Abstract
Background
A broad vaccination coverage is crucial for preventing the spread of Covid-19 and reduce serious illness or death. The aim of this study was to examine social inequalities in Covid-19 vaccination uptake as of 17th May 2021 among Swedish adults aged ≥ 60 years.
Methods
The study population comprised a general population cohort aged 60 years or older (n = 350,805), representative of the Swedish population. Data were collected through the nationwide linked multi-register observational study SCIFI-PEARL, and associations between sociodemographic determinants and Covid-19 vaccination uptake were analysed using logistic regression. Intersectional analyses of sociodemographic heterogeneity were performed by taking several overlapping social dimensions into account. Data availability extended to 17 May 2021.
Findings
The overall vaccination coverage was 87·2% by 17th May 2021. Younger age, male sex, lower income, living alone, and being born outside Sweden, were all associated with a lower uptake of vaccination. The lowest Covid-19 vaccination uptake was seen in individuals born in low-or middle-income countries, of which only 60% had received vaccination, with an odds ratio (OR) of not being vaccinated of 6·05 (95% CI: 5·85–6·26) compared to individuals born in Sweden. These associations persisted after adjustments for possible confounding factors. The intersectional analyses showed even larger variations in vaccination in cross-classified sociodemographic subgroups (ranging from 44% to 97%) with marked differences in uptake of vaccination within sociodemographic groups.
Interpretation
The uptake of Covid-19 vaccine during the spring of 2021 in Sweden varied substantially both between and within sociodemographic groups. The use of an intersectional approach, taking several overlapping social dimensions into account at the same time rather than only using one-dimensional measures, contributes to a better understanding of the complexity in the uptake of vaccination.
Funding
SciLifeLab / Knut & Alice Wallenberg Foundation, Swedish Research Council, Swedish government ALF-agreement, FORMAS.
Keywords: COVID-19, Vaccination, Social determinants of health, Sociodemographic factors, Intersectionality, Public health
Research in context.
Evidence before this study
Only few previous studies have investigated socioeconomic disparities in the uptake of a Covid-19-vaccine in a general population, but none of these have used an intersectional analysis approach. We searched PubMed on November 2nd 2021 using the terms (“vaccination” OR “immunisation” OR “vaccine”) AND (“Covid-19” OR “Covid” OR “SARS-CoV-2”) AND (“uptake” OR “acceptance” OR “coverage” OR “recipient”) AND (“sociodemographic” OR “socioeconomic” OR “demographic” OR “social”); we used no language selection; articles were selected based on the abstract and title. We found that most articles in this field of research had investigated attitudes and potential acceptance towards Covid-19 vaccination, or uptake in specific groups such as health care workers, assisted living and residential care communities, or specific ethnic minority groups, rather than actual uptake of vaccination in a general population.
Added value of this study
This study, based on high quality Swedish health and population registers, used an intersectional analysis approach taking several overlapping social dimensions into account at the same time rather than only using one-dimensional measures. Our results provide new insights into the understanding of the sociodemographic complexity in Covid-19 vaccine uptake, showing marked differences in the uptake of vaccination not only between, but also within sociodemographic groups.
Implications of all the available evidence
This study adds valuable information to the research field of inequalities in the uptake of Covid-19 vaccines by using an intersectional analysis approach. Previous studies have shown a lower coverage with lower age, in lower income groups, male sex, low educational level groups, and ethnic minority groups when using one-dimensional measures of sociodemographic factors. Besides confirming the mentioned differences in vaccination coverage, this study also showed wide differences in vaccination uptake within sociodemographic groups. To reduce sociodemographic disparities in vaccination coverage it is important to increase the impact of the vaccination program in less privileged population groups.
Alt-text: Unlabelled box
Introduction
Vaccination against Covid-19 has progressed well in most developed countries. In Sweden, the vaccination program started on 27th December 20201 and by 5th November 2021, 85% of the adult population had received their first dose.2 The program was implemented in four sequential stages, where the first stage mainly included older adults in assisted living and residential care, and health- or elderly-care workers, while the second stage included all remaining adults ≥65 years, individuals with functional disabilities, and some specific risk groups. By 25th March 2021, all the 21 regions in Sweden had initiated the second stage of vaccination. Stage three, initiated by the vast majority of regions by 26th April, mainly included individuals aged 60–64 years, and various risk groups aged 18–59 years. Finally, stage four included individuals aged 18–59 years who had not been part of phases 1–33; by May 25th 2021 most of the regions had initiated vaccination in the fourth stage.1
Previous studies internationally have demonstrated socioeconomic differences in vaccination uptake for seasonal influenza,4 differences in attitudes and barriers to childhood vaccinations,5 and willingness among adults to be vaccinated.6 Regarding influenza vaccination, associations have been shown between low socioeconomic status (i.e., measured through income, occupation, highest social class in the household or deprivation index of the residential area) and a lower uptake of vaccination, however, results from different studies vary reflecting on the complexity of this issue.4 Studies on the potential acceptance of a Covid-19 vaccine have generally shown a more positive attitude towards vaccination among women,7 older adults,7 higher educated groups,7, 8, 9 and higher income groups.7 Research has also revealed evident ethnic disparities in vaccine hesitancy.9, 10, 11 However, few studies have investigated social inequalities in actual Covid-19 vaccination uptake. The Public Health Agency of Sweden has encouraged a broad vaccination coverage to better prevent serious illness and death, especially since Covid-19, like many other diseases, has been shown to affect lower socioeconomic groups with more severe disease.12, 13, 14, 15
The Public Health Agency of Sweden has reported some data showing differences in vaccination rates across areas of residence and differences between ethnic groups.12 Their regularly updated statistics also include very basic intersectional (cross-classified) descriptions, which indicate differences between sociodemographic groups, and heterogeneity within such groups. Even though such reports are an important basic first step, it is essential also to apply a scientifically based approach to systematically investigate sociodemographic factors associated with vaccine uptake and how they interact.
A recent study from England on older adults showed that vaccination rates differed by demographic and socioeconomic factors, where ethnic minorities, living in areas of deprivation, and low socioeconomic position were associated with lower vaccination rates, even after adjustments for comorbidities.16 Comparable observations were made in two other British studies using similar data, with lower vaccination coverage among ethnic minorities and those living in deprived areas.17 , 18
Sociodemographic disparities in health are traditionally described in relation to certain determinants of health such as socioeconomic group (or social class), income, education level or household composition.19 However, recent research has indicated that taking several overlapping social dimensions into account at the same time can contribute to a more nuanced understanding of socioeconomic disparities in health.20, 21, 22 In the present study, based on data from the nationwide linked multi-register observational study SCIFI-PEARL (Swedish Covid-19 Investigation for Future Insights – A Population Epidemiology Approach Using Register Linkage),23 we examined sociodemographic determinants associated with Covid-19 vaccination uptake in the general Swedish population aged 60 years or older during the first five months of the Covid-19 vaccination program. Also, heterogeneity within sociodemographic groups was studied by using intersectional analysis models that have previously been suggested for detailed sociodemographic characterisation.20, 21, 22
Methods
Study design and study population
The SCIFI-PEARL project is based on a linked and regularly updated database including all registered individuals in Sweden diagnosed with Covid-19, together with a large general population cohort, described in detail elsewhere.23 The database constitutes a nationwide observational study based on multiple health and population registers linked with high accuracy (close to 100%) and quality using the unique Swedish Personal Identity Number (PIN).24 The general population comparison cohort was obtained from the National Register of the Total Population (RTB) at Statistics Sweden and comprised about ten percent of all individuals of all ages living in Sweden on 1 January 2020. Subjects were randomly sampled from 20 five-year age groups by sex (40 strata) with 25,000 individuals from each stratum except the oldest strata where all individuals were included (N = 972,723). Exact sampling fractions were available. The study design is a register-based cross-sectional study and the study population consists of those individuals in the general population cohort aged ≥60 years who were alive and resident in Sweden by 17 May 2021. Individuals in the general population cohort who died or emigrated before 17 May 2021 were excluded. At the time of this study, data availability extended to 17 May 2021 due to the data delivery processes which defined the study period.
Outcome
The outcome in this study was having received at least one dose of a Covid-19 vaccine as of 17 May 2021. Vaccination data was obtained by linkage to the National Vaccination Register (NVR).
Exposure and covariates
The sociodemographic characteristics included in this study were (with units of measurements for the quantitative variables and the categories for the qualitative variables in parentheses): age (years), sex (male/female), income (SEK per year), household composition (living alone/cohabiting), and country of birth. Data on sociodemographic variables were obtained from the National Register of the Total Population (RTB) and the Statistics Sweden Longitudinal Integrated Database for Health Insurance and Labour Market Studies (LISA). The household disposable income divided by the weighted number of members in the household was provided through the LISA register and categorised into tertiles. Later, the income variable was dichotomised into medium-high (2nd and 3rd tertile) and low (1st tertile) and the quantitative variable age was also used as a dichotomised variable (60–74 years and ≥ 75 years) for the adjusted and the intersectional analyses to avoid getting too few individuals in some of the groups.
The country of birth was categorised into three groups: Sweden, High-income countries (HIC), and Low-middle income countries (LMIC), according to the World Bank classification.25 Household composition was grouped into those who were cohabiting (married, in a registered partnership, or had children in common), or living alone (other forms of households). To improve the understanding of heterogeneity in population groups toward vaccination, an intersectional analysis approach was employed. The intersectional multi-categorical variable consisted of all potential combinations of the above-mentioned sociodemographic variables (i.e., age (two categories), sex (two categories), income (two categories), country of birth (three categories), and household composition (two categories), resulting in 48 strata.
Covariates included region of residence, having a history of Covid-19 (yes/no) and prior comorbidity diagnosed in 2015–2019. Prior comorbidities assessed were cardiovascular diseases (ICD-10 codes: I00-I99), respiratory diseases (J00-J99), psychiatric diseases (F20-F39), cancer (C00-C97), and diabetes (E10, E11, E13, E14), based on hospitalisations and specialist outpatient visits obtained from the National Patient Register (NPR). Data on the history of Covid-19 was obtained from SmiNet (the Public Health Agency of Sweden's national register including all positive SARS-CoV-2 polymerase chain reaction (PCR) test results), or diagnoses in the NPR. Region of residence was categorised into regions with a major city (i.e., Stockholm Region, Västra Götaland Region, and Skåne Region) or not (all other regions).
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Statistical analyses
Differences in means and proportions of sociodemographic factors, comorbidities, region of residence, and history of Covid-19, were analysed by vaccination status with t-test and chi-square tests with a statistical significance level of 5%. Sample weights based on age and sex were used to make estimates based on the study population representative of the Swedish population. Absolute numbers of study subjects are presented unweighted, while all means and proportions are weighted. The statistical packages used included IBM SPSS Statistics for windows version 26.0 and Stata 16.0 (StataCorp. TX, USA).
Logistic regression models (weighted) were used to calculate crude and multivariate-adjusted odds ratios (ORs) with 95% confidence intervals (95% CI) of non-vaccination in several different models. Model 1 was the crude model for age only. Model 2 included crude models for all other sociodemographic exposures one at a time. Model 3 incorporated mutual adjustment for all included exposures. Model 4 had additional adjustment for residing in a region with a major city, history of Covid-19, and comorbidities. Lastly, model 5 included the 48 intersectional strata. There were only very few missing data (n = 42). Subjects with missing data were not included in the models holding these variables. Assessment of the discriminatory accuracy (DA) was made by calculating the area under the receiver operating characteristics curve (AUC) for the models.20, 21, 22 The DA was classified as very weak (AUC 0·5–0·6), weak/moderate (AUC 0·6–0·7), strong (AUC 0·7–0·8) or very strong (AUC >0·8).20, 21, 22
Results
The study population included 350,805 individuals aged ≥ 60 years and the uptake of the first dose of a Covid-19 vaccine was 87·2% (Table 1 ). Individuals in the non-vaccinated group were significantly (p < 0.05) more often younger (78·5% vs. 57·8%), males (49·6% vs. 47·2%), had a lower income (47·5% vs. 30·1%), were born outside Sweden (34·9% vs. 12·2%), lived alone (58·4% vs. 43·1%), lived in a region with a major city (60·9% vs. 46·7%), and/or had a history of Covid-19 (11·3% vs. 5·1%), compared to the vaccinated group. A history of cardiovascular disease, respiratory disease, cancer, or diabetes was more common among the vaccinated, however, psychiatric disease (4·4% vs. 2·8%) was slightly more common among the non-vaccinated.
Table 1.
Distribution of sociodemographic factors, history of Covid-19 and comorbidities in the Swedish population aged ≥ 60 years, by having received at least one dose of Covid-19 vaccination or not, presenting as mean (standard deviation) or percentage.
| Characteristics | Vaccinateda | Non-vaccinateda | p-value | Total |
|---|---|---|---|---|
| (N = 312 915; 87·2%) | (N = 37 890; 12·8%) | N = 350 805 | ||
| Age, mean (SD) | 71·3 (8·6) | 66·9 (7·9) | <0·001 | 70·7 (8·7) |
| Age group | ||||
| 60–74 years | 57·8% | 78·5% | <0·001 | 60·4% |
| ≥ 75 years | 42·2% | 21·5% | 39·6% | |
| Males | 47·2% | 49·6% | <0·001 | 47·5% |
| Incomeb | ||||
| High | 36·4% | 25·4% | <0·001 | 35·0% |
| Medium | 33·5% | 27·1% | 32·7% | |
| Low | 30·1% | 47·5% | 32·3% | |
| Living alone | 43·1% | 58·4% | <0·001 | 45·0% |
| Country of birthc | ||||
| Sweden | 87·8% | 65·1% | <0·001 | 84·9% |
| HIC | 7·6% | 14·4% | 8·5% | |
| LMIC | 4·6% | 20·5% | 6·6% | |
| History of Covid-19 | 5·1% | 11·3% | <0·001 | 5·9% |
| Living in a region holding a major Swedish city | 46·7% | 60·9% | <0·001 | 48·5% |
| Comorbidities (2015–2019) | ||||
| Cardiovascular disease | 33·7% | 23·9% | <0·001 | 32·4% |
| Respiratory disease | 12·5% | 11·0% | <0·001 | 12·3% |
| Cancer | 13·0% | 7·0% | <0·001 | 12·2% |
| Psychiatric disease | 2·8% | 4·4% | <0·001 | 3·0% |
| Diabetes | 8·5% | 7·2% | <0·001 | 8·3% |
All proportions weighted to reflect the Swedish population age and sex distribution in the ≥60 age group.
Income: Disposable income of a family divided by the number of family members; High: 3rd tertile, Medium: 2nd tertile, Low: 1st tertile.
Country of birth: Sweden, HIC: High Income Countries; LMIC: Low-Middle Income Countries.
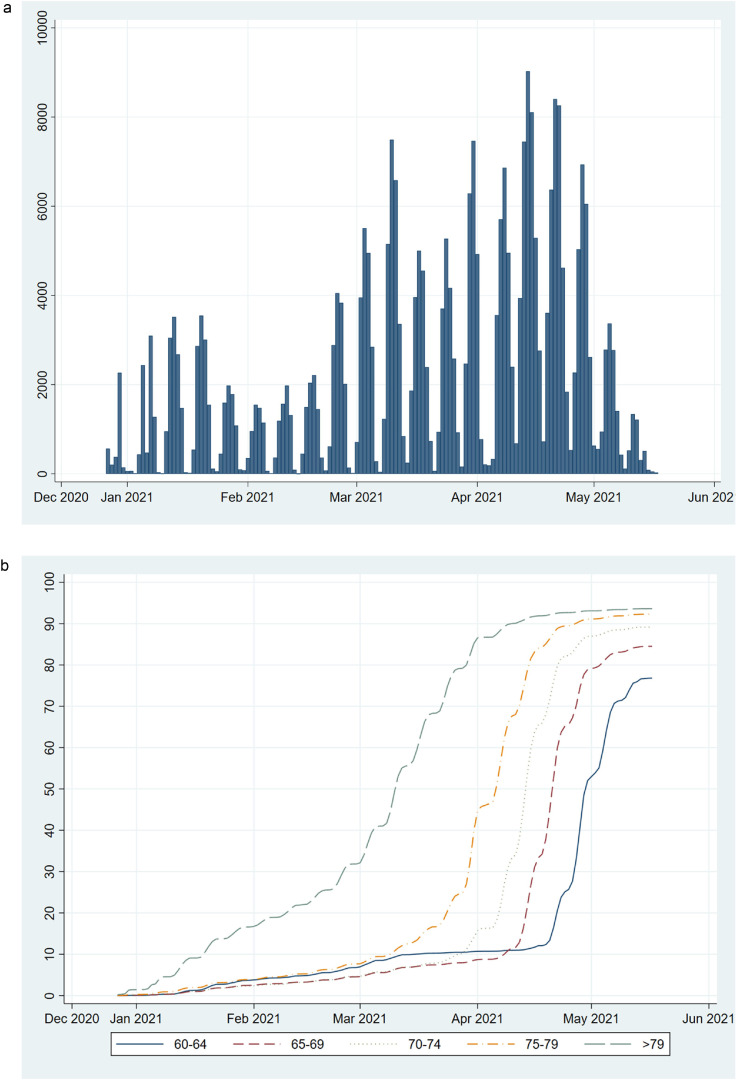
Vaccinations started to pick up in January 2021, with daily vaccinations peaking in mid-April (Figure 1a); the older age groups were vaccinated before the younger (Figure 1b). Coverage steadily increased in all age groups until late April and the beginning of May 2021, earlier in older than in younger age groups, when it started to level off (Figure 1b).
Figure 1.
Daily number of individuals aged ≥ 60 years in the study population who have received at least one dose of a Covid-19 vaccine since the start of vaccination in late December 2020 until 17 May 2021, in total (a) and cumulative uptake (%) of vaccination by age group (b; solid line (blue)=60–64 years; short dashed line (red)=65–69 years; dotted line (grey) =70–74 years; dotted and dashed line (yellow)=75–79 years; long dashed line (green)=>79 years).
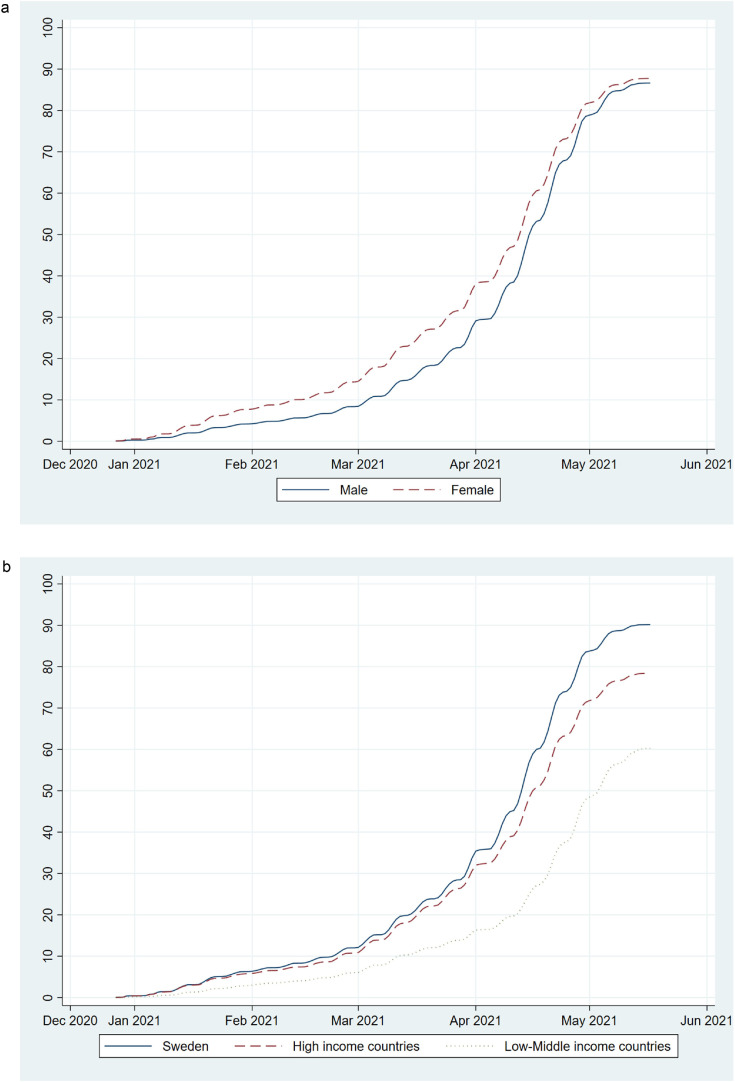
More women than men were vaccinated in the early stages (i. e., December 2020 until March 2021) of the vaccination program (Figure 2 a). Both in the early stages and during the more intense vaccination period (i.e., April 2021), relatively fewer individuals belonging to groups born outside of Sweden were vaccinated, most pronounced among individuals born in low- or middle-income countries. This gap continuously widened and was most pronounced just before the cohort's overall vaccination rates declined, after which, at the end of the study period, relatively more individuals from low- or middle-income countries were vaccinated (Figure 2b).
Figure 2.
Cumulative uptake (%) of Covid-19 vaccination by sex (a; solid line (blue)=Male, dashed line (red)=Female), and by country of birth (b; solid line (blue)=Sweden, dashed line (red)=High income countries, dotted line (grey)=Low- or middle-income countries) in the Swedish population aged ≥ 60 years since the start of vaccination in late December 2020 until 17 May 2021.
Vaccination uptake differed by sociodemographic factors with lower age, being male, having low income, being born outside Sweden (most pronounced for low- or middle-income countries), and living alone all being factors associated with lower uptake of a Covid-19 vaccine and higher ORs of non-vaccination (Table 2 ). For example, in the crude model, having a low income was associated with an OR of 2·10 (95% CI: 2·05–2·15) for non-vaccination compared to having medium or high income. Corresponding ORs among those born in HIC or LMIC were 2·54 (95% CI: 2·44–2·63) and 6·05 (95% CI: 5·85–6·26), respectively, compared to being born in Sweden.
Table 2.
Percentage vaccinated among individuals in the Swedish population aged ≥ 60 years, and adjusted odds ratios (ORs) with 95% Confidence Interval (CI) for non-vaccination by sociodemographic factors i.e., age, sex, income, country of birth, and household composition.
| Characteristics | Vaccinatedg | Model 1c | Model 2d | Model 3e | Model 4f |
|---|---|---|---|---|---|
| % | ORh 95%CI | ORh 95%CI | ORh 95%CI | ORh 95%CI | |
| Age group | |||||
| 60–74 years | 83 | 1·00 | (· ·) | 1·00 | 1·00 |
| ≥ 75 years | 93 | 0·38 (0·37–0·39) | 0·32 (0·31–0·33) | 0·35 (0·34–0·36) | |
| Sex | |||||
| Male | 87 | 1·00 | 1·00 | 1·00 | |
| Female | 88 | 0·91 (0·89–0·93) | 0·84 (0·82–0·86) | 0·81 (0·79–0·84) | |
| Incomea | |||||
| Medium/High | 90 | 1·00 | 1·00 | 1·00 | |
| Low | 81 | 2·10 (2·05–2·15) | 1.99 (1·94–2·05) | 2·16 (2·10–2·23) | |
| Country of birthb | |||||
| Sweden | 90 | 1·00 | 1·00 | 1·00 | |
| HIC | 78 | 2·54 (2·44–2·63) | 2·29 (2·21–2·38) | 2·15 (2·07–2·23) | |
| LMIC | 60 | 6·05 (5·85–6·26) | 4·44 (4·29–4·61) | 3·86 (3·71–4·00) | |
| Living alone | |||||
| No | 90 | 1·00 | 1·00 | 1·00 | |
| Yes | 83 | 1·85 (1·81–1·89) | 1·66 (1·62–1·71) | 1·64 (1·59–1·68) | |
| AUC (ROC-curve) | 0·62 (0·62–0·63) | (· ·)i | 0·73 (0·72–0·73) | 0·74 (0·74–0·75) |
Income: Disposable income of a family divided by the number of family members; Medium/High: 2nd and 3rd tertile, Low: 1st tertile.
Country of birth: Sweden, HIC: High Income Countries; LMIC: Low-Middle Income Countries.
Model 1: Only including age in the model.
Model 2: Crude odds ratios models for each sociodemographic factor.
Model 3: Mutually adjusted.
Model 4: Model 3 + adjusted for living in region with major city, comorbidities, and having a history of Covid-19.
Proportions weighted to reflect the Swedish population age and sex distribution in the ≥ 60 years age group.
Odds ratios (ORs) and 95% Confidence Interval (CI).
AUC Model 2: Sex 0·50 (0·50–0·51); Income 0·57 (0·56–0·57); Country of birth 0·62 (0·61–0·62); Living alone 0·56 (0·56–0·57).
In the mutually adjusted model 3, the ORs of non-vaccination were generally reduced relative to the crude, especially for those born in LMIC compared to those born in Sweden (OR 4·44 (95% CI: 4·29–4·61)), suggesting that part of these differences was related to and explained by other sociodemographic factors. There was increased discriminatory accuracy by including all sociodemographic factors as compared to the model only including age (i.e., an AUC increase from 0·62 to 0·73). After additional adjustment for region, comorbidities, and history of Covid-19 in model 4, ORs for sociodemographic differences in vaccination rates were only slightly affected, and the AUC showed a slight increase (to 0·74). Additional adjustment for educational level did not further affect these results (data not shown).
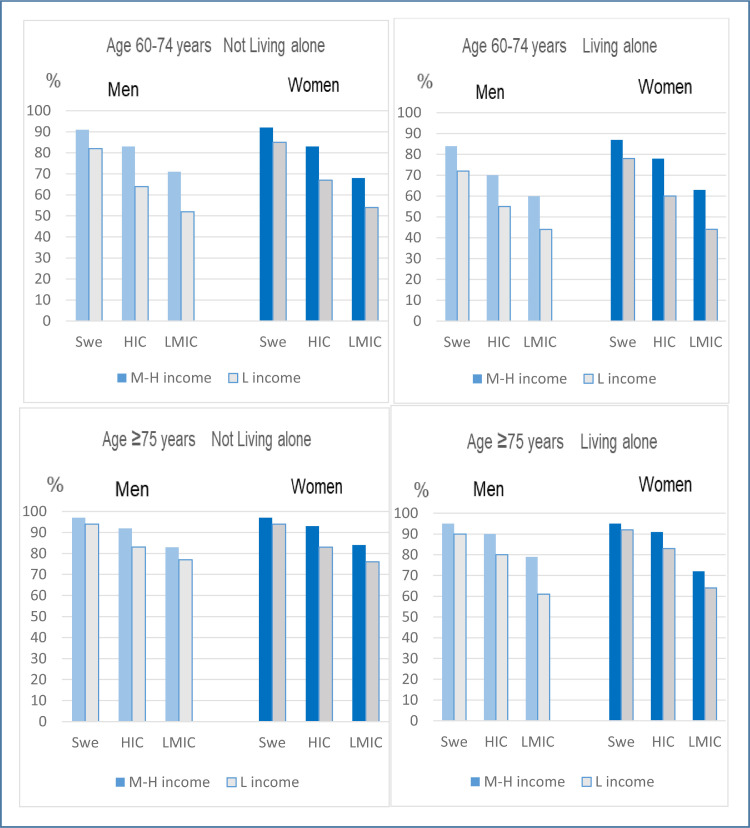
The analysis of 48 intersectional strata, using a multi-categorical variable for all combinations of the sociodemographic variables included in Table 2, showed large variations in the uptake of Covid-19 vaccination ranging from 44% to 97%, depending on other socioeconomic dimensions (Figure 3 ). For example, among those with low income the uptake of vaccination ranged from 44% to 94%. The ten combinations with the lowest uptake of vaccination were generally characterised by lower age (nine of ten strata), having a low income (eight of ten strata), and being born outside Sweden (most pronounced for those born in a low- or middle-income country) (Table 3 ). The majority of these strata included living alone (seven of ten). The ten combinations with the highest uptake of vaccination were most often characterised by higher age, by medium/high income (seven of ten strata), and by being born in Sweden (eight of ten strata). Although the variability across strata in this model provides an important additional layer of multi-dimensional socioeconomic insight, the AUC for this model was not higher than for Model 3, which would be expected if the simpler model with only main effects did not fit data well, thus indicating that there was no evident intersectional interaction between the sociodemographic variables (Table 3).
Figure 3.
Proportion vaccinated (i.e., having received at least one dose of a Covid-19 vaccine) in 48 intersectional strata defined by sex, country of birth (Swe: Sweden, HIC: High income countries, LMIC: Low-middle income countries), income (L income: low income [1st tertile], M-H income: Medium/high income [2nd and 3rd tertile]), age (60–74 years: top panels, ≥75 years: bottom panels), and cohabiting status (not living alone: left panels, living alone: right panels). The proportions are weighted to reflect the Swedish population age and sex distribution in the ≥60 age groups.
Table 3.
Percentage vaccinated among individuals in the Swedish population aged ≥ 60 years, and adjusted odds ratios (ORs) with 95% Confidence Interval (CI) for non-vaccination related to intersectional strata based on sociodemographic factors i.e., age, sex, income, country of birth, and household composition. The twenty intersectional strata with the lowest and highest ORs, respectively, of non-vaccination are marked in grey.
| Intersectional strata | N | Vaccinatede | Model 5 |
|---|---|---|---|
| % | OR (95% CI)f | ||
| Men/Med-High inc/Co/Born Swe/Age ≥75 | 35652 | 97 | 0·30 (0·27–0·33) |
| Women/Med-High inc/Co/Born Swe/Age ≥75 | 20289 | 97 | 0·32 (0·29–0·35) |
| Women/Med-High inc/LA/Born Swe/Age ≥75 | 16142 | 95 | 0·49 (0·44–0·55) |
| Men/Med-High inc/LA/Born Swe/Age ≥75 | 15989 | 95 | 0·57 (0·51–0·63) |
| Women/Low inc/Co/Born Swe/Age ≥75 | 7312 | 94 | 0·61 (0·54–0·70) |
| Men/Low inc/Co/Born Swe/Age ≥75 | 13384 | 94 | 0·63 (0·56–0·71) |
| Women/Med-High inc/Co/Born HIC/Age ≥75 | 1625 | 93 | 0·71 (0·56–0·89) |
| Women/Med-High inc/Co/Born Swe/Age 60–74 | 32985 | 92 | 0·89 (0·84–0·93) |
| Men/Med-High inc/Co/Born HIC/Age ≥75 | 2863 | 92 | 0·84 (0·69–1·02) |
| Women/Low inc/LA/Born Swe/Age ≥75 | 50940 | 92 | 0·81 (0·76–0·86) |
| Women/Med-High inc/LA/Born HIC/Age ≥75 | 1402 | 91 | 0·93 (0·71–1·22) |
| Men/High-Med incb/Coc/Born Swed/age 60–74 | 35173 | 91 | 1·00a |
| Men/Low inc/LA/Born Swe/Age ≥75 | 20629 | 90 | 1·12 (1·04–1·22) |
| Men/Med-High inc/LA/Born HIC/Age ≥75 | 1209 | 90 | 1·13 (0·85–1·51) |
| Women/Med-High inc/LA/Born Swe/Age 60–74 | 15460 | 87 | 1·53 (1·45–1·62) |
| Women/Low inc/Co/Born Swe/Age 60–74 | 2667 | 85 | 1·69 (1·52–1.89) |
| Men/Med-High inc/LA/Born Swe/Age 60–74 | 15523 | 84 | 1·83 (1·73–1·93) |
| Women/Med-High inc/Co/Born LMIC/Age ≥75 | 214 | 84 | 1·89 (1·23–2·89) |
| Women/Low inc/LA/Born HIC/Age ≥75 | 6233 | 83 | 1·98 (1·78–2·19) |
| Men/Low inc/Co/Born HIC/Age ≥75 | 1323 | 83 | 2·02 (1·63–2·49) |
| Men/ Med-High inc /Co/Born LMIC/Age ≥75 | 606 | 83 | 1·97 (1·50–2·58) |
| Women/Low inc/Co/Born HIC/Age ≥75 | 804 | 83 | 2·02 (1·60–2·55) |
| Men/Med-High inc/Co/Born HIC/age 60–74 | 2322 | 83 | 2·09 (1·87–2·33) |
| Women/Med-High inc/Co/Born HIC/Age 60–74 | 2865 | 83 | 2·06 (1·87–2·28) |
| Men/Low inc/Co/Born Swe/Age 60–74 | 2226 | 82 | 2·20 (1.97–2·45) |
| Men/Low inc/LA/Born HIC/Age ≥75 | 2240 | 80 | 2·53 (2·15–2·96) |
| Men/Med-High inc/LA/Born LMIC/Age ≥75 | 167 | 79 | 2·64 (1·61–4·31) |
| Women/Low inc/LA/Born Swe/Age 60–74 | 9393 | 78 | 2·71 (2·55–2·88) |
| Women/Med-High inc/LA/Born HIC/Age 60–74 | 1686 | 78 | 2·86 (2·55–3·21) |
| Men/Low inc/Co/Born LMIC/Age ≥75 | 1183 | 77 | 2·91 (2·41–3·52) |
| Women/Low inc/Co/Born LMIC/Age ≥75 | 413 | 76 | 3·12 (2·39–4·06) |
| Women/High-Med inc/LA/Born LMIC/Age ≥75 | 130 | 72 | 3·72 (2·24–6·17) |
| Men/Low inc/LA/Born Swe/Age 60–74 | 8106 | 72 | 3·90 (3·68–4·14) |
| Men/Med-High inc/Co/Born LMIC/Age 60–74 | 2620 | 71 | 4·10 (3·75–4·49) |
| Men/Med-High inc/LA/Born HIC/Age 60–74 | 1202 | 70 | 4·28 (3·78–4·85) |
| Women/Med-High inc/Co/Born LMIC/Age 60–74 | 1755 | 68 | 4·58 (4·14–5·08) |
| Women/Low inc/Co/Born HIC/Age 60–74 | 575 | 67 | 4·95 (4·17–5·87) |
| Women/Low inc/LA/Born LMIC/Age ≥75 | 2429 | 64 | 5·54 (4·92–6·24) |
| Men/Low inc/Co/Born HIC/Age 60–74 years | 598 | 64 | 5·67 (4·80–6·70) |
| Women/Med-High inc/LA/Born LMIC/Age 60–74 years | 1092 | 63 | 5·91 (5·23–6·68) |
| Men/Low inc/LA/Born LMIC/Age ≥75 years | 1047 | 61 | 6·28 (5·22–7·56) |
| Women/Low inc/LA/Born HIC/Age 60–74 years | 1853 | 60 | 6·47 (5·87–7·13) |
| Men/Med-High inc/LA/Born LMIC/Age 60–74 years | 784 | 60 | 6·64 (5·76–7·65) |
| Men/Low inc/LA/Born HIC/Age 60–74 years | 1272 | 55 | 8·05 (7·18–9·01) |
| Women/Low inc/Co/Born LMIC/Age 60–74 years | 1229 | 54 | 8·34 (7·44–9·34) |
| Men/Low inc/Co/Born LMIC/Age 60–74 years | 1612 | 52 | 8·94 (8·08–9·90) |
| Women/Low inc/LA/Born LMIC/Age 60–74 years | 2074 | 44 | 12·32 (11·26–13·50) |
| Men/Low inc/LA/Born LMIC/Age 60–74 years | 1466 | 44 | 12·54 (11·25–13·94) |
| AUC (ROC-curve) | 0·73 (0·72–0·73) |
Reference group.
Income: Disposable income of a family divided by the number of family members; Medium/High: 2nd and 3rd tertile, Low: 1st tertile.
Cohabiting (Co), Living alone (LA).
Country of birth: Sweden; HIC: High-income country; LMIC: Low-middle income country.
Proportions weighted to reflect the Swedish population age and sex distribution in the ≥60 age group.
Odds ratios (ORs) and 95% confidence intervals (CI).
Discussion
In this nationwide observational register-based study on individuals aged 60 years or older representative of the general population in Sweden, we showed consistent social inequalities in Covid-19 vaccination uptake. Low income, male sex and being born outside of Sweden were all associated with a lower vaccination uptake in the crude and multivariable-adjusted models. Adjustment for region, history of Covid-19 and comorbidities did not change these associations, and thus cannot explain the social patterning in vaccination uptake. The intersectional analyses showed large variations in vaccination coverage in cross-classified sociodemographic subgroups with marked differences also within sociodemographic groups.
Our findings are consistent with the few published studies and reports on sociodemographic differences in the uptake of Covid-19 vaccination. In accordance with reports produced by the Public Health Agency of Sweden,12 we found that vaccination rates vary in relation to country of birth. However, our results extending beyond the early period of vaccination that has largely been the focus of the few studies in this field, show that this association persists across additional strata of sociodemographic characteristics, and after adjusting for region, comorbidities, and having a history of Covid-19. We confirm results from English studies including older adults showing that individuals in ethnic minority groups and those in a less advantaged socioeconomic position were less often vaccinated.16 – 18 Additionally, in another study (i.e., preprint) on primary-care patients, those with a pre-existing medical condition were equally or more likely to be vaccinated unless they had severe mental illness.26 This finding is in line with the results from our study, showing that cardiovascular disease, respiratory disease, cancer and diabetes was more prevalent among the vaccinated, and psychiatric disease was more common among the non-vaccinated. Earlier surveys of the potential acceptance of a Covid-19 vaccine have shown a more positive attitude in higher educated7, 8, 9 and higher income groups,7 but also among women.7 However, women were only marginally more often vaccinated compared to men in our study. Consistent with the results in the mentioned surveys showing large disparities in vaccine hesitancy across ethnic groups9, 10, 11 the lowest Covid-19 vaccination uptake in the present study was seen in individuals born in low-or middle-income countries, of which only 60% had received vaccination compared to individuals born in Sweden.
There were four sequential vaccination stages in Sweden and people at older age were prioritized for vaccination, which we also could confirm showing that older individuals were vaccinated before the younger. Taking part of the Covid-19 vaccination program is an individual decision and different reasons for non-participation have been reported. These mostly include uncertainties and fears connected to side-effects, lack of information, wanting to wait until later, lack of institutional trust or not believing in the risk of becoming seriously ill from Covid-19.27 , 28 While vaccination against Covid-19 is free of charge in Sweden and despite easily accessible vaccination, there are likely also issues related to availability in certain settings. To increase vaccination acceptance and uptake, earlier research has indicated that communication and interventions should be targeted to a range of sub-populations with different socio-cultural and educational characteristics and that there is no “one size fits all” approach to address vaccine hesitancy.11 According to Bagasra et al., such strategies should encompass reduction of potential structural barriers to the uptake of vaccination through feasible access to vaccination sites, reduced language barriers, open communication about concerns of side effects, and strategies to increase trust in the scientific community.28 In Sweden, various initiatives to lower the threshold for vaccination have been suggested and partly implemented, aiming both to increase accessibility (e.g., drop-in vaccination at schools and workplaces, collaborations with assemblies and unions, targeting geographical areas with low vaccination rates) as well as education efforts and outreach activities (e.g., letters, text messages, cultural interpreters, and social media campaigns).29
Previous research in other areas using an intersectional analysis approach have contributed to a more nuanced understanding of sociodemographic differences when studying self-rated health, but also when looking at the dispensation of antibiotics and smoking.20, 21, 22 To our knowledge, the intersectional approach has not been used in vaccination studies when trying to understand and dissect disparities. An intersectional analysis approach presenting multiple cross-classified strata might more easily be interpreted and useful in public health compared to the use of e.g., interaction terms. The results from our analyses more clearly showed wider differences across groups defined by an intersectional classification than in the analyses using one-dimensional classification. As there was no clear indication of statistical interaction, we regard this as being the result of additive effects of the sociodemographic factors that defined the intersectional strata.20 While the overall pattern showed that those with the lowest uptake of Covid-19 vaccination were characterised by lower age, being born outside of Sweden, or on low income, the proportion vaccinated connected to the intersectional strata holding these factors varied highly depending on the associations to other sociodemographic factors in the strata. Thus, intersectional stratification considering several overlapping social dimensions at the same time, adds information on the sociodemographic distribution of vaccination uptake that could be useful for targeting specific groups. The discriminatory accuracy of the intersectional model was modest to strong, indicating that the variables included in the model were reasonably good but not excellent at discriminating vaccinated individuals from non-vaccinated.
This study has several strengths. Firstly, it includes a large representative sample of the Swedish population; the large study population also allows for intersectional analyses using multiple strata. Secondly, all data are register-based with high quality and completeness, resulting in very little missing data. The vaccination data in particular are comprehensive, since reporting to the vaccination register for Covid-19 is mandatory and regulated by law.30 This is of particular importance for determining the extent of non-vaccination in different population groups. Thirdly, our dataset includes information on several sociodemographic characteristics, as well as potential confounding factors. However, despite the large sample size, it was sometimes necessary to combine these factors into broader categories. This may have caused some of the finer differences in non-vaccination between groups to remain undetected. Another minor weakness of the study design we chose is that individuals in the cohort who died or emigrated before 17 May 2021 were excluded from the analyses, and some of these may have been vaccinated before death or emigration. However, in our study, only very few individuals died or emigrated after vaccination. Lastly, the results indicate that some groups with lower vaccination rates participated to a greater extent towards the end of the study period when vaccination rates had substantially declined, indicating a potential reduction in differences in vaccination rate between sociodemographic groups over time. Due to the data delivery processes, data availability only extends to 17 May 2021, and we are unable to assess if vaccination rates in certain groups indeed increased over time after this date.
In conclusion, the results from the present study, addressing a general population sample representative of the older adults in the Swedish population, revealed large sociodemographic differences in Covid-19 vaccination uptake, similar to those seen in the few previous studies made within this field of research. In addition, the intersectional analyses which has not previously been used in vaccination studies when trying to understand and dissect sociodemographic disparities, provides an important additional layer of insight and contributes to a better understanding of the complexity in the uptake of vaccination. Finally, since the Covid -19 vaccination program in Sweden, as well as worldwide, now addresses the whole adult population and since sociodemographic disparities in vaccination uptake may be even more pronounced in younger age groups, further research in this field is now urgently needed.
Contributors
MS: Literature search, Conceptualization, Methodology, Data analysis, Data interpretation, Writing – Original Draft, Writing – Review & Editing. LL: Literature search, Data interpretation, Writing – Review & Editing. CN: Conceptualization, Methodology, Data analysis, Data interpretation, Writing – Review & Editing. HL: Data Curation, Software, Visualization, Data analysis, Writing – Review & Editing. AS: Conceptualization, Methodology, Data interpretation, Writing – Review & Editing. SL: Data interpretation, Writing – Review & Editing. MG: Investigation, Funding acquisition, Writing – Review & Editing. NH: Conceptualization, Methodology, Data interpretation, Writing – Review & Editing. MR: Conceptualization, Methodology, Data analysis, Data interpretation, Writing – Review & Editing. FN: Investigation, Funding acquisition, Conceptualization, Methodology, Data interpretation, Writing – Review & Editing. MR and CN accessed and verified the underlying data. All authors approved the final version of the manuscript.
Declaration of interests
Dr. Nyberg reports prior employment at AstraZeneca until 2019, and ownership of some AstraZeneca shares. Dr. Gisslén reports personal fees (DSMB) from AstraZeneca, personal fees from Gilead, personal fees from GSK/ViiV, personal fees from MSD, other from Gilead, other from GSK/ViiV, personal fees from Biogen, personal fees from Novocure, personal fees from Amgen, personal fees from Novo Nordisk, outside the submitted work. Dr. Hammar reports ownership of AstraZeneca shares and consulting with Sobi. Dr Leach reports consulting for Scandinavian Biopharma. MD. Spetz, MD. Lundberg, Dr. Nwaru, Dr. Santosa, Dr. Li, Dr. Rosvall have nothing to disclose.
Acknowledgments
Ethics committee approval
The study has ethical approval from the Swedish Ethics Review Authority (EPM), no. 2020-01800, 2020-05829, 2021-00267, 2021-00829, 2021-02106, 2021-04098.
Data availability statement
The data in this study are pseudonymized individual-level data from Swedish healthcare registers and are not publicly available according to Swedish legislation. They can be obtained from the respective Swedish public data holders on the basis of ethics approval for the research in question, subject to relevant legislation, processes and data protection.
Acknowledgments
This study was made possible by funding from the SciLifeLab National COVID-19 Research Program, financed by the Knut and Alice Wallenberg Foundation, and the Swedish Research Council. The underlying SCIFI-PEARL study currently has basic funding by a grant from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement and from FORMAS (Research Council for Environment, Agricultural Sciences and Spatial Planning), a Swedish Research Council for Sustainable Development.
References
- 1.Sveriges Kommuner och Regioner [SKR]. Regionernas planering avseende vaccinering mot covid-19, delrapport 6 [The planning regarding vaccination against Covid-19 for regions, report 6]. Stockholm, Sweden, SKR; 2021. https://skr.se/download/18.5bb54e0c179a302981232fd/1621980314035/Regionernas_planering_%20vaccinering_covid-19_delrapport%206.pdf
- 2.Folkhälsomyndigheten [FHM], Statistik för vaccination mot Covid-19 [Statistics regarding vaccination against Covid-19]. Available online: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/statistik-over-registrerade-vaccinationer-covid-19/. Accessed November 8 2021
- 3.Folkhälsomyndigheten [FHM]. Nationell plan för vaccination mot Covid-19 [National plan for vaccination against Covid-19]. Stockholm, Sweden, FHM; 2021. https://www.folkhalsomyndigheten.se/contentassets/43a1e203f7344a399367b816e2c7144c/nationell-plan-vaccination-covid-19-delrapport-3.pdf
- 4.Nagata J.M., Hernández-Ramos I., Kurup A.S., Albrecht D., Vivas-Torrealba C., Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13:388. doi: 10.1186/1471-2458-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocquier A., Ward J., Raude J., Peretti-Watel P., Verger P. Socioeconomic differences in childhood vaccination in developed countries: a systematic review of quantitative studies. Expert Rev Vaccines. 2017;16:1107–1118. doi: 10.1080/14760584.2017.1381020. [DOI] [PubMed] [Google Scholar]
- 6.Baeyens J.P., Lang P., Michel J. Willingness to vaccinate and to be vaccinated in adults. Aging Clin Exp Res. 2009;21:244–249. doi: 10.1007/BF03324913. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus J.V., Ratzan S., Palayew A., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanath K., Bekalu M., Dhawan D., Pinnamaneni D., Lang J., McLoud R. Individual and social determinants of COVID-19 vaccine uptake. BMC Public Health. 2021;21:818. doi: 10.1186/s12889-021-10862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C., Tu P., Terry T.C. Moving the needle on racial disparity: COVID-19 vaccine trust and hesitancy. Vaccine. 2022;40:5–8. doi: 10.1016/j.vaccine.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams L., Flowers P., McLeod J., Young D., Rollins L., The Catalyst Project Team Social patterning and stability of intention to accept a COVID-19 vaccine in Scotland: will those most at risk accept a vaccine? Vaccines. 2021;9:17. doi: 10.3390/vaccines9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folkhälsomyndigheten [FHM]. Covid-19 vaccinationstäckning och födelseland [COVID-19 vaccination coverage and country of birth]. Stockholm, Sweden, FHM; 2021. https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/c/covid-19-vaccinationstackning-och-fodelseland-/?pub=92033
- 13.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drefahl S., Wallace M., Mussino E., et al. A population-based cohort study of socio-demographic risk factors for COVID-19 deaths in Sweden. Nat Commun. 2020;11:5097. doi: 10.1038/s41467-020-18926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health organization (WHO) WHO Regional Office for Europe; 2020. Health Inequity and the Effects of COVID19: Assessing, Responding to and Mitigating the Socioeconomic Impact on Health to Build a Better Future. Copenhagen: Available online: https://apps.who.int/iris/bitstream/handle/10665/338199/WHO-EURO-2020-1744-41495-56594-eng.pdf. Accessed November 3 2021. [Google Scholar]
- 16.Nafilyan V., Dolby T., Razieh C., et al. Sociodemographic inequality in COVID-19 vaccination coverage among elderly adults in England: a national linked data study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-053402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry M., Akbari A., Cottrell S., et al. Inequalities in coverage of COVID-19 vaccination: a population register based cross-sectional study in Wales, UK. Vaccine. 2021;39:6256–6261. doi: 10.1016/j.vaccine.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glampson B., Brittain J., Kaura A., et al. Assessing COVID-19 vaccine uptake and effectiveness through the north west London vaccination program: retrospective cohort study. JMIR Public Health Surveill. 2021;7:e30010. doi: 10.2196/30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marmot M. Social determinants of health inequalities. Lancet. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 20.Wemrell M., Karlsson N., Perez Vicente R., Merlo J. An intersectional analysis providing more precise information on inequities in self-rated health. Int J Equity Health. 2021;20:54. doi: 10.1186/s12939-020-01368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wemrell M., Lenander C., Hansson K., Perez R.V., Hedin K., Merlo J. Socio-economic disparities in the dispensation of antibiotics in Sweden 2016-2017: an intersectional analysis of individual heterogeneity and discriminatory accuracy. Scand J Public Health. 2021;18 doi: 10.1177/1403494820981496. 1403494820981496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelsson Fisk S., Lindström M., Perez-Vicente R., Merlo J. Understanding the complexity of socioeconomic disparities in smoking prevalence in Sweden: a cross-sectional study applying intersectionality theory. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-042323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyberg F., Franzén S., Lindh M., et al. Swedish Covid-19 investigation for future insights – a population epidemiology approach using register linkage (SCIFI-PEARL) Clin Epidemiol. 2021;13:649–659. doi: 10.2147/CLEP.S312742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson J.F., Otterblad-Olausson P., Pettersson B.U., Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The World Bank. World Bank country and lending groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519. Accessed November 3 202
- 26.MacKenna B, Curtis HJ, Morton CE, et al. Trends, regional variation, and clinical characteristics of COVID-19 vaccine recipients: a retrospective cohort study in 23.4 million patients using OpenSAFELY. medRxiv [preprint] 2021 https://www.medrxiv.org/content/10.1101/2021.01.25.21250356v1
- 27.Folkhälsomyndigheten [FHM]. Acceptans för Covid-19 vaccination [Acceptance of the Covid-19 vaccination]. Stockholm, Sweden, FHM; 2021. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/acceptans-for-vaccination-mot-covid-19/resultat-aprilmaj-2021/
- 28.Bagasra A.B., Doan S., Allen C.T. Racial differences in institutional trust and COVID-19 vaccine hesitancy and refusal. BMC Public Health. 2021;21:2104. doi: 10.1186/s12889-021-12195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Government of Sweden. Tilläggsöverenskommelse om genomförande av vaccinering mot covid-19, 2021, Avseende riktade åtgärder för ökad vaccinationstäckning [Supplementary agreement on implementation of vaccination against Covid-19, 2021, concerning targeting actions for increased coverage of vaccination]. Stockholm, Sweden, The Government of Sweden. 16 Sept 2021. https://www.regeringen.se/4a7325/contentassets/ba89969a45544389b43bce4b6c1885fe/tillaggsoverenskommelse-om-genomforande-av-vaccinering-mot-covid-19-2021-avseende-riktade-atgarder-for-okad-vaccinationstackning.pdf
- 30.Folkhälsomyndigheten [FHM]. Det nationella vaccinationsregistret [The National Vaccination Register]. Available online: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/nationella-vaccinationsregistret/. Accessed November 8 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are pseudonymized individual-level data from Swedish healthcare registers and are not publicly available according to Swedish legislation. They can be obtained from the respective Swedish public data holders on the basis of ethics approval for the research in question, subject to relevant legislation, processes and data protection.