Highlights
-
•
Treatment of NSCLC patients with oligo-progressive disease on systemic treatment remains controversial.
-
•
This phase II trial evaluates SABR to all oligo-progressive lesions in combination to current systemic therapy.
-
•
PFS and OS between patients treated with SABR with continuation of current systemic therapy vs standard of care will be assessed.
-
•
Intention-to-treat analysis of SABR compared to standard of care will be evaluated.
Abbreviations: ICI, Immune Checkpoint Inhibitors; NSCLC, Non-Small Cell Lung Cancer; OS, Overall Survival; PFS, Progression Free Survival; PTC, Planning Target Volume; RECIST, Response Evaluation Criteria in Solid Tumor; SABR, Stereotactic Ablative Radiotherapy; TKIs, Tyrosine Kinase Inhibitors
Keywords: Stereotactic body radiation therapy, Non-Small Cell Lung Cancer, Oligoprogression
Abstract
Background
Management of Non-Small Cell Lung Cancer (NSCLC) patients with oligoprogression remains controversial. There is limited data to support the strategy of Stereotactic Ablative Radiotherapy (SABR) targeting the oligoprogressive disease in combination with ongoing systemic treatment. We aim to assess the benefit of this approach compared to standard of care in the treatment of oligoprogressive NSCLC.
Methods
This phase II study will enroll 68 patients with oligoprogressive NSCLC, defined as 1–5 progressive extracranial lesions ≤5 cm involving ≤3 organs. Patients on active systemic therapy (chemotherapy, immunotherapy, targeted therapy or a combination) will be randomized 1:1 to either continue their current systemic therapy in combination with SABR to all lesions or the standard of care (switch to the next line of treatment, continue same treatment or observation). The co-primary endpoints are progression-free survival (PFS) and overall survival (OS). Secondary endpoints include time to next systemic treatment, patient-reported quality of life, cost effectiveness as well as translational analysis to characterize both adaptive immunity and immunogenic cell death markers in the peripheral blood.
Discussion
There is an unmet need to carefully examine the efficacy, safety and quality of life impact of SABR in the context of oligoprogressive disease. The present study will provide higher level randomized evidence on the role of SABR in oligoprogressive NSCLC.
1. Introduction
Over the last decade, the emergence of precision medicine with immuno-oncology, targeted therapy, and radiotherapy in the treatment armamentarium of patients with advanced non-small cell lung cancer (NSCLC) has led to significant improvements in overall survival (OS) [1]. Immune checkpoint inhibitors (ICIs) represent the treatment backbone of first-line therapy in advanced NSCLC without driver mutation. Despite favorable clinical responses to ICIs, more than half of patients will manifest primary resistance, and the majority will eventually progress [2]. On the other hand, patients with NSCLC harboring a driver mutation such as those of EGFR, ALK or ROS benefit from tyrosine kinase inhibitors (TKIs) as part of first-line therapy [1]. Although the introduction of TKIs has greatly improved the prognosis of these patients, secondary progression to novel genetic alterations almost invariably occurs after a median of 18 months [3].
Oligoprogressive disease is loo loosely defined as a secondary progression to a limited number of sites after a systemic therapy has provided a period of stable disease, partial or complete response [4]. The number of lesions constituting oligoprogression has variably been defined as 1–3 or <5 [4]. It has been hypothesized that oligoprogression results from tumor heterogeneity, whereby progression is observed in drug resistant subclones found in a small number of lesions, while the anti-cancer effect is maintained in most disease sites.
Current management of patients developing oligoprogression remains controversial and is supported only by small retrospective studies [4]. SABR, which is defined as a highly conformal image-guided technique allowing for the precise delivery of high radiation dose over a small number of fractions [5], is often the local therapy of choice over more invasive alternatives. Local radiation has been hypothesized to trigger immunogenic cell death, thereby promoting systemic inflammation and anti-cancer immunity. Specifically, a phenomenon of antigen release with use of high dose per fraction (10–15 Gy) was previously demonstrated, suggesting that SABR may work synergistically with ICI [6]. However, the combination of ICI and SABR has been associated with up to 25 % grade ≥3 toxicity [7], while the combination of TKI and SABR has been associated with up to 40% grade ≥3 toxicity [4]. Therefore, there is a need to examine the efficacy, safety and effect on quality of life of this combined approach.
2. Design and treatment description
2.1. Aim
The objective is to compare the can cancer outcomes of SABR in combination with ongoing systemic therapy vs. standard of care (switch to the next line of treatment, best supportive care or continue current systemic treatment — all without SABR), in NSCLC patients with oligoprogression.
The co-primary endpoints are progression-free survival (PFS) and overall survival (OS). Secondary endpoints include: Quality of life, toxicity, time to next systemic therapy, cost-effectiveness as well as immunological surrogate markers.
2.2. Design
We propose a registry-based randomized phase II trial. Patients are entered on registry, screened for study eligibility and then randomized within the registry (NCT03378856).
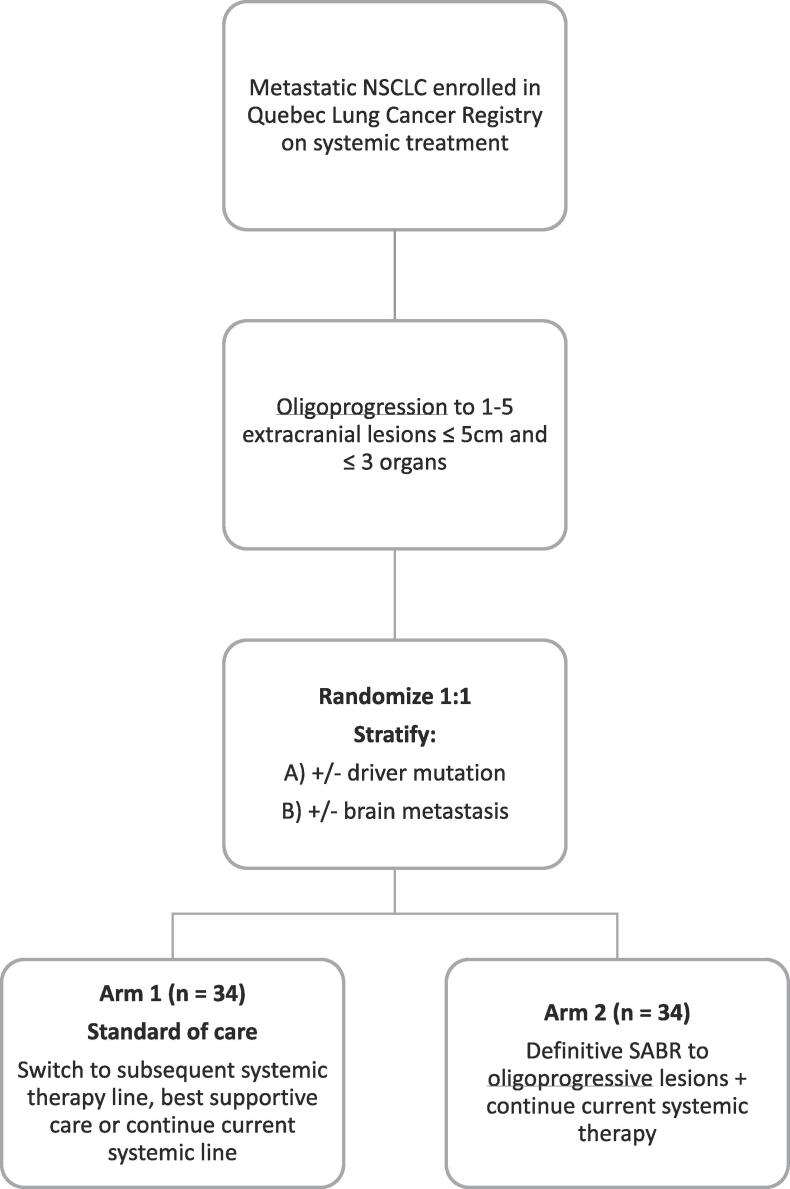
A total of 68 patients with metastatic NSCLC and oligoprogression to 1–5 extracranial lesions undergoing systemic treatment will be randomized 1:1 to the experimental or the standard arm and stratified for the presence of driver mutation and brain metastasis (see Fig. 1). This study is registered on clinicaltrials.gov (NCT04405401).
Fig. 1.
Study diagram.
2.3. Participants
Patients presenting with metastatic NSCLC with progressive disease (as per RECIST criteria v1.1) and an ECOG performance status 0–3 will be included. Oligoprogression is defined as 1–5 extracranial lesions ≤5 cm involving ≤3 organs. Progression at the primary tumor site will be counted within the total of 5 lesions. Brain metastasis will be treated as per standard of care and will not be counted in the maximum number of lesions.
2.4. Treatment plan
a. Arm 1 (Standard arm):
On the standard arm, treatment options could include switching to the next systemic line of therapy, treatment breaks, or persisting with the same systemic treatment. The decision will be at the discretion of the treating physician. Palliative radiotherapy to treat symptomatic lesions or spinal cord compressions is allowed.
b. Arm 2 (Experimental arm):
On the experimental arm, treatment will consist in using SABR on all oligoprogressive lesions while continuing the ongoing systemic therapy.
SABR treatment plan: Patients positioning and immobilization device will be as per treating physician. Positioning should be stable to avoid uncontrolled movement during treatments and maintain treatment accuracy. All patients will undergo planning CT of the region containing the treated lesion. Four-dimensional computed tomography (4D-CT) is required for organs subject to respiratory motion, and internal target volume technique, tracking, abdominal compression, active breathing control, gating, breath hold, etc. should be used where indicated. All treatments will be delivered using intensity modulated and image-guidance (3D/4D cone-beam CT, mega-voltage CT, or stereoscopic kV).
SABR dose: SABR doses will vary based on tumor location and physician’s preference (between 1 and 8 fractions). Table 1 details the suggested dose per tumor site. The prescription isodose line covering 95% of the Planning Target Volume (PTV) may range from 60 to 90% where the maximum dose is 100%. All dose calculations will be performed using corrections for tissue heterogeneity. Constraints for 1 to 5 fraction regimens are derived from Timmerman et al. [8], whereas constraints for 8 fraction regimen are derived from the SUNSET protocol [9].
Table 1.
Recommended doses and fractions for each tumor location.
| Site | Dose (Gy) | Fractions | Frequency |
|---|---|---|---|
| Lung-peripheral | 30–34 | 1 | Single dose |
| 45–54 | 3 | Every 2nd day | |
| Lung-central* | 60 | 8 | Every 2nd day |
| 35–50 | 5 | ||
| Mediastinal/cervical lymph node | 35–40 | 5 | Every 2nd day |
| Liver | 35–50 | 3–5 | Every 2nd day |
| Osseous/Spinal/paraspinal | 24 | 2 | Every day |
| 30 | 3 | Every day | |
| 35 | 5 | Every day | |
| 16–20 | 1 | Single dose | |
| Abdominal-pelvic metastasis (lymph node/adrenal gland) | 30–45 | 3–5 | Every 2nd day |
*Lung-central lesion defined as per RTOG 0813, as tumors within or touching the 2 cm zone around the proximal bronchial tree or immediately adjacent to the mediastinal or pericardial pleura.
Target volume determination: Gross tumor volume will be defined based on the planning CT as well as any other standard multi-modality imaging. With the exception of vertebral body lesions (which could include a clinical target volume as per the consensus guidelines by Cox et al. [10]), no additional margin will be added for microscopic spread in all other sites. A PTV margin of 2–5 mm will be added based on the site of disease, immobilization, and institutional set-up accuracy.
2.5. Study assessments
Patients will be assessed at baseline and at 1-, 3-, 6-, 12-, 18 and 24 months after randomization. Follow-up and data collection will be done as per Table 2. At each assessment, a standard of care CT of the chest, abdomen +/- neck will be obtained for assessment of local, regional and distant recurrence; survival status and toxicity grading will be updated at each visit.
Table 2.
Schedule of assessments.
 |
Clinical follow-up visits can be ±2 weeks of the stated time points.
2.6. Statistical design
a. Primary endpoint: PFS
Previous reports suggest 4–10 months improvement in Progression Free Survival (PFS) after second-line systemic therapy with the addition of local ablation in oligoprogressive NSCLC [11]. These results are biased considering that most studies have assessed the role of SABR in NSCLC in presence of a driver mutation. Assuming a conservative improvement in PFS of 3 months in the experimental arm, a median PFS of 3 months in the standard arm, an accrual period of 4 years followed by a follow-up period of 1 year and using a two-sided alpha of 0.05 and power of 80%, a sample size of 65 patients is required. Taking into account an attrition rate of 5%, a total of 68 patients are needed.
b. Co-primary endpoint: OS
In a recent phase II trial of oligoprogressive NSCLC treated with SABR and erlotinib after progression on first-line therapy, median OS was as high as 20 months, suggesting improved OS compared to when systemic therapy alone is used [4]. Assuming that the median OS of patients on the experimental arm would increase by 6 months compared to those on the standard arm, the proposed sample size would detect this difference with a two-sided alpha of 0.2 and a power of 71%.
2.7. Analytic plan
An intention-to-treat analysis will be performed. PFS, OS and local control will be calculated using the Kaplan-Meier method with differences compared using the stratified log-rank test. Pre-planned subgroup analyses will be made based on the stratification factors. A Cox multivariable regression analysis will be used to determine baseline factors predictive of survival endpoints. All p-values from multilevel analysis will be 2-sided, and levels <0.05 will be considered statistically significant.
2.8. Ancillary study (optional)
Immune monitoring will be performed to understand the difference in both arms for innate and adaptive immunity and expression on immune checkpoint on the peripheral blood. Flow cytometry analysis on peripheral blood mononuclear cells will be performed at time of randomization, at 1- and 3-month post study initiation, and at time of further progression. Monitoring of immunogenic cell death will also be performed. Tumor samples from pathological blocs or optional rebiopsy upon progression will be evaluated for immune micro-environment.
2.9. Planned timeline
Planned timeline is described in Table 2.
2.10. Ethic committee approval
The proposed study has been approved by the Centre Hospitalier de l’Université de Montreal IRB. Informed written consent will be obtained from all the participants.
3. Discussion
The objective of this proposed phase II study is to determine the efficacy of SABR to all progressive sites in combination with ongoing systemic therapy in patients with oligoprogressive NSCLC. The use of SABR as a radical approach in oligometastatic or oligoprogressive disease is particularly interesting given its non-invasive nature, its excellent local control rates approaching 90 % [12], and its safe toxicity profile s* (<5–10% risk of grade ≥3 toxicity) [13], [14]. In the past years, the use of SABR for local ablation in upfront oligometastatic lung cancer has gained significant momentum after two recent randomized phase II trials demonstrated OS benefit from local ablation of all metastatic disease sites [15], [16]. In the specific context of oligoprogression, the rationale for the use of SABR relies on controlling the progressive lesions refractory to current systemic therapy, while maintaining pressure from the ongoing systemic therapy line on the remaining sensitive tumor cells. Contrary to upfront oligometastatic disease, there is currently limited data supporting a survival benefit from the addition of SABR in the treatment of patients with oligoprogressive NSCLC. There is therefore a critical need to carefully examine the efficacy, safety, and effect on quality of life of this approach, as well as to define immunogenic and immunological surrogate makers.
Ethics approval and consent to participate
The proposed study has been approved by the Centre Hospitalier de l’Université de Montreal IRB. Informed written consent will be obtained from all the participants.
Funding
Astra Zeneca provided financial support to the CHUM to implement the registry within which this study takes place.
Authors’ contributions
HB, DR, NB, PW, EF, MPC, TV, MT, MF, LM, CM, BR contributed to the conception and the design of the protocol. CR contributed to the statistical design. MT and AAS wrote this article. All authors have read and approved the manuscript.
Declaration of Competing Interest
HB and BR have received a research grant from Astra Zeneca to support the lung cancer registry within which this study takes place. HB, EF, DR, CM have received research grants from Varian Medical Systems, unrelated to the current work.
Acknowledgments
Acknowledgement
None.
Competing interests
Astra Zeneca.
Study status
Open and currently ongoing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2021.12.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Elkrief A., Joubert P., Florescu M., Tehfe M., Blais N., Routy B. Therapeutic landscape of metastatic non-small-cell lung cancer in Canada in 2020. Curr Oncol. 2020;27(1):52–60. doi: 10.3747/co.27.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton G., Rath B. Immunotherapy for small cell lung cancer: mechanisms of resistance. Expert Opin Biol Ther. 2019;19(5):423–432. doi: 10.1080/14712598.2019.1592155. [DOI] [PubMed] [Google Scholar]
- 3.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 4.Piper-Vallillo A.J., Sequist L.V., Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J Clin Oncol. 2020;38(25):2926–2936. doi: 10.1200/JCO.19.03123. [DOI] [PubMed] [Google Scholar]
- 5.Sahgal A., Roberge D., Schellenberg D., Purdie T.G., Swaminath A., Pantarotto J., et al. The Canadian Association of Radiation Oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol (R Coll Radiol) 2012;24(9):629–639. doi: 10.1016/j.clon.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Golden E.B., Frances D., Pellicciotta I., Demaria S., Helen Barcellos-Hoff M., Formenti S.C. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3(4):e28518. doi: 10.4161/onci.28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke J.J., Lemons J.M., Karrison T.G., Pitroda S.P., Melotek J.M., Zha Y., et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmerman R.D. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215–222. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Giuliani M., Mathew A.S., Bahig H., Bratman S.V., Filion E., Glick D., et al. SUNSET: stereotactic radiation for ultracentral non-small-cell lung cancer-a safety and efficacy trial. Clin Lung Cancer. 2018;19(4):e529–e532. doi: 10.1016/j.cllc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Cox B.W., Spratt D.E., Lovelock M., Bilsky M.H., Lis E., Ryu S., et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83(5):e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Yu H.A., Sima C.S., Huang J., Solomon S.B., Rimner A., Paik P., et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(3):346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo S.S., Fakiris A.J., Chang E.L., Mayr N.A., Wang J.Z., Papiez L., et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 13.Nyman J., Johansson K.-A., Hultén U. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer–mature results for medically inoperable patients. Lung Cancer. 2006;51(1):97–103. doi: 10.1016/j.lungcan.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Onishi H., Shirato H., Nagata Y., Hiraoka M., Fujino M., Gomi K., et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7):S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 15.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 16.Gomez D.R., Blumenschein G.R., Lee J.J., Hernandez M., Ye R., Camidge D.R., et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.