Abstract
Background
A carbapenem-resistant Acinetobacter baumannii outbreak in the COVID intensive care unit of a community hospital was contained using multidrug resistant organism guidelines. The purpose of this study is to report on an outbreak investigation and containment strategy that was used, and to discuss prevention strategy.
Methods
A multidisciplinary approach contained the spread of infection. Strategies implemented included consultation with experts, screening, and reversal of personal protective equipment conservation. Ensuring infection control best practices are maintained remain important efforts to reduce the spread of multidrug resistant organisms.
Results
Five patients with carbapenem-resistant Acinetobacter baumannii were identified from routine clinical cultures within one week and one patient was identified from active surveillance cultures.
Discussion
Personal protective equipment conservation, strategies to prevent health care personnel exposure, and patient surge staffing protocols may have increased the likelihood of multidrug resistant organism transmission. Environmental and behavioral infection control regulations with effective administrative guidance, active surveillance cultures, and antimicrobial stewardship are critical to prevent future outbreaks.
Conclusions
After outbreak containment strategies were implemented, no additional patients were identified with carbapenem-resistant Acinetobacter baumannii. Conventional infection prevention and control strategies were re-instituted. A multidisciplinary approach with continued focus on hand hygiene, environmental cleaning, and correct use of personal protective equipment needs to be put in place to successfully contain and prevent the spread of carbapenem resistant infections.
Key words: Outbreak, Containment, COVID, MDRO, Infection prevention, PPE, Acinetobacter baumannii, Infection control, Antimicrobial resistance
Background
The COVID-19 pandemic brought many challenges with regard to standard infection prevention practices and the proliferation of multidrug resistant organisms (MDROs).1 , 2 Several outbreaks of MDROs within COVID units have been described in the literature.3 During the first few months of the pandemic, personal protective equipment (PPE) availability worldwide was limited and the infection prevention practices were adjusted in order to maintain the access to PPE necessary for patient care. As a result of the acute need for PPE in health care and in the general population, the markets were depleted of the products necessary to protect health care workers from COVID-19 and other potential infectious diseases.4 The Centers for Disease and Control and Prevention (CDC) recommended strategies for conserving and optimizing PPE supply due to the shortage and strategies to extend and reuse PPE were published and advised to the health care industry.5, 6, 7 CDC, Agency for Healthcare Administration (AHCA) and Centers for Medicare and Medicaid (CMS) guidelines for infection control were implemented at all times throughout the pandemic.
Acinetobacter baumannii is a gram-negative, strictly aerobic, non-fastidious, non-fermenting, catalase-positive, oxidase-negative opportunistic pathogen that is a challenging nosocomial organism that is considered an emerging public health threat.8 A. baumannii may cause serious infections including nosocomial pneumonia, bloodstream infections, wound infections, urinary tract infections, and meningitis.8 , 9 Carbapenem resistant Acinetobacter baumannii (CRAB) is listed as an “urgent threat” antibiotic resistant pathogen along with Candida auris, Clostridioides difficile, carbapenem resistant Enterobacteriaceae (CRE), and drug resistant Neisseria gonorrhoeae.10 The threat level was escalated to “urgent” from “serious” in 2019 because of its ability to acquire resistance determinants and the lack of current treatment options.10 11 In 2017, 8,500 infections, 700 deaths, and 281 million dollars of health care costs were attributed to CRAB10. Along with antibiotic resistance, the ability of A. baumannii to survive for prolonged periods in a hospital environment further potentiates nosocomial spread particularly in intensive care units (ICU) and long-term care facilities.12 , 13 The spread of infection can also be facilitated by healthcare personnel who are at increased risk of becoming contaminated with CRAB through contact transmission.14 The majority of patients who develop nosocomial CRAB infection have been exposed to a healthcare facility and have an indwelling device.9 In this study, we report a detailed investigation of a CRAB outbreak in the COVID ICU of a community hospital. We will discuss successful containment strategies that were utilized during the outbreak as well as best practices to prevent future outbreaks.
Methods
The community hospital is a 176-bed acute care facility with an 18 bed ICU compromised of all private rooms with a centralized nursing station, medication room, and nutrition room. CRAB had not been previously identified in this ICU in the years leading up to the outbreak and it has not been identified since the containment strategies were implemented (Table 1). During the pandemic, the ICU was being used for critical care level COVID positive patients. Identification of an index patient with carbapenem resistant A. baumanii in the COVID ICU in November 2020 prompted an outbreak investigation and mounted an immediate containment response. Upon identification of further patients, the outbreak management was expanded.
Table 1.
Incidence rates of all site MDR-Acinetobacter baumannii in the ICU at the community hospital, 2019-2021
| Year | Number of patients | Rate per 1,000 patient days |
|---|---|---|
| 2018 | 0 | 0 |
| 2019 | 0 | 0 |
| 2020 | 6 | 1.66 |
| 2021 | 0 | 0 |
Cohorting and isolation
The response began with immediate closure to new admissions or transfers as well as cohorting of all exposed patients in the unit. Entry points were restricted in the ICU and staff entry was limited including consulting physicians utilizing telehealth. Positive CRAB patients were placed on a designated wing with dedicated nursing and respiratory staff. All patients that had been transferred out of the COVID ICU were maintained on contact transmission based precautions as well as airborne isolation due to their COVID diagnosis. The remaining patients in the ICU were considered potentially exposed and were moved to clean rooms but remained in the ICU until active surveillance cultures (ASC) resulted. CRAB patients were placed on a new, expanded, enhanced contact and airborne transmission based precautions which included isolation gowns, gloves, respirators, and eye protection. Gown conservation strategies that had been implemented due to anticipated PPE shortages caused by COVID-19 were immediately reversed. This change now required isolation gowns and gloves to be discarded after each patient encounter according to pre-pandemic conventional infection prevention and control practices.
Active surveillance cultures
Following the identification of multiple patients with CRAB in the ICU, containment began with planning point prevalence screenings using active surveillance cultures (ASC) on the remaining patients in the unit. Upon conducting a literature review it was determined that the best body site for screening for A. baumanii had not been well documented.15 After consulting with the hospital microbiology department, the infection prevention team determined that the best immediate action was to collect throat swabs from all patients within the COVID ICU. Since the initial positive clinical specimen had been a sputum culture from a ventilated patient in the COVID ICU ward, it was thought that shared respiratory therapy practices within the ward may have been the source of transmission. Non-invasive groin and axillae screening had been described in the literature and was additionally performed after consultation with the local and state Department of Health and CDC experts in order to identify additional cases.16 While the most conclusive site of A. baumannii active surveillance screening has not been determined due to the low yield of single site collections, our study used 2 different sites of both axillae and groin to assess for potential colonization.16 , 17 Two sites were agreed upon by the multidisciplinary team due to pandemic surge constraints in the laboratory.
Screening for new cases through ASC was performed on all patients in the unit as well as 2 additional hospital units that had received patient transfers before the COVID ICU was closed. Nurses were given specific instructions when collecting swab cultures, requiring 5 passes of the swab in each body location moving from axillae to groin. The initial point prevalence testing (PP#1) was multifaceted; initially throat swabs were collected on all patients in the COVID ICU as well as 2 patients that had been moved to the COVID telemetry ward (n = 19). One colonized patient was identified in the COVID-ICU. The following day, after consultation with the state, additional swabs of the axillae and groin were collected on the patients within the COVID ICU as well as non-COVID ICU (n = 28) that shared respiratory, nursing and intensivist staff. A second point prevalence survey (PP#2) was conducted 2 weeks later with sputum cultures collected on all ventilated patients within both the COVID ICU and non-COVID ICU as well as axillae and groin swabs collected on all patients within both wards (n = 22). One additional patient was identified with colonization from ASC, however, it was determined that this patient was not part of the outbreak due to differences in resistance patterns. Each week thereafter the third (PP#3, n = 23) and fourth (PP#4, n = 16) point prevalence surveys were conducted on all patients within the COVID ICU and non-COVID ICU by collecting axillae and groin swabs. No additional patients were identified. A follow-up conference call was conducted with CDC experts and it was determined by expert consensus that 2 negative point prevalence surveys were sufficient evidence to stop conducting ASC and to monitor clinical specimens closely for 3 months. No environmental cultures were collected since fomite transmission was not suspected after 2 point prevalence surveys were conducted with no additional cases identified indicating that there was no cross transmission within the unit and the outbreak had been contained.
Laboratory
For each patient, a single BD BBL CultureSwab Plus (Becton Dickinson, Sparks, MD) swab was used to culture the axillae and the groin bilaterally, in that order. The specimens were inoculated within 1 hour of receipt in the Microbiology Laboratory to trypticase soy agar with 5% sheep blood (BAP), chocolate agar (CHOC), Columbia CNA agar (CNA), and MacConkey agar (MAC) plates (Becton Dickinson) and were incubated for 18-24 hours at 35°C in 5% CO2. Culture plates were examined daily for 48 hours for colonies of growth meeting the morphologic criteria consistent with the genus Acinetobacter, and suspect colonies were identified to the species level by use of MALDI-TOF mass spectrometry (bioMerieux, Hazelwood, MO). Antimicrobial susceptibility testing was performed on all isolates identified as A. baumannii complex using the Vitek 2 (bioMerieux, Hazelwood, MO). A. baumannii isolates that were defined as carbapenem resistant were further tested in-house against minocycline and amikacin (Kirby Bauer), and ceftazidime/avibactam (ETEST), and submitted to ARUP Reference Laboratory (500 Chipeta Way, Salt Lake City, UT) for susceptibility testing against polymycin B and cefidericol. The isolates were also sent to the Tennessee Department of Health for confirmation of organism identification, and molecular testing of resistance genes KPC, NDM, OXA-48, VIM, IMP, OXA-42/40-like, OXA-58-like, and OXA-23-like. All isolates submitted were identified as having the molecular type OXA-23-like gene detected.
Environment of care
A. baumanni has been known to survive on surfaces for months.18 Medical equipment was dedicated to the positive CRAB cohort and included an X-ray machine, respiratory equipment, continuous renal replacement therapy (CRRT) machines, computer workstations on wheels (WOWs) and glucometers. Movement of all equipment was limited in and out of the COVID ICU and the need to clean all equipment between every patient was emphasized. In order to ensure all high touch surfaces were manually disinfected multiple times per day, dedicated environmental services (EVS) technicians were assigned to the entire unit for 24/7 coverage for the duration of the outbreak. Additional trash cans were placed in the unit to prevent PPE overflow and cross-contamination. EVS completed terminal cleaning of each room 3 times with manual cleaning and electrostatic spraying of a hypochlorite solution. Additionally, EVS added the use of a quaternary ammonia disinfectant on floors. Manual cleaning was monitored using fluorescent markers and direct observation. Vaporized hydrogen peroxide was utilized as a final terminal cleaning method in all of the ICU patient rooms.
Patient care
Patient care interventions included increasing daily chlorhexidine gluconate (CHG) bathing on all ICU patients to twice daily for patient decolonization. The respiratory therapists changed the ventilator circuits, tubing, suction and canister daily. Medication preparation practices that had been performed outside the patient room were moved back inside the room at the bedside on a clean bedside table. Equipment that had been externalized was moved back inside the patient room and all supplies stored around the sinks were removed due to potential contamination risk.
Education
Healthcare worker transmission of COVID was a main focus of education. Double gloving and gown conservation were thought to have been practiced for convenience and due to health care workers' fear of acquring COVID. Hand hygiene, PPE, and equipment disinfection educational rounds were completed by Infection Control. Considerable focus was placed on appropriate glove use, necessary use of alcohol based hand rub as well as soap and water hand washing between patient care tasks. Huddles and rounding were conducted, emphasizing the need to disinfect shared equipment. Targeted education on the proper use, donning and doffing of PPE was completed by Infection Control. Monitoring was conducted during daily rounding with real time coaching and feedback by Infection Control, Quality, Nurse Manager, Respiratory Manager, and Clinical Managers to ensure containment strategies were being practiced across all shifts. Daily huddles on MDRO transmission and prevention strategies were conducted with the COVID-19 ICU team and all ancillary departments were educated on outbreak protocols in place. The leadership team was debriefed multiple times during the outbreak and daily during morning leadership huddles to keep the entire hospital abreast of issues identified and potential needs. Infection Control collaborated with the Florida Department of Health Hospital Acquired Infection (HAI) Program and CDC experts to review and approve the outbreak action plan.
Results
Outbreak investigation
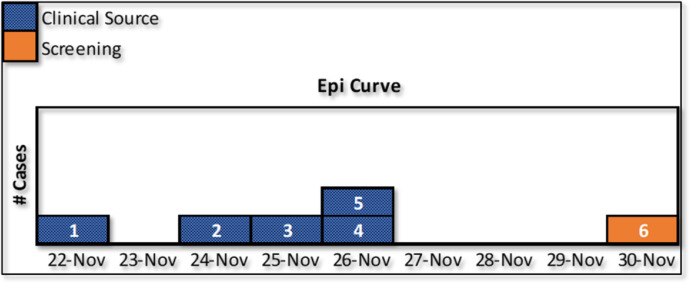
The Manager of Infection Control identified patients on a line listing (Table 2 ) and an epidemic curve through clinical cultures and ASC (Fig 1 ). All patients had been mechanically ventilated. The first case of CRAB was identified in a clinical sputum culture of a ventilated COVID patient on November 22, 2020 nine days after the patient was admitted to the hospital and the COVID ICU. Within the next 4 days, 4 more patients were identified with clinical cultures growing the same organism with very similar resistance patterns. The second patient had been admitted for 3 days and grew positive blood and sputum cultures with CRAB. A third patient was soon identified with positive CRAB blood cultures. Both the second and third patients were acutely ill with COVID-19, acquired bloodstream infections with CRAB, and expired within 3 days of the positive cultures. A fourth patient was a non-COVID-19 patient that happened to be located in the COVID ICU due to acuity of illness. This patient's CRAB source was in the urine and then subsequently identified in the sputum. The fifth patient had been in the ICU since September 23, 2020 and initially had an MDRO Escherichia coli in the sputum before acquisition of CRAB. The sixth patient was identified through ASC in groin and axillae and later also grew CRAB in the sputum. There were 108 total screening specimens collected throughout the 4 point prevalence ASC. The rest of the containment strategies were in place for 3 months after the last point prevalence survey although PPE conservation was never reinstated in the ICU and targeted infection control rounds continued.
Table 2.
Line listing of a CRAB outbreak in COVID ICU
| Age | Hospital admission date | ICU admission date | ICU admission reason | COVID-19 status | Date of first CR-AB isolate collection | CR-AB isolate source(s) | Patient outcomes |
|---|---|---|---|---|---|---|---|
| 62 | 11/9/2020 | 11/13/2020 | Worsening viral PNA* related to COVID-19 | Positive | 11/22/2020 | Sputum | Deceased |
| 62 | 11/1/2020 | 11/3/2020 | Worsening viral PNA* related to COVID-19 | Positive | 11/24/2020 | Sputum | Deceased |
| 82 | 11/1/2020 | 11/11/2020 | Worsening viral PNA* related to COVID-19 | Positive | 11/25/2020 | Sputum and blood | Deceased |
| 69 | 9/23/2020 | 10/3/2020 | Worsening viral PNA* related to COVID-19 | Positive | 11/26/2020 | Sputum | Deceased |
| 49 | 10/7/2020 | 10/29/2020 | Respiratory failure due to re-expansion of pulmonary edema | Negative | 11/26/2020 | Sputum and urine | Deceased |
| 59 | 11/14/2020 | 11/15/2020 | Worsening viral PNA related to COVID-19 | Positive | 11/30/2020 | Throat, Sputum | Deceased |
Pneumonia.
Fig 1.
Epidemic curve of CRAB outbreak in COVID ICU.
Discussion
Patient care during the COVID-19 pandemic included a reversal of standard infection prevention and control containment strategies for MDROs. The first CRAB patient had multiple risk factors for MDRO and is believed to be the initial source of the outbreak. PPE conservation strategies with lapses in infection control during pandemic surge were the potential causes of transmission. On March 10, 2020, the infection preventionists at our organization educated staff on limited re-use of N95 respirators for patients that did not require contact transmission based precautions. Isolation gowns were not to be re-used for any suspected persons under investigation or positive COVID patients. PPE in our organization was highly controlled and continuously tracked utilizing burn rate calculators that predicted community surge and the supply needs of the health care system. On March 25, 2020, the CDC presented a COCA call describing best practices for optimization strategies for health care personnel PPE.19 Infection preventionists, supply chain, and administration continuously monitored the need for implementation of PPE conservation. The CDC guidelines for crisis capacity contingency strategies included “extending the use of isolation gowns such that the same gown is worn by the same provider when interacting with more than one patient housed in the same location and known to be infected with the same infectious disease (i.e., COVID-19 patients residing in an isolation cohort).”10 Following this guidance, once an MDRO patient was identified, the gown conservation was to be immediately ceased. However, nosocomial transmission may have occurred prior to laboratory identification of an MDRO specimen when gown conservation was still in place. Furthermore, the long length of stay of COVID-19 inpatients, along with the incidence of secondary infections in this population, and the increased usage of antimicrobial agents in critically ill COVID patients may have contributed to MDRO formation through antibiotic pressure.
In order to decrease their own exposure to COVID, health care personnel began performing medication preparation outside of patient rooms prior to entry. Additionally, equipment such as intravenous pumps and ventilators were placed outside the patient rooms. These actions may have contributed to fomite transmission of MDROs if hand hygiene or equipment disinfection was not adequately performed. A. baumanni has been found on healthcare personnel hands particularly in the ICU.20 Significant education was necessary to continuously reinforce infection prevention and control standards. Decreased compliance with conventional infection control practices and modifications in patient care were thought to have occurred during periods of patient surge when healthcare personnel were limiting their own exposure. Pandemic patient care strategies such as team nursing, increased use of agency personnel, and the staff's augmented responsibilities may have also contributed to potential nosocomial transmission. Swift identification of a cluster of multidrug resistant organisms by clinicians and immediate action by the Manager of Infection Conrol was crucial in the containment of this outbreak.
The strategies to prevent future outbreaks are the same common principles that were utilized to contain this outbreak. These practices must continue despite obstacles imposed by the COVID-19 pandemic. Continued efforts to prevent future outbreaks include administrative support and adherance to the most fundamental infection control measures such as hand hygiene, transmission based precautions, and environmental disinfection. Point prevalence surveys using ASC can also be part of a tiered approach to containment. Another adjunct strategy to consider is a screening protocol for high risk admissions.21 Previously, ASC have only been suggested as an additional measure because they have not been shown to decrease cross-transmission of CRAB in the endemic setting.21 , 15 However, modifying screening protocols could make ASC an effective tool and MDRO prevention strategy which includes screening multiple body sites. A single swab test has only been found to be up to 30% sensitive in detecting A. baumannii.22 New methods, including using a sponge to sample 2 sites simultaneously, have been found to be more than 80% sensitive in detection.23 Including both a swab of the buccal mucosa and sponge sample of the skin has been found to be the most effective with 99% sensitivity in CRAB detection.17 CDC partners also suggested using skin swabs for the point prevalence studies. High-risk patient settings or populations in areas with endemic MDROs could consider updating their ASC protocol with more sensitive methods and apply these methods to each patient admission. This screening effort should involve active participation from the laboratory to ensure that medical staff, infection control, and administration are immediately notified when an MDRO is identified. Finally, the importance of antimicrobial stewardship is evident and needed to suppress the emergence and spread of MDROs.
Conclusion
The first highly resistant CRAB specimen was detected in a sputum culture on a COVID-19 ICU patient in November 2020. In subsequent days, several other patients with varying demographics in the COVID-19 dedicated ICU tested positive for CRAB in multiple sources. Adherence to pre-pandemic infection control standards and single-use PPE, may have prevented this outbreak. Adjuvant measures to contain the outbreak including MDRO management best practices such as vaporized hydrogen peroxide for no-touch environmental disinfection, dedicated environmental cleaning staff, and point prevalence surveillance studies using ASC have all been described in previous outbreaks.24 , 25 Once conventional infection prevention and control standards were re-instituted, we were able to contain the outbreak of CRAB in a COVID-19 ICU. MDROs are increasing at an alarming rate. Over the last decade, carbapenem non-susceptible A. baumannii infections are becoming more common among hospital settings and present a significant clinical challenge as treatment choices for these organisms are limited.8 , 26 Preventing the spread of resistant pathogens such as carbapenem resistant A. baumannii is also considered an important public health concern.10, 11 Identifying and detecting resistant microorganisms, as well as providing ongoing infection prevention and control education and monitoring should be prioritized by community hospitals.
Acknowledgments
We would like to thank the entire team at Memorial Hospital Miramar and the Memorial Healthcare System who embodied the vision of patient centered care and improving the health of the community during each COVID surge.
Footnotes
Funding/support: None.
Conflicts of interest: All authors report no conflicts of interest and no external financial support.
References
- 1.Polly M., de Almeida B.L., Lennon R.P., Cortês M.F., Costa S.F., Guimarães T. Impact of the COVID-19 pandemic on the incidence of multidrug-resistant bacterial infections in an acute care hospital in Brazil. Am J Infect Control. 2021;S0196-6553 doi: 10.1016/j.ajic.2021.09.018. 00618-0Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rangel K., Chagas T., De-Simone S.G. Acinetobacter baumannii infections in times of COVID-19 pandemic. Pathogens. 2021;10:1006. doi: 10.3390/pathogens10081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez S., Innes G.K., Walters M.S., et al. Increase in hospital-acquired carbapenem-resistant Acinetobacter baumannii infection and colonization in an acute care hospital during a surge in COVID-19 admissions - New Jersey, February-July 2020. MMWR. Morbidity and mortality weekly report. 2020;69:1827–1831. doi: 10.15585/mmwr.mm6948e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen J., Rodgers Y. Contributing factors to personal protective equipment shortages during the COVID-19 pandemic. Prev Med. 2020;141 doi: 10.1016/j.ypmed.2020.106263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; 2021. Interim infection prevention and control recommendations for healthcare personnel during the Coronavirus Disease 2019 (COVID-19) pandemic.https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html [Google Scholar]
- 6.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; 2021. Strategies for optimizing the supply of isolation gowns.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/isolation-gowns.html [Google Scholar]
- 7.Messonnier N., Appiah G.D., Bell M., Kuhar D.T., Delaney L. Clinician Outreach and Communication Activity (COCA) Call. Emergency Preparedness and Response. Centers for Disease Control and Prevention; 2020. COVID-19 Update: optimization strategies for healthcare personal protective equipment (PPE)https://emergency.cdc.gov/coca/calls/2020/callinfo_032520.asp [Google Scholar]
- 8.Balkhair A., Al-Muharrmi Z., Al'Adawi B., et al. Prevalence and 30-day all-cause mortality of carbapenem-and colistin-resistant bacteraemia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: Description of a decade-long trend. Int J Infect Dis. 2019;85:10–15. doi: 10.1016/j.ijid.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Bulens S.N., Yi S.H., Walters M.S., et al. Carbapenem-nonsusceptible Acinetobacter baumannii, 8 US metropolitan areas, 2012-2015. Emerg Infect Dis. 2018;24(4):727–734. doi: 10.3201/eid2404.171461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; 2019. Antibiotic resistant threats in the United States, 2019.https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf [Google Scholar]
- 11.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; 2019. Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs)https://www.cdc.gov/hai/containment/guidelines.html [Google Scholar]
- 12.Peleg A.Y., Hooper D.C. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peleg A.Y., Seifert H., Paterson D.L. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan D.J., Liang S.Y., Smith C.L., et al. Frequent multidrug-resistant Acinetobacter baumannii contamination of gloves, gowns, and hands of healthcare workers. Infect Control Hosp Epidemiol. 2010;31:716. doi: 10.1086/653201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tacconelli E., Cataldo M.A., Dancer S.J., et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin Microbiol Infect. 2014;20:1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 16.Buser G.L., Cassidy P.M., Cunningham M.C., et al. Failure to communicate: transmission of extensively drug-resistant blaOXA-237-containing Acinetobacter baumannii—multiple Facilities in Oregon, 2012–2014. Infect Control Hosp Epidemiol. 2017;38:1335–1341. doi: 10.1017/ice.2017.189. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/isolation-gowns.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nutman A., Temkin E., Lellouche J., Ben David D., Schwartz D., Carmeli Y. Detecting carbapenem-resistant Acinetobacter baumannii (CRAB) carriage: Which body site should be cultured? Infect Control Hosp Epidemiol. 2020;41:965–967. doi: 10.1017/ice.2020.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer A., Schwebke I., Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furuno J.P., Hebden J.N., Standiford H.C., et al. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008;36:468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montoya A., Schildhouse R., Goyal A., et al. How often are health care personnel hands colonized with multidrug- resistant organisms? A systematic review and meta-analysis. Am J Infect Control. 2019;47:693–703. doi: 10.1016/j.ajic.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Maragakis L.L., Tucker M.G., Miller R.G., Carroll K.C., Perl T.M. Incidence and prevalence of multidrug-resistant Acinetobacter using targeted active surveillance cultures. JAMA. 2008;299:2513–2514. doi: 10.1001/jama.299.21.2513. [DOI] [PubMed] [Google Scholar]
- 22.Marchaim D., Navon-Venezia S., Schwartz D., et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol. 2007;45:1551–1555. doi: 10.1128/JCM.02424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi Y., Onuoha E.O., Adams-Haduch J.M., et al. Screening for Acinetobacter baumannii colonization by use of sponges. J Clin Microbiol. 2011;49:154–158. doi: 10.1128/JCM.01043-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C.H., Lin L.C., Chang Y.J., Chen Y.M., Chang C.Y., Huang C.C. Infection control programs and antibiotic control programs to limit transmission of multi-drug resistant Acinetobacter baumannii infections: evolution of old problems and new challenges for institutes. Int J Environ Res Public Health. 2015;12:8871–8882. doi: 10.3390/ijerph120808871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Agata E., Thayer V., Schaffner W. An outbreak of Acinetobacter baumannii: the importance of cross-transmission. Infection Control & Hospital Epidemiology. 2000;21:588–591. doi: 10.1086/501808. [DOI] [PubMed] [Google Scholar]
- 26.Adams-Haduch JM, Onuoha EO, Bogdanovich T, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49:3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]