Abstract
Background
Although the incidence of traumatic brain injury (TBI) has decreased since the beginning of the coronavirus disease 2019 (COVID-19) pandemic and severe acute respiratory syndrome coronavirus 2 is still evolving, the number of TBI cases has still greatly increased in multiple countries. In the present systematic review and meta-analysis, we evaluated the epidemiological characteristics of patients with TBI before and during the COVID-19 pandemic.
Methods
We conducted a systematic literature search of original studies, short reports, and research letters from databases on studies that contained data about the severity, mortality, presence of neurological deficits, radiological diagnosis, cause of injury, and type of management of TBI during a specified period within the pandemic compared with before the pandemic.
Results
A total of 18,490 subjects from 13 studies were included in the present study. The results of the meta-analysis showed a higher TBI mortality rate during the COVID-19 pandemic in the low-to-middle income countries (odds ratio, 1.65; 95% confidence interval, 1.12–2.41; P < 0.05; I2 = 40.8%; P = 0.18). The proportion of subdural hemorrhage was decreased, and the proportion of subarachnoid hemorrhage was increased in low- to middle-income and high-income countries, respectively. The proportion of assaults as the cause of TBI had increased during the pandemic (odds ratio, 1.40; 95% confidence interval, 1.06–1.86; P = 0.02; I2 = 20.8%; P = 0.28). We did not find any significant differences in the incidence of surgical intervention for TBI during the pandemic.
Conclusions
Our results have indicated that during the COVID-19 pandemic, the TBI mortality rate had increased in low- to middle-income countries. The rate of assault as the cause of TBI had also increased during the pandemic.
Key words: COVID-19, Epidemiology, Neurological trauma, Traumatic brain injury
Abbreviations and Acronyms: CI, Confidence interval; COVID-19, Coronavirus disease 2019; HIC, High-income country; LMIC, Low- to middle-income country; OR, Odds ratio; PECO, Population, exposure, comparison, outcome; PRISMA, Preferred reporting items for systematic reviews and meta-analyses; PROSPERO, International Prospective Register of Systematic Reviews; RTA, Road traffic accident; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SAH, Subarachnoid hemorrhage; SDH, Subdural hematoma; TBI, Traumatic brain injury
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has infected millions of people worldwide. The rapid spread of the disease has also continued to burden healthcare providers by postponing necessary interventions for patients who require, not only elective procedures, but also emergency interventions.1, 2, 3 Furthermore, the rapidly evolving nature of SARS-CoV-2 with distinct epidemiological features has required clinicians and stakeholders to adjust the healthcare and workflow settings to prevent COVID-19 complications.
During the COVID-19 outbreak, the incidence of neurological trauma has markedly declined as the practice of lockdowns, self-isolation, quarantine, and travel restrictions have been implemented.4 , 5 Rigorous social distancing has been implemented that has shifted our standard methods of living. Many public places have been closed, workplaces have become remote, and people have only been seeking medical services if they had experienced an emergency.6 , 7 Thus, the number of motor vehicle accidents, amount of public mobility, and number of outdoor activities have decreased, attributing to the declining number of neurological trauma cases.
Despite the reduced number of trauma-related cases, some people have still experienced traumatic brain injury (TBI).4 The altered behavior of the population could have significantly affected the epidemiological characteristics of neurological trauma cases, including TBI. Thus, we evaluated the severity of TBI, mortality, presence of neurological deficits, radiology diagnosis, cause of injury, and the type of management of TBI during versus before the pandemic.
Methods
The present systematic review was reported in accordance with the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.8 , 9 A detailed protocol was registered in PROSPERO (International Prospective Register of Systematic Reviews; registry no. CRD42021251648).
Eligibility Criteria
The present study followed the design structure of PECO (population, exposure, comparison, outcome) to define the inclusion and exclusion criteria for exposure and outcomes.10 The inclusion criteria were original research articles, letters, and short reports. The outcomes of interest was the severity of TBI, mortality, neurological deficits, radiology diagnosis, cause of injury, and type of management during the COVID-19 pandemic compared with before the pandemic. We excluded case reports, non–research letters, editorials, invited commentaries, reviews, abstract-only reports, and preprint reports. No language restriction was applied in the present study.
Search Strategy and Study Selection
A systematic search of PubMed and the Cochrane Collaboration Central Register of Controlled Clinical Trials was performed from inception to December 20, 2021 by 2 of us (F.A.D. and A.F.) independently (Appendix). The following search terms were used: (“Brain Injuries, Traumatic"[MeSH] OR Traumatic Brain Injury OR “Brain Concussion"[MeSH] OR “Brain Injuries"[MeSH] OR Brain Injury OR “Accidents, Traffic"[MeSH]) AND (Pandemic OR COVID-19 OR “COVID-19"[MeSH]). We used the “related articles” feature and manually searched the reference lists of the included reports to expand the search and obtain additional studies. Duplicate results were removed after the initial search. We attempted to contact the authors of the reports for which the study data were incomplete or full-text reports were unavailable.
Data Extraction
Data extraction was conducted independently by 2 of us (F.A.D. and A.F.). The standardized form included author, year, study design, sample size, age, gender, control period, and pandemic period. Different perceptions in the extraction of the data and in the elimination of possible duplicates were resolved through discussion and consensus.
We used Newcastle-Ottawa scale to assess the quality of included studies.11 The following aspects were evaluated for each study and cohort selection: the comparability of the cohort according to the design or analysis, the method of exposure, and the determination of the outcomes of interest. Discrepancies of perception were resolved by discussion.
Outcomes and Effect Measures
The primary outcome of the present study was the severity of TBI and mortality during the COVID-19 pandemic compared with before the pandemic. The pooled estimates are presented using the odds ratio (OR).
The secondary outcome was the presence of neurological deficits, radiology diagnosis, cause of injury, and type of management. The radiology diagnosis included epidural hematoma, subdural hematoma (SDH), subarachnoid hemorrhage (SAH), contusion, and skull fracture. The cause of injury included road traffic accidents (RTAs), high falls, low and mechanical falls, and assaults. The type of management was surgical versus nonsurgical management.
We also conducted a subset outcomes analysis according to the country's income level using the World Bank classification.12 The countries in which the studies were conducted were divided into low- to middle-income countries (LMICs) and high-income countries (HICs). A minimum of 2 studies were required for the creation of a subset of outcomes.
Statistical Analysis
All statistical analyses were performed using R, version 4.0.4 (The R Foundation, Vienna, Austria) using the mada and meta packages.13 We used a meta-analysis of the proportion to calculate the differences in percentages of the investigated outcome. ORs were calculated using the Mantel-Haenszel method random effects model. Also, the Haldane-Anscombe correction was applied to studies with a 0 number of cases.14) An I 2 value of >50% and P value of < 0.05 were considered as significant for heterogeneity.15 The random effects model was used, regardless of the heterogeneity value.
Results
Study Characteristics
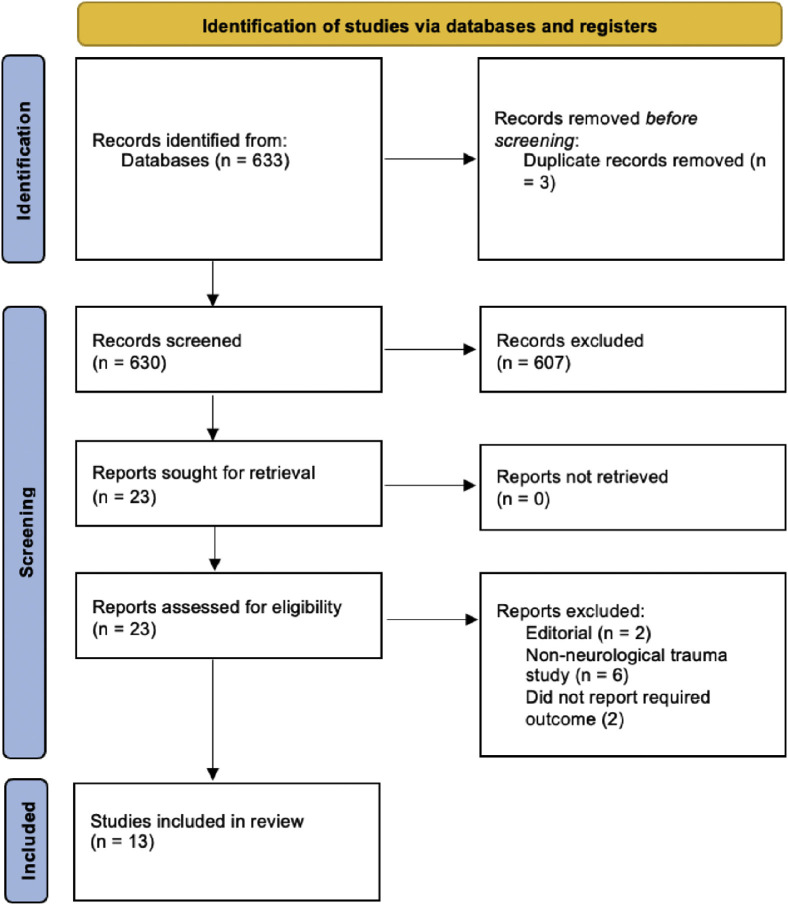
A total of 18,490 subjects from 13 studies were included in the present meta-analysis (Figure 1 ).4 , 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The characteristics of the included studies are presented in Table 1 . A summary of the meta-analysis findings is presented in Table 2, Table 3, Table 4 .
Figure 1.
PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart for the present study.
Table 1.
Characteristics of Included Studies
| Investigator | Study Design | Country | Sample Size (n) | Age (years) | Male Gender (%) | Control Period | Pandemic Period | NOS Score |
|---|---|---|---|---|---|---|---|---|
| Karthigeyan et al.,16 2021 | Single-center, retrospective; prospective observational | India | 3372 | 33.8 | 83.6 | September 16, 2019 to March 24, 2020 | March 25 to September 30, 2020 | 9 |
| Goyal et al.,17 2020 | Single-center, retrospective observational | India | 284 | 33.45 | 75.7 | March 25 to September 15, 2019 | March 25 to September 25, 2020 | 9 |
| Grassner et al.,4 2021 | Multicenter, retrospective observational | Austria, Czech Republic, Switzerland | 506 | 61.74 (NS) | 57.4 (NS) | March 16 to April 15, 2017, 2018, 2019 | March 16 to April 15, 2020 | 7 |
| Horan et al.,18 2021 | Single-center, retrospective observational | Ireland | 658 | 64.7 | 63.07 | March 1 to March 31, 2019 | March 1 to March 31, 2020 | 8 |
| Hecht et al.,19 2020 | Single-center, retrospective observational | Germany | 92 | 59.7 (NS) | 50.3 (NS) | February 1 to April 15, 2019 | February 1 to April 15, 2020 | 7 |
| Lam et al.,20 2021 | Single-center, retrospective observational | Taiwan | 11,931 | 44 | 55.7 | January 20 to April 30, 2019; May 11 to July 31, 2019 | January 20 to April 30, 2020; May 11 to July 31, 2021 | 7 |
| Lara-Reyna et al.,21 2020 | Single-center, retrospective; prospective, observational | USA | 150 | 66.2 | 66 | November 2019 to February 2020 | March to April 2020 | 8 |
| Luostarinen et al.,22 2020 | Single-center, retrospective observational | Finland | 123 | 61.4 | 78.9 | January to May 2019 | January to May 2020 | 7 |
| Manivannan et al.,23 2021 | Single-center, retrospective observational | UK | 597 | 65.9 | 59.7 | April 4 to June 30, 2019 | April 1 to June 30, 2020 | 9 |
| Pinggera et al.,24 2020 | Single-center, retrospective observational | Austria | 122 | 58 | 60 | March 16 to April 5, 2016, 2017, 2018, 2019 | March 16 to April 5, 2020 | 8 |
| Prawiroharjo et al.,25 2020 | Single-center, retrospective observational | Indonesia | 263 | 38.2 | 75.3 | March 16 to June 14, 2019 | March 16 to June 14, 2020 | 7 |
| Rault et al.,26 2021 | Single-center, retrospective observational | France | 158 | 49.1 | 76.6 | March 20, 2019 to March 17, 2020 | March 18 to April 28, 2020 | 7 |
| Santing et al.,27 2020 | Single-center, retrospective; prospective observational | Netherlands | 234 | 62.8 | 54.7 | March 18 to April 6, 2019 | March 18 to April 6, 2020 | 8 |
NOS, Newcastle-Ottawa scale; NS, not stated.
Table 2.
Summary of Meta-Analysis Findings on General Outcomes of Traumatic Brain Injury
| Outcome | OR (95% CI); P Value | Heterogeneity (%; P Value) | % | Studies (n) |
|---|---|---|---|---|
| Moderate to severe TBI | ||||
| LMICs | 1.10 (0.90–1.36); P > 0.05 | 18.9; P = 0.29 | 49 versus 50 | 3 |
| HICs | 1.14 (0.80–1.61); P > 0.05 | 44; P = 0.10 | 17 versus 17 | 7 |
| Overall | 1.13 (0.96–1.34); P = 0.14 | 39%; P = 0.09 | 22 versus 22 | 10 |
| Mortality | ||||
| LMICs | 1.65 (1.12–2.41); P < 0.05∗ | 40.8%; P = 0.18 | 43 versus 50 | 3 |
| HICs | 0.48 (0.14–1.69); P > 0.05 | 47.7; P = 0.15 | 20 versus 8 | 3 |
| Overall | 1.08 (0.58–2.01); P = 0.81 | 73.9; P = 0.001 | 38 versus 46 | 6 |
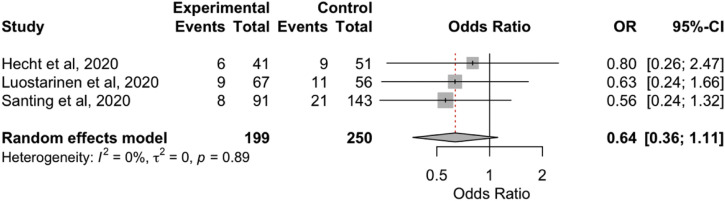
| Neurological deficit, overall | 0.64 (0.36–1.11); P = 0.11 | 0; P = 0.88 | 15 versus 9 | 3 |
| Surgery | ||||
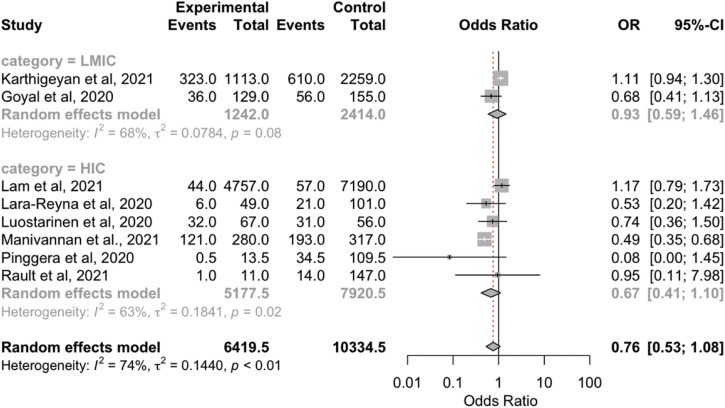
| LMICs | 0.93 (0.59–1.46); P > 0.05 | 68.2; P = 0.08 | 28 versus 29 | 2 |
| HICs | 0.67 (0.41–1.10); P > 0.05 | 63.2; P = 0.02 | 4 versus 4 | 6 |
| Overall | 0.76 (0.53–1.08); P = 0.13 | 73.6; P < 0.001 | 10 versus 9 | 8 |
OR, odds ratio; CI, confidence interval; TBI, traumatic brain injury; LMICs, low- to middle-income countries; HICs, high-income countries.
Statistically significant.
Table 3.
Summary of Meta-Analysis Findings on Radiological Diagnosis
| Radiological Diagnosis | OR (95% CI); P Value | Heterogeneity (%; P Value) | % | Studies (n) |
|---|---|---|---|---|
| EDH | ||||
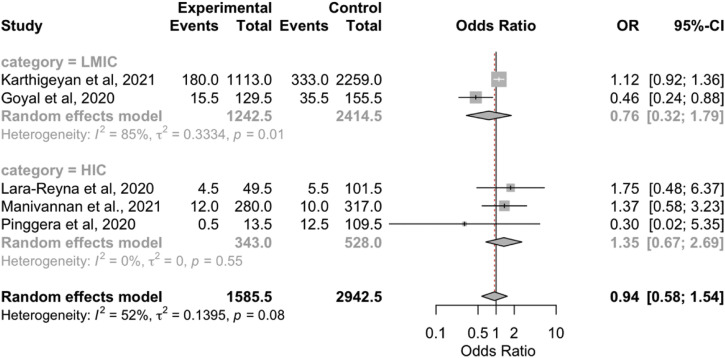
| LMICs | 0.76 (0.32–1.79); P > 0.05 | 84.8; P = 0.1 | 16 versus 15 | 2 |
| HICs | 1.35 (0.67–2.69); P > 0.05 | 0; P = 0.55 | 5 versus 5 | 3 |
| Overall | 0.94 (0.58–1.54); P = 0.81 | 51.7; P = 0.08 | 13 versus 13 | 5 |
| SDH | ||||
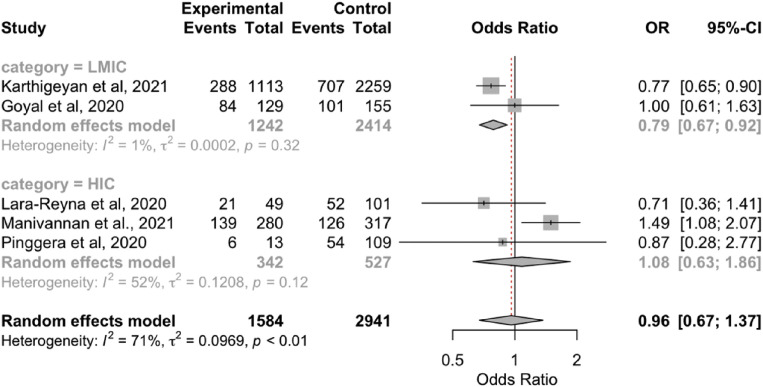
| LMICs | 0.79 (0.67–0.92); P < 0.05∗ | 0.7; P = 0.32 | 33 versus 30 | 2 |
| HICs | 1.08 (0.63–1.86); P > 0.05 | 52; P = 0.12 | 44 versus 49 | 3 |
| Overall | 0.96 (0.67–1.37); P = 0.82 | 70.8; P = 0.01 | 35 versus 34 | 5 |
| SAH | ||||
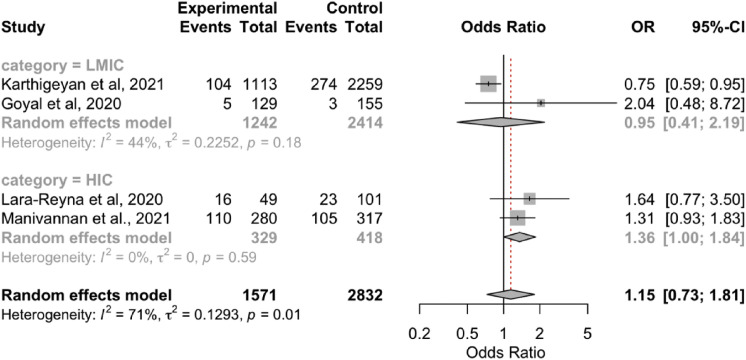
| LMICs | 0.95 (0.41–2.19); P > 0.05 | 44.5; P = 0.18 | 11 versus 9 | 2 |
| HICs | 1.36 (1.00–1.84); P < 0.05∗ | 0; P = 0.59 | 31 versus 38 | 2 |
| Overall | 1.15 (0.73–1.81); P = 0.56 | 71.4; P = 0.01 | 14 versus 15 | 4 |
| Contusion | ||||
| LMICs | 1.04 (0.90–1.19); P > 0.05 | 0; P = 0.57 | 54 versus 55 | 2 |
| HICs | 1.07 (0.76–1.51); P > 0.05 | 0; P = 0.77 | 18 versus 24 | 2 |
| Overall | 1.04 (0.92–1.18); P = 0.54 | 0; P = 0.93 | 48 versus 48 | 4 |
| Skull fracture | ||||
| LMICs | 0.94 (0.78–1.13); P > 0.05 | 0; P = 0.75 | 17 versus 16 | 2 |
| HICs | 0.71 (0.18–2.74); P > 0.05 | 66.3; P = 0.05 | 29 versus 30 | 2 |
| Overall | 1.01 (0.68–1.51); P = 0.94 | 53.6; P = 0.07 | 18 versus 17 | 4 |
OR, odds ratio; CI, confidence interval; EDH, epidural hematoma; LMICs, low- to middle-income countries; HICs, high-income countries; SDH, subdural hematoma; SAH, subarachnoid hemorrhage.
Statistically significant.
Table 4.
Summary of Meta-Analysis Findings on Cause of Traumatic Brain Injury
| Cause of TBI | OR (95% CI); P Value | Heterogeneity (%; P Value) | % | Studies (n) |
|---|---|---|---|---|
| RTA | ||||
| LMICs | 1.04 (0.79–1.38); P > 0.05 | 48.1; P = 0.15 | 62 versus 60 | 3 |
| HICs | 0.89 (0.42–1.91); P > 0.05 | 73.4; P < 0.01 | 14 versus 9 | 5 |
| Overall | 1.00 (0.72–1.38); P = 0.99 | 63.4; P = 0.01 | 48 versus 43 | 8 |
| High fall, overall | 1.17 (0.93–1.48); P = 0.18 | 0; P = 0.43 | 37 versus 42 | 3 |
| Low and mechanical falls, overall | 0.63 (0.39–1.03); P = 0.07 | 42.7; P = 0.17 | 52 versus 47 | 3 |
| Assault | ||||
| LMICs | 1.83 (1.37–2.45); P < 0.05∗ | 0; P = 0.78 | 4 versus 7 | 2 |
| HICs | 1.14 (0.81–1.60); P > 0.05 | 0; P = 0.59 | 32 versus 40 | 4 |
| Overall | 1.40 (1.06–1.86); P = 0.02∗ | 20.8; P = 0.28 | 6 versus 8 | 6 |
TBI, traumatic brain injury; CI, confidence interval; OR, odds ratio; RTA, road traffic accident; HICs, high-income countries; LMICs, low- to middle-income countries.
Statistically significant.
Severity and Mortality of TBI Cases
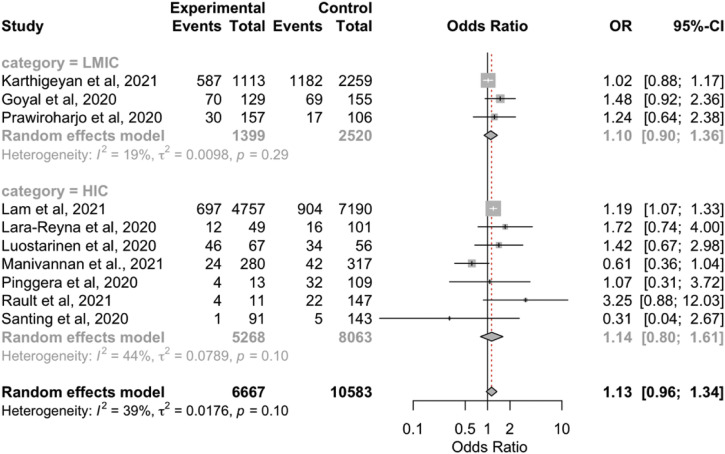
No statistically significant differences were found in the moderate to severe TBI case rates between the COVID-19 outbreak and before the pandemic (control group; OR, 1.13; 95% CI, 0.96–1.34; P = 0.14; I 2 = 39%; P = 0.09; Supplementary Figure 1). Similarly, although the rates of moderate to severe TBI cases were greater during the pandemic in both LMICs and HICs, the differences were not statistically significant (Table 2).
Supplementary Figure 1.
Forest plot of traumatic brain injury severity. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
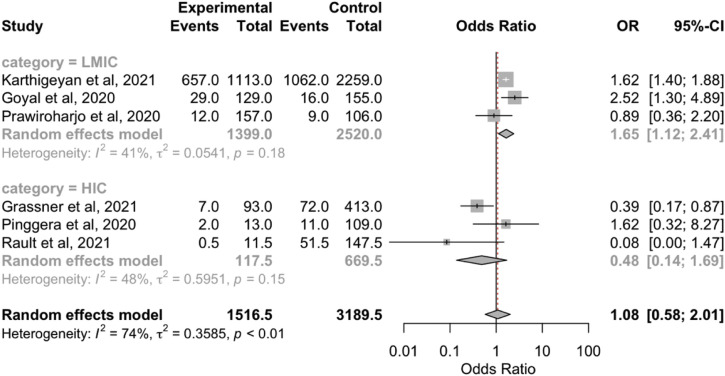
The overall aggregated risk estimates for TBI mortality showed no significant differences between before the pandemic and during the COVID-19 pandemic (OR, 1.08; 95% CI, 0.58–2.01; P = 0.81; I 2 = 73.9%; P = 0.001; Supplementary Figure 2). However, the TBI mortality rate within the LMIC group was significantly greater during the pandemic (OR, 1.65; 95% CI, 1.12–2.41; P < 0.05; I 2 = 40.8%; P = 0.18; Table 2). We did not find any differences in the occurrence of neurological deficits between the 2 periods (Supplementary Figure 3).
Supplementary Figure 2.
Forest plot of traumatic brain injury mortality. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
Supplementary Figure 3.
Forest plot of neurological deficit. CI, confidence interval; OR, odds ratio.
Radiological Findings
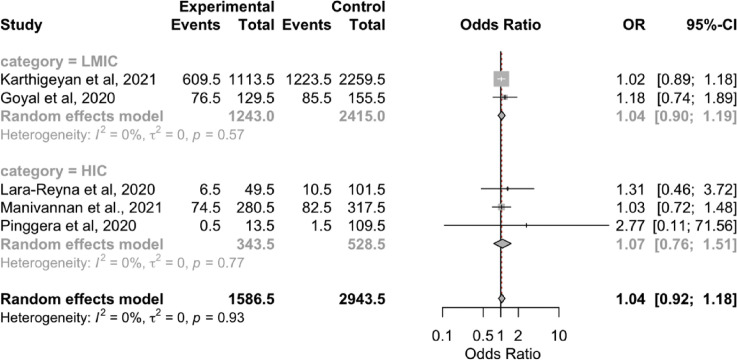
The overall results showed no significant differences in the radiological findings (epidural hematoma, SDH, SAH, contusion, skull fracture; Supplementary Figure 4, Supplementary Figure 5, Supplementary Figure 6, Supplementary Figure 7, Supplementary Figure 8) between the 2 periods. However, the subgroup analyses showed that the rate of SDH was lower during the pandemic in the LMICs (OR, 0.79; 95% CI, 0.67–0.92; P < 0.05; I 2 = 0.7%; P = 0.32). In addition, the rate of SAH was lower during the pandemic in the HICs (OR, 1.36; 95% CI, 1.00–0.84; P < 0.05; I 2 = 0%; P = 0.59; Table 3).
Supplementary Figure 4.
Forest plot of traumatic brain injury surgical intervention. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
Supplementary Figure 5.
Forest plot of epidural hematoma. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
Supplementary Figure 6.
Forest plot of subdural hemorrhage. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
Supplementary Figure 7.
Forest plot of subarachnoid hemorrhage. CI, confidence interval; HIC, high-income country; LMIC, low-to middle-income country; OR, odds ratio.
Supplementary Figure 8.
Forest plot of contusion. CI, confidence interval; HIC, high-income country; LMIC, low- to middle-income country; OR, odds ratio.
Cause of Injury
The proportion of RTAs as a cause of TBI was similar between the 2 periods. Similarly, our results showed no significant differences in the rates of high falls and low and mechanical falls. In contrast, the results from our present analysis indicated that the overall rate of assault as a cause of TBI was greater during the pandemic (OR, 1.40; 95% CI, 1.06–1.86; P = 0.02; I 2 = 20.8%; P = 0.28). Furthermore, the proportion of assault was greater in LMICs (OR, 1.83; 95% CI, 1.37–2.45; P < 0.05; I 2 = 0%; P = 0.78; Table 4).
Management of TBI
The management of TBI was categorized into surgical and nonsurgical management. We found that the rate of surgical TBI treatment was similar between before and during the COVID-19 pandemic (OR, 0.76; 95% CI, 0.53–1.08; P = 0.13; I 2 = 73.6%; P < 0.01; Table 2). A subgroup analysis also showed no significant differences in operative treatment between before the pandemic and during the pandemic in the LMICs and HICs (Table 2).
Discussion
In the present study, we conducted pooled analyses of the epidemiological characteristics of TBI cases during and before the COVID-19 pandemic. Because our meta-analysis included studies from multiple countries, we divided the countries for a subset analysis by the countries' income level.
Our results showed a similar proportion of moderate to severe TBI cases during the pandemic in both the LMIC and the HIC groups, with a moderate level of heterogeneity. Thus, the healthcare management flow only slightly influenced the pattern of TBI across the centers. In addition, the massive lockdown regulations implemented by governments could have potentially caused patients with mild TBI be reluctant to visit hospitals. However, we found that many patients with mild TBI had presented to peripheral centers and were treated appropriately.16 Although most of the studies had demonstrated a greater proportion of moderate to severe TBI cases during the pandemic, we noted a moderate level of heterogeneity in the results. A study by Manivannan et al.23 showed a lower proportion of moderate and severe TBI cases linked to a reduced number of RTAs. The limitations on the use of mass transportation during the COVID-19 pandemic could have caused some people to switch to using bicycles to go to work, home, public places, and other places, which might account for the reduced proportion of severe TBI caused by motorized vehicles.28, 29, 30, 31
Our overall analysis showed no significant differences in TBI mortality between the 2 periods. However, the TBI mortality rate was significantly greater in the LMICs. This discrepancy in the results could have been caused by the relatively greater number of COVID-19 cases in the developing countries, in addition to the limited number of medical supplies that eventually burdened the healthcare systems.32 Pinggera et al.,24 in their study of the Austrian population, reported that all acute neuroemergency cases were considered positive for COVID-19, with all the related consequences until proved otherwise. This policy was implemented with the aim of preventing delays in treating emergency cases caused by the lengthy COVID-19 assessments such that patient mortality could be avoided.24 In contrast, studies in India and Indonesia showed an increase in TBI mortality during the pandemic.17 The discrepancy between the results could also be attributed to differences in the precaution strategies that authorities have implemented to mitigate COVID-19 transmission. In addition, some studies indicated a possible causative link between the higher severity of TBI cases, which, consequently, led to significantly greater mortality during the pandemic, and the postponement of some elective surgeries during the peak of the pandemic spread in India and the requirement that surgical procedures for those who tested positive for COVID-19 should be postponed until the test results were negative, where possible.3 , 28 Ultimately, the overall result has emphasized that adequate resource allocation is required among developing countries to reduce the increasing rate of TBI deaths.
The radiological manifestations in TBI might reflect the pattern of causes and the consequences of altered neurosurgical management within the hospital due to the pandemic. Overall, our pooled analyses found no significant differences in the radiological findings among TBI patients between the 2 periods. However, our subgroup analyses demonstrated a lower incidence of SDH in the LMICs and a higher incidence of SAH in the HICs. This pattern might have resulted from the marked reduction of hospital admissions for the elderly because some patients did not present with acute symptoms and COVID-19 affects elderly groups severely—urging them to stay at home to prevent viral transmission.17 , 33, 34, 35 In addition, the reduced number of hospital visits could have also influenced the pattern of SAH findings owing to the decreased hypertensive control among patients with a history of cerebrovascular disease.36 , 37
Although the manifestation of TBI during the pandemic had altered, this could also have resulted from differences in the pattern of the mechanism of the injury itself. Overall, we did not find any differences in the proportion of RTAs and falls. In some of the HICs, the rate of RTA-related trauma had increased during the pandemic.21 This could have been influenced by various factors that correlate with public mobility. A decrease in traffic as the result of country lockdowns and quarantines could yield empty roads and, thus, trigger speed-related vehicle collisions.28 , 38 However, this shifted pattern was also found to vary across countries and was influenced by geographic differences, urban planning, and seasonal variations. States with hilly areas with narrow and curvaceous roads were more likely to have a higher rate of RTAs.38 , 39
Although most of the included studies had reported a decrease in surgical volumes, our pooled analysis found no significant differences in surgical interventions between the 2 periods (Supplementary Figure 4). A reduction in the surgical volume for TBI cases might have occurred from the measures taken by the authorities to maximize the resources required to manage the pandemic. However, we also noted that some studies had reported a greater proportion of surgical interventions, which affected our aggregated results and the heterogeneity.16 , 20 This was likely because these studies had included patients from a period in which the lockdown regulations had been lifted. Recommendations, reviews, and viewpoints from many surgical bodies have emerged to guide hospitals to reallocate resources by postponing any elective cases and mobilizing more staff to join the COVID-19 care team.4 , 40 In addition, the decreasing proportion of surgeries has also been attributed to the conversion of intensive care units from caring for elective patients to caring for patients with severe COVID-19.1 , 41 Moreover, some hospitals were converted to dedicated COVID-19 isolation centers because the number of patients outweighed the availability of the hospitals.42 Outpatient care management for nonemergency cases also gradually transitioned to telemedicine because many spaces were dedicated to use for COVID-19 patients.28 , 43 , 44 Taken together, these measures will eventually compromise the necessity for early management of TBI patients, causing them to progress to more severe TBI.
The present study had several limitations. Because the epidemiological pattern of TBI could vary greatly owing to differences in geographic factors, we have attempted to describe the current overall representation of how the pandemic influenced patients with TBI. In addition, the paucity of reported studies meant that we performed subset analyses according to the countries' income level. Also, because some countries had different regulations for requiring lockdown periods, this could have affected the pattern of admitted patients with TBI. Ultimately, more studies from different centers are required to strengthen the evidence illustrating the results of the measures taken to manage neurosurgical trauma cases at different centers.
Conclusions
Compared with before the pandemic, the TBI mortality rate had increased during the COVID-19 pandemic in the LMICs. In addition, the radiological findings showed a reduced rate of SDH in LMICs and an increased incidence of SAH in HICs. Our overall analysis showed an increased rate of assaults as the cause of TBI, especially in LMICs. We did not find any significant difference in surgical volumes between the 2 periods.
CRediT authorship contribution statement
Fachreza Aryo Damara: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Galih Ricci Muchamad: Investigation, Resources, Writing – original draft. Anton Anton: Writing – original draft. Alfya Nandika Ramdhani: Writing – original draft. Ivan Christian Channel: Writing – original draft. Ahmad Faried: Conceptualization, Resources, Supervision, Writing – review & editing.
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Data. Appendix
- Search terms
- ((“Brain Injuries, Traumatic"[Mesh] OR Traumatic Brain Injury[tiab] OR “Brain Concussion"[Mesh] OR “Brain Injuries"[Mesh] OR Brain Injury[tiab] OR “Accidents, Traffic"[Mesh]) AND (Pandemic OR COVID-19 OR “COVID-19"[Mesh]))
- R programming script
- library(meta)
- library(mada)
- library(readxl)
- #Odds ratio for Severity outcome
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “Severity")
- View(Document_name)
- data <- Document_name
- OR_outcome <- metabin(Document_name$modsevere_pandemic, Document_name$total_pandemic, Document_name$modsevere_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- forest(OR_outcome, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Mortality outcome
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “Mortality")
- View(Document_name)
- data2 <- Document_name
- OR_mortality <- metabin(Document_name$mortality_pandemic, Document_name$total_pandemic, Document_name$mortality_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_mortality)
- forest(OR_mortality, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Neurological deficit outcome
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “Neurological_deficit")
- View(Document_name)
- data3 <- Document_name
- OR_ND <- metabin(Document_name$ND_pandemic, Document_name$total_pandemic, Document_name$ND_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_ND)
- forest(OR_ND, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Epidural hematoma
- Document_name<- read_excel(“Document location/Document_name.xlsx",sheet = “EDH")
- View(Document_name)
- Document_name$EDH_prepandemic <- Document_name$EDH_prepandemic + 0.5
- Document_name$total_prepandemic <- Document_name$total_prepandemic + 0.5
- Document_name$EDH_pandemic <- Document_name$EDH_pandemic + 0.5
- Document_name$total_pandemic <- Document_name$total_pandemic + 0.5
- OR_EDH <- metabin(Document_name$EDH_pandemic, Document_name$total_pandemic, Document_name$EDH_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_EDH)
- forest(OR_EDH, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Subdural hematoma
- Document_name<- read_excel(“Document location/Document_name.xlsx",sheet = “SDH")
- View(Document_name)
- OR_SDH <- metabin(Document_name$SDH_pandemic, Document_name$total_pandemic, Document_name$SDH_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_SDH)
- forest(OR_SDH, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Subarachnoid hemorrhage
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “SAH")
- View(Document_name)
- Document_name$SAH_prepandemic <- Document_name$SAH_prepandemic + 0.5
- Document_name$total_prepandemic <- Document_name$total_prepandemic + 0.5
- Document_name$SAH_pandemic <- Document_name$SAH_pandemic + 0.5
- Document_name$total_pandemic <- Document_name$total_pandemic + 0.5
- OR_SAH <- metabin(Document_name$SAH_pandemic, Document_name$total_pandemic, Document_name$SAH_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_SAH)
- forest(OR_SAH, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Contusion
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “contusion")
- View(Document_name)
- Document_name$contusion_prepandemic <- Document_name$contusion_prepandemic + 0.5
- Document_name$total_prepandemic <- Document_name$total_prepandemic + 0.5
- Document_name$contusion_pandemic <- Document_name$contusion_pandemic + 0.5
- Document_name$total_pandemic <- Document_name$total_pandemic + 0.5
- OR_contusion <- metabin(Document_name$contusion_pandemic, Document_name$total_pandemic, Document_name$contusion_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_contusion)
- forest(OR_contusion, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Skull fracture
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “skull_fracture")
- View(Document_name)
- OR_SF <- metabin(Document_name$SF_pandemic, Document_name$total_pandemic, Document_name$SF_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_SF)
- forest(OR_SF, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Road traffic accident
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “RTA")
- View(Document_name)
- OR_rta <- metabin(Document_name$rta_pandemic, Document_name$total_pandemic, Document_name$rta_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_rta, digits = 2)
- forest(OR_rta, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for High fall
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “high fall")
- View(Document_name)
- OR_fall <- metabin(Document_name$fall_pandemic, Document_name$total_pandemic, Document_name$fall_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_fall, digits = 2)
- forest(OR_fall, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Low fall
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “low or mechanical fall")
- View(Document_name)
- OR_lowfall <- metabin(Document_name$lowfall_pandemic, Document_name$total_pandemic, Document_name$lowfall_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_lowfall, digits = 2)
- forest(OR_lowfall, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Assault/Violence
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “assault")
- View(Document_name)
- OR_assault <- metabin(Document_name$assault_pandemic, Document_name$total_pandemic, Document_name$assault_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_assault, digits = 2)
- forest(OR_assault, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
- #Odds ratio for Surgical management
- Document_name<- read_excel(“Document location/Document_name.xlsx”, sheet = “surgery")
- View(Document_name)
- Document_name$surgery_prepandemic <- Document_name$surgery_prepandemic + 0.5
- Document_name$total_prepandemic <- Document_name$total_prepandemic + 0.5
- Document_name$surgery_pandemic <- Document_name$surgery_pandemic + 0.5
- Document_name$total_pandemic <- Document_name$total_pandemic + 0.5
- OR_surgery <- metabin(Document_name$surgery_pandemic, Document_name$total_pandemic, Document_name$surgery_prepandemic, Document_name$total_prepandemic, sm = “OR”, method = “I", comb.fixed = F, comb.random = T, data = Document_name, studlab = Document_name$study_id)
- print(OR_surgery, digits = 2)
- forest(OR_surgery, digits = 2, rightcols = c(“effect”, “ci”), xlab = " Odds Ratio”, col.predict.lines = “red”, col.random = “red")
References
- 1.COVIDSurg Collaborative Delaying surgery for patients with a previous SARS-CoV-2 infection. Br J Surg. 2020;107:e601–e602. doi: 10.1002/bjs.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVIDSurg Collaborative; GlobalSurg Collaborative Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748–758. doi: 10.1111/anae.15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grassner L., Petr O., Warner F.M., et al. Trends and outcomes for non-elective neurosurgical procedures in central Europe during the COVID-19 pandemic. Sci Rep. 2021;11:6171. doi: 10.1038/s41598-021-85526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P.D., Kelly K.A., Reynolds R.A., et al. Tracking the volume of neurosurgical care during the coronavirus disease 2019 pandemic. World Neurosurg. 2020;142:e183–e194. doi: 10.1016/j.wneu.2020.06.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman B.G., Whitaker R., Mahendraratnam N., et al. State variation in effects of state social distancing policies on COVID-19 cases. BMC Public Health. 2021;21:1239. doi: 10.1186/s12889-021-11236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olney A.M., Smith J., Sen S., Thomas F., Unwin H.J.T. Estimating the effect of social distancing interventions on COVID-19 in the United States. Am J Epidemiol. 2021;190:1504–1509. doi: 10.1093/aje/kwaa293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page M.A.-O., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotfaliany M., Akbarpour S., Zafari N., et al. World Bank Income Group, health expenditure or cardiometabolic risk factors? A further explanation of the wide gap in cardiometabolic mortality between worldwide countries: an ecological study. Int J Endocrinol Metab. 2018;16:e59946. doi: 10.5812/ijem.59946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doebler P., Holling H. Meta-analysis of diagnostic accuracy with mada. R Packag. 2015;1:15. doi: 10.1007/s11336-014-9430-0. [DOI] [PubMed] [Google Scholar]
- 14.Valenzuela C. [2 solutions for estimating odds ratios with zeros] Rev Med Chil. 1993;121:1441–1444. [PubMed] [Google Scholar]
- 15.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karthigeyan M., Dhandapani S., Salunke P., et al. The collateral fallout of COVID19 lockdown on patients with head injury from north-west India. Acta Neurochir (Wien) 2021;163:1053–1060. doi: 10.1007/s00701-021-04723-4. [DOI] [PubMed] [Google Scholar]
- 17.Goyal N., Swain S.K., Gupta K., Chaturvedi J., Arora R.K., Sharma S.K. "Locked up inside home"—head injury patterns during coronavirus disease of 2019 pandemic. Surg Neurol Int. 2020;11:395. doi: 10.25259/SNI_675_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horan J., Duddy J.C., Gilmartin B., et al. The impact of COVID-19 on trauma referrals to a national neurosurgical centre. Ir J Med Sci. 2021:1–13. doi: 10.1007/s11845-021-02504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht N., Wessels L., Werft F.O., Schneider U.C., Czabanka M., Vajkoczy P. Need for ensuring care for neuro-emergencies-lessons learned from the COVID-19 pandemic. Acta Neurochir (Wien) 2020;162:1795–1801. doi: 10.1007/s00701-020-04437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam C., Yen J.C., Wu C.C., Lin H.Y., Hsu M.H. Effects of the COVID-19 pandemic on treatment efficiency for traumatic brain injury in the emergency department: a multicenter study in Taiwan. J Clin Med. 2021;10:5315. doi: 10.3390/jcm10225314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara-Reyna J., Yaeger K.A., Rossitto C.P., et al. "Staying home"—early changes in patterns of neurotrauma in New York City during the COVID-19 pandemic. World Neurosurg. 2020;143:e344–e350. doi: 10.1016/j.wneu.2020.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luostarinen T., Virta J., Satopää J., et al. Intensive care of traumatic brain injury and aneurysmal subarachnoid hemorrhage in Helsinki during the COVID-19 pandemic. Acta Neurochir (Wien) 2020;162:2715–2724. doi: 10.1007/s00701-020-04583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manivannan S., Sharouf F., Mayo I., et al. Management of neurotrauma during COVID-19: a single centre experience and lessons for the future. Brain Inj. 2021;35:957–963. doi: 10.1080/02699052.2021.1934731. [DOI] [PubMed] [Google Scholar]
- 24.Pinggera D., Klein B., Thomé C., Grassner L. The influence of the COVID-19 pandemic on traumatic brain injuries in Tyrol: experiences from a state under lockdown. Eur J Trauma Emerg Surg. 2021;47:653–658. doi: 10.1007/s00068-020-01445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prawiroharjo P., Pangeran D., Supriawan H., et al. Increasing traumatic brain injury incidence during COVID-19 pandemic in the emergency department of Cipto Mangunkusumo National General Hospital—a national referral hospital in Indonesia. Neurology. 2020;95(suppl 2):S11. doi: 10.1212/01.wnl.0000719968.10580.81. [DOI] [PubMed] [Google Scholar]
- 26.Rault F., Terrier L., Leclerc A., et al. Decreased number of deaths related to severe traumatic brain injury in intensive care unit during the first lockdown in Normandy: at least one positive side effect of the COVID-19 pandemic. Acta Neurochir (Wien) 2021;163:1829–1836. doi: 10.1007/s00701-021-04831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santing J.A.L., van den Brand C.L., Jellema K. Traumatic brain injury during the SARS-CoV-2 pandemic. Neurotrauma Rep. 2020;1:5–7. doi: 10.1089/neur.2020.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal N., Venkataram T., Singh V., Chaturvedi J. Collateral damage caused by COVID-19: change in volume and spectrum of neurosurgery patients. J Clin Neurosci. 2020;80:156–161. doi: 10.1016/j.jocn.2020.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiba H., Lewis M., Benjamin E.R., et al. "Safer at home": the effect of the COVID-19 lockdown on epidemiology, resource utilization, and outcomes at a large urban trauma center. J Trauma Acute Care Surg. 2021;90:708–713. doi: 10.1097/TA.0000000000003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamine T.H., Rembisz A., Barron R.J., Baldwin C., Kromer M. Decrease in trauma admissions with COVID-19 pandemic. West J Emerg Med. 2020;21:819–822. doi: 10.5811/westjem.2020.5.47780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doucette M.L., Tucker A., Auguste M.E., et al. Initial impact of COVID-19's stay-at-home order on motor vehicle traffic and crash patterns in Connecticut: an interrupted time series analysis. Inj Prev. 2021;27:3–9. doi: 10.1136/injuryprev-2020-043945. [DOI] [PubMed] [Google Scholar]
- 32.Dhandapani M., Dhandapani S. Challenges posed by COVID-19 and neurosurgical nursing strategies in developing countries. Surg Neurol Int. 2020;11:441. doi: 10.25259/SNI_677_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W.J., Liang W.H., Zhao Y., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batista S.R., Souza A.S.S., Nogueira J., et al. Protective behaviors for COVID-19 among Brazilian adults and elderly living with multimorbidity: the ELSI-COVID-19 initiative. Cad Saude Publica. 2020;36(suppl 3):e00196120. doi: 10.1590/0102-311X00196120. [DOI] [PubMed] [Google Scholar]
- 35.Li G., Liu Y., Jing X., et al. Mortality risk of COVID-19 in elderly males with comorbidities: a multi-country study. Aging (Albany NY) 2020;13:27–60. doi: 10.18632/aging.202456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haegens N.M., Gathier C.S., Horn J., Coert B.A., Verbaan D., van den Bergh W.M. Induced hypertension in preventing cerebral infarction in delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2018;49:2630–2636. doi: 10.1161/STROKEAHA.118.022310. [DOI] [PubMed] [Google Scholar]
- 37.McGurgan I.J., Clarke R., Lacey B., et al. Blood pressure and risk of subarachnoid hemorrhage in China. https://doi.org/10.1161/STROKEAHA.118.022239 [e-pub ahead of print]. Stroke. [DOI] [PMC free article] [PubMed]
- 38.Inada H., Ashraf L., Campbell S. COVID-19 lockdown and fatal motor vehicle collisions due to speed-related traffic violations in Japan: a time-series study. Inj Prev. 2021;27:98–100. doi: 10.1136/injuryprev-2020-043947. [DOI] [PubMed] [Google Scholar]
- 39.Saladie O., Bustamante E., Gutierrez A. COVID-19 lockdown and reduction of traffic accidents in Tarragona province, Spain. Transp Res Interdiscip Perspect. 2020;8:100218. doi: 10.1016/j.trip.2020.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.COVIDSurg Collaborative COVID-19-related absence among surgeons: development of an international surgical workforce prediction model. BJS Open. 2021;5:zraa021. doi: 10.1093/bjsopen/zraa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajunaid K., Alqurashi A., Alatar A., et al. Neurosurgical procedures and safety during the COVID-19 pandemic: a case-control multicenter study. World Neurosurg. 2020;143:e179–e187. doi: 10.1016/j.wneu.2020.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glasbey J.C., Nepogodiev D., Simoes J.F.F., et al. Elective cancer surgery in COVID-19—free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39:66–78. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung C., Wadhwa H., Sklar M., et al. Telehealth adoption across neurosurgical subspecialties at a single academic institution during the COVID-19 pandemic. World Neurosurg. 2021;150:e539–e549. doi: 10.1016/j.wneu.2021.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert W.A., Leclair N.K., Knopf J., et al. Predictors of telemedicine utilization in a pediatric neurosurgical population during the COVID-19 pandemic. World Neurosurg. 2021;153:e308–e314. doi: 10.1016/j.wneu.2021.06.120. [DOI] [PMC free article] [PubMed] [Google Scholar]