (25%) due to poor WHO performance status, patient choice or unknown reasons. None of the included studies reported about missing data. No concerns regarding measurement of outcome bias were found, due to the solid OS outcome.
Table 3.
ROBINS-I risk of bias assessment.
 |
Meta-analysis
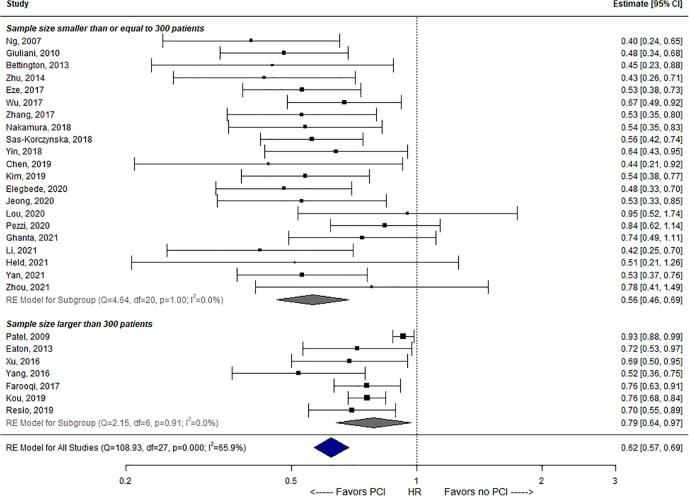
Data on median and 2-year OS estimates are presented in Table 2. Weighted for study sample size, the mean estimate across studies for crude (univariable) median OS was 27.8 versus 18.8 months for PCI versus no-PCI groups. The weighted crude 2-year OS was mean 50.9% versus 26.9% after PCI versus no-PCI. Adjusted HRs of PCI versus no-PCI in LS-SCLC among the 28 studies are presented in Fig. 2. Twenty-three (82%) of 28 studies observed a statistically significant aHR (i.e. 95% CI upper limit < 1) in favor of PCI as opposed to no PCI. Five (18%) of 28 studies observed no significant association between PCI and OS, and no study observed an adverse association between PCI and OS. The pooled aHR across all 28 studies was 0.62 (95% CI: 0.57–0.69). Substantial statistical heterogeneity in aHR estimates among the 28 cohorts was observed (I2 = 65.9%).
Table 2.
Overall survival (OS) outcomes and adjusted hazard ratios of included studies.
| Study, year | Median OS PCI (m) | Median OS No-PCI (m) | 2-year OS PCI | 2-year OS No-PCI | Adjusted hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Ng, 2007 [31] | 21 | 14 | 38% | 18% | 0.40 (0.24–0.64) |
| Patel, 2009 [13] | 24* | 20* | 42% | 23% | 0.93 (0.88–0.99) |
| Giuliani, 2010 [24] | 23* | 10* | 20%* | 48%* | 0.48 (0.33–0.67) |
| Bettington, 2013 [32] | NR | NR | NR | NR | 0.45 (0.23–0.88) |
| Eaton, 2013 [15] | 20* | 16* | 33% | 12% | 0.72 (0.53–0.97) |
| Zhu, 2014 [33] | 48* | NRE | 93% | 63% | 0.43 (0.26–0.71) |
| Xu, 2016 [34] | 36 | 26 | 70% | 52% | 0.69 (0.50–0.95) |
| Yang, 2016 [35] | NR | NR | NR | NR | 0.52 (0.36–0.75) |
| Eze, 2017 [36] | 26 | 14 | 50%* | 10%* | 0.53 (0.38–0.73) |
| Farooqi, 2017 [14] | 28* | 22* | 63%* | 47%* | 0.76 (0.63–0.91) |
| Wu, 2017 [37] | NR | NR | NR | NR | 0.67 (0.49–0.92) |
| Zhang, 2017 [38] | 32 | 23 | 70% | 46% | 0.53 (0.35–0.80) |
| Nakamura, 2018 [39] | 32* | 18* | 36% | 16% | 0.54 (0.36–0.82) |
| Sas-Korczynska, 2018 [40] | 26 | 15 | 52% | 30% | 0.56 (0.42–0.74) |
| Yin, 2018 [41] | NR | NR | 40% | 25% | 0.64 (0.43–0.95) |
| Chen, 2019 [42] | NR | NR | NR | NR | 0.44 (0.22–0.97) |
| Kim, 2019 [43] | 31* | 16* | 59% | 36% | 0.54 (0.38–0.77) |
| Kou, 2019 [44] | 20* | 14* | 40%* | 23%* | 0.76 (0.69–0.85) |
| Resio, 2019 [45] | NRE | 60 | 60% | 82% | 0.70 (0.55–0.89) |
| Elegbede, 2020 [46] | NR | NR | NR | NR | 0.48 (0.33–0.70) |
| Jeong, 2020 [47] | NR | NR | NR | NR | 0.53 (0.33–0.84) |
| Lou, 2020 [48] | 46 | 49 | 74% | 78% | 0.95 (0.52–1.75) |
| Pezzi, 2020 [16] | 27 | 25 | 60% | 58% | 0.84 (0.60–1.11) |
| Ghanta, 2021 [49] | 36* | 24* | 63%* | 50%* | 0.74 (0.49–1.11) |
| Li, 2021 [50] | 36* | 20* | 70%* | 43%* | 0.42 (0.25–0.70) |
| Held, 2021 [51] | 55 | 24 | 52%* | 27%* | 0.51 (0.21–1.28) |
| Yan, 2021 [52] | 36* | 23* | 70%* | 38%* | 0,53 (0.37–0.76) |
| Zhou, 2021 [53] | 76 | 36 | NR | NR | 0.78 (0.41–1.49) |
| Unweighted median | 31.5 | 21.0 | 59.0% | 38.0% | – |
| Weighted mean | 27.8 | 18.8 | 50.9% | 26.9% | – |
| *: Extracted from Kaplan-Meier curve. m: months. NR: not reported. NRE: not reached. | |||||
Fig. 2.
Forest plot of the pooled analysis of 28 studies on the effect of PCI on overall survival in patients with LS-SCLC.
Subgroup analyses
Results from study-level subgroup analyses are presented in Table 4. A statistically significant difference in pooled aHR estimates was found for 21 studies with a sample size of ≤ 300 patients versus 7 studies with > 300 patients (i.e. pooled aHR 0.56 versus 0.79, respectively, p < 0.001; Fig. 2). This subgroup stratification accounted for 60.7% of the overall heterogeneity (R2). Between other subgroups of studies (i.e. based on publication year, mean age, brain MRI at baseline, total radiation dose, primary study determinant, treatment for primary tumor, response to chemotherapy, median follow-up), no statistically significant differences in pooled aHRs were identified.
Table 4.
Results from study-level subgroup analyses for prognostic value of PCI versus no PCI on overall survival.
| Factor | n† | Stratified HR (95% CI) | p value | I2 | R2 |
|---|---|---|---|---|---|
| Publication year: | 0.989 | 61.5% | 0.0% | ||
| Before 2018 | 12 | 0.66 (0.53–0.82) | |||
| In or after 2018 | 16 | 0.63 (0.52–0.78) | |||
| Sample size: | <0.001* | 41.0% | 60.7% | ||
| ≤300 patients | 21 | 0.56 (0.46–0.69) | |||
| >300 patients | 7 | 0.79 (0.64–0.97) | |||
| Mean age: | 0.432 | 61.5% | 0.0% | ||
| ≤65 years | 15 | 0.61 (0.50–0.76) | |||
| >65 years | 12 | 0.69 (0.55–0.86) | |||
| Country of origin: | 0.154 | 62.5% | 12.3% | ||
| Eastern | 10 | 0.56 (0.42–0.75) | |||
| Western | 18 | 0.69 (0.59–0.82) | |||
| Brain MRI at baseline: | 0.906 | 65.3% | 0.0% | ||
| No or not reported | 16 | 0.67 (0.56–0.81) | |||
| Yes | 12 | 0.62 (0.48–0.80) | |||
| Total radiation dose: | 56.2% | 26.5% | |||
| 26 Gy (EQD2α/β=10) | 13 | 0.57 (0.44–0.73) | Ref | ||
| >26 Gy (EQD2α/β=10) | 6 | 0.58 (0.41–0.82) | 0.801 | ||
| Not reported | 9 | 0.77 (0.62–0.94) | 0.020* | ||
| Primary study determinant: | 63.0% | 13.8% | |||
| PCI | 21 | 0.69 (0.59–0.81) | Ref | ||
| Thoracic radiotherapy | 4 | 0.50 (0.30–0.83) | 0.110 | ||
| Both | 3 | 0.52 (0.32–0.86) | 0.165 | ||
| Treatment for primary tumor: | 49.9% | 38.9% | |||
| Chemoradiotherapy | 17 | 0.58 (0.47–0.71) | Ref | ||
| Surgery | 6 | 0.65 (0.45–0.93) | 0.364 | ||
| Both or not reported | 5 | 0.80 (0.63–1.02) | 0.008* | ||
| Response to chemotherapy: | 0.151 | 60.9% | 10.9% | ||
| Only complete or partial | 15 | 0.58 (0.47–0.73) | |||
| Not reported | 13 | 0.72 (0.60–0.88) | |||
| Median follow-up: | 60.3% | 0.0% | |||
| ≤30 months | 11 | 0.69 (0.54–0.87) | Ref | ||
| >30 months | 7 | 0.59 (0.42–0.81) | 0.522 | ||
| Not reported | 10 | 0.64 (0.50–0.82) | 0.818 |
HR: hazard ratio. USA: United States of America. p value significance of difference between stratified HR as compared to reference (Ref) subgroup. I2: residual heterogeneity/unaccounted variability in the meta-regression model. R2: amount of heterogeneity accounted for by including the factor in the meta-regression model. 95% CI: 95% confidence interval. †: number of studies.
Results from study-level subgroup analyses with respect to HR adjustments are presented in Supplementary Table 2. Reported aHRs among 15 studies that adjusted for tumor size or T-stage were significantly higher in comparison with the aHRs among 12 studies that lacked adjustment for tumor size or T-stage (pooled aHR 0.74 versus 0.54, respectively, p = 0.002). In the other studied subgroups based on the type of HR adjustment no significant difference between the pooled aHRs was observed.
Discussion
In many countries including the USA and The Netherlands a significant declining trend of PCI administration over the past decade has been reported not only in ES-SCLC, but also in LS-SCLC patients [12], [23]. Level 1b randomized clinical trial data has likely been an explanation for the decreased use of PCI in ES-SCLC with MRI surveillance as alternative [19]. Over the past 25 years, no such prospective data was published on the impact of PCI in LS-SCLC. The current meta-analysis based on 28 retrospective studies demonstrated a pooled adjusted HR of PCI versus no PCI for OS of 0.62 (95% CI: 0.57–0.69). Importantly, none of these available studies were randomized or prospective by design. However, since even studies in more recent years support this apparent beneficial effect of PCI on OS for patients with LS-SCLC PCI remains an important standard treatment modality when the aim is to prolong survival.
Subgroup analysis demonstrated that aHR estimates for PCI have not significantly changed in more recent years (i.e. since 2018 compared to before 2018). This could be related to the fact that the techniques to plan and deliver PCI has not substantially changed over the last decades. Rather, two explanations for the observed heterogeneity in HR estimates among studies were revealed statistically. First, when stratifying studies with a sample size > 300 patients versus ≤ 300 patients, treatment with PCI in studies with > 300 patients appeared somewhat less (but still significantly) associated with favorable OS in LS-SCLC when compared to studies with ≤ 300 patients (pooled aHR 0.79 versus 0.56, p < 0.001). A possible explanation could be that smaller sample sizes more likely resulted in overoptimism of the effect of PCI due to a higher chance of model-overfitting (i.e. adding too many variables in the multivariable model) compared to studies with a larger sample size.
The apparent survival advantage of PCI in LS-SCLC is thought to arise from preventing or delaying manifestation of brain metastases, as supported by reported 3-year incidence rates of brain metastasis decreasing from 53% to 23% [24]. This advantageous effect of PCI must be weighed against its disadvantages. The key EORTC trial conducted by Slotman et al. demonstrated a negative acute effect on health-related quality of life in the first 3 months after PCI, mainly due to fatigue and hair loss [25]. In addition, among others the phase II RTOG 0212 trial that compared different total doses of PCI demonstrated that PCI is associated with late adverse events such as chronic neurotoxicity (60% after 12 months) and neurologic deterioration (62% after 12 months) [26]. However, that trial had no comparative group of patients with no-PCI.
Alternative approaches to conventional PCI have been proposed. First, in a randomized phase III trial in ES-SCLC MRI surveillance instead of PCI (with MRI surveillance as well) has been shown to result in comparable overall survival with a potential increased sparing of neurocognitive functioning [19]. Importantly, in that trial 83% of patients in the MRI surveillance group still required radiotherapy to the brain due to detection of brain metastases during follow-up [19]. However, no such trial has been completed in LS-SCLC. Second, PCI with hippocampal avoidance (HA-PCI) is a new treatment option to reduce neurocognitive side effects. A Dutch multicenter randomized phase III trial NCT01780675 (including 168 patients) did not reveal a lower probability of cognitive decline (measured by total recall on the revised Hopkins Verbal Learning Test) in patients with SCLC treated with HA-PCI versus conventional PCI [27]. However, the randomized phase III PREMER trial (including 150 SCLC patients) did demonstrate a beneficial effect on cognitive function for HA-PCI using the delayed free recall, free and cued selective reminding, and total recall [28]. Therefore, the role of HA-PCI has not been sufficiently demonstrated and results of ongoing trials like the phase III NRG CC003 trial evaluating HA-PCI are to be awaited.
The results of this meta-analysis were merely based on retrospective comparative data, which stresses the importance of prospective randomized trials. The ongoing phase III MAVERICK trial that started in 2020 investigates the effect of MRI surveillance alone versus MRI surveillance with PCI on OS in both ES-SCLC and LS-SCLC patients. This trial aims to include 668 participants and besides OS as primary objective, also brain metastasis-free survival, cognitive failure-free survival and toxicities will be investigated [29]. In addition, EORTC recently initiated the phase III PRIMALung trial, in which 600 patients with either ES-SCLC and LS-SCLC will be randomized to MRI surveillance versus MRI surveillance plus PCI [30]. The primary endpoint is OS and secondary endpoints include cognitive failure-free survival, quality of life, and safety profiling of PCI.
This systematic review and meta-analysis has several limitations inherent to drawbacks of the included studies. Firstly, all included studies had a retrospective design causing confounding and selection bias. In calculating aHRs in multivariable survival models, studies attempted to minimize confounding bias but several studies only adjusted for a small number of confounders. Secondly, a large variety in patient selection across the studies was observed in terms of what response to chemotherapy was allowed (not reported/complete/partial/no response). Imbalances mostly due to a larger number of patients with no response to chemotherapy in the no-PCI group, may have partly biased the HR estimates falsely disfavoring no-PCI. Thirdly, publication bias could be present in literature, for example because investigators (and reviewers) would not expect outcomes of PCI to be different from the meta-analysis conducted by Aupérin et al [6]. However, this meta-analysis was strengthened by the large amount of studies (n = 28), patient numbers (n = 18,575) and small residual (unexplained) heterogeneity after meta-regression analyses.
In conclusion, this meta-analysis of 28 studies demonstrated that patients with LS-SCLC who underwent PCI had a 38% decreased risk of death over time compared to patients who did not receive PCI. These results support the effective role of PCI in standard clinical practice in patients with LS-SCLC with no progression after systemic treatment, and underline the need for (ongoing) prospective trials before considering alternative strategies.
Funding statement
No external funding was involved in this study.
Data availability statement
All data generated and analyzed during this study are included in this published article (and its Supplementary information files).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.02.002.
Contributor Information
Joost J.C. Verhoeff, Email: J.J.C.Verhoeff-10@umcutrecht.nl.
Peter S.N. van Rossum, Email: P.S.N.vanRossum-2@umcutrecht.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Houston K.A., Mitchell K.A., King J., White A., Ryan B.M. Histologic Lung Cancer Incidence Rates and Trends Vary by Race/Ethnicity and Residential County. J Thorac Oncol. 2018;13(4):497–509. doi: 10.1016/j.jtho.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N., Forjaz G., Mooradian M.J., Meza R., Kong C.Y., Cronin K.A., et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020;383(7):640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstraw P., Chansky K., Crowley J., et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Dingemans A.-M.-C., Früh M., Ardizzoni A., Besse B., Faivre-Finn C., Hendriks L.E., et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32(7):839–853. doi: 10.1016/j.annonc.2021.03.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aupérin A., Arriagada R., Pignon J.-P., Le Péchoux C., Gregor A., Stephens R.J., et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. N Engl J Med. 1999;341(7):476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 7.Arriagada R., Le Chevalier T., Borie F., Riviere A., Chomy P., Monnet I., et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–190. doi: 10.1093/jnci/87.3.183. [DOI] [PubMed] [Google Scholar]
- 8.Ohonoshi T., Ueoka H., Kawahara S., Kiura K., Kamei H., Hiraki Y., et al. Comparative study of prophylactic cranial irradiation in patients with small cell lung cancer achieving a complete response: a long-term follow-up result. Lung Cancer. 1993;10(1-2):47–54. doi: 10.1016/0169-5002(93)90308-k. [DOI] [PubMed] [Google Scholar]
- 9.Gregor A. Prophylactic cranial irradiation in small-cell lung cancer: is it ever indicated? Oncology (Williston Park). 1998;12(1 Suppl 2):19-24. doi:176779 [pii]. [PubMed]
- 10.Aroney R.S., Aisner J., Wesley M.N., et al. Value of prophylactic cranial irradiation given at complete remission in small cell lung carcinoma. Cancer Treat Rep. 1983;67(7):675–682. [PubMed] [Google Scholar]
- 11.Laplanche A., Monnet I., Santos-Miranda J.A., Bardet E., Le Péchoux C., Tarayre M., et al. Controlled clinical trial of prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Lung Cancer (Amsterdam, Netherlands). 1998;21(3):193–201. doi: 10.1016/s0169-5002(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 12.Tomassen M.L., Aarts M.J., Peters M., van Lindert A., De Ruysscher D.K.M., Verhoeff J.J.C., et al. Prophylactic cranial irradiation in patients with small cell lung cancer in The Netherlands: A population-based study. Clin Transl Radiat Oncol. 2021;27:157–163. doi: 10.1016/j.ctro.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S., Macdonald O.K., Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer. 2009;115(4):842–850. doi: 10.1002/cncr.24105. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi A.S., Holliday E.B., Allen P.K., Wei X., Cox J.D., Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: Do all patients benefit? Radiother Oncol. 2017;122(2):307–312. doi: 10.1016/j.radonc.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton B.R., Kim S., Marcus D.M., Prabhu R., Chen Z., Ramalingam S.S., et al. Effect of prophylactic cranial irradiation on survival in elderly patients with limited-stage small cell lung cancer. Cancer. 2013;119(21):3753–3760. doi: 10.1002/cncr.28267. [DOI] [PubMed] [Google Scholar]
- 16.Pezzi T.A., Fang P., Gjyshi O., Feng L., Liu S., Komaki R., et al. Rates of Overall Survival and Intracranial Control in the Magnetic Resonance Imaging Era for Patients With Limited-Stage Small Cell Lung Cancer With and Without Prophylactic Cranial Irradiation. JAMA Netw Open. 2020;3(4):e201929. doi: 10.1001/jamanetworkopen.2020.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamesaya N., Wakuda K., Omae K., Miyawaki E., Kotake M., Fujiwara T., et al. Efficacy of prophylactic cranial irradiation in patients with limited-disease small-cell lung cancer who were confirmed to have no brain metastasis via magnetic resonance imaging after initial chemoradiotherapy. Oncotarget. 2018;9(25):17664–17674. doi: 10.18632/oncotarget.24830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farris M.K., Wheless W.H., Hughes R.T., Soike M.H., Masters A.H., Helis C.A., et al. Limited-Stage Small Cell Lung Cancer: Is Prophylactic Cranial Irradiation Necessary? Pract Radiat Oncol. 2019;9(6):e599–e607. doi: 10.1016/j.prro.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T., Yamanaka T., Seto T., Harada H., Nokihara H., Saka H., et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–671. doi: 10.1016/S1470-2045(17)30230-9. [DOI] [PubMed] [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 22.Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
- 23.Gjyshi O., Ludmir E.B., Pezzi T.A., Boyce-Fappiano D., Dursteler A.E., Mitin T., et al. Evolving Practice Patterns in the Use of Prophylactic Cranial Irradiation for Extensive-Stage Small Cell Lung Cancer. JAMA Netw Open. 2019;2(8):e199135. doi: 10.1001/jamanetworkopen.2019.9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliani M., Sun A., Bezjak A., Ma C., Le L.W., Brade A., et al. Utilization of prophylactic cranial irradiation in patients with limited stage small cell lung carcinoma. Cancer. 2010;116(24):5694–5699. doi: 10.1002/cncr.25341. [DOI] [PubMed] [Google Scholar]
- 25.Slotman B.J., Mauer M.E., Bottomley A., Faivre-Finn C., Kramer G.W.P.M., Rankin E.M., et al. Prophylactic Cranial Irradiation in Extensive Disease Small-Cell Lung Cancer: Short-Term Health-Related Quality of Life and Patient Reported Symptoms—Results of an International Phase III Randomized Controlled Trial by the EORTC Radiation Oncology and Lung Cancer Groups. JCO. 2009;27(1):78–84. doi: 10.1200/JCO.2008.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolfson A.H., Bae K., Komaki R., Meyers C., Movsas B., Le Pechoux C., et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belderbos J.S.A., De Ruysscher D.K.M., De Jaeger K., Koppe F., Lambrecht M.L.F., Lievens Y.N., et al. Phase 3 Randomized Trial of Prophylactic Cranial Irradiation With or Without Hippocampus Avoidance in SCLC ( NCT01780675) J Thorac Oncol. 2021;16(5):840–849. doi: 10.1016/j.jtho.2020.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez de Dios N., Couñago F., Murcia-Mejía M., Rico-Oses M., Calvo-Crespo P., Samper P., et al. Randomized Phase III Trial of Prophylactic Cranial Irradiation With or Without Hippocampal Avoidance for Small-Cell Lung Cancer (PREMER): A GICOR-GOECP-SEOR Study. J Clin Oncol. 2021;39(28):3118–3127. doi: 10.1200/JCO.21.00639. [DOI] [PubMed] [Google Scholar]
- 29.Chad G Rusthoven. MRI Brain Surveillance Alone Versus MRI Surveillance and Prophylactic Cranial Irradiation (PCI): A Randomized Phase III Trial in Small-Cell Lung Cancer (MAVERICK). https://clinicaltrials.gov/ct2/show/NCT04155034. Updated 2021.
- 30.Faivre-Finn Corinne AL. PRophylactic Cerebral Irradiation or Active MAgnetic Resonance Imaging Surveillance in Small-cell Lung Cancer Patients (PRIMALung Study). https://clinicaltrials.gov/ct2/show/NCT04790253.
- 31.Ng M., Chong J., Milner A., MacManus M., Wheeler G., Wirth A., et al. Tolerability of Accelerated Chest Irradiation and Impact on Survival of Prophylactic Cranial Irradiation in Patients with Limited-stage Small Cell Lung Cancer: Review of a Single Institution's Experience. J Thoracic Oncol. 2007;2(6):506–513. doi: 10.1097/JTO.0b013e318060095b. [DOI] [PubMed] [Google Scholar]
- 32.Bettington C.S., Tripcony L., Bryant G., Hickey B., Pratt G., Fay M. A retrospective analysis of survival outcomes for two different radiotherapy fractionation schedules given in the same overall time for limited stage small cell lung cancer. J Med Imaging Radiat Oncol. 2013;57(1):105–112. doi: 10.1111/j.1754-9485.2012.02470.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhu H., Guo H., Shi F., Zhu K., Luo J., Liu X., et al. Prophylactic cranial irradiation improved the overall survival of patients with surgically resected small cell lung cancer, but not for stage I disease. Lung Cancer. 2014;86(3):334–338. doi: 10.1016/j.lungcan.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Xu J., Yang H., Fu X., Jin B.o., Lou Y., Zhang Y., et al. Prophylactic Cranial Irradiation for Patients with Surgically Resected Small Cell Lung Cancer. J Thoracic Oncol. 2017;12(2):347–353. doi: 10.1016/j.jtho.2016.09.133. [DOI] [PubMed] [Google Scholar]
- 35.Yang C.-F., Chan D.Y., Speicher P.J., Gulack B.C., Wang X., Hartwig M.G., et al. Role of Adjuvant Therapy in a Population-Based Cohort of Patients with Early-Stage Small-Cell Lung Cancer. JCO. 2016;34(10):1057–1064. doi: 10.1200/JCO.2015.63.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eze C., Roengvoraphoj O., Niyazi M., Hildebrandt G., Fietkau R., Belka C., et al. Treatment Response and Prophylactic Cranial Irradiation Are Prognostic Factors in a Real-life Limited-disease Small-cell Lung Cancer Patient Cohort Comprehensively Staged With Cranial Magnetic Resonance Imaging. Clin Lung Cancer. 2017;18(4):e243–e249. doi: 10.1016/j.cllc.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Wu A.J., Gillis A., Foster A., Woo K., Zhang Z., Gelblum D.Y., et al. Patterns of failure in limited-stage small cell lung cancer: Implications of TNM stage for prophylactic cranial irradiation. Radiother Oncol. 2017;125(1):130–135. doi: 10.1016/j.radonc.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J., Fan M., Liu D.i., Zhao K.-L., Wu K.-L., Zhao W.-X., et al. Hypo- or conventionally fractionated radiotherapy combined with chemotherapy in patients with limited stage small cell lung cancer. Radiat Oncol. 2017;12(1) doi: 10.1186/s13014-017-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura M., Onozawa M., Motegi A., Hojo H., Zenda S., Nakamura N., et al. Impact of prophylactic cranial irradiation on pattern of brain metastases as a first recurrence site for limited-disease small-cell lung cancer. J Radiat Res. 2018;59(6):767–773. doi: 10.1093/jrr/rry066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sas-Korczyńska B., Elzbieta L., Chudyba A., Skóra T., Sokolowski A. The retrospective evaluation of prophylactic cranial irradiation in patients treated for limited stage small-cell lung cancer -a single centre study. Nowotwory J Oncol. 2018;68(5–6):232–239. doi: 10.5603/NJO.2018.0037. [DOI] [Google Scholar]
- 41.Yin K., Song D., Zhang H., Cai F., Chen J., Dang J. Efficacy of surgery and prophylactic cranial irradiation in stage II and III small cell lung cancer. J Cancer. 2018;9(19):3500–3506. doi: 10.7150/jca.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M., Hu X., Bao Y., et al. Comparison Of Long Term Results Between Matched Chemoradiotherapy And Surgery For Limited Stage Small Cell Lung Cancer. Cancer Manag Res. 2019;11:9049–9055. doi: 10.2147/CMAR.S222882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim T.G., Pyo H., Ahn Y.C., Noh J.M., Oh D. Role of prophylactic cranial irradiation for elderly patients with limited-disease small-cell lung cancer: inverse probability of treatment weighting using propensity score. J Radiat Res. 2019;60(5):630–638. doi: 10.1093/jrr/rrz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kou P., Wang H., Yang D., Zhang Y., Yu J. Application of prophylactic cranial irradiation in limited-stage small-cell lung cancer: which patients could benefit? Future Oncol. 2019;15(1):23–31. doi: 10.2217/fon-2018-0481. [DOI] [PubMed] [Google Scholar]
- 45.Resio B.J., Hoag J., Chiu A., Monsalve A., Dhanasopon A.P., Boffa D.J., et al. Prophylactic cranial irradiation is associated with improved survival following resection for limited stage small cell lung cancer. J Thorac Dis. 2019;11(3):811–818. doi: 10.21037/jtd.2019.01.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elegbede A.A., Gibson A.J., Fu H., Dean M.L., Ezeife D.A., Lau H., et al. Real-World Adherence to Guideline-Recommended Treatment for Small Cell Lung Cancer. Am J Clin Oncol. 2020;43(4):236–242. doi: 10.1097/COC.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 47.Jeong J., Jeon W., Ahn S., Kim Y., Oh I., Park C., et al. Treatment time to the end of thoracic radiotherapy has more predictive power for survival than radiation dose intensity in patients with limited-stage small-cell lung cancer receiving concurrent chemoradiation of more than 45 Gy. Oncol Lett. 2020 doi: 10.3892/ol10.3892/ol.2019.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lou Y., Zhong R., Xu J., Qiao R., Teng J., Zhang Y., et al. Does surgically resected small-cell lung cancer without lymph node involvement benefit from prophylactic cranial irradiation? Thorac Cancer. 2020;11(5):1239–1244. doi: 10.1111/1759-7714.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghanta S., Keller A., Rodríguez-López J.L., Patel A., Beriwal S. Utility of Prophylactic Cranial Irradiation for Limited Stage Small Cell Lung Cancer in the Modern Era with Magnetic Resonance Imaging Surveillance. Clin Oncol (R Coll Radiol) 2021;33(8):e323–e330. doi: 10.1016/j.clon.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Li J., Ding C., Yang C., Wang S., Qiao X. Prophylactic cranial irradiation confers favourable prognosis for patients with limited-stage small cell lung cancer in the era of MRI: A propensity score-matched analysis. J Med Imaging Radiat Oncol. 2021;65(6):778–785. doi: 10.1111/1754-9485.13269. [DOI] [PubMed] [Google Scholar]
- 51.Held M.K., Hansen O., Schytte T., Hansen K.H., Bahij R., Nielsen M., et al. Outcomes of prophylactic cranial irradiation in patients with small cell lung cancer in the modern era of baseline magnetic resonance imaging of the brain. Acta Oncol. 2022;61(2):185–192. doi: 10.1080/0284186X.2021.1974553. [DOI] [PubMed] [Google Scholar]
- 52.Yan M., Toh T.S., Lindsay P.E., Weiss J., Hueniken K., Yeung C., et al. Limited-stage small cell lung cancer: Outcomes associated with prophylactic cranial irradiation over a 20-year period at the Princess Margaret Cancer Centre. Clin Transl Radiat Oncol. 2021;30:43–49. doi: 10.1016/j.ctro.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou N., Bott M., Park B.J., Vallières E., Wilshire C.L., Yasufuku K., et al. Predictors of survival following surgical resection of limited-stage small cell lung cancer. J Thorac Cardiovasc Surg. 2021;161(3):760–771.e2. doi: 10.1016/j.jtcvs.2020.10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.