Abstract
Background
Protein kinase C (PKC) has become a major focus among cell biologists interested in second-messenger signal transduction and much has been learned about differences in the cellular localization and function of its different isotypes. In this study we systematically address the genomic locations and gene structures of the human PKC gene module.
Results
We first carried out fine chromosomal mapping of all nine PKC genes by fluorescence in situ hybridization (FISH), using cosmid and BAC probes. The PKC genes are found to be dispersed throughout the genome, and in some positions distinct from those previously reported: PKCα is at 17q24, PKCβ at 16p12, PKCγ at 19q13.4, PKCδ at 3p21.2, PKCε at 2p21, PKCζ at 1p36.3, PKCη at 14q22-23, PKCθ at 10p15 and PKCι at 3q26. For PKCι, an additional FISH signal mapped on Xq21.3 revealed a pseudogene (derived by retrotransposition). PKCγ, ζ, and θ are found to map to the most distal positions on the chromosomes, potentially implicating telomere position effects in their expression. Using the complete human genome draft sequence and bioinformatics tools, we then carried out a systematic analysis of PKC gene structure, including determination of the occurrence of single-nucleotide polymorphisms corresponding to the PKC loci.
Conclusion
This resource of genomic information now facilitates investigation of the PKC gene module in structural chromosomal abnormalities and human disease locus mapping studies.
Background
The human protein kinase C (PKC) family of serine/threonine kinases comprises nine distinct isotypes with essential roles in cellular activation and differentiation in diverse cell types. They are, for example, known to phosphorylate several cellular proteins, leading to modulation of surface antigens, activation of other protein kinases, and induction of several key transcription factors, including aberrant signaling responses contributing towards malignant transformation [1]. Like many other signaling effectors, PKC is not a single entity but constitutes, at the gene level, nine different isotypes - PKCα, β, γ, δ, ε, ζ, η, θ and ι. PKC became the focus of attention among cell biologists interested in signal transduction and tumorigenesis after it was discovered that it is activated not only by the inositol phospholipid-derived second messenger diacylglycerol, but also by phorbol esters and other tumor promoters [2]. Members of the PKC family have been implicated in numerous cellular responses in a large variety of cell types [3]. PKC isotypes can be grouped into three subfamilies on the basis of their domain structure: the first subfamily includes the Ca2+-dependent PKCα, β and γ isotypes. These kinases contain, in addition to the catalytic C3 protein kinase domain that is shared by all members of the family, a phospholipid- and diacylglycerol/phorbol ester-binding C1 domain and a Ca2+-binding C2 domain. PKCδ, ε, η and θ contain the conserved C1 domain but only a C2-like domain, defective in Ca2+ binding. PKCζ and ι completely lack the C2 domain and their C1 domain contains only one of the two cysteine-rich zinc-finger-like motifs present in all other isotypes (for a review see [4]).
The reasons for the heterogeneity of PKC isotypes are not yet fully understood. However, the differences between these distinct PKC isotypes with respect to cofactor dependencies, subcellular localization, and tissue distribution (including levels of expression in a given cell type), suggest that they may be independently regulated through coupling to distinct receptor signaling pathways and possess different substrate specificities [4]. In this context, the expression of more than one PKC isotype in a cell potentially provides it with the ability to mount heterogeneous responses to diverse extracellular signals. However, many gaps remain in our knowledge of the detailed functions of PKC in certain important cell types such as T cells. Nevertheless, the potential relevance of PKC isotype-specific function(s) in the etiology of a number of human diseases is much discussed ([2] and see below).
To date, human and other mammalian PKC loci have not been systematically characterized, and very little is known regarding structural differences between the isotype genes. We previously focused on the genomic structure of the PKCθ gene [5,6] and its function (for a review see [2]). We now report the complete chromosomal localization and analysis of the structure of the PKC loci. In a critical first step, we systematically defined the chromosomal locations of all nine human PKC loci using cloned contigs of cosmid and/or bacterial artificial chromosome (BAC) probes and fine chromosomal mapping by fluorescence in situ hybridization (FISH). In addition, making use of the complete human genome draft sequence and bioinformatics tools, we have determined the gene organization of the human PKC gene loci as a resource of structural, functional, and positional candidate genes usage. Given the multiplicity of reported PKC functions in numerous cell types, this structural genomic information on the PKC gene module may assist investigations of biological processes regulating cell proliferation, differentiation and survival.
Results and discussion
Chromosomal fine mapping of the nine PKC loci by FISH analysis
Genomic clones were isolated under high-stringency hybridization conditions from human genomic libraries using cDNA probes for the nine human PKC isotypes. Genomic PKC clones confirmed by polymerase chain reaction (PCR) (up to five clones per gene) were subsequently used to determine the human chromosomal location of the PKC gene module using FISH. Fluorescent doublets were observed in the majority of the metaphases on each of the two sister chromatids at the positions indicated in Figure 1a, which shows the composite karyotype of the nine distinct PKC genes. No other chromosomal site exhibited significant fluorescent signals (data not shown). The fluorescence intensity of up to eight signal-carrying chromosomes was measured to create a highly significant average green-to-red profile. This profile was linearly interpolated to the size of standard chromosomal ideograms, allowing an objective assignment of fluorescent signals to a chromosomal band. The diagrammatic presentation of fluorescent spot distribution on metaphase human chromosomes is shown in Figure 1b.
Figure 1.

Chromosomal mapping of the nine human PKC gene loci by FISH to metaphase spreads. (a) Composite karyotype of the nine distinct PKC genes assigned by FISH (in green) to human metaphase spreads (DAPI/PI banding). The PKC gene product label indicates the assignment of the averaged fluorescence peak to the corresponding chromosome band. (b) Ideograms depicting the distribution of fluorescent spots (indicated by vertical bars at the side of the ideograms). Each mark represents the localization of one hybridization spot; n = number of metaphases scored.
Thus, the chromosomal location of all members has been fine-mapped to confirm and/or correct the existing data set. As Table 1 shows, out of the nine human PKC members, five isotypes - PKCα, β, δ, ζ and ι - had not been correctly assigned to individual human chromosomes in the existing literature [5,7,8,9,10,11,12,13]. A comparison of our chromosomal locations for PKC genes with the genome-draft assignments (HUGO), confirmed our results overall; however, our detailed FISH analysis provided enhanced mapping resolution.
Table 1.
Critical chromosomal regions of the PKC gene module
| PKC | HUGO | Literature | FISH | Mouse |
| alpha | 17q22-q23.2 | 17q22-23.2 | 17q24 | 11 |
| beta | 16p12-p13.1 | 16p11.2 | 16p12 | |
| gamma | 19q13.2-13.4 | 19q13.4 | 19q13.4 | 7 |
| delta | 3p21 | 3p | 3p21.2 | 14 |
| epsilon | 2p21 | 2p21 | 2p21 | |
| zeta | 1p36.2-36.33 | 1 | 1p36.3 | |
| eta | 14q | 14q22-23 | 14q22-23 | 12 |
| theta | 10p15 | 10p15 | 10p15 | 2 |
| iota | Xq21.3 (pseudo) | Xq21.3 (pseudo) | 3q26 |
In particular, the reported localization of the PKCι gene locus to the X chromosome (Xq21.3 [13] and HUGO, see Table 1) could not be confirmed, as we detected an additional FISH signal at 3q26. Once isolated from the human genome draft sequence, the Xq21.3 chromosomal DNA sequence harboring PKCι was revealed to be a processed pseudogene similar to the authentic gene at 3q26. This PKCι Xq21.3 pseudogene contains an uninterrupted open reading frame (ORF) and no introns, consistent with an origin through retrotransposition. Interestingly, it is identical to the PKCι gene sequence except for one point mutation at the stop codon (Figure 2), which elongates the ORF by 27 amino acids. The PKCι Xq21.3 pseudogene may therefore be expressed. Because of the extremely high identity of the two prospective PKCι mRNAs, this possibility could only be investigated further by using an antibody designed to detect the additional 27 amino acid sequence of a putative pseudogene protein product, and such an antibody is not yet available.
Figure 2.

Amino-acid sequence of the PKCι Xq21.3 pseudogene. The uninterrupted ORF is in red. The point mutation reverting the wild-type TGA stop codon to CGA is shown in blue.
In addition to the PKCι locus at chromosome 3q26 (and the Xq21.3 pseudogene) another PKCι genomic sequence is found in the draft human genome, which had been mapped (in the database) to chromosome 12 (BAC KlonRP11-147C2). An alignment of intron placement in the chromosome 12 and chromosome 3 PKCι genes indicated the highest possible homology in their genomic organization; for example, the chromosome 12 PKCι gene contains (as well as the 17 introns) 18 exons identical in size and sequence to those of the 3q26 PKCι gene identified by FISH (data not shown). As no FISH signal for a PKCι sequence could be observed on chromosome 12, this apparent 'highly conserved gene duplication' is most probably the result of an in silico error in the assembly of the draft sequence, confirming the existence of errors in the existing sequence.
PKCγ, ζ and θ are found to map to the most distal parts of their chromosomes (PKCγ at 19q13.4, PKCζ at 1p36.3 and PKCθ at 10p15), suggesting that there might be a telomeric position effect modifying these genes' expression throughout the replicative lifespan of human cells. However, there is no experimental evidence on this at present.
Genomic organization of the PKC gene module
Using HUGO and bioinformatics tools, we have dissected the genomic organization, that is the characterization of the exon/intron structure, of the nine PKC isotype genes (Figure 3). The PKC loci range in size from approximately 24.4 kilobases (kb) (PKCγ) to 480 kb (PKCα), and are composed of 14 to 18 coding exons and 13 to 17 introns, varying in size from 94 to 188,435 base pairs (bp). The exons are small to intermediate in size, ranging from 32 to 381 nucleotides. All intron-exon junctions appear to comply with the GT-AG rule. Considering the common evolutionary origin of this gene family (as shown by a cDNA-based phylogenetic tree of PKC and its closest relative, PKD, Figure 3a), it is not surprising that some of their exon structures are identical (and nearly homologous in their amino-acid sequence) within the PKC subfamilies (Figure 3b). The AUG translation initiation site for the ORFs of PKCα, β, γ, ε, ζ and η is located in exon 1, for PKCδ, θ and ι one intron is located in the 5'-untranslated region (5'-UTR) and only the subsequent exons determine the functional domains of the PKC proteins. Nevertheless, the existence of additional exons within the 5'-UTR cannot be excluded at this point.
Figure 3.

The evolutionary relationships and structures of the nine distinct human PKC genes. (a) A cDNA-based phylogenetic tree of PKC isotypes and their closest relative, PKD. (b) Evolutionary comparison between the PKC genomic gene sequences revealed subfamily-specific genomic organization. Within the subfamilies (grouped in rows), the color-coding indicates different collections of conserved exon sizes between loci.
These structural genomic data can be used to represent the phylogenetic relationships of the PKC genes. The organization of the regulatory and catalytic subdomains of PKC has been remarkably preserved during evolution: using the exon structure of PKCα as a reference, exons 10-15 within the catalytic domain share the highest similarity in size, organization and primary structure among conventional (c)PKC and novel (n)PKCs, but not atypical (a)PKCs. aPKCζ, and ι are very distinct from cPKC and nPKC within the exon structure of the catalytic domain. Along this line, aPKCι and aPKCs appear particularly conserved between each other in both their regulatory and catalytic domain structures. Within the regulatory domain, the subfamilies cPKC, nPKC and aPKC are clearly distinct from each other, and there is even a split within the nPKCs into the D-forms, PKCδ and θ, and the E-forms, PKCε and η. Also, cPKCα and cPKCβ appear more closely related to each other than to cPKCγ. Overall, the data suggest that gene duplication followed by intron loss and/or insertion and exon shuffling have been important in the evolution of the PKC gene family. This careful in silico dissection of the gene fine structure is also a prerequisite to an efficient search for potential abnormalities in the PKC genes.
Alternative processing of PKC gene transcripts
The existence of alternative splice variants of PKC within certain tissues is an attractive option for providing further heterogeneity in PKC, reflecting the diverse pathways of signal transduction within these cells.
Alternative splicing is currently known for at least one human PKC isotype. Two distinct PKCβ cDNA clones have been isolated that encode sequences of 671 and 673 amino acids, respectively, which differ from each other only in the carboxy-terminal regions of approximately 50 amino acids [14,15]. Characterization of the PKCβ chromosomal gene provided direct evidence of the existence of two adjacent carboxy-terminal exons that could be alternatively spliced to generate two types of PKCβ [16]. Importantly, these splice variants, PKCβI and βII, appear to be expressed in a tissue-selective manner, suggesting that their functions are different.
PKC splicing variants are known in rodents
A mouse variant of PKCδ - PKCδII - has recently been described [17]. This has a 78 bp (26 amino acid) insertion into the caspase-3-sensitive site in the V3 domain of the original PKCδI sequence, which renders the PKCδII isoform insensitive to caspase-3. PKCδI is detected in most tissues, whereas PKCδII was expressed selectively in the testis, ovary, thymocytes, brain and kidney [18].
In addition, a carboxy-terminal truncated form of PKCδIII exists in rats, which has an 83 bp out-of-frame insertion at the same site in the V3 region [18]. Genomic DNA analysis revealed that the difference between mouse PKCδII and rat PKCδIII is due to a different sequence at the critical 5' donor splice sites (data not shown). PKCδIII, which represents just the regulatory domain, might show a dominant-negative effect against PKCδI, and thus alternative splicing involving this variant might modulate signaling pathways.
Two forms of rat PKCζ RNA, with different 5' ends, have been reported. The major form (PKCζ wild type) is expressed ubiquitously, whereas the smaller form - protein kinase M ζ (PKMζ) - which encodes just the catalytic domain of the enzyme without most of its regulatory domain, is predominant in normal brain and certain rat prostate tumors. The rat PKCζ gene locus appears to contain two alternative promoters from which it can be transcribed to give two transcripts with 5'-end heterogeneity [19].
Finally, a PKCθII cDNA clone has been isolated from mouse testis, encoding a unique 5' sequence of 20 amino acids and the PKCθI (= wild type) sequence of 444 amino acids. The transcription of PKCθII RNA is initiated from the PKCθII-specific exon, which is located between exons 7 and 8 of the PKCθI gene, indicating that alternative splicing is the mechanism by which PKCθII is generated. PKCθII is expressed exclusively in the testis in an age-dependent manner with sexual maturation. Consistent with its lack of a C1 regulatory domain, PKCθII is constitutively active and may have a crucial role in spermatogenesis [20].
The determination of single-nucleotide polymorphisms within the human PKC gene module
The authentic PKC gene loci present in a single copy in the human genome have been analyzed in detail for single-nucleotide polymorphisms (SNPs) using resources from the National Center for Biotechnology Information website [21]. Among the 11 SNPs within the coding regions of the PKC gene module currently known from these databank searches, only two (one each in PKCδ and PKCη, respectively) give rise to non-synonymous codons and create missense mutations (Table 2). These missense mutations do not exert an effect on kinase function in any obvious way, even though an effect cannot be ruled out at this time. Overall, the human PKC genes appear not to be highly variable regions as characterized by SNP analysis; however, the available exonic SNPs appear sufficient to be used for haplotyping purposes in linkage and/or association studies.
Table 2.
SNPs of the PKC gene module
| PKC | cDNA | Protein | ||||
| Position | GenBank accession | Position | Protein accession | Codon position | RefSNP Cluster ID(rs#) | |
| beta | C 2167 T (3'-UTR) | X06318.1 | Untranslated region | rs424741 | ||
| delta | C 1182 T (exon 12) | L07860.1 | S 375 F | NP_006245.1 | 2 | rs1056998 |
| A 445 G (exon 5) | L07860.1 | Q 129 Q | NP_006245.1 | 3 | rs1135095 | |
| epsilon | T 1587 C (exon 11) | X65293.1 | H 524 H | NP_005391.1 | 3 | rs1143691 |
| C 1776 T (exon 12) | X65293.1 | S 587 S | NP_005391.1 | 3 | rs1143692 | |
| eta | G 678 A (exon 5) | M55284.1 | R 172 H | NP_006246.1 | 2 | rs1042559 |
| A 1837 G (exon 13) | M55284.1 | N 558 N | NP_006246.1 | 3 | rs1088680 | |
| G 2285 C (3'-UTR) | M55284.1 | Untranslated region | rs1139537 | |||
| theta | T 2206 A (3'-UTR) | L07032.1 | Untranslated region | rs1065081 | ||
| T 2554 C (3'-UTR) | L07032.1 | Untranslated region | rs663532 | |||
| C 2636 T (3'-UTR) | L07032.1 | Untranslated region | rs2453 | |||
Data retrieved from [21].
Chromosomal location of fine-mapped PKC gene loci with human disease loci
Using the Cancer Genome Anatomy Project [22], a comparison of the chromosomal location of these nine fine-mapped PKC loci with human disease loci revealed chromosomal aberrations/breakpoints in these regions in, for example, patients with malignant disease (Figure 4). In particular, the locations 17q24 (PKCα), 19q13 (PKCγ), 3p21 (PKCδ), 2p21 (PKCε), 1p36 (PKCζ) and 3q26 (PKCι) are noteworthy, as deletions or balanced translocations involving these defined chromosomal regions are frequently described in naturally occurring malignancies. Thus, in theory, PKC-family genes could be affected as possible disease candidate genes in accidental recombinations during intrachromosomal rearrangement or even interchromosomal translocations, as in the deleterious joining of abl sequences to the immunoglobulin or bcr loci that leads to malignancy (via gain-of-function). However, many other genes involved in the regulation of cell activation, proliferation and apoptosis are also included in these chromosomal regions. Nevertheless, our mapping results suggest that some PKC isotypes could be candidate genes involved in human cancer.
Figure 4.
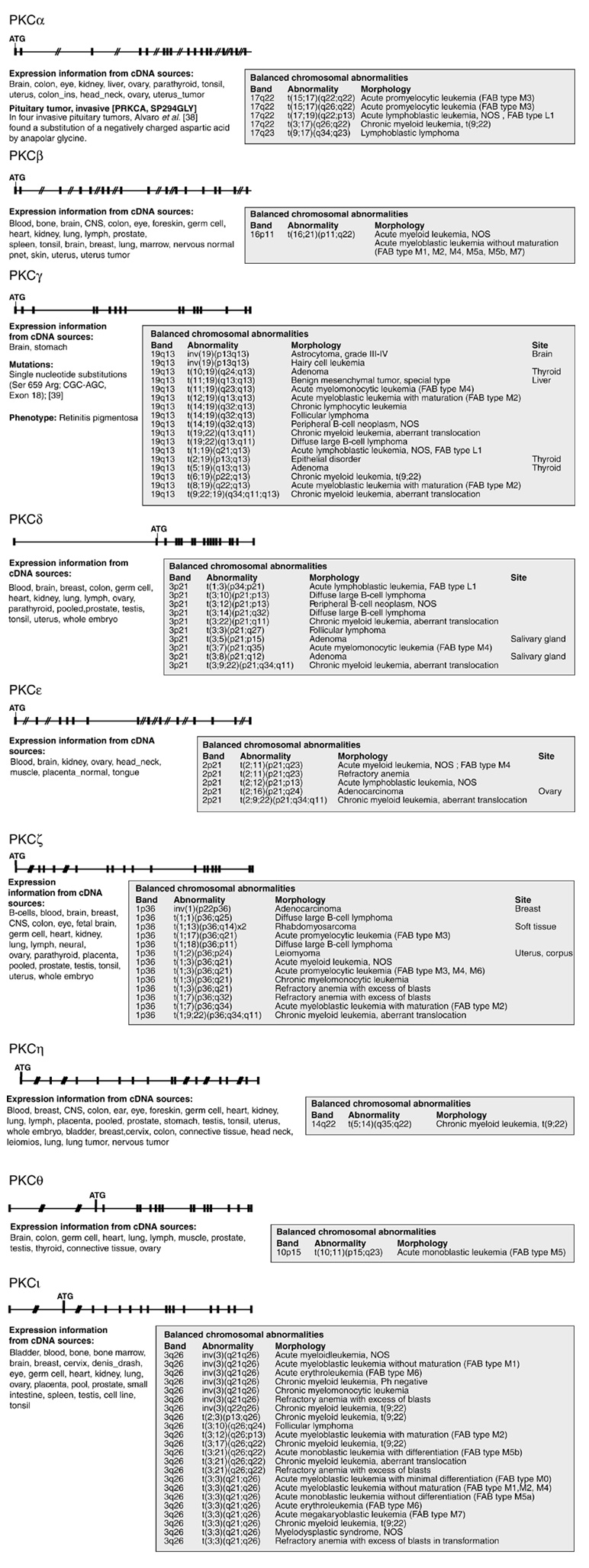
Comparison of the human PKC gene loci with chromosomal localization of balanced aberrations in tumors using data from the Cancer Genome Anatomy Project [37]. In addition to the chromosomal abnormality data listed to the right of each gene locus structure diagram, information on the tissue expression of the gene (from expressed sequence tag (EST) databases) and on known human mutations [38,39] is given. Several of the PKC loci are noteworthy, as deletions or translocation events in this chromosomal region are described in the breakpoint map of recurrent chromosomal rearrangements in human neoplasia [22,37]. Cytogenetic studies on recurrent balanced chromosomal aberrations in all hematological malignancies and solid tumors published are collected in [37].
Members of the PKC family have been consistently implicated in aberrant signaling responses contributing to malignant transformation, for example, as intracellular receptors for tumor-promoting phorbol esters, which have been shown to protect various cells, including T cells, from apoptosis [23,24]. As a result of this potentially transforming capacity of PKC-family genes, the aberrantly high expression levels of distinct PKC isotypes found in most tumor cell lines argue for a functional link between PKC and oncogenesis (G.B., unpublished work). Chronic recruitment of PKCθ (by an undefined mechanism) into the membrane fraction of malignant cells has been reported in cell lines derived from patients with T-cell leukemia [25]. Other, more definitive, results show that both Bcr-Abl and PKCι activity are necessary for resistance to apoptosis in hematopoietic K562 cells, supporting a functional role for PKCι in the survival of leukemia cells [26]. Recent studies, including our own [23,27], have directly linked distinct PKC isotypes to molecular pathways regulating apoptosis. But in spite of the putative role of PKCs in cellular growth control and differentiation, no clinical example of a causative role of PKCs in primary cell malignancy has yet been published. It remains to be seen whether the genetic dissection of naturally occurring malignant somatic cell mutants will eventually establish a link between PKCs and clinical disease.
A directed search for potential loss-of-function (and most likely recessive) mutations in the PKC loci, potentially associated with distinct genetic syndromes, will now be initiated. Along with information from biochemical work on signal transduction, tissue-specific expression patterns and phenotypes of the currently established mouse loss-of-function PKC isotype knockout lines ([28,29,30,31,32,33] and G.B., unpublished work), the genomic fine mapping reported here will enable genetic studies in defined groups of patients to search for functional PKC polymorphisms or mutations associated with familial genetic defects or abnormalities. Given the enormous increase in genetic and molecular databases, bioinformatics approaches should continue to improve and develop into useful tools for evaluating hypotheses, with only the very promising ones being subjected to empirical testing. This human genetic, and therefore long-term, approach may aid in delineating the basic physiological and possible pathophysiological functions of the PKC gene module, and may illuminate whether and eventually how PKCs are involved in human genetic disease.
Materials and methods
Cosmid and BAC clones were isolated from human single-chromosome libraries from the Human Genome Mapping Project (HGMP) Resource Center (Los Alamos National Laboratory and the Laurence Livermore National Laboratory) as described in [34,35]. FISH analysis was carried out essentially as described in [5,36]. Comparative genomic hybridization (CGH) analysis software (MacProbe 3.3) was used to statistically determine the chromosomal localization of the hybridized probe. Long-range PCR was carried out using the DNA polymerase Elongase™ as described by the manufacturer (Gibco/BRL, Gaithersburg, USA).
FISH and detection by immunofluorescence was carried out on metaphase chromosome preparations from phytohemagglutinin-stimulated human lymphocytes from healthy probands. For each slide (area 18 × 18 mm), a total of 200 ng biotinylated DNA probe. Post-hybridization washes were carried out to a stringency of 0.1 × SSC at 45°C. The biotinylated probe was detected using FITC-avidin conjugates (Vector Laboratories, Burlingame, USA) and one round of signal amplification. The data were recorded as a digital fluorescence image and processed by the MacProbe 3.3 software (PSI, London, UK) on a Leitz Aristoplan microscope equipped with integrating color CCD camera (Xbyion Electronic Systems, Cedar Knolls, USA). For each probe a minimum of 20 metaphases were analyzed by recording fluorescent spots on chromosomes and comparing them with images of DAPI-banded chromosomes.
Acknowledgments
Acknowledgements
This work was supported by a grant from the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) (P14394-BIO). We are grateful to A. Janecke and F. Überall (Innsbruck) and M. Leitges (Hannover) for critical discussions. All experiments comply with the current laws of Austria.
References
- Berry N, Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990;189:205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Altman A, Isakov N, Baier G. Protein kinase Cθ: a new essential superstar on the T-cell stage. Immunol Today. 2000;21:567–573. doi: 10.1016/s0167-5699(00)01749-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The protein kinase C family and lipid mediators for transmembrane signaling and cell regulation. Alcohol Clin Exp Res. 2001;25:3S–7S. doi: 10.1097/00000374-200105051-00003. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Erdel M, Baier-Bitterlich G, Duba C, Isakov N, Altman A, Utermann G, Baier G. Mapping of the human protein kinase C-theta (PRKCQ) gene locus to the short arm of chromosome 10 (10p15) by FISH. Genomics. 1995;25:595–597. doi: 10.1016/0888-7543(95)80068-w. [DOI] [PubMed] [Google Scholar]
- Kofler K, Kochl S, Parson W, Erdel M, Utermann G, Baier G. Molecular characterization of the human protein kinase C theta gene locus (PRKCQ). Mol Gen Genet. 1998;259:398–403. doi: 10.1007/s004380050829. [DOI] [PubMed] [Google Scholar]
- Latos-Bielenska A, Klett C, Just W, Hameister H. Refinement of localization of the human genes for myeloperoxidase (MPO), protein kinase C, alpha polypeptide, PRKCA, and the DNA fragment D17S21 on chromosome 17q. Hereditas. 1991;115:69–72. doi: 10.1111/j.1601-5223.1991.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Francke U, Darras BT, Zander NF, Kilimann MW. Assignment of human genes for phosphorylase kinase subunits alpha (PHKA) to Xq12-q13 and beta (PHKB) to 16q12-q13. Am J Hum Genet. 1989;45:276–282. [PMC free article] [PubMed] [Google Scholar]
- Huppi K, Siwarski D, Goodnight J, Mischak H. Assignment of the protein kinase C delta polypeptide gene (PRKCD) to human chromosome 3 and mouse chromosome 14. Genomics. 1994;19:161–162. doi: 10.1006/geno.1994.1028. [DOI] [PubMed] [Google Scholar]
- Chen X, Knauf JA, Gonsky R, Wang M, Lai EH, Chissoe S, Fagin JA, Korenberg JR. From amplification to gene in thyroid cancer: a high-resolution mapped bacterial-artificial-chromosome resource for cancer chromosome aberrations guides gene discovery after comparative genome hybridization. Am J Hum Genet. 1998;63:625–637. doi: 10.1086/301973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher N, Zisman Y, Berent E, Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol Cell Biol. 1991;11:126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask B, Fertitta A, Christensen M, Youngblom J, Bergmann A, Copeland A, de Jong P, Mohrenweiser H, Olsen A, Carrano A, et al. Fluorescence in situ hybridization mapping of human chromosome 19: cytogenetic band location of 540 cosmids and 70 genes or DNA markers. Genomics. 1993;15:133–145. doi: 10.1006/geno.1993.1021. [DOI] [PubMed] [Google Scholar]
- Mazzarella R, Ciccodicola A, Esposito T, Arcucci A, Migliaccio C, Jones C, Schlessinger D, D'Urso M, D'Esposito M. Human protein kinase C Iota gene (PRKCI) is closely linked to the BTK gene in Xq21.3. Genomics. 1995;26:629–631. doi: 10.1016/0888-7543(95)80190-w. [DOI] [PubMed] [Google Scholar]
- Ono Y, Kikkawa U, Ogita K, Fujii T, Kurokawa T, Asaoka Y, Sekiguchi K, Ase K, Igarashi K, Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236:1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Ono Y, Kurokawa T, Fujii T, Kawahara K, Igarashi K, Kikkawa U, Ogita K, Nishizuka Y. Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett. 1986;206:347–352. doi: 10.1016/0014-5793(86)81010-9. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Khan WA, Halpern AE, Obeid LM, Hannun YA. Selective regulation of expression of protein kinase C beta isoenzymes occurs via alternative splicing. J Biol Chem. 1993;268:10627–10635. [PubMed] [Google Scholar]
- Sakurai Y, Onishi Y, Tanimoto Y, Kizaki H. Novel protein kinase C delta isoform insensitive to caspase-3. Biol Pharm Bull. 2001;24:973–977. doi: 10.1248/bpb.24.973. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Ren Y, Ohmori S, Sakai K, Tamaki N, Saito N. cDNA cloning of an alternative splicing variant of protein kinase C delta (PKC deltaIII), a new truncated form of PKCdelta, in rats. Biochem Biophys Res Commun. 2000;269:557–563. doi: 10.1006/bbrc.2000.2331. [DOI] [PubMed] [Google Scholar]
- Marshall BS, Price G, Powell G. Rat protein kinase C zeta gene contains alternative promoters for generation of dual transcripts with 5'-end heterogeneity. DNA Cell Biol. 2000;19:707–719. doi: 10.1089/104454900750058071. [DOI] [PubMed] [Google Scholar]
- Niino YS, Irie T, Takaishi M, Hosono T, Huh N, Tachikawa T, Kuroki T. PKCθII, a new isoform of protein kinase C specifically expressed in the seminiferous tubules of mouse testis. J Biol Chem. 2001;276:36711–36717. doi: 10.1074/jbc.M104348200. [DOI] [PubMed] [Google Scholar]
- National Center for Biotechnology Information dbSNP homepage http://www.ncbi.nlm.nih.gov/SNP
- Mitelman F, Mertens F, Johansson B. A breakpoint map of recurrent chromosomal rearrangements in human neoplasia. Nat Genet. 1997;15:417–474. doi: 10.1038/ng0497supp-417. [DOI] [PubMed] [Google Scholar]
- Bertolotto C, Maulon L, Filippa N, Baier G, Auberger P. Protein kinase C theta and epsilon promote T-cell survival by a RSK-dependent phosphorylation and inactivation of BAD. J Biol Chem. 2000;275:37246–37250. doi: 10.1074/jbc.M007732200. [DOI] [PubMed] [Google Scholar]
- Villalba M, Bushway P, Altman A. Protein kinase C-theta mediates a selective T cell survival signal via phosphorylation of BAD. J Immunol. 2001;166:5955–5963. doi: 10.4049/jimmunol.166.10.5955. [DOI] [PubMed] [Google Scholar]
- Meller N, Elitzur Y, Isakov N. Protein kinase C-theta (PKC-theta) distribution analysis in hematopoietic cells: proliferating T cells exhibit high proportions of PKCtheta in the particulate fraction. Cell Immunol. 1999;193:185–193. doi: 10.1006/cimm.1999.1478. [DOI] [PubMed] [Google Scholar]
- Murray NR, Fields AP. Atypical protein kinase C iota protects human leukemia cells against drug-induced apoptosis. J Biol Chem. 1997;272:27521–27524. doi: 10.1074/jbc.272.44.27521. [DOI] [PubMed] [Google Scholar]
- Bauer B, Krumbock N, Fresser F, Hochholdinger F, Spitaler M, Simm A, Uberall F, Schraven B, Baier G. Complex formation and cooperation of PKCθ and Akt1/PKBα in the NF-κB transactivation cascade in Jurkat T cells. J Biol Chem. 2001;276:31627–31634. doi: 10.1074/jbc.M103098200. [DOI] [PubMed] [Google Scholar]
- Roman BB, Geenen DL, Leitges M, Buttrick PM. PKC-beta is not necessary for cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H2264–H2270. doi: 10.1152/ajpheart.2001.280.5.H2264. [DOI] [PubMed] [Google Scholar]
- Bowers BJ, Collins AC, Tritto T, Wehner JM. Mice lacking PKC gamma exhibit decreased anxiety. Behav Genet. 2000;30:111–121. doi: 10.1023/a:1001951104208. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKC gamma mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. doi: 10.1016/0092-8674(93)90614-v. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Chen C, Goda Y, Silva AJ, Stevens CF, Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhovsky A. Immunodeficiency in protein kinase Cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- Francis F, Zehetner G, Hoglund M, Lehrach H. Construction and preliminary analysis of the ICRF human P1 library. Genet Anal Tech Appl. 1994;11:148–157. doi: 10.1016/1050-3862(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Ioannou PA, Amemiya CT, Garnes J, Kroisel PM, Shizuya H, Chen C, Batzer MA, de Jong PJ. A new bacteriophage P1-derived vector for the propagation of large human DNA fragments. Nat Genet. 1994;6:84–89. doi: 10.1038/ng0194-84. [DOI] [PubMed] [Google Scholar]
- Erdel M, Hubalek M, Lingenhel A, Kofler K, Duba HC, Utermann G. Counting the repetitive kringle-IV repeats in the gene encoding human apolipoprotein(a) by fibre-FISH. Nat Genet. 1999;21:357–358. doi: 10.1038/7681. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Anatomy Project: recurrent chromosome aberrations in cancer http://cgap.nci.nih.gov/Chromosomes/RecurrentAberrations
- Alvaro V, Levy L, Dubray C, Roche A, Peillon F, Querat B, Joubert D. Invasive human pituitary tumors express a point-mutated alpha-protein kinase-C. J Clin Endocrinol Metab. 1993;77:1125–1129. doi: 10.1210/jcem.77.5.8077302. [DOI] [PubMed] [Google Scholar]
- Al-Maghtheh M, Vithana EN, Inglehearn CF, Moore T, Bird AC, Bhattacharya SS. Segregation of a PRKCG mutation in two RP11 families. Am J Hum Genet. 1998;62:1248–1252. doi: 10.1086/301819. [DOI] [PMC free article] [PubMed] [Google Scholar]


